Abstract
Using the aldehyde amine condensation procedure and the triphenylamine group as the skeleton structure, the new triphenylamine-aromatic aldehyde-succinylhydrazone probe molecule DHBYMH was created. A newly created acylhydrazone probe was structurally characterized by mass spectrometry (MS), NMR, and infrared spectroscopy (FTIR). Fluorescence and UV spectroscopy were used to examine DHBYMH’s sensing capabilities for metal ions. Notably, DHBYMH achieved a detection limit of 1.62 × 10−7 M by demonstrating exceptional selectivity and sensitivity towards Cu2+ ions in an optimum sample solvent system (DMSO/H2O, (v/v = 7/3); pH = 7.0; cysteine (Cys) concentration: 1 × 10−4 M). NMR titration, high-resolution mass spectrometry analysis, and DFT computation were used to clarify the response mechanism. Ultimately, predicated on DHBYMH’s reversible identification of Cu2+ ions in the presence of EDTA, a molecular logic gate was successfully designed.
1. Introduction
The copper ion (Cu2+) is an essential redox-active trace element for animals and plants, which mainly plays a role in nerve transmission, oxygen delivery, and redox reactions. However, when there is a large amount of Cu2+ in the environment, it will accumulate in the human body through the food chain. Excess Cu2+ accumulation in the body may lead to a variety of diseases [1,2,3]. The normal level of total copper in blood is 15.7–23.6 μM. The Chinese standard for drinking water specifies a limit of 15.7 μM for Cu2+, and the US Environmental Protection Agency (EPA) sets the limit for Cu2+ in drinking water at 20.0 μM [4]. Therefore, the convenient and rapid detection and treatment of copper ions are of great importance for environmental protection and human health [5,6].
Traditional methods for analyzing Cu2+ include spectrophotometry [7], atomic absorption spectroscopy [8], electrochemical analysis [9], inductively coupled plasma mass spectrometry [10], and isotope dilution [11]. However, these methods are limited by expensive equipment, complex operation procedures, professional personnel, and difficulty in real-time on-site monitoring. Therefore, it is necessary to find a convenient, low-cost, and real-time method for detecting ions. Compared to traditional Cu2+ analysis detection techniques, fluorescence probe detection has the advantages of good selectivity, low detection limits, low cost, simple operation, real-time monitoring, and fast response [12,13,14,15,16,17,18]. It has become one of the most popular Cu2+ analysis detection methods and a research hotspot in recent years [19,20,21,22].
The triphenylamine unit, with its propeller structure, excellent electron-donating ability, and easily modified molecular structure, can serve as an excellent emitting group for small molecular fluorescence probes and has become a research hotspot in recent years [19]. Based on our previous studies [23,24,25,26,27,28,29,30,31,32], trianiline is modified by introducing 4-bromo-2-hydroxybenzaldehyde through a Suzuki coupling reaction to increase its conjugation degree and electron-giving ability, improving its optical properties. It also undergoes condensation with salicylhydrazine to introduce Schiff base structures, enhancing its coordination ability with copper ions to achieve rapid detection and high selectivity. Ultimately, a novel Schiff base-type small molecular fluorescence probe molecule, N-((4-(diphenylamino)-3-hydroxy-[1,1-biphenyl]-4-yl)methylene)-2-hydroxybenzoylhydrazone (DHBYMH), which has a certain solvent effect and can efficiently and sensitively detect copper ions in different water samples, was designed and synthesized. Its reversible design allows for its use in molecular logic gates.
2. Results and Discussion
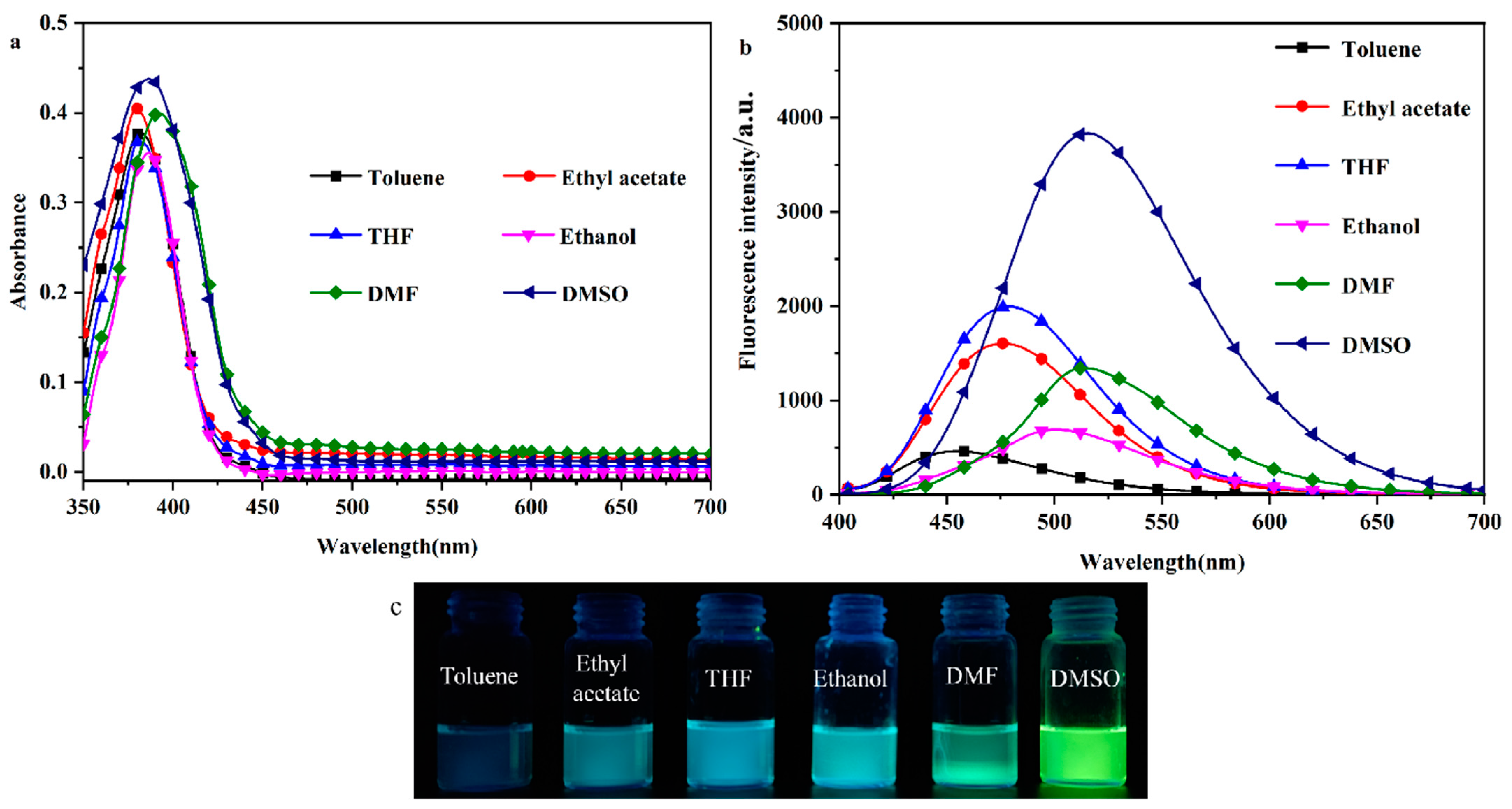
2.1. Solvent–Chromic Effect
To investigate the solvent-induced color change effect of the probe molecule DHBYMH, the UV–Vis absorption spectra and fluorescence emission spectra of DHBYMH (1 × 10−5 M, 2 mL) were observed and recorded in different polar organic solvents (toluene, ethyl acetate, THF, ethanol, DMF, and DMSO). As shown in Figure 1a, there is no significant difference in the UV absorption spectra of the DHBYMH probe, with the maximum absorption wavelength around 385 nm. However, in the fluorescence emission spectra (Figure 1b), the DHBYMH probe exhibits significant differences in the six different polar organic solvents, which manifests as a marked redshift in the maximum emission wavelength with increasing solvent polarity. The maximum emission wavelengths are 454 nm in toluene, 476 nm in ethyl acetate, 479 nm in THF, 501 nm in ethanol, 514 nm in DMF, and 520 nm in DMSO, which are all higher than those of the DHBYMH probe in the same solvents. Across the whole range of solvent polarity, the experimental data do not obey the linear relationship predicted by the Lippert–Mataga equation well, as shown in Figure S2 and Table S1. It is worth noting that the quantum yield (QY) of the DPTYMH probe in DMSO is 25.06%, which is higher than that of other solvents. Figure 1c shows the actual images of DHBYMH (1 × 10−5 M) under UV light (365 nm) in the six organic solvents mentioned above. Table 1 lists the optical data from the DHBYMH probe in the six organic solvents, showing an increase in the Stokes shift with increasing solvent polarity. The main reason for the above phenomenon is that when the DHBYMH probe is excited by light, a π-π* transition occurs. During this transition, the excited state of the probe molecule usually decays or relaxes back to the ground state through non-radiative channels, causing the fluorescence quenching of π-conjugated molecules in the aggregated state and generating the aggregation-caused quenching (ACQ, Figure S1), resulting in an increase in the dipole moment of the excited state, which is higher than that of the ground state. This leads to an increase in the solvent–solute interaction force with increasing solvent polarity, resulting in a decrease in the excited state energy and a significant redshift in the spectrum. After mixing with Cu2+, the maximum absorption wavelength spectrum undergoes a significant redshift and the absorbance intensity increases slightly. The experimental phenomenon can be attributed to intramolecular charge transfer (ICT), which is induced by the π-bridge of the neighboring hydroxyphenyl ring in DHBYMH [33,34,35,36,37].
