Bioaccessibility of Phenolic Compounds, Resistant Starch, and Dietary Fibers from Australian Green Banana during In Vitro Digestion and Colonic Fermentation
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Digestion of Resistant and Non-Resistant Starch and Dietary Fibers in Whole Green Banana
2.2. Phenolic and Bioactivity Changes during In Vitro Digestion
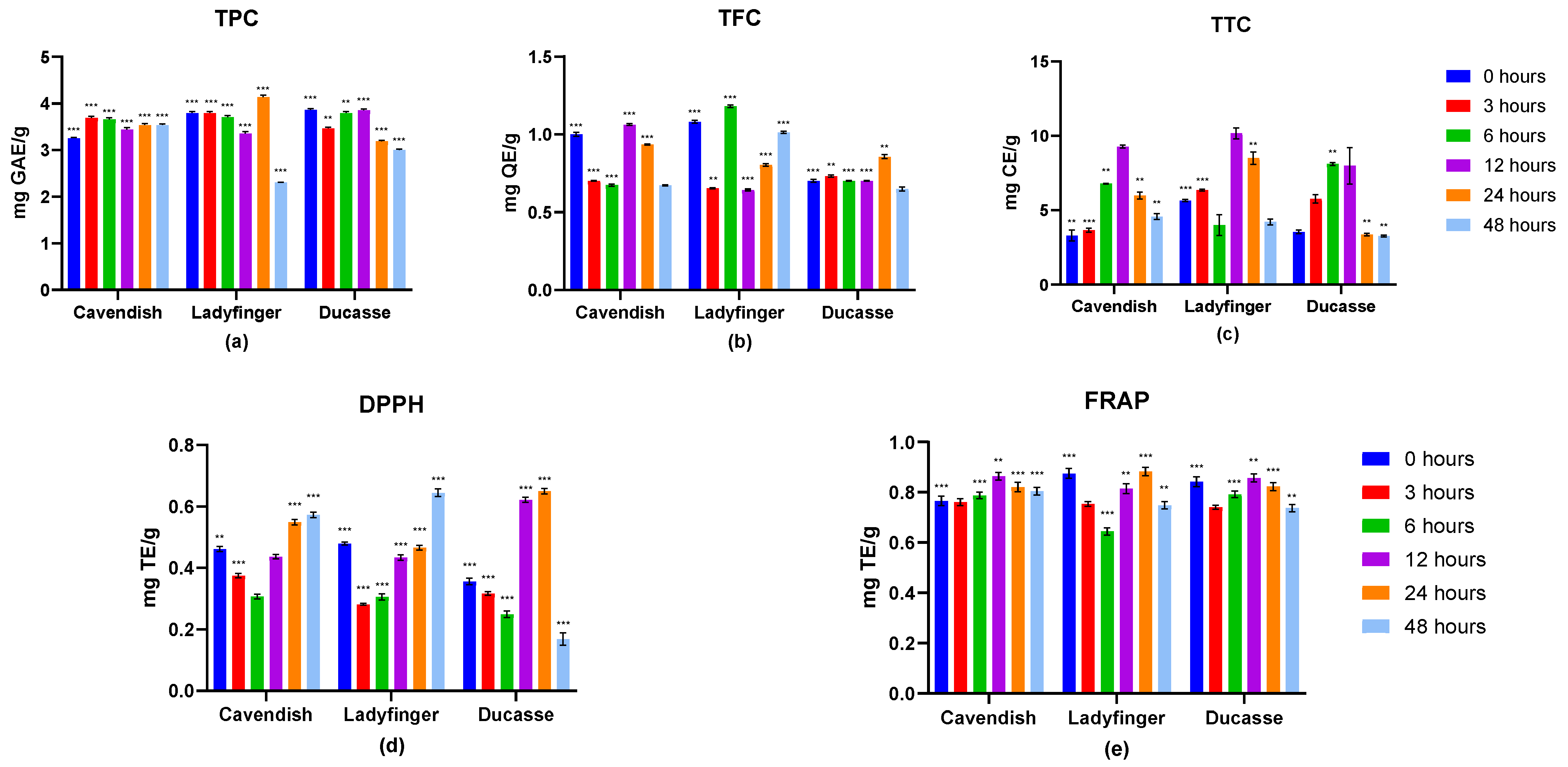
2.2.1. Effects of In Vitro Digestion on TPC, TFC, and TTC
2.2.2. Antioxidant Activities Estimation
2.3. Phenolic and Bioactivity Changes during Colonic Fermentation
2.3.1. Phenolic Evaluation
2.3.2. Antioxidant Capacity Estimation
2.4. Bioaccessibility of Individual Phenolic Compounds in Green Banana
2.5. Recovery and Residual Index of Individual Phenolic Compounds in Green Banana
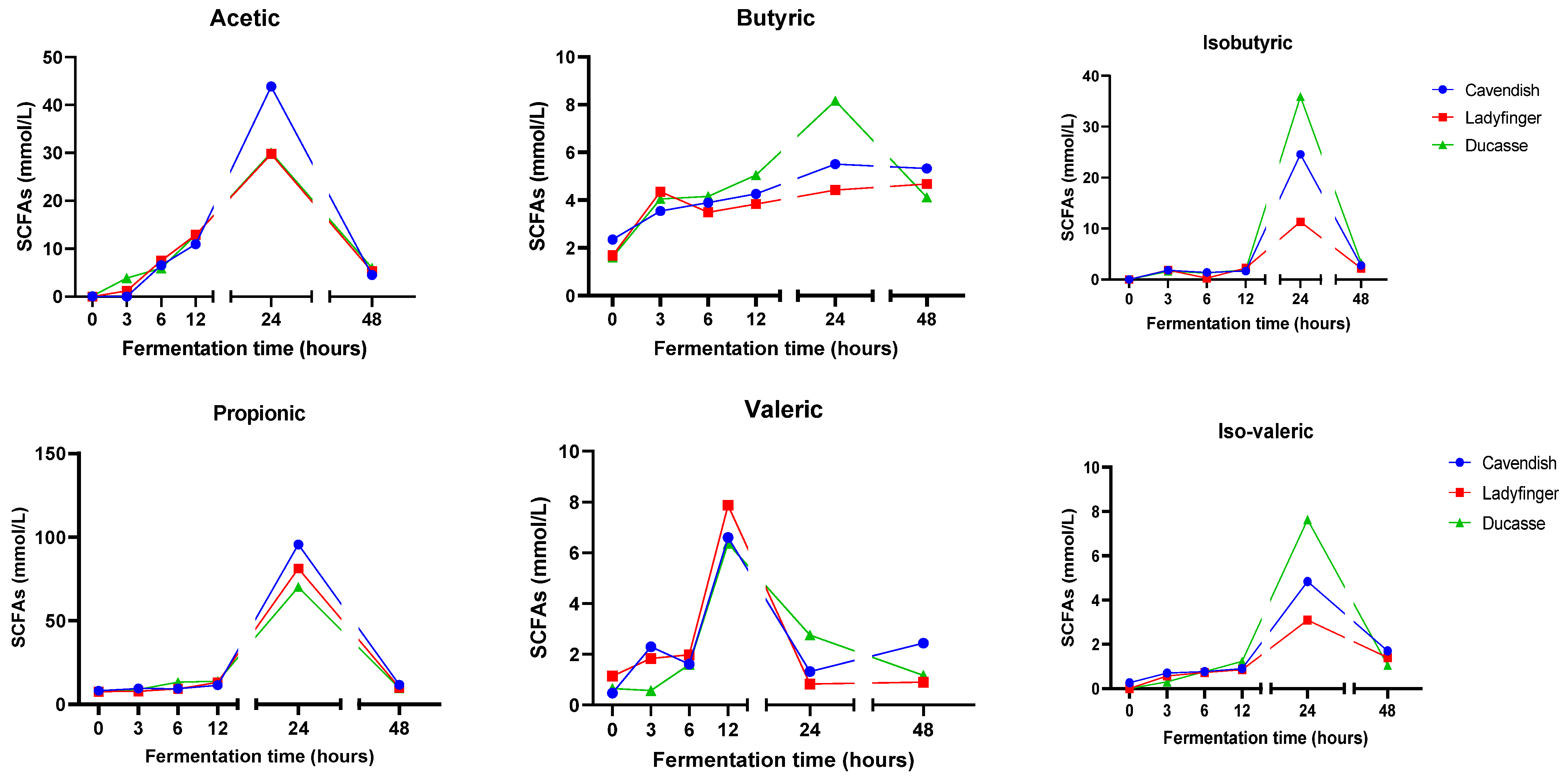
2.6. Short-Chain Fatty Acids (SCFAs)
3. Materials and Methods
3.1. Production of Green Banana Flour
3.2. In Vitro Digestion of Resistant Starch (RS) and Non-Resistant Starch (Non-RS)
3.3. In Vitro Digestion of Total Dietary Fiber (TDF)
3.4. Phenolic Compounds’ Extraction
3.5. In Vitro Gastrointestinal Digestion
3.6. In Vitro Colonic Fermentation
3.7. Estimation of Phenolic Content and Antioxidant Capacity
3.7.1. Total Phenolic Content (TPC)
3.7.2. Total Flavonoid Content (TFC)
3.7.3. Total Condensed Tannins (TTC)
3.7.4. 2,2′-Diphenyl-2-picryl-hydrazyl (DPPH)
3.7.5. Ferric Reducing Antioxidant Power (FRAP)
3.8. Quantification of Phenolic Compounds via High-Performance Liquid Chromatography Photodiode Array (HPLC-PDA)
3.9. Gastrointestinal Digestion and Colonic Fermentation Parameters
3.9.1. Bioaccessibility of Phenolic Compounds
3.9.2. Phenolic Recovery Index
3.9.3. Residual Colonic Digesta Index
3.10. Evaluation of Short-Chain Fatty Acids (SCFAs)
3.11. Statistics Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aurore, G.; Parfait, B.; Fahrasmane, L. Bananas, raw materials for making processed food products. Trends Food Sci. Technol. 2009, 20, 78–91. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic compounds within banana peel and their potential uses: A review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Someya, S.; Yoshiki, Y.; Okubo, K. Antioxidant compounds from bananas (Musa Cavendish). Food Chem. 2002, 79, 351–354. [Google Scholar] [CrossRef]
- Wu, H.; Gu, J.; Amrit, B.K.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Effect of processing on bioaccessibility and bioavailability of bioactive compounds in coffee beans. Food Biosci. 2022, 46, 101373. [Google Scholar] [CrossRef]
- Pavez-Guajardo, C.; Ferreira, S.R.S.; Mazzutti, S.; Guerra-Valle, M.E.; Sáez-Trautmann, G.; Moreno, J. Influence of in vitro digestion on antioxidant activity of enriched apple snacks with grape juice. Foods 2020, 9, 1681. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Reginio, F.C., Jr.; Thuengtung, S.; Ogawa, Y. Changes in bioactive compounds and antioxidant activity of plant-based foods by gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4684–4705. [Google Scholar] [CrossRef] [PubMed]
