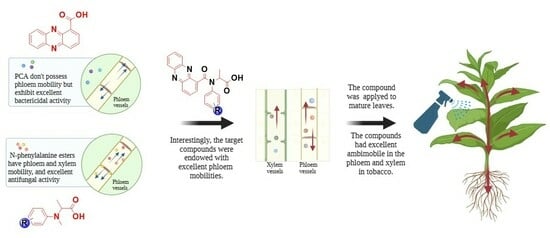
Study on the Design, Synthesis, Bioactivity and Translocation of the Conjugates of Phenazine-1-carboxylic Acid and N-Phenyl Alanine Ester
Abstract
:1. Introduction
2. Result and Discussion
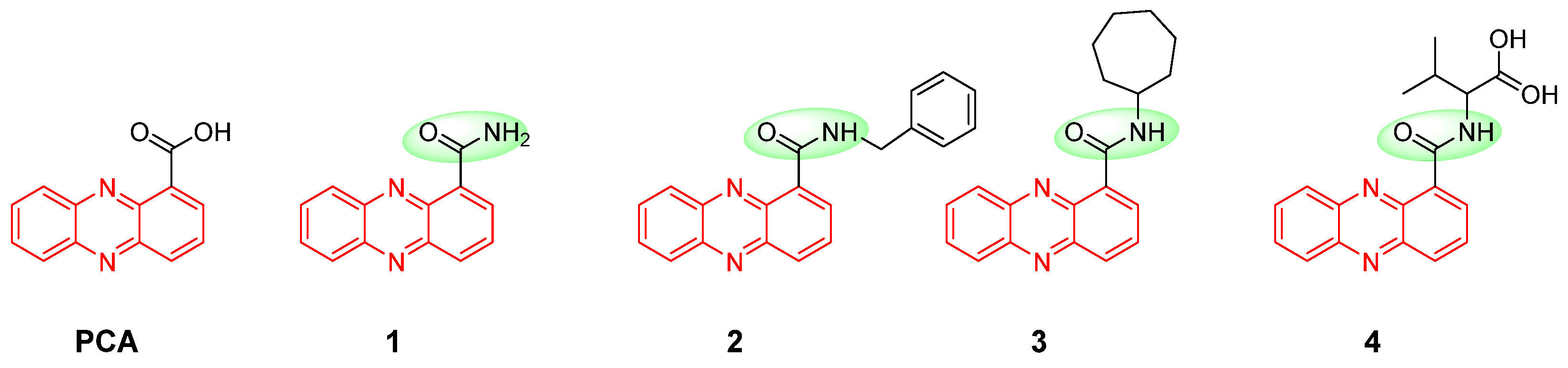
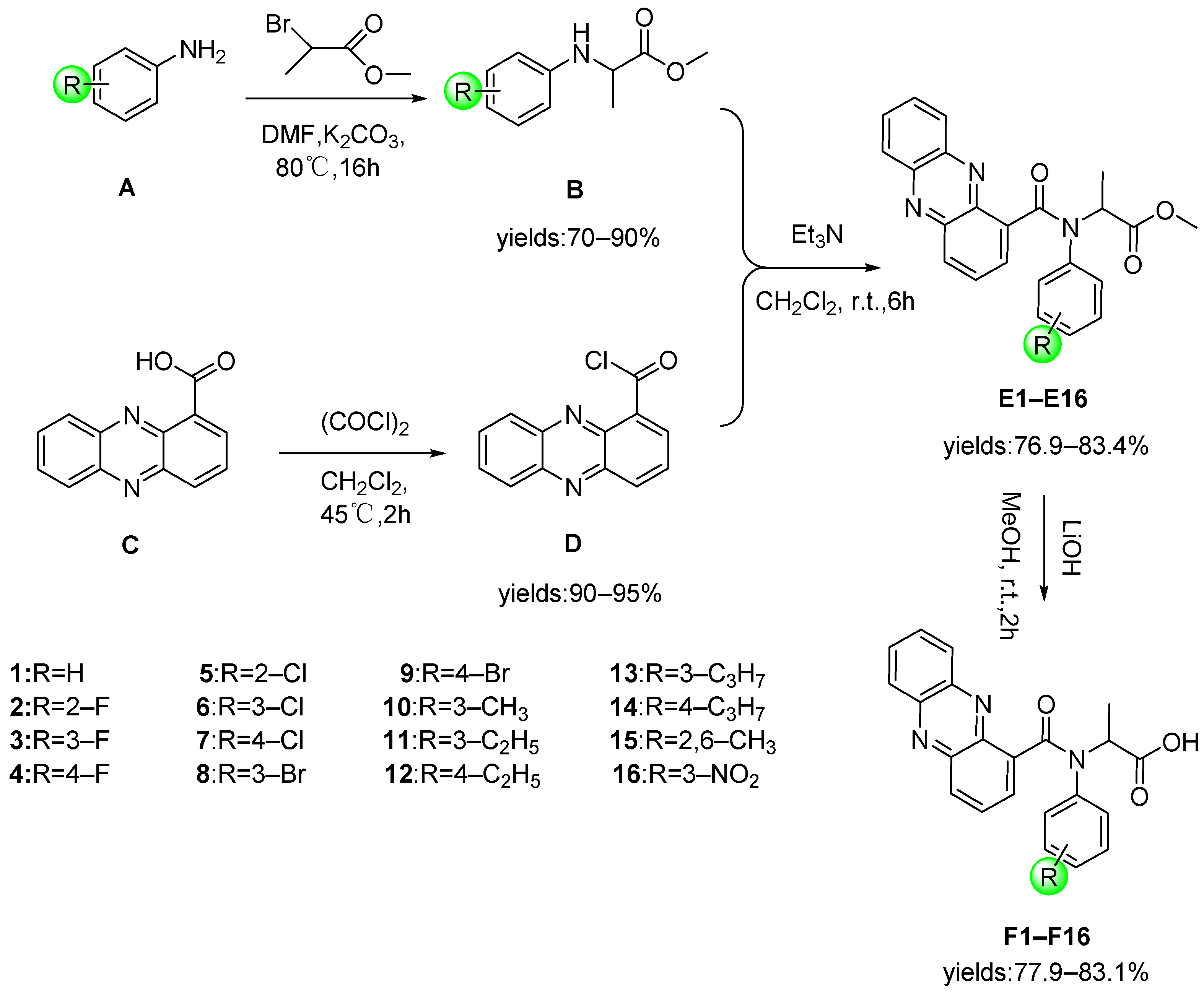
2.1. Chemistry
2.2. Antifungal Activity
2.3. Preliminary Analysis of Structure–Activity Relationship (SAR)
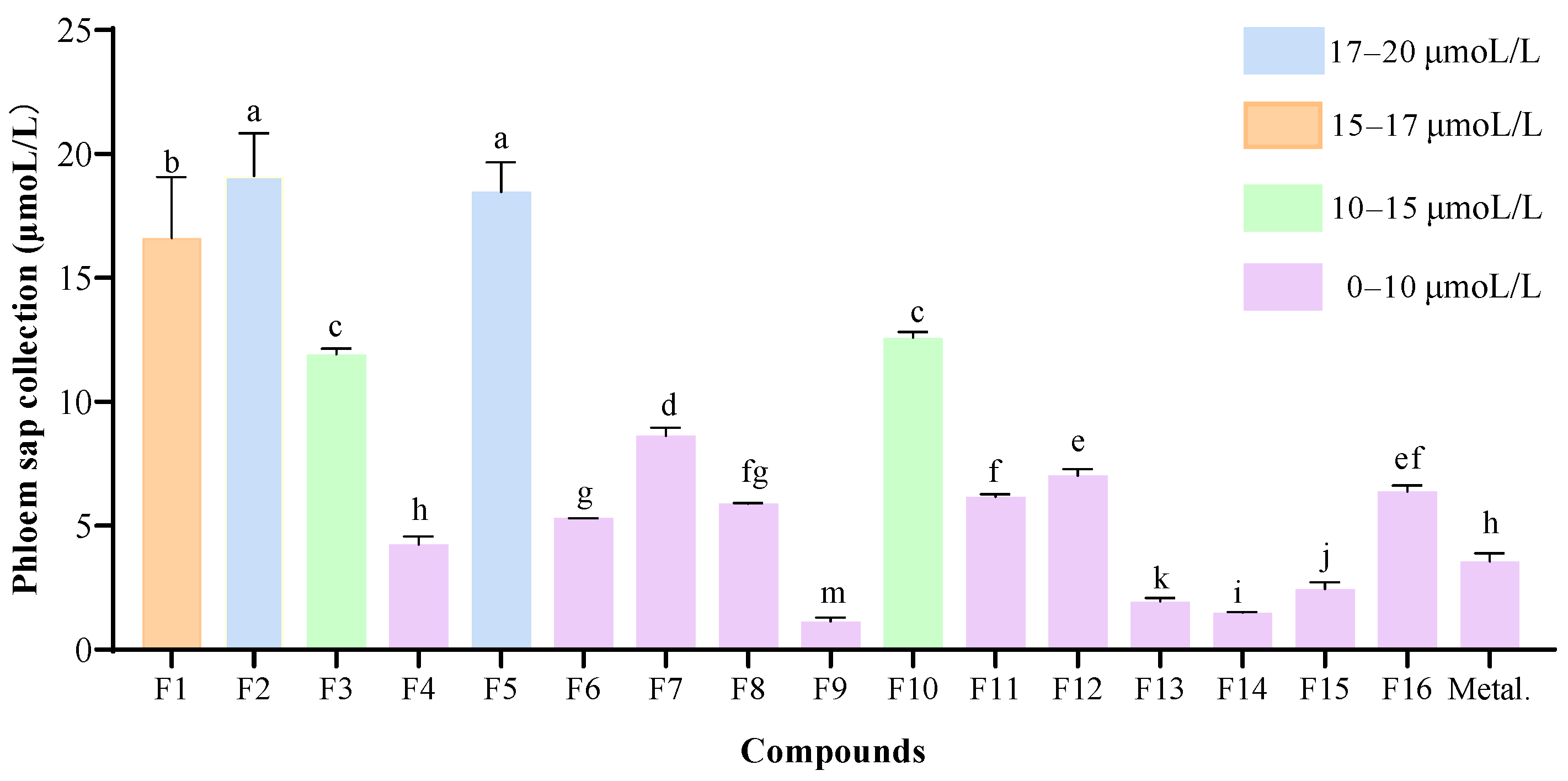
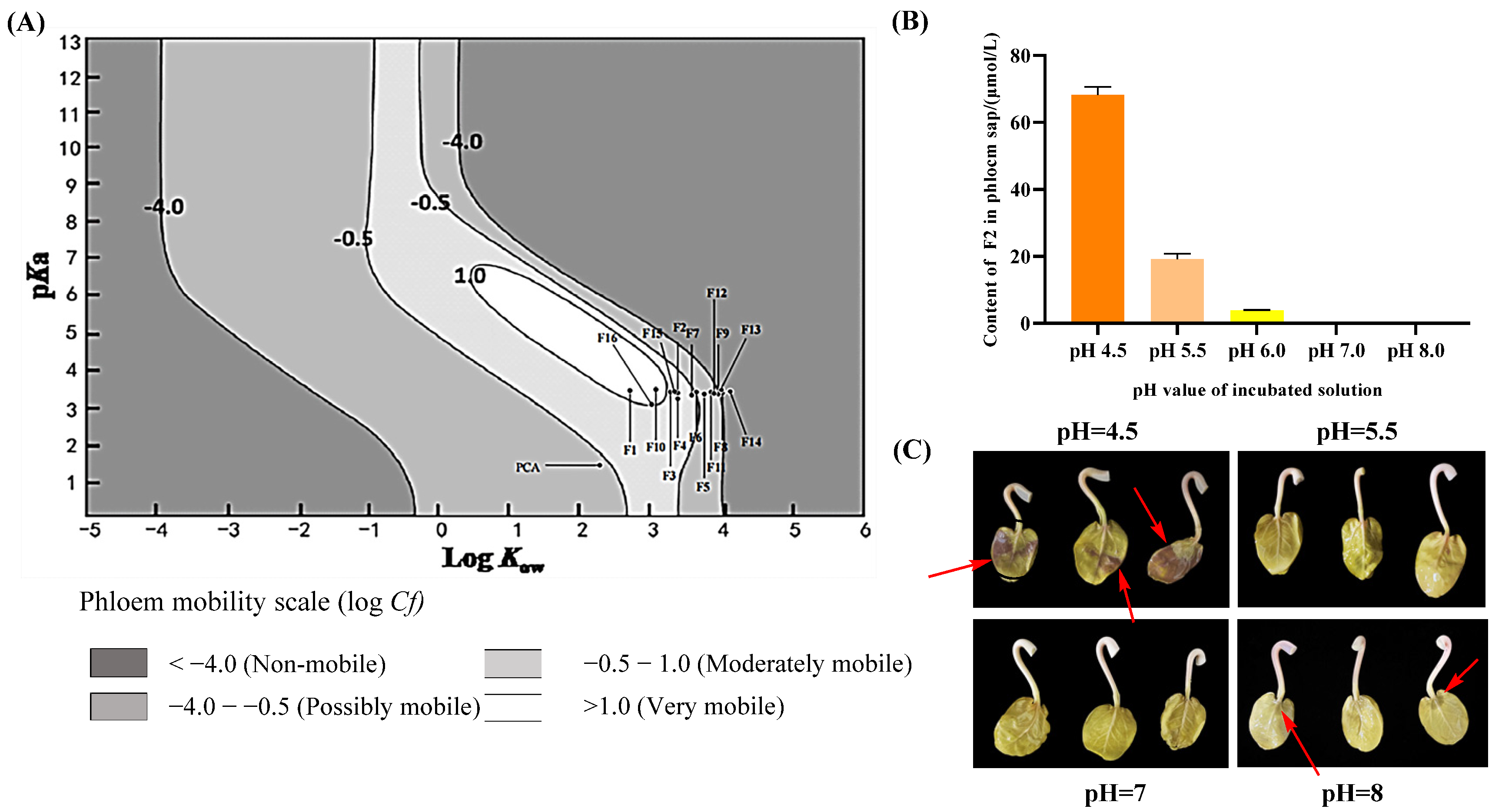
2.4. Phloem Mobility
2.5. Predicting Phloem Mobility and Possible Mobility Mechanism of F Series Compounds
2.6. The Phloem Mobility of Compound F2 Up-Taken through Tobacco Seedlings
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Test Fungus and Plant Materials
3.3. Synthesis
3.3.1. General Procedure for the Preparation of B
3.3.2. General Procedure for the Preparation of Intermediate D
3.3.3. General Procedure for the Preparation of Target Compounds E
3.3.4. General Procedure for the Preparation of Target Compound F
3.4. Antifungal Activity Assay
3.5. Phloem Sap Collection and Analysis
3.5.1. Phloem Sap Collection
3.5.2. Analytical Methods
3.6. Mobile Distribution of Compound F2 in Tobacco
3.6.1. Uptake and Translocation of Compound F2 by Tobacco Seedlings
3.6.2. Extraction and Analysis of Compounds F2
3.6.3. Spike Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeschke, P. Progress of modern agricultural chemistry and future prospects. Pest Manag. Sci. 2016, 72, 433–455. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Cohen, Y.; Weitman, M. Mobility of oxathiapiprolin in and between tomato plants. Pest Manag. Sci. 2023, 79, 1102–1112. [Google Scholar] [CrossRef]
- Edgington, L.V. Structural requirements of systemic fungicides. Annu. Rev. Phytopathol. 1981, 19, 107–124. [Google Scholar] [CrossRef]
- Singh, M.; Mersie, W.; Brlansky, R.H. Phytotoxicity of the fungicide Metalaxyl and its optical isomers. Plant Dis. 2003, 87, 1144–1147. [Google Scholar] [CrossRef]