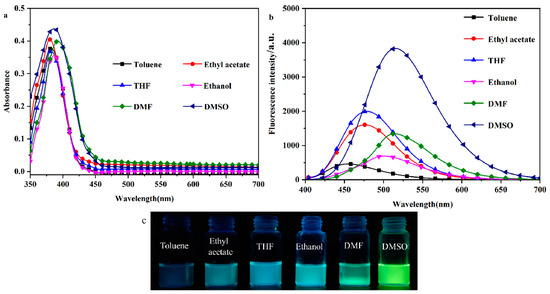
Figure 1.
Absorption spectra (a) and emission spectra (b) of probe DHBYMH in toluene, ethyl acetate, tetrahydrofuran, ethanol DMF, and DMSO (composition of probe DHBYMH: 1.0 × 10−5 M; slit: 5/5). (c) Physical image of DHBYMH (1 × 10−5 M) in organic solvents of different polarity under a UV lamp (365 nm).

Table 1.
Optical data from probe DHBYMH in different polar organic solvents (λabs—absorption maximum and λem—emission maximum).
2.2. Cu2+ Response Behavior of DHBYMH
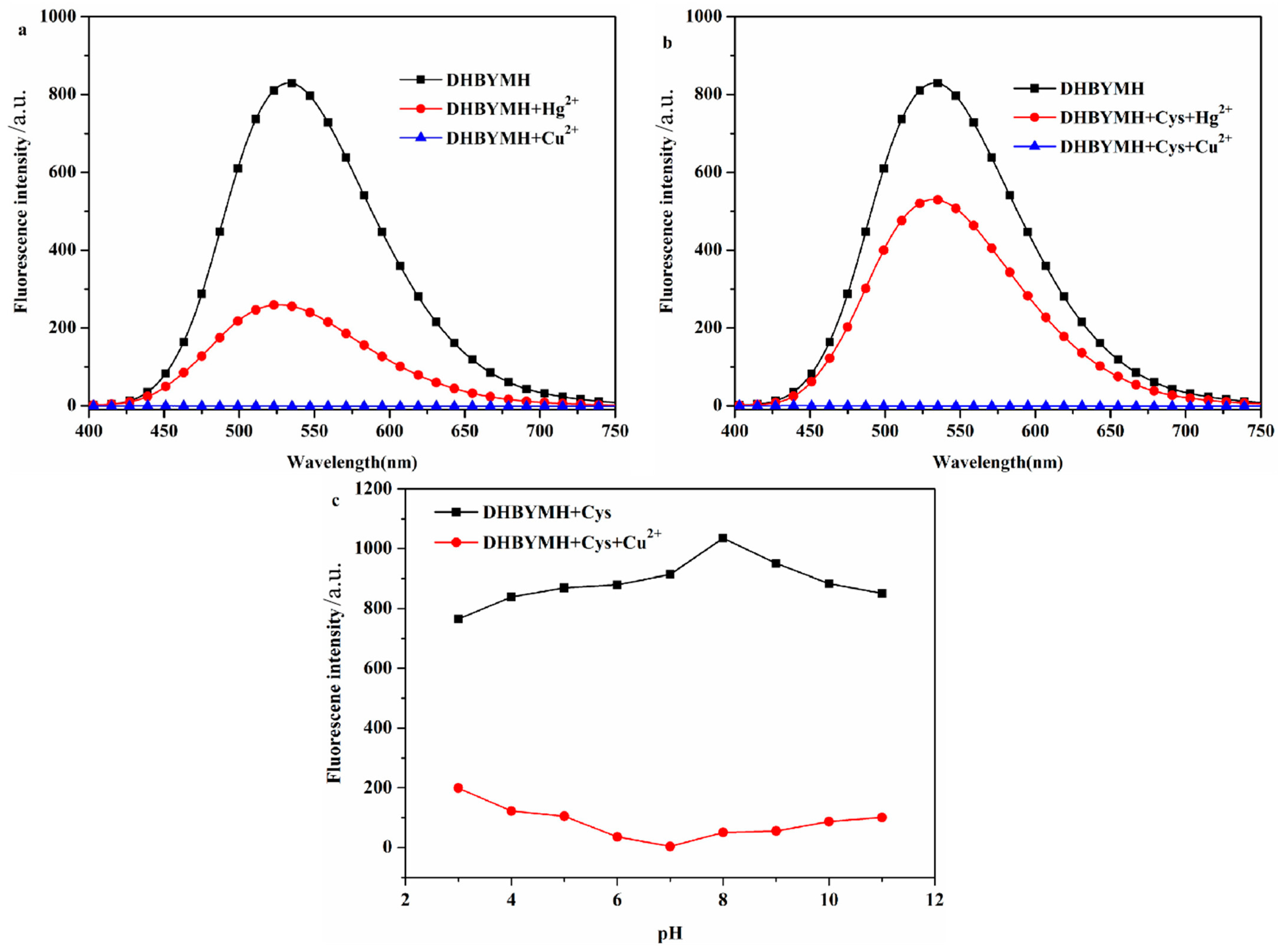
2.2.1. Screening of Test Conditions
In the optimized DMSO/H2O solvent system with a v/v ratio of 7/3, DHBYMH recognizes Cu2+ ions, and Hg2+ ions cause weak interference. However, in the presence of Cys, Hg2+ ions do not interfere with DHBYMH’s recognition of Cu2+ ions. Under the DMSO/H2O, v/v = 7/3, Cys: 1 × 10−4 M system, the ability of DHBYMH to recognize Cu2+ ions under different pH conditions was studied. It was found that in the pH range of 3–11, the probe DHBYMH exhibits fluorescence emission, and upon the addition of Cu2+ ions, a fluorescence quenching phenomenon occurs, with the maximum quenching degree at pH = 7 (Figure 2a–c). Therefore, the pH = 7 solvent system was selected for the exploration of the response time of the probe DHBYMH to Cu2+ ions. As shown in Figure S3, after stirring for 5 s, the probe DHBYMH reaches complete quenching, indicating an extremely fast response time of only 5 s. In summary, subsequent studies on DHBYMH’s recognition of Cu2+ ions were carried out under the conditions of DMSO/H2O, v/v = 7/3, pH = 7.0, Cys: 1 × 10−4 M, with a stirring time of 5 s.
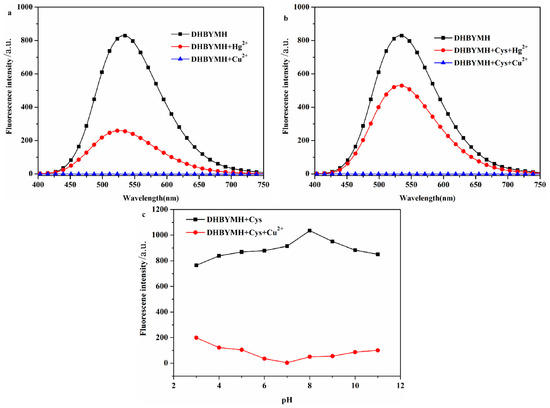
Figure 2.
The probe DHBYMH (1 × 10−5 M, DMSO/H2O, v/v = 7/3) responded to Hg2+ and Cu2+ ions in the absence of Cys (a) and in the presence of Cys (1 × 10−4 M) (b). The fluorescence emission spectra of Cu2+ ion recognition by probe DHBYMH (1 × 10−5 M, DMSO/H2O, v/v = 7/3, pH = 7.0, Cys: 1 × 10−4 M) under different pH conditions (c).
