Efficient Separation of Methanol Single-Micron Droplets by Tailing Phenomenon Using a PDMS Microfluidic Device
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fluid Simulation (CYTOP Surface Treatment)
2.2. Fluid Experiments
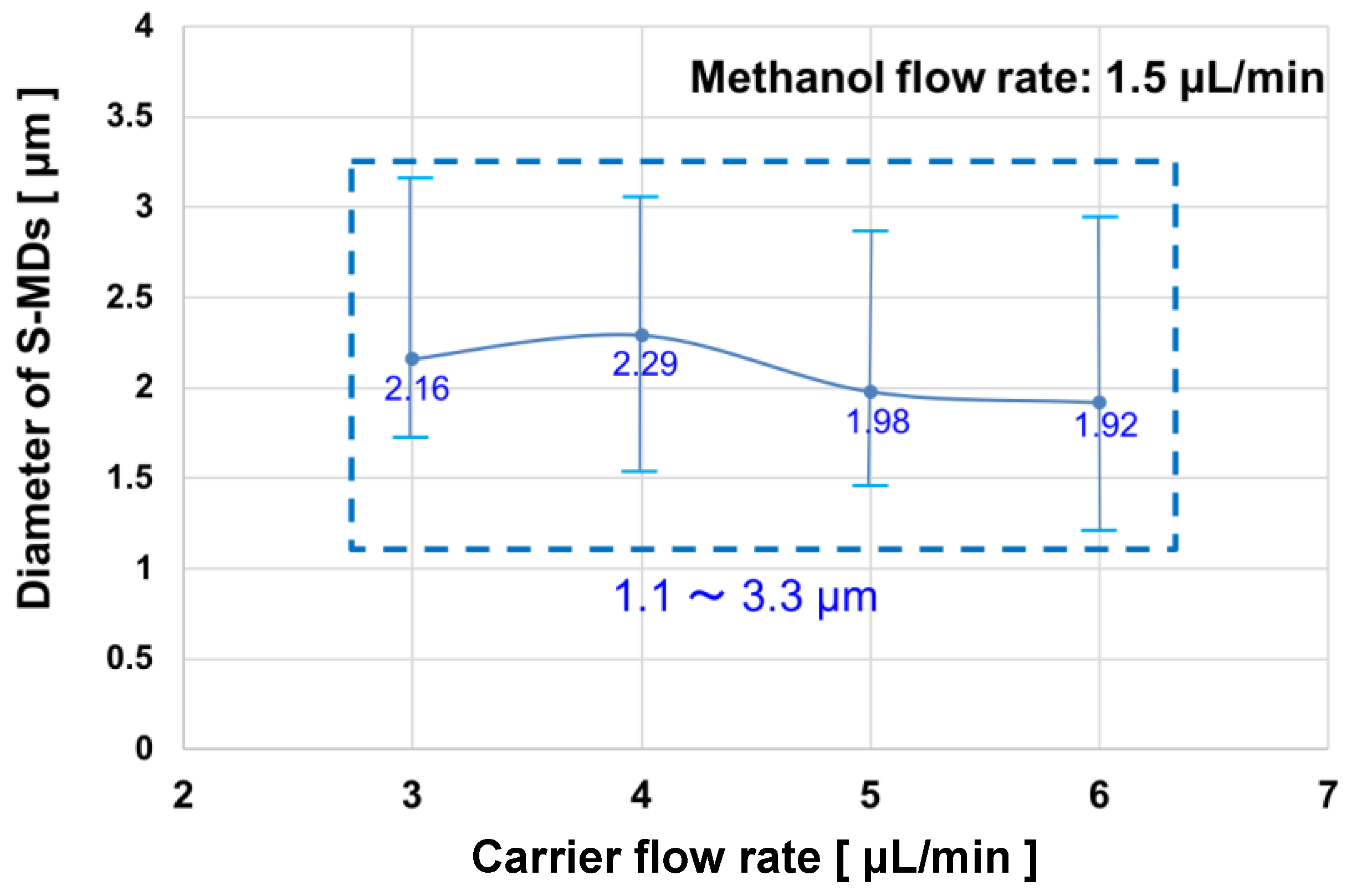
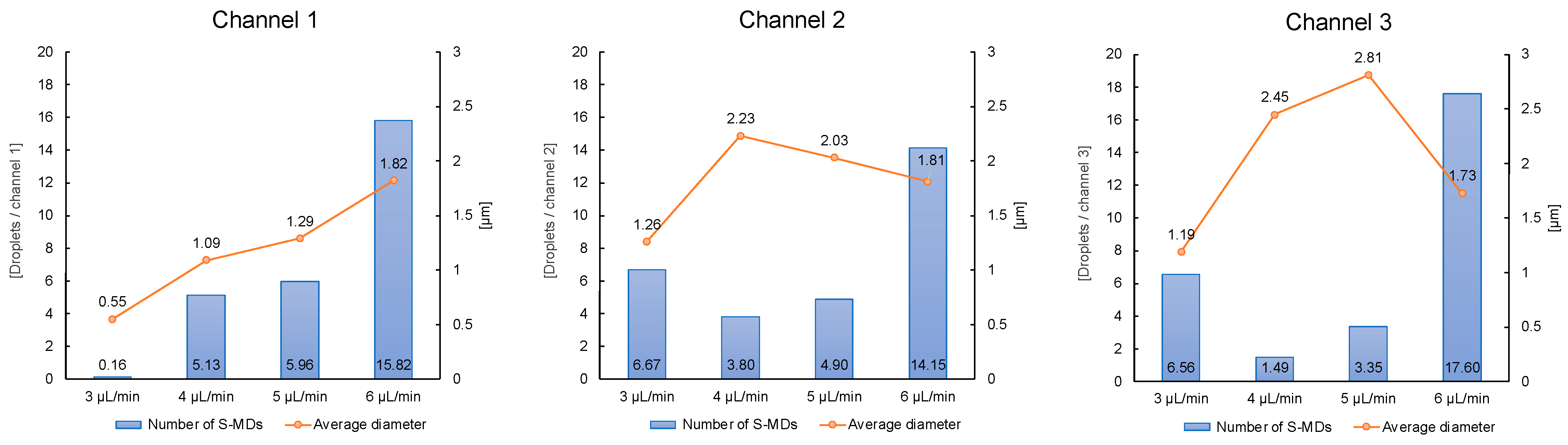
2.3. Relationship between Continuous-Phase Flow Rate and S-MD Diameter
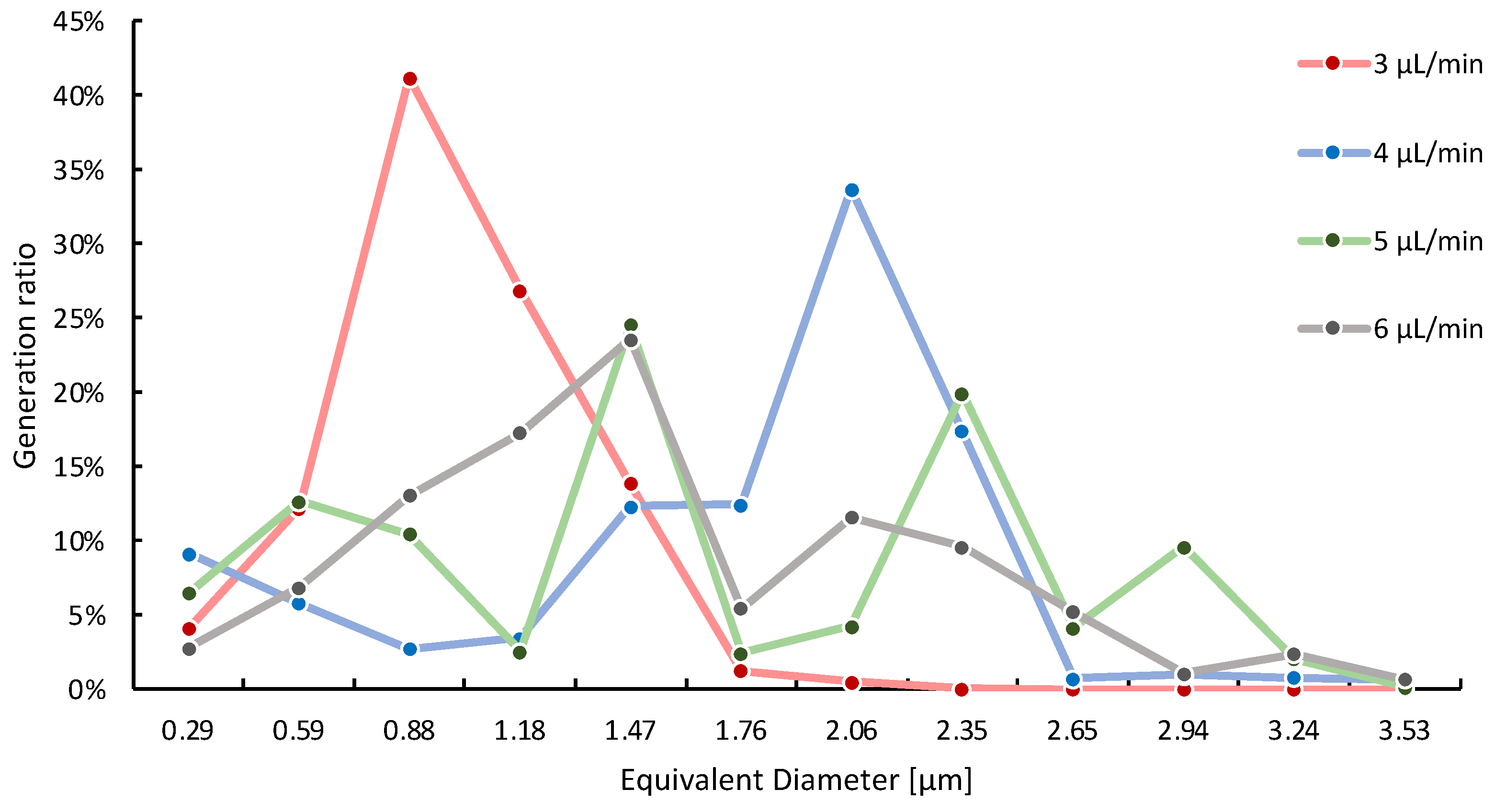
2.4. Drop Diameter Distribution Based on Image Analysis
3. Experimental Section
3.1. Fluid Simulation
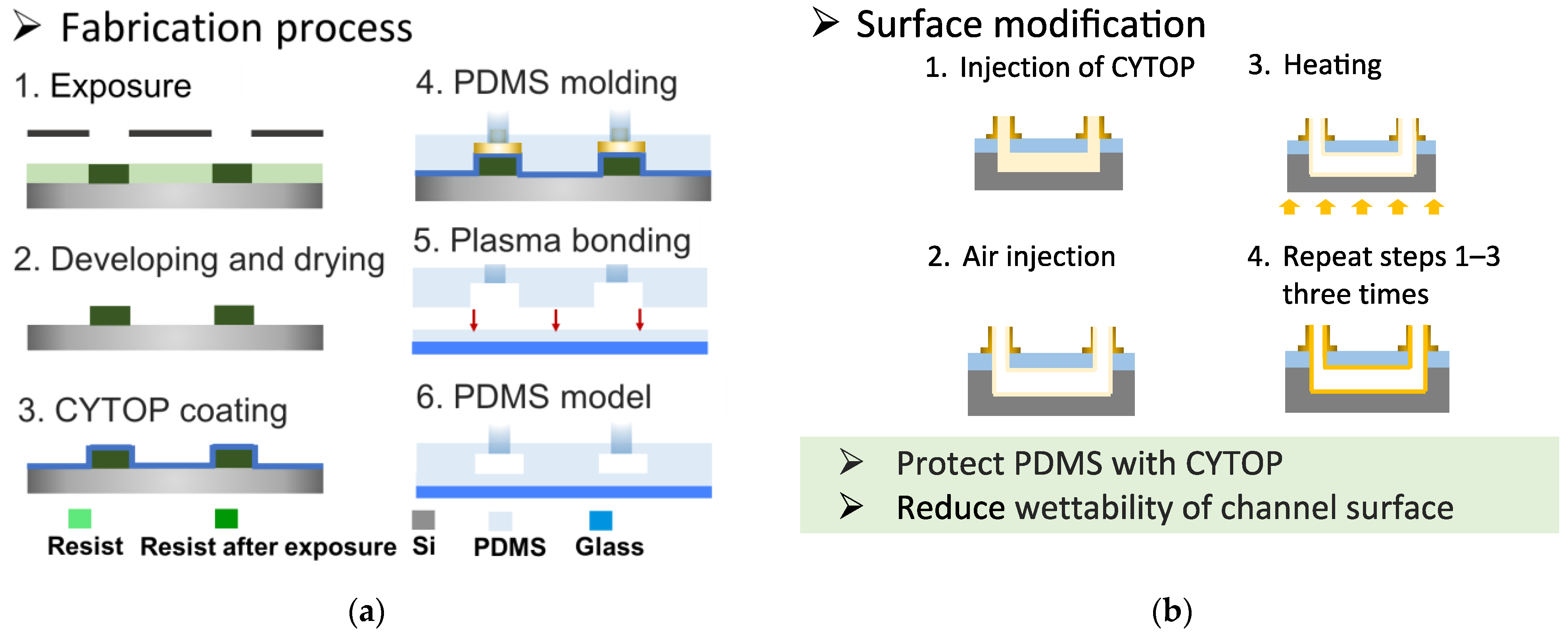
3.2. Device Design and Fabrication
3.3. Fluid Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skeggs, L.T. An automatic method for colorimetric analysis. Am. J. Clin. Pathol. 1957, 27, 311–322. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- Javier, A.; David, J.B. Controlled microfluidic interfaces. Nature 2005, 437, 648–655. [Google Scholar]
- Anton, A.D.; Sandra, M.T. Principles of microfluidic actuation by modulation of surface stresses. Annu. Rev. Fluid Mech. 2005, 37, 425–455. [Google Scholar]
- Leslie, Y.Y.; Hsueh, C.C.; Peggy, P.Y.C.; James, R.F. Microfluidic Devices for Bioapplications. Small 2011, 7, 12–48. [Google Scholar]
- Alireza, Z.; Arezoo, K.; Arthur, J.H.; Pouya, R. Open access tool and microfluidic devices for phenotypic quantification of heart function of intact fruit fly and zebrafish larvae. Comput. Biol. Med. 2021, 132, 104314. [Google Scholar]
