Theoretical Evaluation of Fluorinated Resazurin Derivatives for In Vivo Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Fluorination Reactions
2.2. pH Dependence
2.3. Redox Potentials
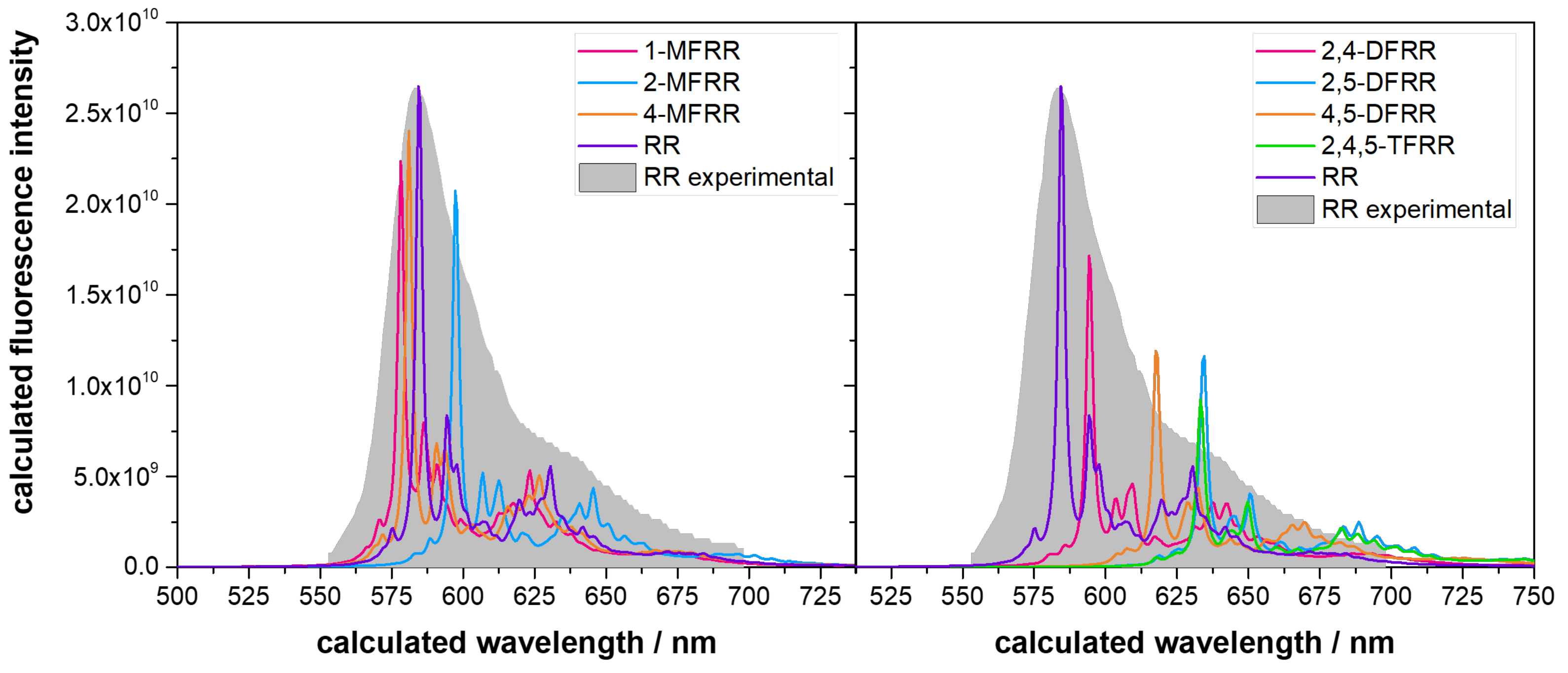
2.4. Fluorescence Spectra
3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansar Ahmed, S.; Gogal, R.M.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.D.; Lancaster, M.V. Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. Am. Biotechnol. Lab. 1993, 11, 48–50. [Google Scholar] [PubMed]
- Kuete, V.; Karaosmanoǧlu, O.; Sivas, H. Anticancer activities of african medicinal spices and vegetables. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 271–297. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in Ccll viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, R.; Oya, M.; Shiomi, T.; Marumo, K.; Okada, Y.; Murai, M. Inhibition of MKP-1 expression potentiates Jnk related apoptosis in renal cancer cells. J. Urol. 2004, 172, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Durrant, D.; Richards, J.E.; Walker, W.T.; Baker, K.A.; Simoni, D.; Lee, R.M. Mechanism of cell death induced by cis-3,4’,5-trimethoxy-3’-aminostilbene in ovarian cancer. Gynecol. Oncol. 2008, 110, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Farinelli, S.; Greene, L. Cell cycle blockers mimosine, ciclopirox, and deferoxamine prevent the death of PC12 cells and postmitotic sympathetic neurons after removal of trophic support. J. Neurosci. 1996, 16, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Yang, S. Inhibition of the p38 MAPK pathway sensitises human colon cancer cells to 5-fluorouracil treatment. Int. J. Oncol. 2011, 38, 1695–1702. [Google Scholar] [CrossRef]
- Yao, K.; Youn, H.; Gao, X.; Huang, B.; Zhou, F.; Li, B.; Han, H. Casein kinase 2 inhibition attenuates androgen receptor function and cell proliferation in prostate cancer cells. Prostate 2012, 72, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- White, M.J.; DiCaprio, M.J.; Greenberg, D.A. Assessment of neuronal viability with Alamar Blue in cortical and granule cell cultures. J. Neurosci. Methods 1996, 70, 195–200. [Google Scholar] [CrossRef]
- Fai, P.B.; Grant, A. A rapid resazurin bioassay for assessing the toxicity of fungicides. Chemosphere 2009, 74, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, S.; Harden, T.J. Bacterial toxicity assessment using Resazurin reduction kinetics: An undergraduate toxicity experiment for the Chemistry-Biology Major. J. Chem. Educ. 1996, 73, 368. [Google Scholar] [CrossRef]
- Zare, M.; Amin, M.M.; Nikaeen, M.; Bina, B.; Pourzamani, H.; Fatehizadeh, A.; Taheri, E. Resazurin reduction assay, a useful tool for assessment of heavy metal toxicity in acidic conditions. Environ. Monit. Assess. 2015, 187, 276. [Google Scholar] [CrossRef] [PubMed]
- Balbaied, T.; Moore, E. Resazurin-based assay for quantifying living cells during alkaline phosphatase (ALP) release. Appl. Sci. 2020, 10, 3840. [Google Scholar] [CrossRef]
- Chen, J.L.; Steele, T.W.J.; Stuckey, D.C. Metabolic reduction of Resazurin; location within the cell for cytotoxicity assays. Biotech. Bioeng. 2018, 115, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Cell Viability Assays; Series Title: Methods in Molecular Biology; Gilbert, D.F., Friedrich, O., Eds.; Springer: New York, NY, USA, 2017; Volume 1601, pp. 1–17. [Google Scholar] [CrossRef]
- Rodríguez-Corrales, J.Á.; Josan, J.S. Resazurin Live Cell Assay: Setup and Fine-Tuning for Reliable Cytotoxicity Results. In Proteomics for Drug Discovery; Series Title: Methods in Molecular, Biology; Lazar, I.M., Kontoyianni, M., Lazar, A.C., Eds.; Springer: New York, NY, USA, 2017; Volume 1647, pp. 207–219. [Google Scholar] [CrossRef]