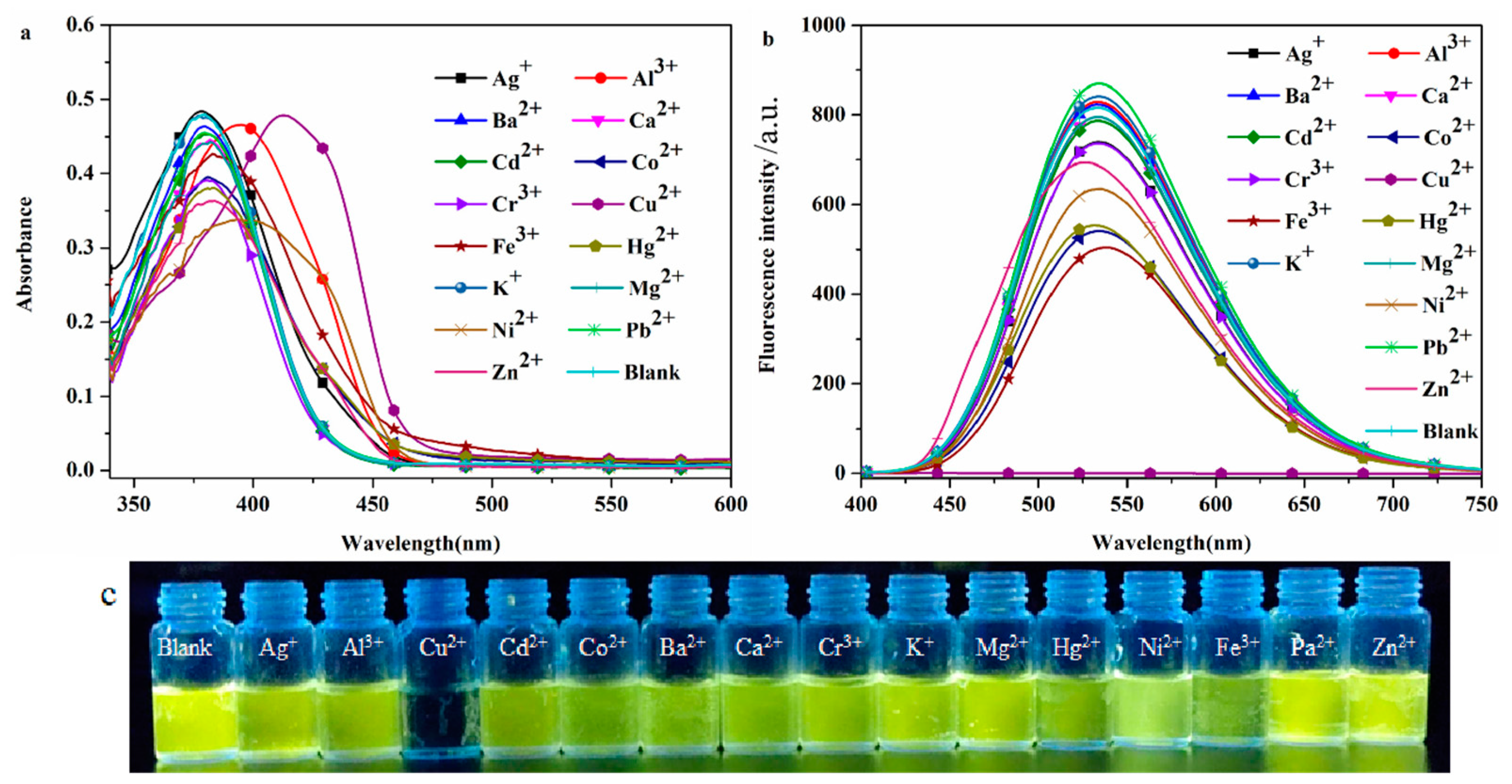
2.2.2. Selective Recognition of Cations
The high selectivity of the probe DHBYMH for the target ion is beneficial for practical applications. To investigate the selectivity of the probe DHBYMH for Cu2+ ions, absorption spectra and emission spectra were used to study the response of DHBYMH to common cations such as Ag+, Ba2+, Al3+, Ca2+, Co2+, Cd2+, Cu2+, Cr3+, Hg2+, Fe3+, Ni2+, K+, Mg2+, Pb2+, and Zn2+. Figure 3a shows that the maximum absorption peak of DHBYMH for Cu2+ ion recognition exhibited a significant redshift compared to other ions. In the emission spectrum (Figure 3b), DHBYMH only showed a significant response to Cu2+ ions, with complete quenching of fluorescence, while there was little response to other common metal ions. This phenomenon can also be observed by the naked eye under UV lamp (365 nm) illumination (Figure 3c). In summary, DHBYMH exhibits high selectivity for Cu2+ ions and has practical application value.
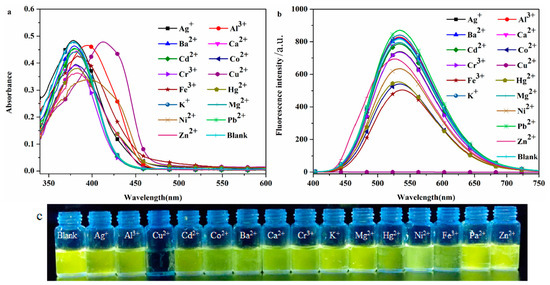
Figure 3.
The UV absorption spectra (a) and fluorescence emission spectra (b) of the probe DHBYMH (1 × 10−5 M, DMSO/H2O, v/v = 7/3, pH = 7.0, Cys: 1 × 10−4 M) for selective recognition of common metal cations (1 × 10−4 M). (c) Physical image of DHBYMH (1 × 10−5 M, DMSO/H2O, v/v = 7/3, pH = 7.0, Cys: 1 × 10−4 M) for selective recognition of common metal cations (1 × 10−4 M) under UV lamp (365 nm) irradiation.
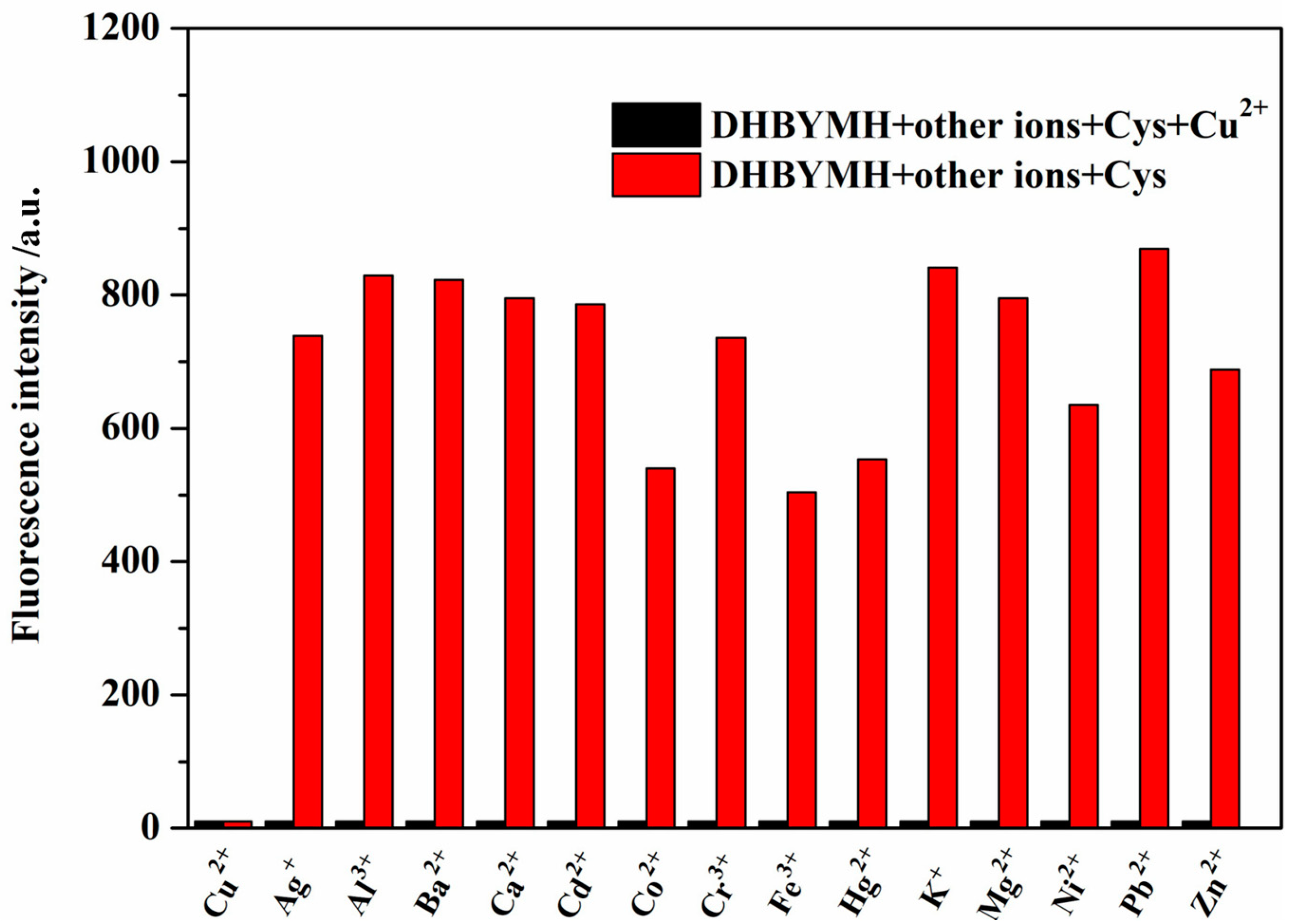
2.2.3. Competitive Recognition of Cu2+ Ions
The better the interference resistance of the probe molecule, the stronger its competitive recognition ability for the target molecule and the higher its practical application value. Therefore, the recognition ability of DHBYMH for Cu2+ ions in the presence of common metal ions such as Ag+, Ba2+, Al3+, Ca2+, Co2+, Cd2+, Cu2+, Cr3+, Hg2+, Fe3+, Ni2+, K+, Mg2+, Pb2+, and Zn2+ was studied. As shown in Figure 4, DHBYMH still showed fluorescence emission in the presence of other common metal ions; however, a fluorescence quenching phenomenon occurred upon the addition of Cu2+ ions. Therefore, DHBYMH has good competitive recognition for Cu2+ ions, allowing for specific detection of Cu2+ ions in environmental systems.
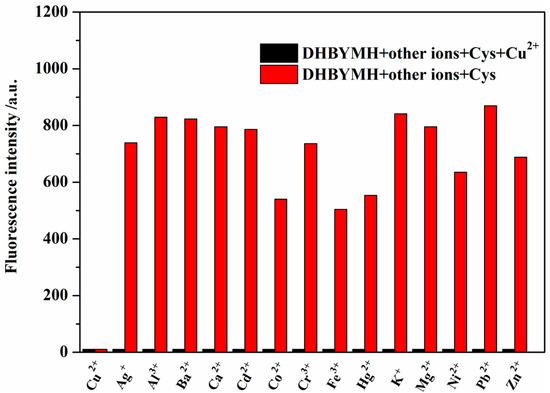
Figure 4.
The detection of Cu2+ (1 × 10−4 M) by probe DHBYMH (1 × 10−5 M, DMSO/H2O, v/v = 7/3, pH = 7.0, Cys: 1 × 10−4 M) in the presence of other common metal ions was tested.
