A 70% Ethanol Neorhodomela munita Extract Attenuates RANKL-Induced Osteoclast Activation and H2O2-Induced Osteoblast Apoptosis In Vitro
Abstract
:1. Introduction
2. Results
2.1. Subsection
2.1.1. The Identification of Compounds of EN
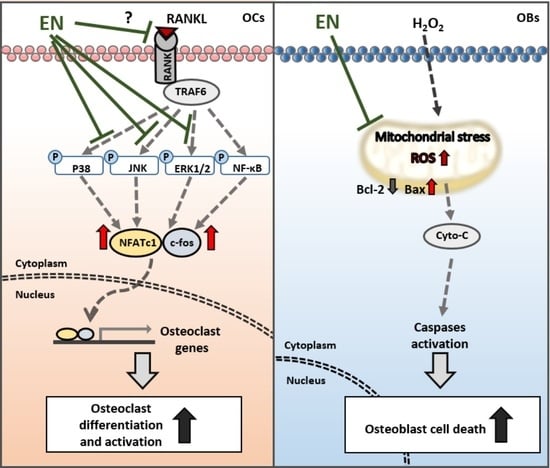
2.1.2. EN Inhibits RANKL-Induced Osteoclast Differentiation and Activation of Raw264.7 Cells
2.1.3. EN Impairs the Catabolic Functions of RANKL-Stimulated Osteoclasts
2.1.4. EN Inhibits RANKL-Induced MAPK Signaling and Subsequent NFATc1 Activation
2.1.5. EN Mitigates H2O2-Induced Cell Death in Saos-2 Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of Neorhodomela munita Extract and Its Compounds
4.2. The Liquid Chromatography–Mass Spectrometry (LC/MS) Analysis
4.3. Cell Culture
4.4. Evaluation of Cell Viability (WST-1)
4.5. Reverse Transcription PCR (RT-PCR)
4.6. Western Blot
4.7. TRAP Staining
4.8. Bone Resorption Assay
4.9. Actin Ring Formation
4.10. Osteoblast Apoptosis Induced by H2O2
4.11. Cell Counting Assay (Trypan Blue Viability Assay)
4.12. TUNEL-Propidium Iodide (PI) Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarafrazi, N.; Wambogo, E.A.; Shepherd, J.A. Osteoporosis or Low Bone Mass in Older Adults: United States, 2017–2018. NCHS Data Brief 2021, 1–8. [Google Scholar] [CrossRef]
- Salari, N.; Darvishi, N.; Bartina, Y.; Larti, M.; Kiaei, A.; Hemmati, M.; Shohaimi, S.; Mohammadi, M. Global prevalence of osteoporosis among the world older adults: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 669. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2022, 33, 2049–2102. [Google Scholar] [CrossRef] [PubMed]
- Rashki Kemmak, A.; Rezapour, A.; Jahangiri, R.; Nikjoo, S.; Farabi, H.; Soleimanpour, S. Economic burden of osteoporosis in the world: A systematic review. Med. J. Islam. Repub. Iran. 2020, 34, 154. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Huang, X.; Wu, J.; Lin, X.; Zhou, X.; Zhu, Z.; Pan, X.; Xu, J.; Qiao, J.; Zhang, T.; et al. The Global Burden of Osteoporosis, Low Bone Mass, and Its Related Fracture in 204 Countries and Territories, 1990–2019. Front. Endocrinol. 2022, 13, 882241. [Google Scholar] [CrossRef]
- Moayyeri, A.; Warden, J.; Han, S.; Suh, H.S.; Pinedo-Villanueva, R.; Harvey, N.C.; Curtis, J.R.; Silverman, S.; Multani, J.K.; Yeh, E.J. Estimating the economic burden of osteoporotic fractures in a multinational study: A real-world data perspective. Osteoporos. Int. 2023, 34, 2121–2132. [Google Scholar] [CrossRef]
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing 2005, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; He, L.; Liang, Y.; Qin, H.; Yu, B.; Chang, L.; Xue, L. TNF-alpha contributes to postmenopausal osteoporosis by synergistically promoting RANKL-induced osteoclast formation. Biomed. Pharmacother. 2018, 102, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Liu, H.; Luo, T.; Liu, D.; Du, J.; Sun, J.; Wang, W.; Han, X.; Yang, K.; Guo, J.; et al. Author Correction: Combination of IL-6 and sIL-6R differentially regulate varying levels of RANKL-induced osteoclastogenesis through NF-kappaB, ERK and JNK signaling pathways. Sci. Rep. 2022, 12, 3746. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Zenz, R.; Eferl, R.; Scheinecker, C.; Redlich, K.; Smolen, J.; Schonthaler, H.B.; Kenner, L.; Tschachler, E.; Wagner, E.F. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res. Ther. 2008, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Nedeva, I.R.; Vitale, M.; Elson, A.; Hoyland, J.A.; Bella, J. Role of OSCAR Signaling in Osteoclastogenesis and Bone Disease. Front. Cell Dev. Biol. 2021, 9, 641162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in Osteoclast Differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.Y.; Yang, Y.; Jung, H. Molecular Mechanisms and Emerging Therapeutics for Osteoporosis. Int. J. Mol. Sci. 2020, 21, 7623. [Google Scholar] [CrossRef]
