Review of the Real-Time Monitoring Technologies for Lithium Dendrites in Lithium-Ion Batteries
Abstract
:1. Introduction
2. Mechanism of Lithium Dendrite Growth
3. Real-Time Monitoring Method of Lithium Dendrites
3.1. In Situ/Operando Characterization
3.1.1. In Situ/Operando X-ray Spectroscopy
3.1.2. In Situ Raman Spectroscopy
3.1.3. In Situ Resonance Spectroscopy
3.1.4. In Situ Microscopy
3.1.5. In Situ/Operando Neutron Technology
3.1.6. Combination of Various In Situ Characterizations
3.1.7. Industrial Application of In Situ Characterizations
3.2. Sensors
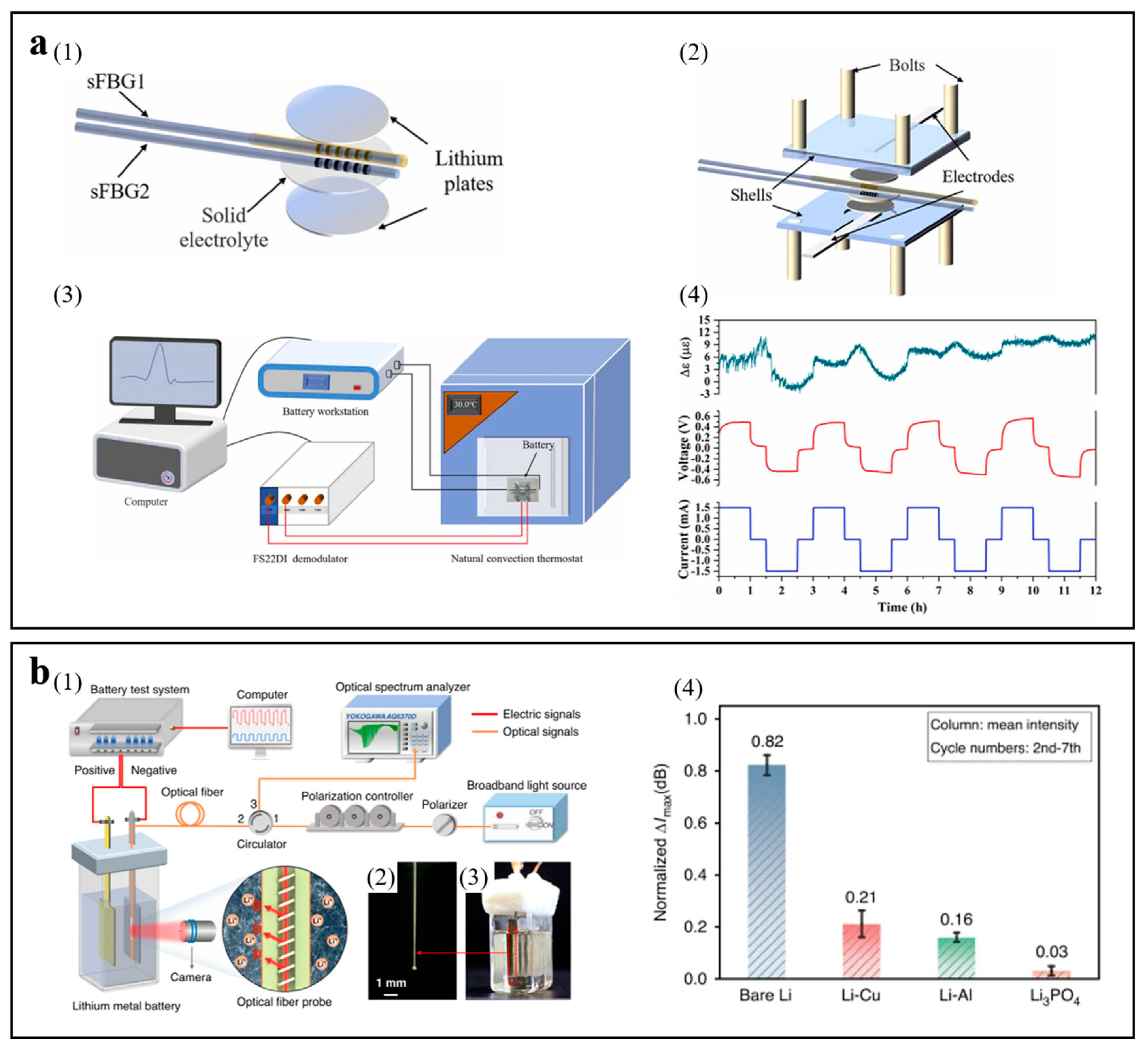
3.2.1. Optical Fiber Sensor
3.2.2. Gas Sensor
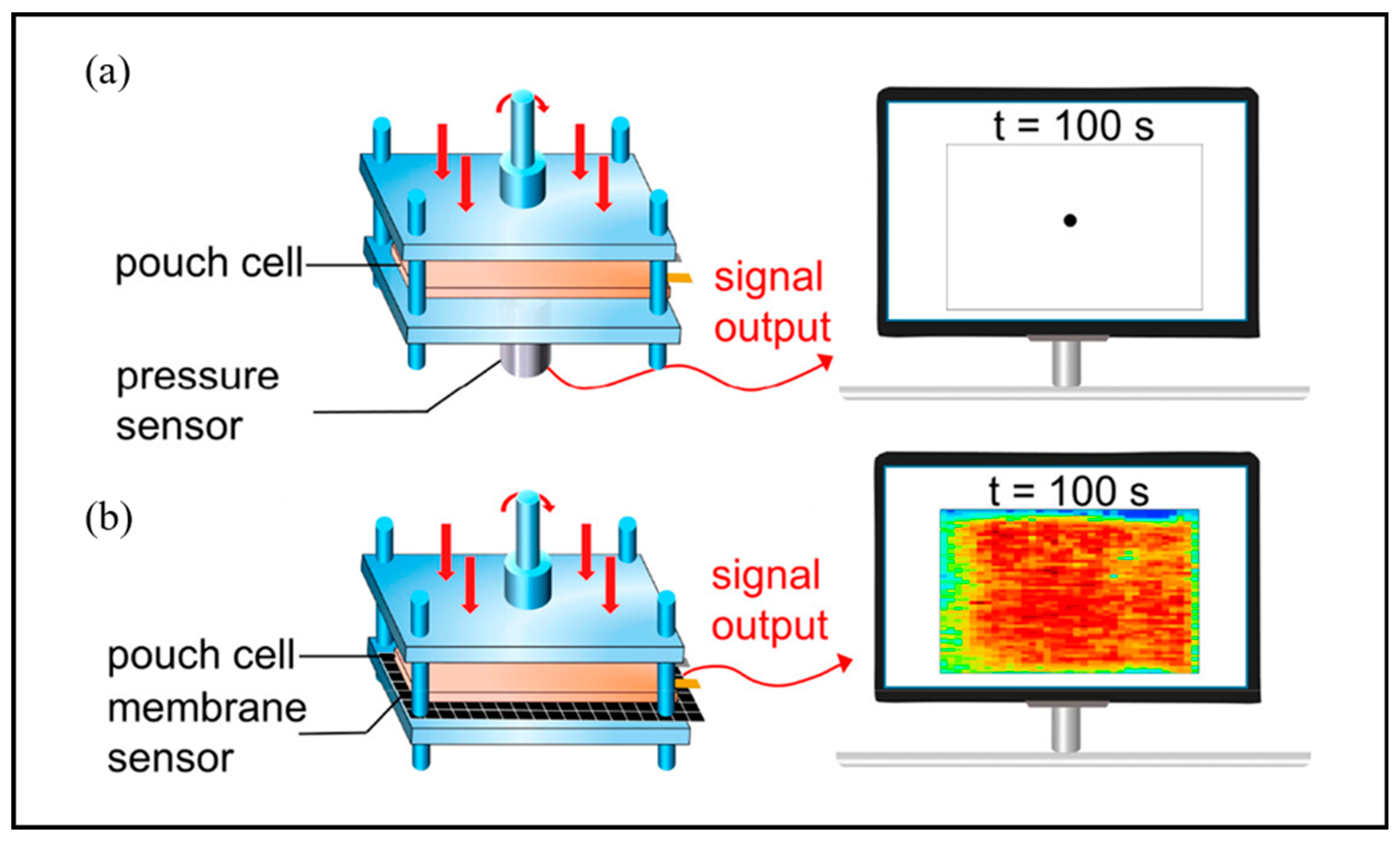
3.2.3. Membrane Sensor
4. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Dong, L.W.; Zhong, S.J.; Zhang, S.H.; Yuan, B.T.; Liu, J.P.; Xie, H.D.; Zhang, C.M.; Liu, Y.P.; Yang, C.H.; Han, J.C.; et al. Toward practical anode-free lithium pouch batteries. Energy Environ. Sci. 2023, 16, 5605–5632. [Google Scholar] [CrossRef]
- Yuan, B.T.; Feng, Y.H.; Qiu, X.H.; He, Y.H.; Dong, L.W.; Zhong, S.J.; Liu, J.P.; Liang, Y.F.; Liu, Y.P.; Xie, H.D.; et al. A safe separator with heat-dispersing channels for high-rate lithium-ion batteries. Adv. Funct. Mater. 2023, 34, 2308929. [Google Scholar] [CrossRef]
- Wan, X.H.; Xu, X.J.; Li, F.K.; Song, X.; Peng, C.X.; Liu, J. Application of nondestructive testing technology in device-scale for lithium-ion batteries. Small Struct. 2023, 5, 2300196. [Google Scholar] [CrossRef]
- Ou, S.Q.; Meng, T.; Xie, Z.Z.; Feng, J.; Wang, Q.S.; Zhou, D.; Liu, Z.F.; Wang, K.; Meng, C.G.; Tong, Y.X. Rational design of silicon nanodots/carbon anodes by partial oxidization strategy with high-performance lithium-ion storage. ACS Appl. Mater. Inter. 2022, 14, 48801–48811. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, H.; Wang, Q.S. Enhanced oxygen evolution reaction and lithium-ion storage performance of MOF-derived NiCo2O4-NiO-Co@graphene composites: Effect of carboxylic ligand group. J. Energy Storage 2024, 84, 110823. [Google Scholar] [CrossRef]
- Dong, L.W.; Zhong, S.J.; Yuan, B.T.; Li, Y.Q.; Liu, J.P.; Ji, Y.P.; Chen, D.J.; Liu, Y.P.; Yang, C.H.; Han, J.C.; et al. Reconstruction of solid electrolyte interphase with SrI2 reactivates dead Li for durable anode-free Li-metal batteries. Angew. Chem. Int. Ed. 2023, 62, e202301073. [Google Scholar] [CrossRef]
- Li, P.; Jeong, J.Y.; Jin, B.J.; Zhang, K.; Park, J.H. Vertically oriented MoS2 with spatially controlled geometry on nitrogenous graphene sheets for high-performance sodium-ion batteries. Adv. Energy Mater. 2018, 8, 1703300. [Google Scholar] [CrossRef]
- Xu, H.Y.; Han, C.; Li, W.T.; Li, H.Y.; Qiu, X.P. Quantification of lithium dendrite and solid electrolyte interphase (SEI) in lithium-ion batteries. J. Power Sources 2022, 529, 231219. [Google Scholar] [CrossRef]
- Yuan, B.T.; He, N.D.; Liang, Y.F.; Dong, L.W.; Liu, J.P.; Han, J.C.; He, W.D.; Liu, Y.P. A surfactant-modified composite separator for high safe lithium ion battery. J. Energy Chem. 2023, 76, 398–403. [Google Scholar] [CrossRef]
- Niu, M.; Dong, L.; Yue, J.; Li, Y.; Dong, Y.; Cheng, S.; Lv, S.; Zhu, Y.; Lei, Z.; Liang, J.; et al. A fast-charge graphite anode with a Li-ion-conductive, electron/solvent-repelling interface. Angew. Chem. Int. Ed. 2024. [Google Scholar] [CrossRef]
- Gireaud, L.; Grugeon, S.; Laruelle, S.; Yrieix, B.; Tarascon, J.M. Lithium metal stripping/plating mechanisms studies: A metallurgical approach. Electrochem. Commun. 2006, 8, 1639–1649. [Google Scholar] [CrossRef]
