An Innovative Methodology to Characterize, at the Molecular Scale, Interactions in Polysaccharide Aqueous Solutions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Probe Stock Solution
2.3. Preparation of Polysaccharide Solutions
2.4. Fluorescence Measurements
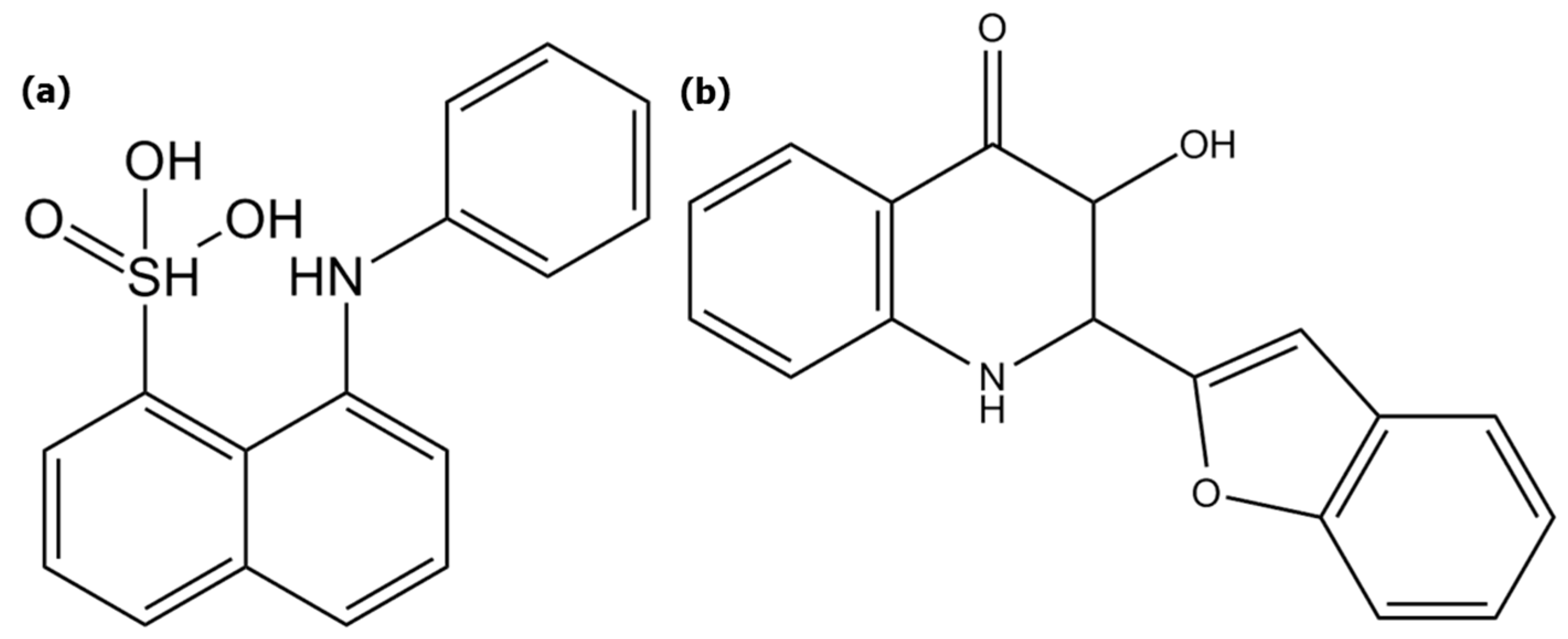
2.4.1. Hydrophobic Micro-Domains and Dipole-dipole Interactions Analyses Using the ANS Probe
2.4.2. H-Bond Network Analyses Using the 3HQ-Bf Probe
2.5. Rheological Measurements
3. Development of the Methodology for the Determination of Interactions
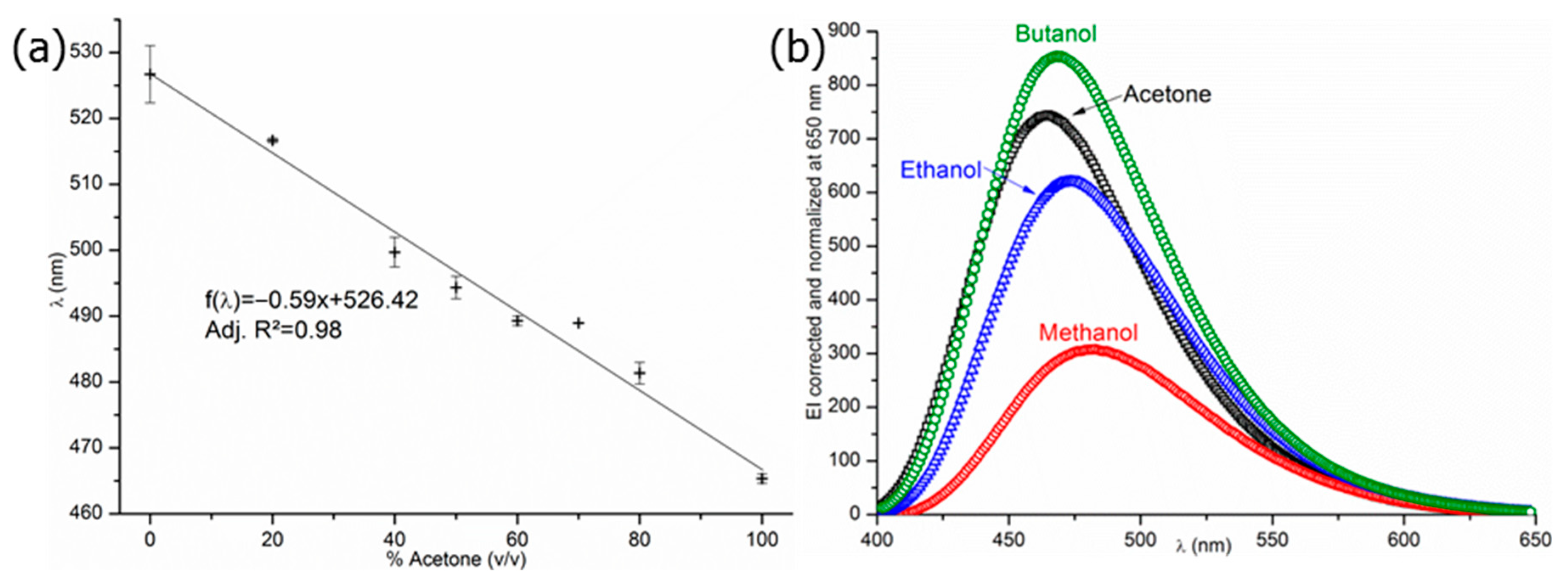
3.1. Probe Responses and Stability Testing
3.2. Selection and Exploitation of the Probes’ Signal and Intensity
3.3. Transposition to a Molecular Interaction Study in Polymer Solutions and Mixtures
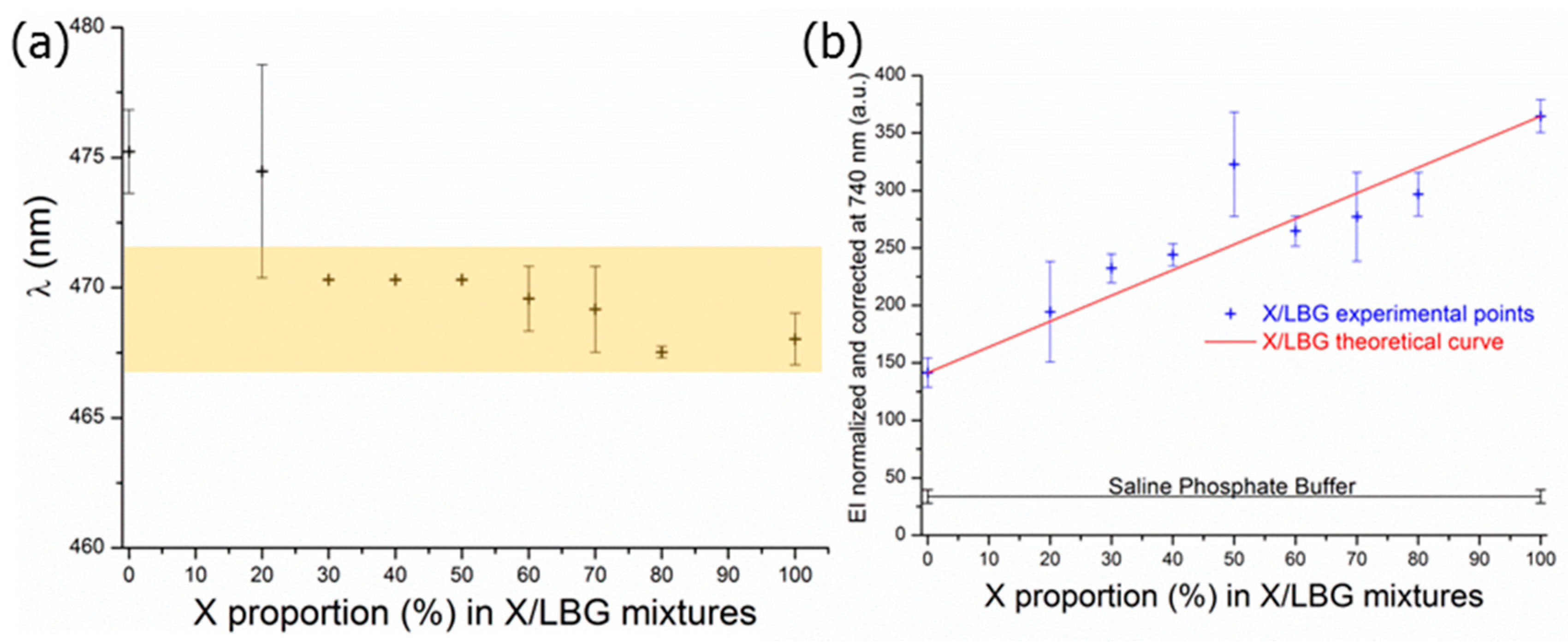
3.3.1. Hydrophobic Micro-Domains and Dipole-dipole Interactions
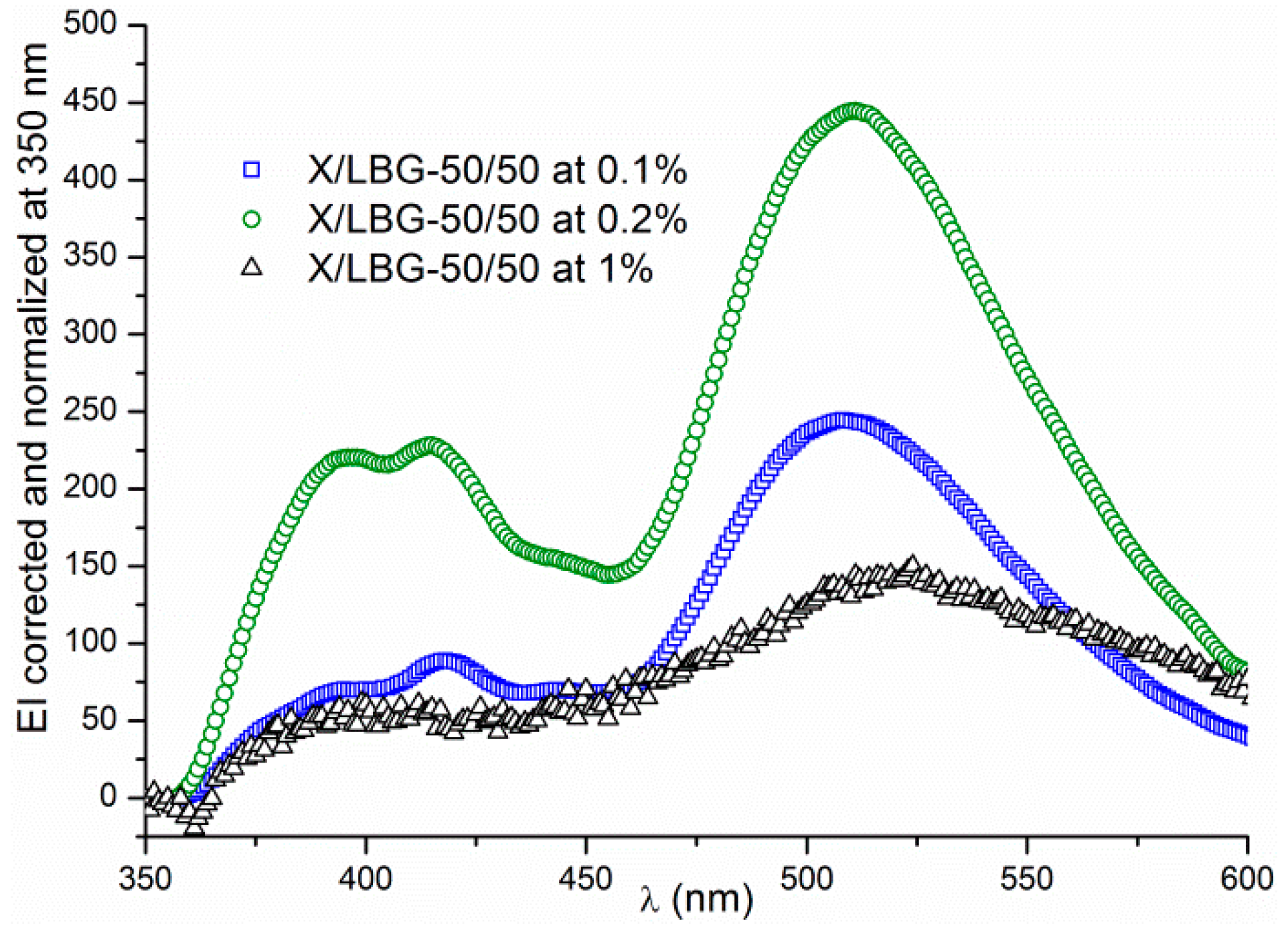
3.3.2. Hydrogen Bond Network
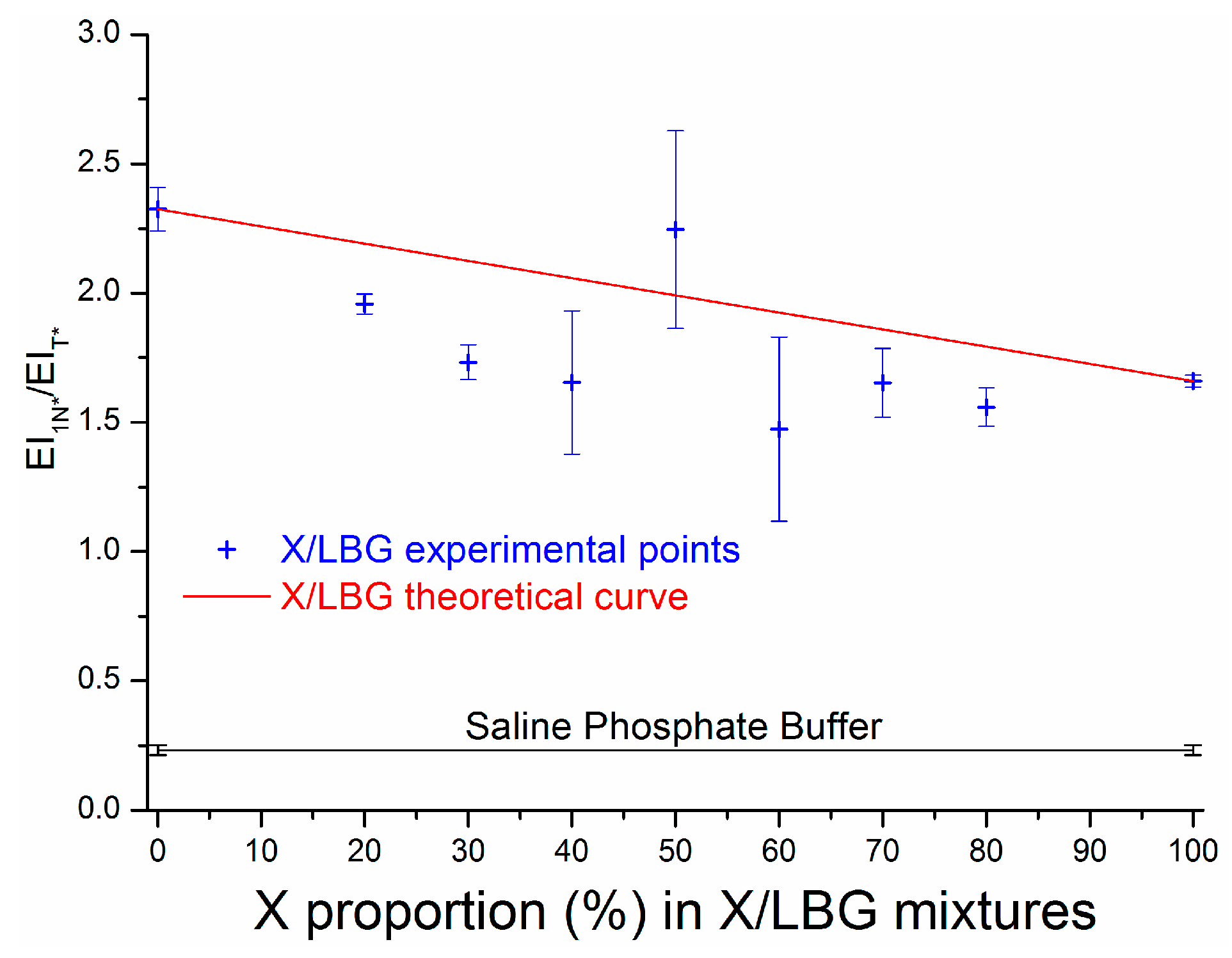
3.3.3. Molecular Interactions in an X/LBG Mixture—A Summary
3.4. Influence of the Chemical Structures of Galactomannans
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.M.; Huber, G.A.; Wang, N.; Minteer, S.D.; McCammon, J.A. Brownian dynamic study of an enzyme metabolon in the TCA cycle: Substrate kinetics and channeling: Brownian Dynamic Study of an Enzyme Metabolon in the TCA Cycle. Protein Sci. 2018, 27, 463–471. [Google Scholar] [CrossRef]
- Takano, Y.; Kusaka, A.; Nakamura, H. Density functional study of molecular interactions in secondary structures of proteins. Biophys. Physicobiol. 2016, 13, 27–35. [Google Scholar] [CrossRef]
- Passos, C.B.; Kuhn, P.S.; Barbosa, M.C. DNA denaturation in the rodlike polyelectrolyte model. Phys. A Stat. Mech. Its Appl. 2014, 413, 481–488. [Google Scholar] [CrossRef]
