Inclusion Complexes of Litsea cubeba (Lour.) Pers Essential Oil into β-Cyclodextrin: Preparation, Physicochemical Characterization, Cytotoxicity and Antifungal Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Complexation Efficiency (CE)
2.2. Physicochemical Properties of Inclusion Complexes
2.2.1. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR)
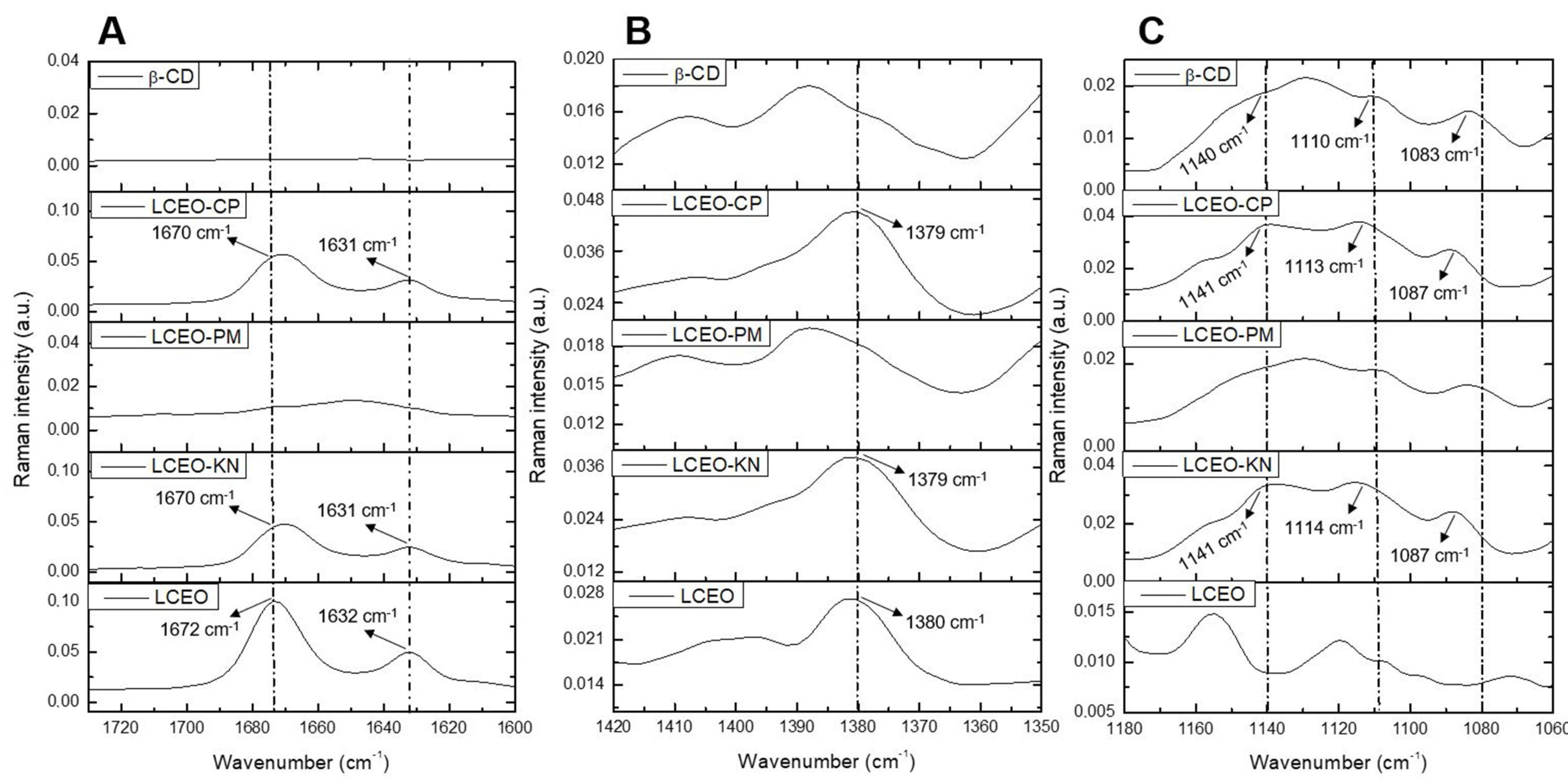
2.2.2. Fourier Transform Raman Spectroscopy (FT-Raman)
2.2.3. Thermal Analysis
2.3. Cytotoxicity of Inclusion Complexes
2.4. Antifungal Activity of Inclusion Complexes
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Inclusion Complexes
3.2.1. Physical Mixture
3.2.2. Kneading
3.2.3. Co-Precipitation
3.3. Complexation Efficiency (CE)
3.4. Physicochemical Properties of Inclusion Complexes
3.4.1. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.4.2. Fourier Transform Raman Spectroscopy (FT-Raman)
3.4.3. Thermal Analysis
3.5. Cytotoxicity of Inclusion Complexes
3.5.1. Cell Cultures
3.5.2. MTT Assay
3.6. Antifungal Activity of Inclusion Complexes
3.6.1. Microorganisms
3.6.2. Agar Dilution Method
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Lee, K.E.; Bajpai, V.K.; Gajurel, P.R.; Gu, K.S.; Kumar, P. Ethnopharmacological properties and medicinal uses of Litsea cubeba. Plants 2019, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-h.; Li, X.-Z.; Zhang, S. Preparation methods and release kinetics of Litsea cubeba essential oil microcapsules. RSC Adv. 2018, 8, 29980–29987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Zheng, L.H.; Zhao, K.; Chen, Y.; Yi, Z. Insecticidal activities of constituents of Litsea cubeba fruit extracts effective against the maize weevil (Coleoptera: Curculionidae). J. Insect Sci. 2017, 17, 1–6. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Ying, G.; Yang, M.; Nian, Y.; Wei, F.; Kong, W. Antifungal evaluation of plant essential oils and their major components against toxigenic fungi. Ind. Crops Prod. 2018, 120, 180–186. [Google Scholar] [CrossRef]
- Pante, G.C.; Castro, J.C.; Lini, R.S.; Romoli, J.C.Z.; Almeida, R.T.R.; Garcia, F.P.; Nakamura, C.V.; Pilau, E.J.; Abreu Filho, B.A.; Machinski, M. Litsea cubeba essential oil: Chemical profile, antioxidant activity, cytotoxicity, effect against Fusarium verticillioides and fumonisins production. J. Environ. Sci. Health B. 2021, 56, 1–9. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The composition, pharmacological and economic importance of essential oil of Litsea cubeba (Lour.) Pers. Food Sci. Technol. 2022, 42, 1–7. [Google Scholar] [CrossRef]
- Costa, P.; Medronho, B.; Gonçalves, S.; Romano, A. Cyclodextrins enhance the antioxidant activity of essential oils from three Lamiaceae species. Ind. Crops Prod. 2015, 70, 341–346. [Google Scholar] [CrossRef]
- Galvão, J.G.; Silva, V.F.; Ferreira, S.G.; França, F.R.M.; Santos, D.A.; Freitas, L.S.; Alves, P.B.; Araújo, A.A.S.; Cavalcanti, S.C.H.; Nunes, R.S. β-cyclodextrin inclusion complexes containing Citrus sinensis (L.) Osbeck essential oil: An alternative to control Aedes aegypti larvae. Thermochim. Acta 2015, 608, 14–19. [Google Scholar] [CrossRef]
