Robust Thiazole-Linked Covalent Organic Frameworks for Water Sensing with High Selectivity and Sensitivity
Abstract
1. Introduction
2. Results and Discussion
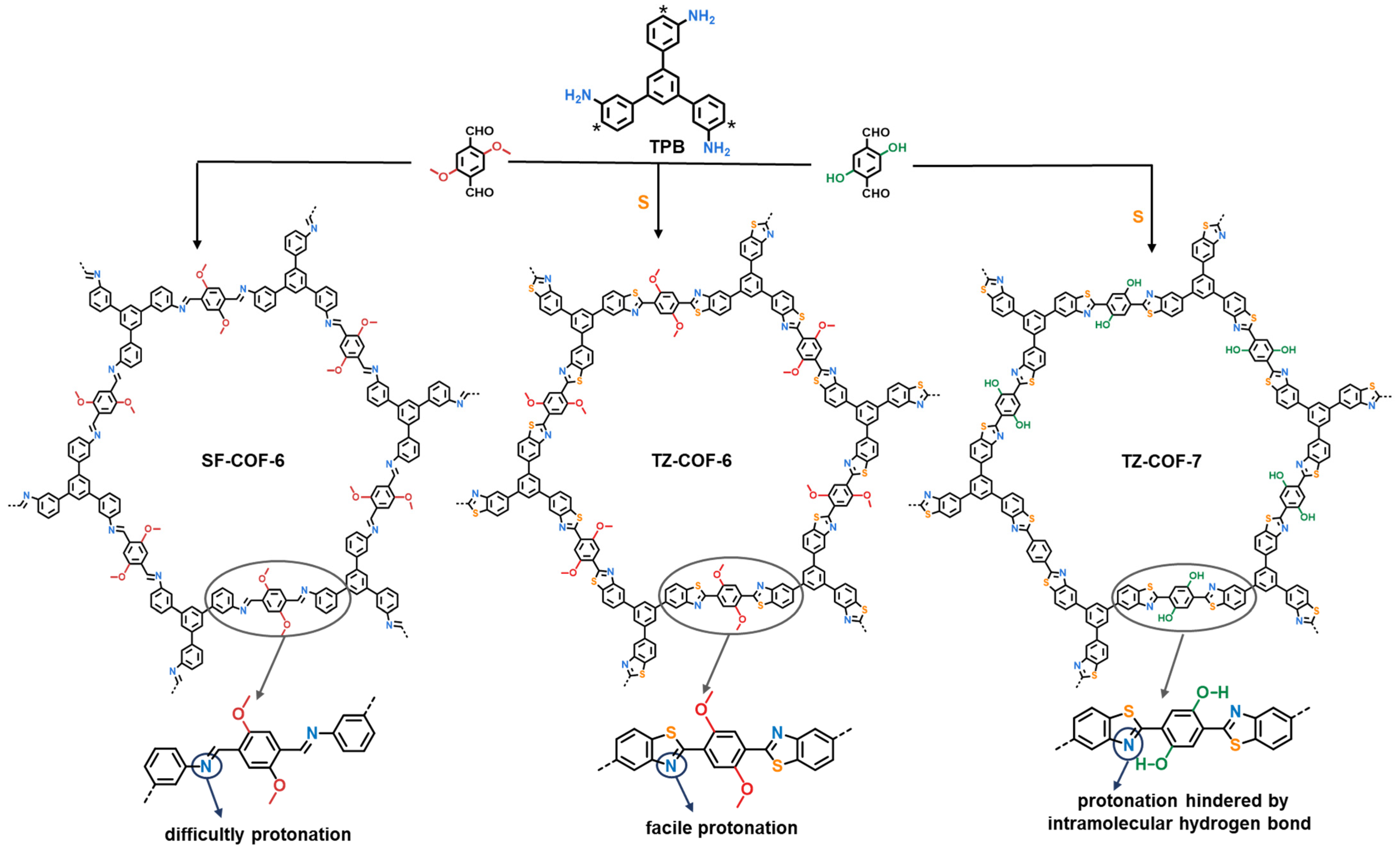
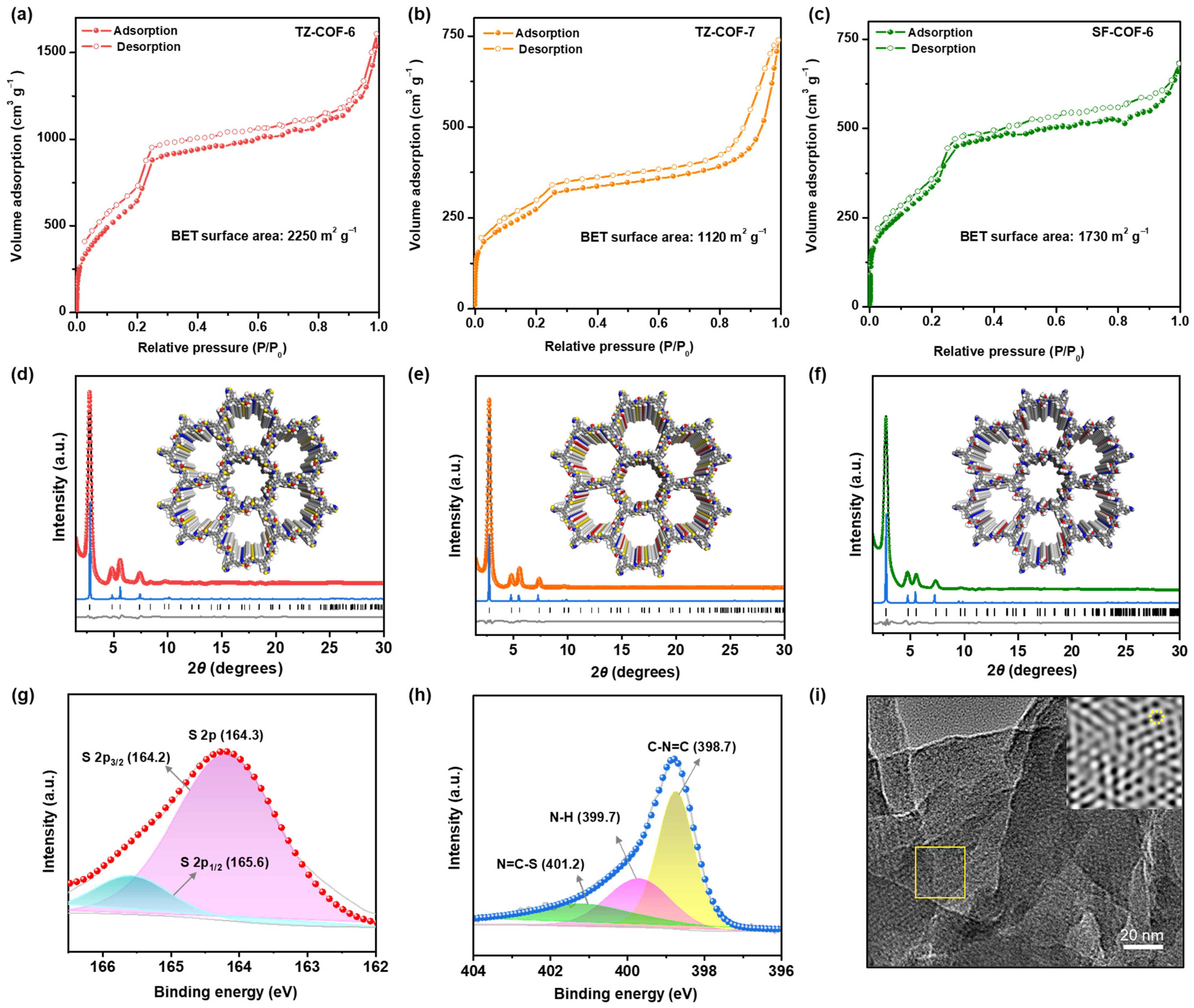
2.1. Characterization of COFs
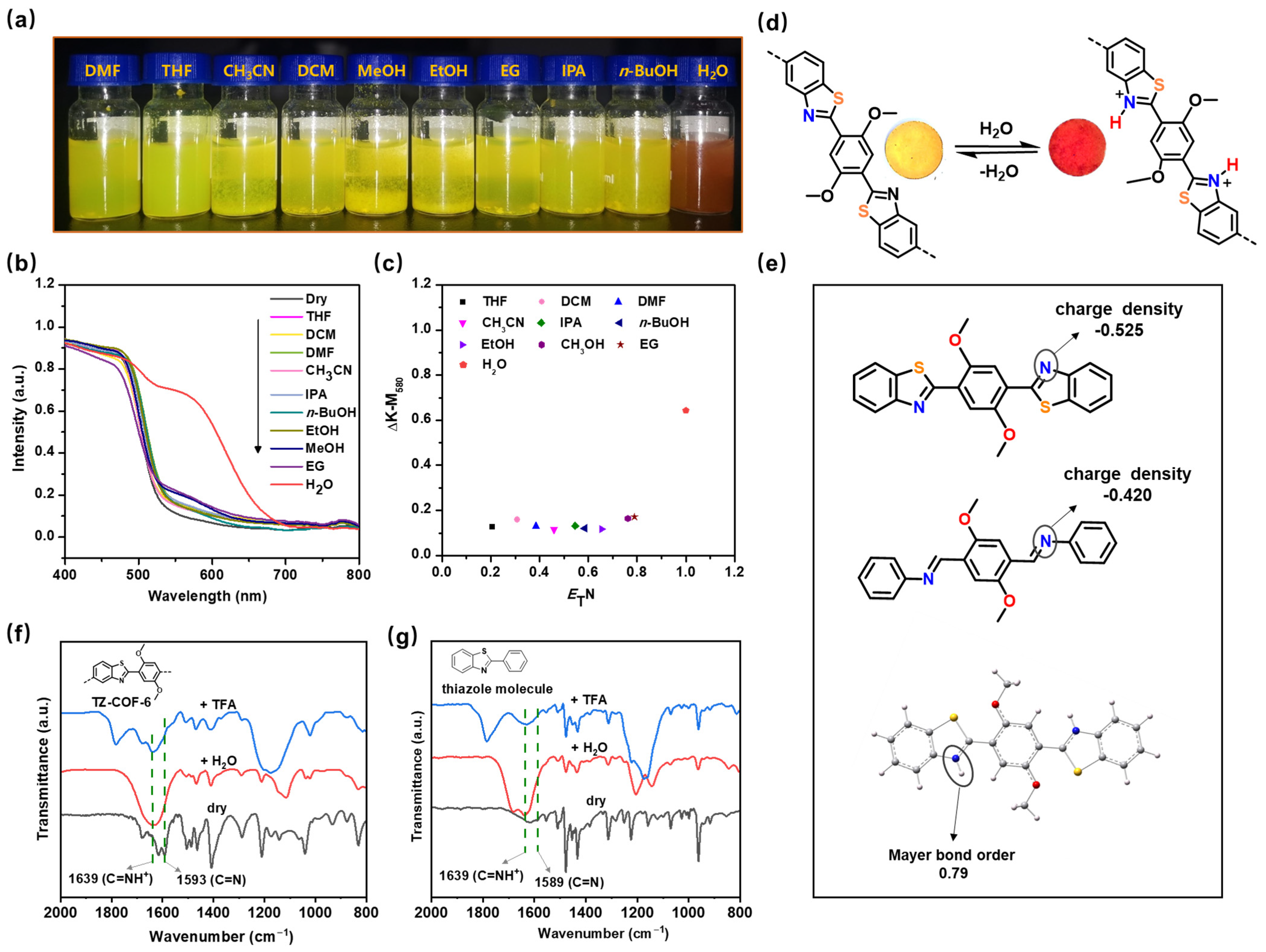
2.2. Hydrochromic Property for Water Sensing
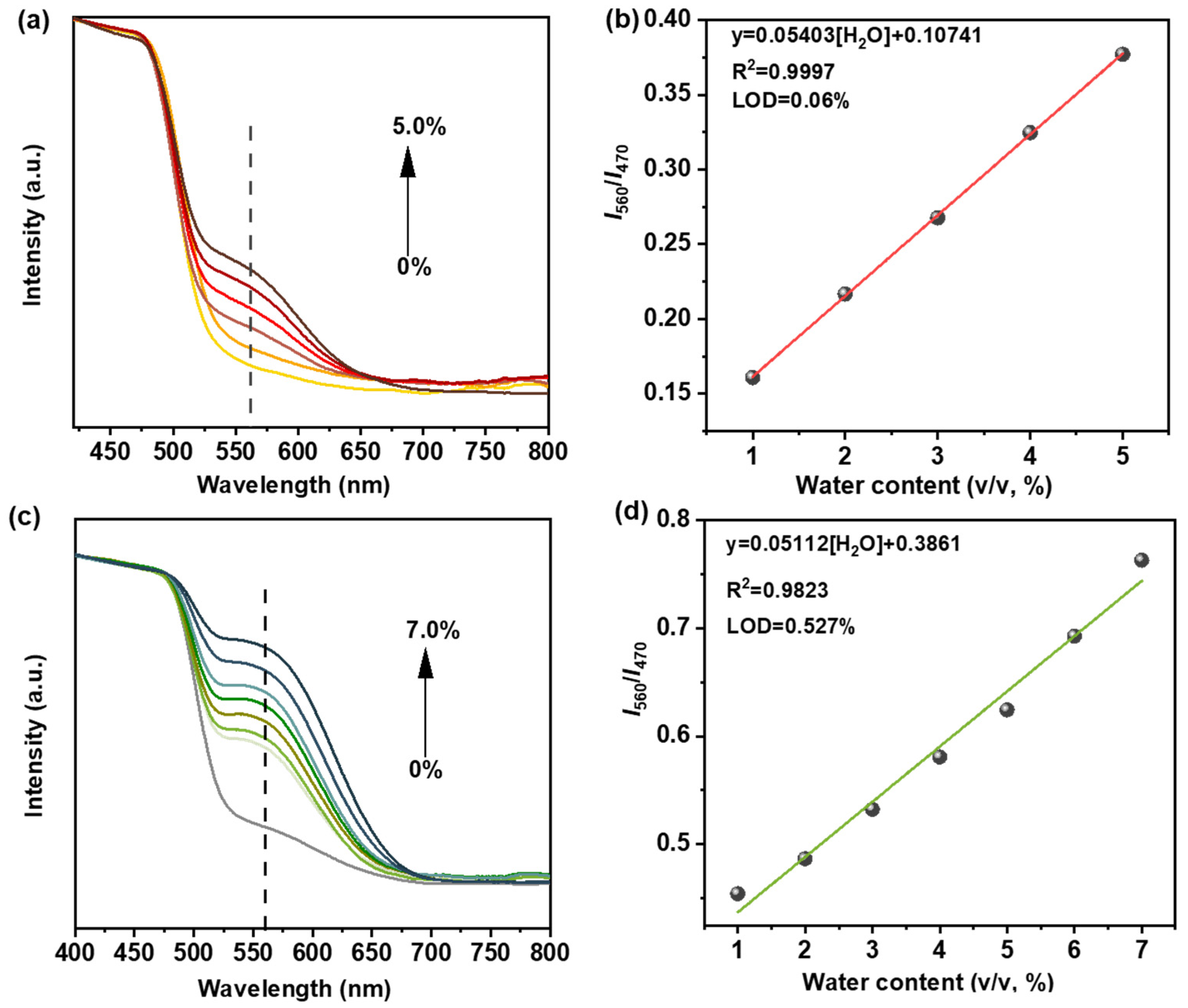
2.3. Detection of Trace Water in Organic Solvents
3. Materials and Methods
3.1. Synthesis of TZ-COF-6, TZ-COF-7, and SF-COF-6
3.2. Water Sensing
3.3. Water Detection in Different Solvents
3.4. Cycle Testing of TZ-COF-6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, Y.; Zhang, W.; Yang, S.; Wang, L.; Yu, G. Research progress in donor-acceptor type covalent organic frameworks. Adv. Mater. 2023, 35, 2301190. [Google Scholar] [CrossRef] [PubMed]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.N.; Lee, H.J.; Tran, N. Covalent organic frameworks: From structures to applications. Polymers 2023, 15, 1279. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, G. Controllable synthesis and performance modulation of 2D covalent-organic frameworks. Small 2021, 17, 2100918. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, J.; Huang, Q.; Zhou, W.; Jin, S. Progress in synthesis of highly crystalline covalent organic frameworks and their crystallinity enhancement strategies. Chin. Chem. Lett. 2022, 33, 2856–2866. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Zou, Y.; Yan, Y.; Song, Z.; Shi, X. Achieving a stable COF with the combination of “flat” and “twist” large-size rigid synthons for selective gas adsorption and separation. Chin. Chem. Lett. 2022, 33, 3017–3020. [Google Scholar] [CrossRef]
