An Anthocyanin-Based Eco-Friendly Triboelectric Nanogenerator for pH Monitoring and Energy Harvesting
Abstract
:1. Introduction
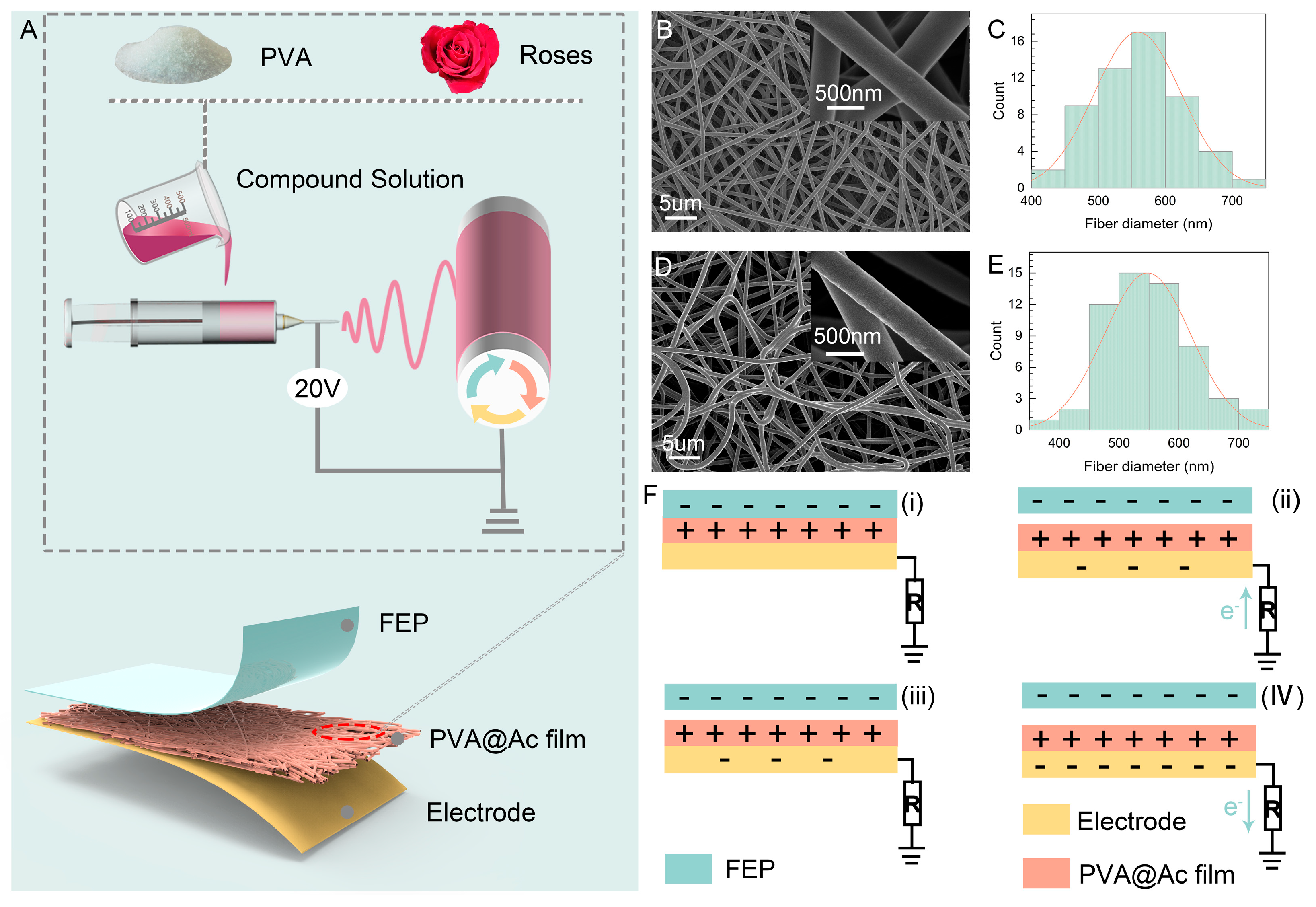
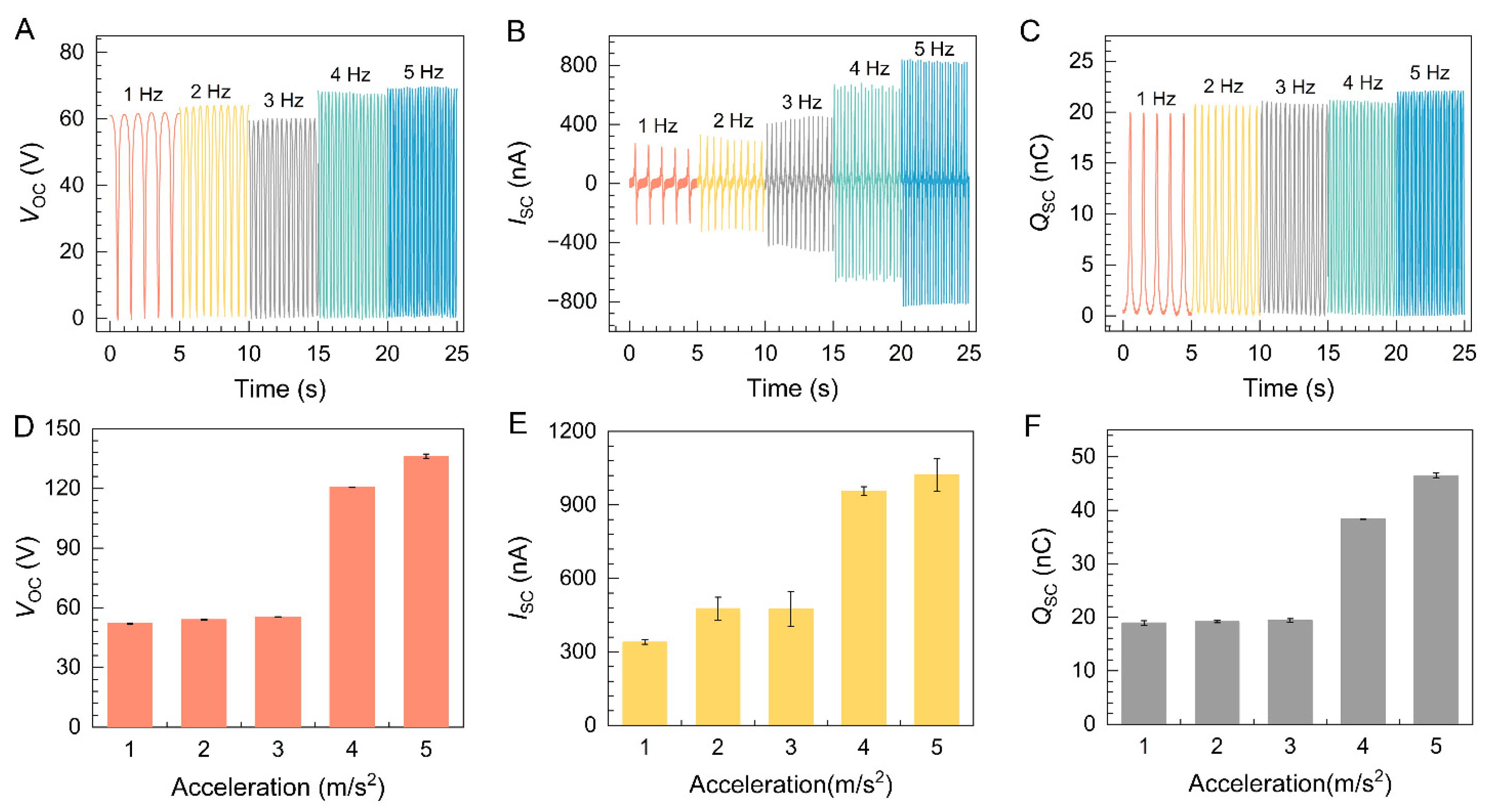
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of pH-TENG
3.3. Scanning Electron Microscopy (SEM)
3.4. pH Sensitivity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, X.; Xu, L.; Lin, X.; Pecht, M. Battery Lifetime Prognostics. Joule 2020, 4, 310–346. [Google Scholar] [CrossRef]
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Xu, S.; Sun, W.; He, Y. On the sustainability of lithium-ion battery industry-A review and perspective. Energy Storage Mater. 2021, 36, 186–212. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, H.; Lin, F. Sustainable Electric Vehicle Batteries for a Sustainable World: Perspectives on Battery Cathodes, Environment, Supply Chain, Manufacturing, Life Cycle, and Policy. Adv. Energy Mater. 2022, 12, 2200383. [Google Scholar] [CrossRef]
- Tanvir, R.U.; Zhang, J.; Canter, T.; Chen, D.; Lu, J.; Hu, Z. Harnessing solar energy using phototrophic microorganisms: A sustainable pathway to bioenergy, biomaterials, and environmental solutions. Renew. Sustain. Energy Rev. 2021, 146, 111181. [Google Scholar] [CrossRef] [PubMed]
- Aghbashlo, M.; Khounani, Z.; Hosseinzadeh-Bandbafha, H.; Gupta, V.K.; Amiri, H.; Lam, S.S.; Morosuk, T.; Tabatabaei, M. Exergoenvironmental analysis of bioenergy systems: A comprehensive review. Renew. Sustain. Energy Rev. 2021, 149, 111399. [Google Scholar] [CrossRef]
- Chen, B.D.; Tang, W.; He, C.; Deng, C.R.; Yang, L.J.; Zhu, L.P.; Chen, J.; Shao, J.J.; Liu, L.; Wang, Z.L. Water wave energy harvesting and self-powered liquid-surface fluctuation sensing based on bionic-jellyfish triboelectric nanogenerator. Mater. Today 2018, 21, 88–97. [Google Scholar] [CrossRef]
- Gao, X.; Xing, F.; Guo, F.; Yang, Y.; Hao, Y.; Chen, J.; Chen, B.; Wang, Z.L. A turbine disk-type triboelectric nanogenerator for wind energy harvesting and self-powered wildfire pre-warning. Mater. Today Energy 2021, 22, 100867. [Google Scholar] [CrossRef]
- Yang, Y.; Wen, J.; Chen, F.; Hao, Y.; Gao, X.; Jiang, T.; Chen, B.; Wang, Z.L. Barycenter Self-Adapting Triboelectric Nanogenerator for Sea Water Wave High-Entropy Energy Harvesting and Self-Powered Forecasting in Marine Meteorology. Adv. Funct. Mater. 2022, 32, 2200521. [Google Scholar] [CrossRef]