- Ukon, Y.; Makino, T.; Kodama, J.; Tsukazaki, H.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. Molecular-Based Treatment Strategies for Osteoporosis: A Literature Review. Int. J. Mol. Sci. 2019, 20, 2557. [Google Scholar] [CrossRef]
- Saag, K.G.; Emkey, R.; Schnitzer, T.J.; Brown, J.P.; Hawkins, F.; Goemaere, S.; Thamsborg, G.; Liberman, U.A.; Delmas, P.D.; Malice, M.P.; et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N. Engl. J. Med. 1998, 339, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.M.; Devogelaer, J.P.; Saag, K.; Roux, C.; Lau, C.S.; Reginster, J.Y.; Papanastasiou, P.; Ferreira, A.; Hartl, F.; Fashola, T.; et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): A multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2009, 373, 1253–1263. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, X.; Wang, T.; Zhai, S. Comparative efficacy of bisphosphonates in short-term fracture prevention for primary osteoporosis: A systematic review with network meta-analyses. Osteoporos. Int. 2016, 27, 3289–3300. [Google Scholar] [CrossRef]
- Trovas, G.P.; Lyritis, G.P.; Galanos, A.; Raptou, P.; Constantelou, E. A randomized trial of nasal spray salmon calcitonin in men with idiopathic osteoporosis: Effects on bone mineral density and bone markers. J. Bone Miner. Res. 2002, 17, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ling, Z.; Feng, X.; Mao, C.; Xu, Z. Herb Medicines against Osteoporosis: Active Compounds & Relevant Biological Mechanisms. Curr. Top. Med. Chem. 2017, 17, 1670–1691. [Google Scholar] [CrossRef]
- Abdel-Naim, A.B.; Alghamdi, A.A.; Algandaby, M.M.; Al-Abbasi, F.A.; Al-Abd, A.M.; Eid, B.G.; Abdallah, H.M.; El-Halawany, A.M. Rutin Isolated from Chrozophora tinctoria Enhances Bone Cell Proliferation and Ossification Markers. Oxidative Med. Cell Longev. 2018, 2018, 5106469. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Pangestuti, R. Biological activities and potential health benefits of fucoxanthin derived from marine brown algae. Adv. Food Nutr. Res. 2011, 64, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Airanthi, M.K.; Hosokawa, M.; Miyashita, K. Comparative antioxidant activity of edible Japanese brown seaweeds. J. Food Sci. 2011, 76, C104–C111. [Google Scholar] [CrossRef] [PubMed]
- Saadaoui, I.; Rasheed, R.; Abdulrahman, N.; Bounnit, T.; Cherif, M.; Al Jabri, H.; Mraiche, F. Algae-Derived Bioactive Compounds with Anti-Lung Cancer Potential. Mar. Drugs 2020, 18, 197. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pages, T.; Viscor, G.; Torres, J.L. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Carvalhal, F.; Cristelo, R.R.; Resende, D.; Pinto, M.M.M.; Sousa, E.; Correia-da-Silva, M. Antithrombotics from the Sea: Polysaccharides and Beyond. Mar. Drugs 2019, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, R.; Cui, Y.; Jia, X.; Liu, T.; Wang, X.; Qu, J. The complete mitochondrial genome and phylogenetic analysis of Neorhodomela munita. Mitochondrial DNA B Resour. 2021, 6, 2746–2747. [Google Scholar] [CrossRef]
- Park, S.H.; Song, J.H.; Kim, T.; Shin, W.S.; Park, G.M.; Lee, S.; Kim, Y.J.; Choi, P.; Kim, H.; Kim, H.S.; et al. Anti-human rhinoviral activity of polybromocatechol compounds isolated from the rhodophyta, Neorhodomela aculeata. Mar. Drugs 2012, 10, 2222–2233. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Jin, D.Q.; Sung, J.Y.; Lee, J.H.; Choi, H.G.; Ha, I.; Han, J.S. Antioxidant and anti-inflammatory activities of the methanolic extract of Neorhodomela aculeate in hippocampal and microglial cells. Biol. Pharm. Bull. 2006, 29, 1212–1216. [Google Scholar] [CrossRef]