- Zhao, L.; Teng, Y.; Luo, Y. Present pollution status and control strategy of pesticides in agricultural soils in China: A review. Soils 2017, 49, 417–427. [Google Scholar]
- Hsu, F.C.; Sun, K.; Kleier, D.A.; Fielding, M.J. Phloem mobility of xenobiotics VI. A phloem-mobile pro-nematicide based on oxamyl exhibiting root-specific activation in transgenic tobacco. Pestic. Sci. 1995, 44, 9–19. [Google Scholar] [CrossRef]
- Triantafyllidis, V.; Hela, D.; Patakioutas, G. Environmental behavior of the fungicide Metalaxyl in experimental tobacco field. J. Environ. Sci. Health Part B 2013, 48, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Damak, M.; Hyder, M.N.; Varanasi, K.K. Enhancing droplet deposition through in-situ precipitation. Nat. Commun. 2016, 7, 12560. [Google Scholar] [CrossRef]
- Chollet, J.F.; Rocher, F.; Jousse, C.; Celine, D.G.; Bashiardes, G.; Bonnemain, J.L. Synthesis and phloem mobility of acidic derivatives of the fungicide fenpiclonil. Pest Manag. Sci. Former. Pestic. Sci. 2004, 60, 1063–1072. [Google Scholar] [CrossRef]
- Yang, W.; Wu, H.; Xu, H.; Hu, A.; Lu, M. Synthesis of glucose–fipronil conjugate and its phloem mobility. J. Agric. Food Chem. 2011, 59, 12534–12542. [Google Scholar] [CrossRef] [PubMed]
- Gisi, U.; Cohen, Y. Resistance to phenylamide fungicides: A case study with Phytophthora infestans involving mating type and race structure. Annu. Rev. Phytopathol. 1996, 34, 549–572. [Google Scholar] [CrossRef] [PubMed]
- Kadish, D.; Cohen, Y. Overseasoning of Metalaxyl-sensitive and Metalaxyl-resistant isolates of Phytophthora infestans in potato tubers. Phytopathology 1992, 82, 887–889. [Google Scholar] [CrossRef]
- Zhu, G.; Huang, F.; Feng, L.; Qin, B.; Yang, Y.; Chen, Y.; Lu, X. Sensitivities of Phytophthora infestans to Metalaxyl, cymoxanil, and dimethomorph. Agric. Sci. China 2008, 7, 831–840. [Google Scholar] [CrossRef]