- Guerin, T.F.; Mondido, M.; McClenn, B.; Peasley, B. Application of resazurin for estimating abundance of contaminant-degrading micro-organisms. Lett. Appl. Microbiol. 2001, 32, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.I.A.; Monteiro, C.A.P.; De Oliveira, W.F.; Santos, B.S.; Fontes, A.; Cabral Filho, P.E. Resazurin-Based Assay to Evaluate Cell Viability After Quantum Dot Interaction. In Quantum Dots; Series Title: Methods in Molecular, Biology; Fontes, A., Santos, B.S., Eds.; Springer: New York, NY, USA, 2020; Volume 2135, pp. 213–221. [Google Scholar] [CrossRef]
- Strotmann, U.; Butz, B.; Bias, W. The Dehydrogenase Assay with Resazurin: Practical Performance as a Monitoring System and Ph-Dependent Toxicity of Phenolic Compounds. Ecotoxicol. Environ. Saf. 1993, 25, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Crane, B.; Hughes, J.P.; Rowley Neale, S.J.; Rashid, M.; Linton, P.E.; Banks, C.E.; Shaw, K.J. Rapid antibiotic susceptibility testing using resazurin bulk modified screen-printed electrochemical sensing platforms. Analyst 2021, 146, 5574–5583. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jang, S. Optimization of a resazurin-based microplate assay for large-scale compound screenings against Klebsiella pneumoniae. 3 Biotech 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Singh, D.; Mishra, K.; Kaur, G.; Dhull, N.; Tomar, M.; Gupta, V.; Kumar, B.; Ganju, L. Rapid antibiotic susceptibility testing by resazurin using thin film platinum as a bio-electrode. J. Microbiol. Methods 2019, 162, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Pace, R.T.; Burg, K.J.L. Toxic effects of resazurin on cell cultures. Cytotechnology 2015, 67, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wang, S.; Hou, W.; Xiao, Y.; Liu, P.; Shi, X.; Shen, M. Comparative study of resazurin reduction and MTT assays for cytocompatibility evaluation of nanofibrous materials. Anal. Methods 2019, 11, 483–489. [Google Scholar] [CrossRef]
- Lavogina, D.; Lust, H.; Tahk, M.J.; Laasfeld, T.; Vellama, H.; Nasirova, N.; Vardja, M.; Eskla, K.L.; Salumets, A.; Rinken, A.; et al. Revisiting the Resazurin-Based Sensing of Cellular Viability: Widening the Application Horizon. Biosensors 2022, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Kawakabe, S.; Arimura, Y. A comparative study of colorimetric cell proliferation assays in immune cells. Cytotechnology 2016, 68, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Zeni, O.; Sannino, A.; Romeo, S.; Micciulla, F.; Bellucci, S.; Scarfi, M.R. Growth inhibition, cell-cycle alteration and apoptosis in stimulated human peripheral blood lymphocytes by multiwalled carbon nanotube buckypaper. Nanomed. J. 2015, 10, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Gartlon, J.; Kinsner, A.; Bal-Price, A.; Coecke, S.; Clothier, R. Evaluation of a proposed in vitro test strategy using neuronal and non-neuronal cell systems for detecting neurotoxicity. In Vitro Toxicol. 2006, 20, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.P.; Parker, T.L.; Walker, D.A.; Alexander, C.; Ashford, M.B.; Gellert, P.R.; Garnett, M.C. Multiplexing Spheroid Volume, Resazurin and Acid Phosphatase Viability Assays for High-Throughput Screening of Tumour Spheroids and Stem Cell Neurospheres. PLoS ONE 2014, 9, e103817. [Google Scholar] [CrossRef] [PubMed]
- Magnani, E.; Bettini, E. Resazurin detection of energy metabolism changes in serum-starved PC12 cells and of neuroprotective agent effect. Brain Res. Protoc. 2000, 5, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Wang, W.H.; Ching, A.; Chen, Y.; Cheng, C.M. Paper-Based Resazurin Assay of Inhibitor-Treated Porcine Sperm. Micromachines 2019, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, Y.; Wang, J.; Yu, W.; Wang, W.; Ma, X. Monitoring of Cell Viability and Proliferation in Hydrogel-Encapsulated System by Resazurin Assay. Appl. Biochem. Biotechnol. 2010, 162, 1996–2007. [Google Scholar] [CrossRef] [PubMed]
