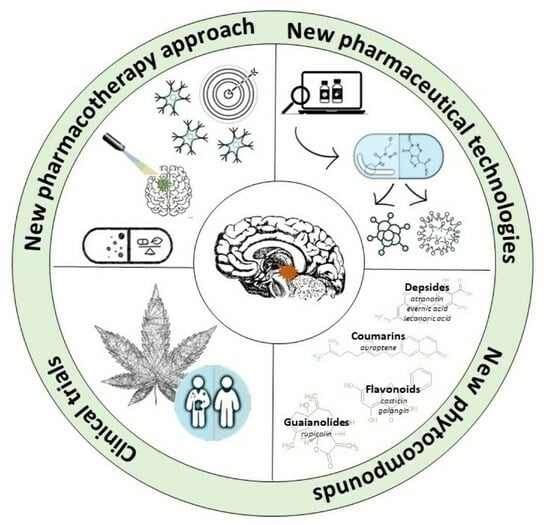
New Avenues and Major Achievements in Phytocompounds Research for Glioblastoma Therapy
Abstract
:1. Introduction
2. Enhancing Temozolomide Response with Phytochemicals
3. Strategies for the Improved Phytocompound Delivery to the Brain—The Advantages of Phyto-Nanocarriers
4. Chemical Modifications of Phytocompounds as a Way to Improve Their Solubility, Bioavailability, and Efficacy
5. Phytocompound-Based Immunotherapy
6. Phytocompounds Used as Photosensitizers in GBM Photodynamic Therapy (PDT)
7. Unraveling the Emerging Role of Less Studied Plant-Derived Substances with Anti-GBM Potential
8. Epidemiological Studies of Dietary Phytocompounds—Novel Trend in GBM Research?
9. Phytocompounds or Phytocompound-Based Products That Reached the Clinical Trials Phase in GBM Research
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silvani, A. New Perspectives: Glioma in Adult Patients. Tumori J. 2023, 109, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Li, N.; Zhang, Z. Emerging Therapies for Glioblastoma: Current State and Future Directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef] [PubMed]
- Mowforth, O.D.; Brannigan, J.; El Khoury, M.; Sarathi, C.I.P.; Bestwick, H.; Bhatti, F.; Mair, R. Personalised Therapeutic Approaches to Glioblastoma: A Systematic Review. Front. Med. 2023, 10, 1166104. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma Multiforme (GBM): An Overview of Current Therapies and Mechanisms of Resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Pellerino, A.; Soffietti, R.; Rudà, R. Blood–Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef]
- Majchrzak-Celińska, A.; Sidhu, A.; Miechowicz, I.; Nowak, W.; Barciszewska, A.-M. ABCB1 Is Frequently Methylated in Higher-Grade Gliomas and May Serve as a Diagnostic Biomarker of More Aggressive Tumors. J. Clin. Med. 2022, 11, 5655. [Google Scholar] [CrossRef] [PubMed]
- Radtke, L.; Majchrzak-Celińska, A.; Awortwe, C.; Vater, I.; Nagel, I.; Sebens, S.; Cascorbi, I.; Kaehler, M. CRISPR/Cas9-Induced Knockout Reveals the Role of ABCB1 in the Response to Temozolomide, Carmustine and Lomustine in Glioblastoma Multiforme. Pharmacol. Res. 2022, 185, 106510. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 22, 351. [Google Scholar] [CrossRef]
- Majchrzak-Celińska, A.; Misiorek, J.O.; Kruhlenia, N.; Przybyl, L.; Kleszcz, R.; Rolle, K.; Krajka-Kuźniak, V. COXIBs and 2,5-Dimethylcelecoxib Counteract the Hyperactivated Wnt/β-Catenin Pathway and COX-2/PGE2/EP4 Signaling in Glioblastoma Cells. BMC Cancer 2021, 21, 493. [Google Scholar] [CrossRef]
- Han, H.S.; Koo, S.Y.; Choi, K.Y. Emerging Nanoformulation Strategies for Phytocompounds and Applications from Drug Delivery to Phototherapy to Imaging. Bioact. Mater. 2022, 14, 182–205. [Google Scholar] [CrossRef]
- Schaff, L.R.; Yan, D.; Thyparambil, S.; Tian, Y.; Cecchi, F.; Rosenblum, M.; Reiner, A.S.; Panageas, K.S.; Hembrough, T.; Lin, A.L. Characterization of MGMT and EGFR Protein Expression in Glioblastoma and Association with Survival. J. Neurooncol. 2020, 146, 163–170. [Google Scholar] [CrossRef]
- Tang, K.; Jin, Q.; Yan, W.; Zhang, W.; You, G.; Liu, Y.; Jiang, T. Clinical Correlation of MGMT Protein Expression and Promoter Methylation in Chinese Glioblastoma Patients. Med. Oncol. 2012, 29, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Limam, S.; Missaoui, N.; Abdessayed, N.; Mestiri, S.; Selmi, B.; Mokni, M.; Yacoubi, M.T. Prognostic Significance of MGMT Methylation and Expression of MGMT, P53, EGFR, MDM2 and PTEN in Glioblastoma Multiforme. Ann. Biol. Clin. 2019, 77, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of Temozolomide Resistance in Glioblastoma—A Comprehensive Review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, B.; Spencer, D.; Pytel, P.; Ahmed, A.U.; Lesniak, M.S. The Role of Glioma Stem Cells in Chemotherapy Resistance and Glioblastoma Multiforme Recurrence. Expert Rev. Neurother. 2015, 15, 741–752. [Google Scholar] [CrossRef]
- Hombach-Klonisch, S.; Mehrpour, M.; Shojaei, S.; Harlos, C.; Pitz, M.; Hamai, A.; Siemianowicz, K.; Likus, W.; Wiechec, E.; Toyota, B.D.; et al. Glioblastoma and Chemoresistance to Alkylating Agents: Involvement of Apoptosis, Autophagy, and Unfolded Protein Response. Pharmacol. Ther. 2018, 184, 13–41. [Google Scholar] [CrossRef] [PubMed]
- Vengoji, R.; Macha, M.A.; Batra, S.K.; Shonka, N.A. Natural Products: A Hope for Glioblastoma Patients. Oncotarget 2018, 9, 22194–22219. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, E.; Banik, K.; Harsha, C.; Sailo, B.L.; Thakur, K.K.; Khwairakpam, A.D.; Vikkurthi, R.; Devi, T.B.; Gupta, S.C.; Kunnumakkara, A.B. Phytochemicals in Cancer Cell Chemosensitization: Current Knowledge and Future Perspectives. Semin. Cancer Biol. 2022, 80, 306–339. [Google Scholar] [CrossRef]
