Obacunone, a Promising Phytochemical Triterpenoid: Research Progress on Its Pharmacological Activity and Mechanism
Abstract
:1. Introduction
2. Methodology
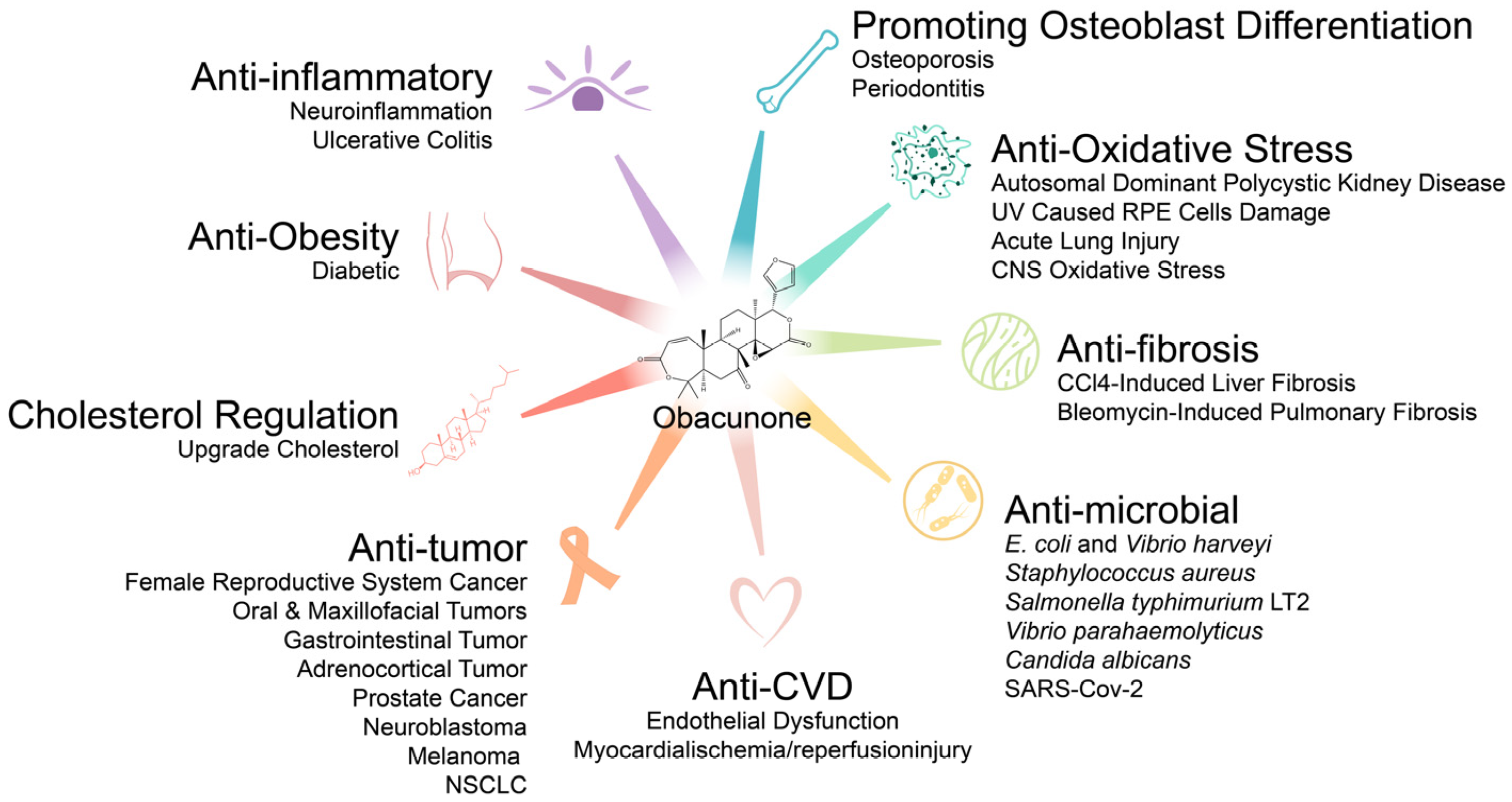
3. Pharmacological Effects of Obacunone
3.1. Antitumor Effects
3.1.1. Antitumor Activity In Vivo
3.1.2. Antitumor Activity In Vitro
Effects on Gastrointestinal Tumors: Colon, Liver, and Pancreatic Cancer Cells
Effects on Prostate Cancer Cells
Effects on Breast Cancer Cells
Effects on Female Reproductive System Cancer Cells
Effects on Neuroblastoma Cells
Effects on Adrenocortical Tumor Cells
Effects on Non-Small Cell Lung Cancer (NSCLC) and Melanoma Cells
Other Effects
3.2. Anti-Inflammatory Effects
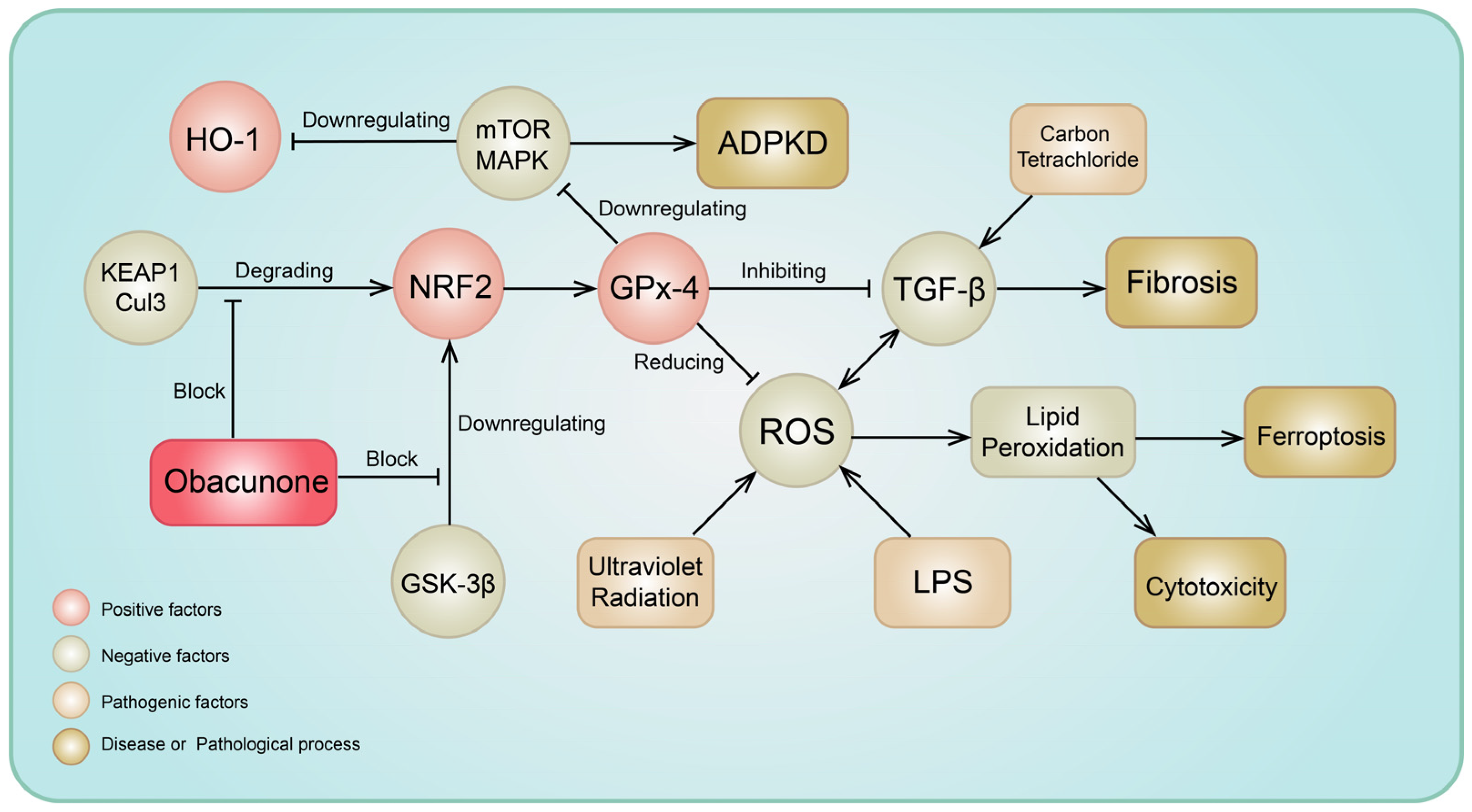
3.3. Antifibrosis Effects
3.4. Antioxidative Stress Activity
3.5. Antimicrobial Effects
3.5.1. Antibacterial Effects
Effects on Escherichia coli and Vibrio harveyi
Effects on Staphylococcus aureus
Effects on Salmonella typhimurium LT2
Effects on Vibrio parahaemolyticus
3.5.2. Antifungal Activity
3.5.3. Potential Antiviral Activity
3.6. Endocrine and Metabolic Effects
3.6.1. Anti-Obesity Effects
3.6.2. Regulation of Cholesterol Metabolism
3.7. Effects on Bones Metabolism
3.8. Effects on Arginase and Ferroptosis
3.9. Other Effects: Insect-Repellent Effects, Insecticidal Effects, and Shortening of Sleep Time in Mice
4. Pharmacokinetic Studies of Obacunone
5. Safety Profile of Obacunone
6. Outlook
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, X.Q.; Zhu, O.H.; Wang, S.C.; Xu, Y.G. Research Progress in Biological Activity and Structure-activity Relationship of Limon. Prog. Pharm. Sci. 2015, 39, 775–780. [Google Scholar]
- National Pharacopoeia Comission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Yang, Y.; Hua, Y.; Chen, W.; Zheng, H.; Wu, H.; Qin, S.; Huang, S. Therapeutic targets and pharmacological mechanisms of Coptidis rhizoma against ulcerative colitis: Findings of system pharmacology and bioinformatics analysis. Front. Pharmacol. 2022, 13, 1037856. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Han, L.T. Study on the molecular mechanism of anti-liver cancer effect of Evodiae fructus by network pharmacology and QSAR model. Front. Chem. 2023, 10, 1060500. [Google Scholar] [CrossRef] [PubMed]
- Salau, V.F.; Erukainure, O.L.; Olofinsan, K.A.; Schoeman, R.L.S.; Matsabisa, M.G. Lippia javanica (Burm. F.) Herbal Tea: Modulation of Hepatoprotective Effects in Chang Liver Cells via Mitigation of Redox Imbalance and Modulation of Perturbed Metabolic Activities. Front. Pharmacol. 2023, 14, 1221769. [Google Scholar] [CrossRef] [PubMed]
- Mbaveng, A.T.; Noulala, C.G.T.; Samba, A.R.M.; Tankeo, S.B.; Fotso, G.W.; Happi, E.N.; Ngadjui, B.T.; Beng, V.P.; Kuete, V.; Efferth, T. Cytotoxicity of botanicals and isolated phytochemicals from Araliopsis soyauxii Engl. (Rutaceae) towards a panel of human cancer cells. J. Ethnopharmacol. 2021, 267, 113535. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, N.; Yang, K.; Liao, H.; Liu, X.; Wu, Y.; Wang, Y.; Peng, X.; Wu, Y. Proteomic Analysis of Staphylococcus aureus Treated with ShangKeHuangShui. Biol. Pharm. Bull. 2024, 47, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, G.; Zhao, Z.; Zuo, Y.; Sun, Z.; Chen, S. Exploring the mechanism of Erchen decoction in the treatment of atherosclerosis based on network pharmacology and molecular docking. Medicine 2023, 102, e35248. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, K.; Liu, B.; Zhou, T.; Tang, Y.; Li, Y. Chaihu Shugan prevents cholesterol gallstone formation by ameliorating the microbiota dysbiosis and metabolic disturbance in mice. Front. Pharmacol. 2024, 14, 1291236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, M.; Li, W.; Zhao, M.; Cao, X.; Li, C.; Zhang, H.; Yang, M.; Liang, L.; Yue, Y.; et al. Inhibition of inflammasome activation via sphingolipid pathway in acute lung injury by Huanglian Jiedu decoction: An integrative pharmacology approach. Phytomedicine 2022, 10, 154469. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.P.; Chen, S.; Ma, Y.Z.; Zhou, A.; Jiang, H.; Wu, P. Evaluation of the Mechanism of Jiedu Huazhuo Quyu Formula in Treating Wilson’s Disease-Associated Liver Fibrosis by Network Pharmacology Analysis and Molecular Dynamics Simulation. Evid.-Based Complement. Altern. Med. 2022, 2022, 9363131. [Google Scholar] [CrossRef]
