A Review of Ultrasonic Treatment in Mineral Flotation: Mechanism and Recent Development
Abstract
1. Introduction
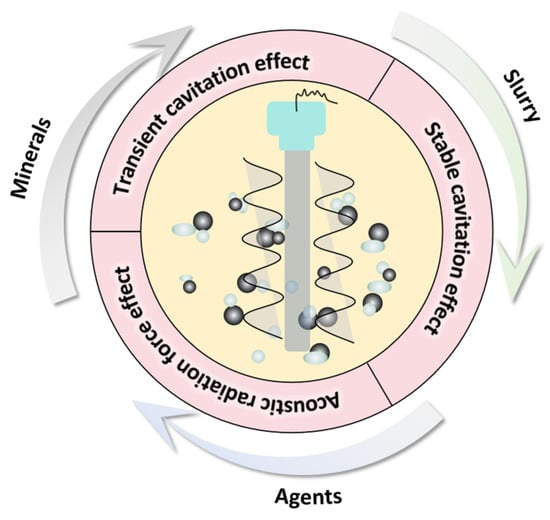
2. Ultrasonic Mechanism
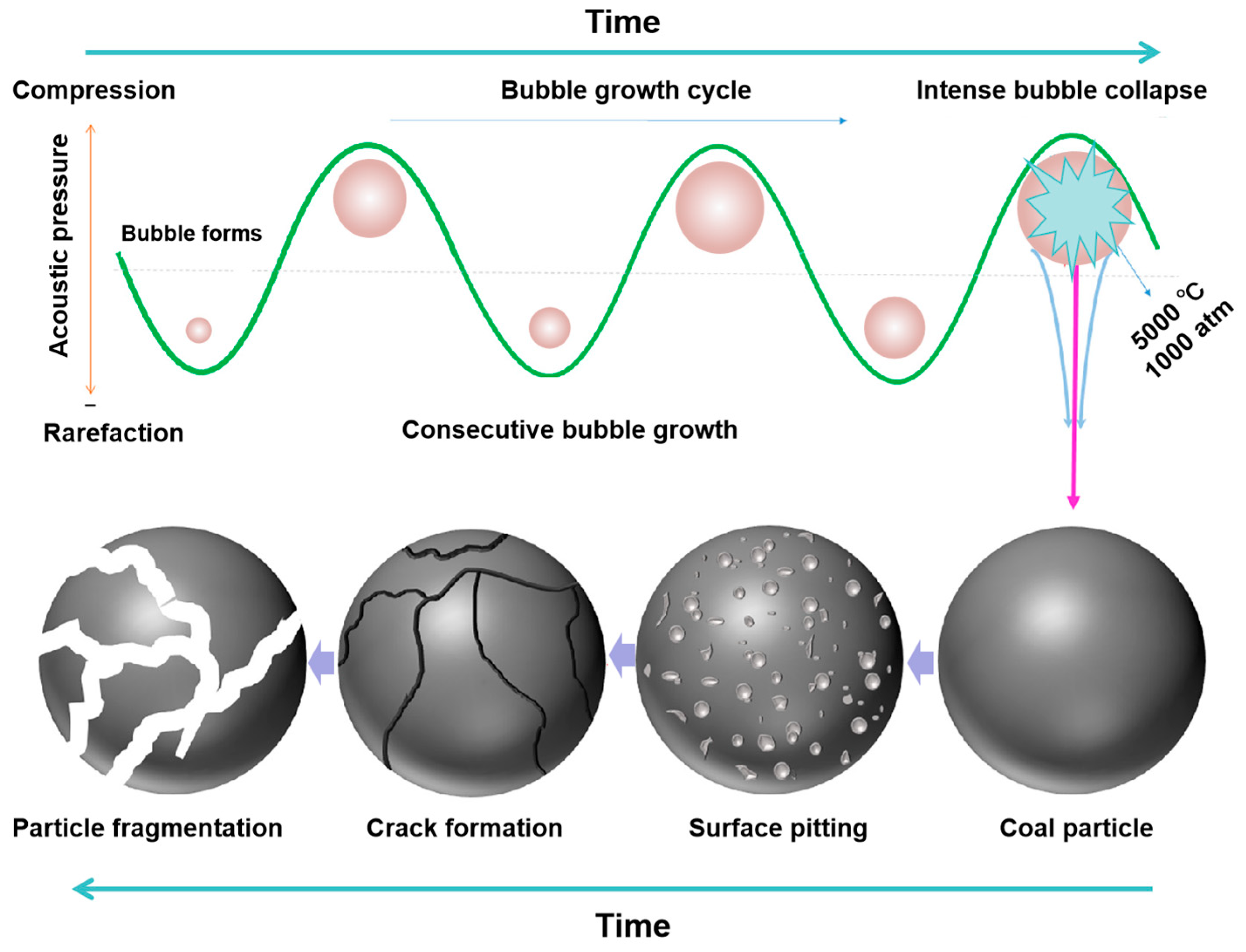
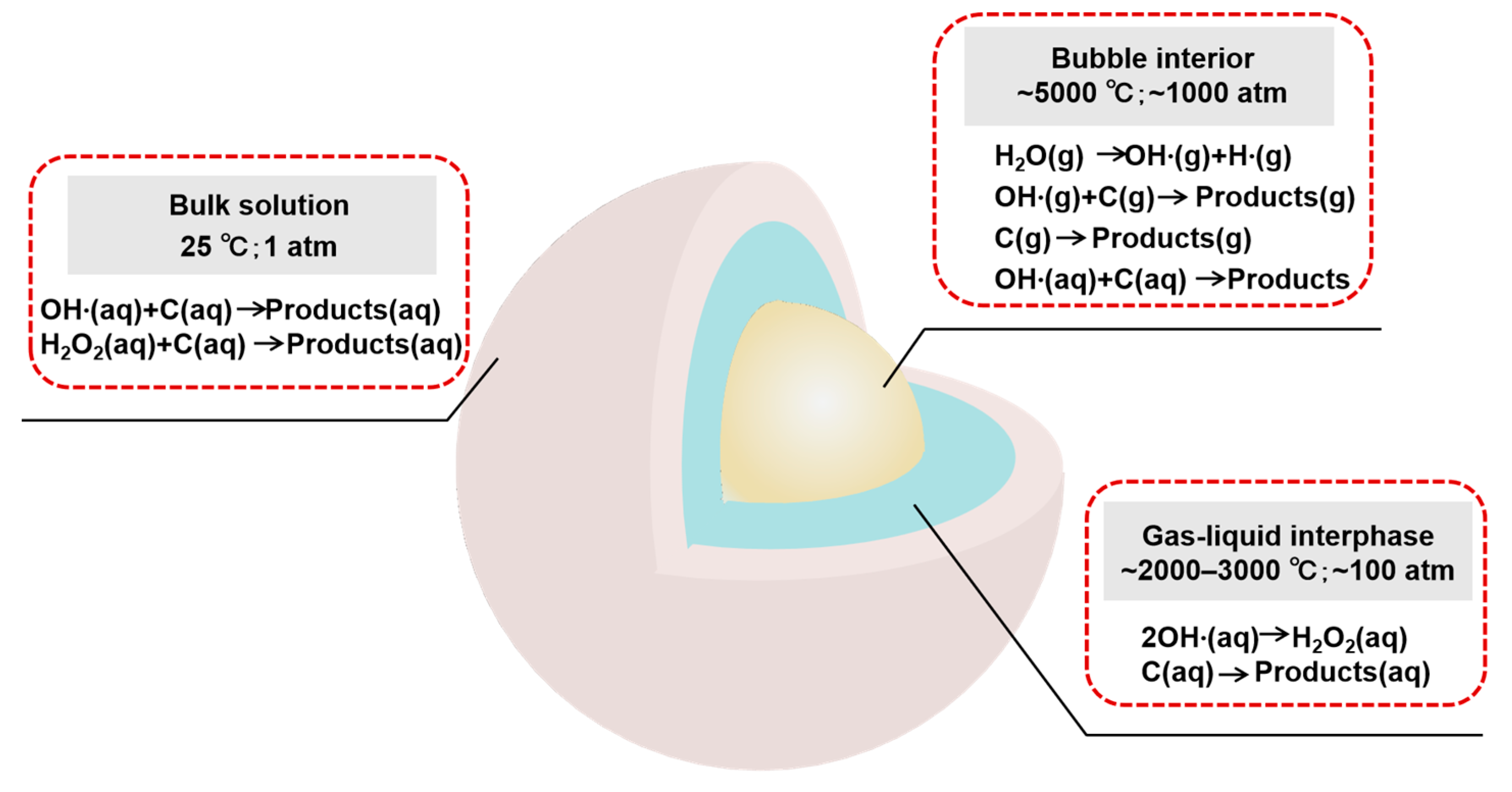
2.1. Ultrasonic Cavitation Effect
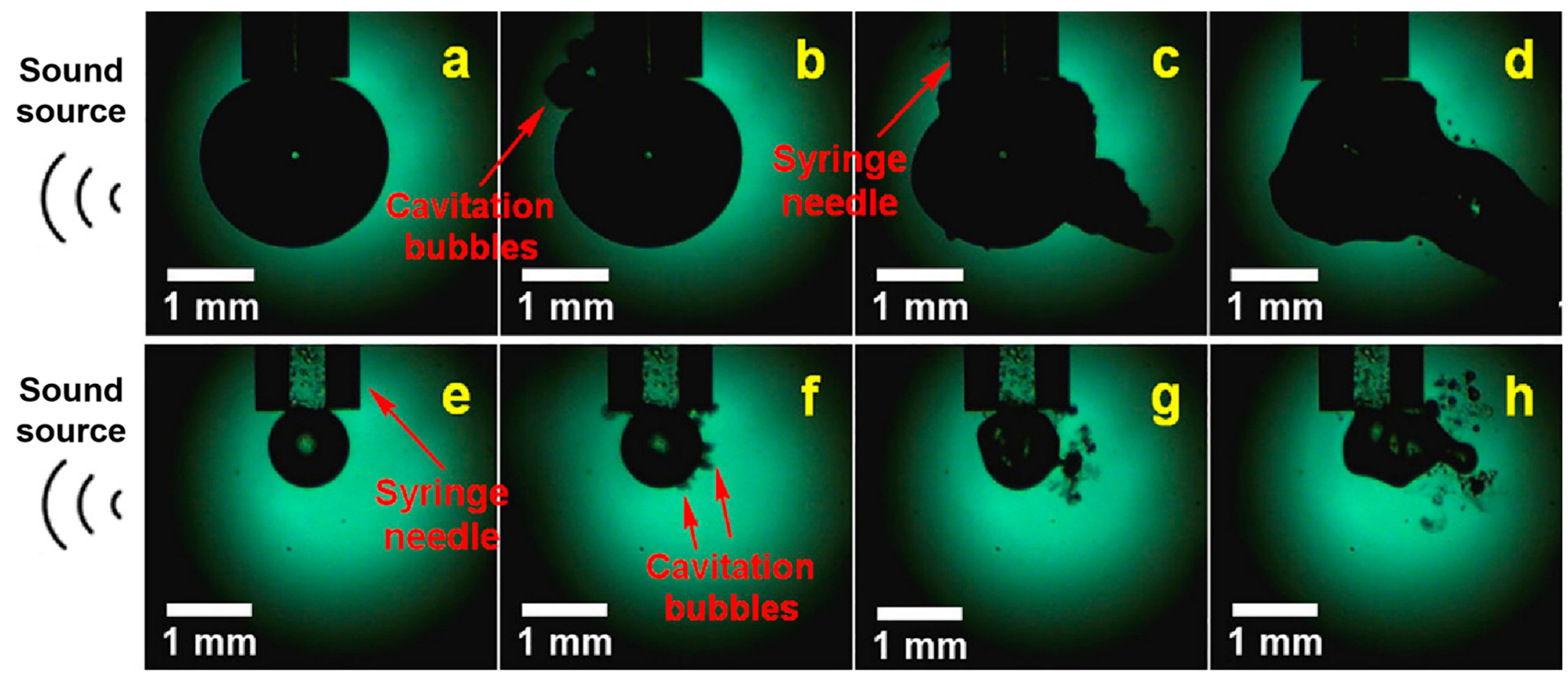
2.1.1. Transient Cavitation Effect
2.1.2. Stable Cavitation Effect
2.2. Acoustic Radiation Force Effect
3. Effect of Main Ultrasonic Parameters on Mineral Flotation
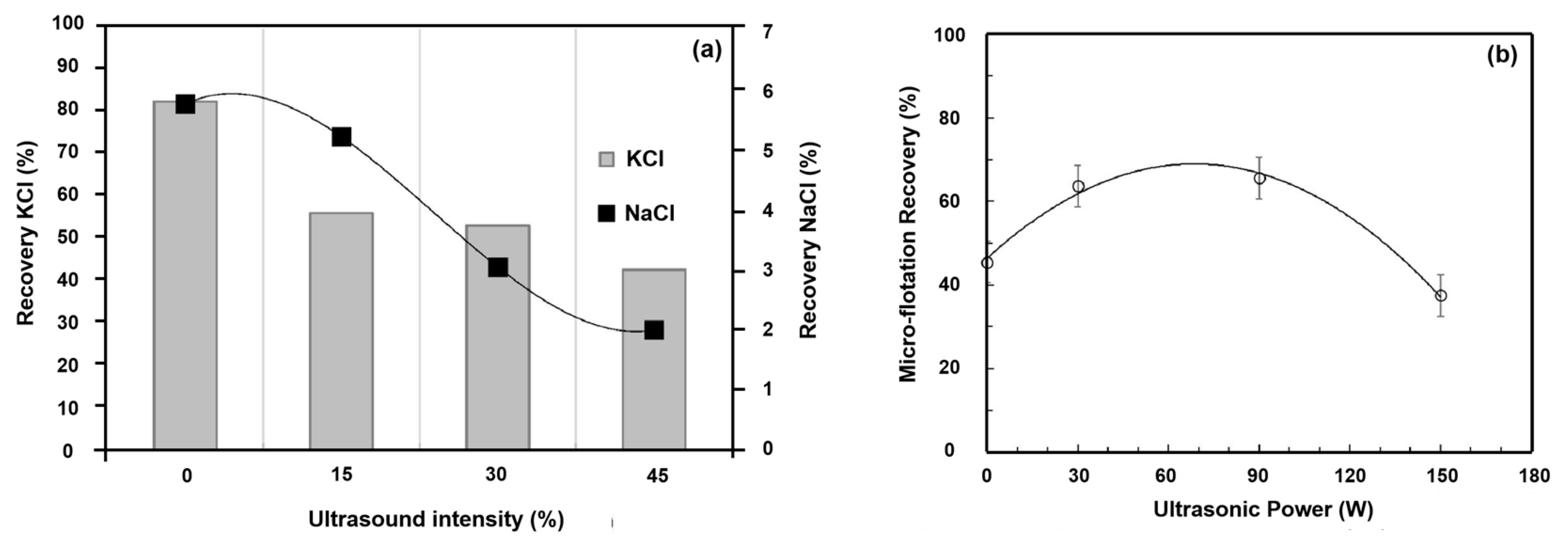
3.1. Ultrasonic Power
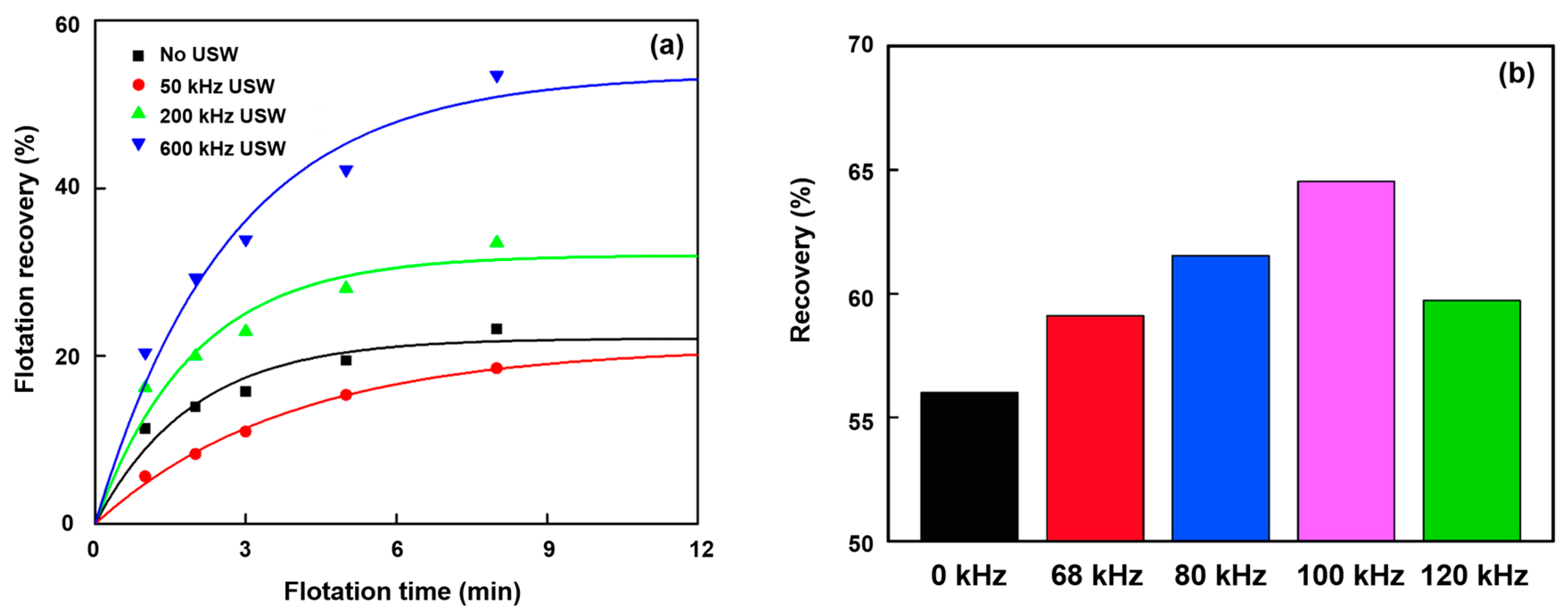
3.2. Ultrasonic Frequency