- Vazquez-Molina, D.A.; Mohammad-Pour, G.S.; Lee, C.; Logan, M.W.; Duan, X.; Harper, J.K.; Uribe-Romo, F.J. Mechanically shaped two-dimensional covalent organic frameworks reveal crystallographic alignment and fast Li-ion conductivity. J. Am. Chem. Soc. 2016, 138, 9767–9770. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Liu, Y.; Huang, S.; Yang, G. Recent progress of covalent organic frameworks applied in electrochemical sensors. ACS Sens. 2023, 8, 2124–2148. [Google Scholar] [CrossRef]
- Wang, L.; Dong, P.; Zhang, G.; Zhang, F. Review and perspectives of β-keto-enamine-based covalent organic framework for photocatalytic hydrogen evolution. Energy Fuel 2023, 37, 6323–6347. [Google Scholar] [CrossRef]
- Du, Q.; Zou, J.; Huang, Z.; Li, S.; Tan, L.; Ren, X.; Yin, G.; Zheng, Y.; Meng, X. Fabrication of microwave-sensitized nanospheres of covalent organic framework with Apatinib for tumor therapy. Chin. Chem. Lett. 2023, 34, 107763. [Google Scholar] [CrossRef]
- Jiang, S.; Meng, L.; Ma, W.; Pan, G.; Zhang, W.; Zou, Y.; Liu, L.; Xu, B.; Tian, W. Dual-functional two-dimensional covalent organic frameworks for water sensing and harvesting. Mater. Chem. Front. 2021, 5, 4193–4201. [Google Scholar] [CrossRef]
- Yan, X.; Yang, Y.; Li, G.; Zhang, J.; He, Y.; Wang, R.; Lin, Z.; Cai, Z. Thiophene-based covalent organic frameworks for highly efficient iodine capture. Chin. Chem. Lett. 2023, 34, 107201. [Google Scholar] [CrossRef]