- Gao, X.; Xing, F.; Guo, F.; Wen, J.; Li, H.; Yang, Y.; Chen, B.; Wang, Z.L. Strongly enhanced charge density via gradient nano-doping for high performance elastic-material-based triboelectric nanogenerators. Mater. Today 2023, 65, 26–36. [Google Scholar] [CrossRef]
- Li, Y.; Wei, C.; Jiang, Y.; Cheng, R.; Zhang, Y.; Ning, C.; Dong, K.; Wang, Z.L. Continuous Preparation of Chitosan-Based Self-Powered Sensing Fibers Recycled from Wasted Materials for Smart Home Applications. Adv. Fiber Mater. 2022, 4, 1584–1594. [Google Scholar] [CrossRef]
- Fan, F.-R.; Lin, L.; Zhu, G.; Wu, W.; Zhang, R.; Wang, Z.L. Transparent Triboelectric Nanogenerators and Self-Powered Pressure Sensors Based on Micropatterned Plastic Films. Nano Lett. 2012, 12, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Chen, J.; Jing, Q.; Zhou, Y.S.; Wen, X.; Wang, Z.L. Single-Electrode-Based Sliding Triboelectric Nanogenerator for Self-Powered Displacement Vector Sensor System. ACS Nano 2013, 7, 7342–7351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, D.; Li, S.; Zhao, Z.; Zhang, C.; Yin, X.; Liu, L.; Cui, S.; Wang Zhong, L.; Wang, J. Rationally Designed Dual-Mode Triboelectric Nanogenerator for Harvesting Mechanical Energy by Both Electrostatic Induction and Dielectric Breakdown Effects. Adv. Energy Mater. 2020, 10, 2000965. [Google Scholar] [CrossRef]
- Chen, B.D.; Tang, W.; Zhang, C.; Xu, L.; Zhu, L.P.; Yang, L.J.; He, C.; Chen, J.; Liu, L.; Zhou, T.; et al. Au nanocomposite enhanced electret film for triboelectric nanogenerator. Nano Res. 2018, 11, 3096–3105. [Google Scholar] [CrossRef]
- Hu, J.; Iwamoto, M.; Chen, X. A Review of Contact Electrification at Diversified Interfaces and Related Applications on Triboelectric Nanogenerator. Nano-Micro Lett. 2024, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Kulandaivel, A.; Potu, S.; Babu, A.; Madathil, N.; Velpula, M.; Rajaboina, R.K.; Khanapuram, U.K. Advances in Ferrofluid-Based Triboelectric Nanogenerators: Design, Performance, and Prospects for Energy Harvesting Applications. Nano Energy 2023, 120, 109110. [Google Scholar] [CrossRef]
- Rajaboina, R.K.; Khanapuram, U.K.; Vivekananthan, V.; Khandelwal, G.; Potu, S.; Babu, A.; Madathil, N.; Velpula, M.; Kodali, P. Crystalline Porous Material-Based Nanogenerators: Recent Progress, Applications, Challenges, and Opportunities. Small 2024, 20, 2306209. [Google Scholar] [CrossRef]