- Lakkakorpi, P.T.; Vaananen, H.K. Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro. J. Bone Miner. Res. 1991, 6, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, S.; Tomita, T.; Matsui, H.; Ohga, E.; Matsuse, T.; Ouchi, Y. Hydrogen peroxide-induced apoptosis and necrosis in human lung fibroblasts: Protective roles of glutathione. Jpn. J. Pharmacol. 1999, 79, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Takahashi, N.; Yasuda, H.; Mizuno, A.; Itoh, K.; Ueno, Y.; Shinki, T.; Gillespie, M.T.; Martin, T.J.; Higashio, K.; et al. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 2000, 141, 3478–3484. [Google Scholar] [CrossRef] [PubMed]
- Brandstrom, H.; Bjorkman, T.; Ljunggren, O. Regulation of osteoprotegerin secretion from primary cultures of human bone marrow stromal cells. Biochem. Biophys. Res. Commun. 2001, 280, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Iyer, S.; Martin-Millan, M.; Bartell, S.M.; Han, L.; Ambrogini, E.; Onal, M.; Xiong, J.; Weinstein, R.S.; Jilka, R.L.; et al. Estrogen receptor-alpha signaling in osteoblast progenitors stimulates cortical bone accrual. J. Clin. Investig. 2013, 123, 394–404. [Google Scholar] [CrossRef]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef]
- Management of osteoporosis in postmenopausal women: The 2021 position statement of The North American Menopause Society. Menopause 2021, 28, 973–997. [CrossRef] [PubMed]
- Yajun, W.; Jin, C.; Zhengrong, G.; Chao, F.; Yan, H.; Weizong, W.; Xiaoqun, L.; Qirong, Z.; Huiwen, C.; Hao, Z.; et al. Betaine Attenuates Osteoarthritis by Inhibiting Osteoclastogenesis and Angiogenesis in Subchondral Bone. Front. Pharmacol. 2021, 12, 723988. [Google Scholar] [CrossRef] [PubMed]
- Villa, I.; Senesi, P.; Montesano, A.; Ferraretto, A.; Vacante, F.; Spinello, A.; Bottani, M.; Bolamperti, S.; Rubinacci, A.; Luzi, L.; et al. Betaine promotes cell differentiation of human osteoblasts in primary culture. J. Transl. Med. 2017, 15, 132. [Google Scholar] [CrossRef]
- Oyen, J.; Gjesdal, C.G.; Karlsson, T.; Svingen, G.F.; Tell, G.S.; Strand, E.; Drevon, C.A.; Vinknes, K.J.; Meyer, K.; Ueland, P.M.; et al. Dietary Choline Intake Is Directly Associated with Bone Mineral Density in the Hordaland Health Study. J. Nutr. 2017, 147, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Spector, T.D.; Calomme, M.R.; Anderson, S.H.; Clement, G.; Bevan, L.; Demeester, N.; Swaminathan, R.; Jugdaohsingh, R.; Berghe, D.A.; Powell, J.J. Choline-stabilized orthosilicic acid supplementation as an adjunct to calcium/vitamin D3 stimulates markers of bone formation in osteopenic females: A randomized, placebo-controlled trial. BMC Musculoskelet. Disord. 2008, 9, 85. [Google Scholar] [CrossRef]
- Wright Muelas, M.; Roberts, I.; Mughal, F.; O’Hagan, S.; Day, P.J.; Kell, D.B. An untargeted metabolomics strategy to measure differences in metabolite uptake and excretion by mammalian cell lines. Metabolomics 2020, 16, 107. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jia, Y.; Li, J.; Huang, X.; Jiang, L.; Xiang, T.; Xie, Y.; Yang, X.; Liu, T.; Xiang, Z.; et al. Niloticin inhibits osteoclastogenesis by blocking RANKL-RANK interaction and suppressing the AKT, MAPK, and NF-kappaB signaling pathways. Biomed. Pharmacother. 2022, 149, 112902. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, F.; Liu, T.; Xu, J.; Li, J.; Jiang, L.; Wang, X.; Sheng, J. Ellagic acid blocks RANKL-RANK interaction and suppresses RANKL-induced osteoclastogenesis by inhibiting RANK signaling pathways. Chem. Biol. Interact. 2020, 331, 109235. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Bu, H.D.; Park, D.S.; Go, G.M.; Jee, Y.; Shin, T.; Hyun, J.W. Antioxidant activity of ethanol extract of Callophyllis japonica. Phytother. Res. 2005, 19, 506–510. [Google Scholar] [CrossRef]