- Fontanille, M.; Gnanou, Y. Chimie et Physico-Chimie des Polymères; Dunod: Paris, France, 2014. [Google Scholar]
- Jaishankar, A.; Wee, M.; Matia-Merino, L.; Goh, K.K.T.; McKinley, G.H. Probing hydrogen bond interactions in a shear thickening polysaccharide using nonlinear shear and extensional rheology. Carbohydr. Polym. 2015, 123, 136–145. [Google Scholar] [CrossRef]
- Smidsrød, O.; Haug, A.; Larsen, B.; Gronowitz, S.; Hoffman, R.A.; Westerdahl, A. The Effect of Divalent Metals on the Properties of Alginate Solutions. I. Calcium Ions. Acta Chem. Scand. 1965, 19, 329–340. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef]
- Garrett, R.H.; Grisham, C.M.; Lubochinsky, B. Biochimie; De Boeck Université: Paris, France, 2000. [Google Scholar]
- Greene, R.F.; Pace, C.N. Urea and guanidine hydrochloride denaturation of ribonuclease, lysozyme, alpha-chymotrypsin, and beta-lactoglobulin. J. Biol. Chem. 1974, 249, 5388–5393. [Google Scholar] [CrossRef]
- Maréchal, Y.; Chanzy, H. The hydrogen bond network in Iβ cellulose as observed by infrared spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Burnham, N.A.; Dominguez, D.D.; Mowery, R.L.; Colton, R.J. Probing the surface forces of monolayer films with an atomic-force microscope. Phys. Rev. Lett. 1990, 64, 1931–1934. [Google Scholar] [CrossRef]
- Katchalsky, A. Solutions of polyelectrolytes and mechanochemical systems. J. Polym. Sci. 1951, 7, 393–412. [Google Scholar] [CrossRef]
- Manning, G.S. Counterion binding in polyelectrolyte theory. Acc. Chem. Res. 1979, 12, 443–449. [Google Scholar] [CrossRef]