- Fang, Z.; Gao, S.; He, H. Natural products: A bridge between new targets and novel pesticide discovery. J. East China Norm. Univ. (Nat. Sci.) 2023, 2023, 21. [Google Scholar]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.; Farnham, A.W.; Janes, N.F.; Needham, P.H.; Pulman, D.A. Synthetic insecticide with a new order of activity. Nature 1974, 248, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Bowers, W.S.; Fales, H.M.; Thompson, M.J.; Uebel, E.C. Juvenile hormone: Identification of an active compound from balsam fir. Science 1966, 154, 1020–1021. [Google Scholar] [CrossRef] [PubMed]
- Puopolo, G.; Masi, M.; Raio, A.; Andolfi, A.; Zoina, A.; Cimmino, A.; Evidente, A. Insights on the susceptibility of plant pathogenic fungi to phenazine-1-carboxylic acid and its chemical derivatives. Nat. Prod. Res. 2013, 27, 956–966. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, H.; Xu, H.; Zou, Q.; Cheng, C.; Dong, D.; Xu, Y.; Li, R. Phenazine-1-carboxylic acid derivatives: Design, synthesis and biological evaluation against Rhizoctonia solani Kuhn. Bioorg. Med. Chem. Lett. 2010, 20, 7369–7371. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, M.; Xiao, Y.; Hsiang, T.; Hu, C.; Li, J. Systemic fungicidal activity of phenazine-1-carboxylic acid-valine conjugate against tobacco sore shin and its translocation and accumulation in tobacco (Nicotiana tabacum L.). Pest Manag. Sci. 2022, 78, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Hu, C.; Hsiang, T.; Li, J. Amino acid permease RcAAP1 increases the uptake and phloem translocation of an L-valine-phenazine-1-carboxylic acid conjugate. Front. Plant Sci. 2023, 14, 1191250. [Google Scholar] [CrossRef] [PubMed]
- Gozzo, F.; Garlaschelli, L.; Boschi, P.M.; Zagni, A.; Overeem, J.C.; Vries, L. Recent progress in the field of N-acylalanines as systemic fungicides. Pestic. Sci. 1985, 16, 277–286. [Google Scholar] [CrossRef]
- Randall, E.; Young, V.; Sierotzki, H.; Scalliet, G.; Birch, P.R.J.; Cooke, D.E.L.; Csukai, M.; Whisson, S.C. Sequence diversity in the large subunit of RNA polymerase I contributes to Mefenoxam insensitivity in Phytophthora infestans. Mol. Plant Pathol. 2014, 15, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zhao, J.; Liu, X.; Meng, R.; Wu, J.; Han, X.; Wang, W. Monitoring of resistance of Phytophthora infestans on potato to Metalaxyl and the control efficacy of alternative fungicides. Sci. Agric. Sin. 2018, 51, 2700–2710. [Google Scholar]
