Stabilizing BiVO4 Photoanode in Bicarbonate Electrolyte for Efficient Photoelectrocatalytic Alcohol Oxidation
Abstract
:1. Introduction
2. Results
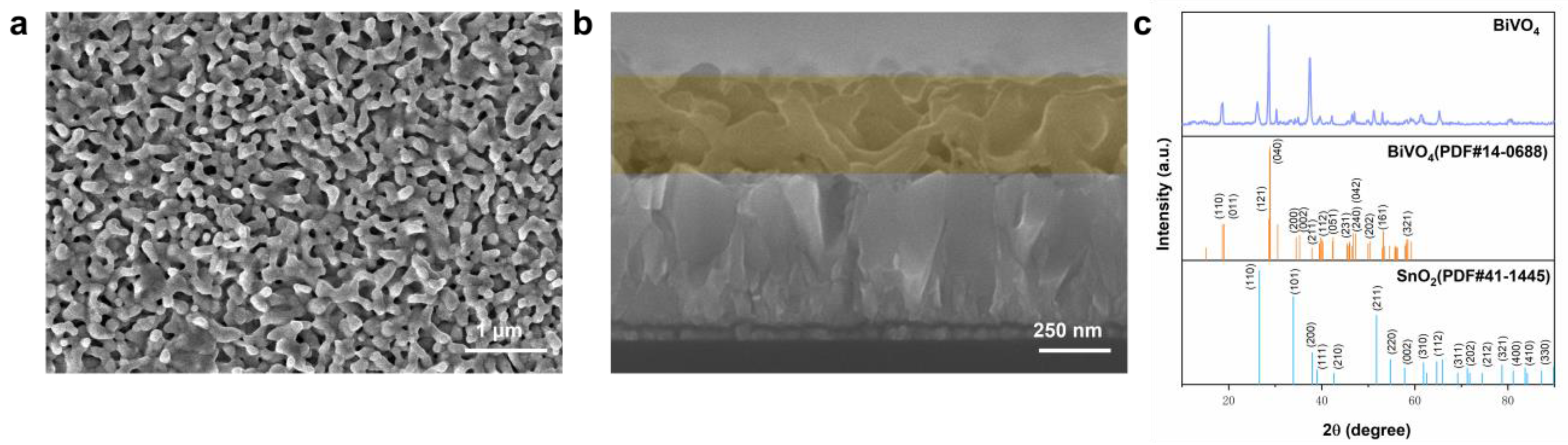
2.1. Structure of a Typical BVO
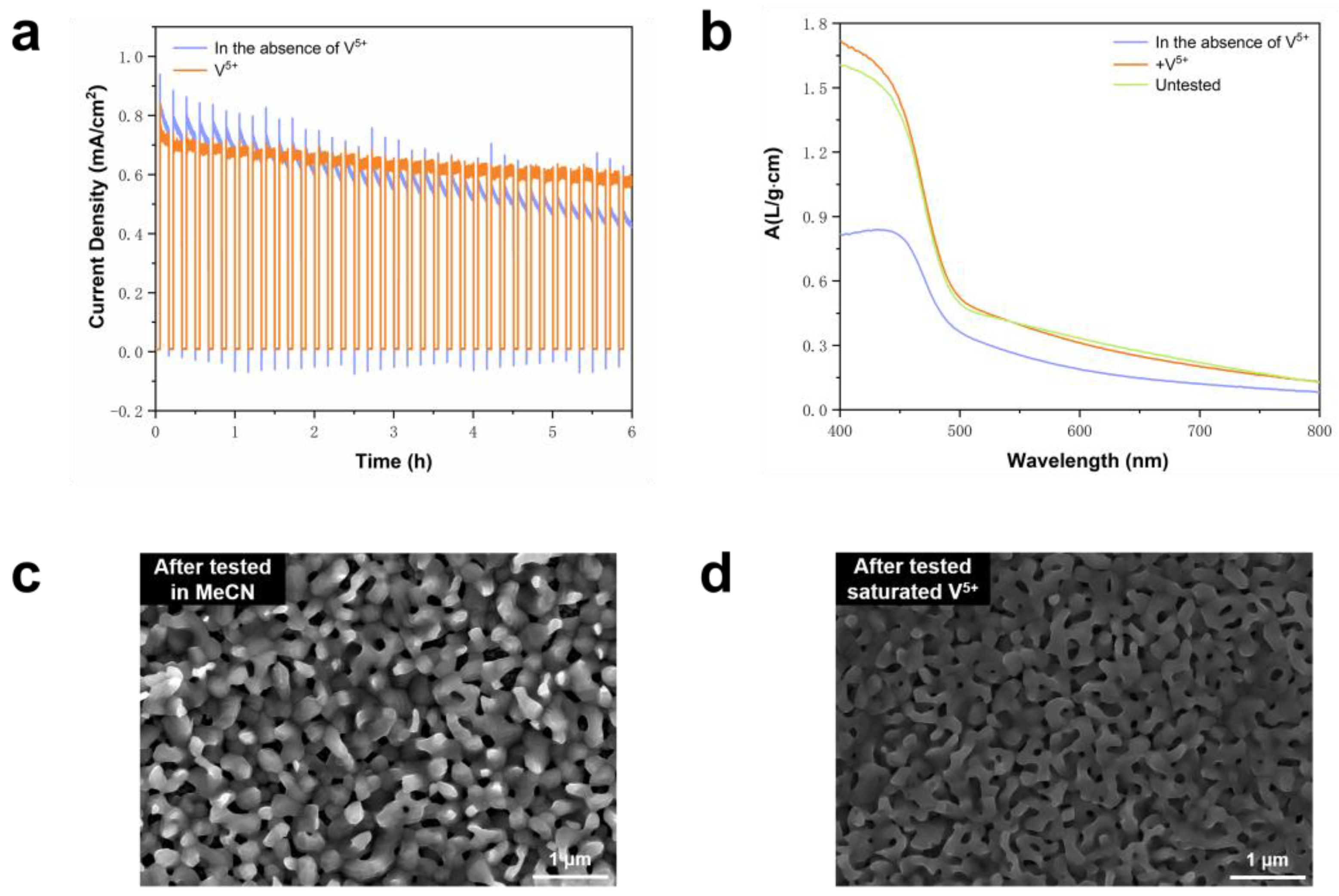
2.2. Introduction of Saturated V5+ for Improved Stability
2.3. BVO Oxidizes Benzyl Alcohol in Different Electrolytes
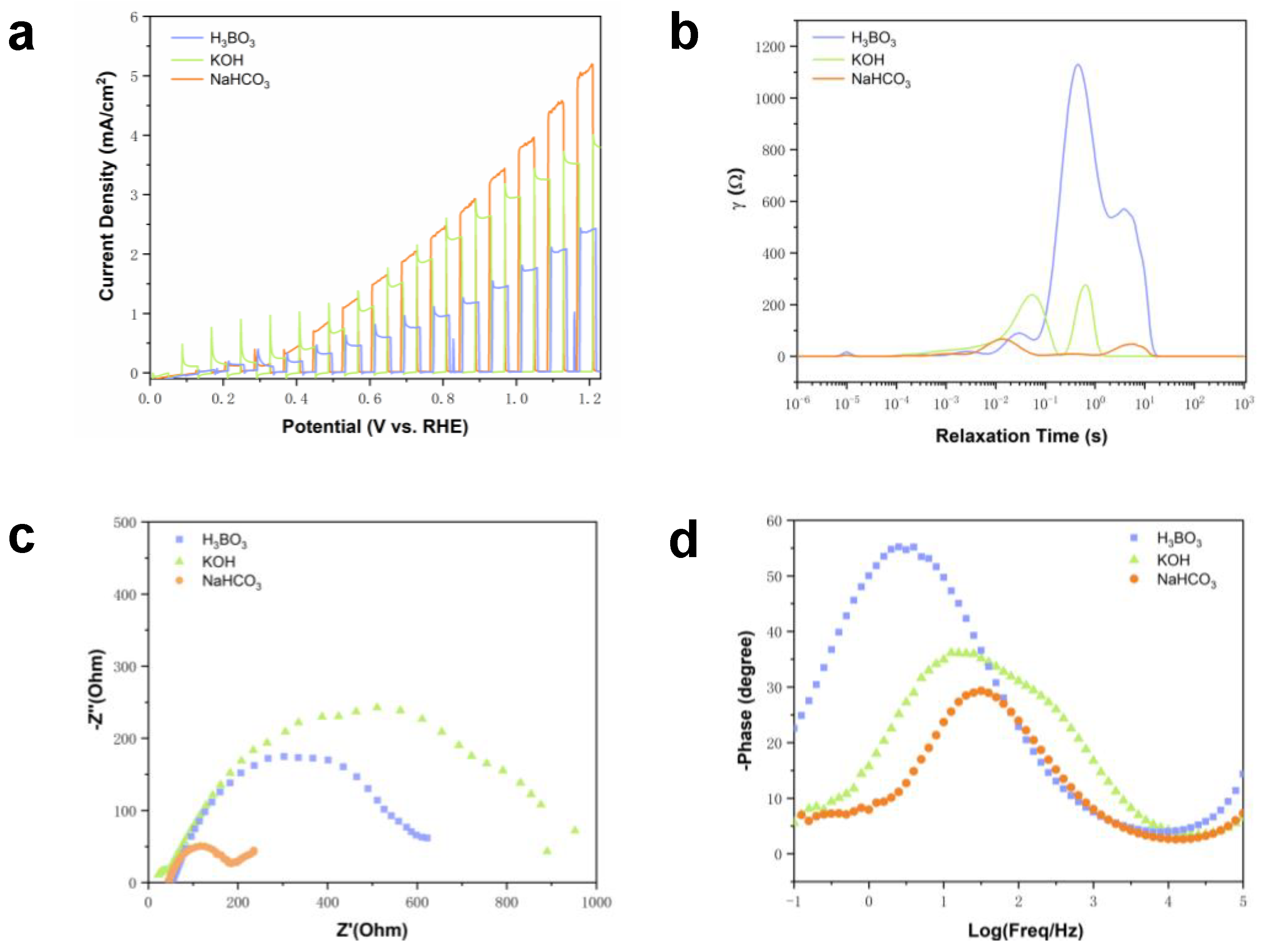
2.4. Influence of Solvent on Electrolyte System
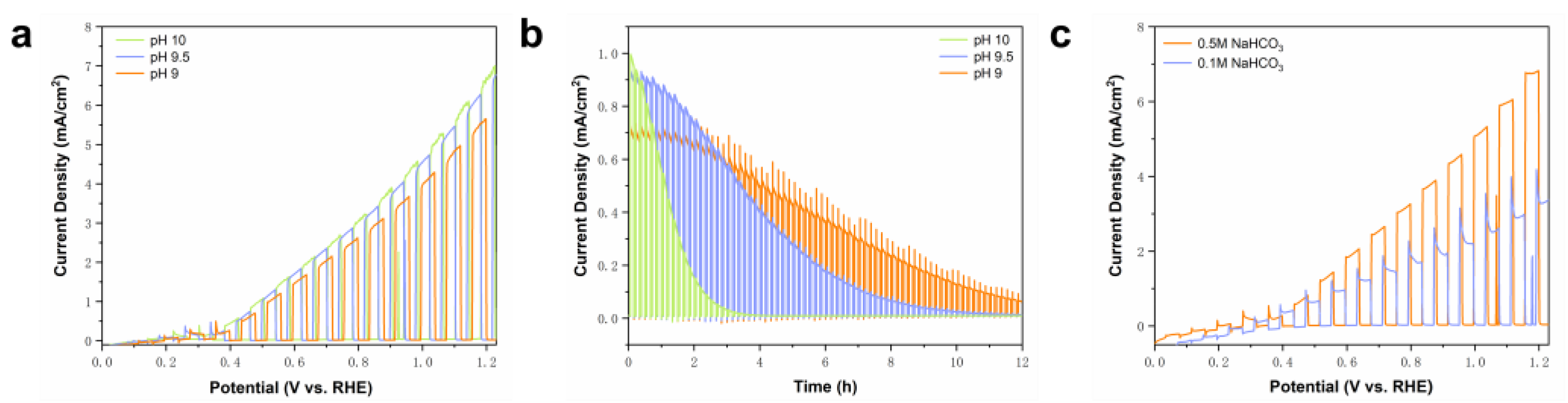
2.5. HCO3− Mediated the Oxidation of Benzyl Alcohol by BVO
3. Discussion
4. Materials and Methods
4.1. All the Chemical Reagents and Manufacturers in the Experiment
4.2. Preparation Method of BVO
4.3. PEC Test Method
4.4. Material Characterization Equipment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, T.W.; Choi, K.-S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]
- Kuang, Y.; Jia, Q.; Nishiyama, H.; Yamada, T.; Kudo, A.; Domen, K. A Front-Illuminated Nanostructured Transparent BiVO4 Photoanode for >2% Efficient Water Splitting. Adv. Energy Mater. 2016, 6, 1501645. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.S. Elaborately Modified BiVO4 Photoanodes for Solar Water Splitting. Adv. Mater. 2019, 31, 1806938. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Nguyen, V.-H.; Nanda, S.; Vo, D.-V.N.; Nguyen, V.H.; Van Tran, T.; Nong, L.X.; Nguyen, T.T.; Bach, L.-G.; Abdullah, B.; et al. BiVO4 Photocatalysis Design and Applications to Oxygen Production and Degradation of Organic Compounds: A Review. Environ. Chem. Lett. 2020, 18, 1779–1801. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Arotiba, O.A. Bismuth Vanadate in Photoelectrocatalytic Water Treatment Systems for the Degradation of Organics: A Review on Recent Trends. J. Electroanal. Chem. 2020, 878, 114724. [Google Scholar] [CrossRef]