- Li, L.L.; Li, S.Y.; Lu, Y.Y. Suppression of dendritic lithium growth in lithium metal-based batteries. Chem. Commun. 2018, 54, 6648–6661. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bao, Z.N.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.Y.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Sheng, J.Z.; Zhang, Q.; Liu, M.S.; Han, Z.Y.; Li, C.; Sun, C.B.; Chen, B.; Zhong, X.W.; Qiu, L.; Zhou, G.M. Stabilized solid electrolyte interphase induced by ultrathin boron nitride membranes for safe lithium metal batteries. Nano Lett. 2021, 21, 8447–8454. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Liu, B.; Shen, Y.H.; Wu, J.K.; Zhao, Z.Q.; Zhong, C.; Hu, W.B. Confronting the challenges in lithium anodes for lithium metal batteries. Adv. Sci. 2021, 8, 2101111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Guo, K.; Wang, C.G.; Gao, H.J. Wrinkling and ratcheting of a thin film on cyclically deforming plastic substrate: Mechanical instability of the solid-electrolyte interphase in Li-ion batteries. J. Mech. Phys. Solids 2019, 123, 103–118. [Google Scholar] [CrossRef]
- Fang, C.C.; Li, J.X.; Zhang, M.H.; Zhang, Y.H.; Yang, F.; Lee, J.Z.; Lee, M.H.; Alvarado, J.; Schroeder, M.A.; Yang, Y.Y.C.; et al. Quantifying inactive lithium in lithium metal batteries. Nature 2019, 572, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, K.; Park, J.H. Dual or multi carbonaceous coating strategies for next-generation batteries. J. Mater. Chem. A 2018, 6, 1900–1914. [Google Scholar] [CrossRef]
- Li, J.W.; Kong, Z.; Liu, X.X.; Zheng, B.C.; Fan, Q.H.; Garratt, E.; Schuelke, T.; Wang, K.L.; Xu, H.; Jin, H. Strategies to anode protection in lithium metal battery: A review. Infomat 2021, 3, 1333–1363. [Google Scholar] [CrossRef]
- Ma, J.; Chen, B.B.; Wang, L.L.; Cui, G.L. Progress and prospect on failure mechanisms of solid-state lithium batteries. J. Power Sources 2018, 392, 94–115. [Google Scholar] [CrossRef]
- Narayanan, S.; Gibson, J.S.; Aspinall, J.; Weatherup, R.S.; Pasta, M. In situ and operando characterisation of Li metal-solid electrolyte interfaces. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100978. [Google Scholar] [CrossRef]
- Huo, H.Y.; Jiang, M.; Mogwitz, B.; Sann, J.; Yusim, Y.; Zuo, T.T.; Moryson, Y.; Minnmann, P.; Richter, F.H.; Singh, C.V.; et al. Interface design enabling stable polymer/thiophosphate electrolyte separators for dendrite-free lithium metal batteries. Angew. Chem. Int. Ed. 2023, 62, e202218044. [Google Scholar] [CrossRef]
- Chen, W.; Hu, Y.; Lv, W.Q.; Lei, T.Y.; Wang, X.F.; Li, Z.H.; Zhang, M.; Huang, J.W.; Du, X.C.; Yan, Y.C.; et al. Lithiophilic montmorillonite serves as lithium ion reservoir to facilitate uniform lithium deposition. Nat. Commun. 2019, 10, 4973. [Google Scholar] [CrossRef] [PubMed]
- Wandt, J.; Marino, C.; Gasteiger, H.A.; Jakes, P.; Eichel, R.A.; Granwehr, J. Operando electron paramagnetic resonance spectroscopy—Formation of mossy lithium on lithium anodes during charge-discharge cycling. Energy Environ. Sci. 2015, 8, 1358–1367. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tsuda, T.; Oshima, Y.; Kuwabata, S. In situ monitoring of lithium metal anodes and their solid electrolyte interphases by transmission electron microscopy. Small Struct. 2021, 2, 2100018. [Google Scholar] [CrossRef]
- Li, Q.; Yi, T.C.; Wang, X.L.; Pan, H.Y.; Quan, B.G.; Liang, T.J.; Guo, X.X.; Yu, X.Q.; Wang, O.W.R.; Huang, X.J.; et al. In-situ visualization of lithium plating in all-solid-state lithium-metal battery. Nano Energy 2019, 63, 103895. [Google Scholar] [CrossRef]
