Abstract
Deferoxamine, an iron chelator used to treat diseases caused by excess iron, has had a Food and Drug Administration-approved status for many years. A large number of studies have confirmed that deferoxamine can reduce inflammatory response and promote angiogenesis. Blood vessels play a crucial role in sustaining vital life by facilitating the delivery of immune cells, oxygen, and nutrients, as well as eliminating waste products generated during cellular metabolism. Dysfunction in blood vessels may contribute significantly to the development of life-threatening diseases. Anti-angiogenesis therapy and pro-angiogenesis/angiogenesis strategies have been frequently recommended for various diseases. Herein, we describe the mechanism by which deferoxamine promotes angiogenesis and summarize its application in chronic wounds, bone repair, and diseases of the respiratory system. Furthermore, we discuss the drug delivery system of deferoxamine for treating various diseases, providing constructive ideas and inspiration for the development of new treatment strategies.
1. Introduction
Iron is an essential trace element in the human body and plays an important role in biological activities such as oxygen transport, oxygen sensing, electron sensing, electron transfer, energy metabolism, and DNA synthesis [1]. However, excessive iron can lead to diseases such as hemochromatosis, thalassemia, myelodysplastic syndrome, aplastic anemia, etc. The iron-chelating drug deferoxamine (DFO) is commonly used in the treatment of such diseases [2]. DFO that has been approved for use by the Food and Drug Administration (FDA) is a natural product extracted from the fermentation liquor of Streptococcus spp. [3].
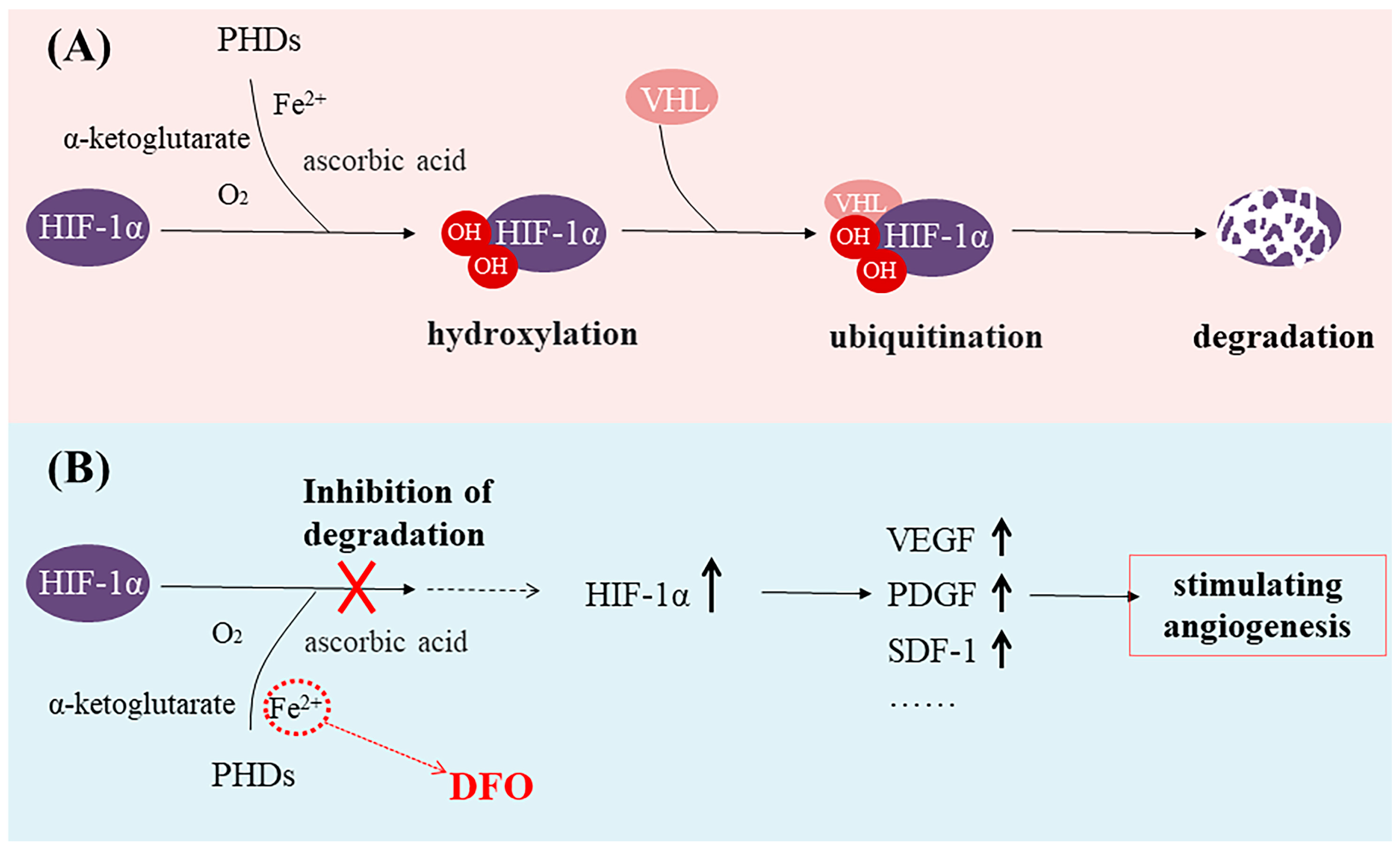
Iron is closely associated with inflammation [4,5,6]. During inflammation, the degradation of ferroportin increases, resulting in reduced iron excretion and elevated intracellular iron concentrations and ultimately leading to iron toxicity in cells and tissues [6,7,8]. DFO can bind to unliganded or incompletely liganded iron, rendering the ion inert and preventing its reaction with peroxides which, in turn, mitigates oxidative damage to tissues and alleviates oxidative stress [9,10]. Thus, DFO exhibits potent anti-inflammatory effects as an iron chelator and represents a promising therapeutic approach for mitigating inflammation in various autoimmune and inflammatory disorders [11]. In addition, studies have shown that Fe(II) in the prolyl hydroxylase domain (PHD) catalytic center can be exchanged or chelated by three hydroxamic acid groups of DFO, making PHD enzymes inactive [12]. Because PHD is a hypoxia-inducible factor (HIF) prolyl hydroxylase, it is known to play an important role in oxygen regulation in the physiological network. Hypoxia-inducible factor-1α (HIF-1α) is an oxygen-sensitive molecule [13,14,15,16]. The expression of HIF-1α is upregulated in hypoxic conditions and subsequently regulates multiple target genes [17,18,19,20,21]. The PHD utilizes O2 and α-ketoglutarate as substrates to hydroxylate two proline residues of HIF-1α [22,23,24]. Then, the Von Hippel–Lindau protein (VHL) swiftly degrades the hydroxylated HIF-1α [25,26] (Figure 1A). Thus, HIF-1α-mediated gene transcription is inhibited [22]. DFO is able to activate and stabilize a hypoxic HIF-1α pathway by rendering PHD inactive [27,28]. Then, upregulated HIF-1α expression can increase the expression of vascular endothelial growth factor (VEGF, a key signaling molecule in the induction of angiogenesis), platelet-derived growth factor (PDGF), stromal cell-derived factor-1 (SDF-1), and other growth factors, thus stimulating angiogenesis [29,30,31,32,33] (Figure 1B). Numerous studies have demonstrated that DFO, functioning as an iron chelator, can effectively induce the accumulation of HIF-1α, subsequently leading to a significant promotion in endothelial tube formation, cell proliferation, and migration [34,35,36].
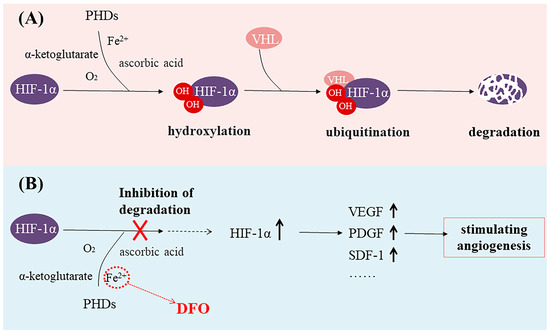
Figure 1.
(A) The prolyl hydroxylase domain (PHD) utilizes O2 and α-ketoglutarate as substrates to hydroxylate two proline residues of hypoxia-inducible factor-1α (HIF-1α), leading to the degradation of HIF-1α. (B) Deferoxamine (DFO) binds to Fe2+, makes PHD enzymes inactive, and stabilizes the expression of HIF-1α.
The blood vasculature is a closed circulatory system and includes networks of arteries, veins, and capillaries [37]. They play a crucial role in sustaining vital life by facilitating the delivery of immune cells, oxygen, and nutrients, as well as eliminating waste products generated during cellular metabolism [38,39,40,41,42]. The endothelial cells (ECs) are enveloped by mural cells to varying degrees to form blood vessels in various circulatory network locations. Endothelial cells (ECs) line the innermost layer of all of these vessels and exhibit a high degree of heterogeneity among different sections of the vasculature. They play an important role in sensing the circulating environment and responding to extrinsic signals [38]. The process of blood vessel development is intricate, with our current understanding indicating that endothelial cells are the earliest differentiated blood vessel cells during embryonic development and play a pivotal role in the formation of blood vessel walls and the establishment of complete blood vessel networks [43,44,45,46]. In adult organisms, ECs rarely proliferate and remain dormant, but they retain the ability to rapidly form new blood vessels in nutrient-deficient, ischemic/hypoxic environments to restore blood flow (providing oxygen and nutrients) in order to support tissue growth and function [47]. VEGF is implicated in multiple steps of vascular EC development [48] and is a key signaling molecule in the induction of angiogenesis. DFO is able to render the PHD inactive to activate and stabilize HIF-1α in order to increase the expression of VEGF in cells (such as stem cells, human dermal fibroblast cells, and human umbilical vein endothelial cells) [27,28]. VEGF can trigger quiescent ECs to become activated [47] in order to promote cell proliferation and migration and thus promote angiogenesis.
Dysfunction in blood vessels may significantly contribute to the development of life-threatening diseases [49]. Anti-angiogenesis therapy and pro-angiogenesis/angiogenesis strategies have been frequently recommended for various diseases [47]. Chronic, non-healing wounds are a persistent medical problem, and reduced blood vessel growth is a key reason many chronic wounds are difficult to heal [50,51,52]. Thus, targeted angiogenesis therapy is playing an increasingly important role as a therapeutic strategy for wound healing [50,53,54]. In the skeletal system, the local vascular system is actively involved in bone formation and bone resorption [55,56]. Angiogenesis plays a central role in bone reconstruction by providing oxygen, minerals, nutrients, and growth factors to the injured microenvironment [57,58]. Angiogenesis also plays a pivotal role in the intricate process of fetal lung development and subsequent tissue regeneration following lung transplantation. Based on the role of DFO in promoting angiogenesis, this review discusses the application of DFO in various diseases and provides constructive ideas and enlightenment for the development of more therapeutic strategies for DFO.
