Efficacy and Functional Mechanisms of a Two-Stage Pretreatment Approach Based on Alkali and Ionic Liquid for Bioconversion of Waste Medium-Density Fiberboard
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of the Optimal Pretreatment Process
2.1.1. Effect of NaOH Pretreatment on the Enzymatic Hydrolysis of MDF
2.1.2. Impact of AAI Pretreatment on MDF Enzymatic Hydrolysis
2.2. Assessment of the Potential of Disused MDF Additive to Affect the Enzymatic Hydrolysis Sugar Yield
2.3. Component MDF Content under Different Pretreatment Conditions
2.4. Chemical Structure Analysis
2.5. Crystal Index Analysis
2.6. Microstructure Analysis
3. Materials and Methods
3.1. Materials
3.2. Pretreatment
3.2.1. NaOH Pretreatment
3.2.2. NaOH + IL Pretreatment (AAI Pretreatment)
3.2.3. Hydrothermal Pretreatment
3.2.4. Microwave + Ionic Liquid Pretreatment
3.3. Composition Analysis
3.4. Enzymatic Hydrolysis of Cellulose
3.5. Physicochemical Characterization of Pretreated MDF Biomass
3.5.1. FTIR
3.5.2. 13C NMR
3.5.3. XRD
3.5.4. SEM
3.5.5. EA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nargotra, P.; Sharma, V.; Gupta, M.; Kour, S.; Bajaj, B.K. Application of ionic liquid and alkali pretreatment for enhancing saccharification of sunflower stalk biomass for potential biofuel-ethanol production. Bioresour. Technol. 2018, 267, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Koupaie, E.H.; Dahadha, S.; Lakeh, A.B.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production—A review. J. Environ. Manag. 2018, 233, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yang, L.; Liu, B.; Tan, L. Hydroxycinnamic acids release during bioconversion of corn stover and their effects on lignocellulolytic enzymes. Bioresour. Technol. 2019, 294, 122116. [Google Scholar] [CrossRef] [PubMed]
- Naveen Chakkaravarthy, A.; Subathra, M.S.P.; Jerin Pradeep, P.; Manoj Kumar, N. Solar irradiance forecasting and energy optimization for achieving nearly net zero energy building. J. Renew. Sustain. Energy 2018, 10, 35103. [Google Scholar] [CrossRef]
- Goshadrou, A. Bioethanol production from cogongrass by sequential recycling of black liquor and wastewater in a mild-alkali pretreatment. Fuel 2019, 258, 116141. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Lu, X.; Zhang, X.; Zhang, S. Cascade utilization of lignocellulosic biomass to high-value products. Green Chem. 2019, 21, 3499–3535. [Google Scholar] [CrossRef]
- Zhong, R. Effect of Urea-Formaldehyde Adhesive to Wood-Based Panels Recyclingand Cellulose Fiber Production; South China Agricultural University: Guangzhou, China, 2016. [Google Scholar]
- Zhou, D.G. Wood-Based Panel Processing; China Forestry Publishing: Beijing, China, 2011. [Google Scholar]