- Chen, Q.H.; Chen, X.M.; Chen, X.H.; Komori, A.; Hung, A.; Li, H. Structure-based multi-ligand molecular modeling to predict the synergistic effects of limonin and obacunone from simiao pill against nitric oxide synthase 3 associated with hyperuricemia. Precis. Med. Res. 2023, 5, 13. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, S.; Chen, X. The pharmacological and pharmacokinetic properties of obacunone from citrus fruits: A comprehensive narrative review. Fitoterapia 2023, 169, 105569. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kohno, H.; Tsukio, Y.; Honjo, S.; Tanino, M.; Miyake, M.; Wada, K. Citrus limonoids obacunone and limonin inhibit azoxymethane-induced colon carcinogenesis in rats. Biofactors 2000, 13, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Maeda, M.; Kohno, H.; Murakami, M.; Kagami, S.; Miyake, M.; Wada, K. Inhibition of azoxymethane-induced colon carcinogenesis in male F344 rats by the citrus limonoids obacunone and limonin. Carcinogenesis 2001, 22, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yu, Z.; Yue, B.; Ren, J.; Zhang, J.; Mani, S.; Wang, Z.; Dou, W. Obacunone reduces inflammatory signalling and tumour occurrence in mice with chronic inflammation-induced colorectal cancer. Pharm. Biol. 2020, 58, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.G.; Porter, J.L.; Binnie, W.H.; Guo, I.Y.; Hasegawa, S. Further studies on the anticancer activity of citrus limonoids. J. Agric. Food Chem. 2004, 52, 4908–4912. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Harris, E.D.; Patil, B.S. Antiproliferative effects of citrus limonoids against human neuroblastoma and colonic adenocarcinoma cells. Nutr. Cancer 2006, 56, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. Obacunone and obacunone glucoside inhibit human colon cancer (SW480) cells by the induction of apoptosis. Food Chem. Toxicol. 2011, 49, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.D.; Kwon, H.C.; Yang, M.C.; Lee, K.H.; Choi, S.U.; Lee, K.R. Isolation of limonoids and alkaloids from Phellodendron amurense and their multidrug resistance (MDR) reversal activity. Arch. Pharm. Res. 2007, 30, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Zhao, B.; Wang, C.; Zhang, J.; Wu, J.; Wang, T.; Xu, A. Subchronic Toxicity Studies of Cortex Dictamni Extracts in Mice and Its Potential Hepatotoxicity Mechanisms in Vitro. Molecules 2018, 23, 2486. [Google Scholar] [CrossRef]
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. Apoptosis mediated cytotoxicity of citrus obacunone in human pancreatic cancer cells. Toxicol. In Vitro 2011, 25, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lenon, G.B.; Yang, A.W.H. Phellodendri Cortex: A Phytochemical, Pharmacological, and Pharmacokinetic Review. Evid.-Based Complement. Altern. Med. 2019, 2019, 7621929. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. Cytotoxicity of obacunone and obacunone glucoside in human prostate cancer cells involves Akt-mediated programmed cell death. Toxicology 2015, 329, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jesudhasan, P.R.; Jayaprakasha, G.K.; Pillai, B.S.; Patil, B.S. Grapefruit bioactive limonoids modulate E. coli O157:H7 TTSS and biofilm. Int. J. Food Microbiol. 2010, 140, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jayaprakasha, G.K.; Patil, B.S. Limonoids and their anti-proliferative and anti-aromatase properties in human breast cancer cells. Food Funct. 2013, 4, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, M. Role of Plant Secondary Metabolites as Modulators of Multidrug Resistance in Cancer Therapy. In Plant Secondary Metabolites; Springer Nature: Singapore, 2022; pp. 415–435. [Google Scholar]
- Jung, H.; Sok, D.E.; Kim, Y.; Min, B.; Lee, J.; Bae, K. Potentiating effect of obacunone from Dictamnus dasycarpus on cytotoxicity of microtuble inhibitors, vincristine, vinblastine and taxol. Planta Med. 2000, 66, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Pan, Z. Inhibitory Effect of Obacunone on Corticosterone Synthesis in Adrenocortical Tumor Cells by Affecting Mitochondrial Function. J. Nangjing Univ. Tradit. Chin. Med. 2021, 37, 251–257. [Google Scholar]