2. Chronic Wounds
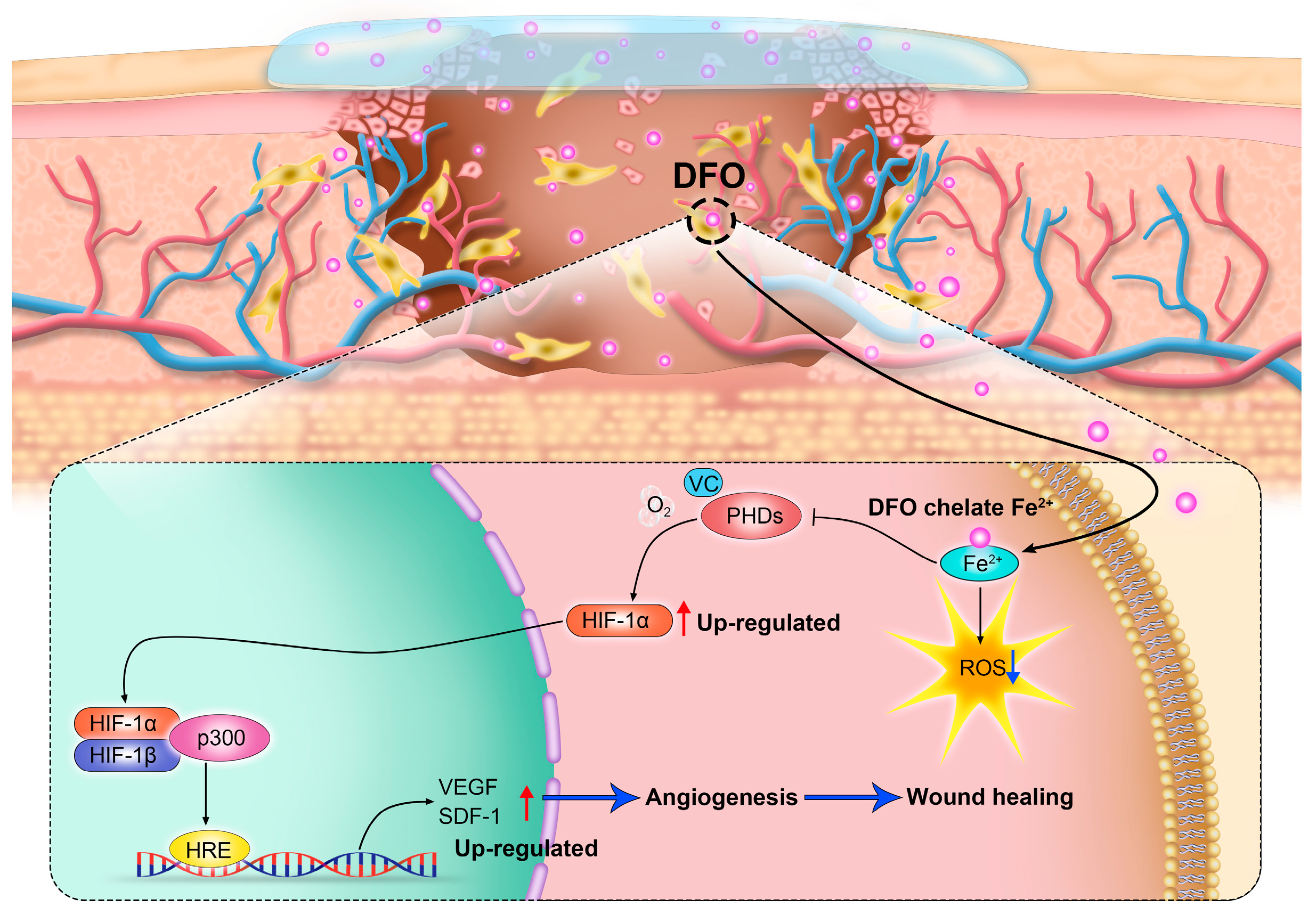
Wound healing typically moves through four overlapping stages: hemostasis/coagulation, inflammation, proliferation, and maturation/remodeling [59]. Chronic wounds fail to proceed through a normal, orderly, and timely repair sequence, resulting in delayed wound healing or even non-healing wounds [50]. Chronic wounds are classified by their etiology into four categories: arterial, diabetic, pressure, and venous ulcers [59,60]. Chronic wounds are often companied by high levels of proinflammatory cytokines, persistent infections, the formation of drug-resistant microbial biofilms, and senescent cells that do not respond to repair stimuli [59]. Over the years, chronic wounds have caused great suffering for patients. Non-healing chronic wounds impose physical, psychological, social, and financial burdens on individuals and the broader health system [61]. Reduced angiogenesis is one of the primary causes of the non-healing nature of chronic wounds [50,62]. During the healing process, angiogenesis is an important behavior in the phase of proliferation. Stimulated by moderate hypoxia, cytokines, and protein hydrolases, endothelial cells are activated to proliferate and migrate toward pro-angiogenic signals (such as VEGF and PDGF) to induce angiogenesis [63]. In addition, pericytes and smooth muscle cells can stabilize neovascularization [50]. The new blood vessel network delivers oxygen and nutrients to the damaged tissue and maintains cell function. It can also provide the wound site with cytokines and other substances necessary to repair the damaged tissue. Therefore, promoting angiogenesis and rebuilding tissue blood flow are promising therapeutic targets of new therapies to promote chronic wound healing [50]. Numerous studies have demonstrated that the promotion of angiogenesis can enhance the healing process of chronic wounds [64,65,66,67]. It is worth noting that chronic wounds have a common characteristic: the local deposition of free iron [68]. By chelating iron deposited at the wound site, DFO not only mitigates oxidative stress but also activates the HIF-1α/VEGF pathway (as mentioned previously), thereby facilitating neovascularization and, ultimately, promoting the healing of chronic wounds [69] (Figure 2).
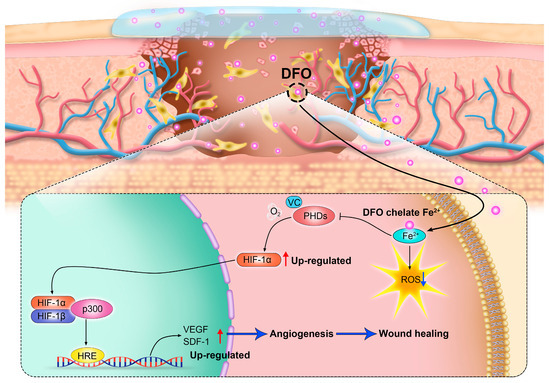
Figure 2.
By regulating the hypoxia-inducible factor-1α (HIF-1α) signaling pathway, deferoxamine (DFO) promotes angiogenesis and accelerates wound healing.
2.1. Diabetic Wounds
Persistent hyperglycemia has been shown to detrimentally impact vascular function and elevate susceptibility to infection. Therefore, diabetic wounds frequently do not follow the four stages of wound healing and often develop into chronic wounds [70]. Diabetic foot ulcers are a classic chronic wound [59,60]. Increasing evidence suggests that defective angiogenesis significantly contributes to a delay in diabetic wound healing because damaged blood vessels are unable to deliver critical oxygen and nutrients to the wounded tissue [71]. Thus, promoting angiogenesis is crucial in diabetic wound healing. This process depends on the proliferation and migration of endothelial cells in response to cytokines such as VEGF. As mentioned above, DFO can stimulate the HIF-1α/VEGF pathway to stimulate angiogenesis; therefore, DFO is expected to promote diabetic wound healing.
A recent study investigated the effect of DFO on diabetic wounds. The researchers showed that DFO was able to enhance angiogenesis and accelerate wound healing in diabetic patients by accumulating HIF-1α and regulating endothelial cell function [36]. Dominik Duscher et al. compared the efficacy of the hydroxylase inhibitor dimethyl oxalate (DMOG) and DFO in ameliorating diabetes-related skin wound healing defects by augmenting HIF-1α activation both in vitro and in vivo. The findings demonstrated that DFO effectively stabilized HIF-1α expression in the presence of hypoxia and hyperglycemia, surpassing the impact of DMOG on wound healing and angiogenesis in aged and diabetic mice. These results highlight the significant therapeutic potential of local administration of DFO for diabetic wounds [72].
During the past few years, researchers have worked to use an appropriate approach to enable DFO to perform better in treating diabetic wounds and reducing its side effects. Thus, researchers have taken an interest in utilizing wound dressings loaded with DFO to treat diabetic wounds. Hao Chen et al. utilized DFO-loaded hydrogel nanofibrous scaffolds and a DFO-loaded photo-crosslinked gelatin hydrogel to exploit their potential in promoting diabetic wound healing. The incorporation of DFO into the wound dressing created an optimal microenvironment for cell viability, adhesion, and proliferation. Moreover, the sustained release of DFO significantly enhanced neovascularization. Ultimately, both in vitro and in vivo experiments demonstrated the safety and efficacy of these strategies [73,74]. In addition, the co-delivery of various drugs which have complementary bioactivity provides a better therapeutic strategy for treating diabetic wounds [75]. Due to the synergistic effect of combining DFO and liposome nanoparticles, drug delivery can be enhanced and maintained, thereby amplifying the therapeutic response. Asif Qayoom et al. developed lecithin-based DFO nanoparticles which exhibit superior potential in treating diabetic wounds compared to using DFO alone [76]. Lingzhi Kong et al. demonstrated the synergistic effect of bioglass (BG) (which has been shown to promote vascular regeneration by modulating the expression of VEGF through the inclusion of Si ions) and DFO in promoting revascularization and developed an injectable hydrogel incorporating both BG and DFO for the treatment of chronic diabetic wounds. The findings revealed that the hydrogel exhibited superior efficacy in enhancing wound healing compared to either BG or DFO alone [3]. Bacterial infection and insufficient angiogenesis are the main factors that hinder the healing of diabetic ulcers. Therefore, antimicrobial and angiogenic treatment strategies are key to treating diabetic ulcer wounds. Shan Gao et al. loaded a microneedle patch with the antibacterial drug tetracycline hydrochloride and DFO at the same time, and the prepared microneedle patch not only had good antibacterial properties but also promoted angiogenesis, thus promoting the healing of diabetic ulcer wounds [77]. The in-depth investigation of DFO in diabetic ulcer treatment underscores the pivotal role of angiogenesis in wound repair, thereby providing valuable insights for advancing wound healing therapies [78,79].
2.2. Burn Wounds
Burn wounds may secrete a large amount of exudate, increasing excessive inflammation and leading to wound infection, scar formation, and even damage to new blood vessels. Eventually, these wounds may progress into chronic non-healing wounds [80]. The combined action of VEGF, PDGF, and other factors could effectively improve cell (cells involved in skin wound healing and inflammation) function, including proliferation, migration, differentiation, collagen remodeling, etc. Therefore, therapeutic strategies that regulate growth factors at the wound site may promote skin tissue regeneration in burn wounds [81]. Angiogenesis provides nutrients and oxygen to damaged tissues, is essential for maintaining normal cell function, and is an important part of tissue regeneration [47]. Oxidative stress and inflammation may mediate cellular damage and tissue destruction, as the burn wound continues to progress after the abatement of the initial insult [82,83]. Intervening in oxidative stress-induced excitation damage can prevent the progression of partial-thickness second-degree burns to a deep partial-thickness burn or of a deep second-degree burn becoming a third-degree burn [82]. Trace metals such as iron and copper may induce vital cellular injuries via lipid peroxidation [84,85]. Amina El Ayadi et al. treated porcine brass comb burn models with the Livionex formulation (LF) lotion (containing ethylenediaminetetraacetic acid as a metal chelator), and the experimental results showed that the application of LF lotion onto burn wounds provided protection oxidative damage and inflammation and prevented subsequent burn wound progression [82]. Therefore, it is imperative to devise therapeutic strategies that promote angiogenesis and prevent excessive inflammation. DFO can inhibit the activity of PHD, upregulate the expression of HIF-1α, and subsequently stimulate the expression of various growth factors (such as VEGF, PDGF, and SDF-1) [29,30,31,32,33]. In addition, DFO, as an iron-chelating agent, can chelate free iron at the wound site, which is expected to prevent excessive inflammation at the burn wound site and promote wound healing [86,87]. Wu Hongfu et al. developed a hydrogel based on the anti-inflammatory effect of glycyrrhizic acid (GA) and the angiogenic effect of DFO. They demonstrated that the hydrogel effectively reduced pro-inflammatory mediators (TNF-α and IL-6) and upregulated anti-inflammatory mediators (TGF-β3) while promoting proliferation, migration, and angiogenesis of human umbilical vein endothelial cells (HUVECs). Finally, the evaluation of a deep second-degree burn wound model in rats demonstrated that the synthetic hydrogel expedited burn wound healing, providing substantiation for its potential application in treating burn wounds through anti-inflammatory and angiogenesis-promoting mechanisms [80].