- Cai, J.; Jiang, J.; Jiang, J.; Tao, Y.; Gao, X.; Ding, M.; Fan, Y. Fabrication of Transparent and Flexible Digital Microfluidics Devices. Micromachines 2022, 13, 498. [Google Scholar] [CrossRef]
- Thorsen, T.; Roberts, R.W.; Arnold, F.H.; Quake, S.R. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett. 2001, 86, 4163–4166. [Google Scholar] [CrossRef]
- Wyatt, S.; Catherine, D.; Gabriel, P.L. Microfluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Won, D.J.; Lee, J.; Kim, J. On-demand, parallel droplet merging method with non-contact droplet pairing in droplet-based microfluidics. Microfluid. Nanofluid. 2016, 20, 1. [Google Scholar] [CrossRef]
- Link, D.R.; Anna, S.L.; Weitz, D.A.; Stone, H.A. Geometrically mediated breakup of drops in microfluidic devices. Phys. Rev. Lett. 2004, 92, 054503. [Google Scholar] [CrossRef]
- Albert, L.; Rohit, K.; Arun, M.; Jamie, H.D. Mixing Crowded Biological Solutions in Milliseconds. Anal. Chem. 2005, 77, 7618–7625. [Google Scholar]
- Ansgar, H.; Dan, B.; Graeme, W.; Min, Y.; Andrew, J.M.; Chris, A.; Florian, H. Static microdroplet arrays: A microfluidic device for droplet trapping, incubation and release for enzymatic and cell-based assays. Lab. Chip. 2009, 9, 692–698. [Google Scholar]
- Kinga, M.; Francesca, R.; Wilhelm, T.S.H. Single-Cell Analysis Using Droplet Microfluidics. Adv. Biosys. 2020, 4, 1900188. [Google Scholar]
- Liu, D.; Sun, M.; Zhang, J.; Hu, R.; Fu, W.; Xuanyuan, T.; Liu, W. Single-cell droplet microfluidics for biomedical applications. Analyst 2022, 147, 2294. [Google Scholar] [CrossRef]