- Melong, R.; Tamokoue Kengne, P.C.; Dzoyem, J.P.; Fusi, A.A.; Allemann, E.; Delie, F.; Bochet, C.G.; Beifuss, U.; Kapche, G. New cytotoxic obacunone-type limonoid and others constituents from the stem bark of Carapa procera DC (Meliaceae). Nat. Prod. Res. 2022, 36, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Kim, S.; Cho, M.; Yun, H.M. Limonoid Triterpene, Obacunone Increases Runt-Related Transcription Factor 2 to Promote Osteoblast Differentiation and Function. Int. J. Mol. Sci. 2021, 22, 2483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, T.; Wang, H.; Jiang, Y.; Peng, S. Obacunone attenuates high glucose-induced oxidative damage in NRK-52E cells by inhibiting the activity of GSK-3beta. Biochem. Biophys. Res. Commun. 2019, 513, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; He, J.; Shao, G.; Hu, J.; Li, X.; Zhou, H.; Li, M.; Yang, B. Obacunone Retards Renal Cyst Development in Autosomal Dominant Polycystic Kidney Disease by Activating NRF2. Antioxidants 2021, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jayaprakasha, G.K.; Patil, B.S. Obacunone exhibits anti-proliferative and anti-aromatase activity in vitro by inhibiting the p38 MAPK signaling pathway in MCF-7 human breast adenocarcinoma cells. Biochimie 2014, 105, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M. Upregulation of Cholesterol Metabolism by a Limonoid Compound Obacunone through Activation of Sterol Regulatory Element Binding Protein-1 Cleavage in Human Keratinocytes. Ph.D. Thesis, Sejong University, Seoul, Republic of Korea, 2014. [Google Scholar]
- Huang, D.R.; Dai, C.M.; Li, S.Y.; Li, X.F. Obacunone protects retinal pigment epithelium cells from ultra-violet radiation-induced oxidative injury. Aging 2021, 13, 11010–11025. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wang, L.; Zhao, L.; Lu, Y.Y.; Zeng, K.W.; Zhao, M.B.; Jiang, Y.; Tu, P.F.; Guo, X.Y. Rapid identification, isolation, and evaluation on anti-neuroinflammatory activity of limonoids derivatives from the root bark of Dictamnus dasycarpus. J. Pharm. Biomed. Anal. 2021, 200, 114079. [Google Scholar] [CrossRef]
- Luo, X.; Yue, B.; Yu, Z.; Ren, Y.; Zhang, J.; Ren, J.; Wang, Z.; Dou, W. Obacunone Protects Against Ulcerative Colitis in Mice by Modulating Gut Microbiota, Attenuating TLR4/NF-kappaB Signaling Cascades, and Improving Disrupted Epithelial Barriers. Front. Microbiol. 2020, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dang, Z.; Wang, X.; Chen, Y.; Dong, P.; Liu, G.; Tan, W.; Gui, Z.; Bu, F.; Lin, F.; et al. Obacunone alleviates chronic pelvic pain and pro-inflammatory depolarization of macrophage induced by experimental autoimmune prostatitis in mice. Biochem. Biophys. Rep. 2023, 36, 101565. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hou, R.; Liu, F.; Liu, H.; Fei, Q.; Han, Y.; Cai, R.; Peng, C.; Qi, Y. Obacunone causes sustained expression of MKP-1 thus inactivating p38 MAPK to suppress pro-inflammatory mediators through intracellular MIF. J. Cell Biochem. 2018, 119, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, W.; Xie, Q.; Xu, Y. Obacunone activates the Nrf2-dependent antioxidant responses. Protein Cell 2016, 7, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Luo, L.; Li, H.; Zhang, L.; Yan, Y.; Fang, M.; Yu, J.; Gao, X.; Liu, Y.; Huang, C.; et al. Nomilin and its analogue obacunone alleviate NASH and hepatic fibrosis in mice via enhancing antioxidant and anti-inflammation capacity. Biofactors 2023, 49, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, W.; Wang, L.; Ma, L.; Zhai, D.; Wang, F.; Shi, R.; Liu, C.; Xu, Q.; Chen, G.; et al. Obacunone Attenuates Liver Fibrosis with Enhancing Anti-Oxidant Effects of GPx-4 and Inhibition of EMT. Molecules 2021, 26, 318. [Google Scholar] [CrossRef] [PubMed]
- Han, G.Y.; Kim, T.J.; Kim, Y.S.; Kang, S.S.; Mar, W.C. Anti-hepatic Fibrosis Effects of Phylligenin from F orsythia suspensa, Obacunone from Phellodendron am urense and Vanillic Acid from M agnolia denudata in Human Hepatic Cells LX-2. Fall Gen. Meet. Acad. Conf. 2008, 2008, 216. [Google Scholar]
- Qiu, S.; yang, L.; Tan, R.Z.; Liu, J. Effects of obacunone on renal interstitial fibrosis and ferroptosis in unilateral ureteral obstruction model mice. China Pharm. 2023, 34, 554–559. [Google Scholar]
- Liu, B.; Jiang, H.; Lu, J.; Baiyun, R.; Li, S.; Lv, Y.; Li, D.; Wu, H.; Zhang, Z. Grape seed procyanidin extract ameliorates lead-induced liver injury via miRNA153 and AKT/GSK-3beta/Fyn-mediated Nrf2 activation. J. Nutr. Biochem. 2018, 52, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.; Villarroel, M.J.; Roger, I.; Morell, A.; Milara, J.; Cortijo, J. Obacunone Photoprotective Effects against Solar-Simulated Radiation-Induced Molecular Modifications in Primary Keratinocytes and Full-Thickness Human Skin. Int. J. Mol. Sci. 2023, 24, 11484. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef]
- Li, J.; Deng, S.H.; Li, J.; Li, L.; Zhang, F.; Zou, Y.; Wu, D.M.; Xu, Y. Obacunone alleviates ferroptosis during lipopolysaccharide-induced acute lung injury by upregulating Nrf2-dependent antioxidant responses. Cell Mol. Biol. Lett. 2022, 27, 29. [Google Scholar] [CrossRef] [PubMed]