- Sun, Y.; Song, J.; Kong, L.; Sha, B.; Tian, X.; Liu, X.; Hu, T.; Chen, P.; Zhang, S. Design, synthesis and evaluation of novel bis-substituted aromatic amide dithiocarbamate derivatives as colchicine site tubulin polymerization inhibitors with potent anticancer activities. Eur. J. Med. Chem. 2022, 229, 114069. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Nie, D.; Yu, D.; Wu, Q.; Yu, L.; Yao, Z.; Du, X.; Li, J. Synthesis, fungicidal activity and phloem mobility of phenazine-1-carboxylic acid-alanine conjugates. Pestic. Biochem. Phys. 2017, 143, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, L.; Jin, S.; Dong, Y.; Lu, H.; Zhang, J. Synthesis and fungicidal activity of diamide compounds based on the metabolite of benalaxyl. Chin. J. Org. Chem. 2017, 37, 157. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Xiao, C.; Lai, D.; Yao, G.; Xu, H. Systemicity of fludioxonil derivatives with a carboxylic acid function and their control effect against Fusarium wilt of banana. J. Plant Protect. 2022, 48, 789–797. [Google Scholar]
- Kleier, D.A. Phloem mobility of xenobiotics. V. Structural requirements for phloem-systemic pesticides. Pestic. Sci. 1994, 42, 1–11. [Google Scholar] [CrossRef]
- Kleier, D.A.; Hsu, F.C. Phloem mobility of xenobiotics. VII. The design of phloem systemic pesticides. Weed Sci. 1996, 44, 749–756. [Google Scholar] [CrossRef]
- Ca, J.; Xiong, Y.; Zhu, X.; Hu, J.; Wang, Y.; Li, J.; Wu, J.; Wu, Q. An Exploration of the Effect of the Kleier Model and Carrier-Mediated Theory to Design Phloem-Mobile Pesticides Based on Researching the N-Alkylated Derivatives of Phenazine-1-Carboxylic Acid-Glycine. Molecules 2022, 27, 4999. [Google Scholar] [CrossRef]