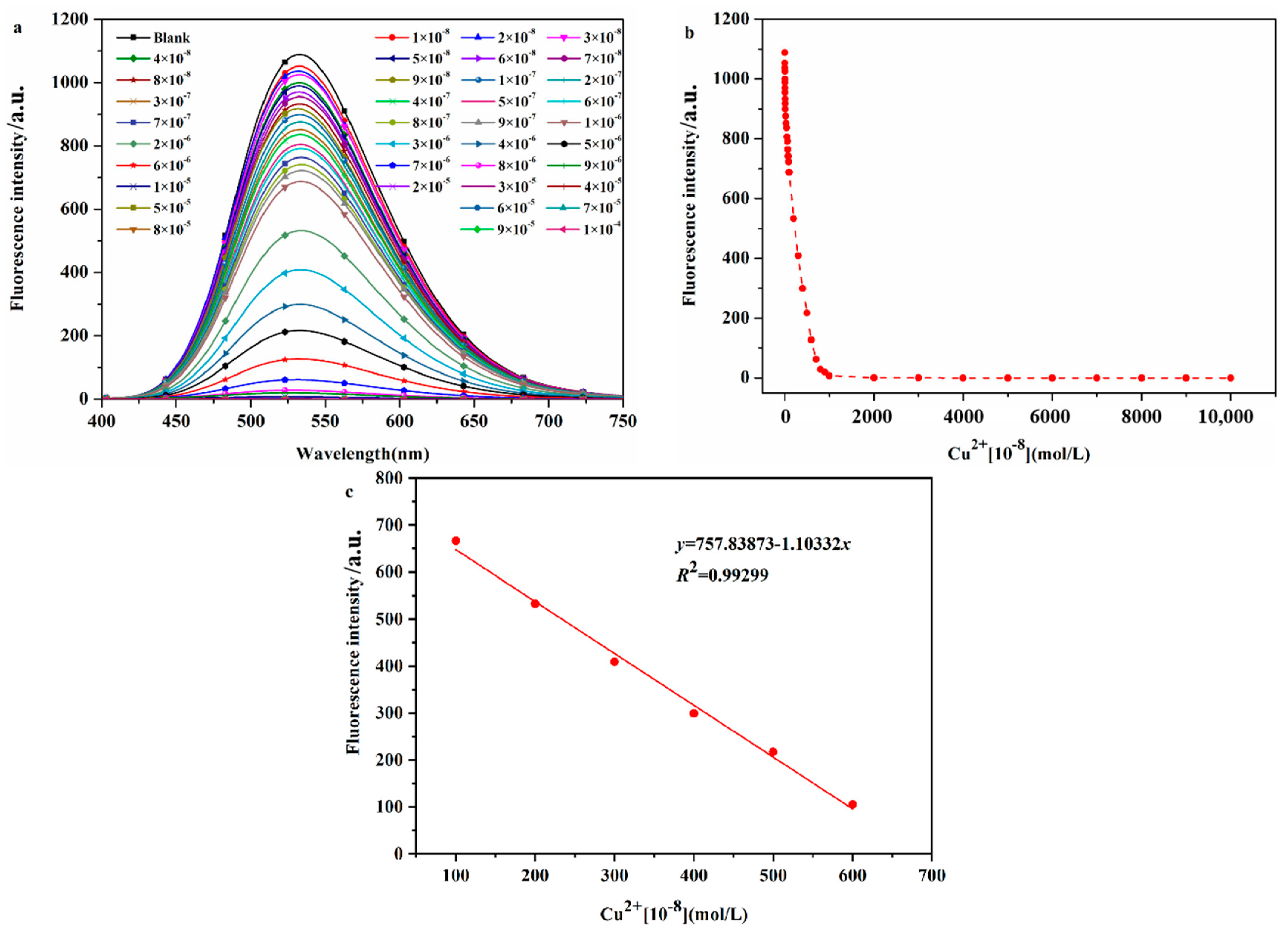
2.2.4. Determination of the Detection Limit of Probe DHBYMH for Cu2+ Ion Recognition
The sensitivity of a probe molecule for the target ion is determined by its detection limit, and a high-sensitivity probe molecule has higher practical application value. Therefore, the detection limit of DHBYMH for Cu2+ ions was determined, as shown by the experimental results in Figure 5. As shown in Figure 5a, the fluorescence intensity of the probe DHBYMH (1 × 10−5 M) gradually decreased with increasing Cu2+ ion concentrations (0~1 × 10−4 M) until complete quenching occurred. Figure 5b shows the fluorescence dot plot of DHBYMH at an emission wavelength of 533 nm for different concentrations of Cu2+ ions. In the 1 × 10−6 to 6 × 10−6 M concentration range, the fluorescence intensity of DHBYMH showed a good linear relationship with the Cu2+ ion concentration, with a regression equation of y = 1037.019 − 1.01083x and a correlation coefficient of 0.99299 (Figure 5c). Based on the 3σ rule, the detection limit of DHBYMH for Cu2+ ions was calculated to be 1.62 × 10−7 M, which is lower than the limit of 20.0 μM for Cu2+ ions in drinking water set by the U.S. Environmental Protection Agency (EPA), indicating that this probe has good practical application value (compared with other studies, Table S2).
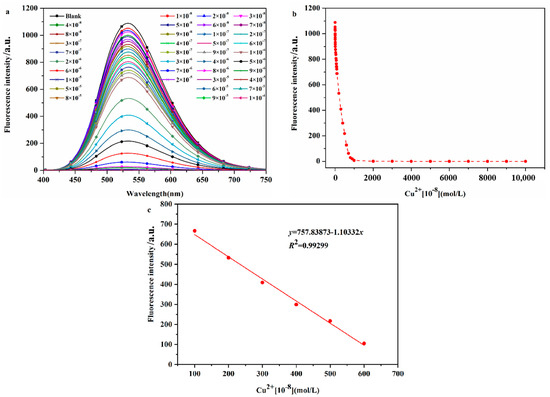
Figure 5.
The fluorescence spectra (a) of different concentrations of Cu2+ ions (0~1 × 10−4 M) recognized by probe DHBYMH (1 × 10−5 M, DMSO/H2O, v/v = 7/3, pH = 7.0, Cys: 1 × 10−4 M), and the fluorescence dot diagram (b) of Cu2+ ions recognized by probe DHBYMH at 533 nm and at concentrations of 1 × 10−6~6 × 10−6 M with intervals defined by curve fitting (c).
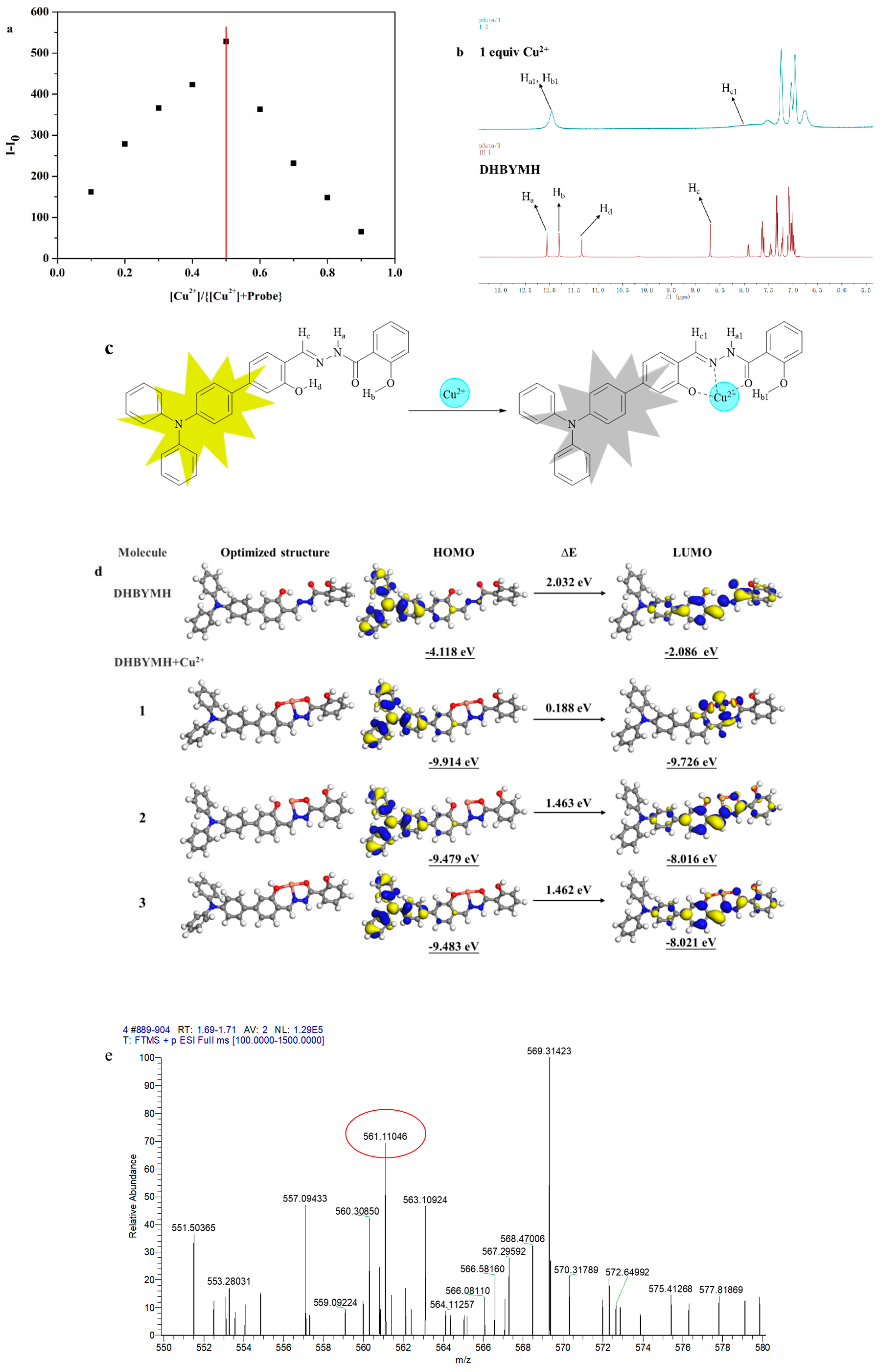
2.2.5. The Mechanism of Cu2+ Ion Recognition
The mechanism of Cu2+ ion recognition by the probe DHBYMH was studied. The first step involved a Job’s plot experiment (Figure 6a), which determined the coordination ratio of the probe DHBYMH to Cu2+ ions to be 1:1. In the second step, a nuclear magnetic resonance (NMR) titration experiment was conducted, and the results showed that the protons Ha, Hb, Hc, and Hd on the DHBYMH molecule experienced chemical shifts upon recognition by Cu2+ ions. Ha shifted from 12.05 ppm to 11.96 ppm, Hb shifted from 11.81 ppm to 11.96 ppm, the amino group’s Ha and hydroxyl group’s Hb merged into a single peak, Hc shifted from 8.70 ppm to 8.15 ppm, and the peak of Hd at 11.34 ppm disappeared. Based on this analysis, the Cu2+ ion recognition mechanism of DHBYMH involves Cu2+ coordinating with the N atom in the carbon–nitrogen double bond and the O atom in the carbonyl group to form a stable five-membered ring structure while also coordinating with the O atom in the hydroxyl group on the benzene ring connected to the triphenylamine group to form a stable six-membered ring structure (Figure 6b,c).
