The Role of mRNA Alternative Splicing in Macrophages Infected with Mycobacterium tuberculosis: A Field Needing to Be Discovered
Abstract
:1. Introduction
2. Alternative Splicing
3. Global Changes in Gene Expression upon Infection with Mtb
3.1. Alternative Splicing Causes Changes in Protein Activity
3.2. TB-Specific Alternatively Spliced Isoforms
3.3. The Expression of Splicing Factors Changes in Cells Infected with Mtb
4. Alternative Splicing in Host Response to Mtb Infection
4.1. The Role of IL-4/IL-4δ2 Ratio in Mtb Infection
4.2. IL-7 and IL-7 R Isoforms Play Different Roles in Control of Mtb Infection
4.3. IL12-Rβ1 Is Essential for Host Defence against Mtb Infection
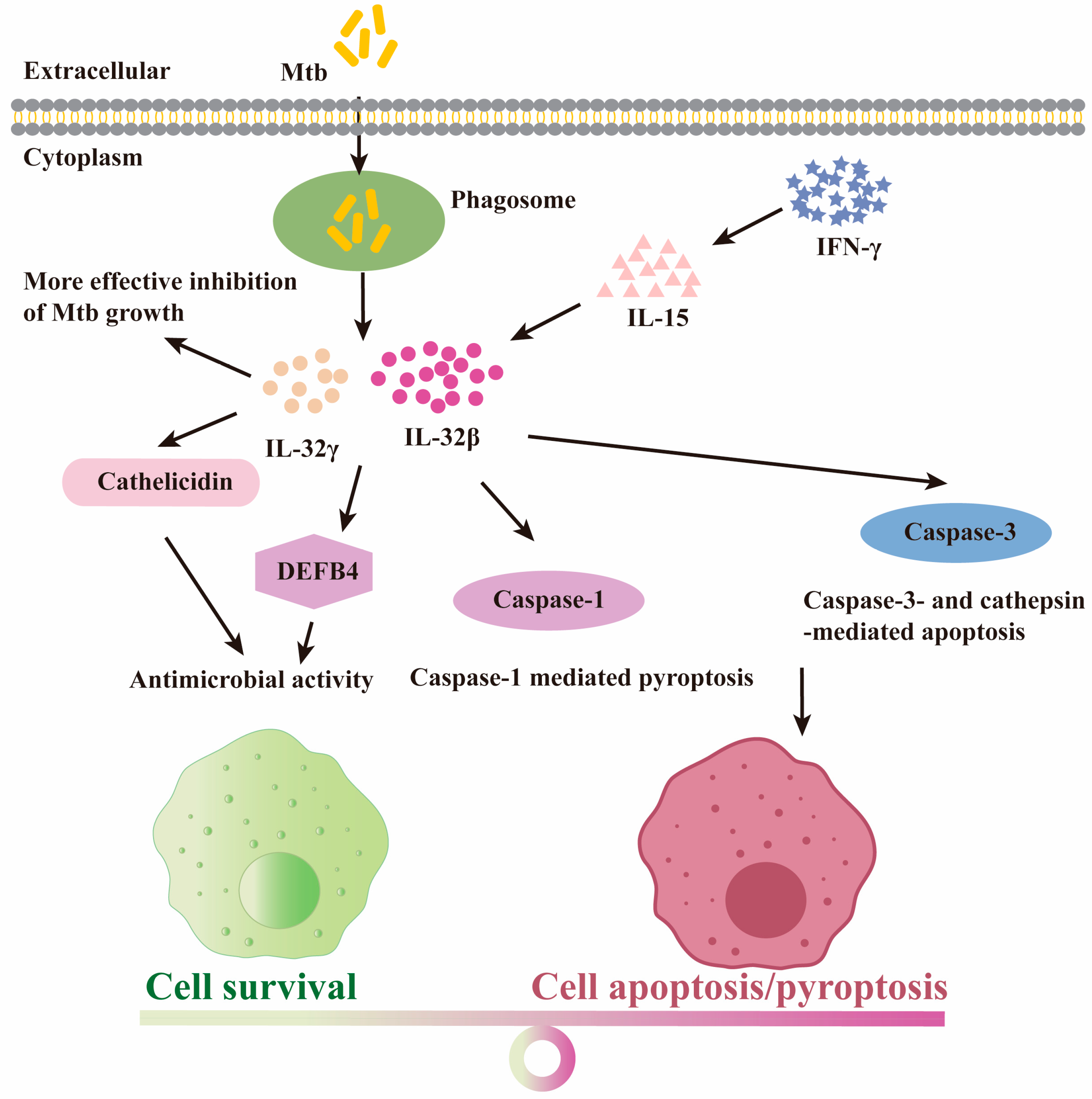
4.4. The Protective Role for IL-32 Isoforms upon Mtb Infection
5. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bagcchi, S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe 2023, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol. Rev. 2015, 240, 252–268. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023.
