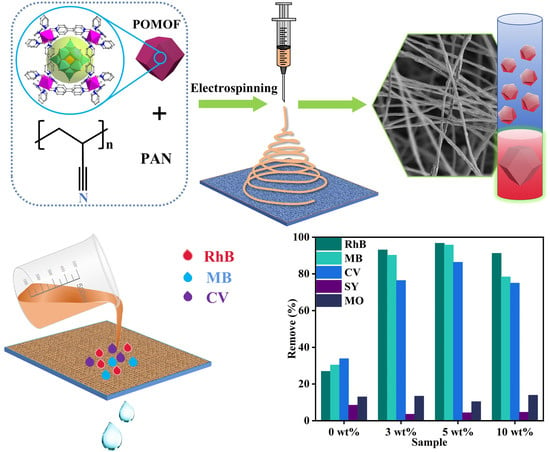
Rapid, Massive, and Green Synthesis of Polyoxometalate-Based Metal–Organic Frameworks to Fabricate POMOF/PAN Nanofiber Membranes for Selective Filtration of Cationic Dyes
Abstract
Share and Cite
Li, J.; Yu, Z.; Zhang, J.; Liu, C.; Zhang, Q.; Shi, H.; Wu, D. Rapid, Massive, and Green Synthesis of Polyoxometalate-Based Metal–Organic Frameworks to Fabricate POMOF/PAN Nanofiber Membranes for Selective Filtration of Cationic Dyes. Molecules 2024, 29, 1493. https://doi.org/10.3390/molecules29071493
Li J, Yu Z, Zhang J, Liu C, Zhang Q, Shi H, Wu D. Rapid, Massive, and Green Synthesis of Polyoxometalate-Based Metal–Organic Frameworks to Fabricate POMOF/PAN Nanofiber Membranes for Selective Filtration of Cationic Dyes. Molecules. 2024; 29(7):1493. https://doi.org/10.3390/molecules29071493
Chicago/Turabian StyleLi, Jianping, Zhaoke Yu, Jiaming Zhang, Chengjie Liu, Qi Zhang, Hongfei Shi, and Dai Wu. 2024. "Rapid, Massive, and Green Synthesis of Polyoxometalate-Based Metal–Organic Frameworks to Fabricate POMOF/PAN Nanofiber Membranes for Selective Filtration of Cationic Dyes" Molecules 29, no. 7: 1493. https://doi.org/10.3390/molecules29071493
APA StyleLi, J., Yu, Z., Zhang, J., Liu, C., Zhang, Q., Shi, H., & Wu, D. (2024). Rapid, Massive, and Green Synthesis of Polyoxometalate-Based Metal–Organic Frameworks to Fabricate POMOF/PAN Nanofiber Membranes for Selective Filtration of Cationic Dyes. Molecules, 29(7), 1493. https://doi.org/10.3390/molecules29071493