- Raps, S.P.; Lai, J.C.K.; Hertz, L.; Cooper, A.J.L. Glutathione is present in high concentrations in cultured astrocytes but not in cultured neurons. Brain Res. 1989, 493, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Lafon-Cazal, M.; Pietri, S.; Culcasi, M.; Bockaert, J. NMDA-dependent superoxide production and neurotoxicity. Nature 1993, 364, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.W.; Norman, J.P.; Litzburg, A.; Gelbard, H.A. Antioxidants are required during the early critical period, but not later, for neuronal survival. J. Neurosci. Res. 2004, 78, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Yang, H.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Limonoids from Dictamnus dasycarpus protect against glutamate-induced toxicity in primary cultured rat cortical cells. J. Mol. Neurosci. 2010, 42, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kietzmann, T.; Samoylenko, A.; Immenschuh, S. Transcriptional regulation of heme oxygenase-1 gene expression by MAP kinases of the JNK and p38 pathways in primary cultures of rat hepatocytes. J. Biol. Chem. 2003, 278, 17927–17936. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Baba, M.; Nakatsuka, D.; Ishikawa, Y.; Aburatani, H.; Furuta, K.; Ishikawa, T.; Hatanaka, H.; Suzuki, M.; Watanabe, Y. Role of heme oxygenase-1 protein in the neuroprotective effects of cyclopentenone prostaglandin derivatives under oxidative stress. Eur. J. Neurosci. 2003, 17, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.; Kapurniotu, A.; Frank, R.W.; Gessner, A.; Mischke, R.; Flieger, O.; Juttner, S.; Brunner, H.; Bernhagen, J. Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J. Mol. Biol. 1998, 280, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Anetzberger, C.; Pirch, T.; Jung, K. Heterogeneity in quorum sensing-regulated bioluminescence of Vibrio harveyi. Mol. Microbiol. 2009, 73, 267–277. [Google Scholar] [CrossRef]
- Chen, X.; Schauder, S.; Potier, N.; Van Dorsselaer, A.; Pelczer, I.; Bassler, B.L.; Hughson, F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002, 415, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, Y.; Li, T.; Sun, Z. Research progress on anti-pathogen activity and mechanism of limonoids. Nat. Prod. Res. Dev. 2020, 32, 1078–1085. [Google Scholar]
- Rhen, M.; Maskell, D.; Mastroeni, P.; Threlfall, J. Salmonella: Molecular Biology and Pathogenesis; Horizon Scientific Press: Poole, UK, 2007. [Google Scholar]
- Bajaj, V.; Lucas, R.L.; Hwang, C.; Lee, C.A. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol. Microbiol. 1996, 22, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Obacunone represses Salmonella pathogenicity islands 1 and 2 in an envZ-dependent fashion. Appl. Environ. Microbiol. 2012, 78, 7012–7022. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.J.; Olczak, A.; Maier, S.; Soni, S.; Gunn, J. Respiratory hydrogen use by Salmonella enterica serovar Typhimurium is essential for virulence. Infect. Immun. 2004, 72, 6294–6299. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H. In Silico Approach Gives Insights into Ig-like Fold Containing Proteins in Vibrio parahaemolyticus: A Focus on the Fibrillar Adhesins. Toxins 2022, 14, 133. [Google Scholar] [CrossRef]
- Han, Y.M.; Kim, J.H. Antifungal Effect of Obacunone on Candida albicans. J. Pharm. Soc. Korea 2013, 57, 383–387. [Google Scholar]
- Zhao, W.; Wolfender, J.-L.; Hostettmann, K.; Xu, R.; Qin, G. Antifungal alkaloids and limonoid derivatives from Dictamnus dasycarpus. Phytochemistry 1998, 47, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Giofre, S.V.; Napoli, E.; Iraci, N.; Speciale, A.; Cimino, F.; Muscara, C.; Molonia, M.S.; Ruberto, G.; Saija, A. Interaction of selected terpenoids with two SARS-CoV-2 key therapeutic targets: An in silico study through molecular docking and dynamics simulations. Comput. Biol. Med. 2021, 134, 104538. [Google Scholar] [CrossRef] [PubMed]
- Simjanoska, M.; Eftimov, T.; Cicimov, V.; Velichkovska, M.; Kralevska, A. Finding Potential Inhibitors of COVID-19. In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies, Virtual, 11–13 February 2021; pp. 110–117. [Google Scholar]
- Basak, H.K.; Saha, S.; Ghosh, J.; Paswan, U.; Karmakar, S.; Pal, A.; Chatterjee, A. Sequence Analysis, Structure Prediction of Receptor Proteins and In Silico Study of Potential Inhibitors for Management of Life Threatening COVID-19. Lett. Drug Des. Discov. 2022, 19, 108–122. [Google Scholar]