- Sun, C.; Sheng, D.; Wang, B.; Feng, X. Covalent organic frameworks for extracting water from air. Angew. Chem. Int. Ed. 2023, 62, e202303378. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P.; Tang, K.C.; Chou, P.T. Excited-state proton coupled charge transfer modulated by molecular structure and media polarization. Chem. Soc. Rev. 2013, 42, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Jiang, S.; Zhang, W.; Xu, B.; Tian, W. Covalent organic frameworks with electron-rich and electron-deficient structures as water sensing scaffolds. Macromol. Rapid Commun. 2020, 41, 2000003. [Google Scholar] [CrossRef]
- Weatherly, C.A.; Woods, R.M.; Armstrong, D.W. Rapid analysis of ethanol and water in commercial products using ionic liquid capillary gas chromatography with thermal conductivity detection and/or barrier discharge ionization detection. J. Agric. Food Chem. 2014, 62, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.H.; Debbie Soo, X.Y.; Goh, W.P.; Png, Z.M.; Zhu, Q.; Xu, J. Thioxanthylium cations: Highly reversible hydrochromic mate-rials with tunable color and moisture sensitivity. Chem. Eur. J. 2022, 28, e202201975. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, T.; Zeng, T.; Zhang, X.; Yao, X.; Liu, S.; Shi, Z.; Wen, W.; Zhao, Y.; Jiang, S.; et al. Symmetry-breaking dynamics in a tautomeric 3D covalent organic framework. Nat. Commun. 2023, 14, 4215. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Shui, F.; Yi, M.; Zhang, Z.; Liu, X.; Zhang, L.; You, Z.; Yang, R.; Yang, S.; et al. Superhigh intrinsic proton conductivity in densely carboxylic covalent organic framework. Chin. Chem. Lett. 2023, 34, 107917. [Google Scholar] [CrossRef]