- Bueno, C.; Villegas, M.L.; Bertolotti, S.G.; Previtali, C.M.; Neumann, M.G.; Encinas, M.V. The Excited-State Interaction of Resazurin and Resorufin with Aminesin Aqueous Solutions. Photophysics and Photochemical Reaction. J. Photochem. Photobiol. C Photochem. 2002, 76, 385–390. [Google Scholar] [CrossRef]
- Njoku, D.I.; Guo, Q.; Dai, W.; Chen, J.L.; Mao, G.; Sun, Q.; Sun, H.; Peng, Y.K. The multipurpose application of resazurin in micro-analytical techniques: Trends from the microbial, catalysis and single molecule detection assays. TrAC Trends Anal. Chem. 2023, 167, 117288. [Google Scholar] [CrossRef]
- Arroyo, A.D.; Guzman, A.E.; Kachur, A.V.; Popov, A.V.; Delikatny, E.J. Development of 18F-Labeled Resazurin Derivatives for the Detection of Tumor Metabolic Activity Using Cerenkov Imaging. Front. Phys. 2021, 9, 652179. [Google Scholar] [CrossRef]
- Kachur, A.V.; Arroyo, A.D.; Popov, A.V.; Saylor, S.J.; Delikatny, E.J. Synthesis of F-18 labeled resazurin by direct electrophilic fluorination. J. Fluor. Chem. 2015, 178, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Da̧browski, J.M.; Urbanska, K.; Arnaut, L.G.; Pereira, M.M.; Abreu, A.R.; Simões, S.; Stochel, G. Biodistribution and Photodynamic Efficacy of a Water-Soluble, Stable, Halogenated Bacteriochlorin against Melanoma. ChemMedChem 2011, 6, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Szaciłowski, K.; Macyk, W.; Drzewiecka-Matuszek, A.; Brindell, M.; Stochel, G. Bioinorganic Photochemistry: Frontiers and Mechanisms. Chem. Rev. 2005, 105, 2647–2694. [Google Scholar] [CrossRef] [PubMed]
- Romero-Garcia, S.; Lopez-Gonzalez, J.S.; Báez-Viveros, J.L.; Aguilar-Cazares, D.; Prado-Garcia, H. Tumor cell metabolism: An integral view. Cancer Biol. Ther. 2011, 12, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Neagu, M.; Constantin, C.; Popescu, I.D.; Zipeto, D.; Tzanakakis, G.; Nikitovic, D.; Fenga, C.; Stratakis, C.A.; Spandidos, D.A.; Tsatsakis, A.M. Inflammation and Metabolism in Cancer Cell-Mitochondria Key Player. Front. Oncol. 2019, 9, 348. [Google Scholar] [CrossRef]
- Duque-Prata, A.; Serpa, C.; Caridade, P.J.S.B. Full theoretical protocol for the design of metal-free organic electron donor-spacer-acceptor systems. Phys. Chem. Chem. Phys. 2023, 25, 27854–27865. [Google Scholar] [CrossRef]
- Duque-Prata, A.; Pinto, T.B.; Serpa, C.; Caridade, P.J.S.B. Performance of Functionals and Basis Sets in Calculating Redox Potentials of Nitrile Alkenes and Aromatic Molecules using Density Functional Theory. ChemistrySelect 2023, 8, e202300205. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Hopkins, E.; Sanvictores, T.; Sharma, S. Physiology, Acid Base Balance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Clarke, F.H.; Cahoon, N.M. Ionization constants by curve fitting: Determination of partition and distribution coefficients of acids and bases and their ions. J. Pharm. Sci. 1987, 76, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Csizmadia, F.; Tsantili-Kakoulidou, A.; Panderi, I.; Darvas, F. Prediction of Distribution Coefficient from Structure. 1. Estimation Method. J. Pharm. Sci. 1997, 86, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.L.; Jurs, P.C. Estimation of pKa for organic oxyacids using calculated atomic charges. J. Comput. Chem. 1993, 14, 1460–1467. [Google Scholar] [CrossRef]
- Szegezdi, J.; Csizmadia, F. Prediction of Dissociation Constant Using Microconstants, 2004. Available online: https://docs.chemaxon.com/display/docs/attachments/attachments_1814016_1_Prediction_of_dissociation_constant_using_microconstants.pdf (accessed on 29 December 2023).