- Miyoshi, J.H.; Castro, J.C.; Fenelon, V.C.; Garcia, F.P.; Nakamura, C.V.; Nogueira, A.C.; Ueda-Nakamura, T.; Souza, H.M.; Mangolim, C.S.; Moura-Costa, G.F.; et al. Essential oil characterization of Ocimum basilicum and Syzygium aromaticum free and complexed with β-cyclodextrin. Determination of its antioxidant, antimicrobial, and antitumoral activities. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 117–132. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Medina Neto, A.; Matioli, G. Curcumin-β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, A.H.; Szeleszczuk, L.; Bethanis, K.; Christoforides, E.; Dudek, M.K.; Zielinska-Pisklak, M.; Pisklak, D.M. 17-β-Estradiol—β-Cyclodextrin Complex as Solid: Synthesis, Structural and Physicochemical Characterization. Molecules 2023, 28, 3747. [Google Scholar] [CrossRef] [PubMed]
- Santana, N.A.; Silva, R.C.S.; Fourmentin, S.; Anjos, K.F.L.; Ootan, M.A.; Silva, A.G.; Araújo, B.G.P.; Correia, M.T.S.; Silva, M.V.; Machado, G. Synthesis, characterization and cytotoxicity of the Eugenia brejoensis essential oil inclusion complex with β-cyclodextrin. J. Drug Deliv. Sci. Technol. 2020, 60, 1–8. [Google Scholar] [CrossRef]
- Halahlah, A.; Kavetsou, E.; Pitterou, I.; Grigorakis, S.; Loupassaki, S.; Tziveleka, L.-A.; Kikionis, S.; Ioannou, E.; Detsi, A. Synthesis and characterization of inclusion complexes of rosemary essential oil with various β-cyclodextrins and evaluation of their antibacterial activity against Staphylococcus aureus. J. Drug Deliv. Sci. Technol. 2021, 65, 1–11. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Preparation and antibacterial activity of Litsea cubeba essential oil/dandelion polysaccharide nanofiber. Ind. Crops Prod. 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, C.; Cheng, X.; Li, G.; Yang, S.; Zhu, X. β-cyclodextrin inclusion complex containing Litsea cubeba essential oil: Preparation, optimization, physicochemical, and antifungal characterization. Coatings 2020, 10, 850. [Google Scholar] [CrossRef]
- Răileanu, M.; Todan, L.; Voicescu, M.; Ciuculescu, C.; Maganu, M. A way for improving the stability of the essential oils in an environmental friendly formulation. Mater. Sci. Eng. C. 2013, 33, 3281–3288. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, B.; Sheng, A.; Yang, F.; Wang, Z. Spectroscopic and theoretical study on inclusion complexation of beta-cyclodextrin with permethrin. J. Mol. Struct. 2010, 981, 194–203. [Google Scholar] [CrossRef]
- Jentzsch, P.V.; Ramos, L.A.; Ciobotă, V. Handheld Raman spectroscopy for the distinction of essential oils used in the cosmetics industry. Cosmetics 2015, 2, 162–176. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nawaz, H.; Naz, S.; Mukhtar, R.; Rashid, N.; Bhatti, I.A.; Saleem, M. Raman spectroscopy for the characterization of different fractions of hemp essential oil extracted at 130 °C using steam distillation method. Spectrochim. Acta A. 2017, 182, 168–174. [Google Scholar] [CrossRef]
- Rocha Neto, A.C.; Rocha, A.B.O.; Maraschin, M.; Di Piero, R.M.; Almenar, E. Factors affecting the entrapment efficiency of β-cyclodextrins and their effects on the formation of inclusion complexes containing essential oils. Food Hydrocoll. 2018, 77, 509–523. [Google Scholar] [CrossRef]