- Tagde, P.; Tagde, P.; Tagde, S.; Bhattacharya, T.; Garg, V.; Akter, R.; Rahman, M.H.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; et al. Natural Bioactive Molecules: An Alternative Approach to the Treatment and Control of Glioblastoma Multiforme. Biomed. Pharmacother. 2021, 141, 111928. [Google Scholar] [CrossRef]
- De Oliveira Júnior, R.G.; Christiane Adrielly, A.F.; Da Silva Almeida, J.R.G.; Grougnet, R.; Thiéry, V.; Picot, L. Sensitization of Tumor Cells to Chemotherapy by Natural Products: A Systematic Review of Preclinical Data and Molecular Mechanisms. Fitoterapia 2018, 129, 383–400. [Google Scholar] [CrossRef]
- Gautam, M.; Gabrani, R. Combinatorial Effect of Temozolomide and Naringenin in Human Glioblastoma Multiforme Cell Lines. Nutr. Cancer 2022, 74, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Netto, J.B.; Melo, E.S.A.; Oliveira, A.G.S.; Sousa, L.R.; Santiago, L.R.; Santos, D.M.; Chagas, R.C.R.; Gonçalves, A.S.; Thomé, R.G.; Santos, H.B.; et al. Matteucinol Combined with Temozolomide Inhibits Glioblastoma Proliferation, Invasion, and Progression: An in Vitro, in Silico, and in Vivo Study. Braz. J. Med. Biol. Res. 2022, 55, e12076. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Li, H.; Yi, D.; Sun, Y.; Bai, Y.; Zhong, S.; Song, Y.; Zhao, G.; Chen, Y. Cordycepin Augments the Chemosensitivity of Human Glioma Cells to Temozolomide by Activating AMPK and Inhibiting the AKT Signaling Pathway. Mol. Pharm. 2018, 15, 4912–4925. [Google Scholar] [CrossRef] [PubMed]
- Vibhavari, R.J.A.; Rao, V.; Cheruku, S.P.; Kumar, B.H.; Maity, S.; Nandakumar, K.; Kumar, L.; Mehta, C.H.; Nayak, U.; Chamallamudi, M.R.; et al. Enhancing Temozolomide Antiglioma Response by Inhibiting O6-Methylguanine-DNA Methyltransferase with Selected Phytochemicals: In Silico and in Vitro Approach. 3 Biotech 2023, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Meteoglu, I.; Erdemir, A. Genistein and Temozolomide-Loaded Polymeric Nanoparticles: A Synergistic Approach for Improved Anti-Tumor Efficacy Against Glioblastoma. Process Biochem. 2021, 110, 9–18. [Google Scholar] [CrossRef]
- Chang, K.-F.; Huang, X.-F.; Chang, J.T.; Huang, Y.-C.; Lo, W.-S.; Hsiao, C.-Y.; Tsai, N.-M. Cedrol, a Sesquiterpene Alcohol, Enhances the Anticancer Efficacy of Temozolomide in Attenuating Drug Resistance via Regulation of the DNA Damage Response and MGMT Expression. J. Nat. Prod. 2020, 83, 3021–3029. [Google Scholar] [CrossRef] [PubMed]
- Çetin, A.; Biltekin, B.; Degirmencioglu, S. Ellagic Acid Enhances the Antitumor Efficacy of Bevacizumab in an In Vitro Glioblastoma Model. World Neurosurg. 2019, 132, e59–e65. [Google Scholar] [CrossRef] [PubMed]
- Altundağ, E.M.; Jannuzzi, A.T.; Özbilenler, C.; Ustürk, S.; Altınoğlu, G. Synergistic Role of Thymoquinone and 5-Fluorouracil in U-251MG Glioblastoma Cell Line. Turk. J. Biochem. 2023, 49, 82–89. [Google Scholar] [CrossRef]
- Cetin, A.; Biltekin, B.; Ozevren, H. Antitumor Activity of Irinotecan with Ellagic Acid in C6 Glioma Cells. Rev. Assoc. Méd. Bras. 2022, 68, 939–944. [Google Scholar] [CrossRef]
- Qu, H.; Song, X.; Song, Z.; Jiang, X.; Gao, X.; Bai, L.; Wu, J.; Na, L.; Yao, Z. Berberine Reduces Temozolomide Resistance by Inducing Autophagy via the ERK1/2 Signaling Pathway in Glioblastoma. Cancer Cell Int. 2020, 20, 592. [Google Scholar] [CrossRef]
- Kamani, M.; Ghanbari, A.; Taghadosi, M.; Mansouri, K.; Jalili, C. Harmine Augments the Cytotoxic and Anti-Invasive Potential of Temozolomide Against Glioblastoma Multiforme Cells. Jundishapur J. Nat. Pharm. Prod. 2022, 17, e115464. [Google Scholar] [CrossRef]
- Jeong, S.; Jung, S.; Park, G.-S.; Shin, J.; Oh, J.-W. Piperine Synergistically Enhances the Effect of Temozolomide against Temozolomide-Resistant Human Glioma Cell Lines. Bioengineered 2020, 11, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Xu, T.; Wang, Y.; Zhou, Y.; Yu, D.; Wang, Z.; He, L.; Chen, Z.; Zhang, Y.; Davidson, D.; et al. Cannabidiol Inhibits Human Glioma by Induction of Lethal Mitophagy through Activating TRPV4. Autophagy 2021, 17, 3592–3606. [Google Scholar] [CrossRef] [PubMed]
- Sumorek-Wiadro, J.; Zając, A.; Bądziul, D.; Langner, E.; Skalicka-Woźniak, K.; Maciejczyk, A.; Wertel, I.; Rzeski, W.; Jakubowicz-Gil, J. Coumarins Modulate the Anti-Glioma Properties of Temozolomide. Eur. J. Pharmacol. 2020, 881, 173207. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wang, D.; Li, L.; Wang, J.; Li, Q.; Duan, L.; Yin, H.; Wang, X.; Liu, Y.; Yuan, G.; et al. Biochanin A Sensitizes Glioblastoma to Temozolomide by Inhibiting Autophagy. Mol. Neurobiol. 2022, 59, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Z.; Dai, X.; Zhang, L.; Li, M. Apigenin and Temozolomide Synergistically Inhibit Glioma Growth Through the PI3K/AKT Pathway. Cancer Biother. Radiopharm. 2021, 39, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhou, Y.; Zhang, H.; Pan, J.; Yang, F.; Zhang, R.; Ahmad, N.; Yang, J.; Sun, M. Morusin Enhances Temozolomide Efficiency in GBM by Inducing Cytoplasmic Vacuolization and Endoplasmic Reticulum Stress. J. Clin. Med. 2022, 11, 3662. [Google Scholar] [CrossRef] [PubMed]
