Synthetic Approaches Toward Phosphorus-Containing BODIPY and Squaraine Dyes: Enhancing Versatility of Small-Molecule Fluorophores
Abstract
1. Introduction
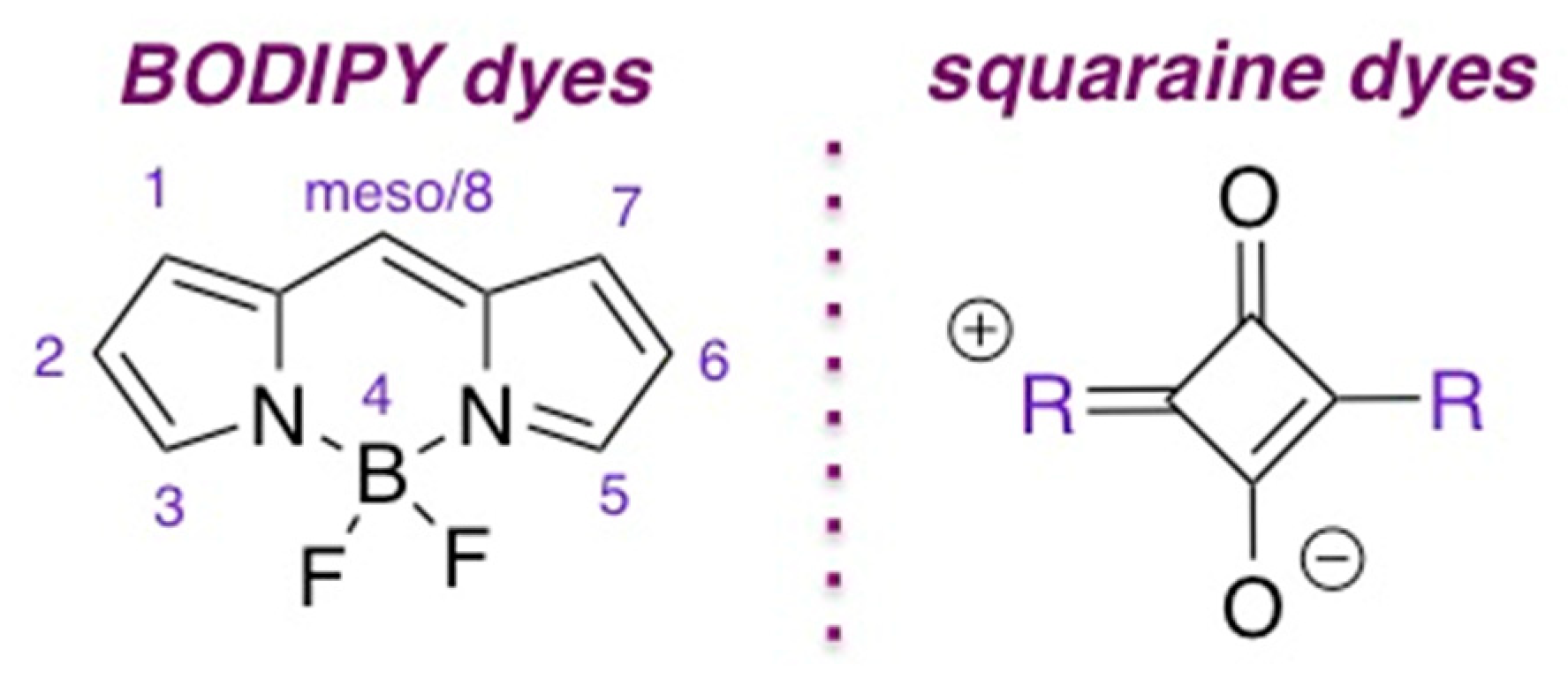
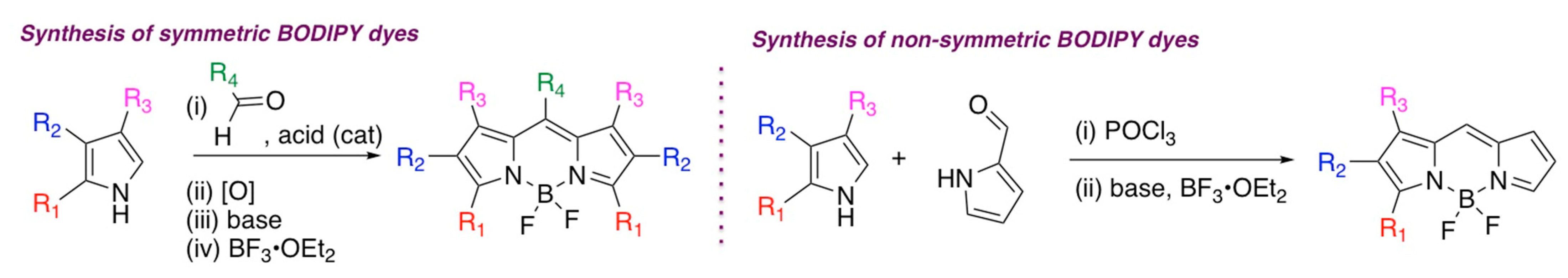
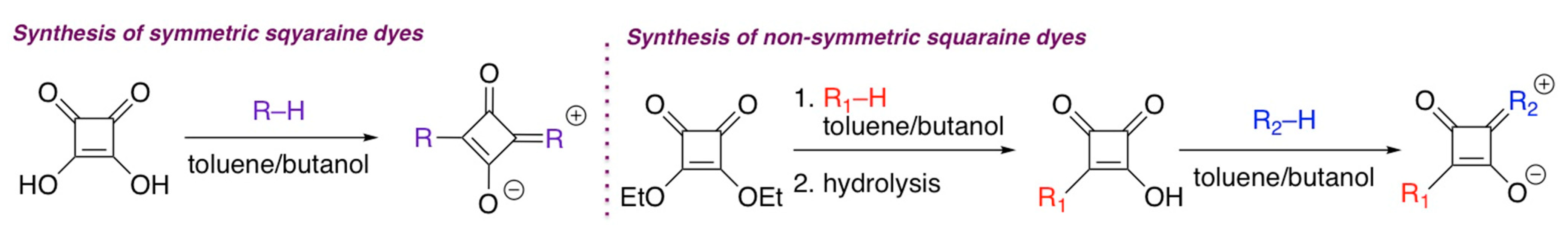