- Wu, Y.; Jiang, L.; Lin, Y.; Qian, L.; Xu, F.; Lang, X.; Fan, S.; Zhao, Z.; Li, H. Novel crude glycerol pretreatment for selective saccharification of sugarcane bagasse via fast pyrolysis. Bioresour. Technol. 2019, 294, 122094. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.K.; Chakraborty, S. Mixing effects on the kinetics of enzymatic hydrolysis of lignocellulosic sunn hemp fibres for bioethanol production. Chem. Eng. J. 2018, 377, 120103. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Meng, Z.; Wang, D. Boosting the fermentable sugar yield and concentration of corn stover by magnesium oxide pretreatment for ethanol production. Bioresour. Technol. 2018, 269, 400–407. [Google Scholar] [CrossRef]
- Sakuragi, K.; Igarashi, K.; Samejima, M. Application of ammonia pretreatment to enable enzymatic hydrolysis of hardwood biomass. Polym. Degrad. Stab. 2018, 148, 19–25. [Google Scholar] [CrossRef]
- Farkas, C.; Rezessy-Szabo, J.M.; Gupta, V.K.; Truong, D.H.; Friedrich, L.; Felfoldi, J.; Nguyen, Q.D. Microbial saccharification of wheat bran for bioethanol fermentation. J. Clean. Prod. 2019, 240, 118261–118269. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Chen, X.; Wang, C.Z.; Sun, S.N.; Sun, R.C. Evaluation of the two step pretreatment with ionic liquids and alkali for enhancing enzymatic hydrolysis of Eucalyptus: Chemical and anatomical changes. Biotechnol. Biofuels 2016, 166, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Raj, T.; Gaur, R.; Dixit, P.; Gupta, R.P.; Kagdiyal, V.; Kumar, R.; Tuli, D.K. Ionic liquid pretreatment of biomass for sugars production: Driving factors with a plausible mechanism for higher enzymatic digestibility. Carbohydr. Polym. 2016, 149, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, L.; Liu, C.; Xu, A. Pretreatment of corn straw using the alkaline solution of ionic liquids. Bioresour. Technol. 2018, 260, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Wang, Y.; Wu, N.; Qing, Y.; Li, X.; Wu, W.Q.; Liu, M. Degradation of urea-formaldehyde resin residues by a hydrothermal oxidation method into recyclable small molecular organics. J. Hazard. Mater. 2022, 426, 127783. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Chu, Q.; Hu, J.G.; Bu, Q.; Li, F.Q.; Chen, X.Y.; Shi, A.P. Two-stage alkali-oxygen pretreatment capable of improving biomass saccharification for bioethanol production and enabling lignin valorization via adsorbents for heavy metal ions under the biorefinery concept. Bioresour. Technol. 2019, 276, 161–169. [Google Scholar] [CrossRef]
- Chu, Q.; Song, K.; Wang, J.; Hu, J.; Chen, X. Improving enzymatic saccharification of hardwood through lignin modification by carbocation scavengers and the underlying mechanisms. Bioresour. Technol. 2019, 294, 122216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J. Statistical optimization of sodium hydroxide pretreatment and enzymatic hydrolysis of corn stover powder for enhancing sugar production using response surface methodology. Biomass Convers. Biorefinery 2021, 13, 7111–7125. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Z.; Sun, J.; Li, M.; Wang, S.; Chen, K.; Gao, Z. A microwave-assisted aqueous ionic liquid pretreatment to enhance enzymatic hydrolysis of eucalyptus and its mechanism. Bioresour. Technol. 2019, 272, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Jason, P.; Hallett, W.T. Cutting-edge research for a greener sustainable future. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Lu, H.; Liu, S.; Shi, Y.; Chen, Q. Efficient delignification of sugarcane bagasse by fenton oxidation coupled with ultrasound-assisted naoh for biotransformation from agaricus sinodeliciosus var. Chaidam. Chem. Eng. J. 2022, 448, 137719. [Google Scholar] [CrossRef]
- Peng, M.; Zhu, J.; Luo, Y.; Li, T.; Xia, X.; Qin, C.; Liang, C.; Bian, H.; Yao, S. Enhancement of separation selectivity of hemicellulose from bamboo using freeze–thaw-assisted p-toluenesulfonic acid treatment at low acid concentration and high temperature. Bioresour. Technol. 2022, 363, 127879. [Google Scholar] [CrossRef] [PubMed]
- Roostazadeh, R.; Behzad, T.; Karimi, K. Isolation and characterization of lignin-rich particles as byproducts of bioethanol production from wheat straw to reinforce starch composite films. Ind. Crop. Prod. 2022, 186, 115175. [Google Scholar] [CrossRef]
- Cassoni, A.C.; Mota, I.; Costa, P.; Vasconcelos, M.W.; Pintado, M. Effect of alkaline and deep eutectic solvents pretreatments on the recovery of lignin with antioxidant activity from grape stalks. Int. J. Biol. Macromol. 2022, 220, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wang, R.; Wang, K.; Jiang, J.; Xu, J. Low-condensed lignin and high-purity cellulose production from poplar by synergistic deep eutectic solvent-hydrogenolysis pretreatment. Bioresour. Technol. 2022, 363, 127905. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Kumar, A.; Singhal, B.; Chander Kuhad, R.; Kant Sharma, K. Fungal oxidoreductases and cazymes effectively degrade lignocellulosic component of switchgrass for bioethanol production. Fuel 2022, 328, 125341. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Sheng, Y.; Huang, Q.; Yang, Z.; Shi, Y.; Guo, X.; Ge, S. Influence of typical pretreatment on cotton stalk conversion activity and bio-oil property during low temperature (180–220 °C) hydrothermal process. Fuel 2022, 328, 125250. [Google Scholar] [CrossRef]
- Mafu, L.D.; Neomagus, H.W.J.P.; Everson, R.C.; Carrier, M.; Strydom, C.A.; Bunt, J.R. Structural and chemical modifications of typical south african biomasses during torrefaction. Bioresour. Technol. 2016, 202, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Melkior, T.; Jacob, S.; Gerbaud, G.; Hediger, S.; Bardet, M. Nmr analysis of the transformation of wood constituents by torrefaction. Fuel 2012, 92, 271–280. [Google Scholar] [CrossRef]