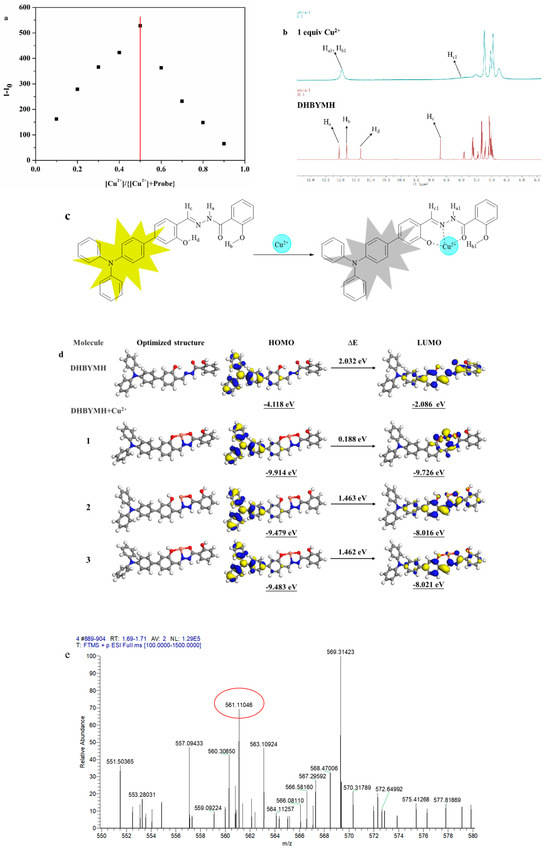
Figure 6.
Job’s curve of Cu2+ ions recognized by probe DHBYMH (a). NMR titration diagram of probe DHBYMH and Cu2+ ions (b). Cu2+ ion recognition mechanism of probe DHBYMH (c). Optimal geometry of DHBYMH and DHBYMH+Cu2+ and electron cloud distribution of HOMO and LUMO levels (d). High-resolution mass spectrometry of DHBYMH+Cu2+ (e).
To validate the above conclusions, density functional theory (DFT) calculations were performed using the DMol 3 module in Materials Studio software 8.0. Figure 6d shows the electronic cloud distribution of the optimized geometric structures of DHBYMH and DHBYMH+Cu2+ in three coordination structures (with structure 1 being the coordination structure mentioned above). The HOMO orbital electronic cloud distribution of DHBYMH and the three coordination structures of DHBYMH+Cu2+ are essentially evenly distributed in the triphenylamine group and the adjacent benzene ring. However, in the LUMO orbital electronic cloud distribution, the electronic cloud distribution of DHBYMH+Cu2+ in structure 1 is more concentrated in the five-membered ring structure and six-membered ring structure formed by DHBYMH and Cu2+ coordination compared to DHBYMH alone and in structures 2 and 3 (Figure 6d). This is due to the paramagnetic properties of Cu2+ in structure 1, which cause changes in the electronic transitions of the probe molecule, leading to fluorescence quenching, which is consistent with the experimental phenomenon. In addition, the HOMO–LUMO band gap of DHBYMH+Cu2+ in the calculated structure 1 is 0.188 eV, which is lower than that of the calculated structures 2 and 3 (1.463 eV, 1.462 eV). The HOMO–LUMO band gap below DHBYMH (2.032 eV) indicates that the molecular structure of DHBYMH+Cu2+ in calculated structure 1 is the most stable among the four structures, that is, Cu2+ ions preferentially form calculated structure 1 when coordinating with DHBYMH. High-resolution mass spectrometry was used to further verify the coordination of DHBYMH with Cu2+ ions in structure 1. The molecular ion peak at m/z 561.11046 is consistent with the molecular weight of structure 1 [DHBYMH+Cu2+–H+], as mentioned in Figure 6e. This further validates the recognition mechanism shown in Figure 6c [38].
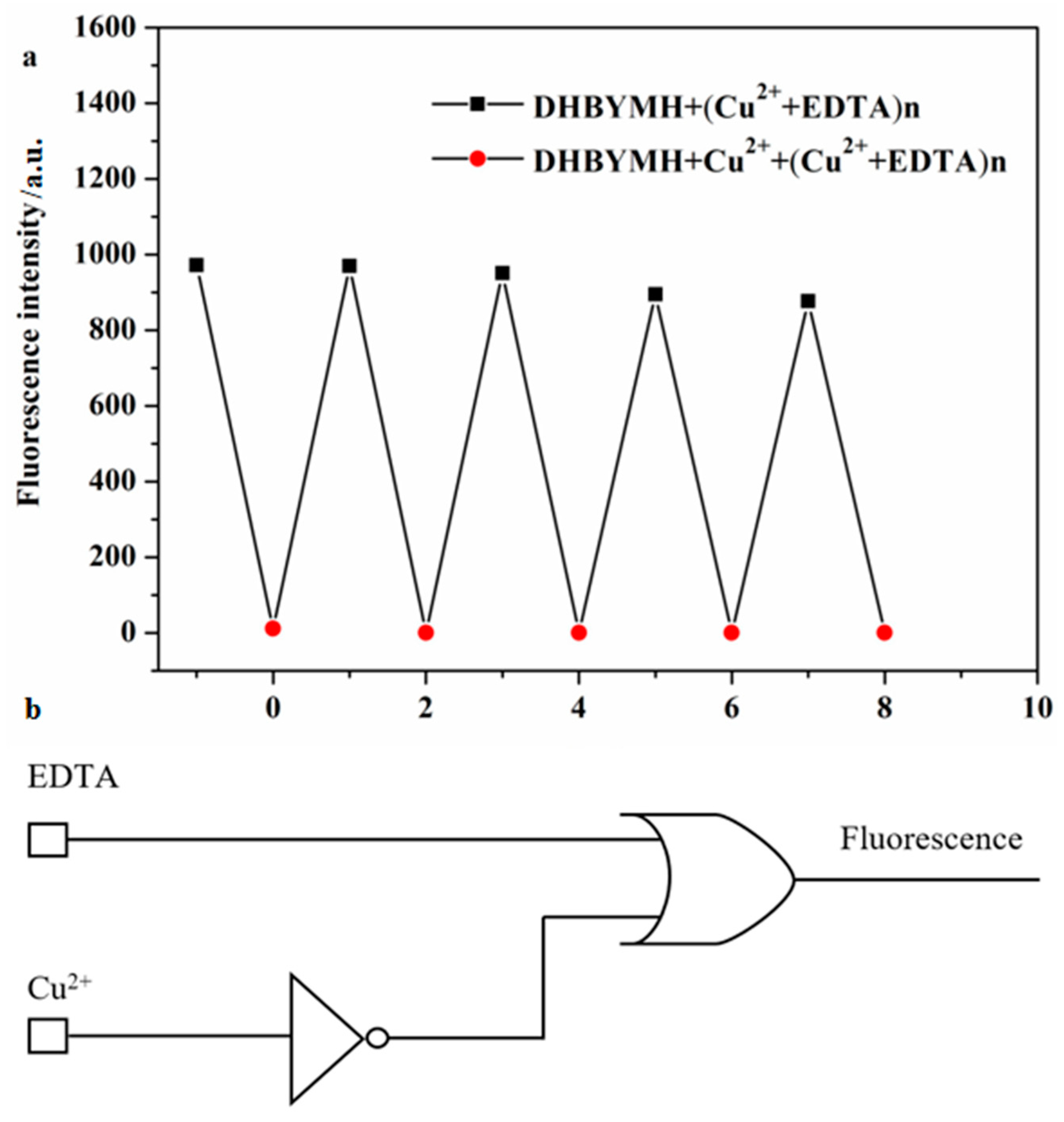
2.2.6. Construction of Molecular Logic Gates
The reversible recognition of target ions by fluorescent probes is beneficial because it enables more economical use of the probe molecules in practical applications. To study the reversible recognition performance of DHBYMH for Cu2+ ions, EDTA (ethylenediaminetetraacetic acid) can be used as a Cu2+ ion chelating agent to remove the Cu2+ ions that have already coordinated with DHBYMH, restoring the original fluorescence properties of DHBYMH. As shown in Figure 7a, the addition of EDTA to the DHBYMH+Cu2+ complex can restore the fluorescence of DHBYMH, and the addition of Cu2+ ions can quench the fluorescence again. After four cycles, DHBYMH still shows obvious fluorescence switching behavior. This indicates that DHBYMH has excellent reversibility and can be reused for detecting Cu2+ ions.