- Ghebreyesus, T.A.; Kasaeva, T. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022.
- van Leth, F.; Van der Werf, M.J.; Borgdorff, M.W. Prevalence of tuberculous infection and incidence of tuberculosis: A re-assessment of the Styblo rule. Bull. World Health Organ. 2008, 86, 20–26. [Google Scholar] [CrossRef]
- Eddo, K.; Alon, M.; Gil, A. Different levels of alternative splicing among eukaryotes. Nucleic Acids Res. 2006, 35, 125–131. [Google Scholar]
- Kalam, H.; Singh, K.; Chauhan, K.; Fontana, M.F.; Kumar, D. Alternate splicing of transcripts upon Mycobacterium tuberculosis infection impacts the expression of functional protein domains. IUBMB Life 2018, 70, 845–854. [Google Scholar] [CrossRef]
- Kim, E.; Goren, A.; Ast, G. Alternative splicing: Current perspectives. Bioessays 2008, 30, 38–47. [Google Scholar] [CrossRef]
- Lee, Y.; Rio, D.C. Mechanisms and Regulation of Alternative Pre-mRNA Splicing. Annu. Rev. Biochem. 2015, 84, 291–323. [Google Scholar] [CrossRef] [PubMed]
- Merkhofer, E.C.; Hu, P.; Johnson, T.L. Introduction to cotranscriptional RNA splicing. Methods Mol. Biol. 2014, 1126, 83–96. [Google Scholar]
- Yan, C.; Wan, R.; Shi, Y. Molecular Mechanisms of pre-mRNA Splicing through Structural Biology of the Spliceosome. Cold Spring Harb. Perspect. Biol. 2019, 11, a032409. [Google Scholar] [CrossRef]
- Gilbert, W. Why genes in pieces? Nature 1978, 271, 501. [Google Scholar] [CrossRef]
- Early, P.; Rogers, J.; Davis, M.; Calame, K.; Bond, M.; Wall, R.; Hood, L. Two mRNAs can be produced from a single immunoglobulin μ gene by alternative RNA processing pathways. Cell 1980, 20, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Werneke, J.M.; Chatfield, J.M.; Ogren, W.L. Alternative mRNA splicing generates the two ribulosebisphosphate carboxylase/oxygenase activase polypeptides in spinach and Arabidopsis. Plant Cell 1989, 1, 815–825. [Google Scholar] [PubMed]
- Watanabe, Y.; Yokobori, S.; Inaba, T.; Yamagishi, A.; Oshima, T.; Kawarabayasi, Y.; Kikuchi, H.; Kita, K. Introns in protein-coding genes in Archaea. FEBS Lett. 2002, 510, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Yokobori, S.I.; Itoh, T.; Yoshinari, S.; Nomura, N.; Sako, Y.; Yamagishi, A.; Oshima, T.; Kita, K.; Watanabe, Y.I. Gain and loss of an intron in a protein-coding gene in Archaea: The case of an archaeal RNA pseudouridine synthase gene. BMC Evol. Biol. 2009, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Artamonova, I.I.; Gelfand, M.S. Comparative Genomics and Evolution of Alternative Splicing: The Pessimists’Science. Chem. Rev. 2007, 107, 3407–3430. [Google Scholar] [CrossRef] [PubMed]
- Alekseyenko, A.V.; Namshin, K.; Lee, C.J. Global analysis of exon creation versus loss and the role of alternative splicing in 17 vertebrate genomes. RNA 2007, 13, 661–670. [Google Scholar] [CrossRef]
- Sharp, P.A. Split genes and RNA splicing. Cell 1994, 77, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Huang, B.O.; Xu, Y.M.; Li, J.; Huang, L.F.; Lin, J.; Zhang, J.; Min, Q.H.; Yang, W.M.; et al. Mechanism of alternative splicing and its regulation. Biomed. Rep. 2015, 3, 152–158. [Google Scholar] [CrossRef]