- Daisy Precilla, S.; Kuduvalli, S.S.; Angeline Praveena, E.; Thangavel, S.; Anitha, T.S. Integration of Synthetic and Natural Derivatives Revives the Therapeutic Potential of Temozolomide against Glioma—An in Vitro and in Vivo Perspective. Life Sci. 2022, 301, 120609. [Google Scholar] [CrossRef]
- Ho, K.-H.; Kuo, T.-C.; Lee, Y.-T.; Chen, P.-H.; Shih, C.-M.; Cheng, C.-H.; Liu, A.-J.; Lee, C.-C.; Chen, K.-C. Xanthohumol Regulates miR-4749-5p-Inhibited RFC2 Signaling in Enhancing Temozolomide Cytotoxicity to Glioblastoma. Life Sci. 2020, 254, 117807. [Google Scholar] [CrossRef]
- Chio, C.-C.; Chen, K.-Y.; Chang, C.-K.; Chuang, J.-Y.; Liu, C.-C.; Liu, S.-H.; Chen, R.-M. Improved Effects of Honokiol on Temozolomide-Induced Autophagy and Apoptosis of Drug-Sensitive and -Tolerant Glioma Cells. BMC Cancer 2018, 18, 379. [Google Scholar] [CrossRef]
- Chio, C.-C.; Tai, Y.-T.; Mohanraj, M.; Liu, S.-H.; Yang, S.-T.; Chen, R.-M. Honokiol Enhances Temozolomide-Induced Apoptotic Insults to Malignant Glioma Cells via an Intrinsic Mitochondrion-Dependent Pathway. Phytomed. Int. J. Phytother. Phytopharm. 2018, 49, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Das, S.; Nandi, S.; Dhara, D.; Mandal, M. Magnolol and Temozolomide Exhibit a Synergistic Anti-Glioma Activity through MGMT Inhibition. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2023, 1869, 166782. [Google Scholar] [CrossRef] [PubMed]
- Cetin, A.; Biltekin, B. Ellagic Acid Enhances Antitumor Efficacy of Temozolomide in an in Vitro Glioblastoma Model. Turk. Neurosurg. 2020, 30, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Bona, N.P.; Pedra, N.S.; Azambuja, J.H.; Soares, M.S.P.; Spohr, L.; Gelsleichter, N.E.; de Meine, B.M.; Sekine, F.G.; Mendonça, L.T.; de Oliveira, F.H.; et al. Tannic Acid Elicits Selective Antitumoral Activity in Vitro and Inhibits Cancer Cell Growth in a Preclinical Model of Glioblastoma Multiforme. Metab. Brain Dis. 2020, 35, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Çetin, A.; Biltekin, B. Combining Ellagic Acid with Temozolomide Mediates the Cadherin Switch and Angiogenesis in a Glioblastoma Model. World Neurosurg. 2019, 132, e178–e184. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-T.; Lee, I.-N.; Chen, C.-H.; Lu, F.-J.; Chung, C.-Y.; Lee, M.-H.; Cheng, Y.-C.; Chen, K.-T.; Peng, J.-Y.; Chen, C.-H. Gallic Acid Enhances the Anti-Cancer Effect of Temozolomide in Human Glioma Cell Line via Inhibition of Akt and P38-MAPK Pathway. Processes 2022, 10, 448. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.; Li, H.; Ji, Y.; Fang, F.; Tang, H.; Qiu, P. A Steroidal Saponin Form Paris Vietnamensis (Takht.) Reverses Temozolomide Resistance in Glioblastoma Cells via Inducing Apoptosis through ROS/PI3K/Akt Pathway. Biosci. Trends 2020, 14, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ercelik, M.; Tekin, C.; Tezcan, G.; Ak Aksoy, S.; Bekar, A.; Kocaeli, H.; Taskapilioglu, M.O.; Eser, P.; Tunca, B. Olea europaea Leaf Phenolics Oleuropein, Hydroxytyrosol, Tyrosol, and Rutin Induce Apoptosis and Additionally Affect Temozolomide against Glioblastoma: In Particular, Oleuropein Inhibits Spheroid Growth by Attenuating Stem-like Cell Phenotype. Life 2023, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, X.; Wu, M.; Wu, J.; Liu, J. Synergistic Effects of Resveratrol and Temozolomide Against Glioblastoma Cells: Underlying Mechanism and Therapeutic Implications. Cancer Manag. Res. 2020, 12, 8341–8354. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Li, A.; Liu, R.; Yang, H.; Xia, X. The Phytochemical Potential for Brain Disease Therapy and the Possible Nanodelivery Solutions for Brain Access. Front. Oncol. 2022, 12, 936054. [Google Scholar] [CrossRef]
- Gostyńska, A.; Czerniel, J.; Kuźmińska, J.; Brzozowski, J.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V.; Stawny, M. Honokiol-Loaded Nanoemulsion for Glioblastoma Treatment: Statistical Optimization, Physicochemical Characterization, and an In Vitro Toxicity Assay. Pharmaceutics 2023, 15, 448. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Mlynarczyk, D.T.; Krajka-Kuźniak, V.; Majchrzak-Celińska, A.; Budzianowska, A.; Tomczak, S.; Budzianowski, J.; Woźniak-Braszak, A.; Pietrzyk, R.; Baranowski, M.; et al. Natural Compounds in Liposomal Nanoformulations of Potential Clinical Application in Glioblastoma. Cancers 2022, 14, 6222. [Google Scholar] [CrossRef]
- Ying, X.; Wang, Y.; Xu, H.; Li, X.; Yan, H.; Tang, H.; Wen, C.; Li, Y. The Construction of the Multifunctional Targeting Ursolic Acids Liposomes and Its Apoptosis Effects to C6 Glioma Stem Cells. Oncotarget 2017, 8, 64129–64142. [Google Scholar] [CrossRef]