2.3. Leg Ulcers as the Main Complications of SCD
Leg ulcers are the main complications of sickle cell disease (SCD); about 2.5–40% of SCD patients have the risk of developing leg ulcers because of chronic hemolysis and poor angiogenesis. Leg ulcers are often difficult to heal [88]. As early as 1968, DFO was FDA-approved for chelation of the excess iron produced by hemolysis in SCD patients [89,90]. In order to achieve effective and localized delivery of DFO for ulcer treatment, Melanie Rodrigues developed a novel transdermal delivery system for DFO (DFO-TDDS) that utilizes reverse micelles to ensure continuous delivery of DFO to the skin surface. Rodrigues’ team initially created excision wounds in a transgenic sickle cell mouse model expressing > 99% human sickle hemoglobin (HbSS-BERK); these were subsequently treated with DFO-TDDS. The findings demonstrated that DFO-TDDS significantly expedited wound healing in HbSS-BERK mice by effectively chelating excessive free iron [91]. Their research makes it possible to translate DFO-TDDS into an effective treatment for patients with sickle cell leg ulcers (SCLUs).
3. Bone Repair
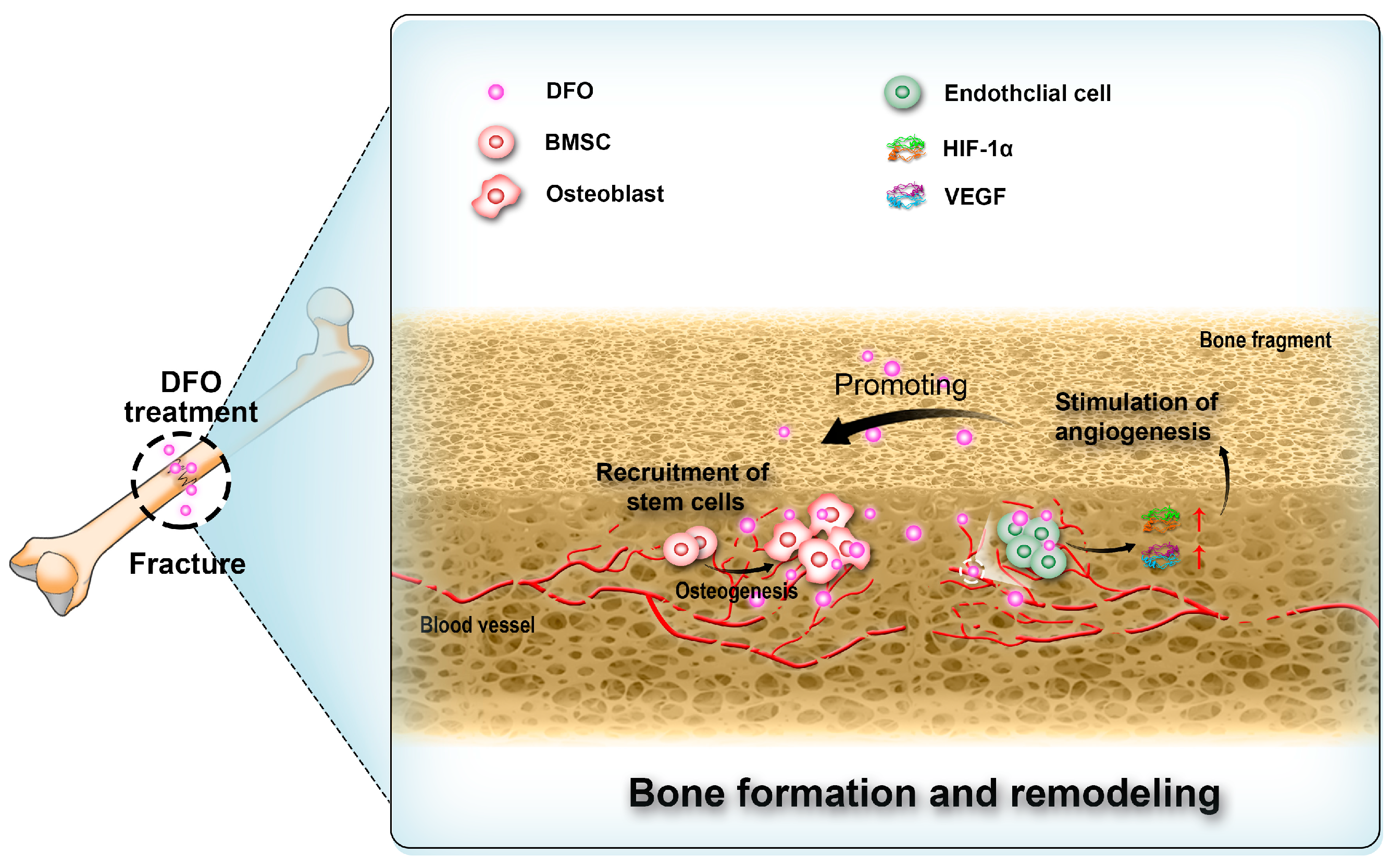
Angiogenesis is critical for bone regeneration [92,93,94,95,96]. Following fractures, a substantial quantity of locally produced angiogenic growth factors stimulates the process of angiogenesis. These vascular networks not only facilitate the supply of oxygen and nutrients [97,98,99,100,101] but also contribute to the recruitment of bone marrow stem cells (BMSCs) for osteoblastic differentiation and provision of essential ions required for subsequent mineralization stages. Thus, they play a pivotal role in bone regeneration [101,102,103]. Prolyl hydroxylase inhibitors have demonstrated efficacy in activating the HIF-1α pathway [104,105,106], thereby effectively promoting angiogenesis (Figure 3). Thus, DFO as a prolyl hydroxylase inhibitor has been proposed for use in bone repair [107,108,109,110]. Rui Shi et al. co-encapsulated DFO-loaded NPs and free DFO in nanofibers through coaxial electrospinning and investigated its effects on cell viability, migration, and osteogenic differentiation. The results suggested that DFO maintained cell viability and promoted the migration of human mesenchymal stem cells. Alkaline phosphatase (ALP) activity, calcium deposition, and the expression of osteogenesis-related markers and HIF-1α were all increased with DFO, indicating that DFO may accelerate bone regeneration [111].
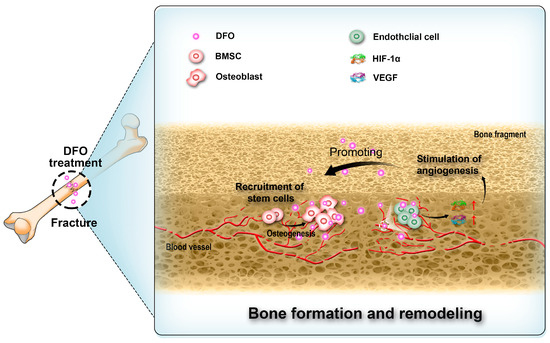
Figure 3.
Deferoxamine (DFO) interacts with endothelial cells, bone marrow stem cells (BMSCs), and osteoblasts in the process of bone regeneration.
3.1. Distraction Osteogenesis
Distraction osteogenesis (DO) is a technique to initiate regeneration by using mechanical strain to enhance the biological response of injured tissue. It is a metabolic-dependent reconstruction process that relies heavily on adequate local blood supply [112]. However, when distraction osteogenesis is used for bone repair after radiotherapy, the distraction osteogenesis therapy is ineffective due to the reduction of blood vessels [113]. Researchers have confirmed that DFO can optimize the quality and quantity of the regeneration tissue in the sites of mandibular distraction by augmenting vascularity [114,115]. Moreover, in a DO model featuring radiation-induced impairment of bone healing, angiogenesis, and biomechanical properties, DFO has demonstrated the ability to restore vascularity to the distraction site, thereby counteracting the detrimental effects caused by radiation therapy (XRT) and facilitating bone regeneration [108,116,117]. The findings presented here enhance the potential utility of vascular enhancement as a means to optimize bone regeneration in DO.
3.2. Steroid-Induced Osteonecrosis of the Femoral Head
Steroids can reduce the expression of VEGF and disrupt vascularization [118]. Therefore, addressing angiogenesis is critical for the treatment of steroid-induced osteonecrosis of the femoral head (ONFH). Jia Li et al. first reported that local DFO administration can improve angiogenesis and bone repair in early-stage models of rabbit ONFH, which may be an efficient, economical, and facile method to treat early-stage ONFH [119].
3.3. Bone Defects
In recent years, researchers have made fresh attempts to use DFO treatment in bone repair. Biomimetic materials produced by 3D printing offer a good treatment method for bone transplantation after major defects, and they also make up for the disadvantages of bone autografting [120]. Although the scaffold-based approach has a great therapeutic potential, it relies on the construction of new blood vessels for regeneration; thus, induction of neovascularization at the site of regeneration is crucial [121]. Justin Drager et al. used 3D technology to print biomimetic materials which were transplanted into a rabbit model of bone segmental defect and, through local injection of DFO, increased the formation of blood vessels at the injured site, creating an environment conducive to bone repair [120]. The findings present a novel concept for the design of bone scaffolds with potential for vascularization.
4. Lung and Airway
4.1. Bronchopulmonary Dysplasia
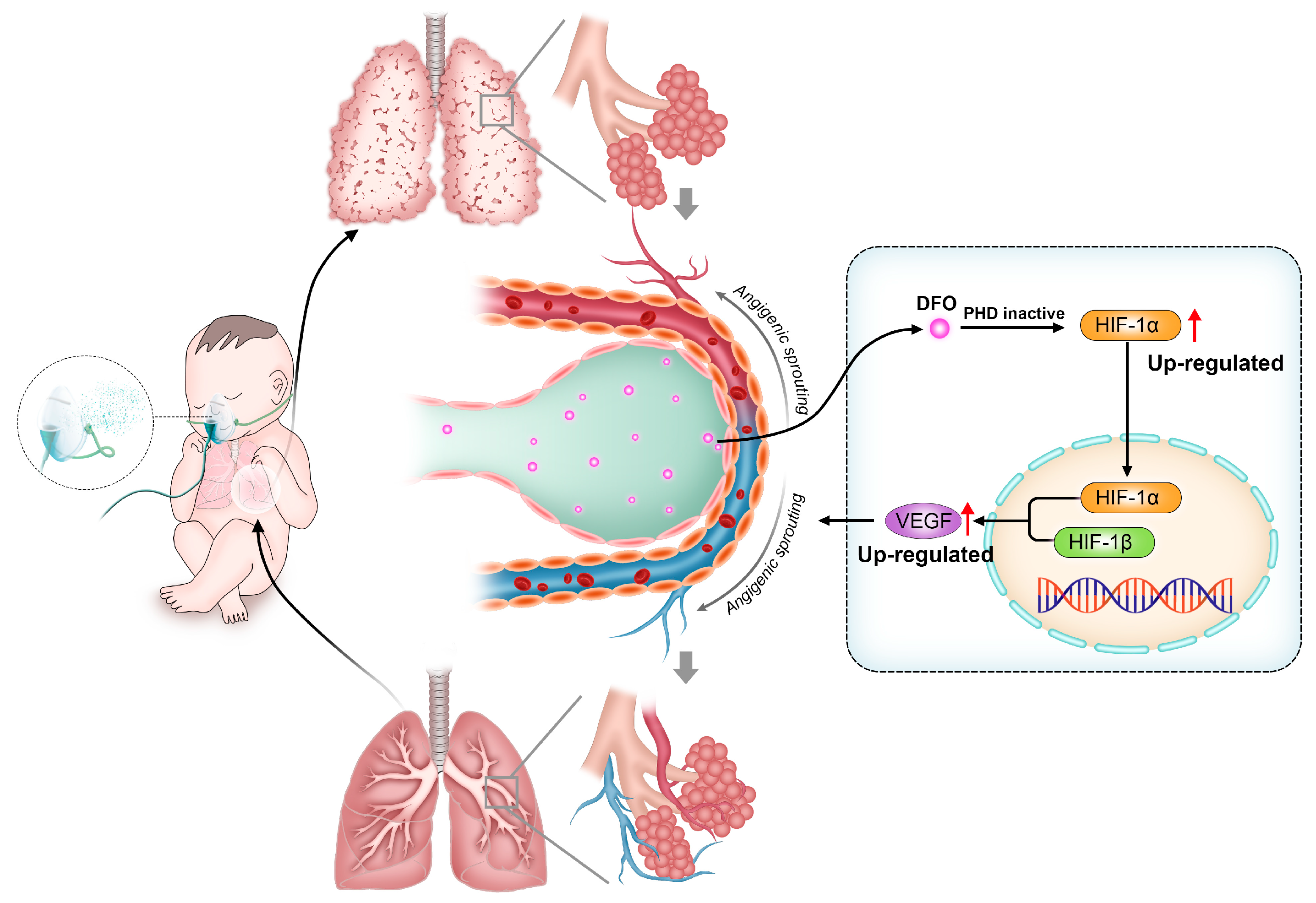
Bronchopulmonary dysplasia (BPD) is a frequent complication in premature infants which seriously affects the health of children. Fetal lungs undergo development in a hypoxic intrauterine environment where HIF-1α plays a crucial role in promoting normal organ growth and maturation. However, premature exposure to oxygen reduces its expression in preterm infants, hindering alveolar and angiogenic processes while disrupting pulmonary development [122,123,124,125]. PHD inhibitors have been shown to promote pulmonary angiogenesis in BPD primate models by increasing HIF-1α and downstream angiogenic factors (Figure 4) [126,127]. As a PHD inhibitor, DFO has been shown to improve lung development in BPD rats by accumulating HIF-1α [128]. Yanru Chen et al. verified, in a mice BPD model, that deferoxamine-loaded aerosol particles (DFO@APs) can release DFO in the alveolar interstitium, thus promoting the reconstruction of microvasculature and, ultimately, inducing lung development for treating BPD [129].