- Xing, Y.; Lee, C. Alternative splicing and RNA selection pressure—Evolutionary consequences for eukaryotic genomes. Nat. Rev. Genet. 2006, 7, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Dai, X.; Wu, J. Alternative splicing: An important mechanism in stem cell biology. World J. Stem Cells 2015, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Dong, H.; Shi, Y.; Bian, L. Mutually exclusive alternative splicing of pre-mRNAs. Wiley Interdiscip. Rev. RNA 2018, 9, e1468. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Pham, M.H.C.; Ko, K.S.; Rhee, B.D.; Han, J. Alternative splicing isoforms in health and disease. Pflügers Arch. 2018, 470, 995–1016. [Google Scholar] [CrossRef]
- Hatje, K.; Kollmar, M. Expansion of the mutually exclusive spliced exome in Drosophila. Nat. Commun. 2013, 4, 2460. [Google Scholar] [CrossRef]
- Sugnet, C.W.; Kent, W.J.; Ares, M., Jr.; Haussler, D. Transcriptome and genome conservation of alternative splicing events in humans and mice. Pac. Symp. Biocomput. 2004, 9, 66–77. [Google Scholar]
- Hong, W.; Zhang, J.; Dong, H.; Shi, Y.; Ma, H.; Zhou, F.; Xu, B.; Fu, Y.; Zhang, S.; Hou, S.; et al. Intron-targeted mutagenesis reveals roles for Dscam1 RNA pairing architecture-driven splicing bias in neuronal wiring. Cell Rep. 2021, 36, 109373. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.D.; Berglund, J.A. Alternative Pre-mRNA Splicing. Acta Physiol. 2013, 1126, 45–54. [Google Scholar]
- Sakabe, N.J.; Souza, S.J.D. Sequence features responsible for intron retention in human. Bmc Genom. 2007, 8, 59. [Google Scholar] [CrossRef]
- Kalam, H.; Fontana, M.F.; Kumar, D. Alternate splicing of transcripts shape macrophage response to Mycobacterium tuberculosis infection. PLoS Pathog. 2017, 13, e1006236. [Google Scholar] [CrossRef]
- Mvubu, N.E.; Pillay, B.; Pillay, M. Infection of pulmonary epithelial cells by clinical strains of M. tuberculosis induces alternate splicing events. Gene 2020, 750, 144755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Niu, C.; Fu, R.Y.; Peng, Z.Y. Mycobacterium tuberculosis H37Rv infection regulates alternative splicing in Macrophages. Bioengineered 2018, 9, 203–208. [Google Scholar] [CrossRef]
- Lyu, M.; Zhou, J.; Zhou, Y.; Chong, W.; Xu, W.; Lai, H.; Niu, L.; Hai, Y.; Yao, X.; Gong, S.; et al. From tuberculosis bedside to bench: UBE2B splicing as a potential biomarker and its regulatory mechanism. Signal Transduct. Target. Ther. 2023, 8, 82. [Google Scholar] [CrossRef]
- Danelishvili, L.; Yamazaki, Y.; Selker, J.; Bermudez, L.E. Secreted Mycobacterium tuberculosis Rv3654c and Rv3655c proteins participate in the suppression of macrophage apoptosis. PLoS ONE 2010, 5, e10474. [Google Scholar] [CrossRef]
- Long, J.C.; Caceres, J.F. The SR protein family of splicing factors: Master regulators of gene expression. Biochem. J. 2009, 417, 15–27. [Google Scholar] [CrossRef]