- Choudhary, P.; Singh, T.; Amod, A.; Singh, S. Evaluation of phytoconstituents of Tinospora cordifolia against K417N and N501Y mutant spike glycoprotein and main protease of SARS-CoV-2—An in silico study. J. Biomol. Struct. Dyn. 2023, 41, 4106–4123. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, S.; Sahoo, S.K. In silico ADMET and molecular docking study on searching potential inhibitors from limonoids and triterpenoids for COVID-19. Comput. Biol. Med. 2020, 124, 103936. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, S.; Bhatia, M.; Rathod, S.; Choudhari, P.; Dhavale, R. Exploration of limonoids for their broad spectrum antiviral potential via DFT, molecular docking and molecular dynamics simulation approach. Nat. Prod. Res. 2024, 38, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Magurano, F.; Sucameli, M.; Picone, P.; Micucci, M.; Baggieri, M.; Marchi, A.; Bucci, P.; Gioacchini, S.; Catinella, G.; Borgonovo, G.; et al. Antioxidant Activity of Citrus Limonoids and Investigation of Their Virucidal Potential against SARS-CoV-2 in Cellular Models. Antioxidants 2021, 10, 1794. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Katsuma, S.; Hirasawa, A.; Tsujimoto, G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun. 2005, 329, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Horiba, T.; Katsukawa, M.; Mita, M.; Sato, R. Dietary obacunone supplementation stimulates muscle hypertrophy, and suppresses hyperglycemia and obesity through the TGR5 and PPARgamma pathway. Biochem. Biophys. Res. Commun. 2015, 463, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Inoue, J.; Hashidume, T.; Shimizu, M.; Sato, R. Anti-obesity and anti-hyperglycemic effects of the dietary citrus limonoid nomilin in mice fed a high-fat diet. Biochem. Biophys. Res. Commun. 2011, 410, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Ai, F.F.; Lin, J.B.; Tang, J.J. Chemical constituents extracted from Dictamnus dasycarpus and their α-glucosidase inhibitory activity. J. China Pharm. Univ. 2019, 50, 41–45. [Google Scholar]
- Tamori, Y.; Masugi, J.; Nishino, N.; Kasuga, M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes 2002, 51, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Korn, B.S.; Hammer, R.E.; Moon, Y.A.; Komuro, R.; Horton, J.D.; Goldstein, J.L.; Brown, M.S.; Shimomura, I. SREBP cleavage-activating protein (SCAP) is required for increased lipid synthesis in liver induced by cholesterol deprivation and insulin elevation. Genes. Dev. 2001, 15, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Espenshade, P.J.; Hughes, A.L. Regulation of sterol synthesis in eukaryotes. Annu. Rev. Genet. 2007, 41, 401–427. [Google Scholar] [CrossRef] [PubMed]
- Grand-Perret, T.; Bouillot, A.; Perrot, A.; Commans, S.; Walker, M.; Issandou, M. SCAP ligands are potent new lipid-lowering drugs. Nat. Med. 2001, 7, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Sun, L.; Feramisco, J.D.; Brown, M.S.; Goldstein, J.L. Cholesterol Addition to ER Membranes Alters Conformation of SCAP, the SREBP Escort Protein that Regulates Cholesterol Metabolism. Mol. Cell 2002, 10, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Z.; Yoshida, C.A.; Furuichi, T.; Amizuka, N.; Ito, M.; Fukuyama, R.; Miyazaki, T.; Kitaura, H.; Nakamura, K.; Fujita, T.; et al. Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Dev. Dyn. 2007, 236, 1876–1890. [Google Scholar] [CrossRef]
- Karsenty, G.; Kronenberg, H.M.; Settembre, C. Genetic control of bone formation. Annu. Rev. Cell Dev. Biol. 2009, 25, 629–648. [Google Scholar] [CrossRef]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.; Komm, B.S.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.L.; Hankenson, K.D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell. Biochem. 2011, 112, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zheng, L.; Li, X.; Huang, F.; Hu, S.; Chen, L.; Jiang, M.; Lin, X.; Jiang, H.; Zeng, Y.; et al. Obacunone targets macrophage migration inhibitory factor (MIF) to impede osteoclastogenesis and alleviate ovariectomy-induced bone loss. J. Adv. Res. 2023, 53, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, X.; Huang, Y.; He, B.; Zhu, J.; Sun, K.; Deng, C.; Guo, Y.; Hao, D.; Jian, B. Obacunone inhibits RANKL/M-CSF-mediated osteoclastogenesis by suppressing integrin- FAK-Src signaling. Cytokine 2023, 164, 156134. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, D.E.; White, R.; Li, D.; Minhas, K.M.; Cernetich, A.; Kim, S.; Burke, S.; Shoukas, A.A.; Nyhan, D.; Champion, H.C.; et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 2003, 108, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.; Gupta, G.; Benjo, A.; Lim, H.K.; Camara, A.; Sikka, G.; Lim, H.K.; Sohi, J.; Santhanam, L.; Soucy, K.; et al. Endothelial arginase II: A novel target for the treatment of atherosclerosis. Circ. Res. 2008, 102, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Cirino, G.; Casini, A.; Napoli, C. Nitric Oxide as a Signaling Molecule in the Vascular System: An Overview. J. Cardiovasc. Pharmacol. 