4. Effect of Ultrasonic Treatment on Mineral Flotation
4.1. Effect of Ultrasonic Treatment on Minerals
4.1.1. Cleaning Effect
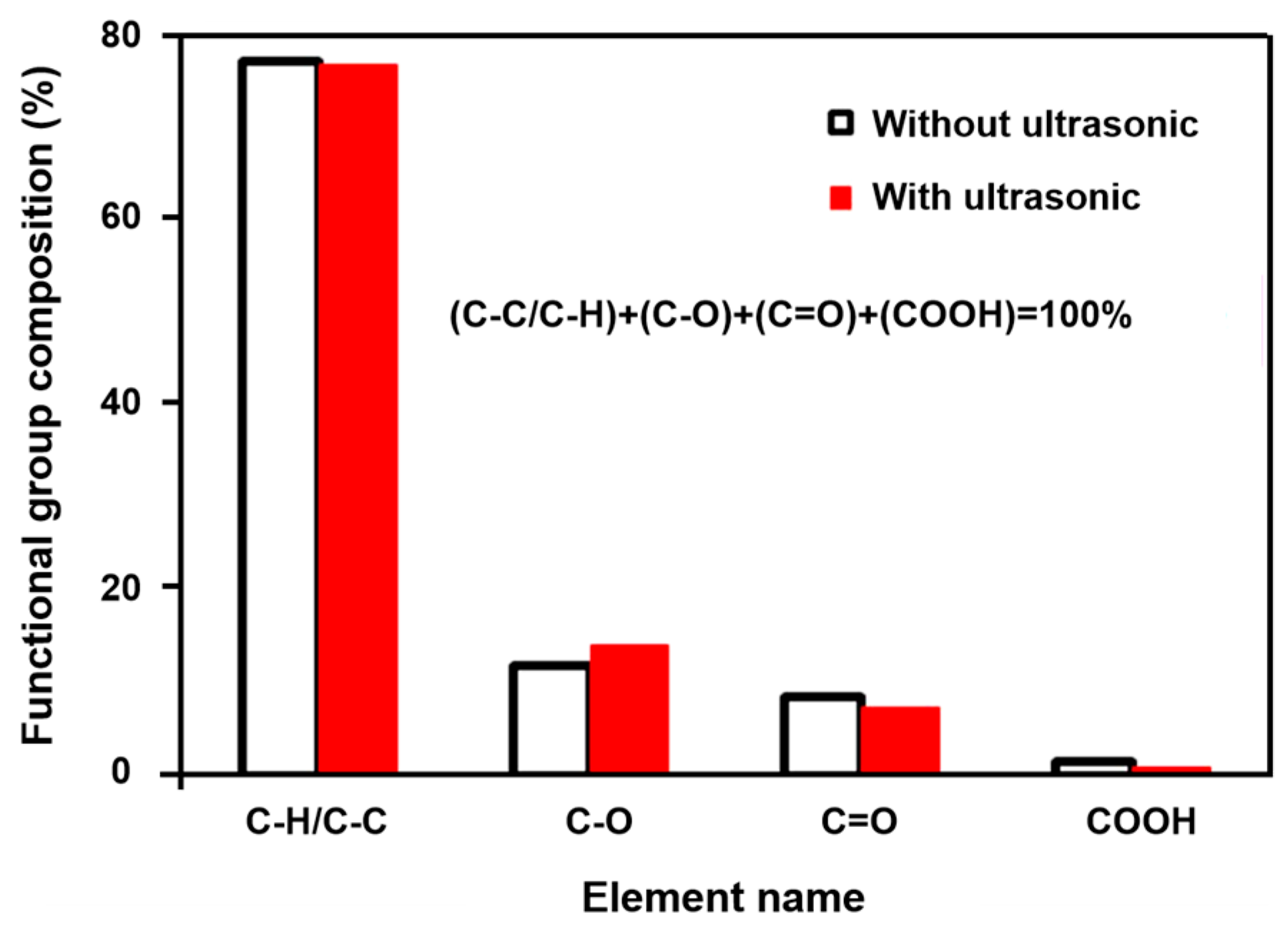
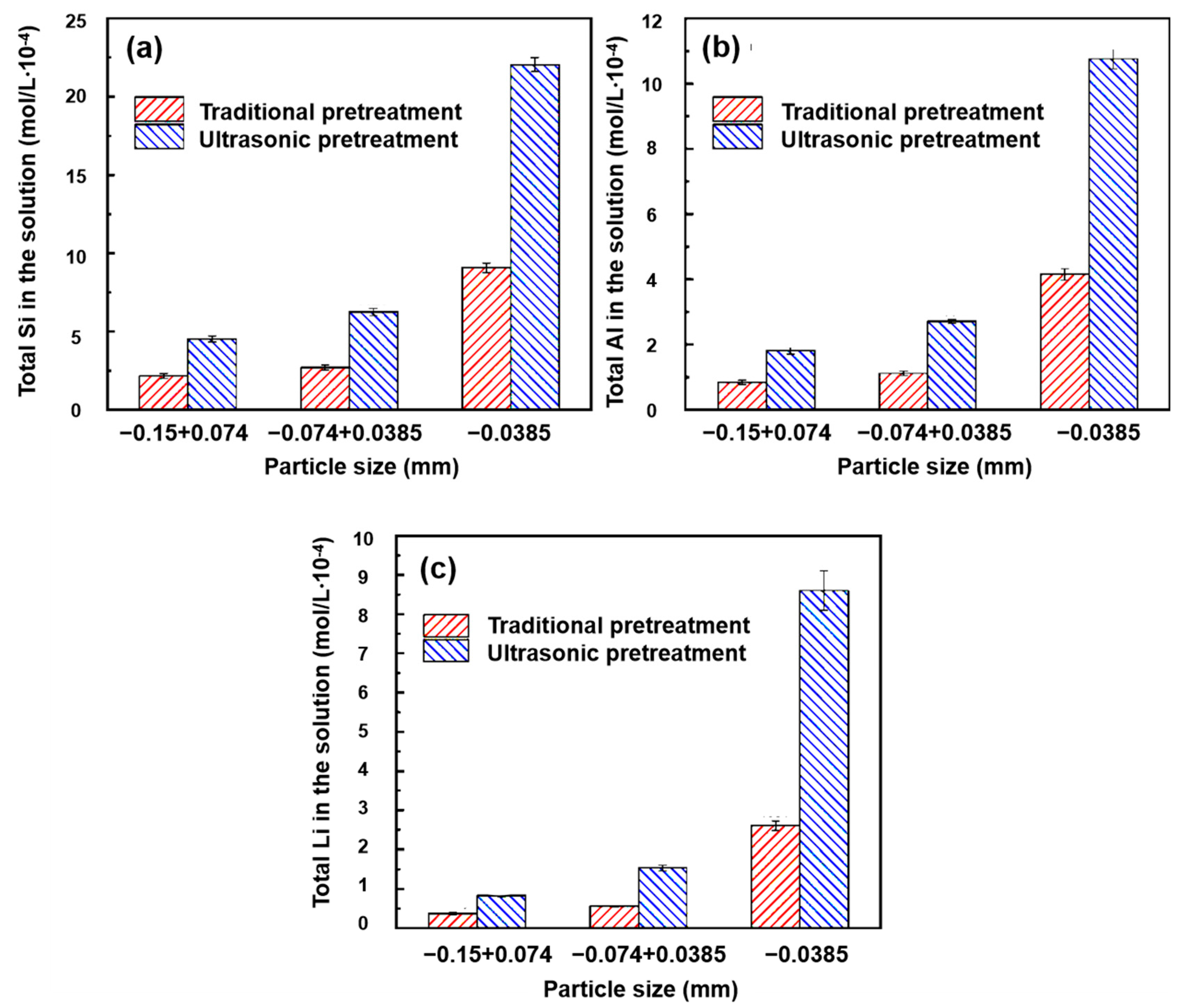
4.1.2. Ultrasonic Corrosion
4.1.3. Desulfuration
4.2. Effect of Ultrasonic Treatment on Flotation Agents
4.2.1. Dispersion and Emulsification
4.2.2. Change in Properties and Microstructure of Pharmaceutical Solution
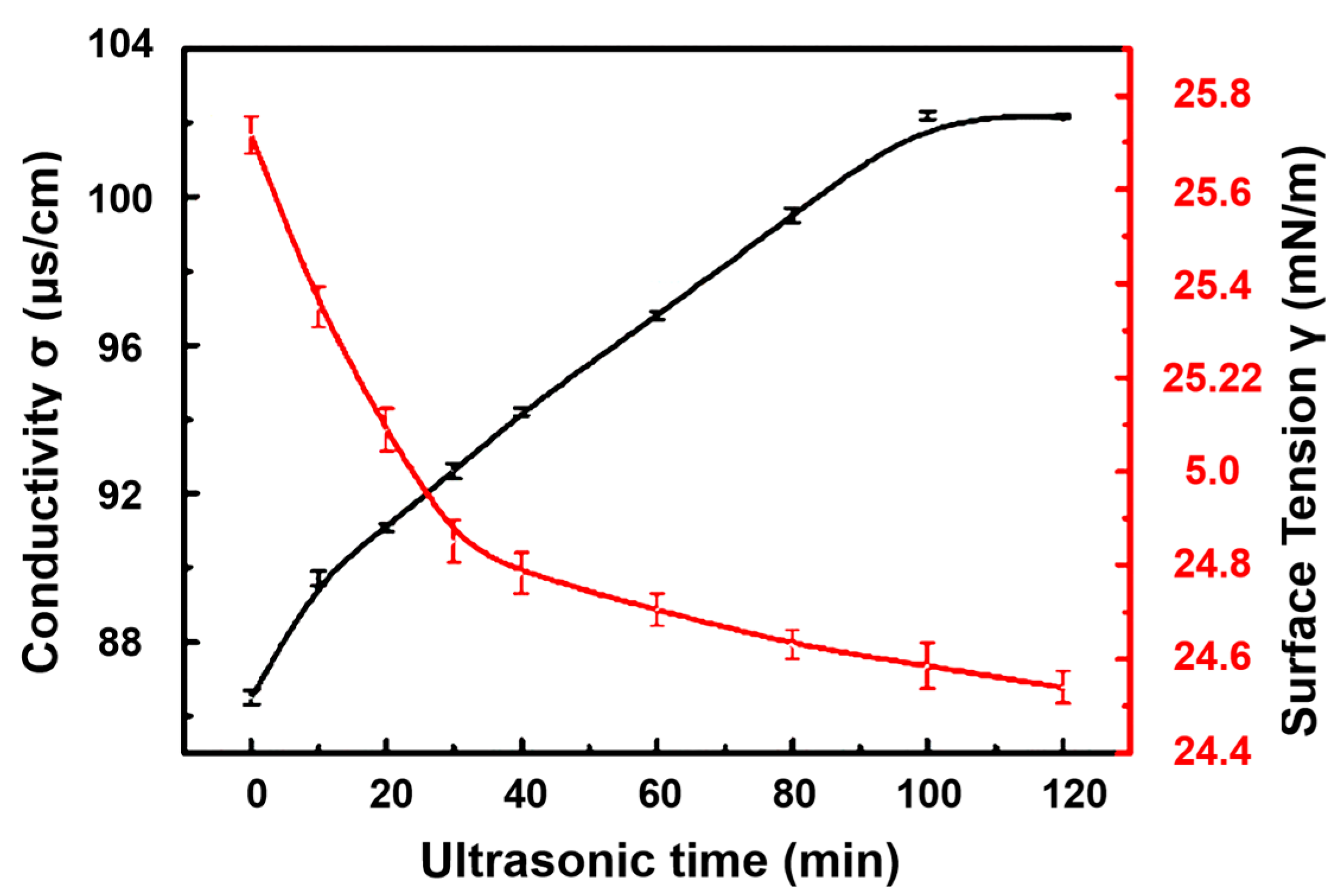
Surface Tension
Conductivity
pH
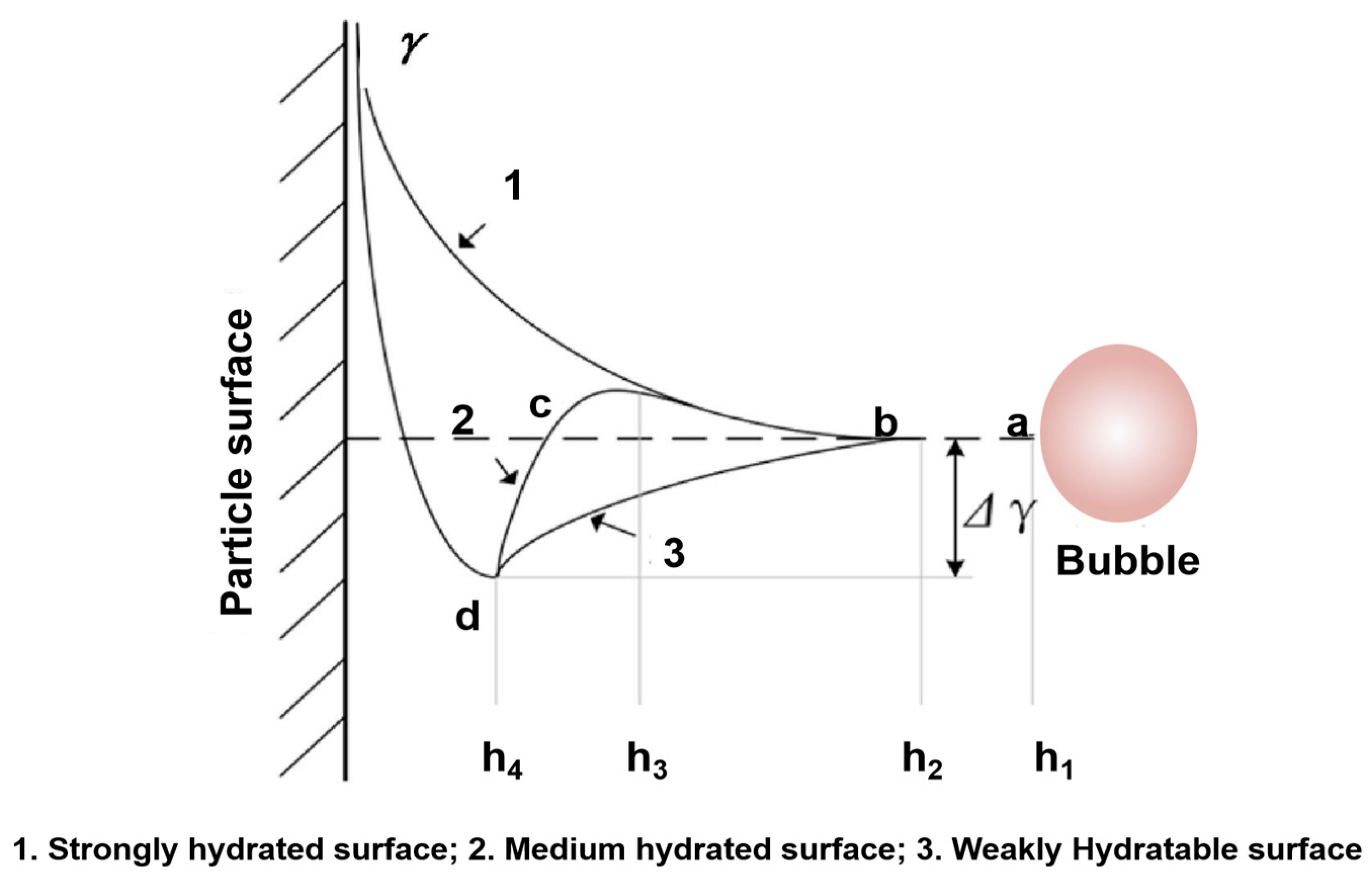
4.3. Effect of Ultrasonic Treatment on Slurry
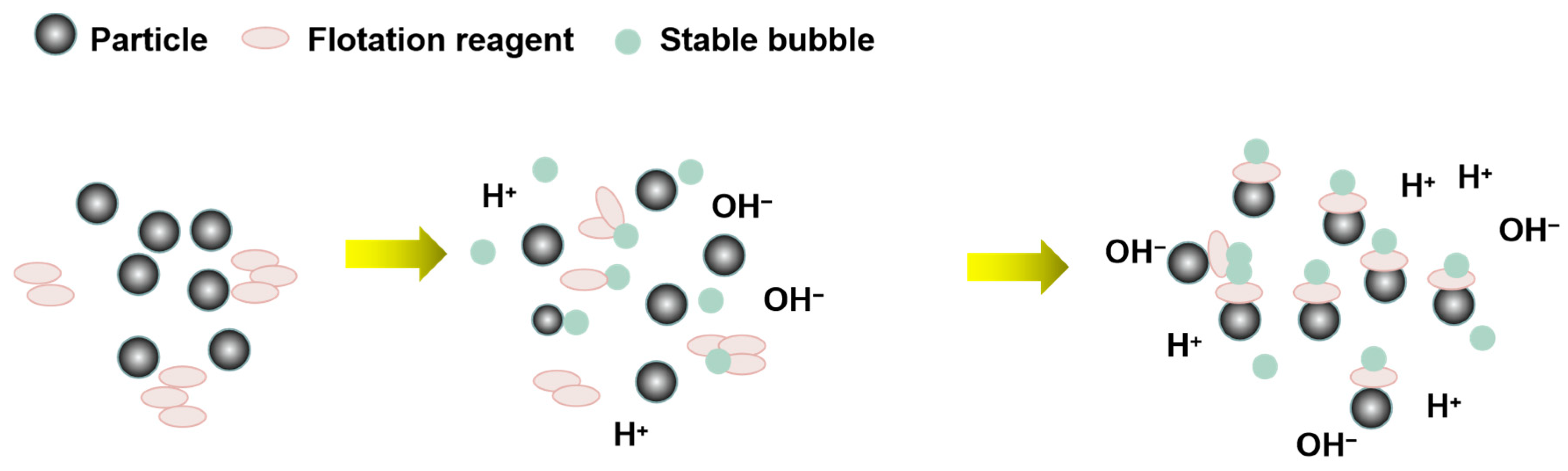