- Szegezdi, J.; Csizmadia, F. A Method for Calculating the pKa Values of Small and Large Molecules, 2007. Available online: https://docs.chemaxon.com/display/docs/attachments/attachments_1814017_1_Calculating_pKa_values_of_small_and_large_molecules.pdf (accessed on 29 December 2023).
- Cooper, G.M.; Adams, K.W. The Cell: A Molecular Approach, 9th ed.; Oxford University Press: New York, NY, USA, 2023. [Google Scholar]
- Bouchard, G.; Carrupt, P.; Testa, B.; Gobry, V.; Girault, H.H. The Apparent Lipophilicity of Quaternary Ammonium Ions Is Influenced by Galvani Potential Difference, Not Ion-Pairing: A Cyclic Voltammetry Study. Pharm. Res. 2001, 18, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Klopman, G.; Li, J.Y.; Wang, S.; Dimayuga, M. Computer Automated log P Calculations Based on an Extended Group Contribution Approach. J. Chem. Inf. Comput. Sci. 1994, 34, 752–781. [Google Scholar] [CrossRef]
- Viswanadhan, V.N.; Ghose, A.K.; Revankar, G.R.; Robins, R.K. Atomic physicochemical parameters for three dimensional structure directed quantitative structure-activity relationships. 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. J. Chem. Inf. Comput. Sci. 1989, 29, 163–172. [Google Scholar] [CrossRef]
- Jacquemin, D.; Planchat, A.; Adamo, C.; Mennucci, B. TD-DFT Assessment of Functionals for Optical 0–0 Transitions in Solvated Dyes. J. Chem. Theory Comput. 2012, 8, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- AAT Bioquest, I. Spectrum [Resorufin]. Available online: https://www.aatbio.com/fluorescence-excitation-emission-spectrum-graph-viewer/resorufin (accessed on 30 January 2024).