- Lai, M.C.; Peng, T.Y.; Tarn, W.Y. Functional interplay between viral and cellular SR proteins in control of post-transcriptional gene regulation. Febs J. 2009, 276, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Arinobu, Y.; Atamas, S.P.; Otsuka, T.; Niiro, H.; Yamaoka, K.; Mitsuyasu, H.; Niho, Y.; Hamasaki, N.; White, B.; Izuhara, K. Antagonistic effects of an alternative splice variant of human IL-4, IL-4delta2, on IL-4 activities in human monocytes and B cells. Cell. Immunol. 1999, 191, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Seah, G.T.; Rook, G.A. High levels of mRNA encoding IL-4 in unstimulated peripheral blood mononuclear cells from tuberculosis patients revealed by quantitative nested reverse transcriptase-polymerase chain reaction; correlations with serum IgE levels. Scand. J. Infect. Dis. 2001, 33, 106–109. [Google Scholar]
- Fletcher, H.A.; Owiafe, P.; Jeffries, D.; Hill, P.; Rook, G.A.; Zumla, A.; Doherty, T.M.; Brookes, R.H.; Vacsel Study, G. Increased expression of mRNA encoding interleukin (IL)-4 and its splice variant IL-4delta2 in cells from contacts of Mycobacterium tuberculosis, in the absence of in vitro stimulation. Immunology 2004, 112, 669–673. [Google Scholar] [CrossRef]
- Demissie, A.; Abebe, M.; Aseffa, A.; Rook, G.; Fletcher, H.; Zumla, A.; Weldingh, K.; Brock, I.; Andersen, P.; Doherty, T.M.; et al. Healthy individuals that control a latent infection with Mycobacterium tuberculosis express high levels of Th1 cytokines and the IL-4 antagonist IL-4delta2. J. Immunol. 2004, 172, 6938–6943. [Google Scholar] [CrossRef]
- Dheda, K.; Chang, J.S.; Huggett, J.F.; Kim, L.U.; Johnson, M.A.; Zumla, A.; Rook, G.A. The stability of mRNA encoding IL-4 is increased in pulmonary tuberculosis, while stability of mRNA encoding the antagonistic splice variant, IL-4delta2, is not. Tuberculosis 2007, 87, 237–241. [Google Scholar] [CrossRef]
- Krawczenko, A.; Kieda, C.; Dus, D. The biological role and potential therapeutic application of interleukin 7. Arch. Immunol. Ther. Exp. 2005, 53, 518–525. [Google Scholar]
- Adankwah, E.; Harelimana, J.D.; Minadzi, D.; Aniagyei, W.; Abass, M.K.; Batsa Debrah, L.; Owusu, D.O.; Mayatepek, E.; Phillips, R.O.; Jacobsen, M. Lower IL-7 Receptor Expression of Monocytes Impairs Antimycobacterial Effector Functions in Patients with Tuberculosis. J. Immunol. 2021, 206, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Rane, L.; Rahman, S.; Magalhaes, I.; Ahmed, R.; Spangberg, M.; Kondova, I.; Verreck, F.; Andersson, J.; Brighenti, S.; Maeurer, M.J. Increased (6 exon) interleukin-7 production after M. tuberculosis infection and soluble interleukin-7 receptor expression in lung tissue. Genes Immun. 2011, 12, 513–522. [Google Scholar] [CrossRef]
- Maeurer, M.J.; Trinder, P.; Hommel, G.; Walter, W.; Freitag, K.; Atkins, D.; Storkel, S. Interleukin-7 or interleukin-15 enhances survival of Mycobacterium tuberculosis-infected mice. Infect. Immun. 2000, 68, 2962–2970. [Google Scholar] [CrossRef] [PubMed]
- Vudattu, N.K.; Magalhaes, I.; Hoehn, H.; Pan, D.; Maeurer, M.J. Expression analysis and functional activity of interleukin-7 splice variants. Genes Immun. 2009, 10, 132–140. [Google Scholar] [CrossRef]
- Crawley, A.M.; Faucher, S.; Angel, J.B. Soluble IL-7R alpha (sCD127) inhibits IL-7 activity and is increased in HIV infection. J. Immunol. 2010, 184, 4679–4687. [Google Scholar] [CrossRef]