4.3.1. Formation of Microbubbles
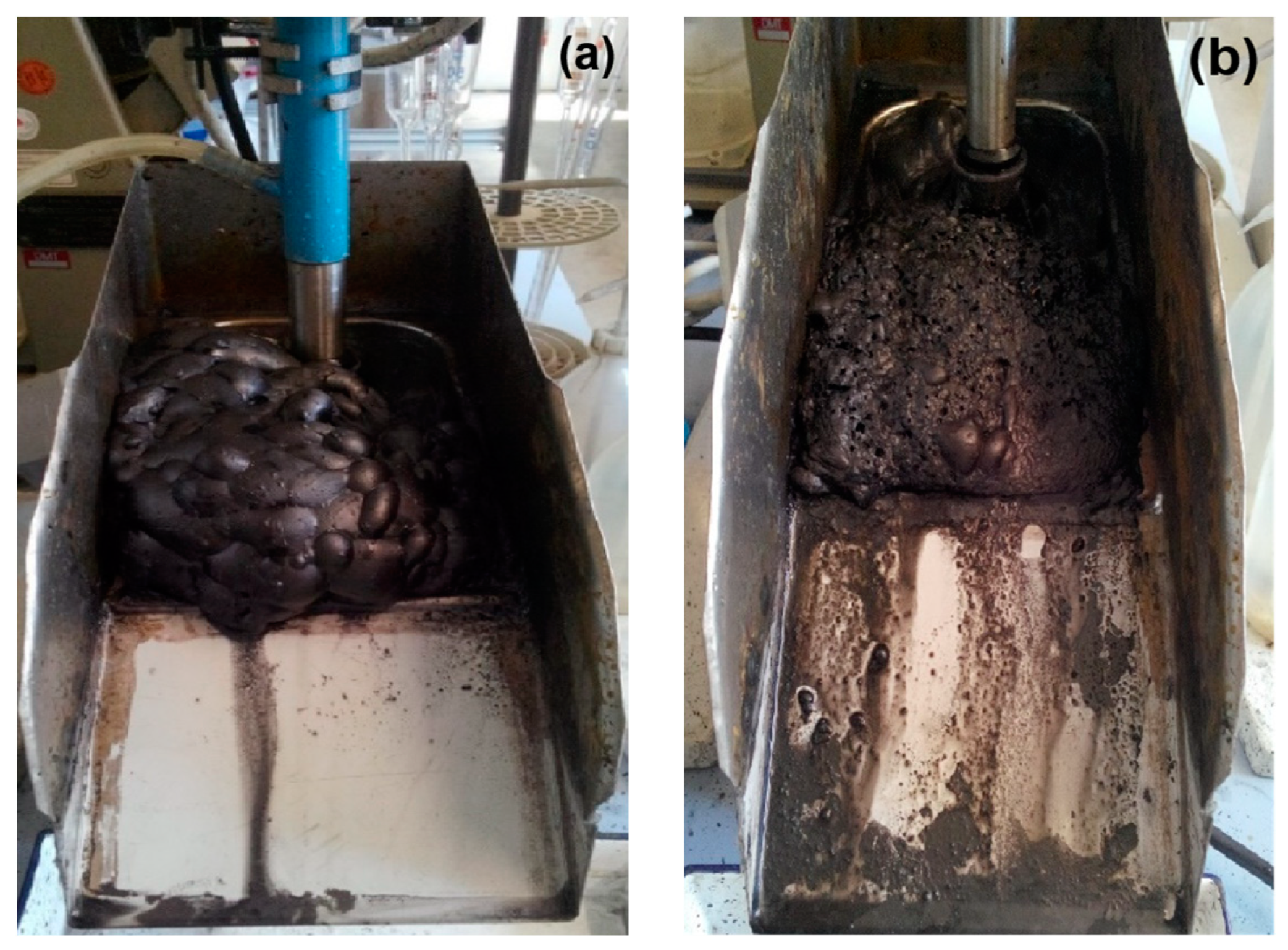
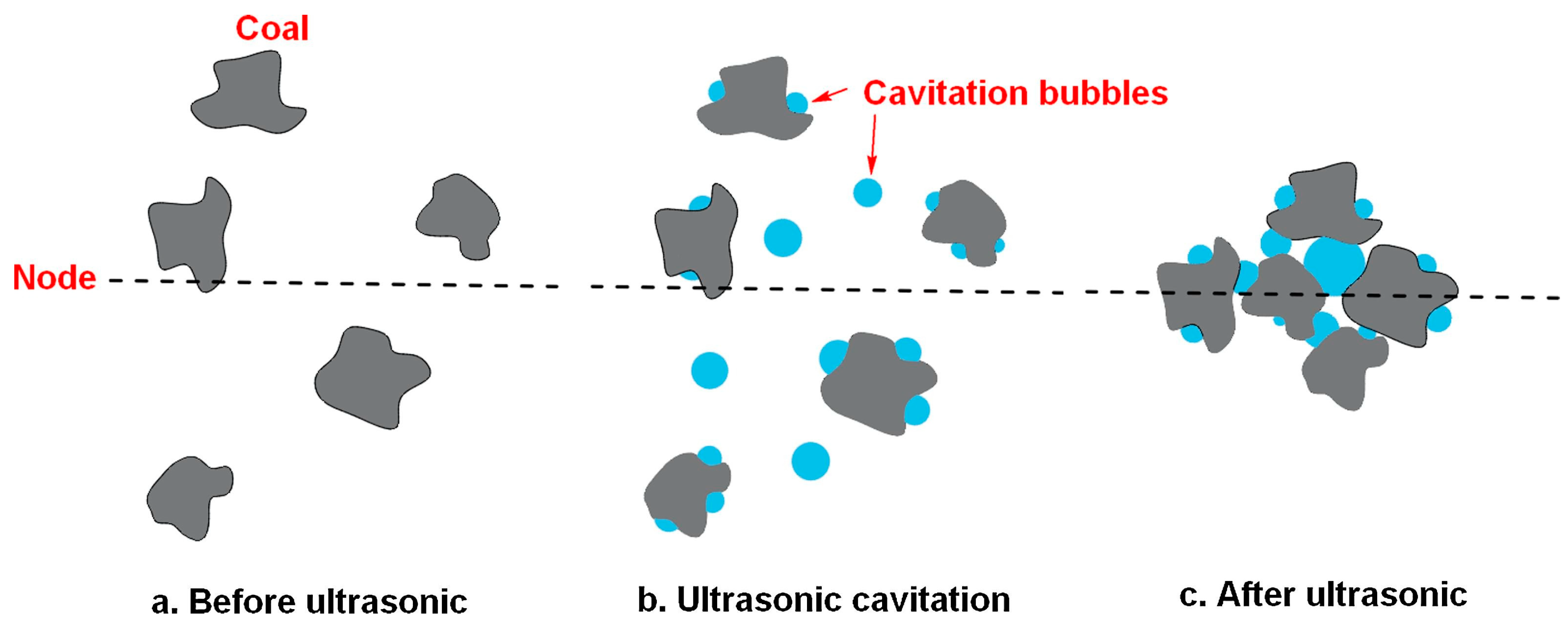
4.3.2. Coalescence
5. Conclusions and Outlook
- (1)
- The different effects of ultrasonic treatment on flotation are realized by precisely controlling the parameter conditions. Different parameter conditions may have different or even opposite effects on the flotation system, such as the dispersion or aggregation of mineral or bubbles. The processing of minerals under different conditions requires different effects of ultrasonic treatment. For example, in fine flotation, it is hoped that particles will be more easily adhered to the flotation bubble through agglomeration, so the conditions required in ultrasonic treatment to lead mineral particles to coalescence should be controlled well. Furthermore, it is hoped that bubbles’ agglomeration will be decreased and the collision probability between mineral particles and bubbles will be increased, so, here, the conditions for the formation of microbubbles by ultrasonic treatment should be controlled. However, at present, there is still a gap in the research into the effect of ultrasonic treatment on mineral flotation performance, which we propose should be a focus of future research.
