Synthesis of Flavonols and Assessment of Their Biological Activity as Anticancer Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
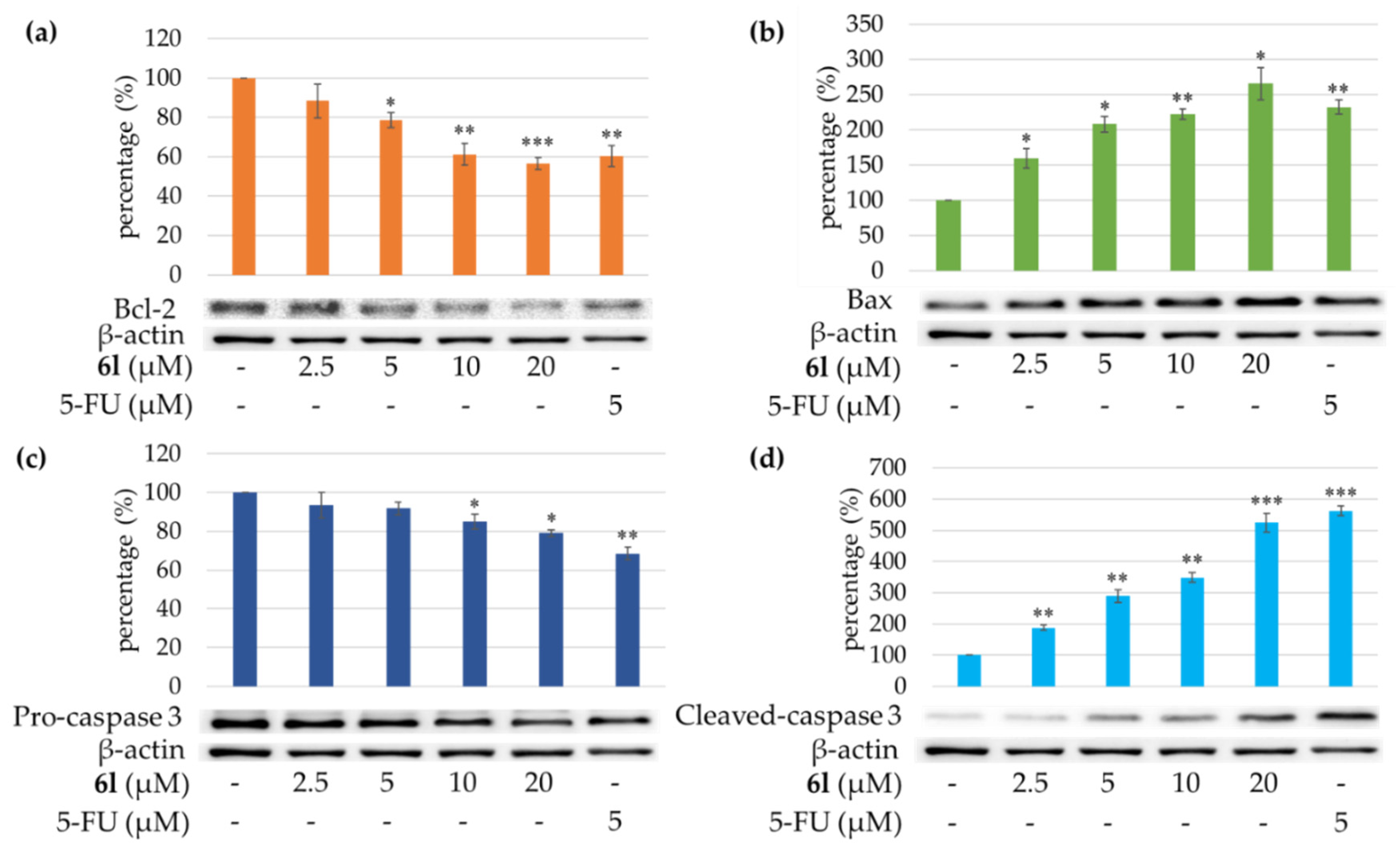
2.2. Pharmacology/Biology
3. Experimental
3.1. General Procedures
3.2. Mass Determination
3.3. Chemistry
3.3.1. General Preparation of Chalcones
3.3.2. General Preparation of Flavonols
3.3.3. 3-Hydroxy-2-phenyl-4H-chromen-4-one (6a)
3.3.4. 2-(2-Fluorophenyl)-3-hydroxy-4H-chromen-4-one (6b)
3.3.5. 2-(2-Chlorophenyl)-3-hydroxy-4H-chromen-4-one (6c)
3.3.6. 2-(2-Bromophenyl)-3-hydroxy-4H-chromen-4-one (6d)
3.3.7. 3-Hydroxy-2-(3-methoxyphenyl)-4H-chromen-4-one (6e)
3.3.8. 2-(3-Fluorophenyl)-3-hydroxy-4H-chromen-4-one (6f)
3.3.9. 2-(3-Chlorophenyl)-3-hydroxy-4H-chromen-4-one (6g)
3.3.10. 2-(3-Bromophenyl)-3-hydroxy-4H-chromen-4-one (6h)
3.3.11. 3-Hydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one (6i)
3.3.12. 2-(4-Fluorophenyl)-3-hydroxy-4H-chromen-4-one (6j)
3.3.13. 2-(4-Chlorophenyl)-3-hydroxy-4H-chromen-4-one (6k)
3.3.14. 2-(4-Bromophenyl)-3-hydroxy-4H-chromen-4-one (6l)
3.3.15. 6-Bromo-3-hydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one (7i)
3.3.16. 6-Bromo-2-(4-fluorophenyl)-3-hydroxy-4H-chromen-4-one (7j)
3.3.17. 6-Bromo-2-(4-chlorophenyl)-3-hydroxy-4H-chromen-4-one (7k)
3.3.18. 6-Bromo-2-(4-bromophenyl)-3-hydroxy-4H-chromen-4-one (7l)
3.4. Pharmacological/Biological Assays
3.4.1. Cell Culture
3.4.2. In Vitro Cytotoxicity Assay
3.4.3. Western Blotting Analysis
3.4.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martens, S.; Preuss, A.; Matern, U. Multifunctional flavonoid dioxygenases: Flavonol and anthocyanin biosynthesis in Arabidopsis thaliana L. Phytochemistry 2010, 71, 1040–1049. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.W.; Murugan, D.; Leong, X.F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: A mini review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Kawai, M.; Hirano, T.; Higa, S.; Arimitsu, J.; Maruta, M.; Kuwahara, Y.; Ohkawara, T.; Hagihara, K.; Yamadori, T.; Shima, Y.; et al. Flavonoids and related compounds as anti-allergic substances. Allergol. Int. 2007, 56, 113–123. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Wang, R. Phenolic contents, cellular antioxidant activity and antiproliferative capacity of different varieties of oats. Food Chem. 2018, 239, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Narang, R.; Singh, V.J.; Pilli, G.; Nayak, S.K. A review on anticancer profile of flavonoids: Sources, chemistry, mechanisms, structure-activity relationship and anticancer activity. Curr. Drug Res. Rev. 2023, 15, 122–148. [Google Scholar] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.C.; Orlikova, B.; Morceau, F.; Diederich, M. Natural and synthetic flavonoids: Structure-activity relationship and chemotherapeutic potential for the treatment of leukemia. Crit. Rev. Food Sci. Nutr. 2016, 56, S4–S28. [Google Scholar] [CrossRef] [PubMed]