- Shrestha, S.; Fonoll, X.; Khanal, S.; Raskin, L. Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: Current status and future perspectives—ScienceDirect. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- BaRdet, M.; Foray, M.F.; Tran, Q.K. High-resolution solid-state cpmas nmr study of archaeological woods. Anal. Chem. 2002, 74, 4386–4390. [Google Scholar] [CrossRef] [PubMed]
- Wikberg, H.; Maunu, S.L. Characterisation of thermally modified hard- and softwoods by 13c cpmas nmr. Carbohydr. Polym. 2004, 58, 461–466. [Google Scholar] [CrossRef]
- Atalla, R.H.; Vanderhart, D.L. The role of solid state 13c nmr spectroscopy in studies of the nature of native celluloses. Solid State Nucl. Magn. Reson. 1999, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Chen, B.; Zeng, A.; Li, Z.; Tang, X.; Sun, Y.; Lin, L.; Zeng, X. Chemical structure change of lignin extracted from bamboo biomass by maleic acid. Int. J. Biol. Macromol. 2022, 221, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qi, J.; Xie, M.; Wang, X.; Xu, J.; Yu, Z.; Zhao, W.; Xiao, Y.; Wei, W. Enhancement of sugar release from sugarcane bagasse through naoh-catalyzed ethylene glycol pretreatment and water-soluble sulfonated lignin. Int. J. Biol. Macromol. 2022, 221, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Morán-Aguilar, M.G.; Calderón-Santoyo, M.; de Souza Oliveira, R.P.; Aguilar-Uscanga, M.G.; Domínguez, J.M. Deconstructing sugarcane bagasse lignocellulose by acid-based deep eutectic solvents to enhance enzymatic digestibility. Carbohydr. Polym. 2022, 298, 120097. [Google Scholar] [CrossRef]
- Gonzalez, M.; Pereira-Rojas, J.; Villanueva, I.; Agüero, B.; Silva, I.; Velasquez, I.; Delgado, B.; Hernandez, J.; Rodriguez, G.; Labrador, H.; et al. Preparation and characterization of cellulose fibers from meghatyrsus maximus: Applications in its chemical derivatives. Carbohydr. Polym. 2022, 296, 119918. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Su, Y.; Wang, Q.; Ling, Z.; Yong, Q. Supramolecular deconstruction of bamboo holocellulose via hydrothermal treatment for highly efficient enzymatic conversion at low enzyme dosage. Int. J. Mol. Sci. 2022, 23, 11829. [Google Scholar] [CrossRef]
- Yin, X.; Cai, T.; Liu, C.; Ma, Y.; Hu, J.; Jiang, J.; Wang, K. A novel solvothermal biorefinery for production of lignocellulosic xylooligosaccharides, fermentable sugars and lignin nano-particles in biphasic system. Carbohydr. Polym. 2022, 295, 119901. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass-Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2011. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Ana. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin, A.; Martin, J.A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
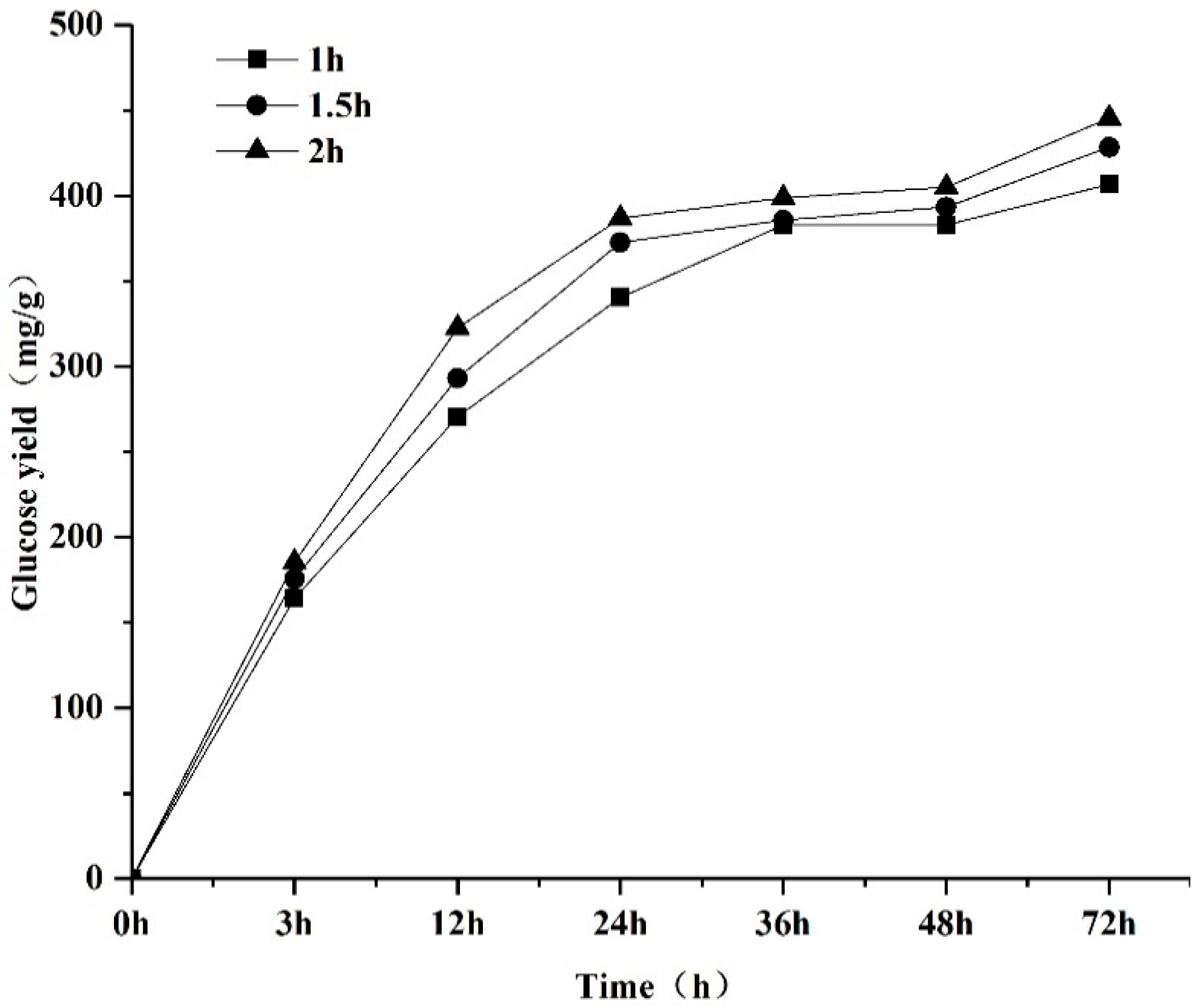




| Run | NaOH Aqueous (%) | Pretreatment Time (h) | Sugar Yield (mg/g) |
|---|---|---|---|
| 1 | 1 | 1.0 | 350.28 |
| 2 | 3 | 1.0 | 360 |
| 3 | 5 | 1.0 | 378.27 |
| 4 | 1 | 1.5 | 338.04 |
| 5 | 3 | 1.5 | 347.85 |
| 6 | 5 | 1.5 | 363.15 |