- Xi, J.; Li, J.; Sun, H.; Ma, T.; Deng, L.; Liu, N.; Huang, X.; Zhang, J. In-situ monitoring of internal temperature and strain of solid-state battery based on optical fiber sensors. Sens. Actuators A Phys. 2022, 347, 113888. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, H.L.; Dang, Y.Z.; Zhuang, Q.C.; Arandiyan, H.; Wang, Y.; Cheng, N.Y.; Sun, H.Y.; Garza, H.H.P.; Zheng, R.G.; et al. Recent advances in in situ and operando characterization techniques for Li7La3Zr2O12-based solid-state lithium batteries. Mater. Horiz. 2023, 10, 1479–1538. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Cho, S.; Nam, J.; Lee, H.; Kim, M. X-ray-based spectroscopic techniques for characterization of polymer nanocomposite materials at a molecular level. Polymers 2020, 12, 1053. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Wu, Y.L.; Chen, Z.Y.; Wang, Y.; Zhu, J.H.; Zhuang, X.D. The value of in situ/operando Raman spectroscopy in all-solid-state Li batteries. J. Mater. Chem. A 2023, 11, 19195–19209. [Google Scholar] [CrossRef]
- Li, H.; Chao, D.L.; Chen, B.; Chen, X.; Chuah, C.; Tang, Y.H.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Revealing principles for design of lean-electrolyte lithium metal anode via in situ spectroscopy. J. Am. Chem. Soc. 2020, 142, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Pecher, O.; Carretero-González, J.; Griffith, K.J.; Grey, C.P. Materials’ methods: NMR in battery research. Chem. Mater. 2017, 29, 213–242. [Google Scholar] [CrossRef]
- Sathiya, M.; Leriche, J.B.; Salager, E.; Gourier, D.; Tarascon, J.M.; Vezin, H. Electron paramagnetic resonance imaging for real-time monitoring of Li-ion batteries. Nat. Commun. 2015, 6, 6276. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, W.L.; Sun, K.; Xu, Y.J.; Sun, Y.; Li, Q.; Hu, H.; Wu, M.B. Developing in situ electron paramagnetic resonance characterization for understanding electron transfer of rechargeable batteries. Nano Res. 2023, 16, 11992–12012. [Google Scholar] [CrossRef]
- Yang, S.J.; Min, X.; Fan, H.; Xiao, J.; Liu, Y.A.; Mi, R.Y.; Wu, X.W.; Huang, Z.H.; Xi, K.; Fang, M.H. In situ characterization of lithium-metal anodes. J. Mater. Chem. A 2022, 10, 17917–17947. [Google Scholar] [CrossRef]
- Ma, X.Y.; Luo, W.; Yan, M.Y.; He, L.; Mai, L.Q. In situ characterization of electrochemical processes in one dimensional nanomaterials for energy storages devices. Nano Energy 2016, 24, 165–188. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Q.; Lv, Z.L.; Zhai, F.F.; Li, Z.L.; Li, S.L.; Zhang, B.T.; Cui, G.L. Multi-scale characterization techniques for polymer-based solid-state lithium batteries. Macromol. Chem. Phys. 2023, 224, 2200351. [Google Scholar] [CrossRef]
- Wan, J.; Yan, H.J.; Wen, R.; Wan, L.J. In situ visualization of electrochemical processes in solid-state lithium batteries. ACS Energy Lett. 2022, 7, 2988–3002. [Google Scholar] [CrossRef]
- Wetjen, M.; Trunk, M.; Werner, L.; Gernhäuser, R.; Märkisch, B.; Révay, Z.; Gilles, R.; Gasteiger, H.A. Quantifying the distribution of electrolyte decomposition products in silicon-graphite electrodes by neutron depth profiling. J. Electrochem. Soc. 2018, 165, A2340–A2348. [Google Scholar] [CrossRef]
- Cao, D.X.; Zhang, Y.X.; Ji, T.T.; Zhu, H.L. In operando neutron imaging characterizations of all-solid-state batteries. MRS Bull. 2023, 48, 1257–1268. [Google Scholar] [CrossRef]