2. General Synthetic Routes to BODIPY and Squaraine Dyes
3. BODIPY and Squaraine Dyes with Phosphorus Bound to Carbon
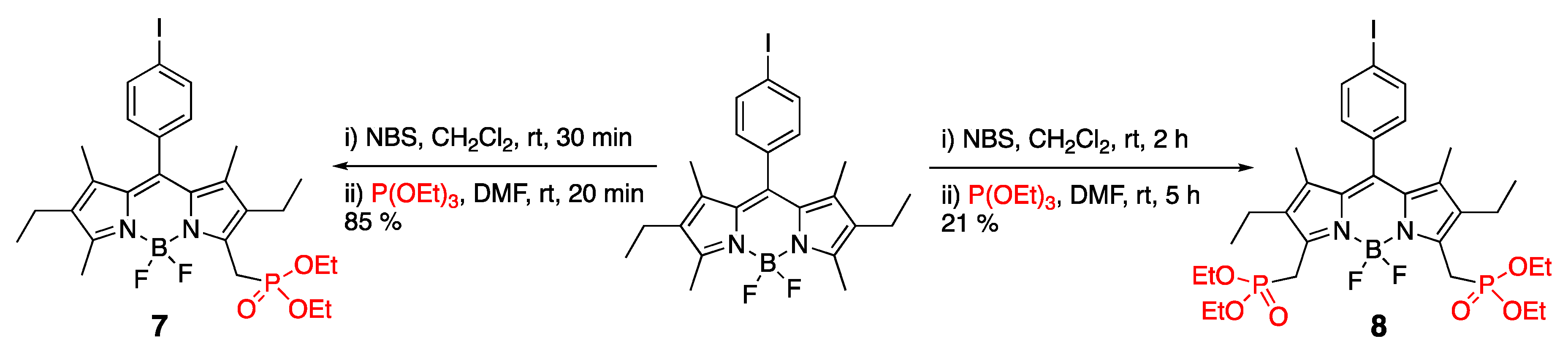
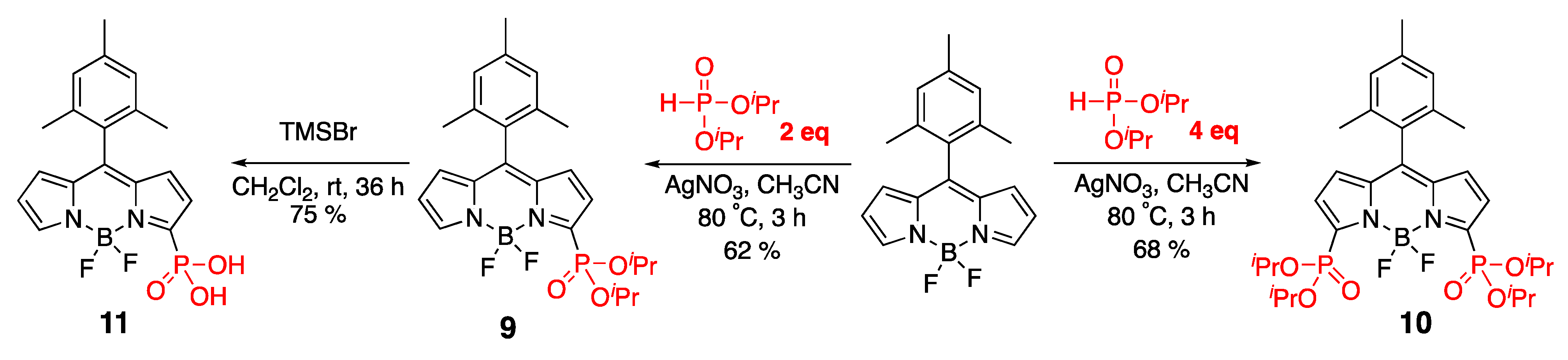
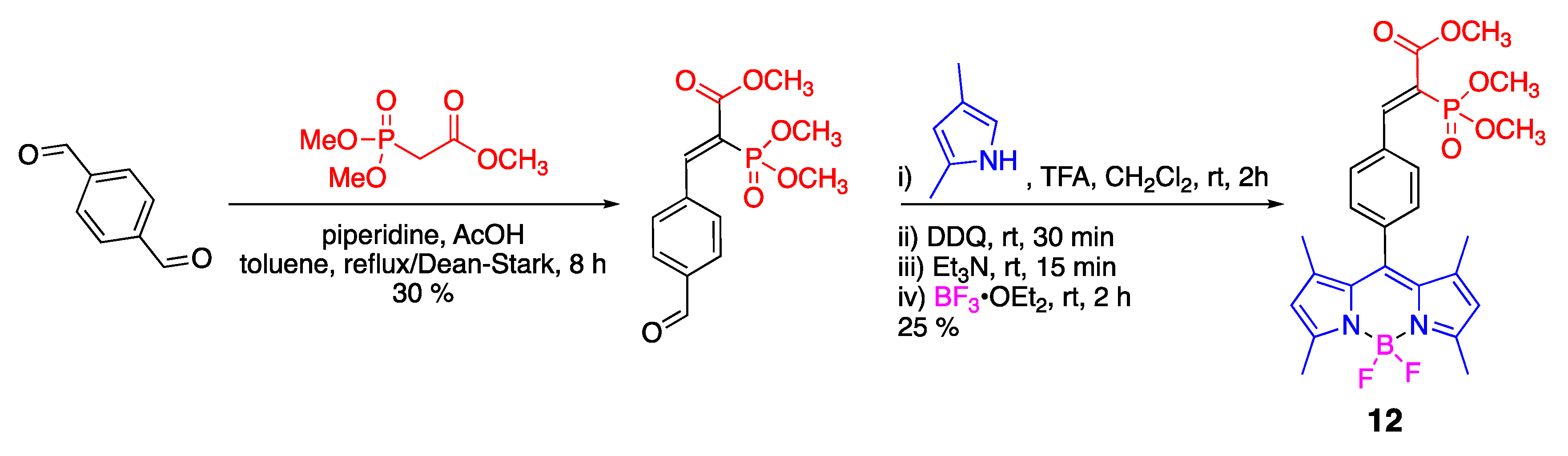
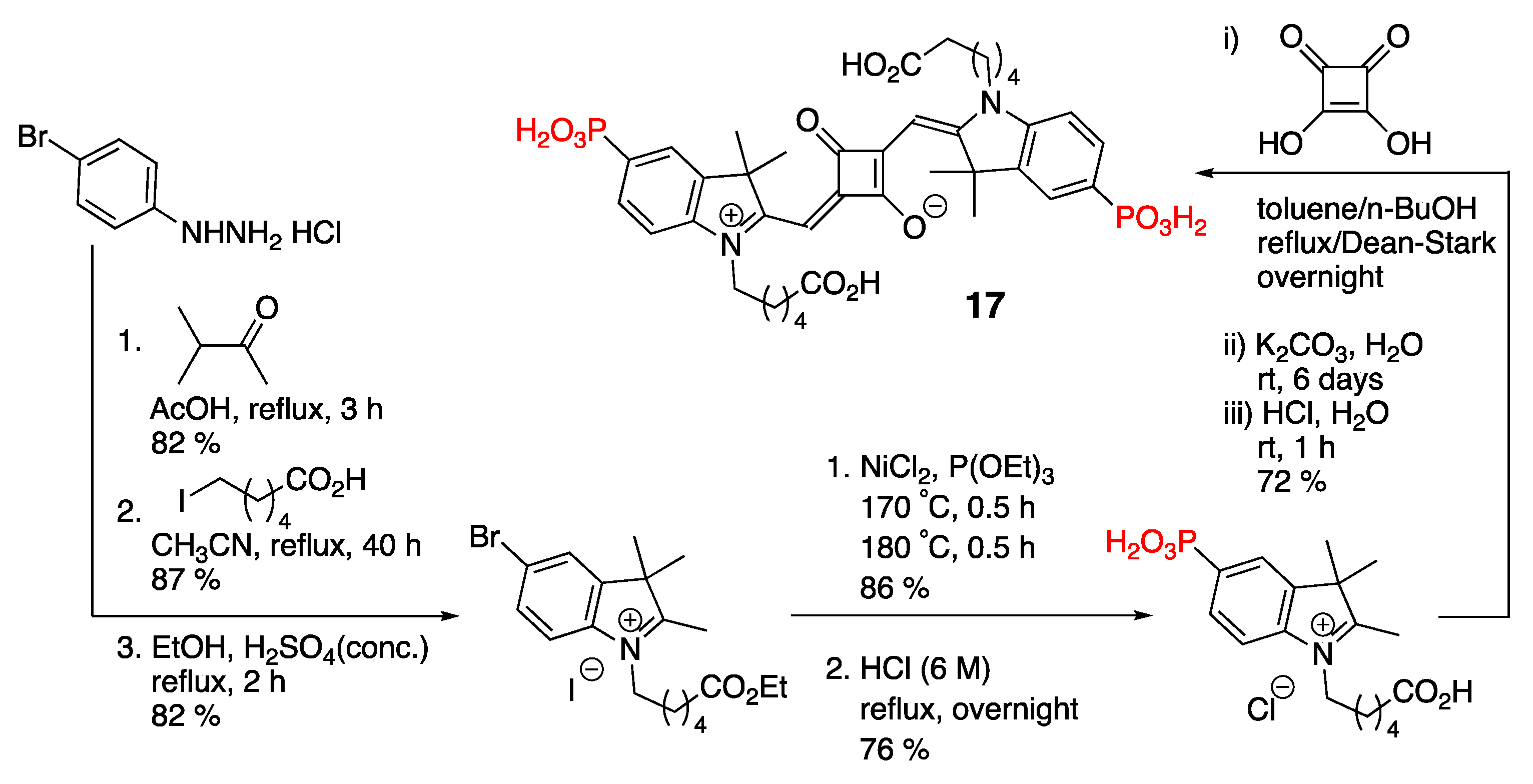