| 7 | 1 | 2.0 | 278.19 |
| 8 | 3 | 2.0 | 294.03 |
| 9 | 5 | 2.0 | 324.36 |
| Samples | Sugar Yield (mg/g) | |
|---|---|---|
| Eucalyptus | Raw | 193.2 |
| Hydrothermal | 205.4 | |
| Hot mill MDF | Raw | 237.8 |
| Hydrothermal | 248.8 | |
| MDF | Raw | 88.5 |
| Hydrothermal | 167.6 | |
| Pretreatment Methods | Sugar Yield (mg/g) | N [%] | C [%] | H [%] | S [%] |
|---|---|---|---|---|---|
| MWI | 281.3 | 3.96 | 45.58 | 6.842 | 0.051 |
| BW | 183.2 | 1.89 | 45.56 | 6.896 | 0.022 |
| BW + IL | 406.1 | 1.54 | 46.24 | 6.761 | 0.058 |
| MDF | 88.5 | 4.60 | 44.69 | 6.791 | 0.000 |
| Eucalyptus | 193.2 | 0.95 | 45.35 | 7.074 | 0.000 |
| Pretreatment Methods | Solid Recovery (%) | Glucan (%) | Xylan (%) | Lignin (%) | Ash (%) | ||
|---|---|---|---|---|---|---|---|
| —— | Enzymatic Hydrolysis Yield | —— | Enzymatic Hydrolysis Yield | ||||
| Untreated | —— | 38.18 ± 0.52 | 23.28 | 27.45 ± 0.17 | 27.25 | 27.35 ± 0.85 | 0.89 |
| NaOH | 60.9 ± 1.0 | 48.91 ± 0.2 | 77.34 | 18.93 ± 0.44 | 43.21 | 23.67 ± 0.41 | 1.48 |
| AAI | 57.6 ± 1.1 | 53.4 ± 0.14 | 83.42 | 16.14 ± 0.21 | 51.74 | 21.4 ± 0.31 | 1.32 |
| Wave Number (cm−1) | Spectral Peak Attribution Analysis |
|---|---|
| 1740 | Carboxyl group, carbonyl group, and acetyl group in hemicellulose C=O stretching vibration. |
| 1596 | Benzene ring skeleton stretching vibration plus C-O stretching vibration. |
| 1515 | C=C stretching vibration of aromatic ring skeleton in lignin. |
| 1355 | Vibration of cellulose and hemicellulose C-H. |
| 1268 | C-H stretching vibration in cellulose, C-O stretching vibration in syringa. |
| 1244 | Stretching vibration of C-O in hemicellulose or lignin. |
| 898 | C-H deformation vibration of β-glycosidic bond. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Hou, X.; Sun, J.; Sun, D.; Gao, Z. Efficacy and Functional Mechanisms of a Two-Stage Pretreatment Approach Based on Alkali and Ionic Liquid for Bioconversion of Waste Medium-Density Fiberboard. Molecules 2024, 29, 2153. https://doi.org/10.3390/molecules29092153
Wang S, Hou X, Sun J, Sun D, Gao Z. Efficacy and Functional Mechanisms of a Two-Stage Pretreatment Approach Based on Alkali and Ionic Liquid for Bioconversion of Waste Medium-Density Fiberboard. Molecules. 2024; 29(9):2153. https://doi.org/10.3390/molecules29092153
Chicago/Turabian StyleWang, Shujie, Xianfeng Hou, Jin Sun, Dan Sun, and Zhenzhong Gao. 2024. "Efficacy and Functional Mechanisms of a Two-Stage Pretreatment Approach Based on Alkali and Ionic Liquid for Bioconversion of Waste Medium-Density Fiberboard" Molecules 29, no. 9: 2153. https://doi.org/10.3390/molecules29092153
APA StyleWang, S., Hou, X., Sun, J., Sun, D., & Gao, Z. (2024). Efficacy and Functional Mechanisms of a Two-Stage Pretreatment Approach Based on Alkali and Ionic Liquid for Bioconversion of Waste Medium-Density Fiberboard. Molecules, 29(9), 2153. https://doi.org/10.3390/molecules29092153