- Gajender; Mazumder, A.; Sharma, A.; Azad, M.A.K. A comprehensive review of the pharmacological importance of dietary flavonoids as hepatoprotective agents. Evid. Based Complement. Alternat. Med. 2023, 2023, 4139117. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Rauf1, A.; Imran, M.; Khan, I.A.; Mujeeb-ur-Rehman; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F.; Andrés, A.C.; Florencia, A.; Carolina, E.; Felicia, R.M. Neuroprotective actions of flavones and flavonols: Mechanisms and relationship to flavonoid structural features. Cent. Nerv. Syst. Agents Med. Chem. 2013, 13, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Zanoaga, O.; Braicu, C.; Jurj, A.; Rusu, A.; Buiga, R.; Berindan-Neagoe, I. Progress in research on the role of flavonoids in lung cancer. Int. J. Mol. Sci. 2019, 20, 4291. [Google Scholar] [CrossRef]
- Kuo, W.T.; Tsai, Y.C.; Wu, H.C. Radiosensitization of non-small cell lung cancer by kaempferol. Oncol. Rep. 2015, 34, 2351–2356. [Google Scholar] [CrossRef]
- Zhang, H.W.; Hu, J.J.; Fu, R.Q. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 11255. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Flavonoids as anticancer agents: Structure-activity relationship study. Curr. Med. Chem. Anticancer Agents 2002, 2, 691–714. [Google Scholar] [CrossRef] [PubMed]
- Alzaabi, M.M.; Hamdy, R.; Ashmawy, N.S. Flavonoids are promising safe therapy against COVID-19. Phytochem. Rev. 2022, 21, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Samec, M.; Koklesova, L. Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed. Pharmacother. 2021, 138, 111430. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.S.; Farghadani, R.; Abidin, S.A.Z.; Othman, I.; Naidu, R. Flavonoids as receptor tyrosine kinase inhibitors in lung cancer. J. Funct. Foods 2023, 110, 105845. [Google Scholar]
- Shih, T.L.; Liu, M.H.; Li, C.W.; Kuo, C.F. Halo-substituted chalcones and azachalcones inhibited lipopolysaccharited-stimulated pro-inflammatory responses through the TLR4-mediated pathway. Molecules 2018, 23, 597. [Google Scholar] [CrossRef] [PubMed]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of chalcones derivatives and their biological activities: A review. ACS Omega, 2022; 7, 27769–27786. [Google Scholar]
- Shen, X.; Zhou, Q.; Xiong, W.; Pu, W.; Zhang, W.; Zhang, G.; Wang, C. Synthesis of 5-subsituted flavonols via the Algar-Flynn-Oyamada (AFO) reaction: The mechanistic implication. Tetrahedron 2017, 73, 4822–4829. [Google Scholar] [CrossRef]
- Bennett, M.; Burke, A.J.; O’Sullivan, W.I. Aspects of the Algar-Flynn-Oyamada (AFO) reaction. Tetrahedron 1996, 52, 7163–7178. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Li, C.W.; Kuo, C.L.; Shih, T.L.; Chen, J.J. Improved synthesis of asymmetric curcuminoids and their assessment as antioxidants. Molecules 2022, 27, 2547. [Google Scholar] [CrossRef]