- Wang, H.B.; Ning, D.; Wang, L.T.; Li, H.; Li, Q.Y.; Ge, M.Z.; Zou, J.Y.; Chen, S.; Shao, H.Y.; Lai, Y.K.; et al. In operando neutron scattering multiple-scale studies of lithium-ion batteries. Small 2022, 18, 2107491. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, S.; Li, H.M.; Wang, K.L.; Jiang, K. An overview on in situ/operando battery sensing methodology through thermal and stress measurements. J. Energy Storage 2023, 64, 107164. [Google Scholar] [CrossRef]
- Jin, Y.; Zheng, Z.K.; Wei, D.H.; Jiang, X.; Lu, H.F.; Sun, L.; Tao, F.B.; Guo, D.L.; Liu, Y.; Gao, J.F.; et al. Detection of micro-scale Li dendrite via H2 gas capture for early safety warning. Joule 2020, 4, 1714–1729. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhang, Z.Y.; Fu, K.; Yang, H.S.; Wang, X.Y.; Yang, K.; Ye, H.; Tan, P. Stress state characterization of Li-ion batteries based on a membrane sensor. Energy Fuels 2023, 37, 13526–13535. [Google Scholar] [CrossRef]
- Yang, H.J.; Guo, C.; Naveed, A.; Lei, J.Y.; Yang, J.; Nuli, Y.N.; Wang, J.L. Recent progress and perspective on lithium metal anode protection. Energy Storage Mater. 2018, 14, 199–221. [Google Scholar] [CrossRef]
- Barton, J.L.; Bockris, J.O.M. The electrolytic growth of dendrites from ionic solutions. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1962, 268, 485–505. [Google Scholar]
- Diggle, J.; Despic, A.; Bockris, J.M. The mechanism of the dendritic electrocrystallization of zinc. J. Electrochem. Soc. 1969, 116, 1503–1514. [Google Scholar] [CrossRef]
- Aogaki, R.; Makino, T. Theory of powdered metal formation in electrochemistry—Morphological instability in galvanostatic crystal growth under diffusion control. Electrochim. Acta 1981, 26, 1509–1517. [Google Scholar] [CrossRef]
- Bruce, P.G.; Vincent, C.A. Steady-state current flow in solid binary electrolyte cells. J. Electroanal. Chem. 1987, 225, 1–17. [Google Scholar] [CrossRef]
- Chazalviel, J.N. Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys. Rev. A 1990, 42, 7355–7367. [Google Scholar] [CrossRef] [PubMed]
- Fleury, V.; Chazalviel, J.N.; Rosso, M. Theory and experimental-evidence of electroconvection around electrochemical deposits. Phys. Rev. Lett. 1992, 68, 2492–2495. [Google Scholar] [CrossRef] [PubMed]
- Brissot, C.; Rosso, M.; Chazalviel, J.N.; Lascaud, S. Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 1999, 81, 925–929. [Google Scholar] [CrossRef]
- Monroe, C.; Newman, J. Dendrite growth in lithium/polymer systems—A propagation model for liquid electrolytes under galvanostatic conditions. J. Electrochem. Soc. 2003, 150, A1377–A1384. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, W.; Zheng, W.T.; Cui, X.Q.; Rojo, T.; Zhang, Q. Towards high-safe lithium metal anodes: Suppressing lithium dendrites via tuning surface energy. Adv. Sci. 2017, 4, 1600168. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.H.; Liu, T.F.; Yuan, Y.F.; Ling, M.; Xu, N.; Liu, Y.Y.; Yan, L.J.; Li, H.; Liu, C.Y.; Lu, Y.Y.; et al. Visualizing lithium dendrite formation within solid-state electrolytes. ACS Energy Lett. 2021, 6, 451–458. [Google Scholar] [CrossRef]
- Wang, H.C.; Gao, H.W.; Chen, X.X.; Zhu, J.P.; Li, W.Q.; Gong, Z.L.; Li, Y.X.; Wang, M.S.; Yang, Y. Linking the defects to the formation and growth of Li dendrite in all-solid-state batteries. Adv. Energy Mater. 2021, 11, 2102148. [Google Scholar] [CrossRef]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.Y.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 16114. [Google Scholar] [CrossRef]
- Zhang, G.B.; Xiong, T.F.; He, L.; Yan, M.Y.; Zhao, K.N.; Xu, X.; Mai, L.Q. Electrochemical in situ X-ray probing in lithium-ion and sodium-ion batteries. J. Mater. Sci. 2017, 52, 3697–3718. [Google Scholar] [CrossRef]