- (2)
- Until now, the applied research on ultrasonic treatment in mineral flotation has mostly been carried out on a laboratory scale, which may provide a theoretical basis for the future industrial applications of ultrasonic treatment. However, when ultrasound is applied on a commercial scale, this will involve very large scale-up ratios and a high degree of uncertainty. In order to effectively utilize this technology and start ultrasonic-based coal beneficiation treatment in practical operations, large-scale pilot studies are very important, which will help to determine amplification parameters and develop suitable ultrasonic-based equipment or reactors. In addition, the high energy loss and safety problems of ultrasonic treatment should be considered and resolved before industrial applications.
- (3)
- Consideration is required of how industrial ultrasonic equipment typically operates at high powers, leading to intense corrosion caused by cavitation. This corrosion cannot be sustained by pipelines and building materials over an extended period, resulting in escalating costs. Furthermore, the operation of industrial ultrasonic equipment generates substantial noise and strong sound waves, which are detrimental to employee well-being and production safety.
- (4)
- Appropriate pretreatment can eliminate the oxide layer on particle surfaces, enhance agent adsorption onto hydrophobic surfaces, and achieve surface hydrophobic modification of fine oxidized coal. The selection of pretreatment methods should consider factors such as the coal slime oxidation degree, feasibility at scale, and power consumption. Additionally, if ultrasonic or other pretreatment methods are combined organically, the flotation performance of fine oxidized coal can be further improved.
- (5)
- Most studies have focused on coal grinding in specific regions, limiting the universality of the process. To enhance its applicability, it is recommended that coal grinding should be investigated across different regions. By examining the variations in coal grinding behavior during ultrasonic flotation, the universality of the process can be further improved.
Author Contributions
Funding
Conflicts of Interest
References
- Zhai, M.; Hu, R.; Wang, Y.; Jiang, S.; Wang, R.; Li, J.; Chen, H.; Yang, Z.; Lü, Q.; Qi, T. Mineral resource science in China: Review and perspective. Geogr. Sustain. 2021, 5, 107–114. [Google Scholar] [CrossRef]
- Zhai, M.G.; Wu, F.Y.; Hu, R.Z.; Jiang, S.Y.; Li, W.C.; Wang, R.C.; Wang, D.H.; Qi, T.; Qin, K.Z.; Wen, H.J. Critical metal mineral resources: Current research status and scientific issues. Bull. Natl. Nat. Sci. Found. China 2019, 33, 106–111. [Google Scholar]
- Han, J.; Chen, P.; Liu, T.; Li, Y. Research and application of fluidized flotation units: A review. J. Ind. Eng. Chem. 2023, 126, 50–68. [Google Scholar] [CrossRef]
- Birinci, M.; Ramazan, G. Characterization and flotation of low-grade boehmitic bauxite ore from Seydiehir (Konya, Turkey). Miner. Eng. 2021, 161, 106714. [Google Scholar] [CrossRef]
- Fang, C.; Yu, S.; Wei, X.; Peng, H.; Ou, L.; Zhang, G.; Wang, J. The cation effect on adsorption of surfactant in the froth flotation of low-grade diasporic bauxite. Min. Eng 2019, 144, 106051. [Google Scholar] [CrossRef]
- Kumar, H.; Luukkanen, S. Selective flotation of fine-grained pentlandite from low grade polymetallic ore. Miner. Eng. 2019, 130, 12–14. [Google Scholar] [CrossRef]
- Agheli, S.; Hassanzadeh, A.; Hassas, B.V.; Hasanzadeh, M. Effect of pyrite content of feed and configuration of locked particles on rougher flotation of copper in low and high pyritic ore types. Int. J. Min. Sci. Technol. 2018, 28, 167–176. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Safari, M.; Hoang, D.H.; Khoshdast, H.; Albijanic, B.; Kowalczuk, P.B. Technological assessments on recent developments in fine and coarse particle flotation systems. Min. Eng. 2022, 180, 107509. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, X.; Gui, X.; Cao, Y.; Xu, M. Effect of kaolinite and montmorillonite on fine coal flotation. Fuel 2017, 195, 284–289. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Sajjady, S.A.; Gholami, H.; Amini, S.; Özkan, S.G. An improvement on selective separation by applying ultrasound to rougher and re-cleaner stages of copper flotation. Minerals 2020, 10, 619. [Google Scholar] [CrossRef]
- Santo, C.E.; Vilar, V.J.P.; Botelho, C.M.S.; Bhatnagar, A.; Kumar, E.; Boaventura, R.A.R. Optimization of coagulation-flocculation and flotation parameters for the treatment of a petroleum refinery effluent from a Portuguese plant. Chem. Eng. J. 2012, 183, 117–123. [Google Scholar] [CrossRef]
- Tsatsis, D.E.; Papachristos, D.K.; Valta, K.A.; Vlyssides, A.G.; Economides, D.G. Enzymatic deinking for recycling of office waste paper. J. Environ. Chem. Eng. 2017, 5, 1744–1753. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Ivey, D.G.; Etsell, T.H. Bi-wetting property of oil sands fine solids determined by film flotation and water vapor adsorption. Fuel 2017, 197, 326–333. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies. A critical review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]
- Mathiazakan, P.; Shing, S.Y.; Ying, S.S.; Kek, H.K.; Tang, M.S.Y.; Show, P.L.; Ooi, C.W.; Ling, T.C. Pilot-scale aqueous two-phase floatation for direct recovery of lipase derived from Burkholderia cepacia strain ST8. Sep. Purif. Technol. 2016, 171, 206–213. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Tahtamouni, T.M.A.; Huong, P.T.; Thang, P.Q. Selective flotation separation of ABS/PC from ESR plastic wastes mixtures assisted by ultrasonic catalyst/H2O2. J. Environ. Chem. Eng. 2019, 7, 103354. [Google Scholar] [CrossRef]
- Irannajad, M.; Salmani Nuri, O.; Mehdilo, A. Surface dissolution-assisted mineral flotation: A review. J. Environ. Chem. Eng. 2019, 7, 103050. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, D.; Sun, W.; Cao, X.; Hu, Y. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, W.; Guo, Y.; Wang, T.; Luo, G.; Wang, H.; He, G. The flotation separation of galena and pyrite using serpentine as depressant. Powder Technol. 2019, 342, 486–490. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y. The effect of saline water on mineral flotation-A critical review. Minerals 2014, 66–68, 13–24. [Google Scholar] [CrossRef]
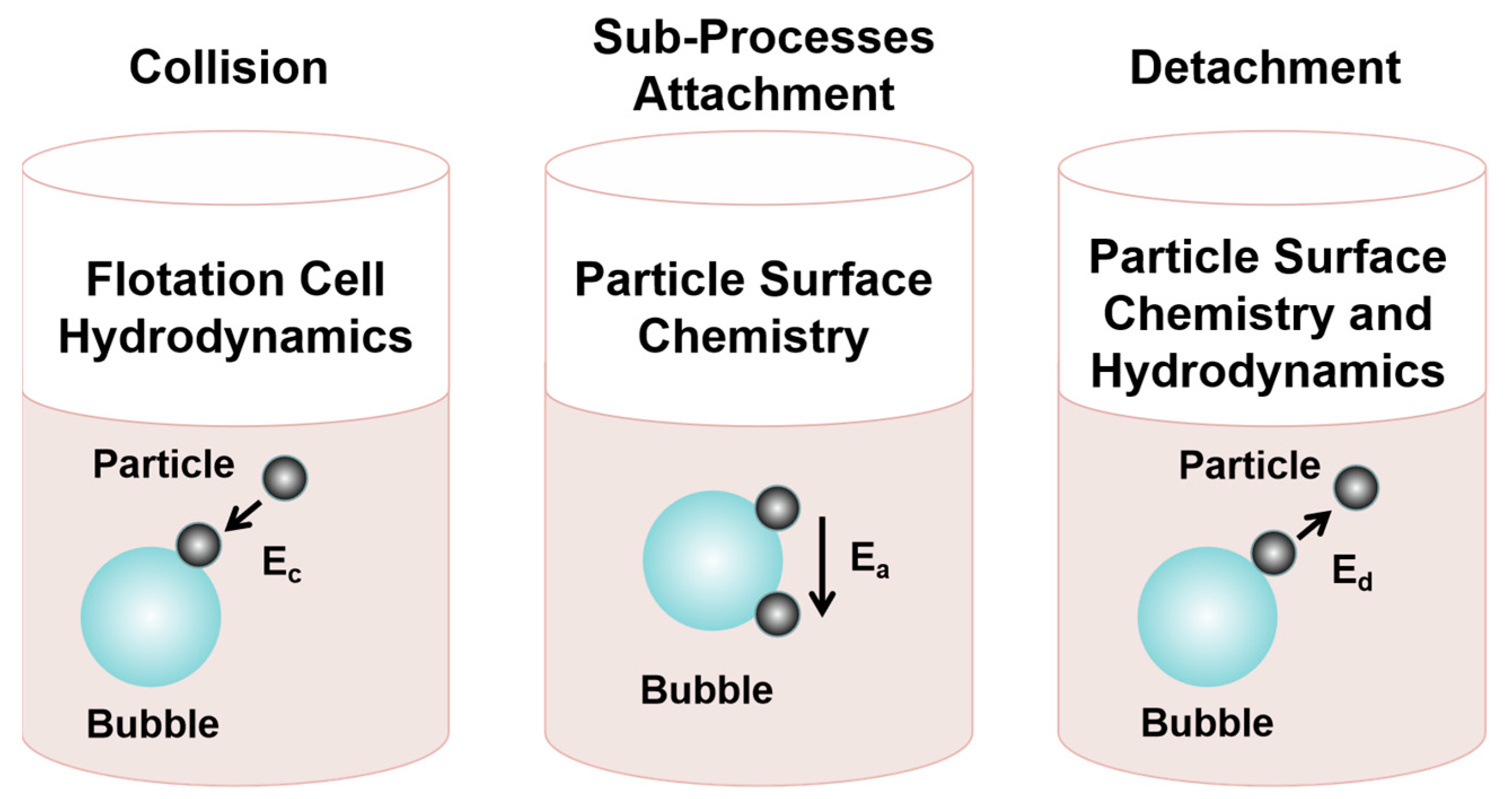
- Brabcová, Z.; Karapantsios, T.; Kostoglou, M.; Basařová, P.; Matis, K. Bubble–particle collision interaction in flotation systems. Colloid Surf. A 2015, 473, 95–103. [Google Scholar] [CrossRef]
- Safari, M.; Deglon, D. Evaluation of an attachment–detachment kinetic model for flotation. Minerals 2020, 10, 978. [Google Scholar] [CrossRef]
- Gautam, S.; Jameson, G.J. The detachment of particles from bubbles at various locations in a turbulent flotation cell. Miner. Eng. 2019, 132, 316–325. [Google Scholar] [CrossRef]
- Yoon, R.; Soni, G.; Huang, K.; Park, S.; Pan, L. Development of a turbulent flotation model from first principles and its validation. Int. J. Miner. Process 2016, 156, 43–51. [Google Scholar] [CrossRef]
- Mesa, D.; Brito-Parada, P.R. Scale-up in froth flotation: A state-of-the-art review. Sep. Purif. Technol. 2019, 210, 950–962. [Google Scholar] [CrossRef]
- Bournival, G.; Ata, S.; Jameson, G.J. Bubble and froth stabilizing agents in froth flotation. Min. Proc. Ext. Met. Rev. 2017, 38, 366–387. [Google Scholar] [CrossRef]
- Han, J.; Li, Y.; Chen, P. A study of bubble size/shape evolution in microbubble countercurrent contacting flotation colum. Asia-Pac. J. Chem. Eng. 2023, 18, 2865. [Google Scholar] [CrossRef]
- Safari, M.; Hoseinian, F.S.; Deglon, D.; Filho, L.S.L.; Pinto, T.C.S. Investigation of the reverse flotation of iron ore in three different flotation cells: Mechanical, oscillating grid and pneumatic. Miner. Eng. 2020, 150, 106283. [Google Scholar] [CrossRef]
- Yarar, B.; Richter, R.B. Flotation: Ullmann’s Encyclopedia of Industrial Chemistry. VHC Verlagsgesellschaft 2016, 23, 23–29. [Google Scholar]