- Frontela-Saseta, C.; López-Nicolás, R.; González-Bermúdez, C.A.; Peso-Echarri, P.; Ros-Berruezo, G.; Martínez-Graciá, C.; Canali, R.; Virgili, F. Evaluation of antioxidant activity and antiproliferative effect of fruit juices enriched with Pycnogenol® in colon carcinoma cells. The effect of in vitro gastrointestinal digestion. Phytother. Res. 2011, 25, 1870–1875. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. In vitro gastrointestinal digestion of pomegranate peel (Punica granatum) flour obtained from co-products: Changes in the antioxidant potential and bioactive compounds stability. J. Funct. Foods 2015, 19, 617–628. [Google Scholar] [CrossRef]
- Brown, I. Complex carbohydrates and resistant starch. Nutr. Rev. 1996, 54, S115. [Google Scholar] [CrossRef]
- Kendall, C.W.C.; Esfahani, A.; Jenkins, D.J.A. The link between dietary fibre and human health. Food Hydrocoll. 2010, 24, 42–48. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Khalili, L.; Keshtiban, A.K.; Homayouni, A. Resistant starch as a bioactive compound in colorectal cancer prevention. In Bioactive Foods in Health Promotion; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, UK, 2016; pp. 773–780. [Google Scholar]
- Perera, A.; Meda, V.; Tyler, R.T. Resistant starch: A review of analytical protocols for determining resistant starch and of factors affecting the resistant starch content of foods. Int. Food Res. J. 2010, 43, 1959–1974. [Google Scholar] [CrossRef]
- Hernández-Aguirre, M.A.; Islas-Hernández, J.J.; Sánchez-Pardo, M.E.; Rodríguez-Ambriz, S.L.; Osorio-Díaz, P. Response surface methodology for optimization of gluten-free bread made with unripe banana flour. J. Food Meas. Charact. 2019, 13, 1652–1660. [Google Scholar] [CrossRef]
- Englyst, H.N.; Cummings, J.H. Digestion of the polysaccharides of some cereal foods in the human small intestine. Am. J. Clin. Nutr. 1985, 42, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Udo, I.I.; Etokakpan, O.U.; Ukwo, S.P.; Ukpong, E.O. Evaluation of the proximate compositions, dietary fibre and resistant starch contents of selected varieties of banana and plantain. J. Food Sci. Technol. 2021, 8, 1–9. [Google Scholar]
- Chen, L.; Liu, R.; Qin, C.; Meng, Y.; Zhang, J.; Wang, Y.; Xu, G. Sources and intake of resistant starch in the Chinese diet. Asia Pac. J. Clin. Nutr. 2010, 19, 274–282. [Google Scholar] [PubMed]
- Langkilde, A.M.; Champ, M.; Andersson, H. Effects of high-resistant-starch banana flour (RS2) on in vitro fermentation and the small-bowel excretion of energy, nutrients, and sterols: An ileostomy study. Am. J. Clin. Nutr. 2002, 75, 104–111. [Google Scholar] [CrossRef] [PubMed]
- da Mota, R.V.; Lajolo, F.M.; Cordenunsi, B.R.; Ciacco, C. Composition and functional properties of banana flour from different varieties. Starch-Stärke 2000, 52, 63–68. [Google Scholar] [CrossRef]
- Faisant, N.; Buleon, A.; Colonna, P.; Molis, C.; Lartigue, S.; Galmiche, J.P.; Champ, M. Digestion of raw banana starch in the small intestine of healthy humans: Structural features of resistant starch. Br. J. Nutr. 1995, 73, 111–123. [Google Scholar] [CrossRef]
- Ring, S.G.; Gee, J.M.; Whittam, M.; Orford, P.; Johnson, I.T. Resistant starch: Its chemical form in foodstuffs and effect on digestibility in vitro. Food Chem. 1988, 28, 97–109. [Google Scholar] [CrossRef]
- Cone, J.W.; Wolters, M.G.E. Some properties and degradability of isolated starch granules. Starch-Stärke 1990, 42, 298–301. [Google Scholar] [CrossRef]