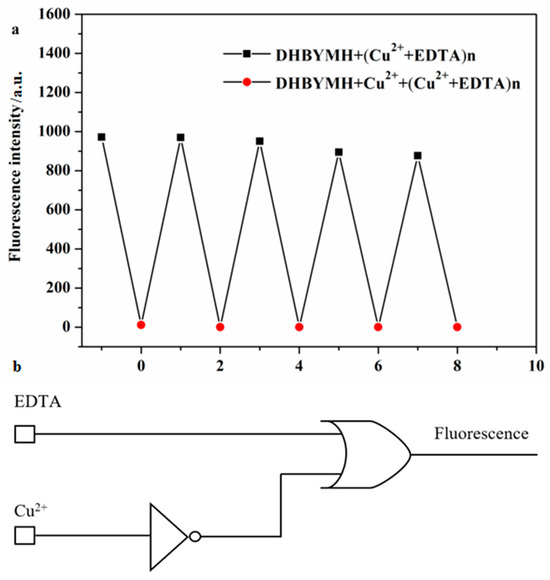
Figure 7.
Reversible recognition of Cu2+ by probe DHBYMH (a). Molecular logic circuit diagram with Cu2+ and EDTA as chemical inputs and fluorescence intensity as chemical output (b).
In recent years, designing molecular logic gates based on chemical substances has been an interesting topic in the study of electronic information technology and communication materials, which also provides new ideas for the practical application of probes [39,40,41]. Therefore, taking advantage of the reversibility of the probe DHBYMH, with Cu2+ ions and EDTA as chemical inputs and fluorescence intensity as the chemical output, a molecular logic gate was constructed. When inputting, Cu2+ ions and EDTA were added as 1, and their absence was 0; when outputting, the fluorescence emission was 1, and the fluorescence quenching was 0. According to the experimental data, the molecular logic gate truth table (Table 2) shows that when both Cu2+ ions and EDTA are absent (Entry 1) or only EDTA is present (Entry 2), DHBYMH shows fluorescence emission at 533 nm, outputting 1; when only Cu2+ ions are present (Entry 3), DHBYMH’s fluorescence is quenched, outputting 0; and when both Cu2+ ions and EDTA are present (Entry 4), DHBYMH shows fluorescence emission at 533 nm, outputting 1. Based on Table 2, a molecular logic circuit (Figure 7b) was designed.

Table 2.
Molecular logic gate truth table (Cu2+ and EDTA as chemical inputs, fluorescence intensity as chemical output).
3. Materials and Methods
3.1. Chemicals and Instruments
X-4 digital micromelting point determination instrument (Beijing TEC Instrument Co., Ltd., Beijing, China), Waters Q-TOF Premier time-of-flight mass spectrometer (WATERS Corporation, Milford, MA, USA), BRUKER 400 MHz nuclear magnetic resonance (Varian Inc., Palo Alto, CA, USA), DZF-6050 vacuum drying oven (Shanghai Hongdu Electronic Technology Co., Ltd., Shanghai, China), CP214 electronic analytical balance (Ohaus Instruments Co., Ltd., Shanghai, China), 78-1 magnetic heating stirrer (Shanghai Shuangjie Experimental Equipment Co., Ltd., Shanghai, China), YRE-2010 rotary evaporator (Gongyi City Yuhua Instrument Co., Ltd., Zhengzhou, China), TMS as an internal standard, CDCl3 or DMSO-d6 as solvents, UV-2450 type ultraviolet–visible spectrophotometer (Shimadzu Corporation, Kyoto, Japan), RF-5301PC fluorescence spectrophotometer (Shimadzu Corporation, Japan), and HORIBA Fluorolog-3 spectrophotometer (HORIBA Instruments Corporation, Irvine, CA, USA). All reagents used were analytical grade. Except for anhydrous ethanol and tetrahydrofuran, which were purified before use, other reagents were not further processed. The metal ions used, except for mercury acetate, were in the form of nitrate, and no further purification was performed before use.
3.2. Design and Synthesis
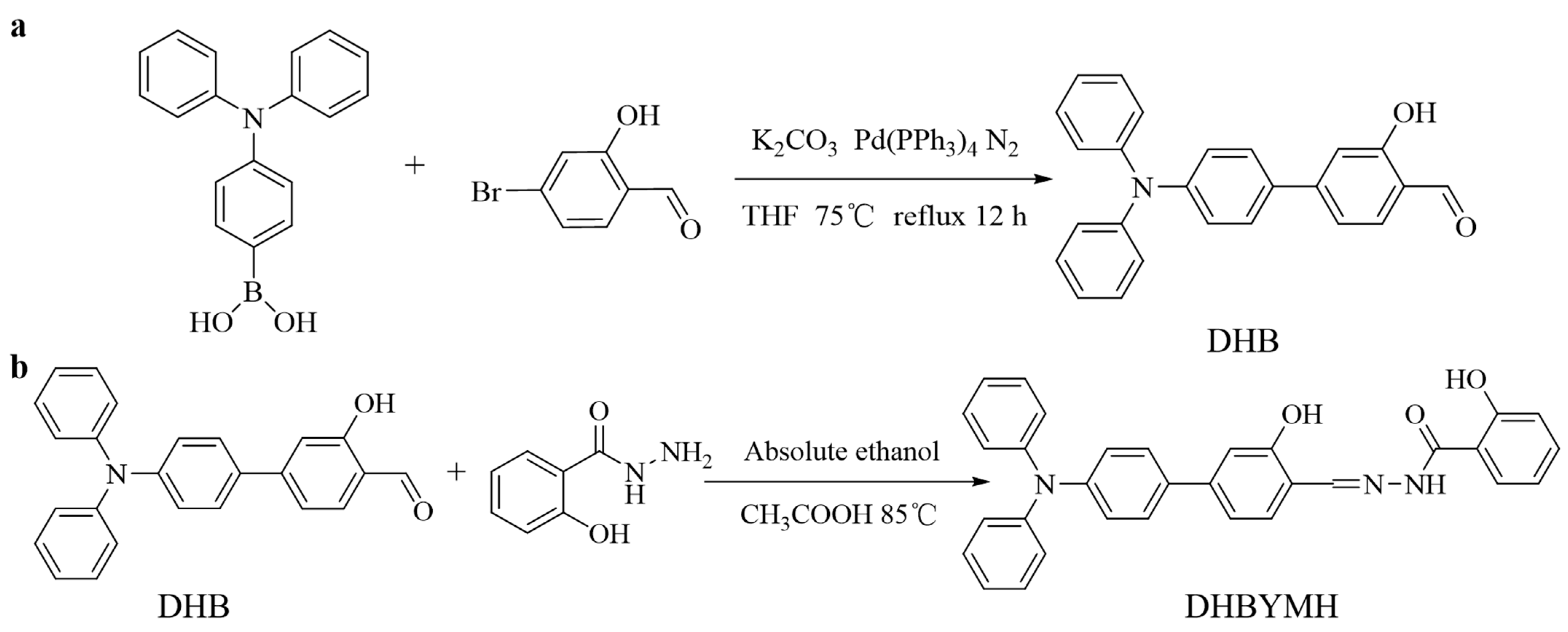
3.2.1. Synthesis of 4-(Diphenylamino)-3-hydroxy-[1,1′-biphenyl]-4-formaldehyde (DHB) [42,43]
Triphenylamine 4-borate (1 mmol), tetrakis (triphenylphosphine) palladium (Pd(PPh3)4, as catalyst), and 15.0 mL THF were added into a round-bottomed flask, and the reaction reflux was carried out for 0.5 h at 75 °C under the protection of N2. Then, 10.0 mL of THF solution dissolved in 4-bromo-2-hydroxybenzaldehyde (1.0 mmol) was added, and the reflux was continued at 75 °C under the protection of N2 for 12 h (Figure 8a). After cooling to room temperature, the mixture was poured into the liquid separation funnel and underwent demulsification with an appropriate amount of brine and then extraction with dichloromethane until the extraction liquid became colorless. The extracted organic phase was then dried with anhydrous sodium sulfate. Finally, the organic phase was separated and purified by column chromatography (elution ratio: ethyl acetate/petroleum ether, 1/30), and a solid greenish-yellow powder was obtained by rotary evaporation with a yield of 69%, which was DHB. M.p. 161–162 °C. FT-IR: 3434 cm−1; 3032 cm−1; 2834 cm−1; 1650 cm−1; 1591 cm−1; 1490 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 10.84 (s, 1H), 10.22 (s, 1H), 7.73 (s, 1H), 7.62 (d, J = 8.8 Hz, 2H), 7.35 (dd, J = 8.4, 7.5 Hz, 4H), 7.23 (d, J = 10.5 Hz, 2H), 7.17–7.06 (m, 6H), 7.02 (d, J = 8.8 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 192.00, 161.57, 148.49, 132.19, 130.80, 130.17, 128.41, 125.19, 124.23, 122.73, 118.01, 114.41.
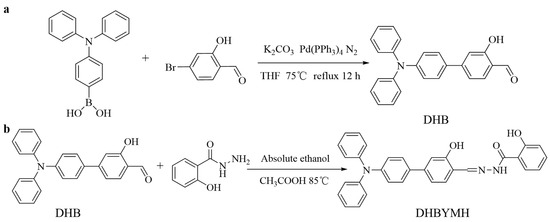
Figure 8.
Synthetic routes of DHB (a) and DHBYMH (b).