3.1. Phosphonate-Containing Dyes
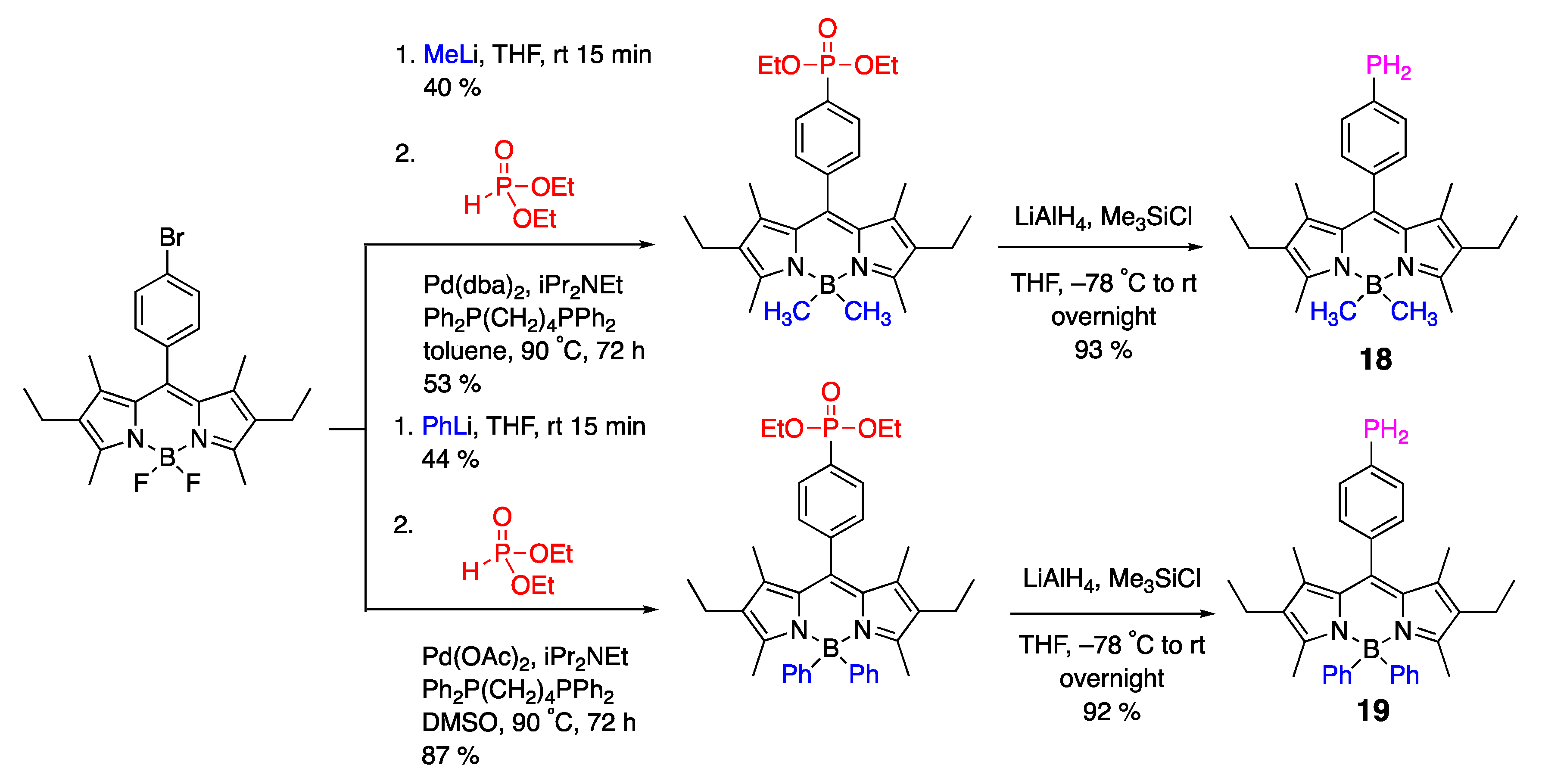
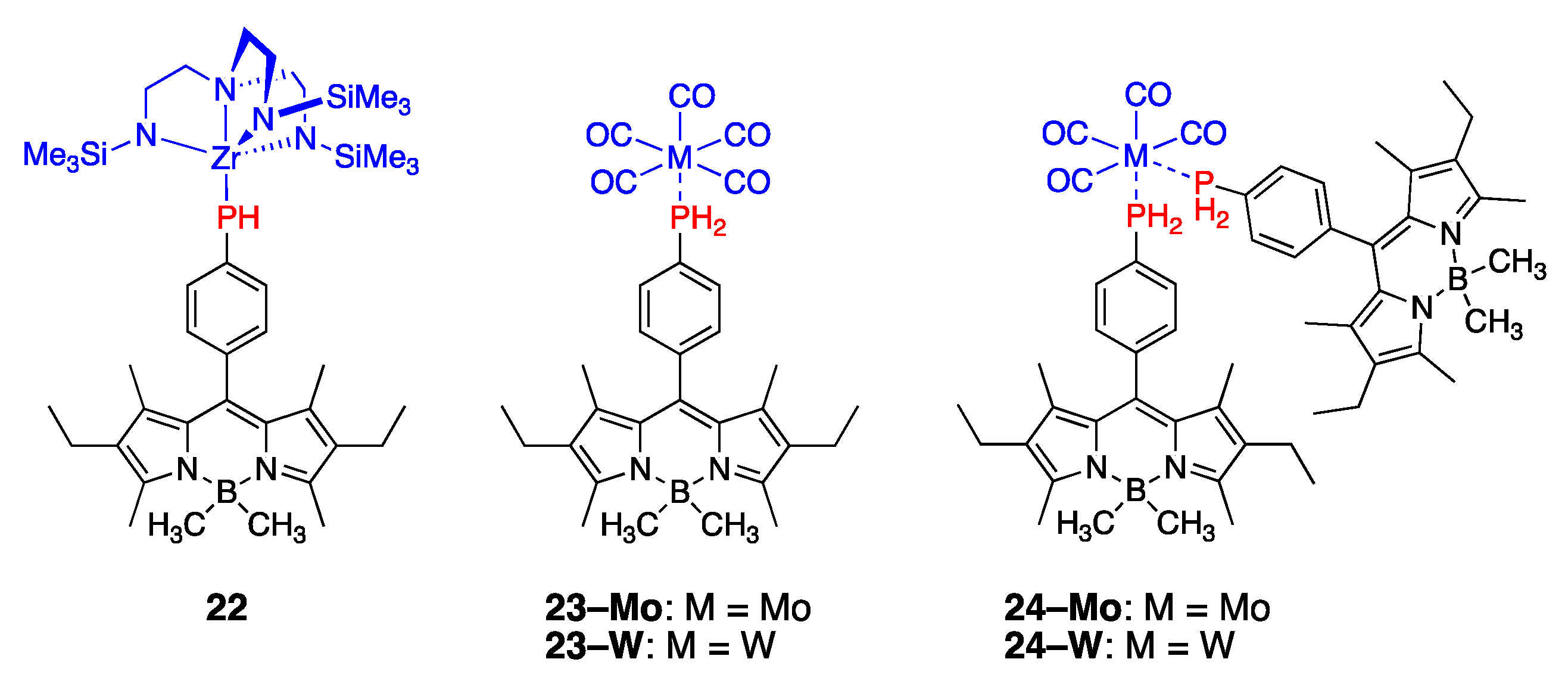
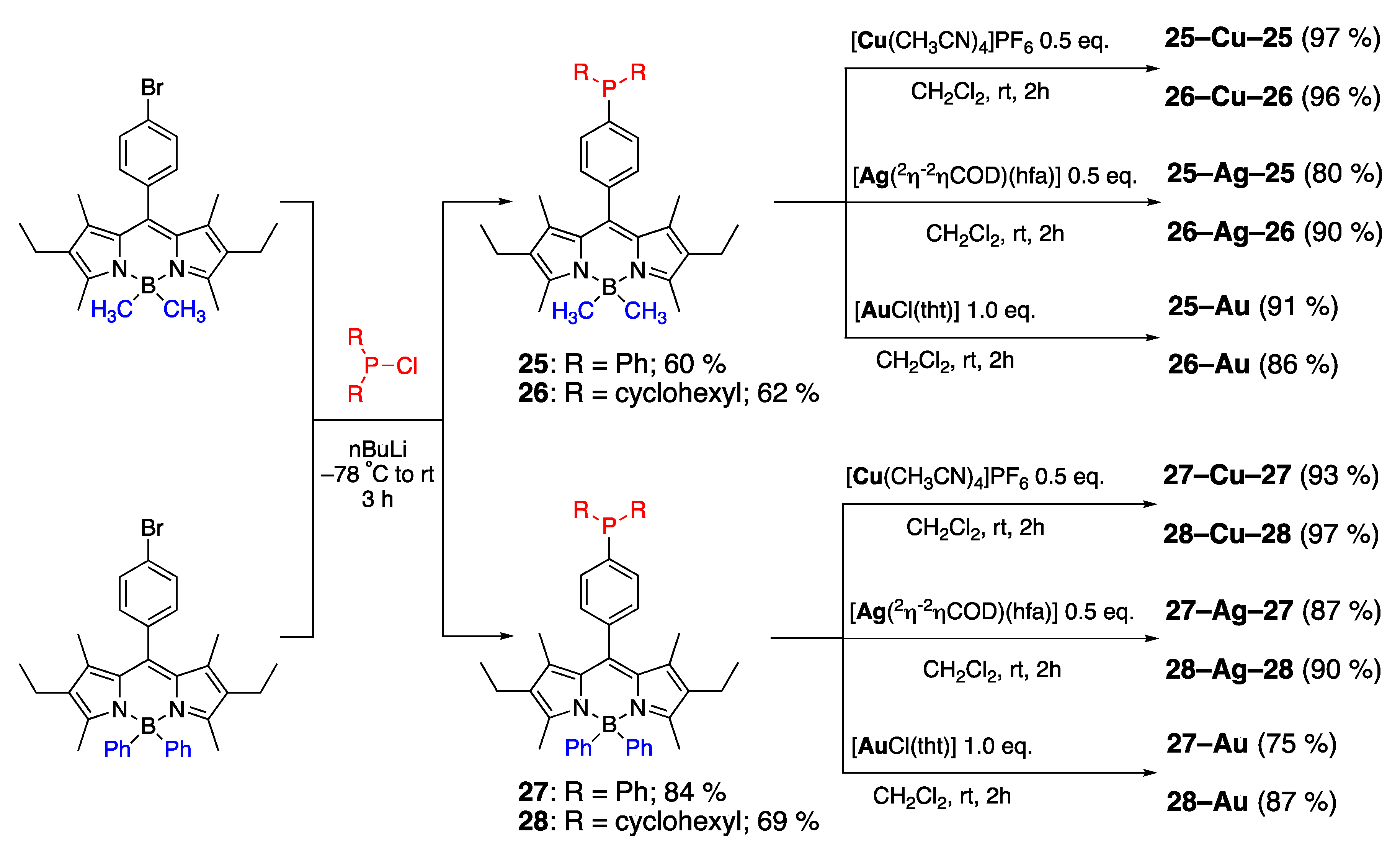
3.2. Phosphine-Containing BODIPY Dyes
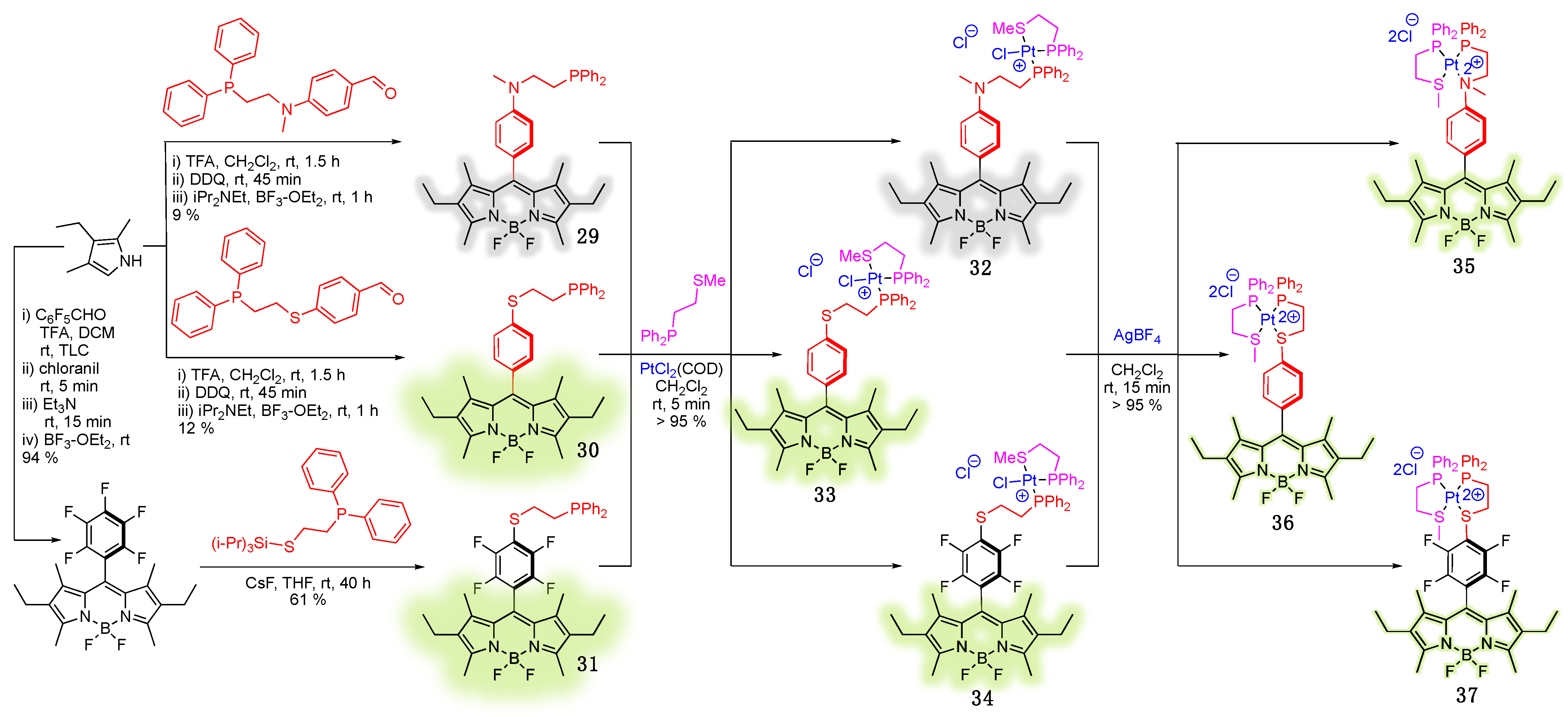
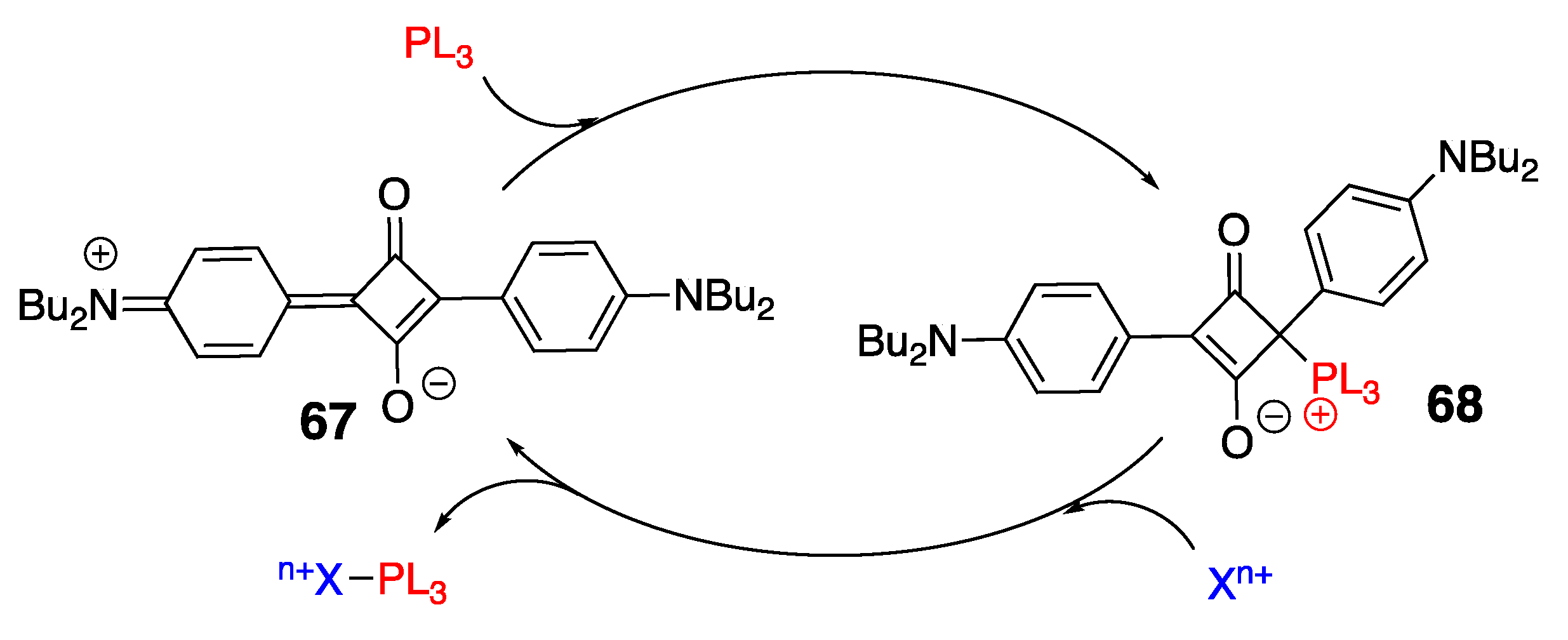