- Raybin, J.; Ren, J.; Chen, X.; Gronheid, R.; Nealey, P.F.; Sibener, S.J. Real-Time Atomic Force Microscopy Imaging of Block Copolymer Directed Self Assembly. Nano Lett. 2017, 17, 7717–7723. [Google Scholar] [CrossRef]
- Vinson, P.K.; Bellare, J.R.; Davis, H.T.; Miller, W.G.; Scriven, L.E. Direct imaging of surfactant micelles, vesicles, discs, and ripple phase structures by cryo-transmission electron microscopy. J. Colloid Interface Sci. 1991, 142, 74–91. [Google Scholar] [CrossRef]
- Meister, A.; Blume, A. (Cryo)Transmission Electron Microscopy of Phospholipid Model Membranes Interacting with Amphiphilic and Polyphilic Molecules. Polymers 2017, 9, 521. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Duportail, G.; Demchenko, A.P.; Mély, Y. Bimodal Distribution and Fluorescence Response of Environment-Sensitive Probes in Lipid Bilayers. Biophys. J. 2004, 86, 2929–2941. [Google Scholar] [CrossRef]
- Pivovarenko, V.G.; Wróblewska, A.; Błażejowski, J. 2-[4-(Dimethylamino)phenyl]-3-hydroxy-4H-chromene-4-one: A H-bond-sensitive fluorescent probe for investigating binary mixtures of organic solvents. Anal. Chim. Acta 2005, 545, 74–78. [Google Scholar] [CrossRef]
- Kato, A.; Nakai, S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim. Biophys. Acta (BBA)—Protein Struct. 1980, 624, 13–20. [Google Scholar] [CrossRef]
- Weber, G.; Young, L.B. Fragmentation of Bovine Serum Albumin by Pepsin. J. Biol. Chem. 1964, 239, 1415–1423. [Google Scholar] [CrossRef]
- Valeur, B.; John WileySons, I. Molecular Fluorescence; Wiley-VCH: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- Haskard, C.A.; Li-Chan, E.C.Y. Hydrophobicity of Bovine Serum Albumin and Ovalbumin Determined Using Uncharged (PRODAN) and Anionic (ANS—) Fluorescent Probes. J. Agric. Food Chem. 1998, 46, 2671–2677. [Google Scholar] [CrossRef]