3.2.2. The Synthesis of N-((4-(Diphenylamino)-3-hydroxy-[1,1-biphenyl]-4-yl)methylene)-2-hydroxybenzohydrazide (DHBYMH)
The DHB (0.5 mmol), salicylaldehyde (0.5 mmol), ethanol (8 mL, as solvent) and glacial acetic acid (0.5 mL, as catalyst) were added into a round-bottomed flask, and the mixture was refluxed at 85 °C for 4 h (Figure 8b). After cooling to room temperature, a solid precipitate was collected by filtration. The obtained solid precipitate was recrystallized using anhydrous ethanol, DHBYMH has been successfully synthesized as a yellow solid powder with a yield of 91%. M.p. 186–187 °C. FT-IR: 3343 cm−1; 3059 cm−1; 3032 cm−1; 1655 cm−1; 1628 cm−1; 1591cm−1; 1489 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 12.05 (s, 1H), 11.80 (s, 1H), 11.34 (s, 1H), 8.70 (s, 1H), 7.93 (s, 1H), 7.73–7.54 (m, 3H), 7.46 (s, 1H), 7.38–7.31 (m, 4H), 7.23 (d, J = 10.7 Hz, 2H), 7.11–7.06 (m, 6H), 7.01 (dd, J = 16.5, 7.7 Hz, 4H). 13C NMR (101 MHz, DMSO-d6) δ 164.95, 159.54, 149.31, 147.38, 134.45, 133.09, 130.62, 130.11, 128.14, 124.92, 123.96, 123.22, 119.50, 117.79, 116.07, 114.00. HR-MS calculated for C32H25N3O3 [M + H]+ 500.19742 found 500.19653; HR-MS for [M + Na]+ 522.17936 found 522.17871.
3.3. DFT Calculations
All DFT calculations were performed using the DMol 3 module in Materials Studio software 8.0 [12,40]. The electron exchange correlation functional was described by the Becke–Lee–Yang–Parr functional within the generalized gradient approximation framework. Relativistic effects were accounted for by employing an effective core potential, and atomic orbitals were represented using a double numerical polarization basis set [14]. To ensure effective convergence, a smearing value of 0.005 Ha was selected to accelerate the convergence process. The energy convergence criteria were set at 2 × 10−5 Ha, while the maximum force and maximum displacement convergence criteria were set at 0.004 Ha/Å and 0.005 Å, respectively. All calculations were performed at Center for Computational Chemistry and Molecular Simulation, College of Chemistry and Chemical Engineering, Southwest Petroleum University.
4. Conclusions
In this paper, based on the excellent properties of triphenylamine groups and hydrazone structure, a novel triphenylamine aldehyde salicylhydrazone molecule, DHBYMH, was successfully synthesized by using “triphenylamine” as the molecular framework and modifying it with a Suzuki coupling reaction and an aldoamine condensation reaction with salicylhydrazine. Its selective response to metal ions was investigated using UV and fluorescence spectra. The optical properties of the probe molecules were studied, and it was found that DHBYMH showed a highly polarity-distorted ICT mechanism based on the π-bridge induced by o-hydroxybenzene, which showed that the maximum fluorescence emission wavelength of the organic solvent showed an obvious redshift phenomenon, with the successive increases in the redshift degree with increasing polarity of the organic solvent. At the same time, the DHBYMH probe molecules also exhibited an ACQ effect, which is mainly due to the aggregation of probe molecules. As the aggregation degree increases, the molecular packing mode changes, resulting in a change in the electron transition of π-conjugated molecules, resulting in fluorescence quenching. DHBYMH achieved a detection limit of 1.62 × 10−7 M by demonstrating exceptional selectivity and sensitivity towards Cu2+ ions in an optimum sample solvent system (DMSO/H2O, (v/v = 7/3); pH = 7.0; cysteine (Cys) concentration: 1 × 10−4 M). The response mechanism was determined by nuclear magnetic resonance titration experiments, high-resolution mass spectrometry, and DFT calculations. Finally, a molecular logic gate was successfully designed based on the reversible recognition of Cu2+ ions by DHBYMH in the presence of EDTA.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29092032/s1, Figure S1: Ultraviolet absorption spectra (a) and fluorescence emission spectra (b) of DHBYMH (1 × 10−5 M) in different ratios of H2O/DMS. (c) Physical images of DHBYMH (1 × 10−5 M) in different ratios of H2O/DMSO under UV lamp (365 nm) irradiation. Figure S2: Lippert−Mataga plot for DHBYMH in various solvents. Figure S3: DHBYMH (1 × 10−5 M, DMSO/H2O, v/v = 7/3, pH = 7.0, Cys: 1 × 10−4 M) recognition of Cu2+ ions response time. Figure S4: FT-IR of DHB; Figure S5: 1H NMR (400 MHz, DMSO-d6) spectrum of DHB; Figure S6: 13C NMR (101 MHz, DMSO-d6) spectrum of DHB; Figure S7: FT-IR of DHBYMH; Figure S8: 1H NMR (400 MHz, DMSO-d6) spectrum of DHBYMH; Figure S9: 13C NMR (101 MHz, DMSO-d6) spectrum of DHBYMH; Figure S10: HR-MS spectra of DHBYMH. Table S1: The Stokes shift of DHBYMH in various solvents with a range of Δf (orientation polarizability) values. Table S2: Comparison of probe DHBYMH with other Cu2+ probes reported in the literature. Refs. [44,45,46] are cited in Supplementary Materials.
Author Contributions
T.S.: conceptualization; methodology; writing—original draft. Z.X.: conceptualization; supervision. X.M., L.Z. and J.G.: funding acquisition; investigation; software. Y.F.: investigation; data curation. T.P.: supervision; resources. F.W.: software. M.Y.: investigation; data curation. J.Z. and L.Z.: supervision; resources; investigation; data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Zunyi Outstanding Youth Science and Technology Innovation Talents Training Project (ZunYouQingKe (2021) 7); Moutai Institute Joint Science and Technology Research and Development Project (ZunShiJiaoHe HZ Zi (2021) 322); Modern Baijiu Brewing Technology Engineering Research Center of Guizhou Universities, Qianjiaoji (2023) No. 028; Zunyi Science and Technology Bureau of Guizhou Province and Moutai Institute Joint Science and Technology Cooperation Fund Project, Zunyi Ke (2023) No. 114; Research Foundation for Scientific Scholars of Moutai Institute (Grant Nos. mygccrc[2022]007, mygccrc[2022]097, mygccrc[2022]022, and mygccrc[2022]031); Guizhou Province Distillers Grain and Agricultural Waste Resource Utilization Engineering Research Center (Grant No. 04500052); A Project on Characteristic Key Laboratory of Guizhou Ordinary Colleges and Universities by the Department of Education of Guizhou Province (grant No.: Qian Jiao He KY Zi [2018] 003); Guizhou Engineering Research Center for Comprehensive Utilization of Distillers’ Grains.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008, 88, 855S–858S. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ma, X.; Zhong, W.; Cao, Y.; Zhao, H.; Leng, X.; Yang, J.; Zhou, H.; She, M. Fluorescent sensing film decorated with ratiometric probe for visual and recyclable monitoring of Cu2+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 249, 119217. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Andreux, P.; Poitry-Yamate, C.; Auwerx, J.; Hanahan, D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 19507–19512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, K.; Liu, X. Distribution and pollution risk assessment of heavy metals in the surface sediment of the intertidal zones of the Yellow River Estuary, China. Mar. Pollut. Bull. 2022, 174, 113286. [Google Scholar] [CrossRef]
- Liu, Z.; Fei, Y.; Shi, H.; Mo, L.; Qi, J. Prediction of high-risk areas of soil heavy metal pollution with multiple factors on a large scale in industrial agglomeration areas. Sci. Total Environ. 2022, 808, 151874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Song, F.; Wei, G.; Wu, R.; Yan, Z.; Zhang, F.; Guang, S.; Xu, H. Molecular design for novel sensing materials with self-screening interference effect (SSIE): Reversible recognizing Cu2+ in aqueous and biologic samples. Sens. Actuators B Chem. 2019, 286, 163–172. [Google Scholar] [CrossRef]
- Hosseini, M.; Hashemimoghaddam, H. Sensitized extraction spectrophotometric determination of Hg(II) with dithizone after its flotation as ion-associate using iodide and ferroin. Talanta 2005, 67, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Danet, A.F.; Bratu, M.; Radulescu, M.; Bratu, A. Portable minianalyzer based on cold vapor atomic absorption spectrometry at 184.9nm for atmospheric mercury determination. Sens. Actuators B Chem. 2009, 137, 12–16. [Google Scholar] [CrossRef]
- Gong, J.; Zhou, T.; Song, D.; Zhang, L.; Hu, X. Stripping Voltammetric Detection of Mercury(II) Based on a Bimetallic Au-Pt Inorganic-Organic Hybrid Nanocomposite Modified Glassy Carbon Electrode. Anal. Chem. 2010, 82, 567–573. [Google Scholar] [CrossRef]
- Schlöglova, K.; Wälle, M.; Heinrich, C.A. LA-ICP-MS analysis of fluid inclusions: Contamination effects challenging micro-analysis of elements close to their detection limit. J. Anal. At. Spectrom. 2017, 32, 1052–1063. [Google Scholar] [CrossRef]
- Wu, J.; Boyle, E.A. Low Blank Preconcentration Technique for the Determination of Lead, Copper, and Cadmium in Small-Volume Seawater Samples by Isotope Dilution ICPMS. Anal. Chem. 1997, 69, 2464–2470. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, Q.; Zhou, D.; An, Y.; Wang, P.; Liao, F. A novel peptide-based fluorescent probe with a large stokes shift for rapid and sequential detection of Cu2+ and CN− in aqueous systems and live cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120257. [Google Scholar] [CrossRef]