- Ding, H.; Mal, A.; Wang, C. Tailored covalent organic frameworks by post-synthetic modification. Mater. Chem. Front. 2020, 4, 113–127. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Cui, E.; Yang, X.; Yuan, X.; Pu, J.; Zhao, Y.; Xie, H.; Hu, J.; Liu, J.; et al. Solvatochromic covalent organic frameworks for the low-level determination of trace water in organic solvents. Sens. Actuators B Chem. 2023, 378, 133134. [Google Scholar] [CrossRef]
- Jhulki, S.; Evans, A.M.; Hao, X.; Cooper, M.W.; Feriante, C.H.; Leisen, J.; Li, H.; Lam, D.; Hersam, M.C.; Barlow, S.; et al. Humidity sensing through reversible isomerization of a covalent organic framework. J. Am. Chem. Soc. 2020, 142, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Ascherl, L.; Evans, E.W.; Gorman, J.; Orsborne, S.; Bessinger, D.; Bein, T.; Friend, R.H.; Auras, F. Perylene-based covalent organic frameworks for acid vapor sensing. J. Am. Chem. Soc. 2019, 141, 15693–15699. [Google Scholar] [CrossRef] [PubMed]
- Bu, R.; Zhang, L.; Liu, X.; Yang, S.; Li, G.; Gao, E. Synthesis and acid-responsive properties of a highly porous vinylene-linked covalent organic framework. ACS Appl. Mater. Interfaces 2021, 13, 26431–26440. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Elewa, A.M.; Huang, S.; Chou, H.; Kuo, S. Dual-function fluorescent covalent organic frameworks: HCl sensing and photocatalytic H2 evolution from water. Adv. Opt. Mater. 2020, 8, 2000641. [Google Scholar] [CrossRef]