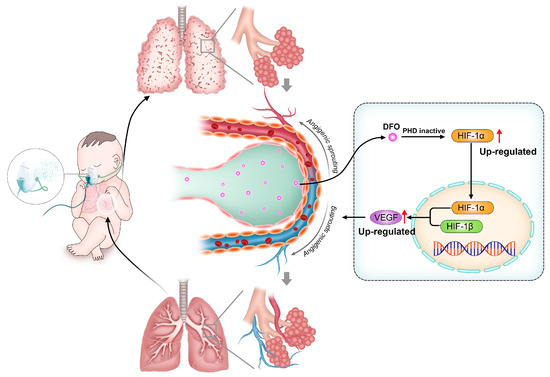
Figure 4.
In bronchopulmonary dysplasia (BPD) models, deferoxamine (DFO) promotes angiogenic sprouting by regulating hypoxia-inducible factor-1α (HIF-1α) signaling pathways.
4.2. Complications of Lung Transplantation
Lung transplantation is often necessary for the treatment of various end-stage lung diseases; however, the occurrence of donor bronchial ischemia poses a significant risk for the development of airway anastomotic complications, potentially leading to severe postoperative complications and transplant failure. Therefore, it is important to promote microvascular repair and alleviate allograft ischemia and hypoxia [130]. Xinguo Jiang et al. developed a DFO nanoparticle and confirmed its ability to improve mouse orthotopic tracheal transplant model complications by producing angiogenic growth factors and reducing ROS production, suggesting that the use of DFO is an effective strategy to reduce postoperative complications following lung and airway transplantation [131].
5. Spinal Cord Injury
Spinal cord injury (SCI) is a serious traumatic disease. As we know, iron overload, reactive oxygen species accumulation, lipid peroxidation, and glutamate accumulation are all associated with spinal cord injury and are also inducers of ferroptosis (ferroptosis is a regulated form of cell death characterized by iron-dependent phospholipid peroxidation) [132,133,134,135,136]. As an iron death inhibitor, DFO can promote the recovery of spinal cord injury by inhibiting iron death [134,137]. Despite the fact that the therapeutic effect of DFO on SCI has been demonstrated in previous studies, the exact mechanism of action is still controversial [138,139]. Guoqing Tang et al. hypothesized that DFO improves spinal cord compression by promoting angiogenesis and demonstrated, in a moderately compressed SCI rat model, that DFO-induced revascularization via activation of the HIF-1α/VEGF pathway is a key mechanism for improving prognosis in spinal cord injury [140]. In addition, the influx of erythrocytes caused by hemorrhage during SCI provides abundant iron sources at the site of the injury, and the increase in iron concentration, iron metabolism, and superoxide metabolism promote each other, producing a large number of free radicals, mediating the oxidative stress response that contributes to secondary injury [141,142]. Many scholars have studied secondary injury responses, among which the inflammatory cascade caused by tumor necrosis factor-α (TNF-α) is considered to be the core of the secondary injury method [143]. Hence, controlling the inflammatory response is essential for treating SCI and preventing further injury. The potential mechanism of DFO in suppressing the inflammatory response following SCI involves chelation of locally produced iron from bleeding, thereby inhibiting TNF-α and interleukin-1β (IL-1β) production by macrophages and microglia. This subsequently promotes the polarization of macrophages from M1 to M2 phenotype, ultimately leading to inhibition of secondary SCI injury [144,145]. Taken together, these results show that DFO treatment reduces the development of inflammation and tissue injury associated with spinal cord trauma. This may accelerate the clinical application of DFO in SCI.
6. Others
During recent years, research on DFO promoting angiogenesis has become increasingly popular. Some researchers use DFO to treat traumatic brain injury. DFO can not only chelate excessive iron from bleeding to prevent oxidative damage through the blood–brain barrier, it can also achieve this through accumulating HIF-1α in order to promote the expression of VEGF, subsequently improving hypoxia tolerance and promoting angiogenesis [146]. DFO has achieved results in the investigation of salivary gland and mammary gland injury reconstruction and in increasing vascularization of islet transplantation due to angiogenesis of DFO [147,148]. In a study of fat transplantation, the experimental results showed that DFO-pretreated adipose fat significantly improved the postoperative weight/volume retention rate, suggesting that DFO promoted angiogenesis in the grafts [149].
7. Drug Delivery System
Since DFO is a low-molecular-weight, water-soluble drug with a short retention time in blood vessels, it is necessary to develop a DFO release system to achieve targeted DFO delivery [150].
The penetration of DFO through the intact cuticle is essential for achieving the objective of preventing and treating diabetic ulcers [65,150]. Hence, Dominik Duscher et al. encapsulated DFO with nonionic surfactants and polymers to form reverse micelles, which were then dispersed within a release-controlling polymer matrix patch. This enabled the delivery of DFO through the hydrophobic stratum corneum, ensuring its targeted delivery to the dermis. The experimental results demonstrated the efficacy of the transdermal drug delivery system in preventing diabetic pressure ulcers and promoting the healing process of existing diabetic wounds [65]. In 2019, Dominik Duscher et al. used state-of-the-art surface micro-texturing technology to develop an enhanced TDDS (eTDDS). Micro-textured surfaces ensure that the patch contacts the wound bed and increases drug release. The results showed that the improved transdermal delivery system not only released DFO continuously but also had a stronger skin penetration ability. Compared with other delivery methods (drip-on aqueous solution and degradable polymer spray application), DFO eTDDS accelerated healing [150]. With the continuous progress of drug delivery systems, in addition to the enhancement of targeted drug penetration [65,150,151], the combination of DFO and local drug delivery systems—which can not only provide therapeutic payloads but also promote wound healing—has attracted widespread attention [152,153]. Electrospinning is an advanced method used for developing wound dressings [154,155,156,157,158]. A wound dressing made from electrospun fibers can maintain a moist environment, absorb wound secretions, or provide adequate oxygen [67,159,160]. These types of porous scaffoldings have a high surface-area-to-volume ratio, use a hydrophilic polymer, and can load drugs and other bioagents as active components. Mohammad Hossein Kazemi et al. used the electrospinning technique to produce a fiber mat loaded with DFO and ciprofloxacin which was verified in vitro to promote wound healing [161].
In addition, the combination of DFO and hydrogels with a porous structure [162,163,164] that can mimic the structure and function of extracellular matrix and promote cell migration, proliferation, and maturation provides a new strategy for the treatment of diabetic ulcers [165,166,167]. For instance, Haijun Shen et al. developed a biomimetic hydrogel containing copper sulfide (CuS) nanoparticles and deferoxamine. DFO and CuS nanoparticles were incorporated into a biomimetic hydrogel which mimics the structure and function of the extracellular matrix. This biomimetic hydrogel can promote cell adhesion and migration, be degraded by cell-secreted matrix metalloproteinases (MMPs), and then release DFO and CuS nanoparticles at the wound site, where they can exert their therapeutic effects. Meanwhile, it can stimulate angiogenesis, effectively eradicate drug-resistant bacteria, and facilitate cell adhesion and migration, all of which are pivotal factors for the healing of diabetic ulcers [78]. An increasing number of studies have shown that changing the administration of DFO can effectively promote angiogenesis and tissue reconstruction [168,169,170]. There is also some evidence that continuous release of DFO or prolongation of the half-life of DFO through the design of a stable drug delivery system can promote cell proliferation and migration and stimulate the formation of blood vessels, providing a theoretical basis for the application of DFO in bone repair [110,171,172,173,174,175,176,177]. Yahong Li et al. used zeolitic imidazolate framework-8 (ZIF-8), which can promote osteogenesis and bone regeneration [178], as a carrier to extend the half-life of DFO. This not only prolonged the drug release but also achieved a synergistic enhancement effect in promoting H-type vessels, angiogenesis, and osteogenic coupling. This provides a new therapeutic strategy, which has a better effect on bone repair, for the regeneration of bone defects of critical size [178]. In the treatment of BPD, DFO is transported into the alveolar interstitium by respiratory delivery, thereby promoting microvascular reconstruction and, ultimately, inducing lung development. In summary, advanced drug delivery systems provide a promising strategy for achieving targeted therapy and improving therapeutic efficacy. This section focuses on exploring the therapeutic effects of diverse delivery systems for DFO in various diseases, aiming to optimize the efficacy of DFO (Table 1).

Table 1.
Different DFO delivery systems.
8. Conclusions and Future Perspectives
Deferoxamine can not only be used as an iron chelator to treat iron overload diseases but can also play an indispensable role in the treatment of angiogenesis deficiency diseases. The intrinsic mechanism of deferoxamine in promoting the therapeutic effect of angiogenesis is closely related to the hypoxia-inducible factor-1α signaling pathway. However, deferoxamine is a drug with a short half-life, small molecular weight, and good water solubility, which limits the durable effect of deferoxamine in angiogenesis. Therefore, in treatment to repair skin and tissue (such as diabetic wounds and burn wounds), researchers coated deferoxamine in various hydrogels or patches and applied them to the repair site. The results showed that the targeted release of deferoxamine can promote endothelial cell proliferation, migration, and angiogenesis, thereby promoting wound healing. In bone regeneration treatment, the combination of biomaterials and deferoxamine can prolong drug release while reducing cytotoxicity. This combination also enriches the function of scaffold materials and plays a role in tissue repair and regeneration, immune regulation, optimization of angiogenesis, and promotion of bone tissue regeneration. In addition, an increasing number of studies have shown that deferoxamine has toxic effects in wound healing, such as visual toxicity and osteotoxicity [179,180,181,182]. Therefore, challenges persist regarding how to control the dosage of deferoxamine and improve the mode of administration. Polyelectrolyte capsules have captured our interest in the context of our current research. These polyelectrolyte capsules are believed to be promising drug delivery systems against cancer and are also utilized in self-healing coatings [183]. They are currently undergoing mass production using automated systems at the first stage. These capsules also constitute a promising platform for deferoxamine drug delivery. In addition, the poly (lactic acid) (PLA) microchamber array (MCA) is a biodegradable and biocompatible controlled drug-release system sensitive to the high-intensity focused ultrasound. The synthesis of such arrays has minimal impact on the drug, preserving the drug’s biological properties. This system can open at therapeutic parameters of ultrasound exposure and complete degradation once the drug is released in full [184]. In conclusion, the combination of different drug delivery systems is expected to maximize the potential advantages of deferoxamine in regenerative medicine treatment.