- Li, T.; Kasahara, T.; He, J.; Dettelbach, K.E.; Sammis, G.M.; Berlinguette, C.P. Photoelectrochemical Oxidation of Organic Substrates in Organic Media. Nat. Commun. 2017, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; Song, R.-J.; Li, J.-H. Recent Advances in Photoelectrochemical Cells (PECs) for Organic Synthesis. Org. Chem. Front. 2020, 7, 1895–1902. [Google Scholar] [CrossRef]
- You, B.; Liu, X.; Liu, X.; Sun, Y. Efficient H2 Evolution Coupled with Oxidative Refining of Alcohols via a Hierarchically Porous Nickel Bifunctional Electrocatalyst. ACS Catal. 2017, 7, 4564–4570. [Google Scholar] [CrossRef]
- Stuart, A.V.; Sutherland, G.B.B.M. Effect of Hydrogen Bonding on the Deformation Frequencies of the Hydroxyl Group in Alcohols. J. Chem. Phys. 1956, 24, 559–570. [Google Scholar] [CrossRef]
- Liao, L.; Ditz, D.; Zeng, F.; Alves Favaro, M.; Iemhoff, A.; Gupta, K.; Hartmann, H.; Szczuka, C.; Jakes, P.; Hausoul, P.J.C.; et al. Efficient Photocatalytic Oxidation of Aromatic Alcohols over Thiophene-based Covalent Triazine Frameworks with A Narrow Band Gap. ChemistrySelect 2020, 5, 14438–14446. [Google Scholar] [CrossRef]
- Yurdakal, S.; Augugliaro, V. Partial Oxidation of Aromatic Alcohols via TiO2 Photocatalysis: The Influence of Substituent Groups on the Activity and Selectivity. RSC Adv. 2012, 2, 8375. [Google Scholar] [CrossRef]
- Samanta, S.; Khilari, S.; Pradhan, D.; Srivastava, R. An Efficient, Visible Light Driven, Selective Oxidation of Aromatic Alcohols and Amines with O2 Using BiVO4/g-C3N4 Nanocomposite: A Systematic and Comprehensive Study toward the Development of a Photocatalytic Process. ACS Sustain. Chem. Eng. 2017, 5, 2562–2577. [Google Scholar] [CrossRef]