- Xia, T.; Wu, Z.; Liang, Y.; Wang, W.; Li, Y.; Sui, Z.; Shan, L.; Li, C.; Fan, R.; Chen, Q. sp2 Carbon-conjugated covalent organic frameworks for efficient photocatalytic degradation and visualized pH detection. Mater. Today Chem. 2022, 25, 100962. [Google Scholar] [CrossRef]
- Gilmanova, L.; Bon, V.; Shupletsov, L.; Pohl, D.; Rauche, M.; Brunner, E.; Kaskel, S. Chemically stable carbazole-based imine covalent organic frameworks with acidochromic response for humidity control applications. J. Am. Chem. Soc. 2021, 143, 18368–18373. [Google Scholar] [CrossRef]
- Wang, K.; Jia, Z.; Bai, Y.; Wang, X.; Hodgkiss, S.E.; Chen, L.; Chong, S.Y.; Wang, X.; Yang, H.; Xu, Y.; et al. Synthesis of stable thiazole-linked covalent organic frameworks via a multicomponent reaction. J. Am. Chem. Soc. 2020, 142, 11131–11138. [Google Scholar] [CrossRef]
- Waller, P.J.; AlFaraj, Y.S.; Diercks, C.S.; Jarenwattananon, N.N.; Yaghi, O.M. Conversion of imine to oxazole and thiazole linkages in covalent organic frameworks. J. Am. Chem. Soc. 2018, 140, 9099–9103. [Google Scholar] [CrossRef]
- Haase, F.; Troschke, E.; Savasci, G.; Banerjee, T.; Duppel, V.; Dörfler, S.; Grundei, M.M.J.; Burow, A.M.; Ochsenfeld, C.; Kaskel, S.; et al. Topochemical conversion of an imine-into a thiazole-linked covalent organic framework enabling real structure analysis. Nat. Commun. 2018, 9, 2600. [Google Scholar] [CrossRef]
- Mou, Y.; Wu, X.; Qin, C.; Chen, J.; Zhao, Y.; Jiang, L.; Zhang, C.; Yuan, X.; Ang, E.H.; Wang, H. Linkage microenvironment of azoles-related covalent organic frameworks precisely regulates photocatalytic generation of hydrogen peroxide. Angew. Chem. Int. Ed. 2023, 62, e202309480. [Google Scholar] [CrossRef]