- Loh, B.; Grant, C.; Hancock, R.E. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1984, 26, 546–551. [Google Scholar] [CrossRef]
- Bam, N.B.; Cleland, J.L.; Yang, J.; Manning, M.C.; Carpenter, J.F.; Kelley, R.F.; Randolph, T.W. Tween protects recombinant human growth hormone against agitation-induced damage via hydrophobic interactions. J. Pharm. Sci. 1998, 87, 1554–1559. [Google Scholar] [CrossRef]
- Freedman, R.B.; Radda, G.K. The interaction of 1-anilino-8-naphthalene sulphonate with erythrocyte membranes. FEBS Lett. 1969, 3, 150–152. [Google Scholar] [CrossRef]
- Stryer, L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. J. Mol. Biol. 1965, 13, 482–495. [Google Scholar] [CrossRef]
- Azzi, A.; Fleischer, S.; Chance, B. Cytochrome c phospholipid interaction: Structural transitions associated with valency changes. Biochem. Biophys. Res. Commun. 1969, 36, 322–327. [Google Scholar] [CrossRef]
- Haynes, D.H. 1-Anilino-8-naphthalenesulfonate: A fluorescent indicator of ion binding and electrostatic potential on the membrane surface. J. Membr. Biol. 1974, 17, 341–366. [Google Scholar] [CrossRef]
- Slavík, J. Anilinonaphthalene sulfonate as a probe of membrane composition and function. Biochim. Biophys. Acta (BBA)—Rev. Biomembr. 1982, 694, 1–25. [Google Scholar] [CrossRef]
- Gasymov, O.K.; Glasgow, B.J. ANS fluorescence: Potential to augment the identification of the external binding sites of proteins. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2007, 1774, 403–411. [Google Scholar] [CrossRef]
- Roy, A.; Comesse, S.; Grisel, M.; Hucher, N.; Souguir, Z.; Renou, F. Hydrophobically Modified Xanthan: An Amphiphilic but Not Associative Polymer. Biomacromolecules 2014, 15, 1160–1170. [Google Scholar] [CrossRef]