- Long, B.; Ding, Z.; Wang, X. Carbon Nitride for the Selective Oxidation of Aromatic Alcohols in Water under Visible Light. ChemSusChem 2013, 6, 2074–2078. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.S.; Jeon, I.S.; Jang, S.H.; Park, S.W.; Lee, S.; Cheong, W.J. Hydrogen Bonding in Aromatic Alcohol-Water Clusters: A Brief Review. Bull. Korean Chem. Soc. 2003, 24, 695–702. [Google Scholar] [CrossRef]
- Klinova McMillan, N.; Lopez, D.A.; Leem, G.; Sherman, B.D. BiVO4 Photoanodes for TEMPO-Mediated Benzyl Alcohol Oxidation in Organic Media. ChemPlusChem 2022, 87, e202200187. [Google Scholar] [CrossRef] [PubMed]
- ElMetwally, A.E.; Sayed, M.S.; Shim, J.-J.; Knecht, M.R.; Bachas, L.G. Plasmon-Enhanced Photocatalytic Oxidation of Benzyl Alcohol to Benzaldehyde Using BiVO4/BiOBr/Au Nanosheets. ACS Appl. Nano Mater. 2023, 6, 5909–5917. [Google Scholar] [CrossRef]
- Xu, C.; Yang, F.; Deng, B.; Zhuang, Y.; Li, D.; Liu, B.; Yang, W.; Li, Y. Ti3C2/TiO2 Nanowires with Excellent Photocatalytic Performance for Selective Oxidation of Aromatic Alcohols to Aldehydes. J. Catal. 2020, 383, 1–12. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Z.; Xiang, X.; Yan, D.; Ye, J. Selective Activation of Benzyl Alcohol Coupled with Photoelectrochemical Water Oxidation via a Radical Relay Strategy. ACS Catal. 2020, 10, 4906–4913. [Google Scholar] [CrossRef]
- Lee, D.K.; Choi, K.-S. Enhancing Long-Term Photostability of BiVO4 Photoanodes for Solar Water Splitting by Tuning Electrolyte Composition. Nat. Energy 2017, 3, 53–60. [Google Scholar] [CrossRef]
- Jie, Y.; Iocozzia, J.; Huang, J.; Meng, K.; Lai, Y.; Lin, Z. HCO3− Mediated Highly Efficient Photoelectrochemical Dioxygenation of Arylalkenes: Triple Roles of HCO3−-Derived Radicals. Energy Environ. Sci. 2023, 11, 772–799. [Google Scholar] [CrossRef]
