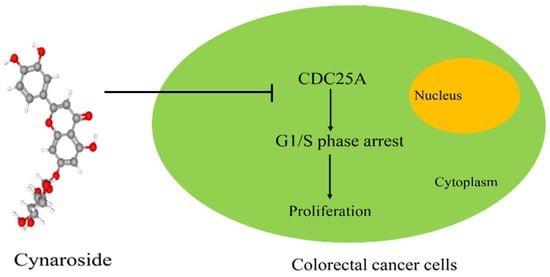
Cynaroside Induces G1 Cell Cycle Arrest by Downregulating Cell Division Cycle 25A in Colorectal Cancer
Abstract
:1. Introduction
2. Results
2.1. Cynaroside Suppresses the Proliferation and Colony Formation of CRC Cells
2.2. Cynaroside Suppresses the Proliferative Potential of CRC Cells In Vivo
2.3. Cynaroside Suppresses the Proliferative Potential of CRC Cells In Vivo
2.4. CDC25A Is a Key Target of Cynaroside
2.5. Cynaroside Suppresses CRC Cell DNA Replication and Induces CRC Cell G1/S-Phase Arrest In Vitro
2.6. Overexpression of CDC25A Attenuates the Inhibitory Impacts of Cynaroside on the Proliferative Potential of CRC Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. qRT-PCR
4.3. Cell Count Kit-8 (CCK-8) Assay
4.4. Colony Formation Assay
4.5. 5-Ethynyl-2′-deoxyuridine (Edu) Assay
4.6. Flow Cytometry Analysis
4.7. In Vivo Assay
4.8. Western Blot
4.9. Hematoxylin–Eosin Staining and Immunohistochemistry
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| DEGs | Differentially expressed genes |
| CDC25A | Cell division cycle |
| TCM | Traditional Chinese medicine |
| FBS | Fetal bovine serum |
| BCA | Bicinchoninic acid |
| CDK4 | Cyclin-dependent kinase 4 |
References
- Abrahami, D.; McDonald, E.G.; E Schnitzer, M.E.; Barkun, A.N.; Suissa, S.; Azoulay, L. Proton pump inhibitors and risk of colorectal cancer. Gut 2022, 71, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Johdi, N.A.; Sukor, N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front. Immunol. 2020, 11, 1624. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; He, M.; Zhang, M.; Sun, Q.; Zeng, S.; Chen, L.; Yang, H.; Liu, M.; Ren, S.; Meng, X.; et al. Colorectal Cancer, Gut Microbiota and Traditional Chinese Medicine: A Systematic Review. Am. J. Chin. Med. 2021, 49, 805–828. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, X.; Zhang, R.; Liu, S.; Xiang, Y.; Zhang, M.; Chen, X.; Pan, T.; Yan, L.; Feng, J.; et al. Combinative treatment of beta-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 2020, 10, 5107–5119. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Zhang, Y.; Song, F.; Ding, Y. Chinese herbal medicine promote tissue differentiation in colorectal cancer by activating HSD11B2. Arch. Biochem. Biophys. 2020, 695, 108644. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liu, Z.; Chen, H.; Zhang, M.; Ma, X.; Han, Q.; Lu, D.; Wang, C. Protective effect of cynaroside on sepsis-induced multiple organ injury through Nrf2/HO-1-dependent macrophage polarization. Eur. J. Pharmacol. 2021, 911, 174522. [Google Scholar] [CrossRef]
- Tabrez, S.; Rahman, F.; Ali, R.; Alouffi, A.S.; Akand, S.K.; Alshehri, B.M.; Alshammari, F.A.; Alam, A.; Alaidarous, M.A.; Banawas, S.; et al. Cynaroside inhibits Leishmania donovani UDP-galactopyranose mutase and induces reactive oxygen species to exert antileishmanial response. Biosci. Rep. 2021, 41, BSR20203857. [Google Scholar] [CrossRef]
- Shao, J.; Wang, C.; Li, L.; Liang, H.; Dai, J.; Ling, X.; Tang, H. Luteoloside Inhibits Proliferation and Promotes Intrinsic and Extrinsic Pathway-Mediated Apoptosis Involving MAPK and mTOR Signaling Pathways in Human Cervical Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1664. [Google Scholar] [CrossRef]
- Ji, J.; Wang, Z.; Sun, W.; Li, Z.; Cai, H.; Zhao, E.; Cui, H. Effects of Cynaroside on Cell Proliferation, Apoptosis, Migration and Invasion though the MET/AKT/mTOR Axis in Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 12125. [Google Scholar] [CrossRef]
- Lara-Chica, M.; Correa-Sáez, A.; Jiménez-Izquierdo, R.; Garrido-Rodríguez, M.; Ponce, F.J.; Moreno, R.; Morrison, K.; Di Vona, C.; Arató, K.; Jiménez-Jiménez, C.; et al. A novel CDC25A/DYRK2 regulatory switch modulates cell cycle and survival. Cell Death Differ. 2022, 29, 105–117. [Google Scholar] [CrossRef]