3.3. Phosphine Reactions with Squaraine: En Route to Squaraine-Chemodosimeters
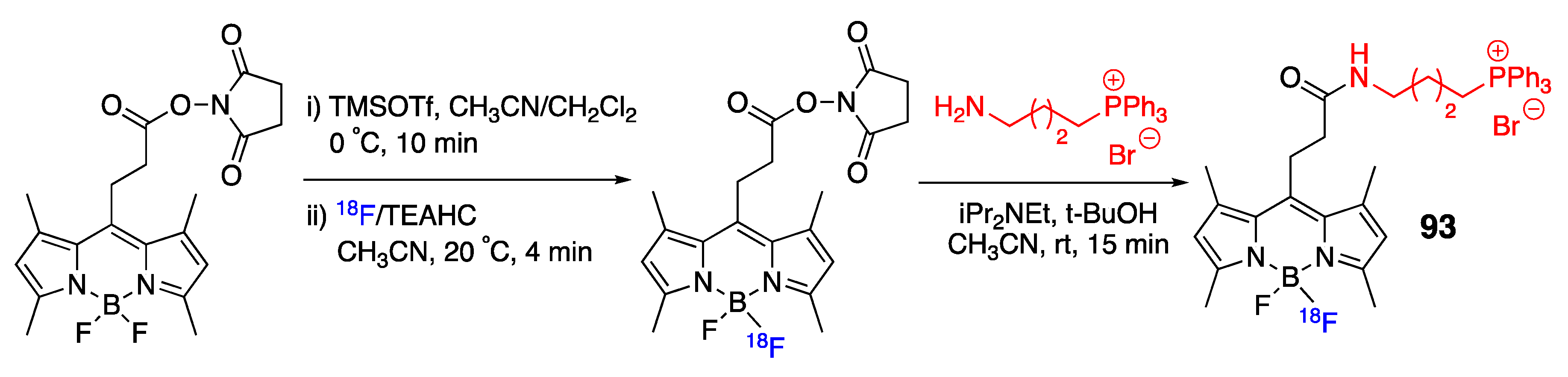
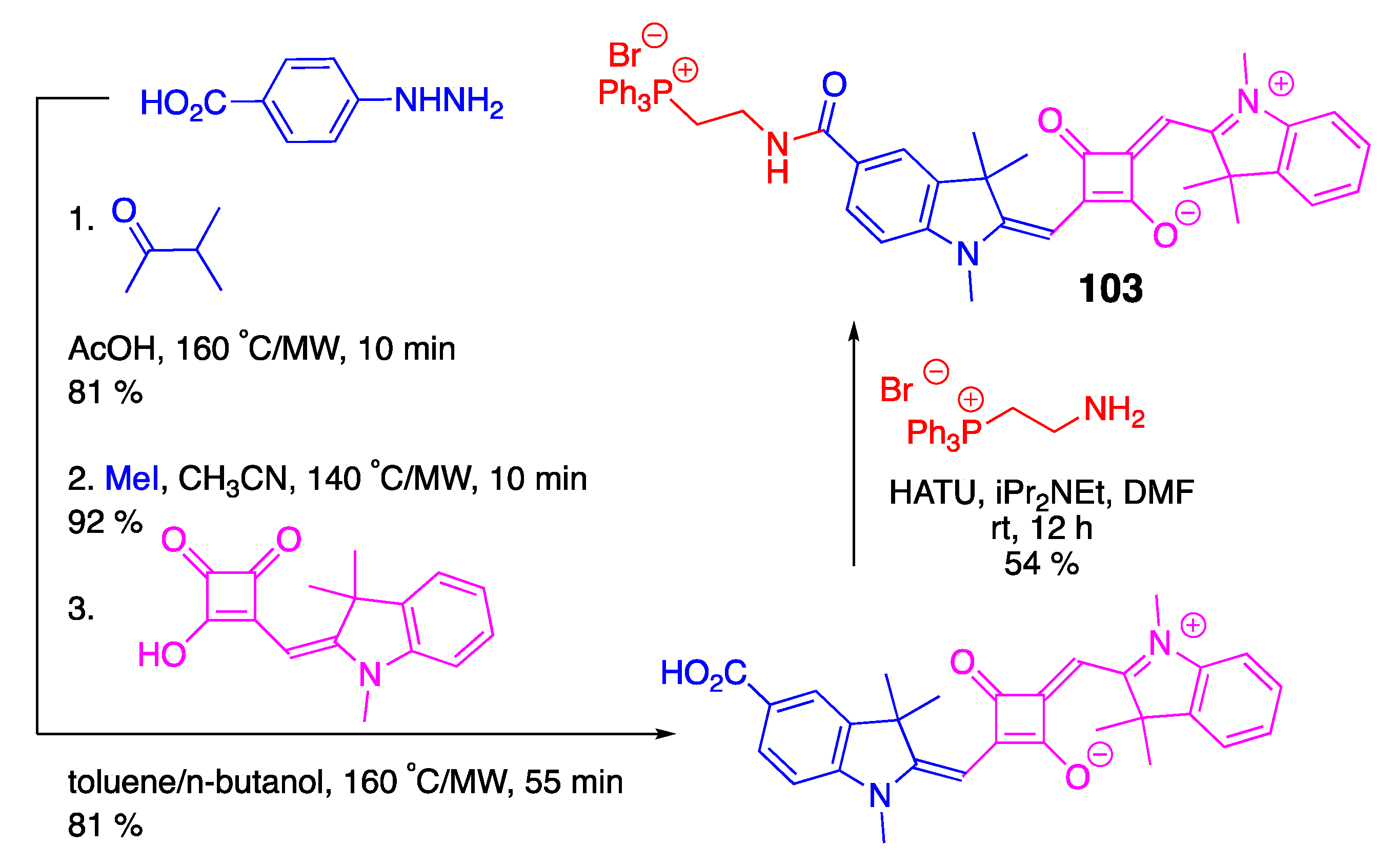
3.4. Phosphonium-Containing BODIPY and Squaraine Dyes
4. Fluorescent Dyes with Phosphorus Not Bound Directly to Carbon