- Haynes, D.H.; Staerk, H. 1-Anilino-8-naphthalenesulfonate: A fluorescent probe of membrane surface structure, composition and mobility. J. Membr. Biol. 1974, 17, 313–340. [Google Scholar] [CrossRef]
- Bilokin’, M.D.; Shvadchak, V.V.; Yushchenko, D.A.; Duportail, G.; Mély, Y.; Pivovarenko, V.G. Dual-Fluorescence Probe of Environment Basicity (Hydrogen Bond Accepting Ability) Displaying no Sensitivity to Polarity. J. Fluoresc. 2009, 19, 545–553. [Google Scholar] [CrossRef]
- Yushchenko, D.A.; Shvadchak, V.V.; Klymchenko, A.S.; Duportail, G.; Mély, Y.; Pivovarenko, V.G. 2-Aryl-3-hydroxyquinolones, a new class of dyes with solvent dependent dual emission due to excited state intramolecular proton transfer. New J. Chem. 2006, 30, 774–781. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Taft, R.W. The solvatochromic comparison method. I. The.beta.-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Abraham, M.H.; Grellier, P.L.; Prior, D.V.; Morris, J.J.; Taylor, P.J. Hydrogen bonding. Part 10. A scale of solute hydrogen-bond basicity using log K values for complexation in tetrachloromethane. J. Chem. Soc. Perkin Trans. 1990, 2, 521. [Google Scholar] [CrossRef]
- Abraham, M.H. Hydrogen bonding. 31. Construction of a scale of solute effective or summation hydrogen-bond basicity. J. Phys. Org. Chem. 1993, 6, 660–684. [Google Scholar] [CrossRef]
- Esquenet, C. Propriétés Structurales et Dynamiques des Solutions de Polyélectrolytes Rigides et Semi-Rigides et de Polysaccharides Associatifs [Université Joseph-Fourier-Grenoble I]. 2003. Available online: https://tel.archives-ouvertes.fr/tel-00005468/ (accessed on 13 March 2024).