- Dai, J.Q.; Yang, C.P.; Wang, C.W.; Pastel, G.; Hu, L.B. Interface engineering for garnet-based solid-state lithium-metal batteries: Materials, structures, and characterization. Adv. Mater. 2018, 30, 1802068. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Kushima, A. Direct observation and quantitative analysis of lithium dendrite growth by in situ transmission electron microscopy. J. Electrochem. Soc. 2021, 168, 020535. [Google Scholar] [CrossRef]
- Hogrefe, C.; Waldmann, T.; Hölzle, M.; Wohlfahrt-Mehrens, M. Direct observation of internal short circuits by lithium dendrites in cross-sectional lithium-ion in situ full cells. J. Power Sources 2023, 556, 232391. [Google Scholar] [CrossRef]
- Song, B.H.; Dhiman, I.; Carothers, J.C.; Veith, G.M.; Liu, J.; Bilheux, H.Z.; Huq, A. Dynamic lithium distribution upon dendrite growth and shorting revealed by operando neutron imaging. ACS Energy Lett. 2019, 4, 2402–2408. [Google Scholar] [CrossRef]
- Giessibl, F.J. Advances in atomic force microscopy. Rev. Mod. Phys. 2003, 75, 949–983. [Google Scholar] [CrossRef]
- Blanc, F.; Leskes, M.; Grey, C.P. In situ solid-state NMR spectroscopy of electrochemical cells: Batteries, supercapacitors, and fuel cells. Acc. Chem. Res. 2013, 46, 1952–1963. [Google Scholar] [CrossRef] [PubMed]
- Dutoit, C.E.; Tang, M.X.; Gourier, D.; Tarascon, J.M.; Vezin, H.; Salager, E. Monitoring metallic sub-micrometric lithium structures in Li-ion batteries by in situ electron paramagnetic resonance correlated spectroscopy and imaging. Nat. Commun. 2021, 12, 1410. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Q.; Shadike, Z.; Lin, R.Q.; Qian, K.; Li, H.; Li, K.K.; Wang, S.W.; Yu, Q.P.; Liu, M.; Ganapathy, S.; et al. Review of recent development of in situ/operando characterization techniques for lithium battery research. Adv. Mater. 2019, 31, 1806620. [Google Scholar] [CrossRef] [PubMed]
- Paolella, A.; Zhu, W.; Xu, G.L.; La Monaca, A.; Savoie, S.; Girard, G.; Vijh, A.; Demers, H.; Perea, A.; Delaporte, N.; et al. Understanding the reactivity of a thin Li1.5Al0.5Ge1.5(PO4)3 solid-state electrolyte toward metallic lithium anode. Adv. Energy Mater. 2020, 10, 2001497. [Google Scholar] [CrossRef]
- Lu, X.M.; Liu, T.C.; Wang, Y.; Du, F.H. Inside-outside lithium deposition achieved by the unusual strategy of constructing the hierarchical lithiophilicity for dendrite-free and durable lithium metal anode. Batter. Supercaps 2022, 5, e202200114. [Google Scholar] [CrossRef]
- Wood, K.N.; Steirer, K.X.; Hafner, S.E.; Ban, C.M.; Santhanagopalan, S.; Lee, S.H.; Teeter, G. Operando X-ray photoelectron spectroscopy of solid electrolyte interphase formation and evolution in Li2S-P2S5 solid-state electrolytes. Nat. Commun. 2018, 9, 2490. [Google Scholar] [CrossRef] [PubMed]
- Bellouard, Y.; Block, E.; Squier, J.; Gobet, J. Plasmon-less surface enhanced Raman spectra induced by self-organized networks of silica nanoparticles produced by femtosecond lasers. Opt. Express 2017, 25, 9587–9594. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, W.; Lei, T.Y.; Jiao, Y.; Wang, H.B.; Wang, X.P.; Rao, G.F.; Wang, X.F.; Chen, B.; Xiong, J. Graphene quantum dots as the nucleation sites and interfacial regulator to suppress lithium dendrites for high-loading lithium-sulfur battery. Nano Energy 2020, 68, 104373. [Google Scholar] [CrossRef]
- Liu, T.C.; Ge, J.X.; Wang, H.C.; Zhang, Y.F.; Wang, Y. Unusual inside-outside Li deposition within three-dimensional honeycomb-like hierarchical nitrogen-doped framework for a dendrite-free lithium metal anode. ACS Appl. Energy Mater. 2021, 4, 2838–2846. [Google Scholar] [CrossRef]