- Sahab-Negah, S.; Ariakia, F.; Jalili-Nik, M.; Afshari, A.R.; Salehi, S.; Samini, F.; Rajabzadeh, G.; Gorji, A. Curcumin Loaded in Niosomal Nanoparticles Improved the Anti-Tumor Effects of Free Curcumin on Glioblastoma Stem-like Cells: An In Vitro Study. Mol. Neurobiol. 2020, 57, 3391–3411. [Google Scholar] [CrossRef]
- Ismail, M.; Yang, W.; Li, Y.; Chai, T.; Zhang, D.; Du, Q.; Muhammad, P.; Hanif, S.; Zheng, M.; Shi, B. Targeted Liposomes for Combined Delivery of Artesunate and Temozolomide to Resistant Glioblastoma. Biomaterials 2022, 287, 121608. [Google Scholar] [CrossRef]
- Majchrzak-Celińska, A.; Kleszcz, R.; Stasiłowicz-Krzemień, A.; Cielecka-Piontek, J. Sodium Butyrate Enhances Curcuminoids Permeability through the Blood-Brain Barrier, Restores Wnt/β-Catenin Pathway Antagonists Gene Expression and Reduces the Viability of Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 11285. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ran, R.; Chen, J.; Kuang, Q.; Tang, J.; Mei, L.; Zhang, Q.; Gao, H.; Zhang, Z.; He, Q. Paclitaxel Loaded Liposomes Decorated with a Multifunctional Tandem Peptide for Glioma Targeting. Biomaterials 2014, 35, 4835–4847. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Hong, W.; Yu, M.; Li, Y.; Zheng, Y.; Ying, X. Multifunctional Targeting Liposomes of Epirubicin Plus Resveratrol Improved Therapeutic Effect on Brain Gliomas. Int. J. Nanomed. 2022, 17, 1087–1110. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak-Celińska, A.; Zielińska-Przyjemska, M.; Wierzchowski, M.; Kleszcz, R.; Studzińska-Sroka, E.; Kaczmarek, M.; Paluszczak, J.; Cielecka-Piontek, J.; Krajka-Kuźniak, V. Methoxy-Stilbenes Downregulate the Transcription of Wnt/β-Catenin-Dependent Genes and Lead to Cell Cycle Arrest and Apoptosis in Human T98G Glioblastoma Cells. Adv. Med. Sci. 2021, 66, 6–20. [Google Scholar] [CrossRef]
- Rampogu, S.; Kim, S.M.; Shaik, B.; Lee, G.; Kim, J.H.; Kim, G.S.; Lee, K.W.; Kim, M.O. Novel Butein Derivatives Repress DDX3 Expression by Inhibiting PI3K/AKT Signaling Pathway in MCF-7 and MDA-MB-231 Cell Lines. Front. Oncol. 2021, 11, 712824. [Google Scholar] [CrossRef]
- Sun, M.; Song, L.; Zhou, T.; Gillespie, G.Y.; Jope, R.S. The Role of DDX3 in Regulating Snail. Biochim. Biophys. Acta 2011, 1813, 438–447. [Google Scholar] [CrossRef]
- He, Y.; Zhang, D.; Yang, Y.; Wang, X.; Zhao, X.; Zhang, P.; Zhu, H.; Xu, N.; Liang, S. A Double-Edged Function of DDX3, as an Oncogene or Tumor Suppressor, in Cancer Progression (Review). Oncol. Rep. 2018, 39, 883–892. [Google Scholar] [CrossRef]
- Saeed, M.E.M.; Yücer, R.; Dawood, M.; Hegazy, M.-E.F.; Drif, A.; Ooko, E.; Kadioglu, O.; Seo, E.-J.; Kamounah, F.S.; Titinchi, S.J.; et al. In Silico and In Vitro Screening of 50 Curcumin Compounds as EGFR and NF-κB Inhibitors. Int. J. Mol. Sci. 2022, 23, 3966. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Tarique, M.; Tabrez, S.; Zughaibi, T.A.; Rehan, M. Synergistic Inhibition of Glioblastoma Multiforme through an In-Silico Analysis of Luteolin and Ferulic Acid Derived from Angelica Sinensis and Cannabis Sativa: Advancements in Computational Therapeutics. PLoS ONE 2023, 18, e0293666. [Google Scholar] [CrossRef] [PubMed]
- Sucu, B.O.; Koc, E.B.; Savlug Ipek, O.; Mirat, A.; Almas, F.; Guzel, M.A.; Dogan, B.; Uludag, D.; Karakas, N.; Durdagi, S.; et al. Design and Synthesis of Novel Caffeic Acid Phenethyl Ester (CAPE) Derivatives and Their Biological Activity Studies in Glioblastoma Multiforme (GBM) Cancer Cell Lines. J. Mol. Graph. Model. 2022, 113, 108160. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Kuo, H.-C.; Chu, C.-Y.; Wang, C.-J.; Lin, W.-C.; Tseng, T.-H. Involvement of Tumor Suppressor Protein P53 and P38 MAPK in Caffeic Acid Phenethyl Ester-Induced Apoptosis of C6 Glioma Cells. Biochem. Pharmacol. 2003, 66, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Łaba, A.E.; Ziółkowski, P. Trends in Glioblastoma Treatment Research: An Analysis of Clinical Trials and Literature. Neurol. Neurochir. Pol. 2021, 55, 269–280. [Google Scholar] [CrossRef]
- Huang, Q.; Pan, X.; Zhu, W.; Zhao, W.; Xu, H.; Hu, K. Natural Products for the Immunotherapy of Glioma. Nutrients 2023, 15, 2795. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Ye, J.; Gao, Y.; Liao, H.; Zhou, J.; Feng, Y.; Liu, D.; Meng, Y.; Chen, X.; et al. Construction of Chlorogenic Acid-Containing Liposomes with Prolonged Antitumor Immunity Based on T Cell Regulation. Sci. China Life Sci. 2021, 64, 1097–1115. [Google Scholar] [CrossRef]
- Ye, J.; Yang, Y.; Jin, J.; Ji, M.; Gao, Y.; Feng, Y.; Wang, H.; Chen, X.; Liu, Y. Targeted Delivery of Chlorogenic Acid by Mannosylated Liposomes to Effectively Promote the Polarization of TAMs for the Treatment of Glioblastoma. Bioact. Mater. 2020, 5, 694–708. [Google Scholar] [CrossRef]