- DiMeglio, J.L.; Terry, B.D.; Breuhaus-Alvarez, A.G.; Whalen, M.J.; Bartlett, B.M. Base-Assisted Nitrate Mediation as the Mechanism of Electrochemical Benzyl Alcohol Oxidation. J. Phys. Chem. C 2021, 125, 8148–8154. [Google Scholar] [CrossRef]
- Schanz, T.; Burek, B.O.; Bloh, J.Z. Fate and Reactivity of Peroxides Formed over BiVO4 Anodes in Bicarbonate Electrolytes. ACS Energy Lett. 2023, 8, 1463–1467. [Google Scholar] [CrossRef]
- Kanigaridou, Y.; Petala, A.; Frontistis, Z.; Antonopoulou, M.; Solakidou, M.; Konstantinou, I.; Deligiannakis, Y.; Mantzavinos, D.; Kondarides, D.I. Solar Photocatalytic Degradation of Bisphenol A with CuOx/BiVO4: Insights into the Unexpectedly Favorable Effect of Bicarbonates. Chem. Eng. J. 2017, 318, 39–49. [Google Scholar] [CrossRef]
- Pop, L.-C.; Sfaelou, S.; Lianos, P. Cation Adsorption by Mesoporous Titania Photoanodes and Its Effect on the Current-Voltage Characteristics of Photoelectrochemical Cells. Electrochim. Acta 2015, 156, 223–227. [Google Scholar] [CrossRef]
- Sfaelou, S.; Pop, L.-C.; Monfort, O.; Dracopoulos, V.; Lianos, P. Mesoporous WO3 Photoanodes for Hydrogen Production by Water Splitting and PhotoFuelCell Operation. Int. J. Hydrog. Energy 2016, 41, 5902–5907. [Google Scholar] [CrossRef]
- Monfort, O.; Pop, L.-C.; Sfaelou, S.; Plecenik, T.; Roch, T.; Dracopoulos, V.; Stathatos, E.; Plesch, G.; Lianos, P. Photoelectrocatalytic Hydrogen Production by Water Splitting Using BiVO4 Photoanodes. Chem. Eng. J. 2016, 286, 91–97. [Google Scholar] [CrossRef]
- Saito, R.; Miseki, Y.; Sayama, K. Photoanode Characteristics of Multi-Layer Composite BiVO4 Thin Film in a Concentrated Carbonate Electrolyte Solution for Water Splitting. J. Photochem. Photobiol. Chem. 2013, 258, 51–60. [Google Scholar] [CrossRef]