- Carelse, C.; Manuel, M.; Chetty, D.; Taguta, J.; Safari, M.; Youlton, K. The flotation behaviour of liberated Platinum Group minerals in Platreef ore under reduced reagent conditions. Miner. Eng. 2022, 190, 107913. [Google Scholar] [CrossRef]
- Hoseinian, F.S.; Rezai, B.; Kowsari, E.; Safari, M. Effect of impeller speed on the Ni(II) ion flotation. Geosystem Eng. 2019, 22, 161–168. [Google Scholar] [CrossRef]
- Brill, C.; Verster, I.; Franks, G.V.; Forbes, L. Aerosol collector addition in coarse particle flotation—A review. Min. Proc. Ext. Met. Rev. 2022, 44, 522–531. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Q. Hydrodynamics of froth flotation and its effects on fine and ultrafine mineral particle flotation: A literature review. Miner. Eng. 2021, 173, 107220. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, J.; Min, F.; Valdivieso, A.L.; Wang, H. Effect of frother addition mode on coal flotation in downflow flotation column. J. Clean Prod. 2021, 278, 123844. [Google Scholar] [CrossRef]
- Jia, R.; Harris, G.H.; Fuerstenau, D.W. Chemical reagents for enhanced coal flotation. Coal Prep. 2002, 22, 123–149. [Google Scholar] [CrossRef]
- Cebeci, Y. The investigation of the floatability improvement of Yozgat Ayrdam lignite using various collectors. Fuel 2002, 81, 281–289. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Yu, Z. Oil agglomeration and its effect on beneficiation and filtration of low-rank/oxidized coals. Int. J. Miner. Process 2000, 58, 237–252. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Tao, X.; He, H.; Chen, L.; Yang, Z. Enhancing flotation performance of low rank coal by improving its hydrophobicity and the property of oily bubbles using 2-ethylhexanol. Int. J. Miner. Process 2017, 167, 61–67. [Google Scholar] [CrossRef]
- Stoev, S.; Kuzev, L.; Metodiev, M.; Djendova, S. Vibroacoustic improvements of froth flotation. Innov. Flotat. Technol. 1992, 208, 383–407. [Google Scholar]
- Xia, W.; Zhou, C.; Peng, Y. Enhancing flotation cleaning of intruded coal dry-ground with heavy oil. J. Clean Prod. 2017, 161, 591–597. [Google Scholar] [CrossRef]
- Gui, X.; Liu, J.; Cao, Y.; Cheng, G.; Zhang, H.; Wang, Y. Process intensification of fine coal separation using two-stage flotation column. J. Cent. South Univ. 2013, 20, 3648–3659. [Google Scholar] [CrossRef]
- Polat, M.; Polat, H.; Chander, S. Physical and chemical interactions in coal flotation. Int. J. Miner. Process 2003, 72, 199–213. [Google Scholar] [CrossRef]
- Kelebek, S.; Demir, U.; Sahbaz, O.; Ucar, A.; Cinar, M.; Karaguzel, C.; Oteyaka, B. The effects of dodecylamine, kerosene and pH on batch flotation of Turkey’s Tuncbilek coal. Int. J. Miner. Process 2008, 88, 65–71. [Google Scholar] [CrossRef]
- Xia, W.; Niu, C.; Ren, C. Enhancement in floatability of sub-bituminous coal by low-temperature pyrolysis and its potential application in coal cleaning. J. Clean Prod. 2017, 168, 1032–1038. [Google Scholar] [CrossRef]
- Niu, C.; Xia, W.; Xie, G. Effect of low-temperature pyrolysis on surface properties of sub-bituminous coal sample and its relationship to flotation response. Fuel 2017, 208, 469–475. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, L.; Cao, M.; Liu, Q. Slime coatings in froth flotation: A review. Miner. Eng. 2017, 114, 26–36. [Google Scholar] [CrossRef]
- Xia, W.; Xie, G.; Liang, C.; Yang, J. Flotation behavior of different size fractions of fresh and oxidized coals. Powder Technol. 2014, 267, 80–85. [Google Scholar] [CrossRef]
- Xia, W.; Yang, J.; Liang, C. A short review of improvement in flotation of low rank/oxidized coals by pretreatments. Powder Technol. 2013, 237, 1–8. [Google Scholar] [CrossRef]
- Kruszelnicki, M.; Hassanzadeh, A.; Legawiec, K.J.; Polowczyk, I.; Kowalczuk, P.B. Effect ofultrasonicpre-treatment on carbonaceous copper-bearing shale. Ultrason. Sonochem. 2022, 84, 105962. [Google Scholar] [CrossRef]
- Filippov, L.O.; Filippova, I.V.; Barres, O.; Lyubimova, T.P.; Fattalov, O.O. Intensification of the flotation separation of potash ore using ultrasound treatment. Miner. Eng. 2021, 171, 107092. [Google Scholar] [CrossRef]
- Vasumathi, N.; Sarjekar, A.; Chandrayan, H.; Chennakesavulu, K.; Reddy, G.R.; Vijaya Kumar, T.V.; El-Gendy, N.S.; Gopalkrishna, S.J. A mini review onflotationtechniques and reagents used in graphite beneficiation. Int. J. Chem. Eng. 2023, 2023, 1007689. [Google Scholar] [CrossRef]
- Sciallero, C.; Grishenkov, D.; Kothapalli, S.V.V.N.; Oddo, L.; Trucco, A. Acoustic characterization and contrast imaging of microbubbles encapsulated by polymeric shells coated or filled with magnetic nanoparticles. J. Acoust. Soc. Am. 2013, 134, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ru, Y.; Xu, M.; Zhen, K.; Zhang, H. Effects of ultrasonic pretreatment on the flotation performance and surface properties of coking middlings. Sci. Technol. Food Ind. 2018, 40, 734–741. [Google Scholar] [CrossRef]
- Suslick, K.S.; Doktycz, S.J.; Flint, E.B. On the origin of sonoluminescence and sonochemistry. Ultrasonics 1990, 28, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Xun, H.; Hu, J. Study of the effect of ultrasonic treatment on the surface composition and the flotation performance of high-sulfur coal. Fuel Process Technol. 2008, 89, 1337–1344. [Google Scholar] [CrossRef]
- Videla, A.R.; Morales, R.; Saint-Jean, T.; Gaete, L.; Vargas, Y.; Miller, J.D. Ultrasound treatment on tailings to enhance copper flotation recovery. Miner. Eng. 2016, 99, 89–95. [Google Scholar] [CrossRef]
- Cilek, E.C.; Ozgen, S. Improvement of the flotation selectivity in a mechanical flotation cell by ultrasound. Sep. Sci. Technol. 2010, 45, 572–579. [Google Scholar] [CrossRef]
- Gogate, P.R. Cavitational reactors for process intensification of chemical processing applications: A critical review. Chem. Eng. Process. Process Intensif. 2008, 47, 515–527. [Google Scholar] [CrossRef]
- Luque-Garc, A.J.L.; Castro, M.D.L.D. Ultrasound: A powerful tool for leaching. Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Mason, T.J. Ultrasonic cleaning: An historical perspective. Ultrason. Sonochem. 2016, 29, 519–523. [Google Scholar] [CrossRef]
- Yusof, N.S.M.; Babgi, B.; Alghamdi, Y.; Aksu, M.; Madhavan, J.; Ashokkumar, M. Physical and chemical effects of acoustic cavitation in selected ultrasonic cleaning applications. Ultrason. Sonochem. 2016, 29, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.; Ahmed, E.; Ismaiel, A.; Ashokkumar, M.; Xu, X.; Pan, S.; Hu, H. Ultrasonic emulsification: An overview on the preparation of different emulsifiers-stabilized emulsions. Trends Food Sci. Technol. 2020, 105, 363–377. [Google Scholar] [CrossRef]
- Naik, P.K.; Reddy, P.S.R.; Misra, V.N. Interpretation of interaction effects and optimization of reagent dosages for fine coal flotation. Int. J. Miner. Process 2005, 75, 83–90. [Google Scholar] [CrossRef]
- Feng, D.; Aldrich, C. Effect of preconditioning on the flotation of coal. Chem. Eng. Commun. 2007, 192, 972–983. [Google Scholar] [CrossRef]
- Barma, S.D. Ultrasonic-assisted coal beneficiation: A review. Ultrason. Sonochem. 2019, 50, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Truong, V.N.T.; Bu, X.; Xie, G. A review of effects and applications of ultrasound in mineral flotation. Ultrason. Sonochem. 2020, 60, 104739. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.N.; Danstan, J.K.; Hassanzadeh, A.; Vakylabad, A.B.; Chelgani, S.C. Metal extraction from ores and waste materials by ultrasound-assisted leaching—An overview. Miner. Process Ext. M 2022, 45, 28–45. [Google Scholar] [CrossRef]
- Wang, J. Numerical analysis of bubble dynamics in ultrasonic cavitation. Value Eng. 2011, 30, 196–197. [Google Scholar]
- Ruo, F. Ultrasonic Manual: Nanjing; Nanjing University Press: Nanjing, China, 1999. [Google Scholar]
- Herbert, E.; Balibar, S.; Caupin, F. Cavitation pressure in water. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2006, 74, 41603. [Google Scholar] [CrossRef]
- Louisnard, O.; Gomez, F. Growth by rectified diffusion of strongly acoustically forced gas bubbles in nearly saturated liquids. Physical review. E Stat. Nonlinear Soft Matter Phys. 2003, 67, 36610. [Google Scholar] [CrossRef]