- Mukherjee, S.; Baidoo, J.N.E.; Sampat, S.; Mancuso, A.; David, L.; Cohen, L.S.; Zhou, S.; Banerjee, P. Liposomal TriCurin, A Synergistic Combination of Curcumin, Epicatechin Gallate and Resveratrol, Repolarizes Tumor-Associated Microglia/Macrophages, and Eliminates Glioblastoma (GBM) and GBM Stem Cells. Molecules 2018, 23, 201. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.L.C.; Amparo, J.A.O.; da Silva, A.B.; da Silva, K.C.; Braga-de-Souza, S.; Barbosa, P.R.; Lopes, G.P.d.F.; Costa, S.L. Apigenin from Croton betulaster Müll Restores the Immune Profile of Microglia against Glioma Cells. Phytother. Res. PTR 2019, 33, 3191–3202. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Liu, H.; Sun, F.; Du, R.; Kong, J.; Wang, H.; Cheng, H.; Wang, G.; Gao, F.; Liang, P. Intratumor Injection of Thermosensitive Polypeptide with Resveratrol Inhibits Glioblastoma Growth. Tissue Eng. Part C Methods 2023, 29, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Siewert, B.; Stuppner, H. The Photoactivity of Natural Products—An Overlooked Potential of Phytomedicines? Phytomed. Int. J. Phytother. Phytopharm. 2019, 60, 152985. [Google Scholar] [CrossRef] [PubMed]
- Muniyandi, K.; George, B.; Parimelazhagan, T.; Abrahamse, H. Role of Photoactive Phytocompounds in Photodynamic Therapy of Cancer. Molecules 2020, 25, 4102. [Google Scholar] [CrossRef] [PubMed]
- Aziz, B.; Aziz, I.; Khurshid, A.; Raoufi, E.; Esfahani, F.N.; Jalilian, Z.; Mozafari, M.R.; Taghavi, E.; Ikram, M. An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy. Biomedicines 2023, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Kielbik, A.; Wawryka, P.; Przystupski, D.; Rossowska, J.; Szewczyk, A.; Saczko, J.; Kulbacka, J.; Chwiłkowska, A. Effects of Photosensitization of Curcumin in Human Glioblastoma Multiforme Cells. In Vivo 2019, 33, 1857–1864. [Google Scholar] [CrossRef]
- Eom, K.S.; Kim, H.-J.; So, H.-S.; Park, R.; Kim, T.Y. Berberine-Induced Apoptosis in Human Glioblastoma T98G Cells Is Mediated by Endoplasmic Reticulum Stress Accompanying Reactive Oxygen Species and Mitochondrial Dysfunction. Biol. Pharm. Bull. 2010, 33, 1644–1649. [Google Scholar] [CrossRef]
- Werner, M.; Lyu, C.; Stadlbauer, B.; Schrader, I.; Buchner, A.; Stepp, H.; Sroka, R.; Pohla, H. The Role of Shikonin in Improving 5-Aminolevulinic Acid-Based Photodynamic Therapy and Chemotherapy on Glioblastoma Stem Cells. Photodiagn. Photodyn. Ther. 2022, 39, 102987. [Google Scholar] [CrossRef]
- Bassler, M.; Hiller, J.; Wackenhut, F.; Zur Oven-Krockhaus, S.; Frech, P.; Schmidt, F.; Kertzscher, C.; Rammler, T.; Ritz, R.; Braun, K.; et al. Fluorescence Lifetime Imaging Unravels the Pathway of Glioma Cell Death upon Hypericin-Induced Photodynamic Therapy. Chemistry 2023, preprint. [Google Scholar]
- Pevna, V.; Wagnières, G.; Huntosova, V. Autophagy and Apoptosis Induced in U87 MG Glioblastoma Cells by Hypericin-Mediated Photodynamic Therapy Can Be Photobiomodulated with 808 Nm Light. Biomedicines 2021, 9, 1703. [Google Scholar] [CrossRef]
- Bassler, M.C.; Rammler, T.; Wackenhut, F.; zur Oven-Krockhaus, S.; Secic, I.; Ritz, R.; Meixner, A.J.; Brecht, M. Accumulation and Penetration Behavior of Hypericin in Glioma Tumor Spheroids Studied by Fluorescence Microscopy and Confocal Fluorescence Lifetime Imaging Microscopy. Anal. Bioanal. Chem. 2022, 414, 4849–4860. [Google Scholar] [CrossRef] [PubMed]
- Bartusik-Aebisher, D.; Woźnicki, P.; Dynarowicz, K.; Aebisher, D. Photosensitizers for Photodynamic Therapy of Brain Cancers—A Review. Brain Sci. 2023, 13, 1299. [Google Scholar] [CrossRef] [PubMed]
- Afshari, A.R.; Karimi Roshan, M.; Soukhtanloo, M.; Ghorbani, A.; Rahmani, F.; Jalili-Nik, M.; Vahedi, M.M.; Hoseini, A.; Sadeghnia, H.R.; Mollazadeh, H.; et al. Cytotoxic Effects of Auraptene against a Human Malignant Glioblastoma Cell Line. Avicenna J. Phytomed. 2019, 9, 334–346. [Google Scholar]
- Izadi, A.; Soukhtanloo, M.; Mirzavi, F.; Jalili-Nik, M.; Sadeghi, A. Alpha-Lipoic Acid, Auraptene, and Particularly Their Combination Prevent the Metastasis of U87 Human Glioblastoma Cells. Evid. Based Complement. Alternat. Med. 2023, 2023, e8618575. [Google Scholar] [CrossRef]
- Tsiftsoglou, O.S.; Krigas, N.; Gounaris, C.; Papitsa, C.; Nanouli, M.; Vartholomatos, E.; Markopoulos, G.S.; Isyhou, R.; Alexiou, G.; Lazari, D. Isolation of Secondary Metabolites from Achillea grandifolia Friv. (Asteraceae) and Main Compounds’ Effects on a Glioblastoma Cellular Model. Pharmaceutics 2023, 15, 1383. [Google Scholar] [CrossRef]
- Chen, X.-M.; Lu, W.; Zhang, Z.-H.; Zhang, J.-Y.; Tuong, T.M.L.; Liu, L.-L.; Kim, Y.H.; Li, C.-H.; Gao, J.-M. Cassane Diterpenoids from the Aerial Parts of Caesalpinia pulcherrima and Their Antibacterial and Anti-Glioblastoma Activity. Phytochemistry 2022, 196, 113082. [Google Scholar] [CrossRef]