- Da Silva, M.R.; Lucilha, A.C.; Afonso, R.; Dall’Antonia, L.H.; De Andrade Scalvi, L.V. Photoelectrochemical Properties of FTO/m-BiVO4 Electrode in Different Electrolytes Solutions under Visible Light Irradiation. Ionics 2014, 20, 105–113. [Google Scholar] [CrossRef]
- Tian, Z.; Wu, S.; Wang, P.; Cai, Y.; Liang, D.; Ye, Y.; Liu, J.; Liang, C. Aqueous Dispersed Ablated Bismuth Species and Their Potential as Colloidal Bi Precursors in Synthetic Strategies. CrystEngComm 2015, 17, 3015–3022. [Google Scholar] [CrossRef]
- Nassiri, C.; Hadri, A.; Chafi, F.; Rouchdi, M.; Fares, B.; Mzerd, A. Structural, Optical and Electrical Properties of Fe Doped SnO2 Prepared by Spray Pyrolysis. J. Mater. Environ. Sci. 2017, 8, 420–425. [Google Scholar]
- Liu, J.; Wang, H.; Wang, S.; Yan, H. Hydrothermal Preparation of BiVO4 Powders. Mater. Sci. Eng. B 2003, 104, 36–39. [Google Scholar] [CrossRef]
- Gryglewicz, S. Alkaline-Earth Metal Compounds as Alcoholysis Catalysts for Ester Oils Synthesis. Appl. Catal. Gen. 2000, 192, 23–28. [Google Scholar] [CrossRef]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRTtools. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Meng, L.; Bae, S.; Erni, R.; Abbott, D.F.; Li, S.; Triana, C.A.; Mougel, V.; Patzke, G.R. Modulating Carrier Kinetics in BiVO4 Photoanodes through Molecular Co4O4 Cubane Layers. Adv. Funct. Mater. 2023, 33, 2307862. [Google Scholar] [CrossRef]
- Bommineedi, L.K.; Pandit, B.; Sankapal, B.R. Spongy Nano Surface Architecture of Chemically Grown BiVO4: High-Capacitance Retentive Electrochemical Supercapacitor. Int. J. Hydrog. Energy 2021, 46, 25586–25595. [Google Scholar] [CrossRef]