- Chen, Z.; Kheiri, S.; Young, E.W.; Kumacheva, E. Trends in Droplet Microfluidics: From Droplet Generation to Biomedical Applications. Langmuir 2022, 38, 6233–6248. [Google Scholar] [CrossRef]
- Sébastien, S.; Gustave, R.; Shreyansh, J.; Gabriel, A.; Charles, N.B. Cell Culture in Microfluidic Droplets. Chem. Rev. 2022, 122, 7061–7096. [Google Scholar]
- Joachim, D.J.; Tomasz, S.K.; David, B.M.; Marcin, T.; Anna, L.E.; Timo, N.K.; Gianluca, A.; Charlotte, E.H.; Gregory, M.F.; Magdalena, Z.G.; et al. spinDrop: A droplet microfluidic platform to maximise single-cell sequencing information content. Nat. Commun. 2023, 14, 4788. [Google Scholar]
- Wang, J.; Cong, L.; Shi, W.; Xu, W.; Xu, S. Single-Cell Analysis and Classification according to Multiplexed Proteins via Microdroplet-Based Self-Driven Magnetic Surface- Enhanced Raman Spectroscopy Platforms Assisted with Machine Learning Algorithms. Anal. Chem. 2023, 95, 11019–11027. [Google Scholar]
- Chen, C.; Zhao, Y.; Wang, J.; Zhu, P.; Tian, Y.; Xu, M.; Wang, L.; Huang, X. Passive Mixing inside Microdroplets. Micromachines 2018, 9, 160. [Google Scholar] [CrossRef]
- Sylvain, U.; Michel, M.; Alain, W. Copper-free click chemistry for microdroplet’s W/O interface engineering. RSC Adv. 2016, 6, 94942. [Google Scholar]
- Evgenia, Y.B.; Frantisek, F. Droplet microfluidics in (bio)chemical analysis. Analyst 2015, 140, 22–38. [Google Scholar]