- Wu, B.L.; Chen, C.G.; Danilov, D.L.; Chen, Z.Q.; Jiang, M.; Eichel, R.A.; Notten, P.H.L. Dual additives for stabilizing Li deposition and SEI formation in anode-free Li-metal batteries. Energy Environ. Mater. 2023. [Google Scholar] [CrossRef]
- Nie, Y.M.; Wang, J.X.; Zhong, J.; Li, G.C.; Wang, Z.X.; Peng, W.J.; Li, X.H.; Wang, R.H.; Yan, G.C.; Guo, H.J. Li+ attraction-repulsion synergy revealed by in-situ Raman spectroscopy for self-healing lithium metal anodes. Appl. Surf. Sci. 2023, 608, 155205. [Google Scholar] [CrossRef]
- Arai, J.; Nakahigashi, R. Study of Li metal deposition in lithium ion battery during low-temperature cycle using in situ solid-state 7Li nuclear magnetic resonance. J. Electrochem. Soc. 2017, 164, A3403–A3409. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Leissing, M.; Nowak, S.; Hwang, B.J.; Winter, M.; Brunklaus, G. Quantification of dead lithium via in situ nuclear magnetic resonance spectroscopy. Cell Rep. Phys. Sci. 2020, 1, 100139. [Google Scholar] [CrossRef]
- Li, Z.Q.; Huang, X.L.; Kong, L.; Qin, N.; Wang, Z.Y.; Yin, L.H.; Li, Y.Z.; Gan, Q.M.; Liao, K.M.; Gu, S.; et al. Gradient nano-recipes to guide lithium deposition in a tunable reservoir for anode-free batteries. Energy Storage Mater. 2022, 45, 40–47. [Google Scholar] [CrossRef]
- Liu, H.W.; Jiang, W.N.; Chen, W.J.; Lin, Q.Y.; Ren, S.Y.; Su, Y.P.; Tong, R.Y.; Zhang, Y.G. Dendrite growth and inhibition in all-solid-state lithium metal batteries: In situ optical observation. J. Mater. Chem. A 2024, 12, 3575–3579. [Google Scholar] [CrossRef]
- Golozar, M.; Paolella, A.; Demers, H.; Bessette, S.; Lagacé, M.; Bouchard, P.; Guerfi, A.; Gauvin, R.; Zaghib, K. In situ observation of solid electrolyte interphase evolution in a lithium metal battery. Commun. Chem. 2019, 2, 131. [Google Scholar] [CrossRef]
- Tang, C.Y.; Dillon, S.J. In situ scanning electron microscopy characterization of the mechanism for Li dendrite growth. J. Electrochem. Soc. 2016, 163, A1660–A1665. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Li, N.W.; Shi, Y.; Yin, Y.X.; Zeng, X.X.; Li, J.Y.; Li, C.J.; Wan, L.J.; Wen, R.; Guo, Y.G. A flexible solid electrolyte interphase layer for long-life lithium metal anodes. Angew. Chem. Int. Ed. 2018, 57, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.Y.; Shen, Z.Z.; Hu, X.C.; Shi, Y.; Guo, Y.G.; Jia, F.F.; Wang, F.Y.; Wen, R.; Wan, L.J. Tunable structure and dynamics of solid electrolyte interphase at lithium metal anode. Nano Energy 2020, 75, 104967. [Google Scholar] [CrossRef]
- Pu, J.; Zhong, C.L.; Liu, J.H.; Wang, Z.H.; Chao, D.L. Advanced in situ technology for Li/Na metal anodes: An in-depth mechanistic understanding. Energy Environ. Sci. 2021, 14, 3872–3911. [Google Scholar] [CrossRef]
- Lv, S.S.; Verhallen, T.; Vasileiadis, A.; Ooms, F.; Xu, Y.L.; Li, Z.L.; Li, Z.C.; Wagemaker, M. Operando monitoring the lithium spatial distribution of lithium metal anodes. Nat. Commun. 2018, 9, 2152. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Mo, F.J.; Hu, J.M.; Li, S.Y.; Huang, L.Z.; Fang, F.; Sun, D.L.; Sun, G.A.; Wang, F.; Song, Y. Revealing the dynamic evolution of Li filaments within solid electrolytes by operando small-angle neutron scattering. Appl. Phys. Lett. 2022, 121, 163901. [Google Scholar] [CrossRef]
- Liu, G.X.; Wan, J.; Shi, Y.; Guo, H.J.; Song, Y.X.; Jiang, K.C.; Guo, Y.G.; Wen, R.; Wan, L.J. Direct tracking of additive-regulated evolution on the lithium anode in quasi-solid-state lithium-sulfur batteries. Adv. Energy Mater. 2022, 12, 2201411. [Google Scholar] [CrossRef]