- Zheng, S.; Gu, Y.; Li, L.; Zhu, R.; Cai, X.; Bai, H.; Zhang, J. Synthesis and fungicidal activity of tryptophan analogues-the unexpected calycanthaceous alkaloid derivatives. Nat. Prod. Res. 2017, 31, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Huang, D.; Zhu, X.; Zhang, M.; Yao, A.; Wu, Q.; Xu, Z.; Li, J. Design, synthesis, phloem mobility, and bioactivities of a series of phenazine-1-carboxylic acid-amino acid conjugates. Molecules 2018, 23, 2139. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yu, D.; Zhang, M.; Li, X.; Zhu, X.; Xu, Z.; Wu, Q.; Li, J. Phloem mobility of Metalaxyl in Ricinus communis seedling under different culture conditions. Chin. J. Pestic. Sci. 2018, 20, 500–505. [Google Scholar]
- Liu, S.; Bian, Z.; Yang, F.; Li, Z.; Fan, Z.; Zhang, H.; Wang, Y.; Zhang, Y.; Tang, G. Determination of multiresidues of three acid herbicides in tobacco by liquid chromatography/tandem mass spectrometry. J. AOAC Int. 2015, 98, 472–476. [Google Scholar] [CrossRef]


| Compd. b | Average Inhibition Rate ± SD (%) (n = 3) | |||||||
|---|---|---|---|---|---|---|---|---|
| S. S a | B. s | P. p | P. c | A. s | P. a | R. s | P. i | |
| E1 | <10 | <10 | 13.36 ± 2.13 | <10 | <10 | 16.39 ± 1.27 | <10 | <10 |
| E2 | 21.38 ± 3.13 | 14.06 ± 0.08 | <10 | <10 | 22.98 ± 2.76 | <10 | <10 | 10.26 ± 2.16 |
| E3 | 17.76 ± 2.88 | 10.63 ± 1.03 | <10 | <10 | 19.09 ± 1.90 | <10 | <10 | <10 |
| E4 | <10 | 10.00 ± 0.49 | 19.80 ± 1.26 | <10 | 12.30 ± 3.07 | <10 | <10 | <10 |
| E5 | 19.74 ± 4.54 | <10 | <10 | <10 | 21.68 ± 4.28 | <10 | <10 | 12.91 ± 1.85 |
| E6 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| E7 | <10 | <10 | <10 | <10 | <10 | 11.11 ± 0.48 | <10 | <10 |
| E8 | 22.70 ± 3.62 | 14.38 ± 1.37 | <10 | 23.44 ± 1.69 | 15.53 ± 4.77 | <10 | 10.20 ± 5.49 | <10 |
| E9 | <10 | <10 | 10.74 ± 3.11 | <10 | 15.86 ± 3.49 | <10 | <10 | <10 |
| E10 | 29.35 ± 2.80 | <10 | 22.15 ± 0.82 | <10 | 19.49 ± 1.09 | 22.78 ± 1.92 | <10 | <10 |
| E11 | 38.16 ± 1.63 | 45.00 ± 0.74 | 41.61 ± 0.80 | 29.69 ± 1.28 | 44.66 ± 1.43 | 50.90 ± 4.04 | 32.86 ± 2.28 | 24.5 ± 1.79 |
| E12 | 16.45 ± 4.33 | 12.19 ± 0.07 | 22.15 ± 0.68 | <10 | 16.18 ± 1.99 | 25.00 ± 1.40 | 10.20 ± 1.53 | <10 |