- Bertero, T.; Gastaldi, C.; Bourget-Ponzio, I.; Mari, B.; Meneguzzi, G.; Barbry, P.; Ponzio, G.; Rezzonico, R. CDC25A targeting by miR-483-3p decreases CCND-CDK4/6 assembly and contributes to cell cycle arrest. Cell Death Differ. 2013, 20, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, Y.; Yany, C.; Li, K.; Huang, X.; Cao, J. Silencing CDC25A inhibits the proliferation of liver cancer cells by downregulating IL-6 in vitro and in vivo. Int. J. Mol. Med. 2020, 45, 743–752. [Google Scholar] [CrossRef]
- Qi, L.W.; Zhang, Z.; Zhang, C.F.; Anderson, S.; Liu, Q.; Yuan, C.S.; Wang, C.Z. Anti-colon cancer effects of 6-shogaol through G2/M cell cycle arrest by p53/p21-cdc2/cdc25A crosstalk. Am. J. Chin. Med. 2015, 43, 743–756. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, J.; Li, L.-J.; Xue, M.; He, S.-L. Cdc25A inhibits autophagy-mediated ferroptosis by upregulating ErbB2 through PKM2 dephosphorylation in cervical cancer cells. Cell Death Dis. 2021, 12, 1055. [Google Scholar] [CrossRef]
- Delattre, J.-F.; Erdogan, A.S.O.; Cohen, R.; Shi, Q.; Emile, J.-F.; Taieb, J.; Tabernero, J.; André, T.; Meyerhardt, J.A.; Nagtegaal, I.D.; et al. A comprehensive overview of tumour deposits in colorectal cancer: Towards a next TNM classification. Cancer Treat. Rev. 2022, 103, 102325. [Google Scholar] [CrossRef] [PubMed]
- Hitsuda, A.; Dan, R.; Urakawa, A.; Hiraoka, Y.; Murakami, C.; Yamamoto, H.; Tanaka, A.R. 25-hydroxycholesterol–induced cell death via activation of ROCK/LIMK/cofilin axis in colorectal cancer cell spheroids. J. Steroid Biochem. Mol. Biol. 2022, 216, 106037. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Karlitz, J.J.; Yen, T.; Lieu, C.H.; Boland, C.R. The rising tide of early-onset colorectal cancer: A comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. Hepatol. 2022, 7, 262–274. [Google Scholar] [CrossRef]
- Ohshima, K.; Oi, R.; Nojima, S.; Morii, E. Mitochondria govern histone acetylation in colorectal cancer. J. Pathol. 2022, 256, 164–173. [Google Scholar] [CrossRef]
- Patel, S.G.; May, F.P.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Gross, S.A.; Jacobson, B.C.; Shaukat, A.; Robertson, D.J. Updates on Age to Start and Stop Colorectal Cancer Screening: Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2022, 162, 285–299. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Q.; Shao, Y.; Yin, S.; Liu, C.; Liu, Y.; Wang, R.; Wang, T.; Qiu, Y.; Yu, H. Anticancer activities of TCM and their active components against tumor metastasis. Biomed. Pharmacother. 2021, 133, 111044. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, W.-D.; Walters, A.S.; Wang, Q.; Liu, Y.-J.; Chu, F.-Y. Traditional Chinese medicine herbal preparations in restless legs syndrome (RLS) treatment: A review and probable first description of RLS in 1529. Sleep Med. Rev. 2012, 16, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Sosnowska, K.; Tomczykowa, M.; Winnicka, K.; Kasacka, I.; Tomczyk, M. In vivo anti-inflammatory and anti-allergic activities of cynaroside evaluated by using hydrogel formulations. Biomed. Pharmacother. 2020, 121, 109681. [Google Scholar] [CrossRef]
- Witkowska-Banaszczak, E.; Krajka-Kuźniak, V.; Papierska, K. The effect of luteolin 7-glucoside, apigenin 7-glucoside and Succisa pratensis extracts on NF-κB activation and α-amylase activity in Hep G2 cells. Acta Biochim. Pol. 2020, 67, 41–47. [Google Scholar] [CrossRef]
- Velmurugan, B.K.; Lin, J.-T.; Mahalakshmi, B.; Chuang, Y.-C.; Lin, C.-C.; Lo, Y.-S.; Hsieh, M.-J.; Chen, M.-K. Luteolin-7-O-Glucoside Inhibits Oral Cancer Cell Migration and Invasion by Regulating Matrix Metalloproteinase-2 Expression and Extracellular Signal-Regulated Kinase Pathway. Biomolecules 2020, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Shen, S.; Zhao, X.; Gong, X. Luteoloside induces G0/G1 arrest and pro-death autophagy through the ROS-mediated AKT/mTOR/p70S6K signalling pathway in human non-small cell lung cancer cell lines. Biochem. Biophys. Res. Commun. 2017, 494, 263–269. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Quiles, J.L.; Quiles, J.L. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 1: The suppression of cell proliferation, promotion of apoptosis and arrest of the cell cycle. Food Funct. 2018, 9, 2145–2157. [Google Scholar] [CrossRef]