- Zhang, G.; Musgrave, C.B. Comparison of DFT Methods for Molecular Orbital Eigenvalue Calculations. J. Phys. Chem. A 2007, 111, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- McQuarrie, D.A. Statistical Mechanics; Harper and Row: New York, NY, USA, 1976. [Google Scholar]
- Grimme, S. Supramolecular Binding Thermodynamics by Dispersion-Corrected Density Functional Theory. Chem. A Eur. J. 2012, 18, 9955–9964. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density functional for spectroscopy: No long-range self-interaction error, good performance for rydberg and charge-transfer states, and better performance on average than B3LYP for ground states. J. Phys. Chem. A 2006, 110, 13126–13130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistent molecular-orbital methods. IX. An extended gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Isse, A.A.; Gennaro, A. Absolute potential of the standard hydrogen electrode and the problem of interconversion of potentials in different solvents. J. Phys. Chem. B 2010, 114, 7894–7899. [Google Scholar] [CrossRef]
- Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew-Burke-Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Nikolaienko, T.Y.; Bulavin, L.A.; Hovorun, D.M. JANPA: An open source cross-platform implementation of the Natural Population Analysis on the Java platform. Comput. Theor. Chem. 2014, 1050, 15–22. [Google Scholar] [CrossRef]
- Laurent, A.D.; Jacquemin, D. TD-DFT benchmarks: A review. Int. J. Quantum Chem 2013, 113, 2019–2039. [Google Scholar] [CrossRef]
- Coskun, D.; Jerome, S.V.; Friesner, R.A. Evaluation of the Performance of the B3LYP, PBE0, and M06 DFT Functionals, and DBLOC-Corrected Versions, in the Calculation of Redox Potentials and Spin Splittings for Transition Metal Containing Systems. J. Chem. Theory Comput. 2016, 12, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Duque-Prata, A.; Serpa, C.; Caridade, P.J.S.B. Photochemistry of 1-Phenyl-4-Allyl-Tetrazol-5-One: A Theoretical Study Contribution towards Mechanism Elucidation. Appl. Sci. 2021, 11, 4045. [Google Scholar] [CrossRef]
- De Souza, B.; Farias, G.; Neese, F.; Izsá, R. Predicting Phosphorescence Rates of Light Organic Molecules Using Time-Dependent Density Functional Theory and the Path Integral Approach to Dynamics. J. Chem. Theory Comput. 2019, 15, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- De Souza, B.; Neese, F.; Izsá, R. On the theoretical prediction of fluorescence rates from first principles using the path integral approach. J. Chem. Phys. 2018, 148, 034104. [Google Scholar] [CrossRef] [PubMed]
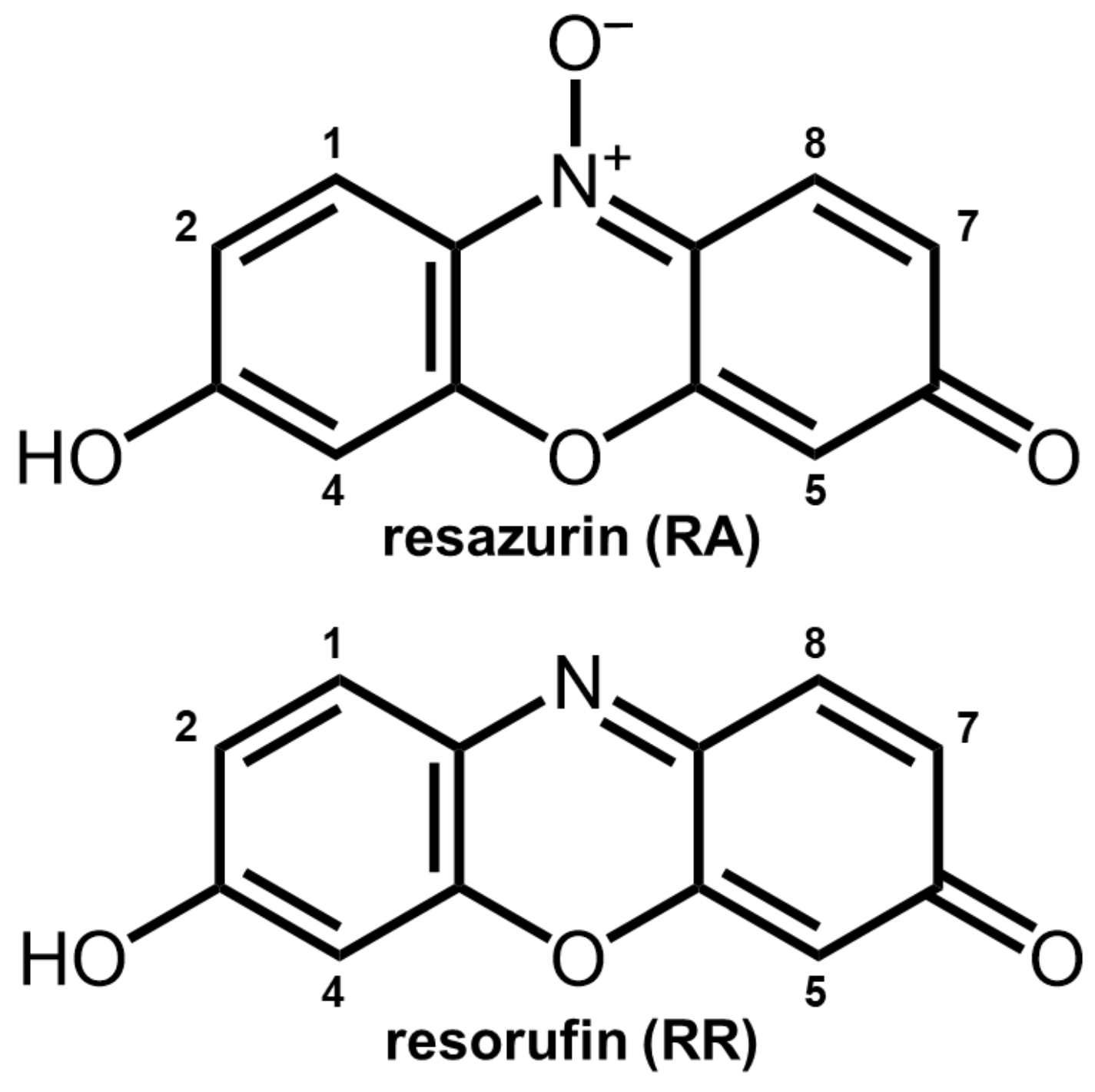
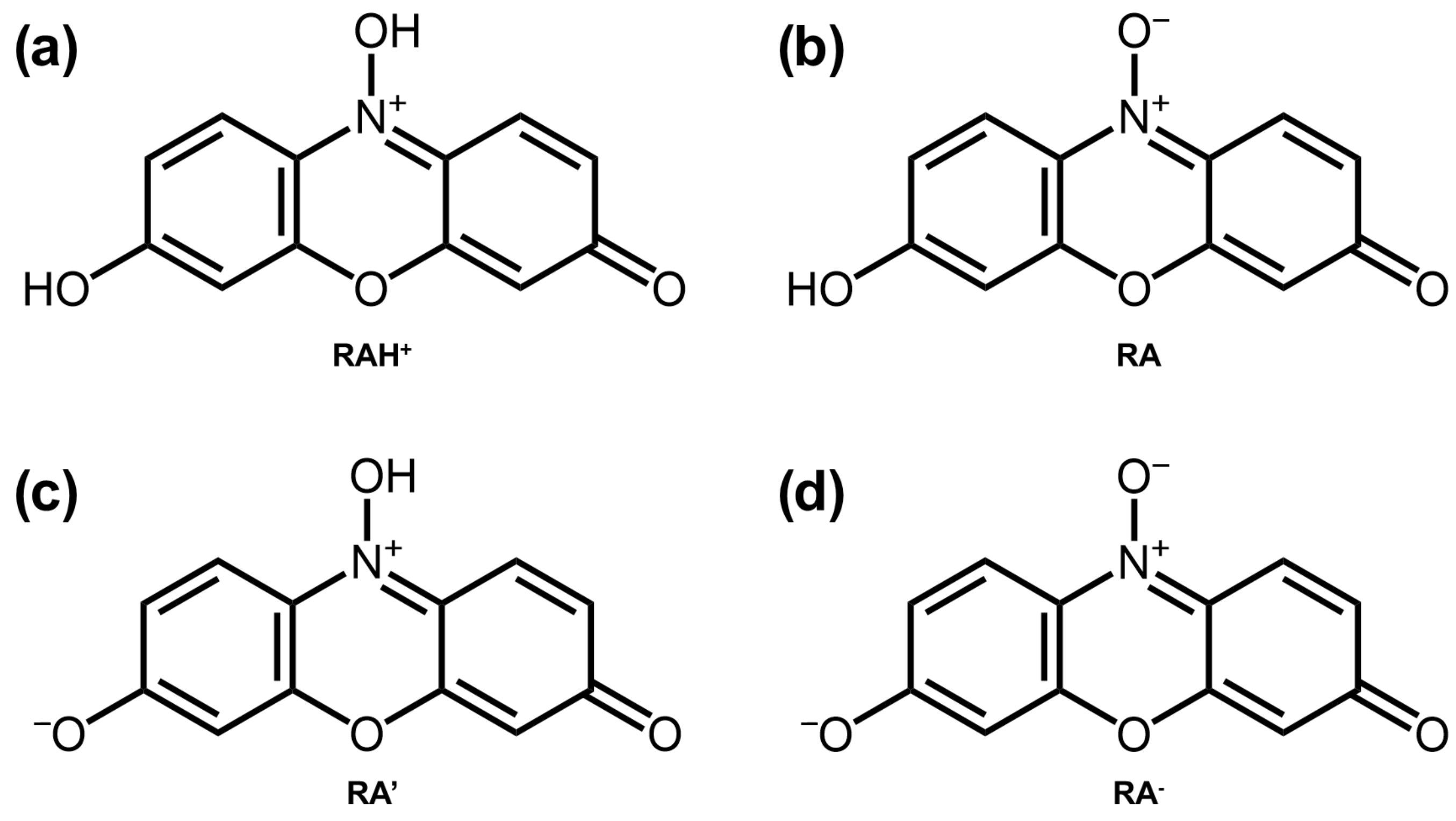
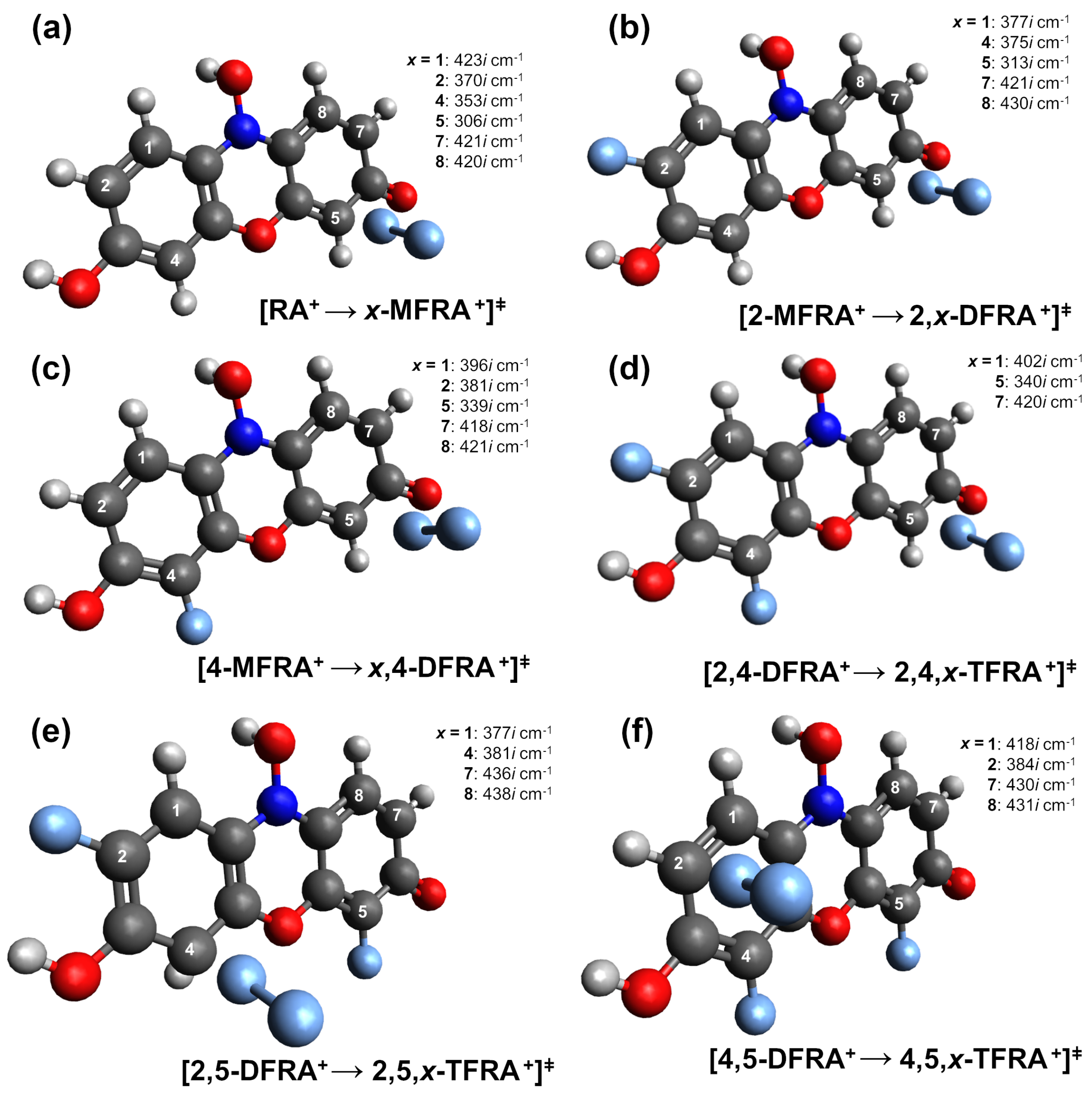