1999, 34, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Park, M.; Lee, J.; Min, B.S.; Ryoo, S. Endothelial nitric oxide synthase activation through obacunone-dependent arginase inhibition restored impaired endothelial function in ApoE-null mice. Vasc. Pharmacol. 2014, 60, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.N.; Zhou, H.Q. Study on the protective effect of obacunone on myocardial injury in rats with myocardial ischemia reperfusion by regulating ferroptosis pathway. Chin. J. Hosp. Pharm. 2023, 43, 1932–1938. [Google Scholar]
- Jayaprakasha, G.K.; Singh, R.P.; Pereira, J.; Sakariah, K.K. Limonoids from Citrus reticulata and their moult inhibiting activity in mosquito Culex quinquefasciatus larvae. Phytochemistry 1997, 44, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Saraf, S. Limonoids: Overview of significant bioactive triterpenes distributed in plants kingdom. Biol. Pharm. Bull. 2006, 29, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Mendel, M.J.; Alford, R.A.; Rajab, M.S.; Bentley, M.D. Antifeedant Effects of Citrus Limonoids Differing in A-Ring Structure on Colorado Potato Beetle (Coleoptera: Chrysomelidae) Larvae. J. Econ. Entomol. 1991, 84, 1158–1162. [Google Scholar] [CrossRef]
- Xiang, P.; Cao, Q.H.; Dong, Q.M.; Yang, X.J.; Tang, J.J.; Bai, H. Furan-site transformations of obacunone as potent insecticidal agents. Heliyon 2018, 4, e01064. [Google Scholar] [CrossRef]
- Wada, K.; Yagi, M.; Matsumura, A.; Sasaki, K.; Sakata, M.; Haga, M. Isolation of limonin and obacunone from phellodendri cortex shorten the sleeping time induced in mice by alpha-chloralose-urethane. Chem. Pharm. Bull. 1990, 38, 2332–2334. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, Y.; Wang, M. Selective and reliable determination of obacunone in rat plasma using solid-phase extraction by liquid chromatography tandem mass spectrometry: Application to a pharmacokinetic study. Biomed. Chromatogr. 2021, 35, e5031. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Xin, S.K.; Han, L.Y.; Zuo, R.; Li, Y.; Gong, M.X.; Wei, X.L.; Zhou, Y.Y.; He, J.; Wang, H.J.; et al. Comparative metabolism of four limonoids in human liver microsomes using ultra-high-performance liquid chromatography coupled with high-resolution LTQ-Orbitrap mass spectrometry. Rapid Commun. Mass. Spectrom. 2015, 29, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, J.; Gao, E.; Zhao, Y.; Qu, W.; Yu, Z. Simultaneous determination of limonin, dictamnine, obacunone and fraxinellone in rat plasma by a validated UHPLC-MS/MS and its application to a pharmacokinetic study after oral administration of Cortex Dictamni extract. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 928, 44–51. [Google Scholar] [CrossRef]
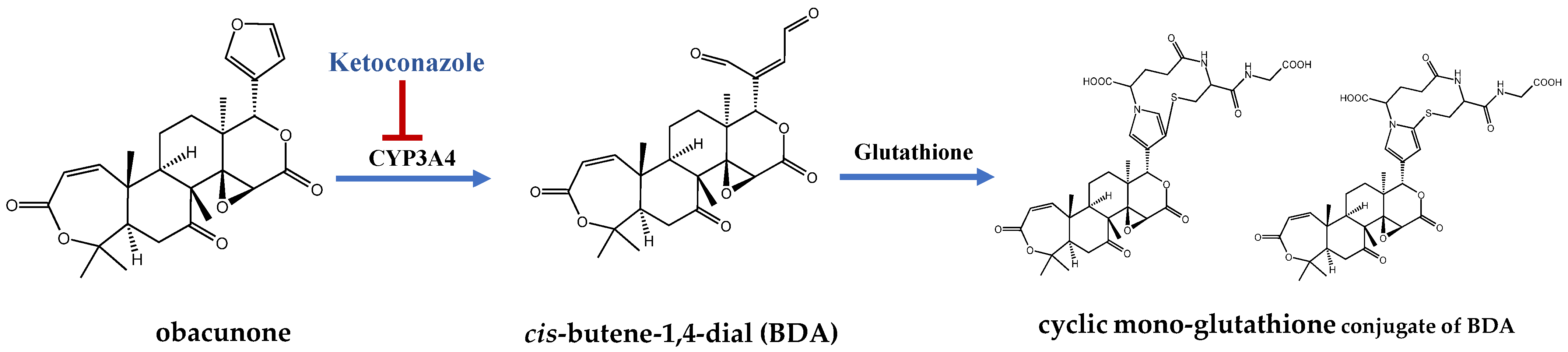
- Lang, X.; Zhang, X.; Wang, D.; Zhou, W. In Vitro and In Vivo Metabolic Activation of Obacunone, A Bioactive and Potentially Hepatotoxic Constituent of Dictamni Cortex. Planta Med. 2020, 86, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Li, Y.; Zuo, R.; Wang, H.J.; Si, N.; Zhao, H.Y.; Han, L.Y.; Yang, J.; Bian, B.L. Species-related difference between limonin and obacunone among five liver microsomes and zebrafish using ultra-high-performance liquid chromatography coupled with a LTQ-Orbitrap mass spectrometer. Rapid Commun. Mass. Spectrom. 2014, 28, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.A. Reactive metabolites in the biotransformation of molecules containing a furan ring. Chem. Res. Toxicol. 2013, 26, 6–25. [Google Scholar] [CrossRef]

| Herbs and Traditional Chinese Medicine Formula | Function of the Traditional Chinese Medicine | Role | |
|---|---|---|---|
| Herb | Dictamnus dasycarpus Turcz. (Chinese name: Bai xian pi) | Clearing heat, drying dampness, dispelling wind, and detoxification. | The qualitative and quantitative indicator components [2]. |
| Phellodendron chinense Schneid. (Chinese name: Huang bo) | Clearing heat, drying dampness, purging fire, removing steam, detoxifying, and treating sores. | The qualitative indicator component [2]. | |
| Coptis chinensis Franch. (Chinese name: Huang lian) | Clearing heat, drying dampness, purging fire, and detoxifying. | The key phytochemical component against ulcerative colitis [3]. | |
| Euodia rutaecarpa (Juss.) Benth. (Chinese name: Wu zhu yu) | Dispelling cold, relieving pain, lowering reversal, stopping nausea, assisting yang, and stopping diarrhea. | The highly active compound for liver cancer [4]. | |