- Behall, K.M.; Scholfield, D.J.; Yuhaniak, I.; Canary, J. Diets containing high amylose vs amylopectin starch: Effects on metabolic variables in human subjects. Am. J. Clin. Nutr. 1989, 49, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.M.L.; do Rio Preto, S.J.; Ciacco, C.F. Factors that affect the enzymatic degradation of natural starch granules-effect of the size of the granules. Starch-Stärke 1992, 44, 422–426. [Google Scholar] [CrossRef]
- Colonna, P.; Leloup, V.; Buleon, A. Limiting factors of starch hydrolysis. Eur. J. Clin. Nutr. 1992, 46, S17–S32. [Google Scholar] [PubMed]
- Gallant, D.J.; Bouchet, B.; Buleon, A.; Perez, S. Physical characteristics of starch granules and susceptibility to enzymatic degradation. Eur. J. Clin. Nutr. 1992, 46, 3–16. [Google Scholar]
- Christl, S.U.; Murgatroyd, P.R.; Gibson, G.R.; Cummings, J.H. Production, metabolism, and excretion of hydrogen in the large intestine. J. Gastroenterol. 1992, 102, 1269–1277. [Google Scholar] [CrossRef]
- Englyst, H.N.; Cummings, J.H. Nutritional classification of starch and its validation by studies in man. In Proceedings of the International Symposium on Nutritional, Chemical and Food Processing Implications of Nutrient Availability, Ettlingen, Germany, 9–12 May 1993; Ettlingen, M., Ed.; Volume 912, pp. 142–145. [Google Scholar]
- Segundo, C.; Román, L.; Gómez, M.; Martínez, M.M. Mechanically fractionated flour isolated from green bananas (M. cavendishii var. nanica) as a tool to increase the dietary fiber and phytochemical bioactivity of layer and sponge cakes. Food Chem. 2017, 219, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Xu, K.; Guo, M.; Mohamedshah, Z.; Ferruzzi, M.G.; Martinez, M.M. Manufacturing the ultimate green banana flour: Impact of drying and extrusion on phenolic profile and starch bioaccessibility. Food Chem. 2019, 297, 124990. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Wójtowicz, A.; Oniszczuk, T.; Matwijczuk, A.; Dib, A.; Markut-Miotła, E. Opuntia fruits as food enriching ingredient, the first step towards new functional food products. Molecules 2020, 25, 916. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agri. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Pekkinen, J.; Rosa, N.N.; Savolainen, O.-I.; Keski-Rahkonen, P.; Mykkänen, H.; Poutanen, K.; Micard, V.; Hanhineva, K. Disintegration of wheat aleurone structure has an impact on the bioavailability of phenolic compounds and other phytochemicals as evidenced by altered urinary metabolite profile of diet-induced obese mice. Nutr. Metab. 2014, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Castro, A.P.; Pérez-Jiménez, J.; Bello-Pérez, L.A.; Tovar, J.; Sáyago-Ayerdi, S.G. Bioaccessibility of phenolic compounds in common beans (Phaseolus vulgaris L.) after in vitro gastrointestinal digestion: A comparison of two cooking procedures. Cereal Chem. 2020, 97, 670–680. [Google Scholar] [CrossRef]
- Sancho, R.A.S.; Pavan, V.; Pastore, G.M. Effect of in vitro digestion on bioactive compounds and antioxidant activity of common bean seed coats. Food Res. Int. 2015, 76, 74–78. [Google Scholar] [CrossRef]
- Pavan, V.; Sancho, R.A.S.; Pastore, G.M. The effect of in vitro digestion on the antioxidant activity of fruit extracts (Carica papaya, Artocarpus heterophillus and Annona marcgravii). LWT-Food Sci. Technol. 2014, 59, 1247–1251. [Google Scholar] [CrossRef]