- Magozwi, D.K.; Dinala, M.; Mokwana, N.; Siwe-Noundou, X.; Krause, R.W.M.; Sonopo, M.; McGaw, L.J.; Augustyn, W.A.; Tembu, V.J. Flavonoids from the genus Euphorbia: Isolation, structure, pharmacological activities and structure–activity relationships. Pharmaceuticals 2021, 14, 428. [Google Scholar] [CrossRef]
- Kurzwernhart, A.; Kandioller, W.; Bächler, S. Structure-activity relationships of targeted RuII(η6-p-cymene) anticancer complexes with flavonol-derived ligands. J. Med. Chem. 2012, 55, 10512–10522. [Google Scholar] [CrossRef] [PubMed]
- Dias, T.A.; Duarte, C.L.; Lima, C.F.; Proença, M.F.; Pereira-Wilson, C. Superior anticancer activity of halogenated chalcones and flavonols over the natural flavonol quercetin. Eur. J. Med. Chem. 2013, 65, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Rasheed, L.; Malik, A. Synthesis and characterization of variably halogenated chalcones and flavonols and their antifungal activity. Asian J. Chem. 2007, 19, 937. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Wang, R.Y.; Li, C.W.; Cho, S.T.; Chang, C.H.; Chen, J.J.; Shih, T.L. Synthesis of cinnamils and quinoxalines and their biological evaluation as anticancer agents. Arch. Pharm. 2022, 355, 2100448. [Google Scholar] [CrossRef]



| Compounds | IC50 (μM) a |
|---|---|
| 6a | 6.34 ± 0.89 ** |
| 6b | 38.17 ± 4.21 * |
| 6c | 24.54 ± 3.13 * |
| 6d | 22.97 ± 2.73 * |
| 6e | 15.07 ± 0.93 ** |
| 6f | 14.64 ± 1.48 * |
| 6g | 10.78 ± 0.97 ** |
| 6h | 8.25 ± 0.77 ** |
| 6i | >100 |
| 6j | 6.13 ± 0.63 ** |
| 6k | 3.14 ± 0.29 ** |
| 6l | 0.46 ± 0.02 *** |
| 7i | 22.04 ± 3.29 * |
| 7j | 19.44 ± 1.82 * |
| 7k | 31.35 ± 6.64 * |
| 7l | 47.58 ± 7.11 * |
| 5-FU b | 4.98 ± 0.41 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, Y.-H.; Hsu, P.-H.; Hu, A.; Cheng, Y.-J.; Shih, T.-L.; Chen, J.-J. Synthesis of Flavonols and Assessment of Their Biological Activity as Anticancer Agents. Molecules 2024, 29, 2041. https://doi.org/10.3390/molecules29092041
Hsieh Y-H, Hsu P-H, Hu A, Cheng Y-J, Shih T-L, Chen J-J. Synthesis of Flavonols and Assessment of Their Biological Activity as Anticancer Agents. Molecules. 2024; 29(9):2041. https://doi.org/10.3390/molecules29092041
Chicago/Turabian StyleHsieh, Yu-Hui, Pei-Hsuan Hsu, Anren Hu, Yang-Je Cheng, Tzenge-Lien Shih, and Jih-Jung Chen. 2024. "Synthesis of Flavonols and Assessment of Their Biological Activity as Anticancer Agents" Molecules 29, no. 9: 2041. https://doi.org/10.3390/molecules29092041
APA StyleHsieh, Y.-H., Hsu, P.-H., Hu, A., Cheng, Y.-J., Shih, T.-L., & Chen, J.-J. (2024). Synthesis of Flavonols and Assessment of Their Biological Activity as Anticancer Agents. Molecules, 29(9), 2041. https://doi.org/10.3390/molecules29092041