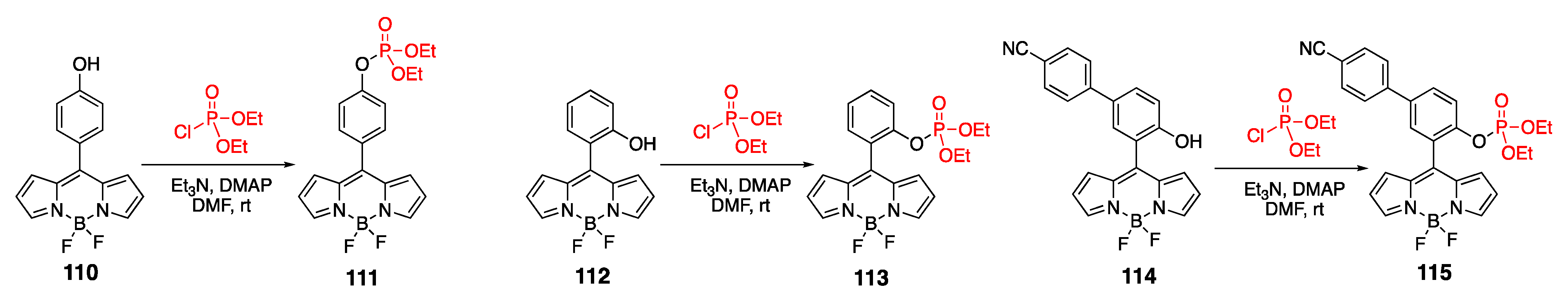
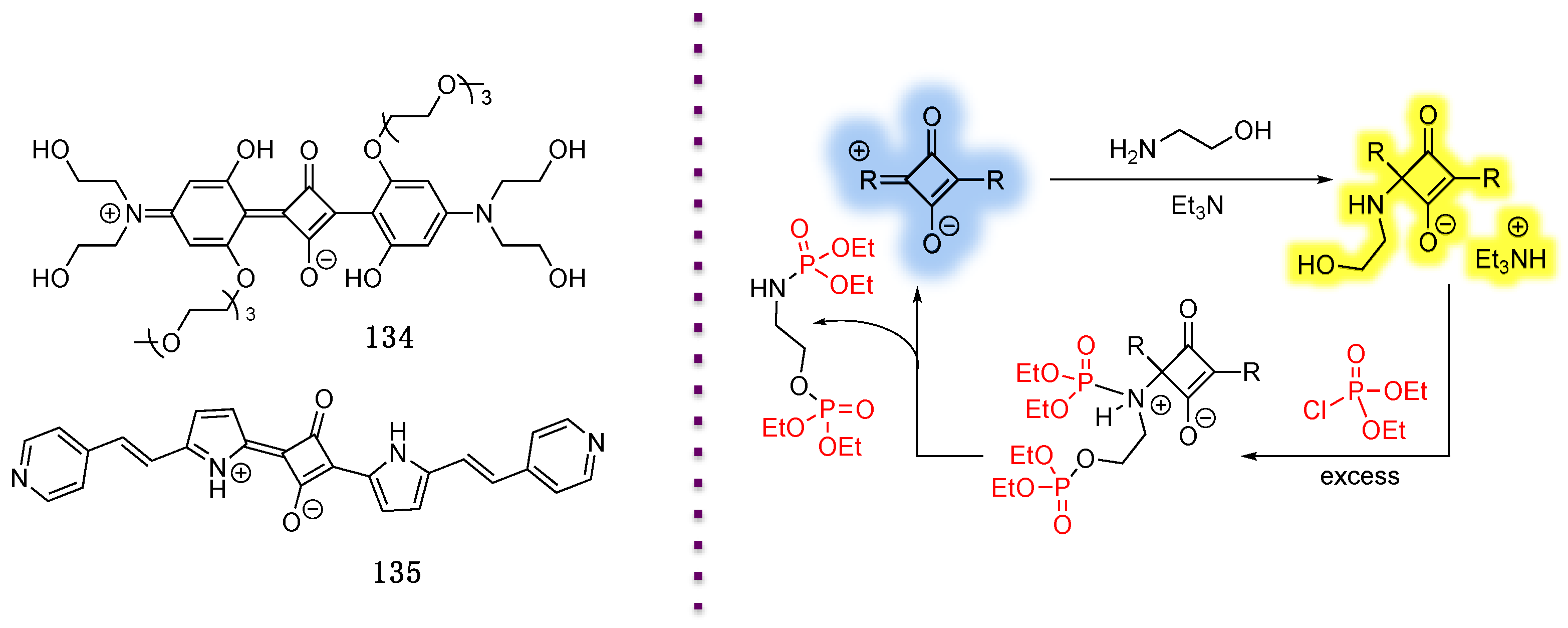
4.1. Phosphate-Containing Groups
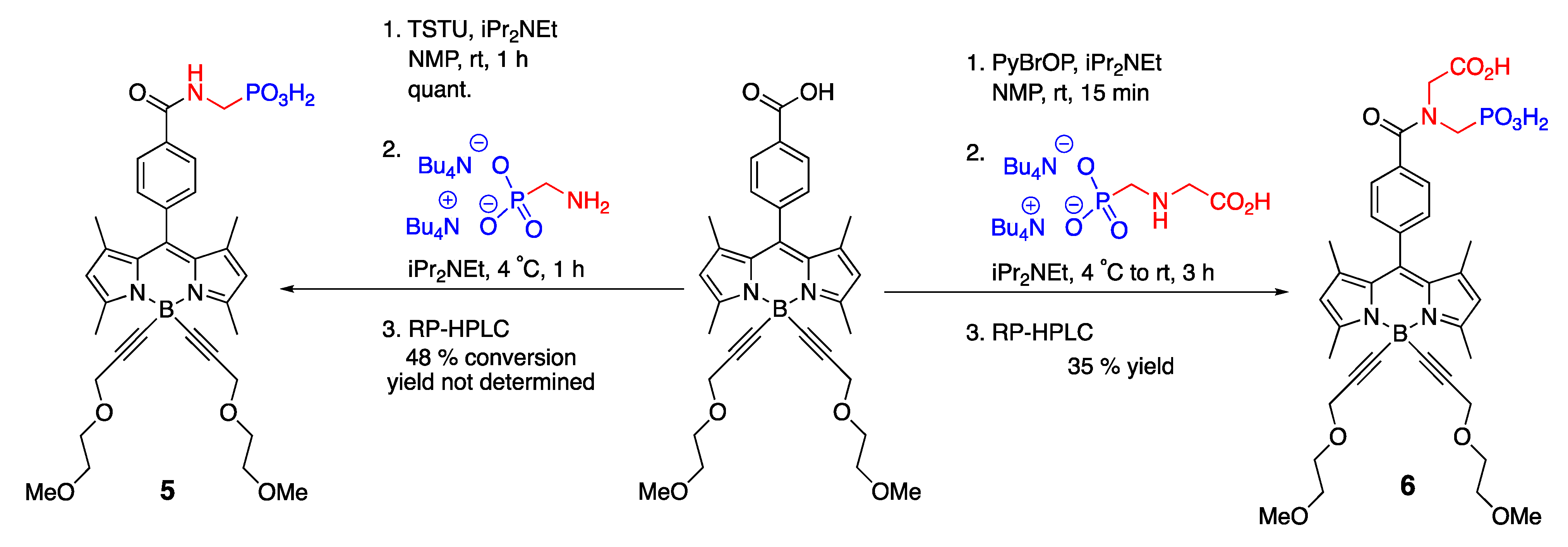
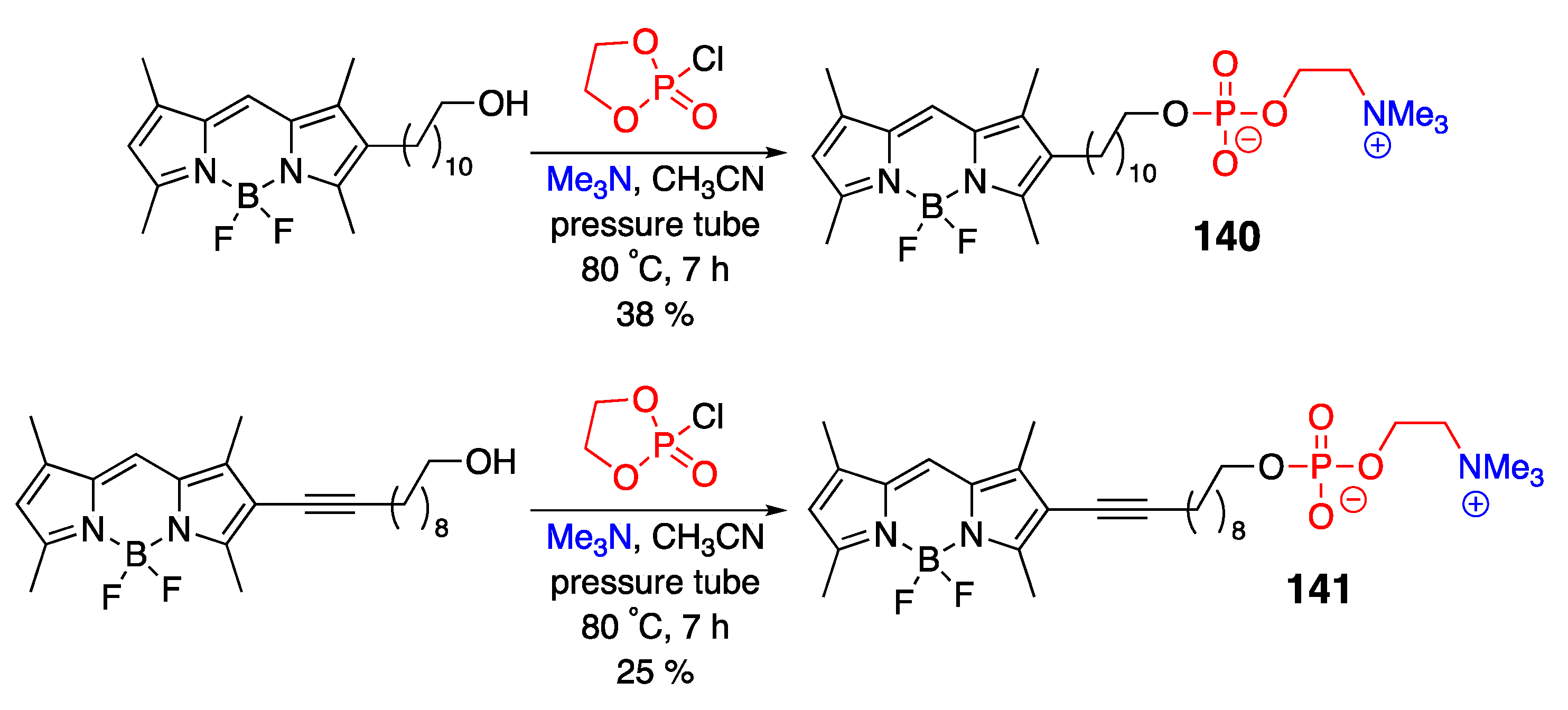
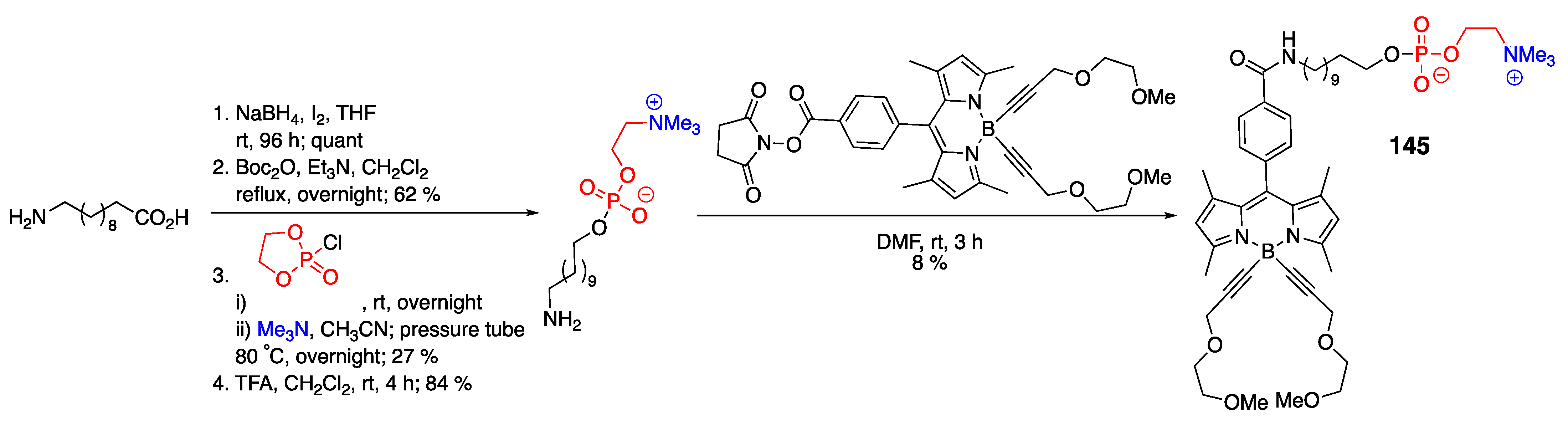
4.1.1. Phosphate-Containing BODIPY: Control of Water Solubility