Author Contributions
H.S. and Y.M. contributed equally to this work. Conceptualization, H.S.; writing—original draft preparation, R.Z. and Y.M.; writing—review and editing, Y.Q., C.Z. and J.C.; supervision, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (no. 82072044) and Qing Lan Project of Jiangsu Province of China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to acknowledge the financial support provided by the National Natural Science Foundation of China (no. 82072044) and sponsored by Qing Lan Project of Jiangsu Province of China.
Conflicts of Interest
The authors declare no conflicts of interest. No competing financial interests exist.
References
- Holden, P.; Nair, L.S. Deferoxamine: An Angiogenic and Antioxidant Molecule for Tissue Regeneration. Tissue Eng. Part B Rev. 2019, 25, 461–470. [Google Scholar] [CrossRef]
- Ikeda, Y.; Tajima, S.; Yoshida, S.; Yamano, N.; Kihira, Y.; Ishizawa, K.; Aihara, K.; Tomita, S.; Tsuchiya, K.; Tamaki, T. Deferoxamine promotes angiogenesis via the activation of vascular endothelial cell function. Atherosclerosis 2011, 215, 339–347. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Z.; Zhao, H.; Cui, H.; Shen, J.; Chang, J.; Li, H.; He, Y. Bioactive Injectable Hydrogels Containing Desferrioxamine and Bioglass for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 30103–30114. [Google Scholar] [CrossRef]
- Roemhild, K.; von Maltzahn, F.; Weiskirchen, R.; Knüchel, R.; von Stillfried, S.; Lammers, T. Iron metabolism: Pathophysiology and pharmacology. Trends Pharmacol. Sci. 2021, 42, 640–656. [Google Scholar] [CrossRef]
- Deng, L.; He, S.; Guo, N.; Tian, W.; Zhang, W.; Luo, L. Molecular mechanisms of ferroptosis and relevance to inflammation. Inflamm. Res. 2023, 72, 281–299. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron Metabolism in Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef]
- Coradduzza, D.; Congiargiu, A.; Chen, Z.; Zinellu, A.; Carru, C.; Medici, S. Ferroptosis and Senescence: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 3658. [Google Scholar] [CrossRef]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef]
- Ouyang, S.; You, J.; Zhi, C.; Li, P.; Lin, X.; Tan, X.; Ma, W.; Li, L.; Xie, W. Ferroptosis: The potential value target in atherosclerosis. Cell Death Dis. 2021, 12, 782. [Google Scholar] [CrossRef]
- Hatcher, H.C.; Singh, R.N.; Torti, F.M.; Torti, S.V. Synthetic and natural iron chelators: Therapeutic potential and clinical use. Future Med. Chem. 2009, 1, 1643–1670. [Google Scholar] [CrossRef]
- Di Paola, A.; Tortora, C.; Argenziano, M.; Marrapodi, M.M.; Rossi, F. Emerging Roles of the Iron Chelators in Inflammation. Int. J. Mol. Sci. 2022, 23, 7977. [Google Scholar] [CrossRef] [PubMed]
- Keberle, H. The biochemistry of desferrioxamine and its relation to iron metabolism. Ann. N. Y. Acad. Sci. 1964, 119, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Fong, G.H.; Takeda, K. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ. 2008, 15, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiang, Z.; Xing, Y.; Li, S.; Shi, S. Mitochondria bridge HIF signaling and ferroptosis blockage in acute kidney injury. Cell Death Dis. 2022, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Wenger, R.H.; Camenisch, G.; Stiehl, D.P.; Katschinski, D.M. HIF prolyl-4-hydroxylase interacting proteins: Consequences for drug targeting. Curr. Pharm. Des. 2009, 15, 3886–3894. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, M.H. Inhibition of hypoxia-inducible factor prolyl hydroxylase domain oxygen sensors: Tricking the body into mounting orchestrated survival and repair responses. J. Med. Chem. 2013, 56, 9369–9402. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.D.; Walters, E.H.; Simpson, J.L.; Keely, S.; Wark, P.A.B.; O’Toole, R.F.; Hansbro, P.M. Hypoxia-inducible factor and bacterial infections in chronic obstructive pulmonary disease. Respirology 2020, 25, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, R.; Zou, J.; Ying, Y.; Luo, Z. Dual Roles of the AMP-Activated Protein Kinase Pathway in Angiogenesis. Cells 2019, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Zhou, W.; Tang, Z. Pathogenesis of diabetic complications: Exploring hypoxic niche formation and HIF-1α activation. Biomed. Pharmacother. 2024, 172, 116202. [Google Scholar] [CrossRef] [PubMed]
- Welsh, S.J.; Koh, M.Y.; Powis, G. The hypoxic inducible stress response as a target for cancer drug discovery. Semin. Oncol. 2006, 33, 486–497. [Google Scholar] [CrossRef]
- Wouters, A.; Boeckx, C.; Vermorken, J.B.; Van den Weyngaert, D.; Peeters, M.; Lardon, F. The intriguing interplay between therapies targeting the epidermal growth factor receptor, the hypoxic microenvironment and hypoxia-inducible factors. Curr. Pharm. Des. 2013, 19, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chang, B.; Pang, Y.; Wang, H.; Zhou, Y. Advances in Hypoxia-Inducible Factor-1α Stabilizer Deferoxamine in Tissue Engineering. Tissue Eng. Part B Rev. 2023, 29, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Heber-Katz, E. Oxygen, Metabolism, and Regeneration: Lessons from Mice. Trends Mol. Med. 2017, 23, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yang, E.G. Recent Advances in Developing Inhibitors for Hypoxia-Inducible Factor Prolyl Hydroxylases and Their Therapeutic Implications. Molecules 2015, 20, 20551–20568. [Google Scholar] [CrossRef] [PubMed]
- Carroll, V.A.; Ashcroft, M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: Implications for targeting the HIF pathway. Cancer Res. 2006, 66, 6264–6270. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Pharmacologic Targeting of Hypoxia-Inducible Factors. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, O.; Mandel, S.; Youdim, M.B.H.; Amit, T. Targeting dysregulation of brain iron homeostasis in Parkinson’s disease by iron chelators. Free Radic. Biol. Med. 2013, 62, 52–64. [Google Scholar] [CrossRef]
- Zanotti, F.; Zanolla, I.; Trentini, M.; Tiengo, E.; Pusceddu, T.; Licastro, D.; Degasperi, M.; Leo, S.; Tremoli, E.; Ferroni, L.; et al. Mitochondrial Metabolism and EV Cargo of Endothelial Cells Is Affected in Presence of EVs Derived from MSCs on Which HIF Is Activated. Int. J. Mol. Sci. 2023, 24, 6002. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wei, W.; Zhang, J.; Zhao, B.; Li, Q.; Jin, P. Mechanism of damage of HIF-1 signaling in chronic diabetic foot ulcers and its related therapeutic perspectives. Heliyon 2024, 10, e24656. [Google Scholar] [CrossRef]
- Li, G.; Ko, C.N.; Li, D.; Yang, C.; Wang, W.; Yang, G.J.; Di Primo, C.; Wong, V.K.W.; Xiang, Y.; Lin, L.; et al. A small molecule HIF-1α stabilizer that accelerates diabetic wound healing. Nat. Commun. 2021, 12, 3363. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia--a key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhong, Z.F.; Wang, S.P.; Vong, C.T.; Yu, B.; Wang, Y.T. HIF-1: Structure, biology and natural modulators. Chin. J. Nat. Med. 2021, 19, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.B.; Zheng, X. Disturbed hypoxic responses as a pathogenic mechanism of diabetic foot ulcers. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. S1), 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Xie, W.; Jankovic, J.; Le, W.; Pan, T. Neuroprotection of deferoxamine on rotenone-induced injury via accumulation of HIF-1 alpha and induction of autophagy in SH-SY5Y cells. Neurochem. Int. 2010, 57, 198–205. [Google Scholar] [CrossRef]
- Martínez-Romero, R.; Martínez-Lara, E.; Aguilar-Quesada, R.; Peralta, A.; Oliver, F.J.; Siles, E. PARP-1 modulates deferoxamine-induced HIF-1alpha accumulation through the regulation of nitric oxide and oxidative stress. J. Cell Biochem. 2008, 104, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Nie, C.; Si, Z.; Ma, Y. Deferoxamine enhances neovascularization and accelerates wound healing in diabetic rats via the accumulation of hypoxia-inducible factor-1α. Diabetes Res. Clin. Pract. 2013, 101, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Street, J.; Bao, M.; deGuzman, L.; Bunting, S.; Peale, F.V., Jr.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [PubMed]
- Marziano, C.; Genet, G.; Hirschi, K.K. Vascular endothelial cell specification in health and disease. Angiogenesis 2021, 24, 213–236. [Google Scholar] [CrossRef]
- Chi, Z.; Chen, L.; Ye, X.; Liu, A.; Yu, G.; Sun, Y. The vasculature niches required for hematopoiesis. J. Mol. Med. 2022, 100, 53–61. [Google Scholar] [CrossRef]
- Blanchard, L.; Girard, J.P. High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis 2021, 24, 719–753. [Google Scholar] [CrossRef]
- Fujioka, T.; Kaneko, N.; Sawamoto, K. Blood vessels as a scaffold for neuronal migration. Neurochem. Int. 2019, 126, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, P.; Gu, Y.; Guo, Q.; Liu, Y. Type H vessels: Functions in bone development and diseases. Front. Cell Dev. Biol. 2023, 11, 1236545. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.R.; Kushner, E.J. Trafficking in blood vessel development. Angiogenesis 2022, 25, 291–305. [Google Scholar] [CrossRef]
- Hirschi, K.K.; Rohovsky, S.A.; D’Amore, P.A. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J. Cell Biol. 1998, 141, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.K.; Rohovsky, S.A.; Beck, L.H.; Smith, S.R.; D’Amore, P.A. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ. Res. 1999, 84, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Iba, T.; Takakura, N. Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int. Immunol. 2020, 32, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef] [PubMed]
- Casie Chetty, S.; Rost, M.S.; Enriquez, J.R.; Schumacher, J.A.; Baltrunaite, K.; Rossi, A.; Stainier, D.Y.; Sumanas, S. Vegf signaling promotes vascular endothelial differentiation by modulating etv2 expression. Dev. Biol. 2017, 424, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Shaabani, E.; Sharifiaghdam, M.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Gene therapy to enhance angiogenesis in chronic wounds. Mol. Ther. Nucleic Acids 2022, 29, 871–899. [Google Scholar] [CrossRef]