- Pelletier, E.; Viebke, C.; Meadows, J.; Williams, P.A. A rheological study of the order-disorder conformational transition of xanthan gum. Biopolymers 2001, 59, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Dahi, A.; Abdellahi, B.M.-L.; Deida, M.F.; Hucher, N.; Malhiac, C.; Renou, F. Chemical and physicochemical characterizations of the water-soluble fraction of the Commiphora Africana exudate. Food Hydrocoll. 2019, 86, 2–10. [Google Scholar] [CrossRef]
- Tapie, N.; Malhiac, C.; Hucher, N.; Grisel, M. Determination of galactose and mannose residues in natural galactomannans using a fast and efficient high-performance liquid chromatography/UV detection. J. Chromatogr. A 2008, 1181, 45–50. [Google Scholar] [CrossRef]
- Alizadeh-Pasdar, N.; Li-Chan, E.C.Y. Comparison of Protein Surface Hydrophobicity Measured at Various pH Values Using Three Different Fluorescent Probes. J. Agric. Food Chem. 2000, 48, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S.; Demchenko, A.P. Multiparametric probing of intermolecular interactions with fluorescent dye exhibiting excited state intramolecular proton transfer. Phys. Chem. Chem. Phys. 2003, 5, 461–468. [Google Scholar] [CrossRef]
- Yushchenko, D.A.; Shvadchak, V.V.; Klymchenko, A.S.; Duportail, G.; Pivovarenko, V.G.; Mély, Y. Modulation of Excited-State Intramolecular Proton Transfer by Viscosity in Protic Media. J. Phys. Chem. A 2007, 111, 10435–10438. [Google Scholar] [CrossRef]
- Tomin, V.I.; Demchenko, A.P.; Chou, P.-T. Thermodynamic vs. Kinetic control of excited-state proton transfer reactions. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 1–18. [Google Scholar] [CrossRef]
- Schorsch, C. Etude Rhéologique et Structurale des Mélanges Gomme Xanthane/Gomme Guar [Université Paris IX.; ENSIA]. 1995. Available online: http://www.theses.fr/1995EIAA0053 (accessed on 13 March 2024).