| E13 | 57.89 ± 1.17 | 49.38 ± 0.71 | 59.06 ± 1.19 | 42.81 ± 0.31 | 38.51 ± 2.15 | 57.83 ± 2.16 | 40.23 ± 1.53 | 26.16 ± 0.77 |
| E14 | 55.59 ± 0.82 | 33.13 ± 0.48 | 22.15 ± 3.34 | 17.19 ± 4.57 | 23.95 ± 3.89 | 27.41 ± 4.74 | 23.23 ± 1.73 | <10 |
| E15 | <10 | <10 | <10 | 12.32 ± 8.89 | 12.64 ± 1.65 | 13.33 ± 1.67 | <10 | <10 |
| E16 | <10 | 10.31 ± 0.89 | <10 | <10 | 19.09 ± 3.36 | <10 | <10 | <10 |
| F1 | 33.70 ± 3.56 | 11.22 ± 0.48 | 33.33 ± 2.22 | <10 | <10 | <10 | 53.14 ± 2.70 | 22.15 ± 2.49 |
| F2 | 44.93 ± 1.33 | 68.98 ± 0.90 | 26.74 ± 1.79 | 10.15 ± 0.20 | 66.08 ± 0.58 | 54.29 ± 0 | 29.04 ± 5.35 | 19.54 ± 1.41 |
| F3 | 46.01 ± 0.39 | <10 | 20.83 ± 1.36 | 22.46 ± 2.41 | <10 | 17.73 ± 0.50 | 20.13 ± 1.88 | 14.66 ± 0.70 |
| F4 | 78.62 ± 1.57 | <10 | 47.22 ± 0.40 | 28.31 ± 1.63 | <10 | 13.30 ± 1.26 | 20.79 ± 0.90 | 26.38 ± 1.88 |
| F5 | 81.16 ± 0.53 | <10 | 40.28 ± 2.44 | 16.92 ± 1.61 | <10 | 13.85 ± 0.58 | 31.35 ± 1.21 | 41.04 ± 1.05 |
| F6 | 81.52 ± 1.17 | 11.88 ± 0.87 | 27.78 ± 0.95 | <10 | <10 | 13.57 ± 1.50 | 32.67 ± 0.87 | 28.99 ± 0.79 |
| F7 | 81.52 ± 1.03 | <10 | 49.31 ± 2.05 | 12.31 ± 3.29 | <10 | 20.22 ± 1.00 | 22.44 ± 1.84 | 27.69 ± 1.42 |
| F8 | 78.99 ± 0.38 | 10.89 ± 1.87 | 25.00 ± 1.16 | <10 | <10 | <10 | 30.36 ± 2.31 | 29.64 ± 2.19 |
| F9 | 81.52 ± 0.89 | 62.71 ± 0.31 | 47.22 ± 1.18 | 13.23 ± 1.68 | 47.95 ± 0.29 | 26.32 ± 0.76 | 26.40 ± 2.27 | 34.85 ± 0.68 |
| F10 | 71.74 ± 0.50 | 11.22 ± 1.40 | 43.75 ± 1.15 | 11.08 ± 2.25 | <10 | 17.45 ± 0.76 | 42.90 ± 2.37 | 25.73 ± 1.36 |
| F11 | 81.16 ± 0.39 | 46.20 ± 0.78 | 35.07 ± 0.26 | 66.77 ± 0.35 | 24.85 ± 0.29 | 22.16 ± 0.58 | 42.57 ± 1.37 | 30.62 ± 1.73 |
| F12 | 70.29 ± 0.40 | 13.86 ± 0.85 | 43.40 ± 1.50 | 11.38 ± 1.75 | <10 | <10 | 24.75 ± 1.52 | 57.65 ± 0.80 |
| F13 | 80.80 ± 0.78 | 16.17 ± 0.44 | 26.39 ± 1.51 | 20.31 ± 2.07 | <10 | 14.13 ± 1.04 | 36.63 ± 0.63 | 39.41 ± 0.33 |
| F14 | 83.70 ± 0.27 | 15.18 ± 1.36 | 37.15 ± 0.26 | 51.38 ± 2.80 | 15.20 ± 1.15 | 21.33 ± 0.76 | 8.58 ± 6.36 | 63.19 ± 0.20 |