- De Wolde, S.D.; Hulskes, R.H.; Weenink, R.P.; Hollmann, M.W.; Van Hulst, R.A. The Effects of Hyperbaric Oxygenation on Oxidative Stress, Inflammation and Angiogenesis. Biomolecules 2021, 11, 1210. [Google Scholar] [CrossRef]
- Rai, V.; Moellmer, R.; Agrawal, D.K. Stem Cells and Angiogenesis: Implications and Limitations in Enhancing Chronic Diabetic Foot Ulcer Healing. Cells 2022, 11, 2287. [Google Scholar] [CrossRef] [PubMed]
- Cucci, L.M.; Satriano, C.; Marzo, T.; La Mendola, D. Angiogenin and Copper Crossing in Wound Healing. Int. J. Mol. Sci. 2021, 22, 10704. [Google Scholar] [CrossRef]
- Qin, Q.; Lee, S.; Patel, N.; Walden, K.; Gomez-Salazar, M.; Levi, B.; James, A.W. Neurovascular coupling in bone regeneration. Exp. Mol. Med. 2022, 54, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wu, S.; Li, Y.; Crane, J.L. Type H blood vessels in bone modeling and remodeling. Theranostics 2020, 10, 426–436. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Liu, K.; Gao, F. Hydrogel scaffolds in bone regeneration: Their promising roles in angiogenesis. Front. Pharmacol. 2023, 14, 1050954. [Google Scholar] [CrossRef] [PubMed]
- Tuckermann, J.; Adams, R.H. The endothelium-bone axis in development, homeostasis and bone and joint disease. Nat. Rev. Rheumatol. 2021, 17, 608–620. [Google Scholar] [CrossRef]
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, D.; Zhao, F. Updates on Recent Clinical Assessment of Commercial Chronic Wound Care Products. Adv. Healthc. Mater. 2023, 12, 2300556. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Lu, X.; Yang, Y.; Yang, Y.; Li, Y.; Kuai, L.; Li, B.; Dong, H.; Shi, J. Microenvironment-Based Diabetic Foot Ulcer Nanomedicine. Adv. Sci. 2023, 10, e2203308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jiang, G.; Gao, Q.; Wang, X.; Wang, Y.; Xu, X.; Yan, W.; Shen, H. Sprayed copper peroxide nanodots for accelerating wound healing in a multidrug-resistant bacteria infected diabetic ulcer. Nanoscale 2021, 13, 15937–15951. [Google Scholar] [CrossRef] [PubMed]
- Duscher, D.; Neofytou, E.; Wong, V.W.; Maan, Z.N.; Rennert, R.C.; Inayathullah, M.; Januszyk, M.; Rodrigues, M.; Malkovskiy, A.V.; Whitmore, A.J.; et al. Transdermal deferoxamine prevents pressure-induced diabetic ulcers. Proc. Natl. Acad. Sci. USA 2015, 112, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, Y.; Tian, J.; Yang, P.; Zhang, X.; Chen, Y.; Hu, Y.; Wu, J. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Sci. Adv. 2020, 6, eaba4311. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, H.S.; Yoo, H.S. Electrospinning strategies of drug-incorporated nanofibrous mats for wound recovery. Drug Deliv. Transl. Res. 2015, 5, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Tchanque-Fossuo, C.N.; Dahle, S.E.; Buchman, S.R.; Isseroff, R.R. Deferoxamine: Potential novel topical therapeutic for chronic wounds. Br. J. Dermatol. 2017, 176, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Thangarajah, H.; Yao, D.; Chang, E.I.; Shi, Y.; Jazayeri, L.; Vial, I.N.; Galiano, R.D.; Du, X.L.; Grogan, R.; Galvez, M.G.; et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc. Natl. Acad. Sci. USA 2009, 106, 13505–13510. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Ma, P.; Wu, H.; Xiao, D.; Zhang, Y.; Sui, X.; Zhang, L.; Dong, A. Functional carbohydrate-based hydrogels for diabetic wound therapy. Carbohydr. Polym. 2023, 312, 120823. [Google Scholar] [CrossRef]
- Wu, H.; Li, F.; Shao, W.; Gao, J.; Ling, D. Promoting Angiogenesis in Oxidative Diabetic Wound Microenvironment Using a Nanozyme-Reinforced Self-Protecting Hydrogel. ACS Cent. Sci. 2019, 5, 477–485. [Google Scholar] [CrossRef]
- Duscher, D.; Januszyk, M.; Maan, Z.N.; Whittam, A.J.; Hu, M.S.; Walmsley, G.G.; Dong, Y.; Khong, S.M.; Longaker, M.T.; Gurtner, G.C. Comparison of the Hydroxylase Inhibitor Dimethyloxalylglycine and the Iron Chelator Deferoxamine in Diabetic and Aged Wound Healing. Plast. Reconstr. Surg. 2017, 139, 695e–706e. [Google Scholar] [CrossRef]
- Chen, H.; Jia, P.; Kang, H.; Zhang, H.; Liu, Y.; Yang, P.; Yan, Y.; Zuo, G.; Guo, L.; Jiang, M.; et al. Upregulating Hif-1α by Hydrogel Nanofibrous Scaffolds for Rapidly Recruiting Angiogenesis Relative Cells in Diabetic Wound. Adv. Healthc. Mater. 2016, 5, 907–918. [Google Scholar] [CrossRef]
- Chen, H.; Guo, L.; Wicks, J.; Ling, C.; Zhao, X.; Yan, Y.; Qi, J.; Cui, W.; Deng, L. Quickly promoting angiogenesis by using a DFO-loaded photo-crosslinked gelatin hydrogel for diabetic skin regeneration. J. Mater. Chem. B 2016, 4, 3770–3781. [Google Scholar] [CrossRef]
- Gao, S.Q.; Chang, C.; Li, J.J.; Li, Y.; Niu, X.Q.; Zhang, D.P.; Li, L.J.; Gao, J.Q. Co-delivery of deferoxamine and hydroxysafflor yellow A to accelerate diabetic wound healing via enhanced angiogenesis. Drug Deliv. 2018, 25, 1779–1789. [Google Scholar] [CrossRef]
- Qayoom, A.; Aneesha, V.A.; Anagha, S.; Dar, J.A.; Kumar, P.; Kumar, D. Lecithin-based deferoxamine nanoparticles accelerated cutaneous wound healing in diabetic rats. Eur. J. Pharmacol. 2019, 858, 172478. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, W.; Zhai, X.; Zhao, X.; Wang, J.; Weng, J.; Li, J.; Chen, X. An antibacterial and proangiogenic double-layer drug-loaded microneedle patch for accelerating diabetic wound healing. Biomater. Sci. 2023, 11, 533–541. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, C.; Meng, Y.; Qiao, Y.; Ma, Y.; Chen, J.; Wang, X.; Pan, L. Biomimetic Hydrogel Containing Copper Sulfide Nanoparticles and Deferoxamine for Photothermal Therapy of Infected Diabetic Wounds. Adv. Healthc. Mater. 2023, 13, e2303000. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, Y.; Guo, P.; Duan, T.; Cheng, W.; Guo, Y.; Zheng, X.; Lu, G.; Lu, Q.; Kaplan, D.L. Injectable Desferrioxamine-Laden Silk Nanofiber Hydrogels for Accelerating Diabetic Wound Healing. ACS Biomater. Sci. Eng. 2021, 7, 1147–1158. [Google Scholar] [CrossRef]
- Wu, H.; Wang, T.; Liang, Y.; Chen, L.; Li, Z. Self-assembled and dynamic bond crosslinked herb-polysaccharide hydrogel with anti-inflammation and pro-angiogenesis effects for burn wound healing. Colloids Surf. B Biointerfaces 2024, 233, 113639. [Google Scholar] [CrossRef]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef]
- El Ayadi, A.; Salsbury, J.R.; Enkhbaatar, P.; Herndon, D.N.; Ansari, N.H. Metal chelation attenuates oxidative stress, inflammation, and vertical burn progression in a porcine brass comb burn model. Redox Biol. 2021, 45, 102034. [Google Scholar] [CrossRef]
- El Ayadi, A.; Wang, C.Z.; Zhang, M.; Wetzel, M.; Prasai, A.; Finnerty, C.C.; Enkhbaatar, P.; Herndon, D.N.; Ansari, N.H. Metal chelation reduces skin epithelial inflammation and rescues epithelial cells from toxicity due to thermal injury in a rat model. Burn. Trauma 2020, 8, tkaa024. [Google Scholar] [CrossRef]
- Wang, C.Z.; Ayadi, A.E.; Goswamy, J.; Finnerty, C.C.; Mifflin, R.; Sousse, L.; Enkhbaatar, P.; Papaconstantinou, J.; Herndon, D.N.; Ansari, N.H. Topically applied metal chelator reduces thermal injury progression in a rat model of brass comb burn. Burns 2015, 41, 1775–1787. [Google Scholar] [CrossRef]
- Welch, K.D.; Davis, T.Z.; Van Eden, M.E.; Aust, S.D. Deleterious iron-mediated oxidation of biomolecules. Free Radic. Biol. Med. 2002, 32, 577–583. [Google Scholar] [CrossRef]
- Beaufay, F.; Quarles, E.; Franz, A.; Katamanin, O.; Wholey, W.Y.; Jakob, U. Polyphosphate Functions In Vivo as an Iron Chelator and Fenton Reaction Inhibitor. mBio 2020, 11, e01017-20. [Google Scholar] [CrossRef]
- Żwierełło, W.; Styburski, D.; Maruszewska, A.; Piorun, K.; Skórka-Majewicz, M.; Czerwińska, M.; Maciejewska, D.; Baranowska-Bosiacka, I.; Krajewski, A.; Gutowska, I. Bioelements in the treatment of burn injuries—The complex review of metabolism and supplementation (copper, selenium, zinc, iron, manganese, chromium and magnesium). J. Trace Elem. Med. Biol. 2020, 62, 126616. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nassar, D.; Batteux, F.; Raymond, K.; Tharaux, P.L.; Aractingi, S. Delayed Healing of Sickle Cell Ulcers Is due to Impaired Angiogenesis and CXCL12 Secretion in Skin Wounds. J. Investig. Dermatol. 2016, 136, 497–506. [Google Scholar] [CrossRef]
- Vichinsky, E.; Onyekwere, O.; Porter, J.; Swerdlow, P.; Eckman, J.; Lane, P.; Files, B.; Hassell, K.; Kelly, P.; Wilson, F.; et al. A randomised comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. Br. J. Haematol. 2007, 136, 501–508. [Google Scholar] [CrossRef]
- Franchini, M.; Gandini, G.; Veneri, D.; Aprili, G. Safety and efficacy of subcutaneous bolus injection of deferoxamine in adult patients with iron overload: An update. Blood 2004, 103, 747–748. [Google Scholar] [CrossRef]
- Rodrigues, M.; Bonham, C.A.; Minniti, C.P.; Gupta, K.; Longaker, M.T.; Gurtner, G.C. Iron Chelation with Transdermal Deferoxamine Accelerates Healing of Murine Sickle Cell Ulcers. Adv. Wound Care 2018, 7, 323–332. [Google Scholar] [CrossRef]
- Wan, C.; Gilbert, S.R.; Wang, Y.; Cao, X.; Shen, X.; Ramaswamy, G.; Jacobsen, K.A.; Alaql, Z.S.; Eberhardt, A.W.; Gerstenfeld, L.C.; et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc. Natl. Acad. Sci. USA 2008, 105, 686–691. [Google Scholar] [CrossRef]
- Bosch-Rué, È.; Díez-Tercero, L.; Buitrago, J.O.; Castro, E.; Pérez, R.A. Angiogenic and immunomodulation role of ions for initial stages of bone tissue regeneration. Acta Biomater. 2023, 166, 14–41. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Flores, M.; Madureira, S.; Zanotto, F.; Monteiro, F.J.; Laranjeira, M.S. Magnetic Bone Tissue Engineering: Reviewing the Effects of Magnetic Stimulation on Bone Regeneration and Angiogenesis. Pharmaceutics 2023, 15, 1045. [Google Scholar] [CrossRef]
- Longoni, A.; Li, J.; Lindberg, G.C.J.; Rnjak-Kovacina, J.; Wise, L.M.; Hooper, G.J.; Woodfield, T.B.F.; Kieser, D.C.; Lim, K.S. Strategies for inclusion of growth factors into 3D printed bone grafts. Essays Biochem. 2021, 65, 569–585. [Google Scholar] [CrossRef]