- Yang, J.I.; Yeh, C.C.; Lee, J.C.; Yi, S.C.; Huang, H.W.; Tseng, C.N.; Chang, H.W. Aqueous extracts of the edible Gracilaria tenuistipitata are protective against H2O2-induced DNA damage, growth inhibition, and cell cycle arrest. Molecules 2012, 17, 7241–7254. [Google Scholar] [CrossRef]
- Kane, D.J.; Sarafian, T.A.; Anton, R.; Hahn, H.; Gralla, E.B.; Valentine, J.S.; Ord, T.; Bredesen, D.E. Bcl-2 inhibition of neural death: Decreased generation of reactive oxygen species. Science 1993, 262, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Giardino, I.; Edelstein, D.; Brownlee, M. BCL-2 expression or antioxidants prevent hyperglycemia-induced formation of intracellular advanced glycation endproducts in bovine endothelial cells. J. Clin. Investig. 1996, 97, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Mao, Y.; Sun, X.; Li, X.; Muhammad, I.; Gu, W.; Zhang, D.; Zhou, Y.; Ni, Z.; Ma, J.; et al. Attenuation of Oxidative Stress-Induced Osteoblast Apoptosis by Curcumin is Associated with Preservation of Mitochondrial Functions and Increased Akt-GSK3beta Signaling. Cell Physiol. Biochem. 2017, 41, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Chen, W.; Gu, X.; Shan, R.; Zou, J.; Liu, G.; Shahid, M.; Gao, J.; Han, B. Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Oncotarget 2017, 8, 14680–14692. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.B.; Diab, D.L. Long-term use of bisphosphonates in osteoporosis. J. Clin. Endocrinol. Metab. 2010, 95, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D. A review of the efficacy and safety of denosumab in postmenopausal women with osteoporosis. Ther. Adv. Musculoskelet. Dis. 2011, 3, 271–282. [Google Scholar] [CrossRef] [PubMed]








| No. | RT (Min.) | Formula | Candidate M.W. | Area (Max.) | Name by Searching ChemSpider Results |
|---|---|---|---|---|---|
| 1 | 0.762 | C6 H13 N O2 | 131.0945 | 341,181,829.574 | 6-Aminocaproic acid |
| 2 | 7.26 | C11 H16 O3 | 196.1097 | 187,512,214.227 | 1-carboxy-3-hydroxyadamantane |
| 3 | 0.705 | C5 H13 N O | 103.0998 | 181,541,412.769 | Choline |
| 4 | 6.522 | C6 H18 O3 Si3 | 222.0556 | 141,499,620.568 | Hexamethylcyclotrisiloxane |
| 5 | 7.431 | C11 H16 O3 | 196.1097 | 117,850,468.790 | 1-carboxy-3-hydroxyadamantane |
| 6 | 0.814 | C5 H11 N O2 | 117.0789 | 112,852,646.944 | Betaine |
| 7 | 6.6 | C10 H12 Br N O4 | 288.9944 | 112,762,617.285 | Amino(5-bromo-2,4-dimethoxyphenyl)acetic acid |
| 8 | 0.785 | C5 H9 N O2 | 115.0633 | 93,627,111.482 | D-(+)-Proline |
| 9 | 8.553 | C11 H16 O2 | 180.1148 | 74,265,194.775 | 2-tert-Butyl-4-methoxyphenol |
| 10 | 1.25 | C6 H6 N2 O | 122.048 | 62,495,868.421 | Nicotinamide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Kim, I.-K.; Moon, H.; Kim, H.; Song, B.-W.; Choi, J.-W.; Kim, S.W.; Lee, S.; Chae, D.-S.; Lim, S. A 70% Ethanol Neorhodomela munita Extract Attenuates RANKL-Induced Osteoclast Activation and H2O2-Induced Osteoblast Apoptosis In Vitro. Molecules 2024, 29, 1741. https://doi.org/10.3390/molecules29081741
Jeong S, Kim I-K, Moon H, Kim H, Song B-W, Choi J-W, Kim SW, Lee S, Chae D-S, Lim S. A 70% Ethanol Neorhodomela munita Extract Attenuates RANKL-Induced Osteoclast Activation and H2O2-Induced Osteoblast Apoptosis In Vitro. Molecules. 2024; 29(8):1741. https://doi.org/10.3390/molecules29081741
Chicago/Turabian StyleJeong, Seongtae, Il-Kwon Kim, Hanbyeol Moon, Hojin Kim, Byeong-Wook Song, Jung-Won Choi, Sang Woo Kim, Seahyoung Lee, Dong-Sik Chae, and Soyeon Lim. 2024. "A 70% Ethanol Neorhodomela munita Extract Attenuates RANKL-Induced Osteoclast Activation and H2O2-Induced Osteoblast Apoptosis In Vitro" Molecules 29, no. 8: 1741. https://doi.org/10.3390/molecules29081741