- Chua, A.O.; Wilkinson, V.L.; Presky, D.H.; Gubler, U. Cloning and characterization of a mouse IL-12 receptor-beta component. J. Immunol. 1995, 155, 4286–4294. [Google Scholar] [CrossRef]
- de Jong, R.; Altare, F.; Haagen, I.A.; Elferink, D.G.; Boer, T.; van Breda Vriesman, P.J.; Kabel, P.J.; Draaisma, J.M.; van Dissel, J.T.; Kroon, F.P.; et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 1998, 280, 1435–1438. [Google Scholar] [CrossRef]
- Ford, N.R.; Miller, H.E.; Reeme, A.E.; Waukau, J.; Bengtson, C.; Routes, J.M.; Robinson, R.T. Inflammatory signals direct expression of human IL12RB1 into multiple distinct isoforms. J. Immunol. 2012, 189, 4684–4694. [Google Scholar] [CrossRef]
- Robinson, R.T.; Khader, S.A.; Martino, C.A.; Fountain, J.J.; Teixeira-Coelho, M.; Pearl, J.E.; Smiley, S.T.; Winslow, G.M.; Woodland, D.L.; Walter, M.J.; et al. Mycobacterium tuberculosis infection induces il12rb1 splicing to generate a novel IL-12Rbeta1 isoform that enhances DC migration. J. Exp. Med. 2010, 207, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.A.; Fountain, J.J.; Miller, H.E.; Cooper, A.M.; Robinson, R.T.; Flynn, J.L. IL12Rβ1ΔTM is a secreted product of il12rb1 that promotes control of extrapulmonary tuberculosis. Infect. Immun. 2015, 83, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Aass, K.R.; Kastnes, M.H.; Standal, T. Molecular interactions and functions of IL-32. J. Leukoc. Biol. 2021, 109, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Dahl, C.A.; Schall, R.P.; He, H.L.; Cairns, J.S. Identification of a novel gene expressed in activated natural killer cells and T cells. J. Immunol. 1992, 148, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Han, S.; Azam, T.; Yoon, D.; Dinarello, C. Interleukin-32: A cytokine and inducer of TNFalpha. Immunity 2005, 22, 131–142. [Google Scholar] [PubMed]
- Kang, J.W.; Park, Y.S.; Lee, D.H.; Kim, M.S.; Bak, Y.; Ham, S.Y.; Park, S.H.; Kim, H.; Ahn, J.H.; Hong, J.T.J.B. Interaction network mapping among IL-32 isoforms. Biochimie 2014, 101, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Azam, T.; Lewis, E.C.; Joosten, L.; Wang, M.; Langenberg, D.; Meng, X.; Chan, E.D.; Yoon, D.Y.; Ottenhoff, T. Mycobacterium tuberculosis Induces Interleukin-32 Production through a Caspase-1/IL-18/Interferon-γ-Dependent Mechanism. PLoS Med. 2006, 3, e277. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Kim, S.H.; Azam, T.; McGibney, M.T.; Huang, H.; Dinarello, C.A.; Chan, E.D. IL-32 is a host protective cytokine against Mycobacterium tuberculosis in differentiated THP-1 human macrophages. J. Immunol. 2010, 184, 3830–3840. [Google Scholar] [CrossRef]
- Bai, X.; Shang, S.; Henao-Tamayo, M.; Basaraba, R.J.; Ovrutsky, A.R.; Matsuda, J.L.; Takeda, K.; Chan, M.M.; Dakhama, A.; Kinney, W.H.; et al. Human IL-32 expression protects mice against a hypervirulent strain of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2015, 112, 5111–5116. [Google Scholar] [CrossRef]
- Koeken, V.; Verrall, A.J.; Ardiansyah, E.; Apriani, L.; Dos Santos, J.C.; Kumar, V.; Alisjahbana, B.; Hill, P.C.; Joosten, L.A.B.; van Crevel, R.; et al. IL-32 and its splice variants are associated with protection against Mycobacterium tuberculosis infection and skewing of Th1/Th17 cytokines. J. Leukoc. Biol. 2020, 107, 113–118. [Google Scholar] [CrossRef]