- Simunovic, F.; Finkenzeller, G. Vascularization Strategies in Bone Tissue Engineering. Cells 2021, 10, 1749. [Google Scholar] [CrossRef]
- Saul, D.; Khosla, S. Fracture Healing in the Setting of Endocrine Diseases, Aging, and Cellular Senescence. Endocr. Rev. 2022, 43, 984–1002. [Google Scholar] [CrossRef]
- Rather, H.A.; Jhala, D.; Vasita, R. Dual functional approaches for osteogenesis coupled angiogenesis in bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109761. [Google Scholar] [CrossRef]
- Wagner, D.R.; Karnik, S.; Gunderson, Z.J.; Nielsen, J.J.; Fennimore, A.; Promer, H.J.; Lowery, J.W.; Loghmani, M.T.; Low, P.S.; McKinley, T.O.; et al. Dysfunctional stem and progenitor cells impair fracture healing with age. World J. Stem Cells 2019, 11, 281–296. [Google Scholar] [CrossRef]
- Irfan, D.; Ahmad, I.; Patra, I.; Margiana, R.; Rasulova, M.T.; Sivaraman, R.; Kandeel, M.; Mohammad, H.J.; Al-Qaim, Z.H.; Jawad, M.A.; et al. Stem cell-derived exosomes in bone healing: Focusing on their role in angiogenesis. Cytotherapy 2023, 25, 353–361. [Google Scholar] [CrossRef]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef]
- Grcevic, D.; Pejda, S.; Matthews, B.G.; Repic, D.; Wang, L.; Li, H.; Kronenberg, M.S.; Jiang, X.; Maye, P.; Adams, D.J.; et al. In Vivo Fate Mapping Identifies Mesenchymal Progenitor Cells. Stem Cells 2012, 30, 187–196. [Google Scholar] [CrossRef]
- Colnot, C. Cellular and molecular interactions regulating skeletogenesis. J. Cell Biochem. 2005, 95, 688–697. [Google Scholar] [CrossRef]
- Shen, X.; Wan, C.; Ramaswamy, G.; Mavalli, M.; Wang, Y.; Duvall, C.L.; Deng, L.F.; Guldberg, R.E.; Eberhart, A.; Clemens, T.L.; et al. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J. Orthop. Res. 2009, 27, 1298–1305. [Google Scholar] [CrossRef]
- Warnecke, C.; Griethe, W.; Weidemann, A.; Jürgensen, J.S.; Willam, C.; Bachmann, S.; Ivashchenko, Y.; Wagner, I.; Frei, U.; Wiesener, M.; et al. Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. Faseb J. 2003, 17, 1186–1188. [Google Scholar] [CrossRef]
- Wang, G.L.; Semenza, G.L. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: Implications for models of hypoxia signal transduction. Blood 1993, 82, 3610–3615. [Google Scholar] [CrossRef]
- Stewart, R.; Goldstein, J.; Eberhardt, A.; Chu, G.T.; Gilbert, S. Increasing vascularity to improve healing of a segmental defect of the rat femur. J. Orthop. Trauma 2011, 25, 472–476. [Google Scholar] [CrossRef]
- Farberg, A.S.; Jing, X.L.; Monson, L.A.; Donneys, A.; Tchanque-Fossuo, C.N.; Deshpande, S.S.; Buchman, S.R. Deferoxamine reverses radiation induced hypovascularity during bone regeneration and repair in the murine mandible. Bone 2012, 50, 1184–1187. [Google Scholar] [CrossRef]
- Zhang, J.; Tong, D.; Song, H.; Ruan, R.; Sun, Y.; Lin, Y.; Wang, J.; Hou, L.; Dai, J.; Ding, J.; et al. Osteoimmunity-Regulating Biomimetically Hierarchical Scaffold for Augmented Bone Regeneration. Adv. Mater. 2022, 34, e2202044. [Google Scholar] [CrossRef]
- Zeng, Y.; Huang, C.; Duan, D.; Lou, A.; Guo, Y.; Xiao, T.; Wei, J.; Liu, S.; Wang, Z.; Yang, Q.; et al. Injectable temperature-sensitive hydrogel system incorporating deferoxamine-loaded microspheres promotes H-type blood vessel-related bone repair of a critical size femoral defect. Acta Biomater. 2022, 153, 108–123. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, J.; Niu, K.; Li, W.; Jiang, N.; Li, J.; Yu, Q.; Wu, C. Electrospun artificial periosteum loaded with DFO contributes to osteogenesis via the TGF-β1/Smad2 pathway. Biomater. Sci. 2021, 9, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Ai-Aql, Z.S.; Alagl, A.S.; Graves, D.T.; Gerstenfeld, L.C.; Einhorn, T.A. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J. Dent. Res. 2008, 87, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wu, X.; Frassica, D.; Yu, B.; Pang, L.; Xian, L.; Wan, M.; Lei, W.; Armour, M.; Tryggestad, E.; et al. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 1609–1614. [Google Scholar] [CrossRef]
- Farberg, A.S.; Sarhaddi, D.; Donneys, A.; Deshpande, S.S.; Buchman, S.R. Deferoxamine enhances bone regeneration in mandibular distraction osteogenesis. Plast. Reconstr. Surg. 2014, 133, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Donneys, A.; Farberg, A.S.; Tchanque-Fossuo, C.N.; Deshpande, S.S.; Buchman, S.R. Deferoxamine enhances the vascular response of bone regeneration in mandibular distraction osteogenesis. Plast. Reconstr. Surg. 2012, 129, 850–856. [Google Scholar] [CrossRef]
- Felice, P.A.; Ahsan, S.; Donneys, A.; Deshpande, S.S.; Nelson, N.S.; Buchman, S.R. Deferoxamine administration delivers translational optimization of distraction osteogenesis in the irradiated mandible. Plast. Reconstr. Surg. 2013, 132, 542e–548e. [Google Scholar] [CrossRef] [PubMed]
- Donneys, A.; Weiss, D.M.; Deshpande, S.S.; Ahsan, S.; Tchanque-Fossuo, C.N.; Sarhaddi, D.; Levi, B.; Goldstein, S.A.; Buchman, S.R. Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone 2013, 52, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, L.; Cui, Q.; Wang, G.J.; Balian, G. Steroid effects on osteogenesis through mesenchymal cell gene expression. Osteoporos. Int. 2005, 16, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, L.; Yu, Z.; Dang, X.; Wang, K. The effect of deferoxamine on angiogenesis and bone repair in steroid-induced osteonecrosis of rabbit femoral heads. Exp. Biol. Med. 2015, 240, 273–280. [Google Scholar] [CrossRef]
- Drager, J.; Ramirez-GarciaLuna, J.L.; Kumar, A.; Gbureck, U.; Harvey, E.J.; Barralet, J.E. Hypoxia Biomimicry to Enhance Monetite Bone Defect Repair. Tissue Eng. Part A 2017, 23, 1372–1381. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Kneser, U.; Arkudas, A. Scaffolds for vascularized bone regeneration: Advances and challenges. Expert Rev. Med. Devices 2012, 9, 457–460. [Google Scholar] [CrossRef]
- van Tuyl, M.; Liu, J.; Wang, J.; Kuliszewski, M.; Tibboel, D.; Post, M. Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, L167–L178. [Google Scholar] [CrossRef]
- Caniggia, I.; Mostachfi, H.; Winter, J.; Gassmann, M.; Lye, S.J.; Kuliszewski, M.; Post, M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ3. J. Clin. Investig. 2000, 105, 577–587. [Google Scholar] [CrossRef]
- Chakraborty, D.; Rumi, M.A.; Soares, M.J. NK cells, hypoxia and trophoblast cell differentiation. Cell Cycle 2012, 11, 2427–2430. [Google Scholar] [CrossRef]
- Asikainen, T.M.; Ahmad, A.; Schneider, B.K.; Ho, W.B.; Arend, M.; Brenner, M.; Günzler, V.; White, C.W. Stimulation of HIF-1alpha, HIF-2alpha, and VEGF by prolyl 4-hydroxylase inhibition in human lung endothelial and epithelial cells. Free Radic. Biol. Med. 2005, 38, 1002–1013. [Google Scholar] [CrossRef]
- Asikainen, T.M.; Waleh, N.S.; Schneider, B.K.; Clyman, R.I.; White, C.W. Enhancement of angiogenic effectors through hypoxia-inducible factor in preterm primate lung in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L588–L595. [Google Scholar] [CrossRef]
- Asikainen, T.M.; Chang, L.Y.; Coalson, J.J.; Schneider, B.K.; Waleh, N.S.; Ikegami, M.; Shannon, J.M.; Winter, V.T.; Grubb, P.; Clyman, R.I.; et al. Improved lung growth and function through hypoxia-inducible factor in primate chronic lung disease of prematurity. Faseb J. 2006, 20, 1698–1700. [Google Scholar] [CrossRef]
- Choi, C.W.; Lee, J.; Lee, H.J.; Park, H.S.; Chun, Y.S.; Kim, B.I. Deferoxamine Improves Alveolar and Pulmonary Vascular Development by Upregulating Hypoxia-inducible Factor-1α in a Rat Model of Bronchopulmonary Dysplasia. J. Korean Med. Sci. 2015, 30, 1295–1301. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, W.; Xiang, X.; Deng, L.; Qian, J.; Cui, W.; Chen, H. Pollen-Inspired Shell-Core Aerosol Particles Capable of Brownian Motion for Pulmonary Vascularization. Adv. Mater. 2023, 35, e2207744. [Google Scholar] [CrossRef] [PubMed]
- Weigt, S.S.; Wallace, W.D.; Derhovanessian, A.; Saggar, R.; Saggar, R.; Lynch, J.P.; Belperio, J.A. Chronic allograft rejection: Epidemiology, diagnosis, pathogenesis, and treatment. Semin. Respir. Crit. Care Med. 2010, 31, 189–207. [Google Scholar] [CrossRef]
- Jiang, X.; Malkovskiy, A.V.; Tian, W.; Sung, Y.K.; Sun, W.; Hsu, J.L.; Manickam, S.; Wagh, D.; Joubert, L.M.; Semenza, G.L.; et al. Promotion of airway anastomotic microvascular regeneration and alleviation of airway ischemia by deferoxamine nanoparticles. Biomaterials 2014, 35, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Koszyca, B.; Manavis, J.; Cornish, R.J.; Blumbergs, P.C. Patterns of immunocytochemical staining for ferritin and transferrin in the human spinal cord following traumatic injury. J. Clin. Neurosci. 2002, 9, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.Y.; Liu, X.L.; Deng, Z.Z.; Wei, D.M.; Zhang, D.; Xi, H.L.; Wang, Q.Y.; He, M.Z.; Yang, Y.L. Ferroptosis is a new therapeutic target for spinal cord injury. Front. Neurosci. 2023, 17, 1136143. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Guo, Y.; Sun, M.; Li, B.; Zhang, Y.; Li, C. Iron is a potential key mediator of glutamate excitotoxicity in spinal cord motor neurons. Brain Res. 2009, 1257, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Hao, J.; Duan, H.Q.; Zhao, C.X.; Sun, C.; Li, B.; Fan, B.Y.; Wang, X.; Li, W.X.; et al. Deferoxamine promotes recovery of traumatic spinal cord injury by inhibiting ferroptosis. Neural Regen. Res. 2019, 14, 532–541. [Google Scholar] [CrossRef]
- Sinis, N.; Di Scipio, F.; Schönle, P.; Werdin, F.; Kraus, A.; Koopmanns, G.; Masanneck, C.; Hermanns, S.; Danker, T.; Guenther, E.; et al. Local administration of DFO-loaded lipid particles improves recovery after end-to-end reconstruction of rat median nerve. Restor. Neurol. Neurosci. 2009, 27, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yang, Q.H.; Yu, X.D.; Jiang, D.M. Additive effect of tetramethylpyrazine and deferoxamine in the treatment of spinal cord injury caused by aortic cross-clamping in rats. Spinal Cord. 2011, 49, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Chen, Y.; Chen, J.; Chen, Z.; Jiang, W. Deferoxamine Ameliorates Compressed Spinal Cord Injury by Promoting Neovascularization in Rats. J. Mol. Neurosci. 2020, 70, 1437–1444. [Google Scholar] [CrossRef]