- Das, S.; Chowdhury, A.; Ali, S.W. A Critical Review on Triboelectric Nanogenerators (TENGs) with Flame Retardant, Antibacterial, Self-Cleaning, or Water Repellent Properties. ACS Appl. Electron. Mater. 2024. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Y.; Chen, B.; Li, H.; Mao, Y. Recent Progress of Bioinspired Triboelectric Nanogenerators for Electronic Skins and Human–Machine Interaction. Nanoenergy Adv. 2024, 4, 45–69. [Google Scholar] [CrossRef]
- Liu, L.; Wu, M.; Zhao, W.; Tao, J.; Zhou, X.; Xiong, J. Progress of Triboelectric Nanogenerators with Environmental Adaptivity. Adv. Funct. Mater. 2024, 34, 2308353. [Google Scholar] [CrossRef]
- Du, T.; Chen, Z.; Dong, F.; Cai, H.; Zou, Y.; Zhang, Y.; Sun, P.; Xu, M. Advances in Green Triboelectric Nanogenerators. Adv. Funct. Mater. 2024, 2313794. [Google Scholar] [CrossRef]
- Chen, Y.; Jie, Y.; Wang, J.; Ma, J.; Jia, X.; Dou, W.; Cao, X. Triboelectrification on natural rose petal for harvesting environmental mechanical energy. Nano Energy 2018, 50, 441–447. [Google Scholar] [CrossRef]
- Roy, S.; Ko, H.-U.; Maji, P.K.; Kim, J. Large amplification of triboelectric property by allicin to develop high performance cellulosic triboelectric nanogenerator. Chem. Eng. J. 2020, 385, 123723. [Google Scholar] [CrossRef]
- Yan, X.; Yu, M.; Ramakrishna, S.; Russell, S.J.; Long, Y.-Z. Advances in portable electrospinning devices for in situ delivery of personalized wound care. Nanoscale 2019, 11, 19166–19178. [Google Scholar] [CrossRef] [PubMed]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Fang, J.; Shao, H.; Ding, X.; Lin, T. High-sensitivity acoustic sensors from nanofibre webs. Nat. Commun. 2016, 7, 11108. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A Review on Electrospun Nanofibers Based Advanced Applications: From Health Care to Energy Devices. Polymers 2021, 13, 3746. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cheng, L.; Yuan, M.; Wang, Z.; Zhang, L.; Qin, Y.; Jing, T. An electrospun nanowire-based triboelectric nanogenerator and its application in a fully self-powered UV detector. Nanoscale 2014, 6, 7842–7846. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, J.; Yu, J.; Li, Z.; Ding, B. The Rising of Fiber Constructed Piezo/Triboelectric Nanogenerators: From Material Selections, Fabrication Techniques to Emerging Applications. Adv. Funct. Mater. 2023, 33, 2303249. [Google Scholar] [CrossRef]
- Fu, Q.; Cui, C.; Meng, L.; Hao, S.; Dai, R.; Yang, J. Emerging cellulose-derived materials: A promising platform for the design of flexible wearable sensors toward health and environment monitoring. Mater. Chem. Front. 2021, 5, 2051–2091. [Google Scholar] [CrossRef]