- Gotoh, K.; Izuka, M.; Arai, J.; Okada, Y.; Sugiyama, T.; Takeda, K.; Ishida, H. In situ 7Li nuclear magnetic resonance study of the relaxation effect in practical lithium ion batteries. Carbon 2014, 79, 380–387. [Google Scholar] [CrossRef]
- Zhou, H.; An, K.; Allu, S.; Pannala, S.; Li, J.L.; Bilheux, H.Z.; Martha, S.K.; Nanda, J. Probing multiscale transport and inhomogeneity in a lithium-ion pouch cell using in situ neutron methods. ACS Energy Lett. 2016, 1, 981–986. [Google Scholar] [CrossRef]
- Lee, B. Review of the present status of optical fiber sensors. Opt. Fiber Technol. 2003, 9, 57–79. [Google Scholar] [CrossRef]
- Han, X.L.; Zhong, H.; Li, K.W.; Xue, X.B.; Wu, W.; Hu, N.; Lu, X.H.; Huang, J.Q.; Xiao, G.Z.; Mai, Y.H.; et al. Operando monitoring of dendrite formation in lithium metal batteries via ultrasensitive tilted fiber Bragg grating sensors. Light-Sci. Appl. 2024, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.J.; Jin, Z.S.; Lin, Z.Y.; Zhou, S.Y.; Du, Y.H.; Chen, Y.L.; Zhou, H.P. A single dual-mode gas sensor for early safety warning of Li-ion batteries: Micro-scale Li dendrite and electrolyte leakage. Chin. Phys. B 2022, 31, 110704. [Google Scholar] [CrossRef]





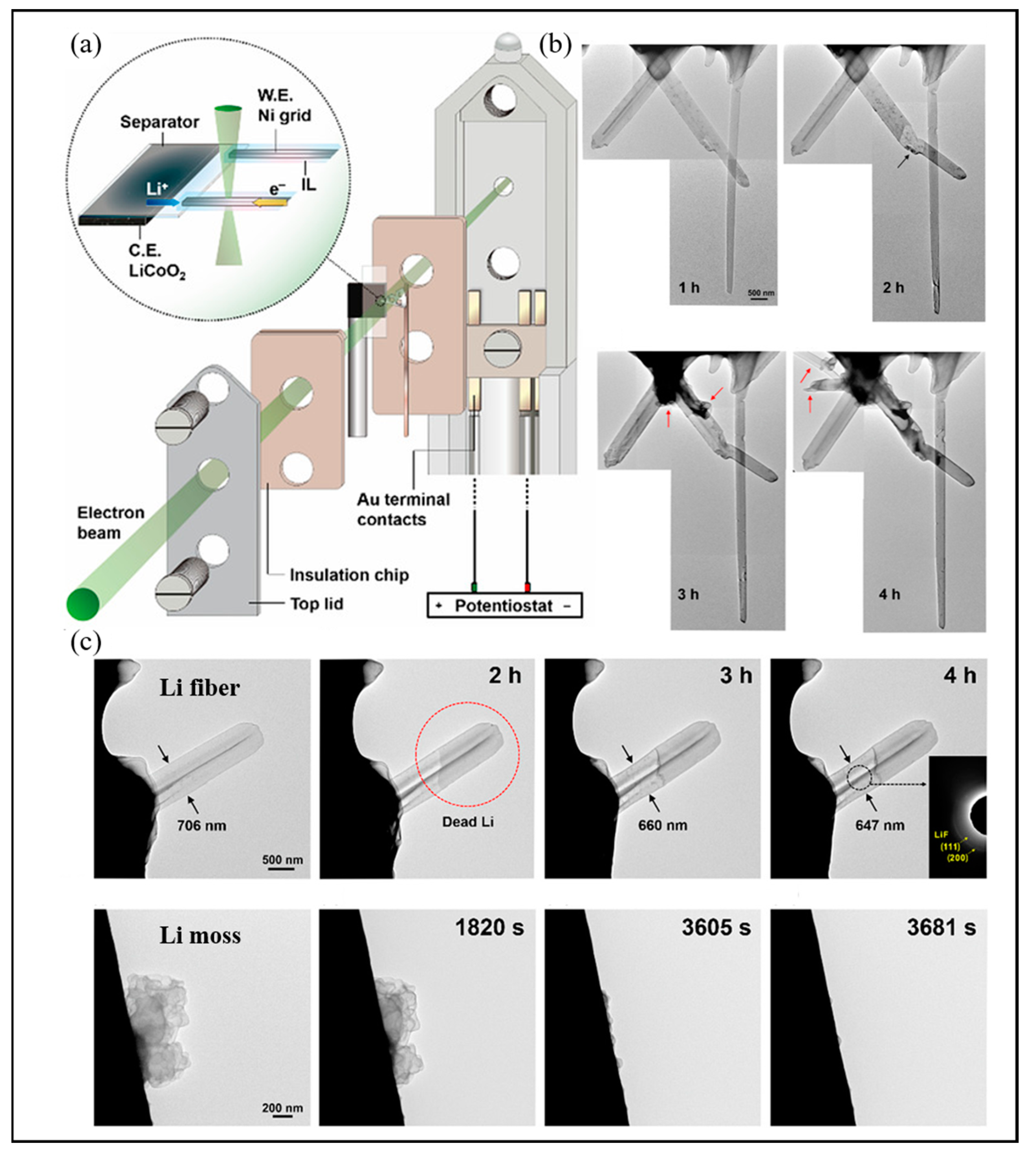

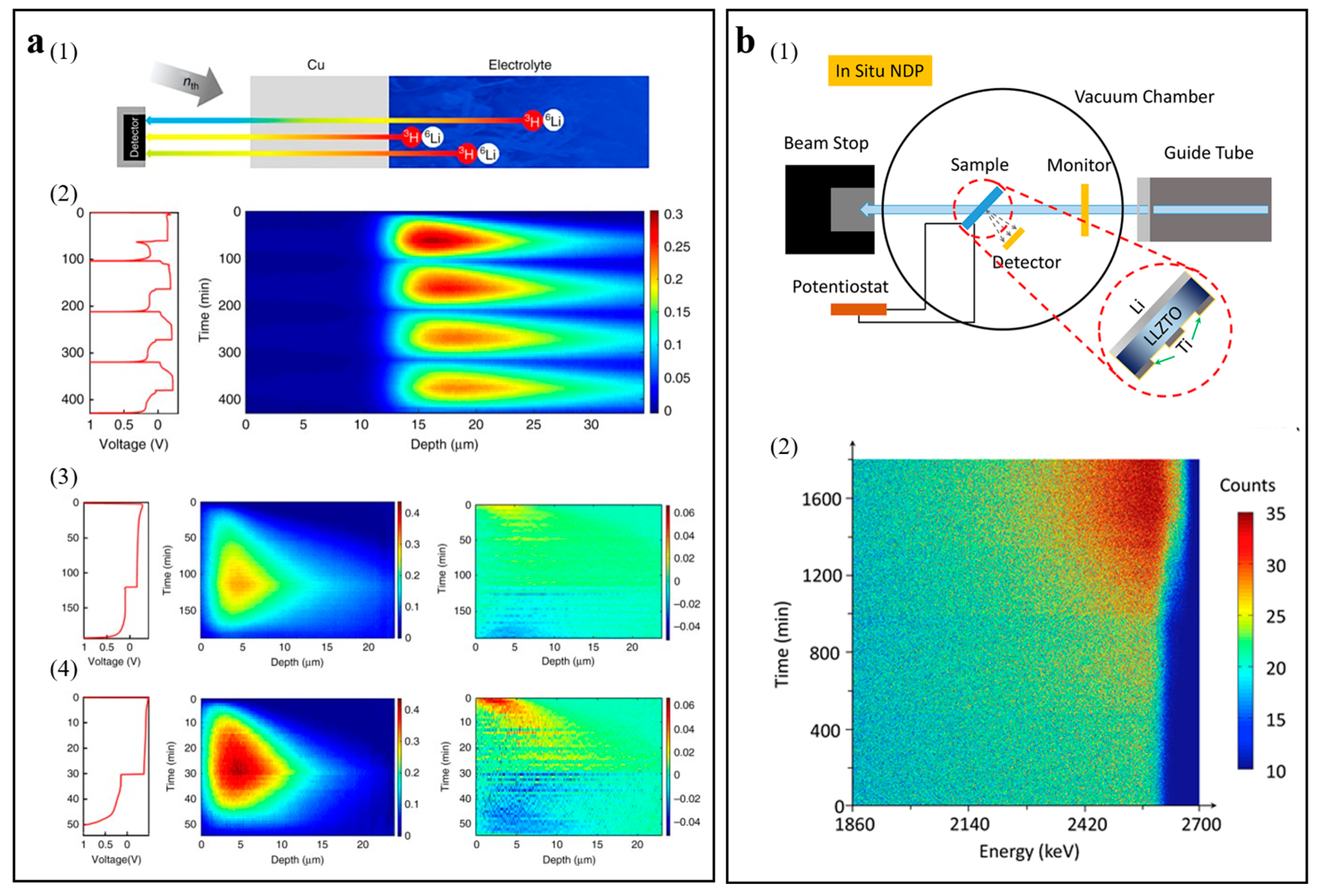
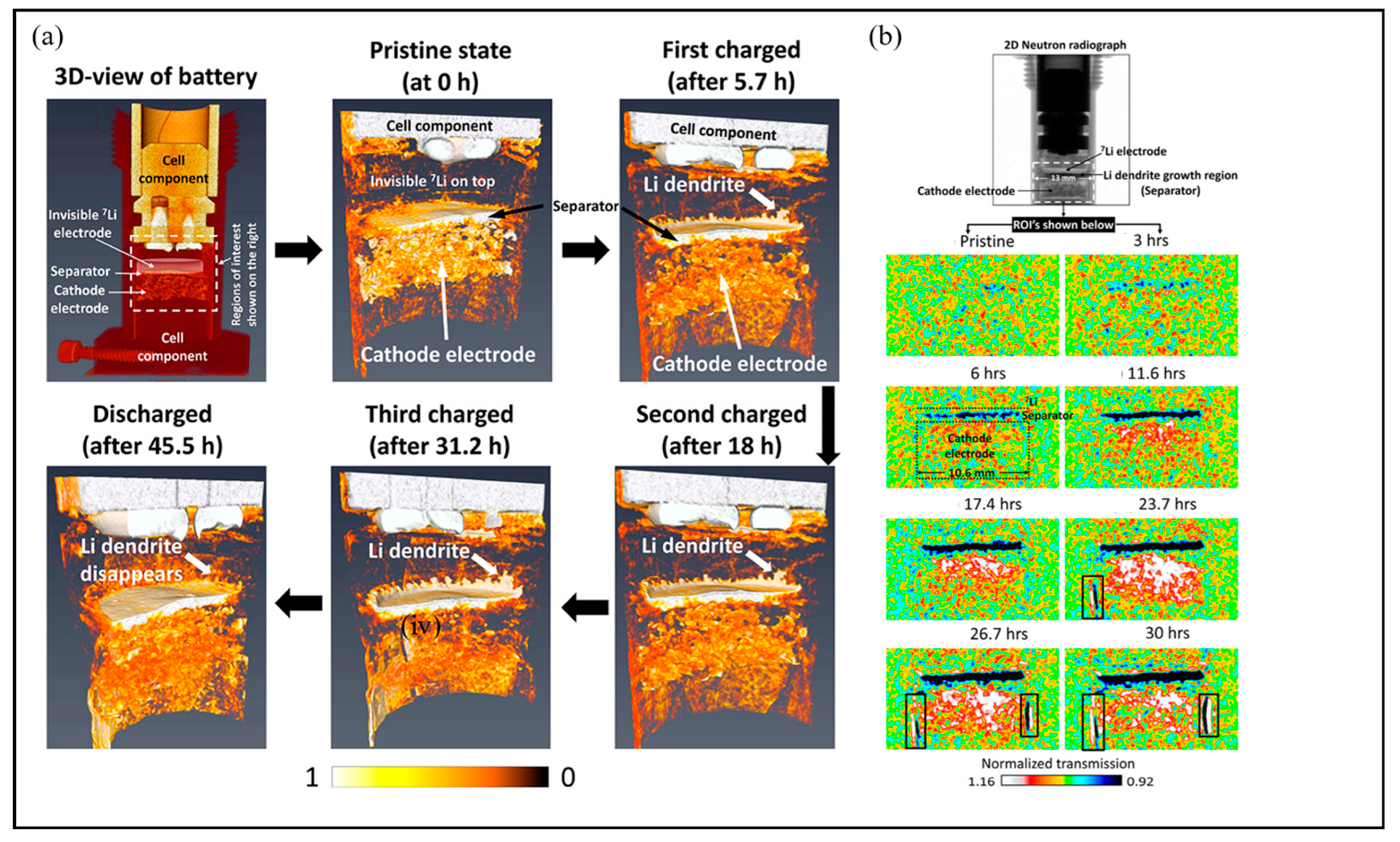
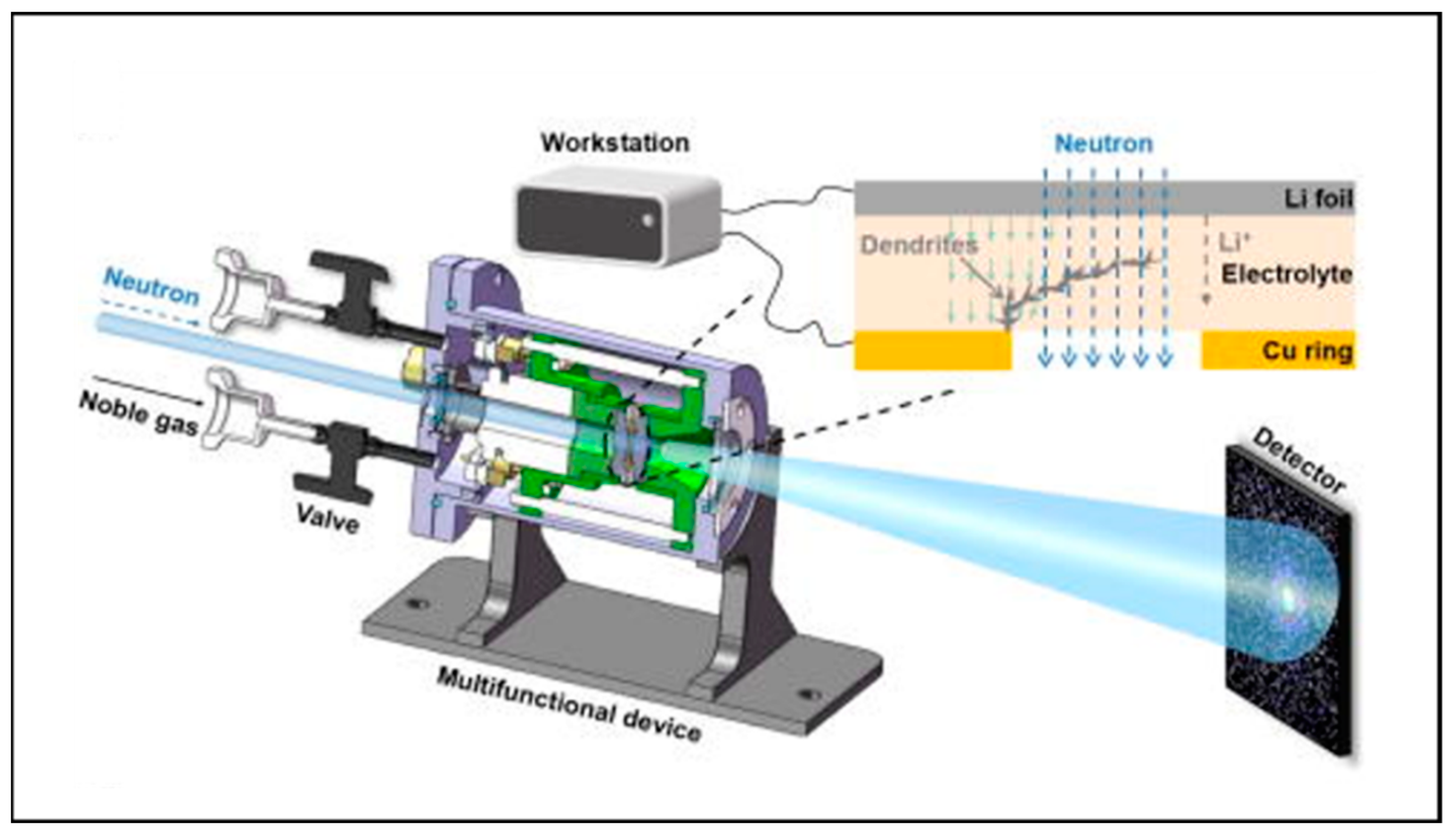
| Technologies | The Main Uses | Merits | Drawback | Ref. |
|---|---|---|---|---|
| In situ XRD | Phase transition and structural change | No destruction, high detection speed, high precision, and no contamination | Not applicable to amorphous materials and not suitable for direct observation morphology | [28] |
| In situ XPS | Elemental composition | Fast speed, no damage, high precision, and analysis depth is a few nanometers | Not applicable to the detection of overall composition | [29] |
| In situ Raman | Crystallinity, chemical structure, phase, and molecular interactions | Non-destructive, applicable to amorphous or weakened crystallized compounds | Not applicable to Li metal | [30,31] |
| In situ NMR | The information of electronic structure is analyzed qualitatively and quantitatively | Non-destructive, high-sensitivity | Expensive and long experiment time | [32] |
| In situ EPR | Detection of unpaired electrons or free radicals | Non-destructive, higher sensitivity, and accurate | Complex operation | [33,34] |
| In situ OM | Morphology evolution | Simple operation, and low cost | Low spatial resolution, the sample size is larger than nanometers, and only studies the sample surface | [35] |
| In situ SEM | Micromorphology | Large depth of field and high-definition | Harsh operating conditions | [36] |
| In situ TEM | Micromorphology | Extra-high resolution | Sample thickness is limited and harsh operating conditions | [37] |
| In situ AFM | Surface morphology and structure information and surface roughness information | Small intrusion, high spatial resolution, flexible application | Slow scanning speed | [38] |
| In situ NDP | Real-time distribution and migration of lithium ions | High sensitivity, strong penetration, and non-destructive to lithium | Under vacuum or pressure atmosphere | [39] |
| In situ NI | Lithium dynamic distribution | Sensitivity to light elements | Expensive | [40] |
| Operando SANS | Structure at the nanoscale | Sensitivity to light elements, identification of isotopes, and strong scattering of magnetic moments | Low neutron source brightness | [41] |