| Lippia javanica (Burm. F.) (Farecha) | Treatment of fever, malaria, cough, cold, chest pain, asthma, bronchitis, and diarrhea. | The key phytochemical component with antioxidant and liver protection properties [5]. | |
| Araliopsis soyauxii Engl. (Rutaceae) | Treatment of lung diseases, malaria, and gonorrhea. | The main active component for antitumor activity [6]. | |
| Traditional Chinese Medicine Formula | ShangKeHuangShui, composed of Coptis chinensis Franch. (Huang lian), Phellodendron chinense Schneid. (Huang bo), Gardenia jasminoides Ellis (Zhi zi), Arnebia euchroma (Royle) Johnst. (Zi Cao), Arnebia euchroma (Royle) Johnst.(Bo he), and Ming fan | Treatment of staphylococcus aureus infection. | The active chemical component against bacterial infection [7]. |
| Erchen decoction, composed of Atractylodes macrocephala Koidz. (Bai zhu), Citrus reticulata Blanco (Ju hong), Poria cocos (Schw.) Wolf (Fu ling), Glycyrrhiza uralensis Fisch. (Gan cao) | Regulation of multiple core target genes in the pathogenesis of atherosclerosis. | The core component for the treatment of atherosclerosis [8]. | |
| Chaihu Shugan San, composed of Bupleurum chinense DC. (Chai hu), Citrus aurantium L. (Zhi qiao), Ligusticum chuanxiong Hort. (Chuan xiong), Aconitum carmichaelii Debx. (Fu zi), Paeonia lactiflora Pall. (Bai shao), Curcuma longa L. (Yu jin), Lygodium japonicum (Thunb.) Sw. (Hai jin sha) | Prevention of cholesterol gallstone formation. | The critical active metabolite in regulating bile acid metabolism [9]. | |
| Huazhuo Quyu Formula, composed of Rheum palmatum L. (Da huang), Coptis chinensis Franch. (Huang lian), Salvia miltiorrhiza Bge. (Dan shen), Curcuma phaeocaulis VaL. (E zhu), Curcuma Longa L. (Jiang huang), Lysimachia christinae Hance (Jin qian cao) | Treatment of Wilson’s Disease-associated liver fibrosis. | The essential active compound that inhibited liver inflammatory processes and vascular hyperplasia regulated the cell cycle and suppressed both the activation and proliferation of hepatic stellate cells [10]. | |
| Huanglian Jiedu decoction, composed of Coptis chinensis Franch. (Huang lian), Scutellaria baicalensis Georgi (Huang qin), Phellodendron chinense Schneid. (Huang bo), Gardenia jasminoides Ellis (Zhi zi) | Treating acute lung injury. | The active compound inhibits inflammasome activation via the sphingolipid pathway [11]. | |
| Simiao pill, composed of Atractylodes lancea (Thunb.) DC. (Cang zhu), Achyranthes bidentata Bl. (Niu xi), Phellodendron chinense Schneid. (Huang bo), Coix lacryma-jobi L.var.ma-yuen (Roman.) Stapf (Yi yi ren) | Treating hyperuricemia. | The synergistic effects of limonin against nitric oxide synthase 3 [12]. | |
| Species | Cell Line | Dose Used (μM) | Impacts | |
|---|---|---|---|---|
| Mouse | Normal Embryo Fibroblast | NIH/3T3 cells [19] | 6.25–200 | No cytotoxicity |
| Mouse | Normal Bone | MC3T3-E1 cells [31] | 1–100 | No cytotoxicity |
| Mouse | Normal Liver | AML12 cells [6] | IC50 > 100 | No cytotoxicity |
| Rat | Normal Kidney | NRK-52E cells [32] | 40–1280 | No cytotoxicity |
| Cricetulus | Normal Ovary | Chinese hamster ovary cells [18] | 10–40 | No cytotoxicity |
| Canine | Normal Kidney | MDCK cells [33] | 0.78–50 | No cytotoxicity |
| Human | Normal Prostate | RWPE-1 cells [24] | 6.25–200 | No cytotoxicity |
| Human | Normal Breast | MCF-12F cells [34] | 6.25–200 | No cytotoxicity |
| Human | Normal Epidermis | HaCaT cells [35] | 25–100 | No cytotoxicity |
| Human | Normal Retina | ARPE-19 cells [36] | 1–50 | No cytotoxicity |
| Types of Cancer Cell Lines | Effect | Mechanism | Reference | |
|---|---|---|---|---|
| Colon cancer | Caco-2 cells | An apparent dose–response antiproliferative effect was observed throughout 24–48 h incubation at 10–50 μM concentration. | Obacunone arrested the cell cycle process, accumulating cells in the G1 and G2 phases. | [18] |
| SW480 cells | The IC50 values of obacunone were 97.02 ± 4.1 μM and 56.22 ± 4.03 μM for 24 h and 72 h, respectively. | Obacunone induced apoptosis by activating the intrinsic apoptosis pathway and activating p21, leading to cell arrest at the G2/M phase of the cell cycle. | [19] | |
| Liver cancer | HepG2 cells | The IC50 values of obacunone on cell number, nuclear intensity, cell membrane permeability, and concentration of reactive oxygen species were 42.87 μM, 54.09 μM, 84.00 μM, and 41.51 μM for 48 h incubation, respectively. | The potential mechanism of hepatotoxicity might be associated with changes in the cell number, nuclear intensity, cell membrane permeability, and concentration of reactive oxygen species, which may induce cell apoptosis. | [21] |