- Ge, X.; Hu, N.; Yan, F.; Wang, Y. Development and applications of electrospun nanofiber-based triboelectric nanogenerators. Nano Energy 2023, 112, 108444. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Lin, P.; Wang, Y.; Yang, L.; Chen, L.; Wang, L.; Chen, B.; Wang, Z.L. Piezotronic Effect on Rashba Spin-Orbit Coupling in a ZnO/P3HT Nanowire Array Structure. ACS Nano 2018, 12, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, W.; Lu, C.; Xu, L.; Yang, Z.; Chen, B.; Jiang, T.; Wang, Z.L. Characteristics of triboelectrification on dielectric surfaces contacted with a liquid metal in different gases. Appl. Phys. Lett. 2017, 110, 201603. [Google Scholar] [CrossRef]
- Rafique, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Rehman, S. Chitosan functionalized poly(vinyl alcohol) for prospects biomedical and industrial applications: A review. Int. J. Biol. Macromol. 2016, 87, 141–154. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef] [PubMed]
- Tirtashi, F.E.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Cellulose/chitosan pH-responsive indicator incorporated with carrot anthocyanins for intelligent food packaging. Int. J. Biol. Macromol. 2019, 136, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Guo, M.; Li, L.; Chen, M.; Jiang, S.; Li, X.; Jiang, S. Extract from Lycium ruthenicum Murr. Incorporating κ-carrageenan colorimetric film with a wide pH–sensing range for food freshness monitoring. Food Hydrocoll. 2019, 94, 1–10. [Google Scholar] [CrossRef]
- Stoll, L.; Silva, A.M.d.; Iahnke, A.O.e.S.; Costa, T.M.H.; Flores, S.H.; Rios, A.d.O. Active biodegradable film with encapsulated anthocyanins: Effect on the quality attributes of extra-virgin olive oil during storage. J. Food Process. Preserv. 2017, 41, e13218. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.H.; Han, G.Y.; Chang, Y.Z.; Song, H.; Hou, W.J.; Chen, Q. Using Stretchable PPy@PVA Composites as a High-Sensitivity Strain Sensor To Monitor Minute Motion. ACS Appl. Mater. Interfaces 2020, 12, 45373–45382. [Google Scholar] [CrossRef] [PubMed]
- Destaye, A.G.; Lin, C.-K.; Lee, C.-K. Glutaraldehyde Vapor Cross-linked Nanofibrous PVA Mat with in Situ Formed Silver Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 4745–4752. [Google Scholar] [CrossRef]
- Slabov, V.; Kopyl, S.; dos Santos, M.P.S.; Kholkin, A.L. Natural and Eco-Friendly Materials for Triboelectric Energy Harvesting. Nano-Micro Lett. 2020, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bu, C.; Li, F.; Yin, K.; Pang, J.; Wang, L.; Wang, K. Research Progress and Prospect of Triboelectric Nanogenerators as Self-Powered Human Body Sensors. ACS Appl. Electron. Mater. 2020, 2, 863–878. [Google Scholar] [CrossRef]