- Wang, P.; Sun, L.; Wu, J.; Yang, X.; Lin, P.; Wang, M. A dual-functional colorimetric and fluorescent peptide-based probe for sequential detection of Cu2+ and S2- in 100% aqueous buffered solutions and living cells. J. Hazard. Mater. 2021, 407, 124388. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, M.; Rajalakshmi, K.; Ahn, D.; Yoon, S.; Nam, Y.; Lee, Y.; Xu, Y.; Song, J.; Lee, K. Tetraphenylethene-based fluorescent probe with aggregation-induced emission behavior for Hg2+ detection and its application. Anal. Chim. Acta 2021, 1148, 238178. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Pang, X.; Wang, Z.; Chai, Q.; Ye, F. A highly sensitive and selective fluorescent probe for determination of Cu (II) and application in live cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 208, 198–205. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Wang, E. A highly selective “turn-on” fluorescent probe for detecting Cu2+ in two different sensing mechanisms. Dye. Pigment. 2019, 163, 533–537. [Google Scholar] [CrossRef]
- Jiang, N.; Gong, X.; Zhong, T.; Zheng, Y.; Wang, G. A highly selective and sensitive “turn-on” fluorescent probe for rapid recognition and detection of Cu2+ in aqueous solution and in living cells. J. Mol. Struct. 2020, 1219, 128573. [Google Scholar] [CrossRef]
- Li, B.; Kou, J.; Mei, H.; Gu, X.; Wang, M.; Xie, X.; Xu, K. A hemicyanine-based “turn-on” fluorescent probe for the selective detection of Cu2+ ions and imaging in living cells. Anal. Methods 2020, 12, 4181–4184. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Zhao, L.; Xu, B. A dual-responsive and highly sensitive fluorescent probe for Cu2+ and pH based on a dansyl derivative. Dye. Pigment. 2020, 180, 108513. [Google Scholar] [CrossRef]
- Yin, J.; Wang, Z.; Zhao, F.; Yang, H.; Li, M.; Yang, Y. A novel dual functional pyrene-based turn-on fluorescent probe for hypochlorite and copper (II) ion detection and bioimaging applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118470. [Google Scholar] [CrossRef]
- Lina, G.; Gao, Y.; Han, L. Detecting Cu2+ and H2O in methanol based on aggregation-induced emission fluorescent enhancement. J. Coord. Chem. 2021, 74, 1284–1297. [Google Scholar] [CrossRef]
- Leng, X.; Wang, D.; Mi, Z.; Zhang, Y.; Yang, B.; Chen, F. Novel Fluorescence Probe toward Cu2+ Based on Fluorescein Derivatives and Its Bioimaging in Cells. Biosensors 2022, 12, 732. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Xie, Z.; He, J.; Wu, F.; Li, H.; Guo, J.; Zhao, J. Synthesis and Application of Acylhydrazone Probe with High Selectivity and Rapid Detection of Mercury Ion. ChemistrySelect 2023, 8, e202203827. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, H.; Liang, H.; Tao, Y.; Zhou, R.; Chu, Y.; Shi, T.; Xie, Z.; Wen, Y. Synthesis of Triazole Functionalized Triphenylamine Cu2+ Fluorescent Probe and Its Application in Detection and HeLa Cells. Chin. J. Org. Chem. 2022, 42, 1463–1473. [Google Scholar] [CrossRef]
- Xue, S.; Xie, Z.; He, J.; Zhang, T.; Xia, B.; Li, Y. Synthesis of Sulfonylhydrazone Probe with High Selectivity and Rapid Identification of Hg(Ⅱ)Ion and Its Application in Adsorption. Chin. J. Appl. Chem. 2021, 5, 760–768. [Google Scholar] [CrossRef]
- Xue, S.; Xie, Z.; Chu, Y.; Shi, W.; Liu, Y.; Zhao, Y. Highly selective and sensitive fluorescent probe possessing AIEE and ICT properties for rapid detection of Pb2+ in aqueous medium and its applications in living cells. Luminescence 2021, 37, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shi, W.; Yue, Y.; Chu, Y.; Xie, Z.; Xue, S. Synthesis of Sulfonylhydrazone Type Probe with High Selectivity for Rapid Detection of Mercury and Its Application in Adsorption and HeLa Cell. Chin. J. Org. Chem. 2021, 41, 1138–1145. [Google Scholar] [CrossRef]
- Yue, Y.; Xie, Z.; Chu, Y.; Xue, S. Synthesis and Optical Properties of Novel Spiro[chromo(2,3-c)-pyrazole-4,1′-isobenzofuran]-3′-one Compounds. Chin. J. Org. Chem. 2020, 40, 501–510. [Google Scholar] [CrossRef]
- Xue, S.; Xie, Z.; Wen, Y.; He, J.; Liu, Y.; Shi, W. Highly Selective and Sensitive Sulfonylhydrazone Type Fluorescent Probe for Rapid Detection of Mercury(II) and Its Application in Logic Gate and Adsorption. ChemistrySelect 2021, 6, 7123–7129. [Google Scholar] [CrossRef]
- Shi, T.; Xie, Z.; Mo, X.; Shi, W.; Qiu, H.; Lan, G.; Liu, Y. Adsorption behaviors of heavy metal ions by different hydrazone-modified sodium alginate in aqueous medium: Experimental and DFT studies. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130754. [Google Scholar] [CrossRef]
- Shi, T.; Xie, Z.; Mo, X.; Feng, Y.; Peng, T.; Song, D. Highly Efficient Adsorption of Heavy Metals and Cationic Dyes by Smart Functionalized Sodium Alginate Hydrogels. Gels 2022, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Xie, Z.; Zhu, Z.; Shi, W.; Liu, Y.; Liu, M. Highly efficient and selective adsorption of heavy metal ions by hydrazide-modified sodium alginate. Carbohydr. Polym. 2022, 276, 118797. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J.; He, J.; Zhang, J.; Zhou, H.; Gao, C. A novel red-emitting fluorescent probe for the highly selective detection of Hg2+ ion with AIE mechanism. Chem. Phys. 2020, 539, 110944. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, X.; Luo, X.; Hu, B.; Huang, W. A highly selective fluorescent probe based on coumarin and pyrimidine hydrazide for Cu2+ ion detection. Inorg. Chem. Commun. 2020, 114, 107823. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Zhi, W.; Huang, Y.; Han, J.; Wang, Y.; Ren, Y.; Ni, L. A new coumarin schiff based fluorescent-colorimetric chemosensor for dual monitoring of Zn2+ and Fe3+ in different solutions: An application to bio-imaging. Sens. Actuators B Chem. 2018, 260, 243–254. [Google Scholar] [CrossRef]
- Qin, J.; Yang, Z.; Wang, G.; Li, C. FRET-based rhodamine–coumarin conjugate as a Fe3+ selective ratiometric fluorescent sensor in aqueous media. Tetrahedron Lett. 2015, 56, 5024–5029. [Google Scholar] [CrossRef]
- Hua, C.; Zheng, H.; Zhang, K.; Xin, M.; Gao, J.; Li, Y. A novel turn off fluorescent sensor for Fe(III) and pH environment based on coumarin derivatives: The fluorescence characteristics and theoretical study. Tetrahedron 2016, 72, 8365–8372. [Google Scholar] [CrossRef]
- Erdemir, S.; Malkondu, S. A switch-on xanthene triphenylamine based fluorescent and colorimetric sensor for the detection of ultra-trace Hg2+ in food samples and living cells. Food Chem. 2022, 376, 131951. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Tong, Q.; Qin, X.; Liao, X.; Li, Q.; Yan, G. A hydrophilic naphthalimide-based fluorescence chemosensor for Cu2+ ion: Sensing properties, cell imaging and molecular logic behavior. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118029. [Google Scholar] [CrossRef]
- Mohanasundaram, D.; Vinoth Kumar, G.G.; Kumar, S.K.; Maddiboyina, B.; Raja, R.P.; Rajesh, J.; Sivaraman, G. Turn-on fluorescence sensor for selective detection of fluoride ion and its molecular logic gates behavior. J. Mol. Liq. 2020, 317, 113913. [Google Scholar] [CrossRef]
- Acharyya, S.; Gharami, S.; Sarkar, D.; Ghosh, P.; Murmu, N.; Mondal, T.K. A thioether containing reversible fluorescence “turn-on” chemosensor for selective detection of zinc(II): Applications in live cell imaging and inhibit logic gate. J. Mol. Struct. 2021, 1224, 129179. [Google Scholar] [CrossRef]
- Lin, H.; Shi, W.; Tian, Y.; Ma, F.; Xu, L.; Ma, J.; Hui, Y.; Xie, Z. A simple and highly selective ‘turn-on’ type fluorescence chemodosimeter for Hg2+ based on 1-(2-phenyl-2H-[1,2,3]triazole-4-carbonyl)thiosemicarbazide. J. Lumin. 2015, 157, 280–284. [Google Scholar] [CrossRef]
- Feng, L.; Shi, W.; Ma, J.; Chen, Y.; Kui, F.; Hui, Y.; Xie, Z. A novel thiosemicarbazone Schiff base derivative with aggregation-induced emission enhancement characteristics and its application in Hg2+ detection. Sens. Actuators B Chem. 2016, 237, 563–569. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Q.-M.; Bai, S.-J.; Wang, H.-C.; Ren, X.; Xu, Y.-X. Ladder-Type Dye with Large Transition Dipole Moment for Solvatochromism and Microphase Visualization. ACS Appl. Mater. Interfaces 2019, 11, 29814–29820. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Li, X.; Nie, S.; Liu, C.; Zhang, Y.; Guo, J.; Liu, C. A dual functional fluorescent probe based on naphthalimide for detecting Cu2+ and pH and its applications. Inorganica Chim. Acta 2023, 554, 121544. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Cao, J.-Q.; Ma, C.-M.; Zhang, Y.-P.; Guo, H.-C.; Xue, J.-J. A novel pyrazoline-based fluorescence probe armed by pyrene and naphthol system for the selective detection of Cu2+ and its biological application. J. Iran. Chem. Soc. 2022, 19, 3451–3461. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).