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: Bioaccessibility and bioactivity. LWT 2020, 117, 108623. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; de Araújo, F.F.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; Catharino, R.R.; Pastore, G.M. Effect of in vitro digestion on the bioaccessibility and bioactivity of phenolic compounds in fractions of Eugenia pyriformis fruit. Food Res. Int. 2021, 150, 110767. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Lu, P.; Barrow, C.; Dunshea, F.R.; Suleria, H.A.R. Bioaccessibility and bioactivities of phenolic compounds from roasted coffee beans during in vitro digestion and colonic fermentation. Food Chem. 2022, 386, 132794. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Macià, A.; Romero, M.-P.; Reguant, J.; Motilva, M.-J. Matrix composition effect on the digestibility of carob flour phenols by an in-vitro digestion model. Food Chem. 2011, 124, 65–71. [Google Scholar] [CrossRef]
- González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Zafrilla, P.; Mulero, J.; Tomás-Barberán, F.A.; Espín, J.C. Identifying the limits for ellagic acid bioavailability: A crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. J. Funct. Foods 2015, 19, 225–235. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Absorption, bioavailability, and metabolism of flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Arranz, S.; Silván, J.M.; Saura-Calixto, F. Nonextractable polyphenols, usually ignored, are the major part of dietary polyphenols: A study on the Spanish diet. Mol. Nutr. Food Res. 2010, 54, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Quatrin, A.; Rampelotto, C.; Pauletto, R.; Maurer, L.H.; Nichelle, S.M.; Klein, B.; Rodrigues, R.F.; Junior, M.R.M.; de Souza Fonseca, B.; de Menezes, C.R. Bioaccessibility and catabolism of phenolic compounds from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Foods 2020, 65, 103714. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Chaudry, F.; Pannala, A.S.; Srai, S.K.; Debnam, E.; Rice-Evans, C. Decomposition of cocoa procyanidins in the gastric milieu. Biochem. Biophys. Res. Commun. 2000, 272, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini-Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compost. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Laib, I.; Kehal, F.; Haddad, N.E.; Boudjemia, T.; Barkat, M. Effect of in vitro gastrointestinal digestion on phenolic compounds and the antioxidant activity of Aloe vera. ASN 2020, 7, 11–25. [Google Scholar] [CrossRef]
- Zheng, G.; Deng, J.; Wen, L.; You, L.; Zhao, Z.; Zhou, L. Release of phenolic compounds and antioxidant capacity of Chinese hawthorn “Crataegus pinnatifida” during in vitro digestion. J. Funct. Foods 2018, 40, 76–85. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Identification of phenolics profile in freeze-dried apple peel and their bioactivities during in vitro digestion and colonic fermentation. Int. J. Mol. Sci. 2023, 24, 1514. [Google Scholar] [CrossRef]