- Rinaudo, M. Relation between the molecular structure of some polysaccharides and original properties in sol and gel states. Food Hydrocoll. 2001, 15, 433–440. [Google Scholar] [CrossRef]
- Dakia, P.A.; Wathelet, B.; Paquot, M. Influence de la teneur en galactose sur les interactions moléculaires et sur les propriétés physico-chimiques des galactomannanes en solution. Biotechnol. Agron. Soc. Environ. 2010, 14, 213–223. [Google Scholar]
- Garnier, C.; Schorsch, C.; Doublier, J.-L. Phase separation in dextran/locust bean gum mixtures. Carbohydr. Polym. 1995, 28, 313–317. [Google Scholar] [CrossRef]
- Renou, F.; Petibon, O.; Malhiac, C.; Grisel, M. Effect of xanthan structure on its interaction with locust bean gum: Toward prediction of rheological properties. Food Hydrocoll. 2013, 32, 331–340. [Google Scholar] [CrossRef]
- Schorsch, C.; Garnier, C.; Doublier, J.-L. Viscoelastic properties of xanthangalactomannan mixtures: Comparison of guar gum with locust bean gum. Carbohydr. Polym. 1997, 34, 165–175. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.-J.; Sun, Z. Conformational Role of Xanthan in its Interaction with Locust Bean Gum. J. Food Sci. 2002, 67, 2609–2614. [Google Scholar] [CrossRef]
- Southwick, J.G.; Lee, H.; Jamieson, A.M.; Blackwell, J. Self-association of xanthan in aqueous solvent-systems. Carbohydr. Res. 1980, 84, 287–295. [Google Scholar] [CrossRef]
- Tinland, B.; Rinaudo, M. Dependence of the stiffness of the xanthan chain on the external salt concentration. Macromolecules 1989, 22, 1863–1865. [Google Scholar] [CrossRef]
- Morris, G.A.; Patel, T.R.; Picout, D.R.; Ross-Murphy, S.B.; Ortega, A.; Garcia de la Torre, J.; Harding, S.E. Global hydrodynamic analysis of the molecular flexibility of galactomannans. Carbohydr. Polym. 2008, 72, 356–360. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Radha, A.; Okuyama, K. Morphology of galactomannans: An X-ray structure analysis of guaran. Carbohydr. Res. 1998, 306, 243–255. [Google Scholar] [CrossRef]
- Dea, I.C.M.; Morris, E.R.; Rees, D.A.; Welsh, E.J.; Barnes, H.A.; Price, J. Associations of like and unlike polysaccharides: Mechanism and specificity in galactomannans, interacting bacterial polysaccharides, and related systems. Carbohydr. Res. 1977, 57, 249–272. [Google Scholar] [CrossRef]
- Morris, E.R.; Rees, D.A.; Young, G.; Walkinshaw, M.D.; Darke, A. Order-disorder transition for a bacterial polysaccharide in solution. A role for polysaccharide conformation in recognition between Xanthomonas pathogen and its plant host. J. Mol. Biol. 1977, 110, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cairns, P.; Miles, M.J.; Morris, V.J.; Brownsey, G.J. X-ray fibre-diffraction studies of synergistic, binary polysaccharide gels. Carbohydr. Res. 1987, 160, 411–423. [Google Scholar] [CrossRef]
- Tako, M.; Asato, A.; Nakamura, S. Rheological Aspects of the Intermolecular Interaction between Xanthan and Locust Bean Gum in Aqueous Media. Agric. Biol. Chem. 1984, 48, 2995–3000. [Google Scholar] [CrossRef]
- Aguni, Y. Contribution à la Compréhension des Mécanismes D’Interaction Xanthane/Galactimannane; Université Le Havre: Le Havre, France, 2009. [Google Scholar]
- Grisel, M.; Aguni, Y.; Renou, F.; Malhiac, C. Impact of fine structure of galactomannans on their interactions with xanthan: Two co-existing mechanisms to explain the synergy. Food Hydrocoll. 2015, 51, 449–458. [Google Scholar] [CrossRef]
- Jouquand, C.; Aguni, Y.; Malhiac, C.; Grisel, M. Influence of chemical composition of polysaccharides on aroma retention. Food Hydrocoll. 2008, 22, 1097–1104. [Google Scholar] [CrossRef]