| Pancreatic cancer | Panc-28 cells | Obacunone demonstrated both time (2, 4, and 6 days) and dose-dependent (50 μM and 100 μM) inhibition of cell proliferation. | The cytotoxicity was associated with tumor suppressor protein (p53) activation and proapoptotic and anti-inflammatory pathways. | [22] |
| Prostate cancer | LNCaP cells | Obacunone had a time- and dose-dependent inhibition of cell proliferation, with more than 60% inhibition of cell viability at 100 μM after 24 and 48 h of incubation. | Obacunone caused cytotoxicity to cells by activating intrinsic apoptosis, suppressing inflammation, and down-regulating androgen receptors and prostate-specific antigens. | [24] |
| 22RV1 cells | Obacunone at the concentrations of 21.25 μM, 42.5 μM, and 85 μM showed intense inhibitory ability, with the highest inhibition rate of 78.9% at 85 μM. The proportion of apoptosis cells was raised progressively in a time-dependent mode after 24 h, 48 h, and 72 h incubation at concentrations of 85 μM of obacunone treatment. | Obacunone effectually controlled proliferation and promoted apoptosis in 22RV1 prostate cancer cells, which were related to twenty-one proposed metabolites, and nicotinate and nicotinamide metabolism, phenylalanine metabolism, tryptophan metabolism, as well as ascorbate metabolism. | [25] | |
| Breast cancer | MCF-7 and MDA-MB-231 cell | (1) Obacunone (200 μM, 72 h) exhibited the cytotoxicity of 44% and 18% of MCF-7 and MDA-MB-231 cells, respectively. (2) Obacunone (20 μM, 30 min) exhibited potent aromatase inhibition activity with an IC50 value of 28.04 μM. (3) Caspase-7 in MCF-7 cells treated with obacunone (200 μM, 72 h) was altered 3.6-fold. | The antiproliferative properties of obacunone were mediated by caspase-7-dependent pathways. | [6,34] |
| Neuroblastoma | SH-SY5Y cells | (1) An apparent dose–response antiproliferative effect was observed throughout 24–48 h at the concentration of 10–50 μM obacunone (25 μM, 36 h). (2) Obacunone (25 μM, 36 h) could induce the caspase 3/7 activity and aneuploidy. | The mechanism of obacunone action was related to apoptosis induction, cell cycle arrest, and aneuploidy. | [18] |
| Adrenocortical tumor | Y1 mouse adrenocortical tumor cells | (1) The inhibitory rate of obacunone (2.5–40 μM for 24 h) on cell growth was about 35%, while 160 μM had a cell inhibition rate of over 60%. (2) The backbone (more than 80 μM for 24 h) had cytotoxicity. | Obacunone inhibited corticosterone synthesis by corticosterone in adrenal cortex cells, which might be related to cell cycle arrest and the expression of steroid synthase on the mitochondrial membrane. | [29] |
| Drug Components | Dosage | Route of Administration | Animal Model | Obacunone Pk/Pd Parameters | Ref. |
|---|---|---|---|---|---|
| Obacunone | 10 mg/kg | Oral | Rat | AUC0-last = 804.21 ± 163.73 ng·h/mL Cmax = 202.75 ± 36.11 ng/mL Tmax = 1–2 h T1/2 = 3.47 ± 0.55 h Vd = 64.68 ± 20.96 L/kg CL = 12.75 ± 2.59 L/kg/h F = 13.59% | [101] |
| Obacunone | 1 mg/kg | Intravenous | Rat | AUC0-last = 591.59 ± 109.41 ng·h/mL Cmax = 628.52 ± 188.41 ng/mL T1/2 = 4.30 ± 1.98 h Vd = 10.79 ± 5.69 L/kg CL = 1.71 ± 0.28 L/kg/h | [101] |
| Dictamnus dasycarpus cortex extract | 0.424 g/kg (44.7 mg/kg Obacunone equivalent) | Oral | Rat | AUC0-last = 1701 ng·h/mL Cmax = 531 ± 56.7 ng/mL Tmax = 1.00 ± 0.00 h | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Gu, J.; Li, J.; Zhang, H.; Wang, M.; Li, Y.; Wang, T.; Wang, J.; Shi, R. Obacunone, a Promising Phytochemical Triterpenoid: Research Progress on Its Pharmacological Activity and Mechanism. Molecules 2024, 29, 1791. https://doi.org/10.3390/molecules29081791
Zhou Y, Gu J, Li J, Zhang H, Wang M, Li Y, Wang T, Wang J, Shi R. Obacunone, a Promising Phytochemical Triterpenoid: Research Progress on Its Pharmacological Activity and Mechanism. Molecules. 2024; 29(8):1791. https://doi.org/10.3390/molecules29081791
Chicago/Turabian StyleZhou, Yuyang, Jifeng Gu, Jiahui Li, Huishan Zhang, Mei Wang, Yuanyuan Li, Tianming Wang, Jiajie Wang, and Rong Shi. 2024. "Obacunone, a Promising Phytochemical Triterpenoid: Research Progress on Its Pharmacological Activity and Mechanism" Molecules 29, no. 8: 1791. https://doi.org/10.3390/molecules29081791
APA StyleZhou, Y., Gu, J., Li, J., Zhang, H., Wang, M., Li, Y., Wang, T., Wang, J., & Shi, R. (2024). Obacunone, a Promising Phytochemical Triterpenoid: Research Progress on Its Pharmacological Activity and Mechanism. Molecules, 29(8), 1791. https://doi.org/10.3390/molecules29081791