- Ayoub, M.; de Camargo, A.C.; Shahidi, F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Suwannachot, J.; Ogawa, Y. In vitro gastrointestinal digestion of crisphead lettuce: Changes in bioactive compounds and antioxidant potential. Food Chem. 2020, 311, 125885. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Bennett, R.N.; Shiga, T.M.; Hassimotto, N.M.A.; Rosa, E.A.S.; Lajolo, F.M.; Cordenunsi, B.R. Phenolics and antioxidant properties of fruit pulp and cell wall fractions of postharvest banana (Musa acuminata Juss.) cultivars. J. Agric. Food Chem. 2010, 58, 7991–8003. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Hu, H.; Hu, Y.; Xie, J. Metabolism of flavonoids in novel banana germplasm during fruit development. Front. Plant Sci. 2016, 7, 1291. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.R.; Hasan, M.M.; Islam, M.R.; Haque, A.R.; Hasan, S.M.K. Formulation of yogurt with banana peel extracts to enhance storability and bioactive properties. J. Food Process. Preserv. 2021, 45, e15191. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Yusoff, N.A.M.; Eldeen, I.M.; Seow, E.M.; Sajak, A.A.B.; Ooi, K.L. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.). J. Food Compos. Anal. 2011, 24, 1–10. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.d.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Pegg, R.B.; Kong, F. Total phenolics content and antioxidant capacities of microencapsulated blueberry anthocyanins during in vitro digestion. Food Chem. 2014, 153, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Thuengtung, S.; Niwat, C.; Tamura, M.; Ogawa, Y. In vitro examination of starch digestibility and changes in antioxidant activities of selected cooked pigmented rice. Food Biosci. 2018, 23, 129–136. [Google Scholar] [CrossRef]
- Nagar, E.E.; Okun, Z.; Shpigelman, A. Digestive fate of polyphenols: Updated view of the influence of chemical structure and the presence of cell wall material. Curr. Opin. Food Sci. 2020, 31, 38–46. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.-H. Degradation kinetics of fisetin and quercetin in solutions as effected by pH, temperature and coexisted proteins. J. Serb. Chem. Soc. 2016, 81, 243–253. [Google Scholar] [CrossRef]
- Luo, J.; Li, M.; Wu, H.; Liu, Z.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Bioaccessibility of phenolic compounds from sesame seeds (Sesamum indicum L.) during in vitro gastrointestinal digestion and colonic fermentation. J. Food Process. Preserv. 2022, 46, e16669. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef]
- Brownlee, I.A.; Dettmar, P.W.; Strugala, V.; Pearson, J.P. The interaction of dietary fibres with the colon. Curr. Nutr. Food Sci. 2006, 2, 243–264. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- de Almeida, S.S.; da Costa, G.B.M.; Barreto, M.S.; Freire, D.M.G.; Lobo, L.A.; Domingues, R.M.C.P.; Moura-Nunes, N.; Monteiro, M.; Perrone, D. Bioaccessibility and gut metabolism of phenolic compounds of breads added with green coffee infusion and enzymatically bioprocessed. Food Chem. 2020, 333, 127473. [Google Scholar] [CrossRef]