- Emerit, J.; Beaumont, C.; Trivin, F. Iron metabolism, free radicals, and oxidative injury. Biomed. Pharmacother. 2001, 55, 333–339. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Sun, D.; Alcock, N.W.; Wen, J. Spinal cord injury increases iron levels: Catalytic production of hydroxyl radicals. Free Radic. Biol. Med. 2003, 34, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Si, Q.; Nakamura, Y.; Kataoka, K. A serum factor enhances production of nitric oxide and tumor necrosis factor-alpha from cultured microglia. Exp. Neurol. 2000, 162, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kroner, A.; Greenhalgh, A.D.; Zarruk, J.G.; Passos Dos Santos, R.; Gaestel, M.; David, S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 2014, 83, 1098–1116. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Li, B.; Duan, H.Q.; Zhao, C.X.; Zhang, Y.; Sun, C.; Pan, B.; Liu, C.; Kong, X.H.; Yao, X.; et al. Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats. Neural Regen. Res. 2017, 12, 959–968. [Google Scholar] [CrossRef]
- Wang, K.; Jing, Y.; Xu, C.; Zhao, J.; Gong, Q.; Chen, S. HIF-1α and VEGF Are Involved in Deferoxamine-Ameliorated Traumatic Brain Injury. J. Surg. Res. 2020, 246, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Lynn, J.V.; Urlaub, K.M.; Ranganathan, K.; Donneys, A.; Nelson, N.S.; Subramanian, C.; Cohen, M.S.; Buchman, S.R. The Role of Deferoxamine in Irradiated Breast Reconstruction: A Study of Oncologic Safety. Plast. Reconstr. Surg. 2019, 143, 1666–1676. [Google Scholar] [CrossRef]
- Dassoulas, K.R.; Mericli, A.F.; Wang, J.S.; Lei, S.S.; Kim, T.; Cottler, P.S.; Lin, K.Y. Treatment With Topical Deferoxamine Improves Cutaneous Vascularity and Tissue Pliability in an Irradiated Animal Model of Tissue Expander-Based Breast Reconstruction. Ann. Plast. Surg. 2019, 82, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, X.; Li, H.; Mu, D. Deferoxamine Mesylate Improves the Survival Rate of Transplanted Fat by Promoting Angiogenesis. Aesthet. Surg. J. 2023, 43, 789–798. [Google Scholar] [CrossRef]
- Duscher, D.; Trotsyuk, A.A.; Maan, Z.N.; Kwon, S.H.; Rodrigues, M.; Engel, K.; Stern-Buchbinder, Z.A.; Bonham, C.A.; Barrera, J.; Whittam, A.J.; et al. Optimization of transdermal deferoxamine leads to enhanced efficacy in healing skin wounds. J. Control. Release 2019, 308, 232–239. [Google Scholar] [CrossRef]
- Schuster, L.; Seifert, O.; Vollmer, S.; Kontermann, R.E.; Schlosshauer, B.; Hartmann, H. Immunoliposomes for Targeted Delivery of an Antifibrotic Drug. Mol. Pharm. 2015, 12, 3146–3157. [Google Scholar] [CrossRef] [PubMed]
- Madhukiran, D.; Jha, A.; Kumar, M.; Ajmal, G.; Bonde, G.V.; Mishra, B. Electrospun nanofiber-based drug delivery platform: Advances in diabetic foot ulcer management. Expert Opin. Drug Deliv. 2021, 18, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, H.; Khute, S.; Sahu, R.; Jangde, R.K. Advanced Drug Delivery System for Management of Chronic Diabetes Wound Healing. Curr. Drug Targets 2023, 24, 1239–1259. [Google Scholar] [CrossRef]
- Tan, G.; Wang, L.; Pan, W.; Chen, K. Polysaccharide Electrospun Nanofibers for Wound Healing Applications. Int. J. Nanomed. 2022, 17, 3913–3931. [Google Scholar] [CrossRef] [PubMed]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 110994. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, P.; Song, P.; Li, N. Electrospinning of botanicals for skin wound healing. Front. Bioeng. Biotechnol. 2022, 10, 1006129. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Feng, Z.; Han, B.; Yu, D.G.; Wang, K. Advances in the Preparation of Nanofiber Dressings by Electrospinning for Promoting Diabetic Wound Healing. Biomolecules 2022, 12, 1727. [Google Scholar] [CrossRef]
- Akhmetova, A.; Heinz, A. Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges. Pharmaceutics 2020, 13, 4. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Bwanga, A.; Makoni, P.A.; Witika, B.A. Applications of Electrospun Drug-Eluting Nanofibers in Wound Healing: Current and Future Perspectives. Polymers 2022, 14, 2931. [Google Scholar] [CrossRef]
- Kazemi, M.H.; Sajadimajd, S.; Gorgin Karaji, Z. In vitro investigation of wound healing performance of PVA/chitosan/silk electrospun mat loaded with deferoxamine and ciprofloxacin. Int. J. Biol. Macromol. 2023, 253, 126602. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Chyzy, A.; Plonska-Brzezinska, M.E. Hydrogel Properties and Their Impact on Regenerative Medicine and Tissue Engineering. Molecules 2020, 25, 5795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, J. Recent advances on gelatin methacrylate hydrogels with controlled microstructures for tissue engineering. Int. J. Biol. Macromol. 2022, 221, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, J.; Zhang, C.; Hu, W.; Wang, L.; et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: An Effective Strategy for Tissue Engineering. Stem Cell Rev. Rep. 2019, 15, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kilian, K.A. Bridging the Gap: From 2D Cell Culture to 3D Microengineered Extracellular Matrices. Adv. Healthc. Mater. 2015, 4, 2780–2796. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, S.; Sivashanmugam, A.; Annapoorna, M.; Janarthanan, R.; Subramania, I.; Shantikumar, V.N.; Jayakumar, R. Injectable deferoxamine nanoparticles loaded chitosan-hyaluronic acid coacervate hydrogel for therapeutic angiogenesis. Colloids Surf. B Biointerfaces 2018, 161, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.H.; Borrelli, M.R.; Adem, S.; Deleon, N.M.D.; Patel, R.A.; Mascharak, S.; Yen, S.J.; Sun, B.Y.; Taylor, W.L.t.; Januszyk, M.; et al. Prophylactic treatment with transdermal deferoxamine mitigates radiation-induced skin fibrosis. Sci. Rep. 2020, 10, 12346. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Q.; Hu, Q.; Zhang, T.; Shi, J.; Kong, L.; Fu, D.; Yang, C.; Zhang, Z. An injectable bioactive dressing based on platelet-rich plasma and nanoclay: Sustained release of deferoxamine to accelerate chronic wound healing. Acta Pharm. Sin. B 2023, 13, 4318–4336. [Google Scholar] [CrossRef]
- Yu, F.X.; Lee, P.S.Y.; Yang, L.; Gao, N.; Zhang, Y.; Ljubimov, A.V.; Yang, E.; Zhou, Q.; Xie, L. The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas. Prog. Retin. Eye Res. 2022, 89, 101039. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Kwee, B.J.; Mooney, D.J.; Duda, G.N. Boon and Bane of Inflammation in Bone Tissue Regeneration and Its Link with Angiogenesis. Tissue Eng. Part. B Rev. 2015, 21, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Zhang, M.; Wu, Z.F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef] [PubMed]
- Donneys, A.; Yang, Q.; Forrest, M.L.; Nelson, N.S.; Zhang, T.; Ettinger, R.; Ranganathan, K.; Snider, A.; Deshpande, S.S.; Cohen, M.S.; et al. Implantable hyaluronic acid-deferoxamine conjugate prevents nonunions through stimulation of neovascularization. NPJ Regen. Med. 2019, 4, 11. [Google Scholar] [CrossRef]
- Li, H.; Luo, B.; Wen, W.; Zhou, C.; Tian, L.; Ramakrishna, S. Deferoxamine immobilized poly(D,L-lactide) membrane via polydopamine adhesive coating: The influence on mouse embryo osteoblast precursor cells and human umbilical vein endothelial cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 701–709. [Google Scholar] [CrossRef]
- Ran, Q.; Yu, Y.; Chen, W.; Shen, X.; Mu, C.; Yuan, Z.; Tao, B.; Hu, Y.; Yang, W.; Cai, K. Deferoxamine loaded titania nanotubes substrates regulate osteogenic and angiogenic differentiation of MSCs via activation of HIF-1α signaling. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Chen, H.; Kang, H.; Qi, J.; Zhao, P.; Jiang, M.; Guo, L.; Zhou, Q.; Qian, N.D.; Zhou, H.B.; et al. Deferoxamine released from poly(lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angiogenesis and osteogenesis. J. Biomed. Mater. Res. A 2016, 104, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, J.; Zhang, X.; Li, Y.; Zhang, S.; Yang, L.; Li, R.; Wan, Q.; Pei, X.; Chen, J.; et al. Drug-Delivery Nanoplatform with Synergistic Regulation of Angiogenesis-Osteogenesis Coupling for Promoting Vascularized Bone Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 17543–17561. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.L.; Chu, C.W.; Chik, K.W.; Pang, L.M.; Shing, M.K.; Li, C.K. Deferoxamine-induced dysplasia of the knee: Sonographic features and diagnostic performance compared with magnetic resonance imaging. J. Ultrasound Med. 2001, 20, 723–728. [Google Scholar] [CrossRef]
- Brittenham, G.M. Iron-chelating therapy for transfusional iron overload. N. Engl. J. Med. 2011, 364, 146–156. [Google Scholar] [CrossRef]
- Miller, S.C.; Pan, H.; Wang, D.; Bowman, B.M.; Kopecková, P.; Kopecek, J. Feasibility of using a bone-targeted, macromolecular delivery system coupled with prostaglandin E(1) to promote bone formation in aged, estrogen-deficient rats. Pharm. Res. 2008, 25, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Miller, S.C.; Kopecková, P.; Kopecek, J. Bone-targeting macromolecular therapeutics. Adv. Drug Deliv. Rev. 2005, 57, 1049–1076. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gai, M.; Rutkowski, S.; He, W.; Meng, S.; Gorin, D.; Dai, L.; He, Q.; Frueh, J. An Automated Device for Layer-by-Layer Coating of Dispersed Superparamagnetic Nanoparticle Templates. Colloid J. 2018, 80, 648–659. [Google Scholar] [CrossRef]
- Sindeeva, O.A.; Gusliakova, O.I.; Inozemtseva, O.A.; Abdurashitov, A.S.; Brodovskaya, E.P.; Gai, M.; Tuchin, V.V.; Gorin, D.A.; Sukhorukov, G.B. Effect of a Controlled Release of Epinephrine Hydrochloride from PLGA Microchamber Array: In Vivo Studies. ACS Appl. Mater. Interfaces 2018, 10, 37855–37864. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).