- Jouquand, C.; Ducruet, V.; Giampaoli, P. Partition coefficients of aroma compounds in polysaccharide solutions by the phase ratio variation method. Food Chem. 2004, 85, 467–474. [Google Scholar] [CrossRef]
- Jouquand, C.; Malhiac, C.; Grisel, M. Determination of specific interactions between aroma compounds and xanthan/galactomannan mixtures. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 43, pp. 421–424. [Google Scholar] [CrossRef]
- Secouard, S.; Grisel, M.; Malhiac, C. Flavour release study as a way to explain xanthan–galactomannan interactions. Food Hydrocoll. 2007, 21, 1237–1244. [Google Scholar] [CrossRef]
- Secouard, S.; Malhiac, C.; Grisel, M.; Decroix, B. Release of limonene from polysaccharide matrices: Viscosity and synergy effect. Food Chem. 2003, 82, 227–234. [Google Scholar] [CrossRef]
- Takemasa, M.; Nishinari, K. Solution Structure of Molecular Associations Investigated Using NMR for Polysaccharides: Xanthan/Galactomannan Mixtures. J. Phys. Chem. B 2016, 120, 3027–3037. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P.; Nagar, B.J.; Naikwadi, N.N.; Variya, B.C. Galactomannan: A versatile biodegradable seed polysaccharide. Int. J. Biol. Macromol. 2013, 60, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Daas, P.J.H.; Schols, H.A.; de Jongh, H.H.J. On the galactosyl distribution of commercial galactomannans. Carbohydr. Res. 2000, 329, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.E. Seed gums. In Thickening and Gelling Agents for Food; Imeson, A.P., Ed.; Springer US: Boston, MA, USA, 1997; pp. 262–283. [Google Scholar] [CrossRef]


| Gum | Mv a ×106 | Protein (% w/w) | DSAc (%) b | DSPy (%) b | Tm (°C) c | M/G Ratio d | Degree of Blocness (DB; %) e |
|---|---|---|---|---|---|---|---|
| X | 1.46 | 4.7 | 35 | 65 | 64 ± 1 | / | / |
| LBG | 1.6 | 1.22 ± 0.03 | / | / | / | 2.7 | 95 |
| GG | 2.67 | 1.33 ± 0.15 | / | / | / | 1.4 | 33 |
| 1N* (λ393 nm) | 2N* (λ414 nm) | |
|---|---|---|
| Raw EI (a.u.) | 179 | 190 |
| 187 | 188 | |
| 182 | 177 | |
| 187 | 172 | |
| 186 | 191 | |
| Mean value | 184 ± 4 | 184 ± 9 |
| Samples | G′ (Pa) | G″ (Pa) | Tanδ | EIANS (a.u *) | EI1N*/EIT* |
|---|---|---|---|---|---|
| X | 0.73 ± 0.10 | 0.59 ± 0.05 | 0.80 ± 0.03 | 365 ± 14 | 1.7 ± 0 |
| LBG | 2.4 × 10−4 ± 0.6 × 10−4 | 1.1 × 10−2 ± 0.1 × 10−2 | 49 ± 11 | 142 ± 13 | 2.3 ± 0.1 |
| X/LBG 50/50 | 3.9 ± 0.7 | 0.60 ± 0.03 | 0.16 ± 0.02 | 323 ± 46 | 2.245 ± 0.382 |
| X/GG 50/50 | 0.16 ± 0.01 | 0.27 ± 0.13 | 1.72 ± 0.94 | 280 ± 26 | 0.794 ± 0.064 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordinier, A.; Petukhov, I.; Hucher, N.; Grisel, M. An Innovative Methodology to Characterize, at the Molecular Scale, Interactions in Polysaccharide Aqueous Solutions. Molecules 2024, 29, 1787. https://doi.org/10.3390/molecules29081787
Cordinier A, Petukhov I, Hucher N, Grisel M. An Innovative Methodology to Characterize, at the Molecular Scale, Interactions in Polysaccharide Aqueous Solutions. Molecules. 2024; 29(8):1787. https://doi.org/10.3390/molecules29081787
Chicago/Turabian StyleCordinier, Alexandre, Igor Petukhov, Nicolas Hucher, and Michel Grisel. 2024. "An Innovative Methodology to Characterize, at the Molecular Scale, Interactions in Polysaccharide Aqueous Solutions" Molecules 29, no. 8: 1787. https://doi.org/10.3390/molecules29081787
APA StyleCordinier, A., Petukhov, I., Hucher, N., & Grisel, M. (2024). An Innovative Methodology to Characterize, at the Molecular Scale, Interactions in Polysaccharide Aqueous Solutions. Molecules, 29(8), 1787. https://doi.org/10.3390/molecules29081787