- Konishi, Y.; Zhao, Z.; Shimizu, M. Phenolic acids are absorbed from the rat stomach with different absorption rates. J. Agric. Food Chem. 2006, 54, 7539–7543. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A. Influence of food matrix on the bioaccessibility of fruit polyphenolic compounds. J. Agric. Food Chem. 2020, 68, 1315–1325. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P.; Elez-Martínez, P.; Martín-Belloso, O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. JFF 2015, 14, 33–43. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Díaz, J.L.; Moreno-Ortega, A.; Roldán-Guerra, F.J.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M.; Pereira-Caro, G. In vitro gastrointestinal digestion and colonic catabolism of mango (Mangifera indica L.) pulp polyphenols. Foods 2020, 9, 1836. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Álvarez, J.A.P.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef]
- Herrera-Cazares, L.A.; Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Gutiérrez-Uribe, J.A.; Campos-Vega, R.; Gaytán-Martínez, M. Influence of extrusion process on the release of phenolic compounds from mango (Mangifera indica L.) bagasse-added confections and evaluation of their bioaccessibility, intestinal permeability, and antioxidant capacity. Food Res. Int. 2021, 148, 110591. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; He, W.; Chen, S.; Chen, J.; Zeng, M.; Qin, F.; He, Z. Interactions of digestive enzymes and milk proteins with tea catechins at gastric and intestinal pH. Int. J. Food Sci. 2017, 52, 247–257. [Google Scholar] [CrossRef]
- Sharma, K.P.; John, P.J.; Goswami, P.; Soni, M. Enzymatic synthesis of gallic acid from tannic acid with an inducible hydrolase of Enterobacter spp. J. Biocatal. Biotransformation 2017, 35, 177–184. [Google Scholar] [CrossRef]
- Attri, S.; Sharma, K.; Raigond, P.; Goel, G. Colonic fermentation of polyphenolics from Sea buckthorn (Hippophae rhamnoides) berries: Assessment of effects on microbial diversity by Principal Component Analysis. Food Res. Int. 2018, 105, 324–332. [Google Scholar] [CrossRef]
- Zabela, V.; Sampath, C.; Oufir, M.; Moradi-Afrapoli, F.; Butterweck, V.; Hamburger, M. Pharmacokinetics of dietary kaempferol and its metabolite 4-hydroxyphenylacetic acid in rats. Fitoterapia 2016, 115, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, L.; Tarcomnicu, I.; Dulea, C.; Attili, N.R.B.N.; Ciuca, V.; Peru, D.; Rizea Savu, S. Confirmation of diosmetin 3-O-glucuronide as major metabolite of diosmin in humans, using micro-liquid-chromatography–mass spectrometry and ion mobility mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 8295–8310. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wu, H.; Li, M.; Liu, Z.; Duan, X.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Assessment of the bioaccessibility of phenolics from Australian grown lettuces by in vitro simulated gastrointestinal digestion and colonic fermentation. Food Biosci. 2022, 48, 101754. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, Y.; Tao, S.; Zhang, G.; Wang, J.; Liu, L.; Zhang, S. Fiber-rich foods affected gut bacterial community and short-chain fatty acids production in pig model. J. Funct. Foods 2019, 57, 266–274. [Google Scholar] [CrossRef]
- Welli, Y.; Agnes, M.; Yudi, P.; Yustinus, M. The effect of application of cavendish Jepara 30 banana pseudostem flour on the production of short-chain fatty acids and cholesterol in caecum digesta of hypercholesterolemic mice. Int. J. Sci. Technol. Res. 2019, 8, 12. [Google Scholar]