- Bai, X.; Ovrutsky, A.R.; Kartalija, M.; Chmura, K.; Kamali, A.; Honda, J.R.; Oberley-Deegan, R.E.; Dinarello, C.A.; Crapo, J.D.; Chang, L.Y.; et al. IL-32 expression in the airway epithelial cells of patients with Mycobacterium avium complex lung disease. Int. Immunol. 2011, 23, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Schenk, M.; Mahapatra, S.; Le, P.; Kim, H.J.; Choi, A.W.; Brennan, P.J.; Belisle, J.T.; Modlin, R.L.J.I. Immunity, Human NOD2 Recognizes Structurally Unique Muramyl Dipeptides from Mycobacterium leprae. Infect. Immun. 2016, 84, 2429–2438. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Yang, W.; Jie, Z.; Elgaili, A.A.; Xie, J.J.O. Mycobacterium tuberculosis PPE32 promotes cytokines production and host cell apoptosis through caspase cascade accompanying with enhanced ER stress response. Oncotarget 2016, 7, 67347–67359. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Kinney, W.H.; Su, W.L.; Bai, A.; Ovrutsky, A.R.; Honda, J.R.; Netea, M.G.; Henao-Tamayo, M.; Ordway, D.J.; Dinarello, C.A.; et al. Caspase-3-independent apoptotic pathways contribute to interleukin-32gamma-mediated control of Mycobacterium tuberculosis infection in THP-1 cells. BMC Microbiol. 2015, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Montoya, D.; Inkeles, M.S.; Liu, P.T.; Realegeno, S.; Teles, R.M.; Vaidya, P.; Munoz, M.A.; Schenk, M.; Swindell, W.R.; Chun, R.; et al. IL-32 is a molecular marker of a host defense network in human tuberculosis. Sci. Transl. Med. 2014, 6, 250ra114. [Google Scholar] [CrossRef] [PubMed]
- Goda, C.; Kanaji, T.; Kanaji, S.; Tanaka, G.; Arima, K.; Ohno, S.; Izuhara, K. Involvement of IL-32 in activation-induced cell death in T cells. Int. Immunol. 2006, 18, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, W.; Xie, J. The Biology and Role of Interleukin-32 in Tuberculosis. J. Immunol. Res. 2018, 2018, 1535194. [Google Scholar] [CrossRef]
- Her, E.; Kim, S.J.; Kim, B.K.; Choi, W.S.; Yoon, D.Y.; Kim, S.H.; Bae, S.Y.; Choi, J.D.; Dinarello, C.A.; Lee, C.K. Identification of the most active interleukin-32 isoform. Immunology 2017, 126, 535–542. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, W.; Yang, H.; Wang, X.; Shi, J.; Zhang, J.; Xie, J. The Role of mRNA Alternative Splicing in Macrophages Infected with Mycobacterium tuberculosis: A Field Needing to Be Discovered. Molecules 2024, 29, 1798. https://doi.org/10.3390/molecules29081798
Hong W, Yang H, Wang X, Shi J, Zhang J, Xie J. The Role of mRNA Alternative Splicing in Macrophages Infected with Mycobacterium tuberculosis: A Field Needing to Be Discovered. Molecules. 2024; 29(8):1798. https://doi.org/10.3390/molecules29081798
Chicago/Turabian StyleHong, Weiling, Hongxing Yang, Xiao Wang, Jingyi Shi, Jian Zhang, and Jianping Xie. 2024. "The Role of mRNA Alternative Splicing in Macrophages Infected with Mycobacterium tuberculosis: A Field Needing to Be Discovered" Molecules 29, no. 8: 1798. https://doi.org/10.3390/molecules29081798