- Fuku, K.; Sayama, K. Efficient Oxidative Hydrogen Peroxide Production and Accumulation in Photoelectrochemical Water Splitting Using a Tungsten Trioxide/Bismuth Vanadate Photoanode. Chem. Commun. 2016, 52, 5406–5409. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Magesh, G.; Kang, H.J.; Banu, M.; Kim, J.H.; Lee, J.; Lee, J.S. Carbonate-Coordinated Cobalt Co-Catalyzed BiVO4/WO3 Composite Photoanode Tailored for CO2 Reduction to Fuels. Nano Energy 2015, 15, 153–163. [Google Scholar] [CrossRef]
- Thalluri, S.M.; Rojas, R.M.; Rivera, O.D.; Hernández, S.; Russo, N.; Rodil, S.E. Chemically Induced Porosity on BiVO4 Films Produced by Double Magnetron Sputtering to Enhance the Photo-Electrochemical Response. Phys. Chem. Chem. Phys. 2015, 17, 17821–17827. [Google Scholar] [CrossRef]
- Cui, J.; Daboczi, M.; Cui, Z.; Gong, M.; Flitcroft, J.; Skelton, J.; Parker, S.C.; Eslava, S. BiVO4 Photoanodes Enhanced with Metal Phosphide Co-Catalysts: Relevant Properties to Boost Photoanode Performance. Small 2024, 20, 2306757. [Google Scholar] [CrossRef]
- Chen, S.-N.; Hoffman, M.Z.; Parsons, G.H. Reactivity of the Carbonate Radical toward Aromatic Compounds in Aqueous Solution. J. Phys. Chem. 1975, 79, 1911–1912. [Google Scholar] [CrossRef]
- Bonini, M.G.; Radi, R.; Ferrer-Sueta, G.; Ferreira, A.M.D.C.; Augusto, O. Direct EPR Detection of the Carbonate Radical Anion Produced from Peroxynitrite and Carbon Dioxide. J. Biol. Chem. 1999, 274, 10802–10806. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.I.; Sharmin, F.A.; Akhter, S.; Bhuiyan, M.A.; Shahriar, M. Investigation of Cytotoxicity and In-Vitro Antioxidant Activity of Asparagus Racemosus Root Extract. Int. Curr. Pharm. J. 2012, 1, 250–257. [Google Scholar] [CrossRef]