| F15 | 35.51 ± 1.16 | <10 | 37.50 ± 1.24 | 10.46 ± 0.74 | 11.70 ± 0.29 | 14.13 ± 0.58 | 43.23 ± 5.87 | 19.54 ± 1.17 |
| F16 | 19.57 ± 1.16 | 60.40 ± 1.38 | <10 | <10 | 29.24 ± 0.29 | 23.55 ± 0.50 | 19.47 ± 0.42 | 12.38 ± 1.09 |
| PCA c | 98.55 ± 0.51 | 100.00 ± 0 | 85.76 ± 0.65 | 68.00 ± 1.06 | 75.73 ± 0.29 | 73.13 ± 0.29 | 93.07 ± 1.66 | 89.25 ± 0.98 |
| Metal. d | 15.58 ± 1.80 | <10 | 80.56 ± 0.71 | <10 | 24.56 ± 0 | 16.62 ± 0.29 | 56.11 ± 1.23 | <10 |
| Compd. | EC50/(μg/mL) | EC50/(μmol/L) | Regression Equation | 95% Confidence Interval/(μg/mL) | Correlation Coefficient (R2) |
|---|---|---|---|---|---|
| F5 | 14.57 | 35.98 | y = 3.2851 + 1.4740x | 13.1904–16.0933 | 0.9961 |
| F6 | 18.11 | 44.72 | y = 2.6425 + 1.8741x | 15.7107–20.8773 | 0.9924 |
| F7 | 6.57 | 16.22 | y = 4.0718 + 1.1352x | 5.5046–7.8456 | 0.9939 |
| F9 | 9.92 | 22.09 | y = 3.5751 + 1.4296x | 9.1232–10.7955 | 0.9972 |
| F11 | 20.43 | 51.20 | y = 2.5061 + 1.9032x | 16.6768–25.0367 | 0.9742 |
| F13 | 16.97 | 41.09 | y = 3.0535 + 1.5830x | 15.5039–18.5692 | 0.9965 |
| F14 | 8.02 | 19.42 | y = 3.8083 + 1.3183x | 6.8062–9.4396 | 0.9939 |
| PCA | 3.87 | 17.28 | y = 4.1864 + 1.3840x | 3.218–4.6567 | 0.9901 |
| Compd. | Regression Equation | Correlation Coefficient (R2) |
|---|---|---|
| F1 | y = 11034099x − 10368260 | 0.9927 |
| F2 | y = 10613651x − 650875 | 0.9990 |
| F3 | y = 1202838x − 5885 | 0.9997 |
| F4 | y = 2065740x − 44534 | 0.9962 |
| F5 | y = 2234165x − 1020903 | 0.9992 |
| F6 | y = 6126714x − 6610569 | 0.9972 |
| F7 | y = 6695972x − 3185573 | 0.9952 |
| F8 | y = 5314495x − 2033452 | 0.9994 |
| F9 | y = 1173264x − 85687 | 0.9980 |
| F10 | y = 7134918x − 6908171 | 0.9983 |
| F11 | y = 12128866x + 5301627 | 0.9998 |
| F12 | y = 6795334x − 1939715 | 0.9991 |
| F13 | y = 11573595x − 985307 | 1.0000 |
| F14 | y = 2148578x − 669546 | 0.9992 |
| F15 | y = 14370508x + 103540 | 0.9998 |
| F16 | y = 3231821x − 2254916 | 0.9991 |
| Metal. | y = 922409x + 211247 | 0.9995 |
| PCA | y = 27002x − 2443 | 0.9971 |
| Compd. | Molecular Weight (g/mol) | LogKow | pKa |
|---|---|---|---|