- Saab, A.M.; Tundis, R.; Loizzo, M.R.; Lampronti, I.; Borgatti, M.; Gambari, R.; Menichini, F.; Esseily, F.; Menichini, F. Antioxidant and antiproliferative activity of Laurus nobilis L. (Lauraceae) leaves and seeds essential oils against K562 human chronic myelogenous leukaemia cells. Nat. Prod. Res. 2012, 26, 1741–1745. [Google Scholar] [CrossRef]
- Bailly, C. Targets and pathways involved in the antitumor activity of citral and its stereo-isomers. Eur. J. Pharmacol. 2020, 871, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Valarini Junior, O.; Dantas, J.H.; Barão, C.E.; Zanoelo, E.F.; Cardozo-Filho, L.; Moraes, F.F. Formation of inclusion compounds of (+)catechin with β-cyclodextrin in different complexation media: Spectral, thermal and antioxidant properties. J. Supercrit. Fluids 2017, 121, 10–18. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.; Mehdizadeh, L.; Najafgholi, H.M.; Pirbalouti, A.G. Chemical composition, antibacterial and antifungal activities of seed essential oil of Ferulago angulate. Int. J. Food Prop. 2018, 21, 158–170. [Google Scholar] [CrossRef]


| Samples | IC50 (ug mL−1) | ||
|---|---|---|---|
| HT-29 | HeLa | Vero | |
| β-CD | >1000 | >1000 | >1000 |
| LCEO-KN | 81.7 ± 7.5 | 88.0 ± 20.8 | 99.0 ± 11.5 |
| LCEO-CP | 74.8 ± 20.9 | 95.0 ± 21.2 | 88.0 ± 5.4 |
| LCEO-PM | 71.5 ± 5.6 | 106.3 ± 11.0 | 90.0 ± 2.6 |
| Samples | Radial Inhibition (%) | |
|---|---|---|
| Aspergillus flavus | Fusarium verticillioides | |
| FC | 0 c ± 0 | 0 d ± 0 |
| β-CD | 0 c ± 0 | 0 d ± 0 |
| LCEO | 10.22 b ± 1.88 | 8.55 c ± 2.43 |
| LCEO-KN | 25.68 a ± 2.44 | 27.41 a ± 2.96 |
| LCEO-CP | 23.92 a ± 0.80 | 23.04 a, b ± 1.61 |
| LCEO-PM | 11.07 b ± 2.25 | 16.20 b ± 2.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pante, G.C.; Castro, J.C.; Lini, R.S.; Romoli, J.C.Z.; Pires, T.Y.; Garcia, F.P.; Nakamura, C.V.; Mulati, A.C.N.; Matioli, G.; Machinski Junior, M. Inclusion Complexes of Litsea cubeba (Lour.) Pers Essential Oil into β-Cyclodextrin: Preparation, Physicochemical Characterization, Cytotoxicity and Antifungal Activity. Molecules 2024, 29, 1626. https://doi.org/10.3390/molecules29071626
Pante GC, Castro JC, Lini RS, Romoli JCZ, Pires TY, Garcia FP, Nakamura CV, Mulati ACN, Matioli G, Machinski Junior M. Inclusion Complexes of Litsea cubeba (Lour.) Pers Essential Oil into β-Cyclodextrin: Preparation, Physicochemical Characterization, Cytotoxicity and Antifungal Activity. Molecules. 2024; 29(7):1626. https://doi.org/10.3390/molecules29071626
Chicago/Turabian StylePante, Giseli Cristina, Juliana Cristina Castro, Renata Sano Lini, Jéssica Cristina Zoratto Romoli, Thiago Yoshioka Pires, Francielle Pelegrin Garcia, Celso Vataru Nakamura, Ana Claúdia Nogueira Mulati, Graciette Matioli, and Miguel Machinski Junior. 2024. "Inclusion Complexes of Litsea cubeba (Lour.) Pers Essential Oil into β-Cyclodextrin: Preparation, Physicochemical Characterization, Cytotoxicity and Antifungal Activity" Molecules 29, no. 7: 1626. https://doi.org/10.3390/molecules29071626