- Singh, V.; Kim, J.; Kang, B.; Moon, J.; Kim, S.; Kim, W.Y.; Byon, H.R. Thiazole-linked covalent organic framework promoting fast two-electron transfer for lithium-organic batteries. Adv. Energy Mater. 2021, 11, 2003735. [Google Scholar] [CrossRef]
- Deng, M.; Sun, J.; Laemont, A.; Liu, C.; Wang, L.; Bourda, L.; Chakraborty, J.; Hecke, K.V.; Morent, R.; Geyter, N.D.; et al. Extending the π-conjugation system of covalent organic frameworks for more efficient photocatalytic H2O2 production. Green Chem. 2023, 25, 3069–3076. [Google Scholar] [CrossRef]
- Ascherl, L.; Evans, E.W.; Hennemann, M.; Nuzzo, D.D.; Hufnagel, A.G.; Beetz, M.; Friend, R.H.; Clark, T.; Bein, T.; Auras, F. Solvatochromic covalent organic frameworks. Nat. Commun. 2018, 9, 3802. [Google Scholar] [CrossRef] [PubMed]
- Nekoeinia, M.; Yousefinejad, S.; Abdollahi-Dezaki, A. Prediction of ETN polarity scale of ionic liquids using a QSPR approach. Ind. Eng. Chem. Res. 2015, 54, 12682–12689. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Kandambeth, S.; Shinde, D.B.; Panda, M.K.; Lukose, B.; Heine, T.; Banerjee, R. Enhancement of chemical stability and crystallinity in porphyrin-containing covalent organic frameworks by intramolecular hydrogen bonds. Angew. Chem. Int. Ed. 2013, 52, 13052–13056. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, C.; Xie, J.; Li, H.; Dai, W.; Deng, Q.; Wang, S. Covalent organic frameworks as a sensing platform for water in organic solvent over a broad concentration range. Anal. Chim. Acta 2020, 1109, 114–121. [Google Scholar] [CrossRef]