| Optical fiber sensor | Detection of strain, temperature, and pressure | Anti-electromagnetic interference, small dimension, low weight, large bandwidth, great sensibility | Expensive and high environmental requirements | [42] |
| Gas sensor | Detection of generated gas | High sensitivity, quick response | Expensive, poor anti-interference | [43] |
| Membrane sensor | Detection of stress distribution | Deformability, simple structure, fast response speed, long service life | Precision limitation, poor anti-interference | [44] |
| Technologies | Ex Situ | In Situ |
|---|---|---|
| XRD | Tests are carried out at the end of the reaction or at specific stages The state of the electrode may change during disassembly, washing, and other operations, affecting the accuracy of XRD peaks | Real-time monitoring of electrode structure changes during reaction or charge and discharge process |
| XPS | Evaluation of electrode/electrolyte interface chemical structure at the end of the reaction | Evaluation of chemical changes at the interface under electrochemical conditions |
| Raman | Only the end product can be tested | Monitoring the intermediate products, the reaction process |
| NMR | Investigating the reactants and products of the reaction | Different components of the same battery can be studied in different charging states Instantaneous states and the dynamic processes occurring in real time can be investigated |
| EPR | Free radicals are detected by trapping them in probe molecules The sample needs to be tested immediately | Real-time monitoring of electron spin signals during electrochemical reactions |
| OM | Morphology of electrode before and after reaction | Monitoring the growth morphology of lithium dendrites in real time |
| SEM | Pretreatments such as sample fixation and slicing may result in changes in the original state of the sample | Real-time observation in close proximity to the actual environment of the sample Capturing the process of sample change under specific conditions |
| TEM | Microstructure before and after reaction | Monitoring the microstructure evolution during the chemical reaction |
| AFM | Morphology and mechanical properties before and after reaction | The evolution of electrode morphology and mechanical properties can be dynamically observed |
| NDP | Analyzing the composition of electrode material | Analyzing the distribution and migration of lithium ions in real time |
| NI | The neutron beam penetrates material for imaging | Monitoring of lithium deposition and distribution during the cycle |
| SANS | Characterization of nanoscale material unevenness | Investigating the dynamic behaviors and the evolution of Li dendrites in real time |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Song, D.; Wu, W.; Yu, Y.; You, J.; Liu, Y. Review of the Real-Time Monitoring Technologies for Lithium Dendrites in Lithium-Ion Batteries. Molecules 2024, 29, 2118. https://doi.org/10.3390/molecules29092118
Liang Y, Song D, Wu W, Yu Y, You J, Liu Y. Review of the Real-Time Monitoring Technologies for Lithium Dendrites in Lithium-Ion Batteries. Molecules. 2024; 29(9):2118. https://doi.org/10.3390/molecules29092118
Chicago/Turabian StyleLiang, Yifang, Daiheng Song, Wenju Wu, Yanchao Yu, Jun You, and Yuanpeng Liu. 2024. "Review of the Real-Time Monitoring Technologies for Lithium Dendrites in Lithium-Ion Batteries" Molecules 29, no. 9: 2118. https://doi.org/10.3390/molecules29092118
APA StyleLiang, Y., Song, D., Wu, W., Yu, Y., You, J., & Liu, Y. (2024). Review of the Real-Time Monitoring Technologies for Lithium Dendrites in Lithium-Ion Batteries. Molecules, 29(9), 2118. https://doi.org/10.3390/molecules29092118