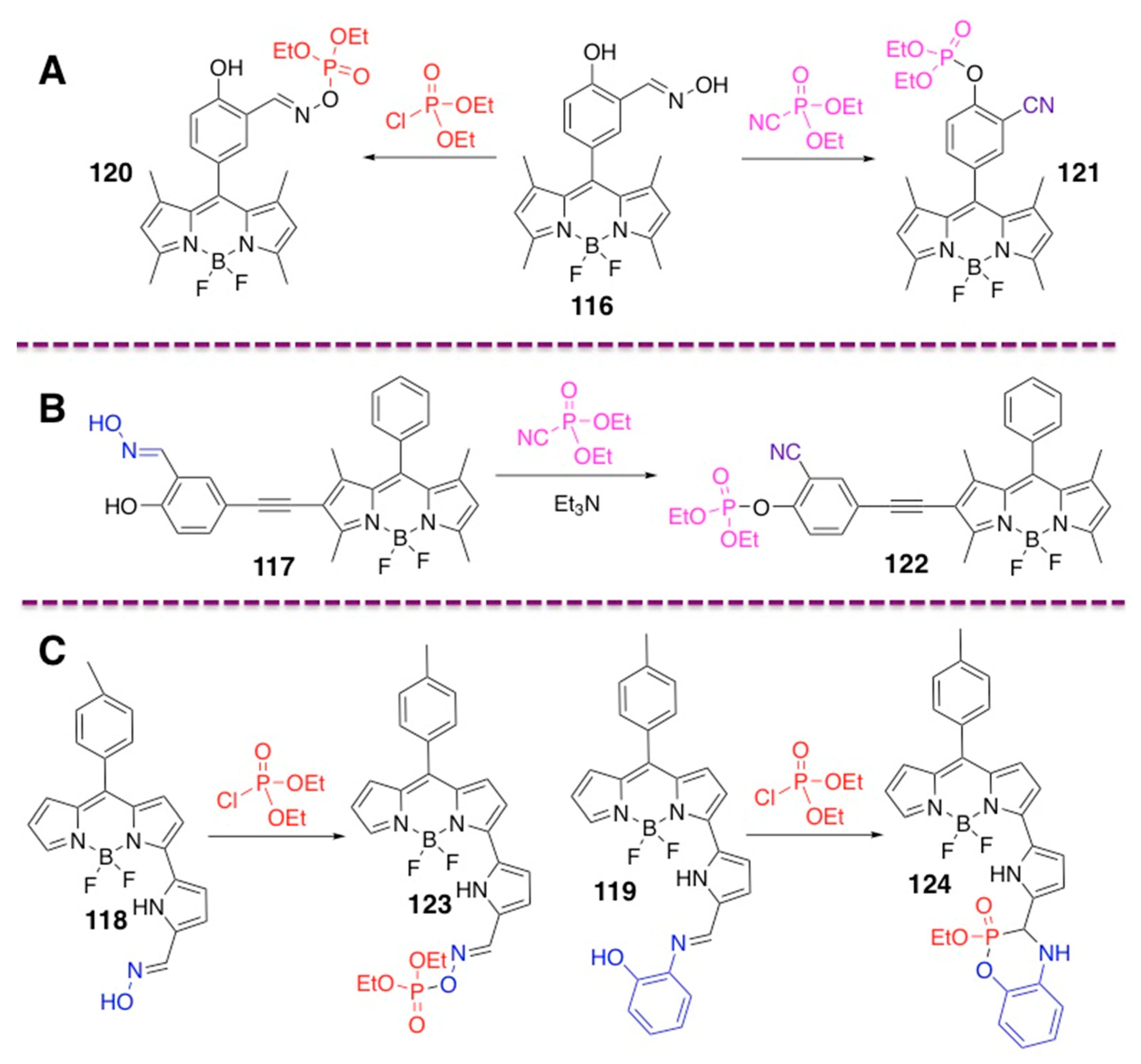
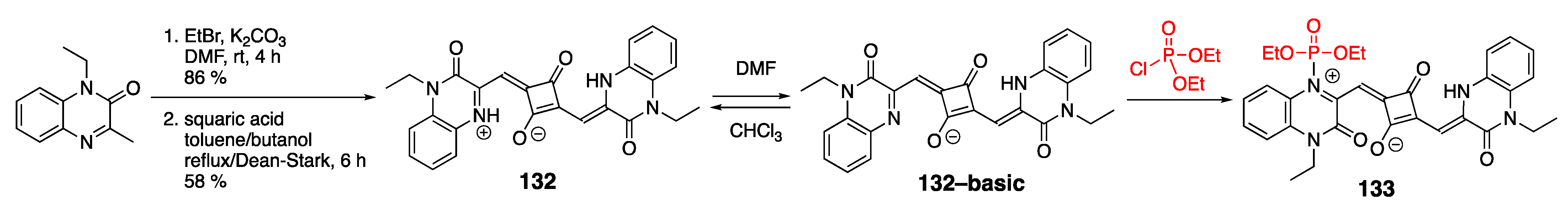
4.1.2. Phosphate-Containing Dyes: Sensing of Organophosphorus Nerve Agents
4.1.3. Phosphate-Containing BODIPYs as Photocaging Agents
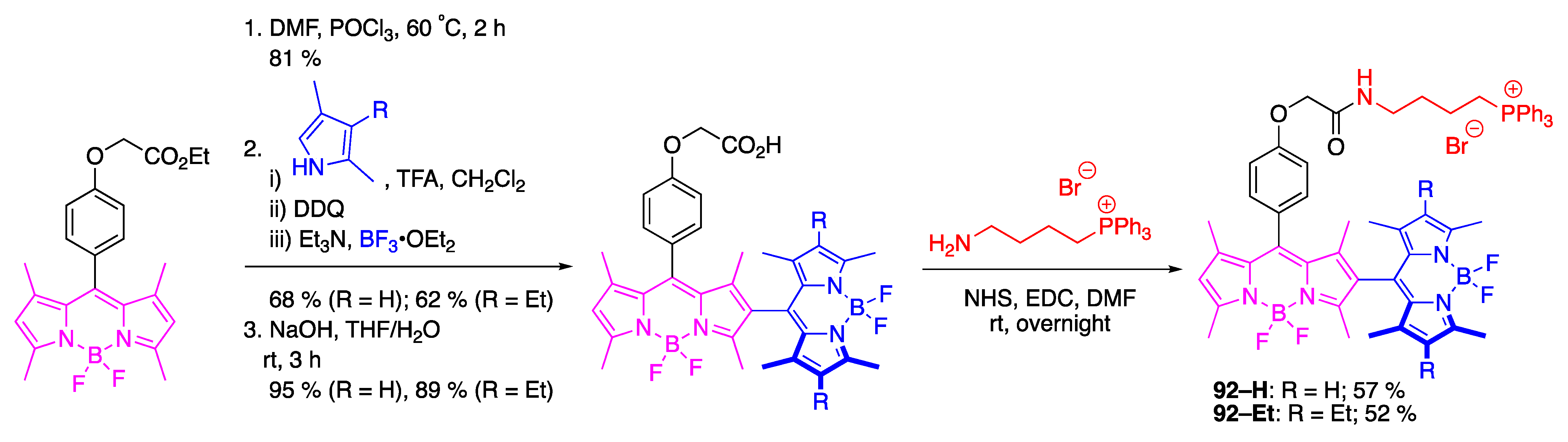