- Yang, H.-Z.; Zhang, J.; Zeng, J.; Liu, S.; Zhou, F.; Zhang, F.; Giampieri, F.; Cianciosi, D.; Forbes-Hernandez, T.Y.; Ansary, J.; et al. Resveratrol inhibits the proliferation of melanoma cells by modulating cell cycle. Int. J. Food Sci. Nutr. 2020, 71, 84–93. [Google Scholar] [CrossRef]
- Amatori, S.; Mazzoni, L.; Alvarez-Suarez, J.M.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Provenzano, A.E.; Persico, G.; Mezzetti, B.; et al. Polyphenol-rich strawberry extract (PRSE) shows in vitro and in vivo biological activity against invasive breast cancer cells. Sci. Rep. 2018, 8, 30917. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Quiles, J.L.; Gil, E.; Bompadre, S.; Simal-Gandara, J.; Battino, M.; Giampieri, F. The Influence of In Vitro Gastrointestinal Digestion on the Anticancer Activity of Manuka Honey. Antioxidants 2020, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Iyirhiaro, G.O.; Im, D.S.; Boonying, W.; Callaghan, S.M.; During, M.J.; Slack, R.S.; Park, D.S. Cdc25A Is a Critical Mediator of Ischemic Neuronal Death In Vitro and In Vivo. J. Neurosci. 2017, 37, 6729–6740. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Gao, S.; Zhuang, Y.; Dong, Y.; Guan, W.; Zhang, K.; Xu, J.; Cui, J. R-Phycoerythrin Induces SGC-7901 Apoptosis by Arresting Cell Cycle at S Phase. Mar. Drugs 2016, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-H.; Chen, Z.-F.; Qin, J.-L.; Liu, Y.-C.; Li, Z.-Q.; Khan, T.-M.; Wang, M.; Jiang, Y.-H.; Shen, W.-Y.; Liang, H. Water-soluble oxoglaucine-Y(iii), Dy(iii) complexes: In vitro and in vivo anticancer activities by triggering DNA damage, leading to S phase arrest and apoptosis. Dalton Trans. 2015, 44, 11408–11419. [Google Scholar] [CrossRef] [PubMed]







| GENE | Sequence (5’ -> 3’) | |
|---|---|---|
| PCNA | Forward Primer | CCTGCTGGGATATTAGCTCCA |
| Reverse Primer | CAGCGGTAGGTGTCGAAGC | |
| CDK4 | Forward Primer | ATGGCTACCTCTCGATATGAGC |
| Reverse Primer | CATTGGGGACTCTCACACTCT | |
| MCM2 | Forward Primer | ATGGCGGAATCATCGGAATCC |
| Reverse Primer | GGTGAGGGCATCAGTACGC | |
| CDC45 | Forward Primer | TTCGTGTCCGATTTCCGCAAA |
| Reverse Primer | TGGAACCAGCGGTATATTGCAC | |
| CDC25A | Forward Primer | GTGAAGGCGCTATTTGGCG |
| Reverse Primer | TGGTTGCTCATAATCACTGCC | |
| E2F1 | Forward Primer | ACGCTATGAGACCTCACTGAA |
| Reverse Primer | TCCTGGGTCAACCCCTCAAG | |
| CDC7 | Forward Primer | GAGGCGTCTTTGGGGATTCAG |
| Reverse Primer | GGTCCTACTTGTAACTGTGCTG | |
| PLK1 | Forward Primer | AAAGAGATCCCGGAGGTCCTA |
| Reverse Primer | GGCTGCGGTGAATGGATATTTC | |
| β-actin | Forward Primer | CATGTACGTTGCTATCCAGGC |
| Reverse Primer | CTCCTTAATGTCACGCACGAT | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, S.; Cao, W.; Zeng, Z.; Wang, L.; Lan, J.; Chen, T. Cynaroside Induces G1 Cell Cycle Arrest by Downregulating Cell Division Cycle 25A in Colorectal Cancer. Molecules 2024, 29, 1508. https://doi.org/10.3390/molecules29071508
Lei S, Cao W, Zeng Z, Wang L, Lan J, Chen T. Cynaroside Induces G1 Cell Cycle Arrest by Downregulating Cell Division Cycle 25A in Colorectal Cancer. Molecules. 2024; 29(7):1508. https://doi.org/10.3390/molecules29071508
Chicago/Turabian StyleLei, Shan, Wenpeng Cao, Zhirui Zeng, Lu Wang, Jinzhi Lan, and Tengxiang Chen. 2024. "Cynaroside Induces G1 Cell Cycle Arrest by Downregulating Cell Division Cycle 25A in Colorectal Cancer" Molecules 29, no. 7: 1508. https://doi.org/10.3390/molecules29071508
APA StyleLei, S., Cao, W., Zeng, Z., Wang, L., Lan, J., & Chen, T. (2024). Cynaroside Induces G1 Cell Cycle Arrest by Downregulating Cell Division Cycle 25A in Colorectal Cancer. Molecules, 29(7), 1508. https://doi.org/10.3390/molecules29071508