- Danneskiold-Samsøe, N.B.; Barros, H.D.d.F.Q.; Santos, R.; Bicas, J.L.; Cazarin, C.B.B.; Madsen, L.; Kristiansen, K.; Pastore, G.M.; Brix, S.; Júnior, M.R.M. Interplay between food and gut microbiota in health and disease. Food Res. Int. 2019, 115, 23–31. [Google Scholar] [CrossRef]
- Molino, S.; Fernández-Miyakawa, M.; Giovando, S.; Rufián-Henares, J.Á. Study of antioxidant capacity and metabolization of quebracho and chestnut tannins through in vitro gastrointestinal digestion-fermentation. J. Funct. Foods 2018, 49, 188–195. [Google Scholar] [CrossRef]
- Gu, C.; Suleria, H.A.R.; Dunshea, F.R.; Howell, K. Dietary lipids influence bioaccessibility of polyphenols from black carrots and affect microbial diversity under simulated gastrointestinal digestion. Antioxidants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; White, P.J. In vitro fermentation of oat flours from typical and high β-glucan oat lines. J. Agric. Food Chem. 2009, 57, 7529–7536. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; Louis, P. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.H.; Sperandio, M.; Di Ciaula, A. Gut microbiota and short chain fatty acids: Implications in glucose homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Prosky, L.; Asp, N.-G.; Furda, I.; Devries, J.W.; Schweizer, T.F.; Harland, B.F. Determination of total dietary fiber in foods and food products: Collaborative study. J. Assoc. Off. Anal. Chem. 1985, 68, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms profiling of australian mango peel by-product polyphenols and their potential antioxidant activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]

| Samples | N-RS% (g/100 g) | RS% (g/100 g) | TS% (g/100 g) | TDF% (g/100 g) |
|---|---|---|---|---|
| Cavendish | 23.39 ± 0.13 a | 31.46 ± 0.17 c | 54.85 ± 0.11 b | 31.28 ± 0.38 b |
| Ladyfinger | 11.89 ± 0.18 c | 48.99 ± 0.21 a | 60.88 ± 0.04 a | 31.01 ± 0.66 b |
| Ducasse | 16.22 ± 0.20 b | 35.22 ± 0.08 b | 51.44 ± 0.12 c | 38.72 ± 0.25 a |
| No | Compound | Oral BIA (%) | Gastric BIA (%) | Intestinal BIA (%) | Colonic BIA (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cavendish | Ladyfinger | Ducasse | Cavendish | Ladyfinger | Ducasse | Cavendish | Ladyfinger | Ducasse | Cavendish | Ladyfinger | Ducasse | ||
| 1 | Gallic acid | 27.63 | 26.98 | 28.96 | 29.08 | 36.19 | 39.58 | 79.11 | 68.99 | 41.50 | 5.56 | 6.75 | 8.25 |
| 2 | Protocatechuic acid | 30.15 | 32.67 | 30.57 | 40.35 | 43.68 | 56.94 | 54.41 | 61.26 | 36.67 | 6.99 | 6.67 | 8.11 |
| 3 | Caftaric acid | 0.00 | 0.00 | 0.00 | 47.62 | 46.79 | 49.24 | 52.38 | 53.21 | 57.06 | 10.65 | 11.63 | 0.00 |
| 4 | P-hydroxybenzoic acid | 28.87 | 31.66 | 28.87 | 32.45 | 40.31 | 36.51 | 45.82 | 37.24 | 42.41 | 6.73 | 8.19 | 6.95 |
| 5 | Catechin | 27.54 | 26.62 | 27.39 | 51.55 | 58.57 | 50.69 | 71.56 | 64.59 | 30.21 | 6.69 | 8.15 | 0.00 |
| 6 | Chlorogenic acid | 33.24 | 32.84 | 30.79 | 38.45 | 41.38 | 54.54 | 49.85 | 48.43 | 55.50 | 8.66 | 0.00 | 12.66 |
| 7 | Caffeic acid | 0.00 | 0.00 | 27.07 | 49.99 | 48.17 | 33.23 | 51.60 | 50.35 | 25.69 | 5.23 | 6.30 | 7.64 |
| 8 | Syringic acid | 27.34 | 30.32 | 29.14 | 33.18 | 36.15 | 32.77 | 50.84 | 39.68 | 34.30 | 4.76 | 5.77 | 0.00 |
| 9 | Epicatechin | 33.09 | 32.49 | 32.63 | 40.70 | 47.76 | 55.47 | 51.62 | 35.17 | 52.27 | 0.00 | 0.00 | 0.00 |