- Ouyang, T.; Ye, Y.-Q.; Tan, C.; Guo, S.-T.; Huang, S.; Zhao, R.; Zhao, S.; Liu, Z.-Q. 1D α-Fe2O3/ZnO Junction Arrays Modified by Bi as Photocathode: High Efficiency in Photoelectrochemical Reduction of CO2 to HCOOH. J. Phys. Chem. Lett. 2022, 13, 6867–6874. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Feng, F.; Huang, R.; Ouyang, T.; Liu, J.; Liu, Z. Activating C–H Bonds by Tuning Fe Sites and an Interfacial Effect for Enhanced Methanol Oxidation. Adv. Mater. 2022, 34, 2208438. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ouyang, T.; Zheng, B.; Dan, M.; Liu, Z. Enhanced Photoelectrocatalytic Activities for CH3OH-to-HCHO Conversion on Fe2O3/MoO3: Fe-O-Mo Covalency Dominates the Intrinsic Activity. Angew. Chem. Int. Ed. 2021, 60, 9546–9552. [Google Scholar] [CrossRef]
- Beck, W.C.; Grossman, E.L.; Morse, J.W. Experimental Studies of Oxygen Isotope Fractionation in the Carbonic Acid System at 15°, 25°, and 40°C. Geochim. Cosmochim. Acta 2005, 69, 3493–3503. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, H.; An, S.; Qin, W.; Kuang, Y.; Liu, D. Stabilizing BiVO4 Photoanode in Bicarbonate Electrolyte for Efficient Photoelectrocatalytic Alcohol Oxidation. Molecules 2024, 29, 1554. https://doi.org/10.3390/molecules29071554
Gong H, An S, Qin W, Kuang Y, Liu D. Stabilizing BiVO4 Photoanode in Bicarbonate Electrolyte for Efficient Photoelectrocatalytic Alcohol Oxidation. Molecules. 2024; 29(7):1554. https://doi.org/10.3390/molecules29071554
Chicago/Turabian StyleGong, Haorui, Sai An, Weilong Qin, Yongbo Kuang, and Deyu Liu. 2024. "Stabilizing BiVO4 Photoanode in Bicarbonate Electrolyte for Efficient Photoelectrocatalytic Alcohol Oxidation" Molecules 29, no. 7: 1554. https://doi.org/10.3390/molecules29071554
APA StyleGong, H., An, S., Qin, W., Kuang, Y., & Liu, D. (2024). Stabilizing BiVO4 Photoanode in Bicarbonate Electrolyte for Efficient Photoelectrocatalytic Alcohol Oxidation. Molecules, 29(7), 1554. https://doi.org/10.3390/molecules29071554