| F1 | 371.40 | 2.74 | 3.447 |
| F2 | 389.39 | 3.38 | 3.322 |
| F3 | 389.39 | 3.30 | 3.374 |
| F4 | 389.39 | 3.38 | 3.239 |
| F5 | 405.84 | 3.70 | 3.299 |
| F6 | 405.84 | 3.67 | 3.365 |
| F7 | 405.84 | 3.64 | 3.285 |
| F8 | 450.29 | 3.99 | 3.363 |
| F9 | 450.29 | 3.97 | 3.303 |
| F10 | 385.42 | 3.13 | 3.481 |
| F11 | 399.45 | 3.82 | 3.461 |
| F12 | 399.45 | 3.87 | 3.415 |
| F13 | 413.48 | 3.98 | 3.442 |
| F14 | 413.48 | 4.05 | 3.408 |
| F15 | 399.45 | 3.37 | 3.396 |
| F16 | 415.39 | 3.09 | 3.156 |
| PCA | 224.22 | 2.34 | 1.59 |
| Metal. | 279.34 | -- | -- |
| Detected Organs of Seeding | Added Content (μmol/L) | Detected Content (μmol/L) | Average Recoveries (%) | Coefficient of Variation (%) |
|---|---|---|---|---|
| Foliage | 400 | 301.21 | 75.30 ± 1.54 | 2.05 |
| 100 | 87.65 | 87.65 ± 3.62 | 3.85 | |
| 40 | 42.40 | 106.00 ± 2.26 | 2.13 | |
| Stem | 400 | 335.75 | 83.94 ± 4.26 | 5.07 |
| 100 | 104.16 | 104.16 ± 2.59 | 2.49 | |
| 40 | 43.93 | 109.83 ± 1.39 | 1.27 | |
| Root | 400 | 309.31 | 77.33 ± 1.44 | 1.86 |
| 100 | 70.67 | 70.67 ± 5.10 | 7.22 | |
| 40 | 29.22 | 73.04 ± 3.27 | 4.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Mao, G.; Xing, G.; Tian, Y.; Hu, Y.; Liao, C.; Li, L.; Zhu, X.; Li, J. Study on the Design, Synthesis, Bioactivity and Translocation of the Conjugates of Phenazine-1-carboxylic Acid and N-Phenyl Alanine Ester. Molecules 2024, 29, 1780. https://doi.org/10.3390/molecules29081780
Wu Y, Mao G, Xing G, Tian Y, Hu Y, Liao C, Li L, Zhu X, Li J. Study on the Design, Synthesis, Bioactivity and Translocation of the Conjugates of Phenazine-1-carboxylic Acid and N-Phenyl Alanine Ester. Molecules. 2024; 29(8):1780. https://doi.org/10.3390/molecules29081780
Chicago/Turabian StyleWu, Yiran, Guoqing Mao, Gaoshan Xing, Yao Tian, Yong Hu, Changzhou Liao, Li Li, Xiang Zhu, and Junkai Li. 2024. "Study on the Design, Synthesis, Bioactivity and Translocation of the Conjugates of Phenazine-1-carboxylic Acid and N-Phenyl Alanine Ester" Molecules 29, no. 8: 1780. https://doi.org/10.3390/molecules29081780