- Niu, Z.; Cheng, W.; Cao, M.; Wang, D.; Wang, Q.; Han, J.; Long, Y.; Han, G. Recent advances in cellulose-based flexible triboelectric nanogenerators. Nano Energy 2021, 87, 106175. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, Y.; Mi, Y.; Zhu, Q.; Meng, J.; Wang, X.; Cao, X.; Wang, N. Modular Design in Triboelectric Sensors: A Review on the Clinical Applications for Real-Time Diagnosis. Sensors 2023, 23, 4194. [Google Scholar] [CrossRef]
- Wang, Z.L. Triboelectric Nanogenerators as New Energy Technology for Self-Powered Systems and as Active Mechanical and Chemical Sensors. ACS Nano 2013, 7, 9533–9557. [Google Scholar] [CrossRef]
- Jung, Y.H.; Kim, J.-H.; Rogers, J.A. Skin-Integrated Vibrohaptic Interfaces for Virtual and Augmented Reality. Adv. Funct. Mater. 2021, 31, 2008805. [Google Scholar] [CrossRef]
- Castaneda-Ovando, A.; de Lourdes Pacheco-Hernandez, M.; Elena Paez-Hernandez, M.; Rodriguez, J.A.; Andres Galan-Vidal, C. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Bu, T.; Zhang, C. Effects of interfacial acid-base on the performance of contact-separation mode triboelectric nanogenerator. Mater. Today Energy 2021, 20, 100686. [Google Scholar] [CrossRef]
- GB/T604 Standard. Available online: https://www.chinesestandard.net/Related.aspx/GBT604-2002?Redirect=YES (accessed on 8 April 2024).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Dong, J.; Li, W.; Gao, X.; Liu, J.; Nan, D. An Anthocyanin-Based Eco-Friendly Triboelectric Nanogenerator for pH Monitoring and Energy Harvesting. Molecules 2024, 29, 1925. https://doi.org/10.3390/molecules29091925
Sun W, Dong J, Li W, Gao X, Liu J, Nan D. An Anthocyanin-Based Eco-Friendly Triboelectric Nanogenerator for pH Monitoring and Energy Harvesting. Molecules. 2024; 29(9):1925. https://doi.org/10.3390/molecules29091925
Chicago/Turabian StyleSun, Wuliang, Junhui Dong, Wenbo Li, Xiaobo Gao, Jun Liu, and Ding Nan. 2024. "An Anthocyanin-Based Eco-Friendly Triboelectric Nanogenerator for pH Monitoring and Energy Harvesting" Molecules 29, no. 9: 1925. https://doi.org/10.3390/molecules29091925
APA StyleSun, W., Dong, J., Li, W., Gao, X., Liu, J., & Nan, D. (2024). An Anthocyanin-Based Eco-Friendly Triboelectric Nanogenerator for pH Monitoring and Energy Harvesting. Molecules, 29(9), 1925. https://doi.org/10.3390/molecules29091925