4.1.4. Alkylphosphocholine-Containing BODIPY
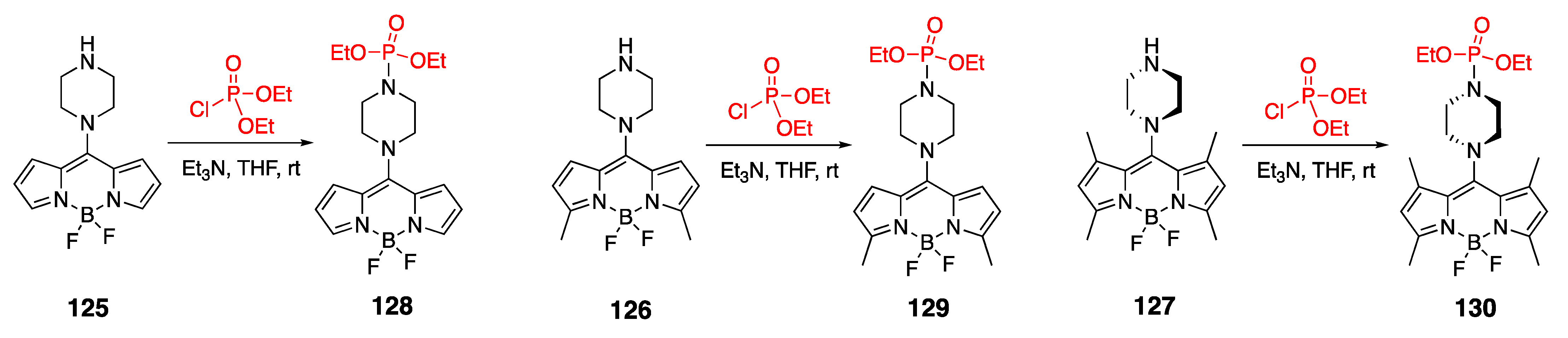
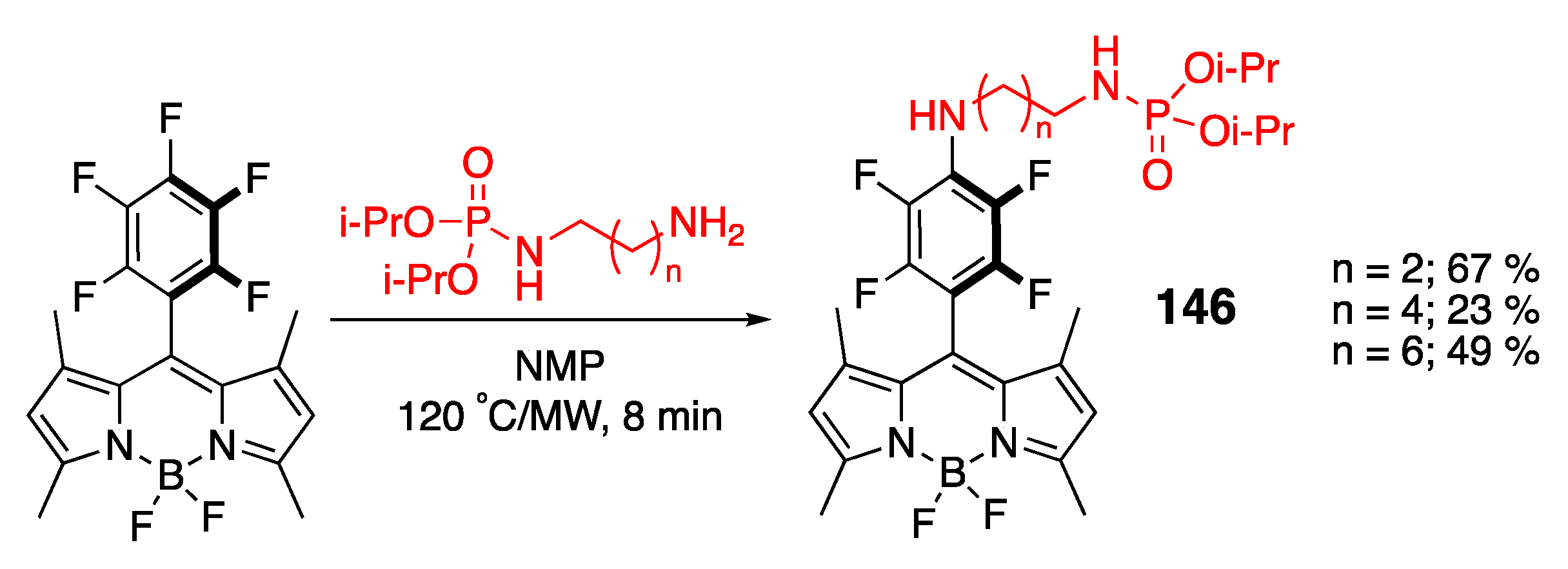
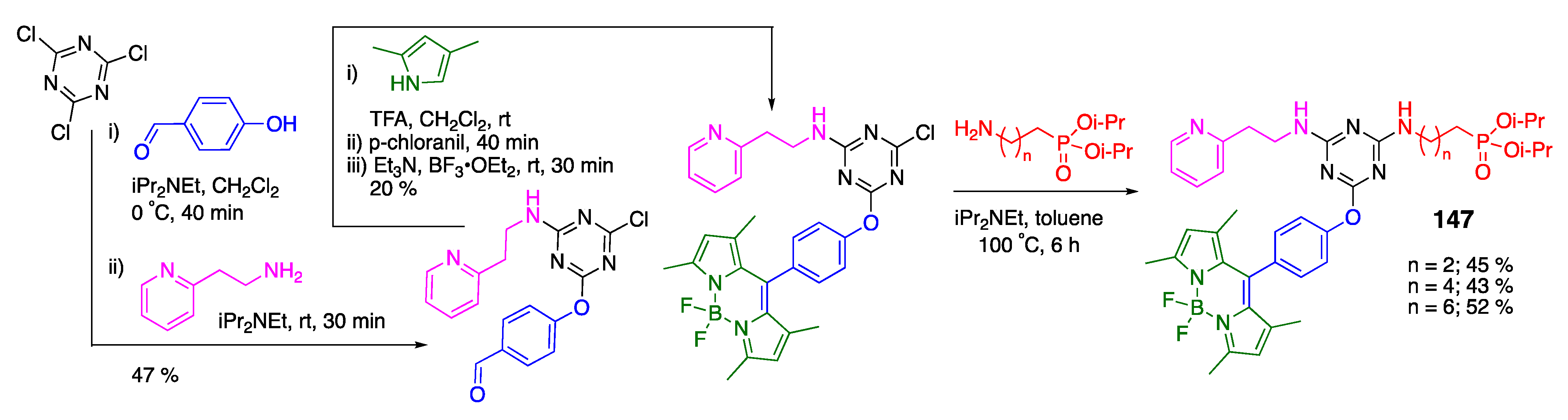
4.2. Phosphoramidate-Containing BODIPY Dyes
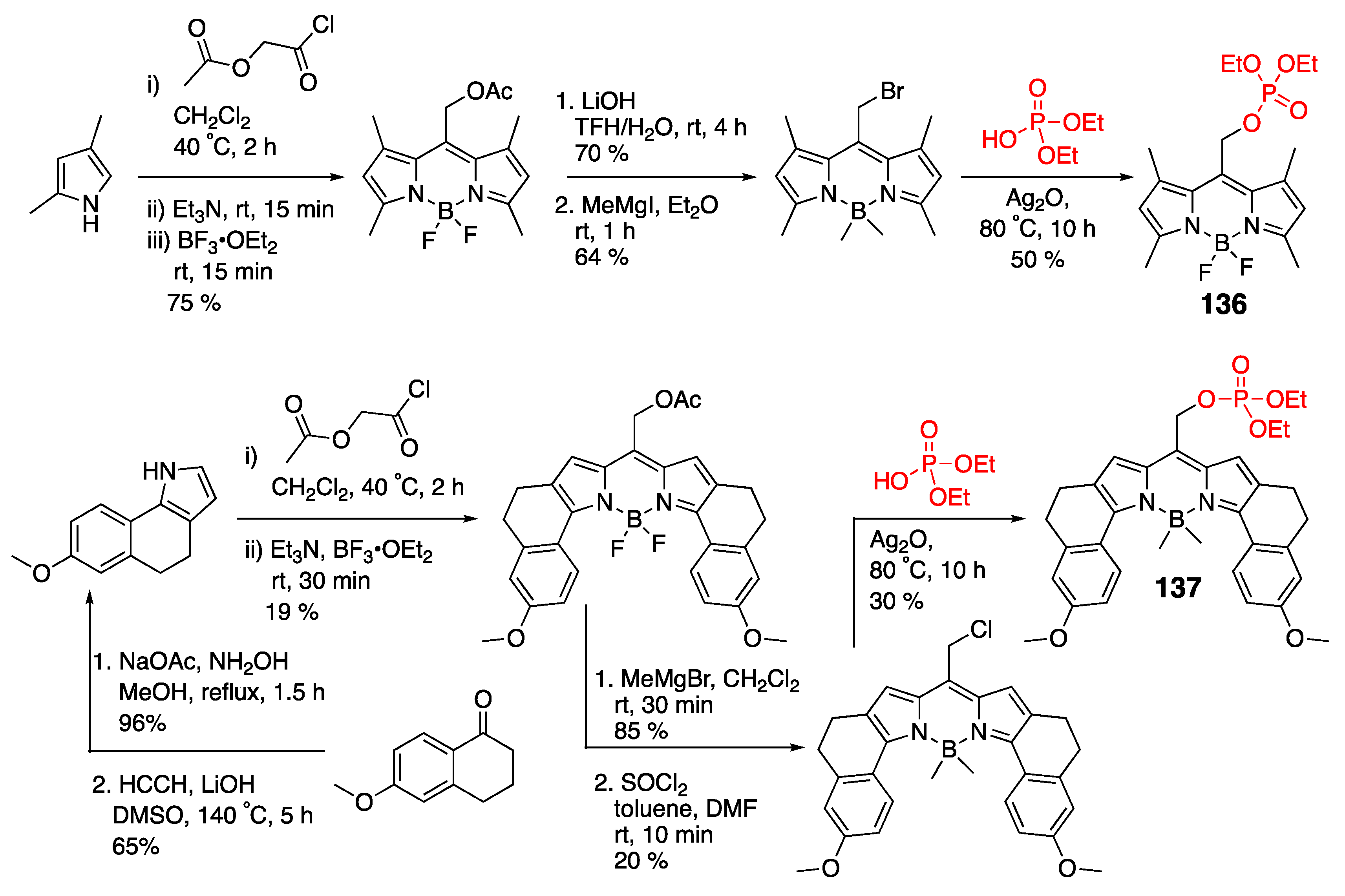
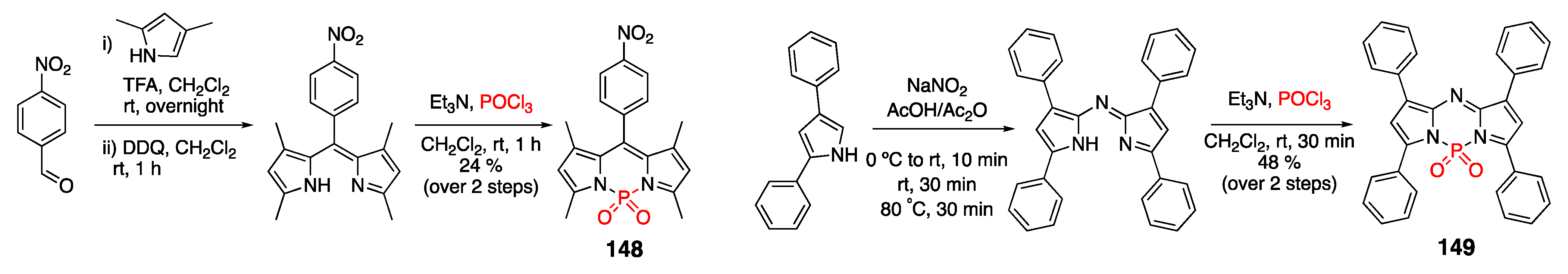