- Qian, H.; Dai, C.; Yang, C.; Yan, X. High-crystallinity covalent organic framework with dual fluorescence emissions and its ratiometric sensing application. ACS Appl. Mater. Interfaces 2017, 9, 24999–25005. [Google Scholar] [CrossRef]
- Wu, J.; Yan, B. A dual-emission probe to detect moisture and water in organic solvents based on green-Tb3+ post-coordinated metal-organic frameworks with red carbon dots. Dalton Trans. 2017, 46, 7098–7105. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Wu, Z.; Ji, N.; Wang, T.; Gu, Y.; Zhao, Z.; Guo, Y.; Wang, X.; Jia, Z.; Tan, B. Robust Thiazole-Linked Covalent Organic Frameworks for Water Sensing with High Selectivity and Sensitivity. Molecules 2024, 29, 1677. https://doi.org/10.3390/molecules29071677
Wang K, Wu Z, Ji N, Wang T, Gu Y, Zhao Z, Guo Y, Wang X, Jia Z, Tan B. Robust Thiazole-Linked Covalent Organic Frameworks for Water Sensing with High Selectivity and Sensitivity. Molecules. 2024; 29(7):1677. https://doi.org/10.3390/molecules29071677
Chicago/Turabian StyleWang, Kewei, Zhaoxia Wu, Na Ji, Tingxia Wang, Yongxin Gu, Zhixiang Zhao, Yong Guo, Xiaoyan Wang, Zhifang Jia, and Bien Tan. 2024. "Robust Thiazole-Linked Covalent Organic Frameworks for Water Sensing with High Selectivity and Sensitivity" Molecules 29, no. 7: 1677. https://doi.org/10.3390/molecules29071677
APA StyleWang, K., Wu, Z., Ji, N., Wang, T., Gu, Y., Zhao, Z., Guo, Y., Wang, X., Jia, Z., & Tan, B. (2024). Robust Thiazole-Linked Covalent Organic Frameworks for Water Sensing with High Selectivity and Sensitivity. Molecules, 29(7), 1677. https://doi.org/10.3390/molecules29071677