- Hua, D.; Zhao, Q.; Yu, Y.; Yu, H.; Yu, L.; Zhou, X.; Wang, Q.; Sun, C.; Shi, C.; Luo, W.; et al. Eucalyptal A Inhibits Glioma by Rectifying Oncogenic Splicing of MYO1B mRNA via Suppressing SRSF1 Expression. Eur. J. Pharmacol. 2021, 890, 173669. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, D.; Xu, X.; Qiu, S.; Luo, S.; Qiu, E.; Rong, Z.; Zhang, J.; Zheng, D. Galangin Inhibits Epithelial-Mesenchymal Transition and Angiogenesis by Downregulating CD44 in Glioma. J. Cancer 2019, 10, 4499–4508. [Google Scholar] [CrossRef]
- Kong, Y.; Feng, Z.; Chen, A.; Qi, Q.; Han, M.; Wang, S.; Zhang, Y.; Zhang, X.; Yang, N.; Wang, J.; et al. The Natural Flavonoid Galangin Elicits Apoptosis, Pyroptosis, and Autophagy in Glioblastoma. Front. Oncol. 2019, 9, 942. [Google Scholar] [CrossRef]
- Xiong, Y.; Lai, X.; Xiang, W.; Zhou, J.; Han, J.; Li, H.; Deng, H.; Liu, L.; Peng, J.; Chen, L. Galangin (GLN) Suppresses Proliferation, Migration, and Invasion of Human Glioblastoma Cells by Targeting Skp2-Induced Epithelial–Mesenchymal Transition (EMT). OncoTargets Ther. 2020, 13, 9235–9244. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Z.; Xu, X.; He, M.; Xiong, H.; Liu, L. Casticin Induces Apoptosis and Cytoprotective Autophagy While Inhibiting Stemness Involving Akt/mTOR and JAK2/STAT3 Pathways in Glioblastoma. Phytother. Res. 2024, 38, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Studzinska-Sroka, E.; Galanty, A.; Bylka, W. Atranorin—An Interesting Lichen Secondary Metabolite. Mini Rev. Med. Chem. 2017, 17, 1633–1645. [Google Scholar] [CrossRef]
- Mutai, C.; Abatis, D.; Vagias, C.; Moreau, D.; Roussakis, C.; Roussis, V. Lupane Triterpenoids from Acacia mellifera with Cytotoxic Activity. Molecules 2007, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, W.; Lou, H. Antifungal Constituents from the Chinese Moss Homalia Trichomanoides. Chem. Biodivers. 2005, 2, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak-Celińska, A.; Kleszcz, R.; Studzińska-Sroka, E.; Łukaszyk, A.; Szoszkiewicz, A.; Stelcer, E.; Jopek, K.; Rucinski, M.; Cielecka-Piontek, J.; Krajka-Kuźniak, V. Lichen Secondary Metabolites Inhibit the Wnt/β-Catenin Pathway in Glioblastoma Cells and Improve the Anticancer Effects of Temozolomide. Cells 2022, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Kaproń, B.; Plech, T.; Żarowski, M.; Cielecka-Piontek, J. Lichen-Derived Compounds and Extracts as Biologically Active Substances with Anticancer and Neuroprotective Properties. Pharmaceuticals 2021, 14, 1293. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Żarowski, M.; Plech, T.; Cielecka-Piontek, J. Permeability of Hypogymnia Physodes Extract Component-Physodic Acid through the Blood-Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System. Cancers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Bańdurska, M.; Rosiak, N.; Szwajgier, D.; Baranowska-Wójcik, E.; Szymański, M.; Gruszka, W.; Cielecka-Piontek, J. Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia Glauca within the Nervous System? Antioxidants 2022, 11, 2069. [Google Scholar] [CrossRef]
- Murugesan, M.; Kandhavelu, M.; Thiyagarajan, R.; Natesan, S.; Rajendran, P.; Murugesan, A. Marine Halophyte Derived Polyphenols Inhibit Glioma Cell Growth through Mitogen-Activated Protein Kinase Signaling Pathway. Biomed. Pharmacother. 2023, 159, 114288. [Google Scholar] [CrossRef]
- Kakouri, E.; Hatziagapiou, K.; Kanakis, C.; Nikola, O.; Lambrou, G.I.; Trigas, P.; Kanaka-Gantenbein, C.; Tarantilis, P.A. Cytotoxic and Antioxidant Activity of a Chemically Characterized Extract of Smilax Aspera Leaves and Stems. Appl. Sci. 2023, 13, 4784. [Google Scholar] [CrossRef]
- Kamarudin, N.A.; Nik Salleh, N.N.H.; Tan, S.C. Gallotannin-Enriched Fraction from Quercus infectoria Galls as an Antioxidant and Inhibitory Agent against Human Glioblastoma Multiforme. Plants 2021, 10, 2581. [Google Scholar] [CrossRef]
- Kleszcz, R.; Majchrzak-Celińska, A.; Baer-Dubowska, W. Tannins in Cancer Prevention and Therapy. Br. J. Pharmacol. 2023. early access. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Chen, F.; He, Y.; Yin, S.; Peng, Y.; Li, W. Phytochemicals and Glioma: Results from Dietary Mixed Exposure. Brain Sci. 2023, 13, 902. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Ghorbani, A. Cancer Therapy with Phytochemicals: Evidence from Clinical Studies. Avicenna J. Phytomed. 2015, 5, 84–97. [Google Scholar] [PubMed]
- Guzmán, M.; Duarte, M.J.; Blázquez, C.; Ravina, J.; Rosa, M.C.; Galve-Roperh, I.; Sánchez, C.; Velasco, G.; González-Feria, L. A Pilot Clinical Study of Δ9-Tetrahydrocannabinol in Patients with Recurrent Glioblastoma Multiforme. Br. J. Cancer 2006, 95, 197–203. [Google Scholar] [CrossRef]