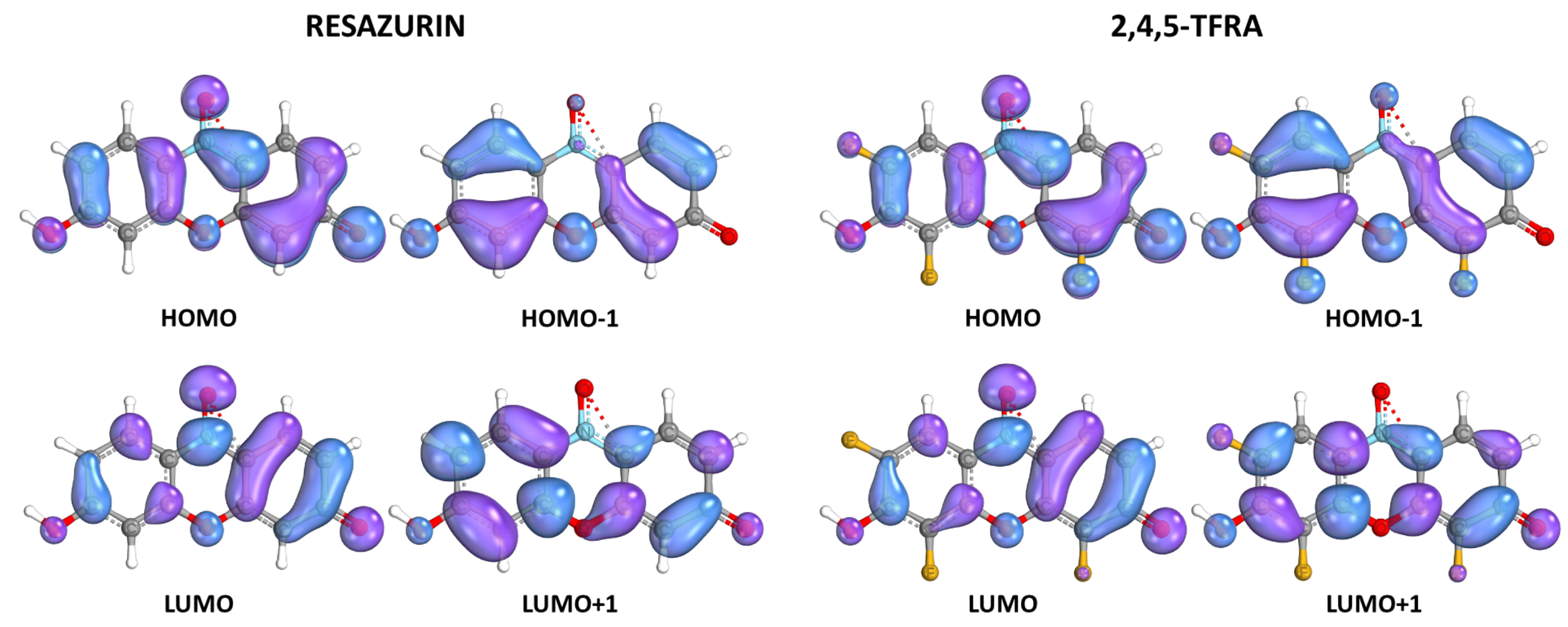
| Carbon # | 2- | 4- | 2,4- | 2,5- | 4,5- | |
|---|---|---|---|---|---|---|
| 1 | −0.16 | −0.24 | −0.17 | −0.25 | −0.24 | −0.17 |
| 2 | −0.27 | −0.26 | −0.26 | |||
| 4 | −0.31 | −0.29 | −0.29 | |||
| 5 | −0.31 | −0.31 | −0.30 | −0.30 | ||
| 7 | −0.16 | −0.15 | −0.15 | −0.15 | −0.16 | −0.16 |
| 8 | −0.22 | −0.22 | −0.22 | −0.22 | −0.21 | −0.21 |
| Compound | Compound | Compound | |||
|---|---|---|---|---|---|
| 1- | 6.3 | 1,2- | 5.1 | 1,2,4- | 4.6 |
| 2- | 3.3 | 1,4- | 5.4 | 1,2,5- | 1.5 |
| 4- | 7.2 | 2,4- | 2.3 | 1,4,5- | 1.2 |
| 5- | 5.5 | 2,5- | 0.0 | 2,4,5- | 0.0 |
| 7- | 0.0 | 2,8- | 2.2 | 2,4,8- | 2.5 |
| 8- | 7.8 | 4,5- | 4.0 | 2,5,8- | 0.4 |
| 4,8- | 6.2 | 4,5,8- | 4.0 |
| Carbon # | 2- | 4- | 2,4- | 2,5- | 4,5- | |
|---|---|---|---|---|---|---|
| 1 | 10.6 | 9.1 | 8.5 | 8.0 | 9.6 | 9.2 |
| 2 | 2.6 | 3.9 | 2.0 | |||
| 4 | 2.7 | 4.4 | 4.7 | |||
| 5 | 1.1 | 1.3 | 2.0 | 2.0 | ||
| 7 | 6.1 | 6.8 | 7.0 | 7.8 | 7.6 | 7.7 |
| 8 | 6.1 | 6.8 | 7.0 | n.o. | 7.7 | 7.8 |
| Compound | ||||||
|---|---|---|---|---|---|---|
| RA | 1649 | −0.10 | 0.07 | 19.55 | 0.76 | 76.63 |
| 1-MFRA | 1694 | −0.17 | 0.00 | 12.52 | 0.05 | 87.43 |
| 2-MFRA | 1701 | −0.98 | 0.00 | 0.95 | 0.17 | 98.88 |
| 4-MFRA | 1699 | −0.91 | 0.00 | 1.26 | 0.20 | 98.54 |
| 2,4-DFRA | 1755 | −1.10 | 0.00 | 0.08 | 0.04 | 99.88 |
| 2,5-DFRA | 1755 | −1.11 | 0.00 | 0.93 | 0.05 | 99.02 |
| 4,5-DFRA | 1748 | −1.04 | 0.00 | 1.23 | 0.06 | 98.71 |