- Ashleigh, B.T.; Graeme, W.; Max, F.; Luis, M.F.; Robert, C.R.W.; Wilhelm, T.S.H. Suzuki–Miyaura coupling reactions in aqueous microdroplets with catalytically active fluorous interfaces. Chem. Commun. 2009, 41, 6225–6227. [Google Scholar]
- Meier, T.A.; Beulig, R.J.; Klinge, E.; Fuss, M.; Ohla, S.; Belder, D. On-chip monitoring of chemical syntheses in microdroplets via surface-enhanced Raman spectroscopy. Chem. Commun. 2015, 51, 8588. [Google Scholar] [CrossRef]
- Qim, Q.X.; Zhuo, C.; Shao, W.L.; Yun, D.W.; Jian, H.X. Micro-PIV measurement and CFD simulation of flow field and swirling strength during droplet formation process in a coaxial microchannel. Chem. Eng. Sci. 2018, 185, 157–167. [Google Scholar]
- Fu, Y.; Bai, L.; Zhao, S.; Zhang, X.; Jin, Y.; Cheng, Y. Simulation of reactive mixing behaviors inside micro-droplets by a lattice Boltzmann method. Chem. Eng. Sci. 2018, 181, 79–89. [Google Scholar] [CrossRef]
- Indrajit, C.; Joshua, R.; Pavel, Y.; Patrick, T.; Alexander, L. Droplet generation at Hele-Shaw microfluidic T-junction. Phys. Fluids 2019, 31, 022010. [Google Scholar]
- Rahimi, M.; Khorrami, A.S.; Rezai, P. Effect of device geometry on droplet size in co-axial flow-focusing microfluidic droplet generation devices. Colloids Surf. A 2019, 570, 510–517. [Google Scholar] [CrossRef]
- Shirin, M.; Javad, K.S.; Mohammad, A.M.; Younes, A. Liquid-liquid extraction of calcium using ionic liquids in spiral microfluidics. Chem. Eng. J. 2019, 356, 492–505. [Google Scholar]