- Lah, T.T.; Majc, B.; Novak, M.; Sušnik, A.; Breznik, B.; Porčnik, A.; Bošnjak, R.; Sadikov, A.; Malavolta, M.; Halilčević, S.; et al. The Cytotoxic Effects of Cannabidiol and Cannabigerol on Glioblastoma Stem Cells May Mostly Involve GPR55 and TRPV1 Signalling. Cancers 2022, 14, 5918. [Google Scholar] [CrossRef]
- Rybarczyk, A.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V. Targeting Nrf2 Signaling Pathway in Cancer Prevention and Treatment: The Role of Cannabis Compounds. Antioxidants 2023, 12, 2052. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, H.; Salles, É.L.; Alptekin, A.; Mehrabian, D.; Rutkowski, M.; Arbab, A.S.; Yeudall, W.A.; Yu, J.C.; Morgan, J.C.; Hess, D.C.; et al. Inhalant Cannabidiol Inhibits Glioblastoma Progression Through Regulation of Tumor Microenvironment. Cannabis Cannabinoid Res. 2023, 8, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, L.; Singer, E.; Dighe, P.; Sidorov, M.; Limbad, C.; Rodriquez-Brotons, A.; Rix, P.; Woo, R.W.L.; Dickinson, L.; Desprez, P.-Y.; et al. Cannabidiol Inhibits RAD51 and Sensitizes Glioblastoma to Temozolomide in Multiple Orthotopic Tumor Models. Neuro-Oncol. Adv. 2022, 4, vdac019. [Google Scholar] [CrossRef]
- Kuźmińska, J.; Sobczak, A.; Majchrzak-Celińska, A.; Żółnowska, I.; Gostyńska, A.; Jadach, B.; Krajka-Kuźniak, V.; Jelińska, A.; Stawny, M. Etoricoxib-Cannabidiol Combo: Potential Role in Glioblastoma Treatment and Development of PLGA-Based Nanoparticles. Pharmaceutics 2023, 15, 2104. [Google Scholar] [CrossRef]
- Volmar, M.N.M.; Cheng, J.; Alenezi, H.; Richter, S.; Haug, A.; Hassan, Z.; Goldberg, M.; Li, Y.; Hou, M.; Herold-Mende, C.; et al. Cannabidiol Converts NF-κB into a Tumor Suppressor in Glioblastoma with Defined Antioxidative Properties. Neuro-Oncolology 2021, 23, 1898–1910. [Google Scholar] [CrossRef] [PubMed]
- Likar, R.; Koestenberger, M.; Stutschnig, M.; Nahler, G. Cannabidiol Μay Prolong Survival in Patients with Glioblastoma Multiforme. Cancer Diagn. Progn. 2021, 1, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, A.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V. The Application of Cannabidiol in the Treatment of Glioblastoma. Acta Pol. Pharm. Drug Res. 2023, 80, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Mathur, A.; Pandey, V.K.; Kakkar, P. Endoplasmic Reticulum Stress-Dependent Activation of TRB3-FoxO1 Signaling Pathway Exacerbates Hyperglycemic Nephrotoxicity: Protection Accorded by Naringenin. Eur. J. Pharmacol. 2022, 917, 174745. [Google Scholar] [CrossRef]
- Twelves, C.; Sabel, M.; Checketts, D.; Miller, S.; Tayo, B.; Jove, M.; Brazil, L.; Short, S.C.; GWCA1208 study group. A Phase 1b Randomised, Placebo-Controlled Trial of Nabiximols Cannabinoid Oromucosal Spray with Temozolomide in Patients with Recurrent Glioblastoma. Br. J. Cancer 2021, 124, 1379–1387. [Google Scholar] [CrossRef]

| Compound | Group | Cell Line | Model | Results | Reference |
|---|---|---|---|---|---|
| Bevacizumab + elagic acid | phenolic acid | C6 | in vitro | Antiproliferative efficacy. Inhibition of MGMT expression and time-dependent inhibition of MDR1. | [27] |
| 5-Fluorouracil + thymoquinone | quinones | U-251MG | in vitro | Reduced cell viability and proliferation in GBM cells. Strong synergistic anticancer effect. | [28] |
| Irinotekan + elagic acid | phenolic acid | C6 | in vitro | Synergistic effect. Reduced cell proliferation by inhibiting the cadherin switch and promoting the antiangiogenic processes. | [29] |
| Temozolomide + berberine | alkalod | U87 U251 | in vitro/ in vivo mice | Enhanced autophagy and apoptosis in TMZ-resistant cells linked with ERK1/2 signaling. In vivo increased GBM sensitivity to TMZ through the ERK1/2 signaling pathway. | [30] |
| Temozolomide + harmine | alkaloid | T98G | in vitro | Decreased cancer cells’ migration, invasion, and adhesion potentials, as well as the expression of metalloproteinases 2 and 9. | [31] |
| Temozlomide + piperine | alkaloid | U251MG T98G | in vitro | Apoptosis induction by activation of caspase-8/-9/-3, MMP loss, and inhibition of cell motility. | [32] |
| Temozlomide + cannabidiol | canabinoid | U87MG | in vivo mice | Controlling tumor size and improving survival. | [33] |
| Temozolomide + osthol | coumarin | T98G | in vitro | Apoptosis, correlated with Bcl-2/Beclin 1 complex formation. | [34] |
| Temozolomide + biohanin A | isoflavone | U251 U87 C6 | in vitro/ in vivo rats/ in silico | Enhanced cells sensitivity to TMZ in vitro and in vivo. Inhibited TMZ-induced autophagy in GBM cells by activating the AMPK/ULK1 pathway in silico. | [35] |
| Temozolomide + apigenine | flavonoid | glioma cells | in vitro/ in vivo mice | Synergistic inhibition of glioma growth through the PI3K/AKT pathway. | [36] |
| Temozolomide + morusin | flavonoid | U87 U251 | in vitro/ in vivo mice | Enhanced endoplasmic reticulum stress, synergistic effect in GBM cells, suppressed tumor progression in an orthotopic xenograft model. | [37] |