| 2,4,5-TFRA | 1804 | −1.19 | 0.00 | 0.08 | 0.01 | 99.91 |
| Compound | Compound | ||||
|---|---|---|---|---|---|
| RA | 1.64 | −0.58 | RR | 1.65 | −0.80 |
| 1-MFRA | 1.68 | −0.53 | 1-MFRR | 1.76 | −0.74 |
| 2-MFRA | 1.69 | −0.52 | 2-MFRR | 1.71 | −0.74 |
| 4-MFRA | 1.73 | −0.51 | 4-MFRR | 1.79 | −0.72 |
| 2,4-DFRA | 1.79 | −0.45 | 2,4-DFRR | 1.87 | −0.66 |
| 2,5-DFRA | 1.67 | −0.41 | 2,5-DFRR | 1.71 | −0.62 |
| 4,5-DFRA | 1.72 | −0.39 | 4,5-DFRR | 1.80 | −0.61 |
| 2,4,5-TFRA | 1.78 | −0.33 | 2,4,5-TFRR | 1.87 | −0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duque-Prata, A.; Serpa, C.; Caridade, P.J.S.B. Theoretical Evaluation of Fluorinated Resazurin Derivatives for In Vivo Applications. Molecules 2024, 29, 1507. https://doi.org/10.3390/molecules29071507
Duque-Prata A, Serpa C, Caridade PJSB. Theoretical Evaluation of Fluorinated Resazurin Derivatives for In Vivo Applications. Molecules. 2024; 29(7):1507. https://doi.org/10.3390/molecules29071507
Chicago/Turabian StyleDuque-Prata, Amílcar, Carlos Serpa, and Pedro J. S. B. Caridade. 2024. "Theoretical Evaluation of Fluorinated Resazurin Derivatives for In Vivo Applications" Molecules 29, no. 7: 1507. https://doi.org/10.3390/molecules29071507
APA StyleDuque-Prata, A., Serpa, C., & Caridade, P. J. S. B. (2024). Theoretical Evaluation of Fluorinated Resazurin Derivatives for In Vivo Applications. Molecules, 29(7), 1507. https://doi.org/10.3390/molecules29071507