- Flannigan, D.J.; Suslick, K.S. Plasma formation during single-bubble cavitation. Nature 2005, 434, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Rooze, J.; Rebrov, E.V.; Schouten, J.C.; Keurentjes, J.T.F. Dissolved gas and ultrasonic cavitation-A review. Ultrason. Sonochem. 2013, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, M.C.; Lin, C.C.; Chang, Y.H.; Chen, L.C. Ultrasonic mixing and closed microwave irradiation-assisted transesterification of soybean oil. Fuel 2010, 89, 3618–3622. [Google Scholar] [CrossRef]
- Stavarache, C.; Vinatoru, M.; Nishimura, R.; Maeda, Y. Fatty acids methyl esters from vegetable oil by means of ultrasonic energy. Ultrason. Sonochem. 2005, 12, 367–372. [Google Scholar] [CrossRef]
- Adewuyi, Y.G. Sonochemistry: Environmental science and engineering applications. Ind. Eng. Chem. Res. 2001, 40, 4681–4715. [Google Scholar] [CrossRef]
- Lei, Z. Enhancement of Ceramic Honeycomb Catalytic Ozonation by Ultrasound for the Degradation of Organic Compound In Water; Harbin Institute of Technology: Harbin, China, 2008. [Google Scholar]
- Chen, L.; Lu, D.; Wang, Y.; Cheng, Z. Research and application of ultrasonic technology in mineral flotation and development trend. Chin. J. Nonferrous Met. 2021, 31, 1042–1056. [Google Scholar]
- Peng-Li, Z.; Shu-Yu, L.; Hua-Ze, Z. The radiation pressure of steady state and transient cavitation bubble. In Proceedings of the 2017 National Acoustical Conference of the Chinese Acoustical Society, Shanghai, China, 3 March 2017; Technical Acoustics Press: Shanghai, China, 2017; pp. 491–492. [Google Scholar]
- Oliveira, H.; Azevedo, A.; Rubio, J. Nanobubbles generation in a high-rate hydrodynamic cavitation tube. Miner. Eng. 2018, 116, 32–34. [Google Scholar] [CrossRef]
- Zhou, Z.A.; Xu, Z.; Finch, J.A.; Masliyah, J.H.; Chow, R.S. On the role of cavitation in particle collection in flotation—A critical review. II. Miner. Eng. 2009, 22, 419–433. [Google Scholar] [CrossRef]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef]
- Xiangyang, L. Study on the Effect Mechanisms of Froth Stability and Particle Motion at the Air-Water Interface on Froth Phase Flotation; China University of Mining and Technology: Xuzhou, China, 2019. [Google Scholar]
- Zhizhuang, S.; Wenqiang, L. The effect of Bjerknes force on motive behaviors of micro-bubble. J. Shaanxi Norm. Univ. (Nat. Sci.) 2013, 41, 24–27. [Google Scholar]
- Doinikov, A.A.; Zavtrak, S.T. On the “bubble grapes” induced by a sound field. Ultrason. Snochemistry 1999, 99, 25–29. [Google Scholar] [CrossRef]
- Moussatov, A.; Granger, C.; Dubus, B. Cone-like bubble formation in ultrasonic cavitation field. Ultrason. Sonochem. 2003, 10, 191–195. [Google Scholar] [CrossRef]
- Mettin, R.; Luther, S.; Ohl, C.D.; Lauterborn, W. Acoustic cavitation structures and simulations by a particle model. Ultrason. Sonochem. 1999, 6, 25–29. [Google Scholar] [CrossRef]
- Gungoren, C.; Baktarhan, Y.; Demir, I.; Ozkan, S.G. Enhancement of galena-potassium ethyl xanthate flotation system by low power ultrasound. Trans. Nonferrous Met. Soc. China 2020, 30, 1102–1110. [Google Scholar] [CrossRef]
- Gungoren, C.; Ozdemir, O.; Wang, X.; Ozkan, S.G.; Miller, J.D. Effect of ultrasound on bubble-particle interaction in quartz-amine flotation system. Ultrason. Sonochem. 2019, 52, 446–454. [Google Scholar] [CrossRef]
- Chen, Y.; Bu, X.; Truong, V.N.T.; Peng, Y.; Xie, G. Study on the effects of pre-conditioning time on the floatability of molybdenite from the perspective of cavitation threshold. Miner. Eng. 2019, 141, 105845. [Google Scholar] [CrossRef]
- Cao, Q.; Cheng, J.; Feng, Q.; Wen, S.; Luo, B. Surface cleaning and oxidative effects of ultrasonication on the flotation of oxidized pyrite. Powder Technol. 2017, 311, 390–397. [Google Scholar] [CrossRef]
- Wang, G.; Joshi, J.B.; Sathe, M.; Jameson, G.J.; Zhou, S.; Evans, G.M. Bubble detachment from a steel ball in turbulent field: Application to mineral flotation systems. Procedia Eng. 2015, 102, 1046–1055. [Google Scholar] [CrossRef]
- Altun, N.E.; Hwang, J.Y.; Hicyilmaz, C. Enhancement of flotation performance of oil shale cleaning by ultrasonic treatment. Int. J. Miner. Process 2009, 91, 1–13. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, Y.; Bu, X.; Xie, G. Effects of 20 kHz ultrasound on coal flotation: The roles of cavitation and acoustic radiation force. Fuel 2019, 256, 115938. [Google Scholar] [CrossRef]
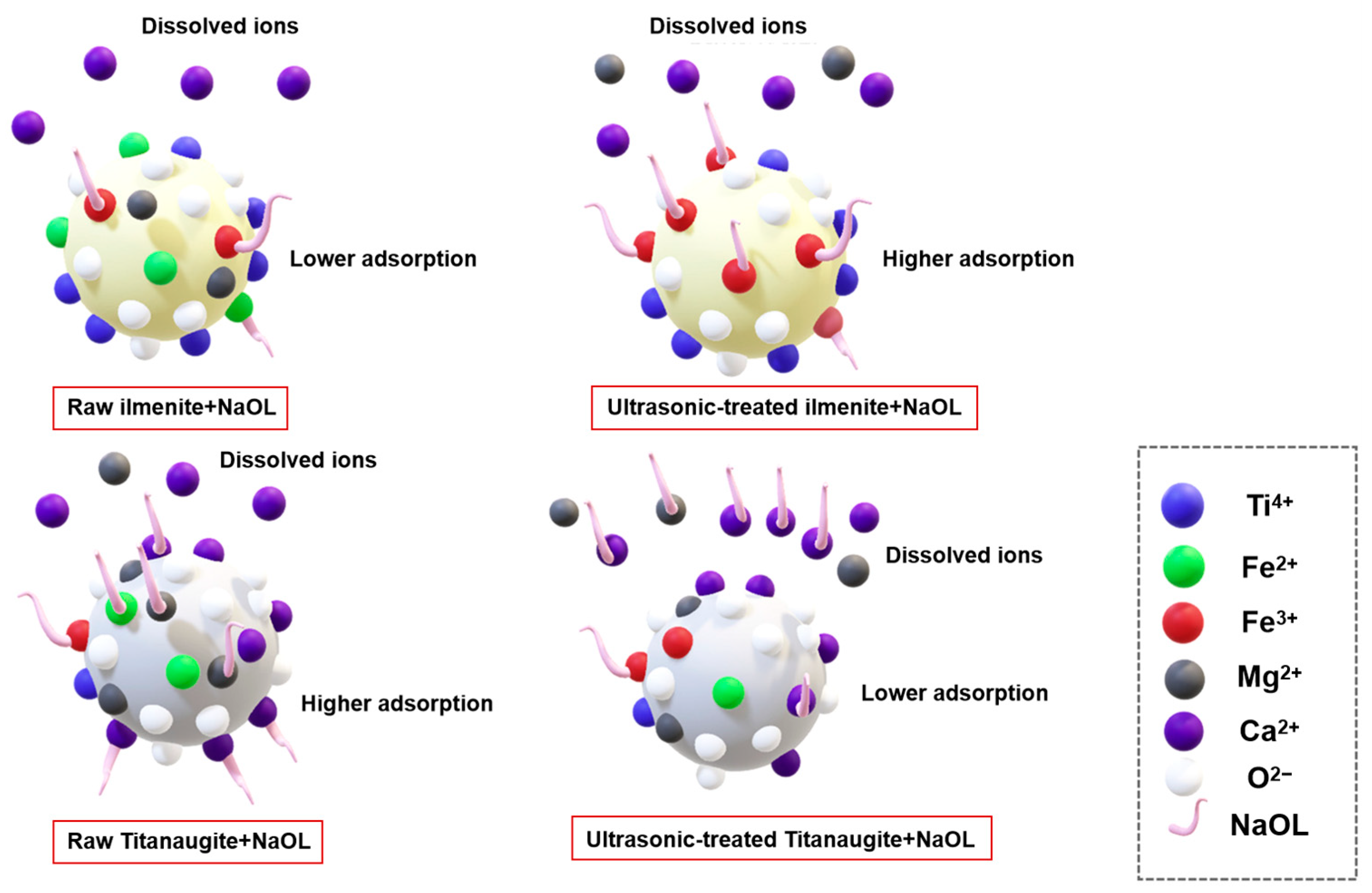
- Shu, K.; Xu, L.; Wu, H.; Wang, Z.; Fang, S. Influence of ultrasound pre-treatment on ilmenite surface chemical properties and collectors’ adsorption behaviour. Ultrason. Sonochem. 2019, 57, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fang, S.; Shu, K.; Xu, Y.; Wang, Z.; Luo, L.; Yang, J.; Xu, L. Selective flotation and adsorption of ilmenite from titanaugite by a novel method: Ultrasonic treatment. Powder Technol. 2020, 363, 38–47. [Google Scholar] [CrossRef]
- Griffing, V. The chemical effects of ultrasonics. J. Chem. Phys. 1952, 20, 939–942. [Google Scholar] [CrossRef]
- Chen, Y.; Chelgani, S.C.; Bu, X.; Xie, G. Effect of the ultrasonic standing wave frequency on the attractive mineralization for fine coal particle flotation. Ultrason. Sonochem. 2021, 77, 105682. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shi, J.; Fang, S.; Zhu, D.; Nie, D.; Cui, R. Study on the influence of ultrasonic and thermal effects on the floatability of calcite in sodium silicate and sodium oleate system. Conserv. Util. Miner. Resour. 2021, 13, 131–138. [Google Scholar]
- Ouyang, J.; Chen, Y.; Wang, Y.; Wen, P. Study of ultrasonic strengthening desulfurization in bauxite flotation. China Mine Eng. 2015, 44, 15–18. [Google Scholar]
- Wang, W.; Zhang, N.; Jin, L. Experiment study on fine coal slime flotation with simultaneous ultrasonic treatment. J. Min. Sci. Technol. 2019, 4, 357–364. [Google Scholar]
- Lu, Y.; Cheng, F. Research on the mechanism of the oxidized pyrrhotite flotation improvement by ultrasonic. Met. Mine 2019, 4, 88–92. [Google Scholar]
- Chang-tao, W.; Run-qing, L.; Shang-yong, L.; Jian-de, G.; Wei, S.; Xin, S. Beneficiation process for ore-washing slime from a fluorite mine in Hunan Province. Min. Metall. Eng. 2019, 39, 43–46. [Google Scholar]
- Guo, T.; Li, S.; Huang, C.; Li, K. Research status and development of beneficiation process of manganese carbonate ore. Met. Mine 2019, 12, 118–123. [Google Scholar]
- Li, Y. Ultrasonic cleaning principle and application. Clean. World 2006, 22, 31–35. [Google Scholar]
- Chen, L.; He, J.; Zhu, L.; Yao, Q.; Sun, Y.; Guo, C.; Chen, H. Efficient recovery of valuable metals from waste printed circuit boards via ultrasound-enhanced flotation. Process Saf. Env. 2023, 169, 869–878. [Google Scholar] [CrossRef]
- Ozkan, S.G.; Kuyumcu, H.Z. Investigation of mechanism of ultrasound on coal flotation. Int. J. Miner Process 2006, 81, 201–203. [Google Scholar] [CrossRef]
- Hassani, F.; Noaparast, M.; Tonkaboni, S.Z.S. A study on the effect of ultrasound irradiation as pretreatment method on flotation of sedimentary phosphate rock with carbonate-silicate gangue. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 2787–2798. [Google Scholar] [CrossRef]
- Barma, S.D.; Tej, S.S.P.; Ramya, B.; Sathish, R. Ultrasound-promoter pretreatment for enhancing the yield and combustible matter recovery of high-ash oxidized coal flotation. Energ. Fuel 2019, 33, 8165–8175. [Google Scholar] [CrossRef]
- Shi, H.; Shi, Y. Effect of supersonic treatment on surface property of fine coal and performance of flotation. Coal Prep. Technol. 2007, 5, 21–23. [Google Scholar]
- Peng, Y.; Mao, Y.; Xia, W.; Li, Y. Ultrasonic flotation cleaning of high-ash lignite and its mechanism. Fuel 2018, 220, 558–566. [Google Scholar] [CrossRef]
- Yin, W.; Sun, C. Research progress on flotation principle of silicate minerals. The Editorial Department of Mining Express. In Proceedings of the Fourth National Mining and Dressing Technology Progress Report; Mining Express: Zhangjiajie, China, 2001; pp. 127–132. [Google Scholar]