| Temozolomide + naringenin | flavonoid | C6 U87MG LN229 HEK-293 T | in vitro/ in vivo rats | Synergistically increased efficacy of TMZ on glioma in vitro and in vivo. | [38] |
| Temozolomide + xantohumol | flavonoid | U87 MG A172 | in vitro | miR-4749-5p targeting RFC2 signaling participates in XN-enhanced TMZ cytotoxicity. | [39] |
| Temozolomide + honokiol | lignan | U87MG GL261 U87MG-R9 | in vitro | Significantly enhanced TMZ-induced insults. Induced greater caspase-3 activation, DNA fragmentation, cell apoptosis, and cell-cycle arrest at the G1 phase. Autophagy and consequent apoptosis in U87-MG-R9. | [40] |
| Temozolomide + honokiol | lignan | U373MG GL261 U87MG | in vitro | Improved TMZ-induced insults to human malignant glioma cells. Enhanced TMZ-induced apoptosis and suppression of proliferation in human glioma cells. | [41] |
| Temozolomide + magnolol | lignan | LN18 U87MG LN229 T98G HEK293 C6 | in vitro/ in vivo | Potentiation of TMZ-induced apoptosis in glioma by inhibiting NF-κB pathway-mediated MGMT activation. | [42] |
| Temozoomide + elagic acid | phenolic acid | C6 | in vitro | Antiproliferative efficacy by inhibiting MGMT expression and activating apoptotic protein, p53, and caspase-3 expression. | [43] |
| Temozolomide + tannic acid | phenolic acid | C6 | in vitro/ in vivo rats | Not cytotoxic to astrocytes. Induced anti-glioma activity, apoptosis, and cell-cycle arrest. Reduced the formation and size of colonies, and cell migration/adhesion. In vivo: decreased tumor volume and increased the area of intratumoral necrosis and infiltration of lymphocytes. | [44] |
| Temozolomide + elagic acid | phenolic acid | C6 | in vitro | Inhibited the cadherin switch and angiogenesis. | [45] |
| Temozolomide + gallic acid | phenolic acid | U87MG | in vitro | Potential augmentation of the anticancer effect of TMZ via the repression of Bcl-2 expression and Akt activation and the enhancement of the p38 MAPK pathway. | [46] |
| Temozolomide + steroidal saponin (N45) | saponin | U87R | in vitro | Induced mitochondrial apoptosis, and decreased drug resistance by downregulation of NF-κB p65. | [47] |
| Temozolomide + oleuropein | secoiridoid | T98G A172 | in vitro | Demonstrated additive effects that can augment the effect of TMZ. | [48] |
| Temozolomide + resveratrol | stilbenoid | RG-2 LN-18 LN-428 | in vitro | Downregulated MGMT overexpression. Inhibition of the STAT3/Bcl-2/survivin signaling pathway. | [49] |
| Compound/Product | ClinicalTrials.gov ID | Description | Study Type/Phase | Status |
|---|---|---|---|---|
| Cannabidiol | NCT05753007 | A Clinical Trial of a Hemp-Derived, High-Cannabidiol Product for Anxiety in Glioblastoma Patients | Phase 2 | Not yet recruiting |
| NCT03607643 | A Study of the Efficacy of Cannabidiol in Patients with Multiple Myeloma, Glioblastoma Multiforme, and GI Malignancies | Phases 1 and 2 | Unknown status | |
| TN-TC11G (Δ9-tetrahydrocannabinol + cannabidiol) | NCT03529448 | TN-TC11G (THC+CBD) Combination with Temozolomide and Radiotherapy in Patients with Newly Diagnosed Glioblastoma | Phases 1 and 2 | Recruiting |
| Cannabis (for smoking) | NCT03246113 | Tolerability of Cannabis in Patients Receiving Concurrent Chemoradiation for Glioblastoma | Phase 1 | Terminated |
| Sativex® (Nabiximols oromucosal spray) | NCT01812603 | A Safety Study of Sativex in Combination with Dose-Intense Temozolomide in Patients with Recurrent Glioblastoma | Phases 1 and 2 | Completed |
| NCT01812616 | A Safety Study of Sativex Compared with Placebo (Both with Dose-Intense Temozolomide) in Recurrent Glioblastoma Patients | Phases 1 and 2 | Completed | |
| NCT05629702 | ARISTOCRAT: Blinded Trial of Temozolomide +/− Cannabinoids | Phase 2 | Recruiting | |
| Chlorogenic Acid | NCT02728349 | Tolerance and Pharmacokinetic Study of Chlorogenic Acid to Advanced Glioblastoma | Phase 1 | Completed |
| Curcumin | NCT01712542 | Curcumin Bioavailability in Glioblastoma Patients | Observational | Completed |
| NCT05768919 | Study of Liposomal Curcumin in Combination with RT and TMZ in Patients with Newly Diagnosed High-Grade Gliomas | Phases 1 and 2 | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majchrzak-Celińska, A.; Studzińska-Sroka, E. New Avenues and Major Achievements in Phytocompounds Research for Glioblastoma Therapy. Molecules 2024, 29, 1682. https://doi.org/10.3390/molecules29071682
Majchrzak-Celińska A, Studzińska-Sroka E. New Avenues and Major Achievements in Phytocompounds Research for Glioblastoma Therapy. Molecules. 2024; 29(7):1682. https://doi.org/10.3390/molecules29071682
Chicago/Turabian StyleMajchrzak-Celińska, Aleksandra, and Elżbieta Studzińska-Sroka. 2024. "New Avenues and Major Achievements in Phytocompounds Research for Glioblastoma Therapy" Molecules 29, no. 7: 1682. https://doi.org/10.3390/molecules29071682