- Daiki, T.; Satsuki, K.; Seito, S.; Dong, H.Y.; Masahiro, F.; Yoshito, N.; Hiroyuki, F.; Tetsushi, S.; Shuichi, S. Efficient Generation of Microdroplets Using Tail Breakup Induced with Multi-Branch Channels. Molecules 2021, 26, 3707. [Google Scholar] [CrossRef]
- Babak, S.; Chil, H.C.; Wu, J. Effects of Surfactants on the Rate of Chemical Reactions. J. Chem. 2014, 908476, 14. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, D.; Zheng, S.; Furuya, M.; Kobayashi, M.; Fujita, H.; Akitsu, T.; Sekiguchi, T.; Shoji, S. Efficient Separation of Methanol Single-Micron Droplets by Tailing Phenomenon Using a PDMS Microfluidic Device. Molecules 2024, 29, 1949. https://doi.org/10.3390/molecules29091949
Tanaka D, Zheng S, Furuya M, Kobayashi M, Fujita H, Akitsu T, Sekiguchi T, Shoji S. Efficient Separation of Methanol Single-Micron Droplets by Tailing Phenomenon Using a PDMS Microfluidic Device. Molecules. 2024; 29(9):1949. https://doi.org/10.3390/molecules29091949
Chicago/Turabian StyleTanaka, Daiki, Shengqi Zheng, Masahiro Furuya, Masashi Kobayashi, Hiroyuki Fujita, Takashiro Akitsu, Tetsushi Sekiguchi, and Shuichi Shoji. 2024. "Efficient Separation of Methanol Single-Micron Droplets by Tailing Phenomenon Using a PDMS Microfluidic Device" Molecules 29, no. 9: 1949. https://doi.org/10.3390/molecules29091949
APA StyleTanaka, D., Zheng, S., Furuya, M., Kobayashi, M., Fujita, H., Akitsu, T., Sekiguchi, T., & Shoji, S. (2024). Efficient Separation of Methanol Single-Micron Droplets by Tailing Phenomenon Using a PDMS Microfluidic Device. Molecules, 29(9), 1949. https://doi.org/10.3390/molecules29091949