| 10 | Coumaric acid | 22.27 | 24.03 | 25.68 | 33.25 | 33.29 | 50.72 | 47.25 | 25.14 | 57.68 | 4.80 | 5.77 | 7.01 |
| 11 | polydatin | 26.03 | 21.57 | 24.82 | 34.91 | 30.55 | 40.06 | 43.39 | 33.44 | 36.76 | 1.23 | 0.00 | 0.00 |
| 12 | Diosmin | 15.79 | 26.45 | 17.20 | 27.76 | 32.72 | 24.79 | 38.51 | 35.99 | 27.30 | 0.13 | 0.25 | 0.18 |
| 13 | Resveratrol | 32.92 | 31.20 | 30.90 | 40.67 | 41.51 | 48.35 | 57.78 | 19.12 | 49.76 | 4.92 | 0.00 | 0.00 |
| 14 | Quercetin | 37.60 | 36.72 | 33.26 | 44.14 | 42.74 | 40.25 | 53.48 | 30.86 | 42.42 | 33.66 | 40.51 | 49.21 |
| 15 | Kaempferol | 20.62 | 13.71 | 22.09 | 34.75 | 33.31 | 36.46 | 33.45 | 28.67 | 35.92 | 0.00 | 0.00 | 0.00 |
| Total phenolic compounds | 27.91 | 26.06 | 27.53 | 40.41 | 41.17 | 43.45 | 51.26 | 38.72 | 42.24 | 22.08 | 18.18 | 15.34 | |
| No | Compound | Colonic Recovery (%) | Residual Colonic Digesta Index (%) | ||||
|---|---|---|---|---|---|---|---|
| Cavendish | Ladyfinger | Ducasse | Cavendish | Ladyfinger | Ducasse | ||
| 1 | Gallic acid | 11.79 | 16.92 | 14.80 | 6.23 | 10.17 | 6.55 |
| 2 | Protocatechuic acid | 16.46 | 18.12 | 15.52 | 9.47 | 11.44 | 7.42 |
| 3 | Caftaric acid | 24.08 | 31.65 | 13.01 | 13.44 | 20.02 | 13.01 |
| 4 | P-hydroxybenzoic acid | 13.18 | 18.50 | 13.32 | 6.45 | 10.31 | 6.36 |
| 5 | Catechin | 15.69 | 8.15 | 9.03 | 9.00 | 0.00 | 9.03 |
| 6 | Chlorogenic acid | 20.43 | 18.45 | 24.40 | 11.77 | 18.45 | 11.74 |
| 7 | Caffeic acid | 5.23 | 6.30 | 14.69 | 0.00 | 0.00 | 7.04 |
| 8 | Syringic acid | 4.76 | 5.77 | 0.00 | 0.00 | 0.00 | 0.00 |
| 9 | Epicatechin | 11.05 | 16.77 | 0.00 | 11.05 | 16.77 | 0.00 |
| 10 | Coumaric acid | 4.80 | 5.77 | 7.01 | 0.00 | 0.00 | 0.00 |
| 11 | polydatin | 2.90 | 2.54 | 1.65 | 1.68 | 2.54 | 1.65 |
| 12 | Diosmin | 0.13 | 0.25 | 0.18 | 0.00 | 0.00 | 0.00 |
| 13 | Resveratrol | 4.92 | 10.29 | 6.71 | 0.00 | 10.29 | 6.71 |
| 14 | Quercetin | 33.66 | 40.51 | 49.21 | 0.00 | 0.00 | 0.00 |
| 15 | Kaempferol | 30.91 | 0.00 | 30.50 | 30.91 | 0.00 | 30.50 |
| Total phenolics | 27.66 | 32.05 | 27.79 | 84.51 | 77.07 | 79.75 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashmil, Y.M.; Dunshea, F.R.; Appels, R.; Suleria, H.A.R. Bioaccessibility of Phenolic Compounds, Resistant Starch, and Dietary Fibers from Australian Green Banana during In Vitro Digestion and Colonic Fermentation. Molecules 2024, 29, 1535. https://doi.org/10.3390/molecules29071535
Bashmil YM, Dunshea FR, Appels R, Suleria HAR. Bioaccessibility of Phenolic Compounds, Resistant Starch, and Dietary Fibers from Australian Green Banana during In Vitro Digestion and Colonic Fermentation. Molecules. 2024; 29(7):1535. https://doi.org/10.3390/molecules29071535
Chicago/Turabian StyleBashmil, Yasmeen M., Frank R. Dunshea, Rudi Appels, and Hafiz A. R. Suleria. 2024. "Bioaccessibility of Phenolic Compounds, Resistant Starch, and Dietary Fibers from Australian Green Banana during In Vitro Digestion and Colonic Fermentation" Molecules 29, no. 7: 1535. https://doi.org/10.3390/molecules29071535
APA StyleBashmil, Y. M., Dunshea, F. R., Appels, R., & Suleria, H. A. R. (2024). Bioaccessibility of Phenolic Compounds, Resistant Starch, and Dietary Fibers from Australian Green Banana during In Vitro Digestion and Colonic Fermentation. Molecules, 29(7), 1535. https://doi.org/10.3390/molecules29071535