- Yuhua, W.; Guangli, Z.; Lei, Z.; Dongfang, L.; Liguang, W.; Yuehao, Z.; Zheng, H. Surface dissolution of spodumene and its role in the flotation concentration of a spodumene ore. Miner. Eng. 2018, 125, 120–125. [Google Scholar]
- Haitao, Z.; Yuhua, W.; Yuehao, Z.; Guangli, Z.; Dongfang, L.; Xiayu, Z. Influence of NaOH and mechanical agitation on the surface and flotation behavior of spodumene. Nonferrous Met. Eng. 2019, 9, 61–68. [Google Scholar]
- Shu, K.; Xu, L.; Wu, H.; Fang, S.; Zhang, Z. Effects of ultrasonic pre-treatment on the flotation of ilmenite and collector adsorption. Miner. Eng. 2019, 137, 124–132. [Google Scholar] [CrossRef]
- Fang, S.; Xu, L.; Wu, H.; Xu, Y.; Wang, Z.; Shu, K.; Hu, Y. Influence of surface dissolution on sodium oleate adsorption on ilmenite and its gangue minerals by ultrasonic treatment. Appl. Surf. Sci. 2020, 500, 144038. [Google Scholar] [CrossRef]
- Longhua, X.; Shuai, F.; Houqin, W. Flotation separation on oxidized ores by surface modification and regulation: A review. J. Guizhou Univ. (Nat. Sci.) 2020, 37, 1–6. [Google Scholar]
- Chu, H.; Chen, L.; Lu, D.; Wang, Y.; Zheng, X. Ultrasonic pretreatment of spodumene with different size fractions and its influence on flotation. Ultrason. Sonochem. 2022, 82, 105889. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kuang, J.; Yuan, W.; Yu, M.; Wang, X. Regulation mechanism of ultrasonication on surface hydrophobicity of scheelite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127412. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Ahmadi, M.; Joshaghani, M. Ultrasonic assisted ash and sulphur removal from bitumen using column flotation technique: Experimental, RSM and ANN methods in modelling and optimization of process. Iran. J. Sci. Technol. Trans. A Sci. 2017, 41, 1149–1163. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, X.; Xu, S.; Li, Z.; Yu, H.; Shen, X. Enhanced desulfurizing flotation of coal using sonoelectrochemical method. Ultrason. Sonochem. 2013, 20, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.Z.; Xun, H.X.; Chen, J.T. Study of enhanced fine coal de-sulphurization and de-ashing by ultrasonic flotation. J. China Univ. Min. Technol. 2007, 17, 358–362. [Google Scholar] [CrossRef]
- Kang, W.; Xun, H.; Kong, X.; Li, M. Effects from changes in pulp nature after ultrasonic conditioning on high-sulfur coal flotation. Min. Sci. Technol. 2009, 19, 498–502. [Google Scholar] [CrossRef]
- Zhang, H.X.; Bai, H.J.; Dong, X.S.; Wang, Z.Z. Enhanced desulfurizing flotation of different size fractions of high sulfur coal using sonoelectrochemical method. Fuel Process Technol. 2012, 97, 9–14. [Google Scholar] [CrossRef]
- Gedanken, A. Doping nanoparticles into polymers and ceramics using ultrasonic radiation. Ultrason. Sonochem. 2007, 14, 418–430. [Google Scholar] [CrossRef]
- Lauterborn, W.; Kurz, T.; Geisler, R.; Schanz, D.; Lindau, O. Acoustic cavitation, bubble dynamics and sonoluminescence. Ultrason. Sonochem. 2007, 14, 484–491. [Google Scholar] [CrossRef]
- Zaidi, S.A.H. Ultrasonically enhanced coal desulphurization. Fuel Process Technol. 1993, 33, 95–100. [Google Scholar] [CrossRef]
- Geng, P.; Gao, S.; Huang, J. Influence of ultrasonic on flotation. Guide Sci.-Tech. Mag. 2011, 52, 52. [Google Scholar]
- Chen, S.; Tao, X.; Tang, L.; Dong, F.; Gui, D. Application of ultrasonic pretreatment for coking coal flotation and its mechanism. Int. J. Coal Prep. Util. 2022, 42, 762–774. [Google Scholar] [CrossRef]
- Burov, V.E.; Poilov, V.Z.; Sazhina, M.M.; Huang, Z. Effect of ultrasound on reagent compositions foaming properties used in mineral flotation. ChemChemTech 2022, 65, 81. [Google Scholar]
- Huang, Z.; Kuang, J.; Zhu, L.; Yuan, W.; Zou, Z. Effect of ultrasonication on the separation kinetics of scheelite and calcite. Miner. Eng. 2021, 163, 106762. [Google Scholar] [CrossRef]
- Ozkan, S.G. Effects of simultaneous ultrasonic treatment on flotation of hard coal slimes. Fuel 2012, 93, 576–580. [Google Scholar] [CrossRef]
- Chen, D.; Liang, D.; Han, D.; Wang, Z. Effect of ultrasonic treatment on the emulsion dispersion of common flotation reagent such as xanthate, aerofloat, calcium hydroxide and oleic acid. Nonferrous Met. (Miner. Process. Sect.) 2011, 3, 50–53. [Google Scholar]
- Li, M.; Wang, F.; Wang, L. Ultrasonic-aided flotation decarbonization of fly ash. Clean Coal Technol. 2018, 24, 46–49. [Google Scholar]
- He, S. Effect of the Foaming Agents Properties by the Combination of Ultrasonic Emulaication and Chemical Modification; Taiyuan University of Technology: Taiyuan, China, 2015. [Google Scholar]
- Hassanzadeh, A.; Gholami, H.; Niedoba, T.; Surowiak, A. Effect of power ultrasound on wettability and collector-less floatability of chalcopyrite, pyrite and quartz. Minerals 2021, 11, 48. [Google Scholar] [CrossRef]
- Chen, J.; Hu, J.; Xie, G.; Ou, Z. Effects of ultrasonic treatment on flotation of high-sulfur coal desulfurization by flotation. Coal Sci. Technol. 1998, 26, 20–22. [Google Scholar]
- Giriûnienë, R.; Garðka, E. The influence of ultrasonic on electrical conductivity of water. Ultragarsas Ultrason. 1997, 28, 25–28. [Google Scholar]
- Özkan, G.; Kuyumcu, H.Z. Design of a flotation cell equipped with ultrasound transducers to enhance coal flotation. Ultrason. Sonochem. 2007, 14, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Drenckhan, W.; Saint-Jalmes, A. The science of foaming. Adv. Colloid. Interfac. 2015, 222, 228–259. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.G. Further investigations on simultaneous ultrasonic coal flotation. Minerals 2017, 7, 177. [Google Scholar] [CrossRef]
- Ozkan, G.S. Beneficiation of magnesite slimes with ultrasonic treatment. Miner. Eng. 2002, 15, 99–101. [Google Scholar] [CrossRef]
- Trujillo, F.J.; Juliano, P.; Barbosa-Cánovas, G.; Knoerzer, K. Separation of suspensions and emulsions via ultrasonic standing waves-A review. Ultrason. Sonochem. 2014, 21, 2151–2164. [Google Scholar] [CrossRef]
- Luo, X.; Cao, J.; Gong, H.; Yan, H.; He, L. Phase separation technology based on ultrasonic standing waves: A review. Ultrason. Sonochem. 2018, 48, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.; Johansson, L.; Juliano, P.; Mcarthur, S.L.; Manasseh, R. Ultrasonic separation of particulate fluids in small and large scale systems: A review. Ind. Eng. Chem. Res. 2013, 47, 16555–16576. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, G.; Chang, J.; Grundy, J.; Liu, Q. A study of coal aggregation by standing-wave ultrasound. Fuel 2019, 248, 38–46. [Google Scholar] [CrossRef]
- Cilek, E.C.; Ozgen, S. Effect of ultrasound on separation selectivity and efficiency of flotation. Miner. Eng. 2009, 22, 1209–1217. [Google Scholar] [CrossRef]
- Gao, K.; Liu, H.; Sun, L.; Zhang, Z. Effect of gas input conditions and ultrasound on the dynamic behavior of flotation bubbles. ACS Omega 2022, 26, 22326–22340. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, N. Flotation bubble size distribution rules with ultrasonic radiation. J. Min. Sci. Technol. 2018, 3, 84–90. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Du, M.; Hu, H.; Zhang, H.; Song, N. A Review of Ultrasonic Treatment in Mineral Flotation: Mechanism and Recent Development. Molecules 2024, 29, 1984. https://doi.org/10.3390/molecules29091984
Zhang H, Du M, Hu H, Zhang H, Song N. A Review of Ultrasonic Treatment in Mineral Flotation: Mechanism and Recent Development. Molecules. 2024; 29(9):1984. https://doi.org/10.3390/molecules29091984
Chicago/Turabian StyleZhang, Huan, Mingming Du, Haijie Hu, Hongli Zhang, and Naijian Song. 2024. "A Review of Ultrasonic Treatment in Mineral Flotation: Mechanism and Recent Development" Molecules 29, no. 9: 1984. https://doi.org/10.3390/molecules29091984
APA StyleZhang, H., Du, M., Hu, H., Zhang, H., & Song, N. (2024). A Review of Ultrasonic Treatment in Mineral Flotation: Mechanism and Recent Development. Molecules, 29(9), 1984. https://doi.org/10.3390/molecules29091984