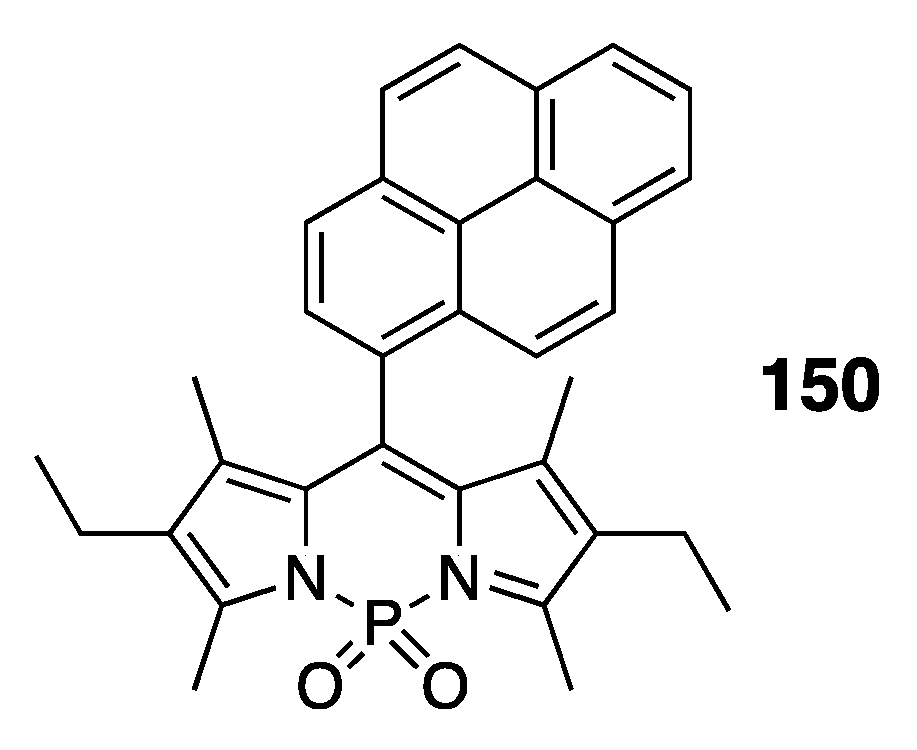
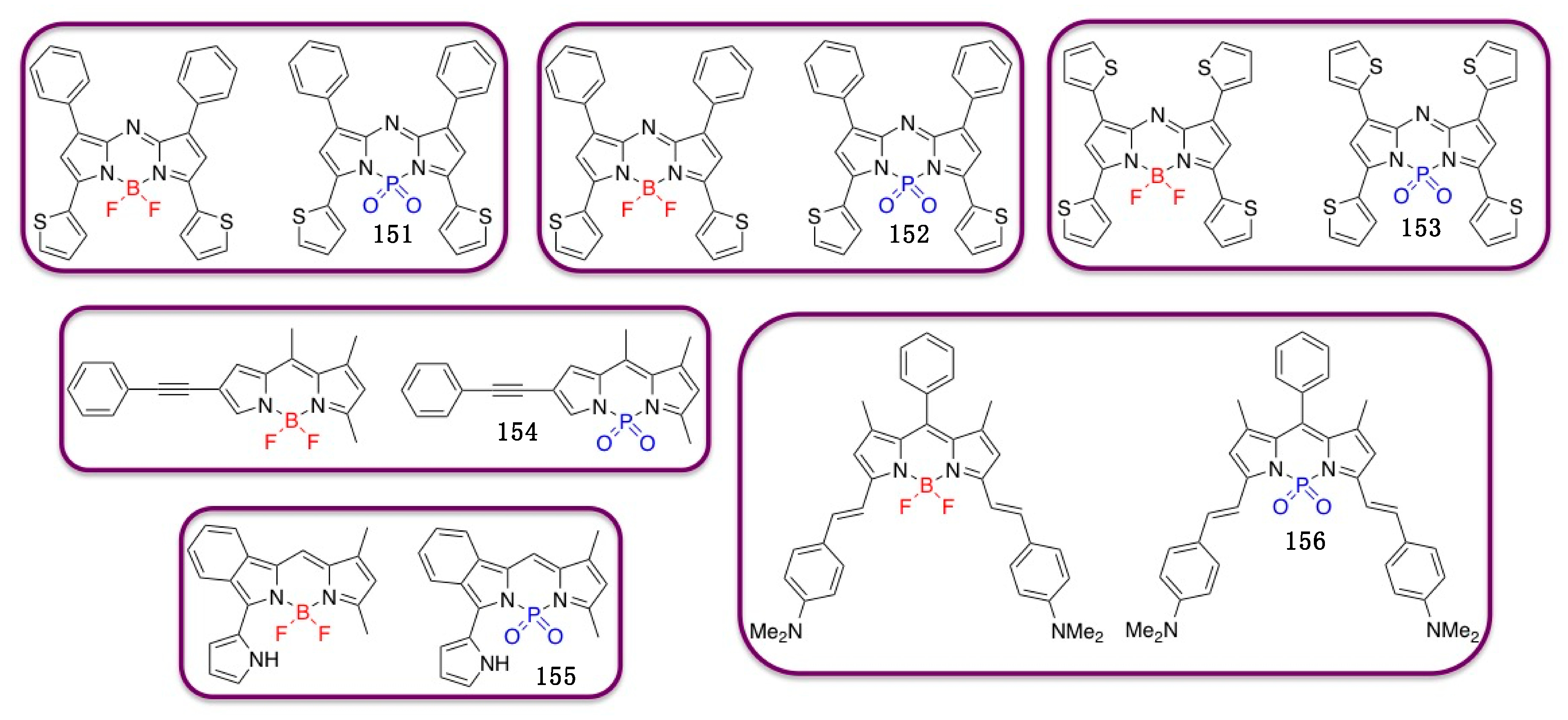
4.3. N–P-Containing Dyes: PODIPYs and PHODIPY
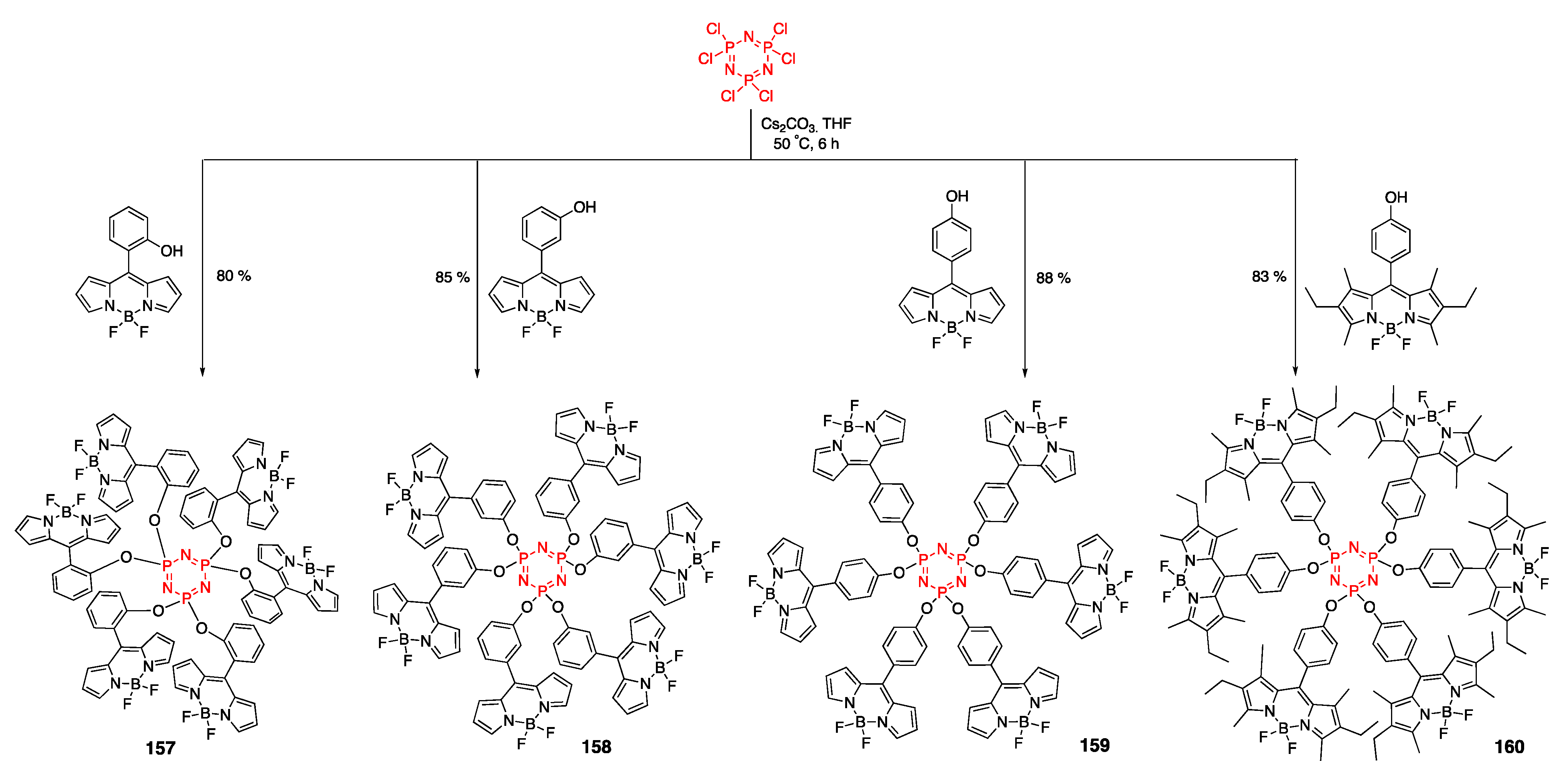
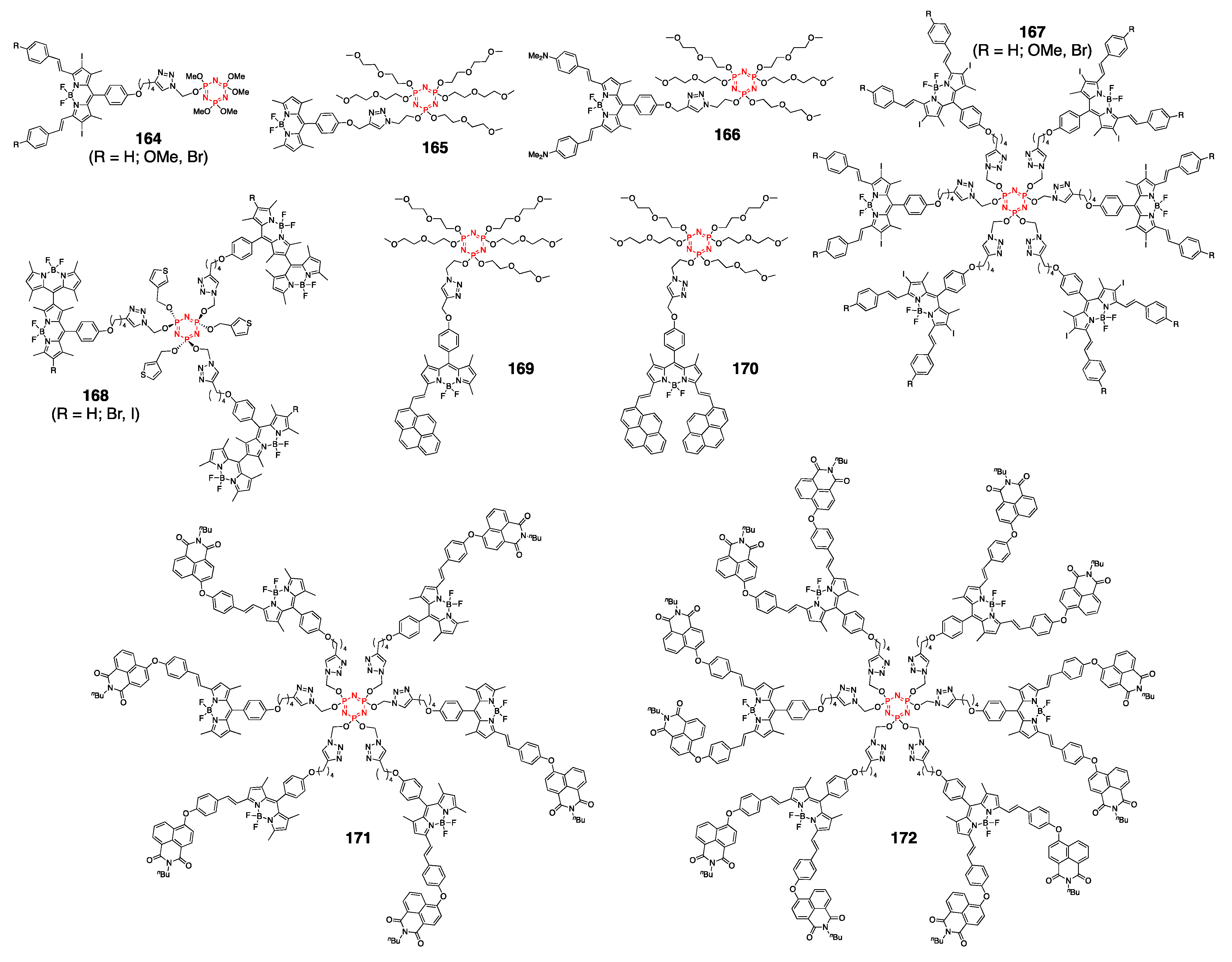
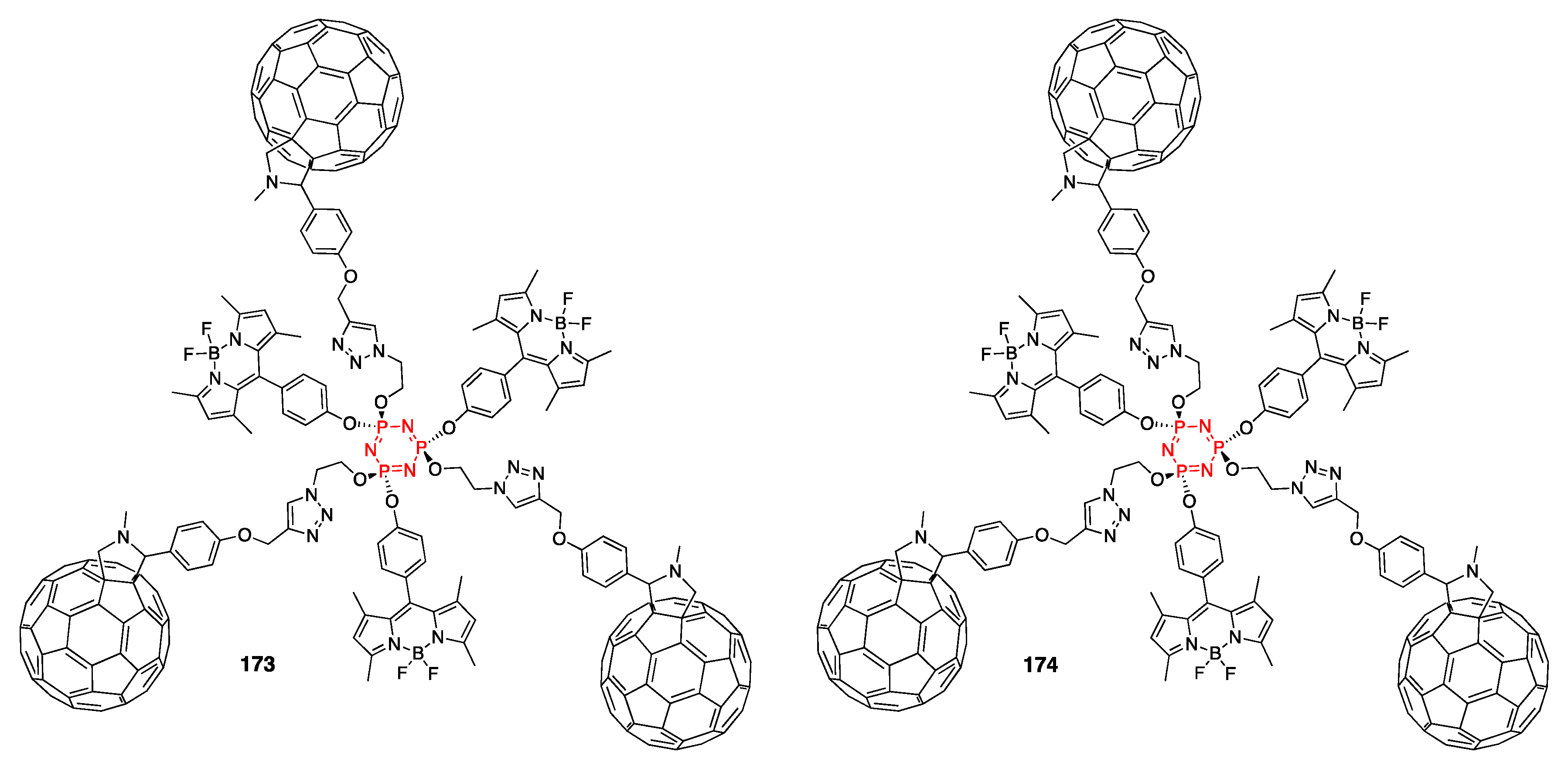
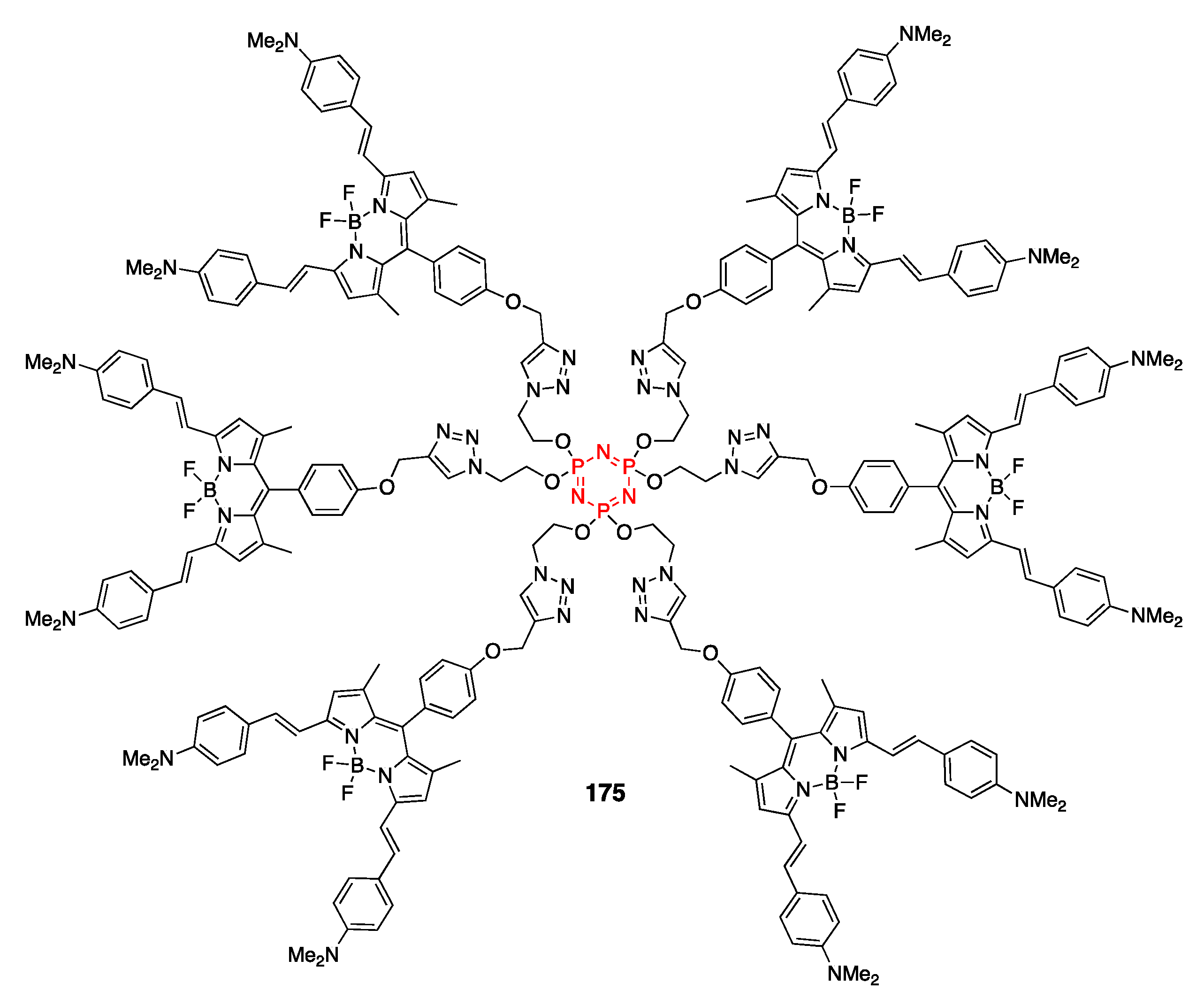
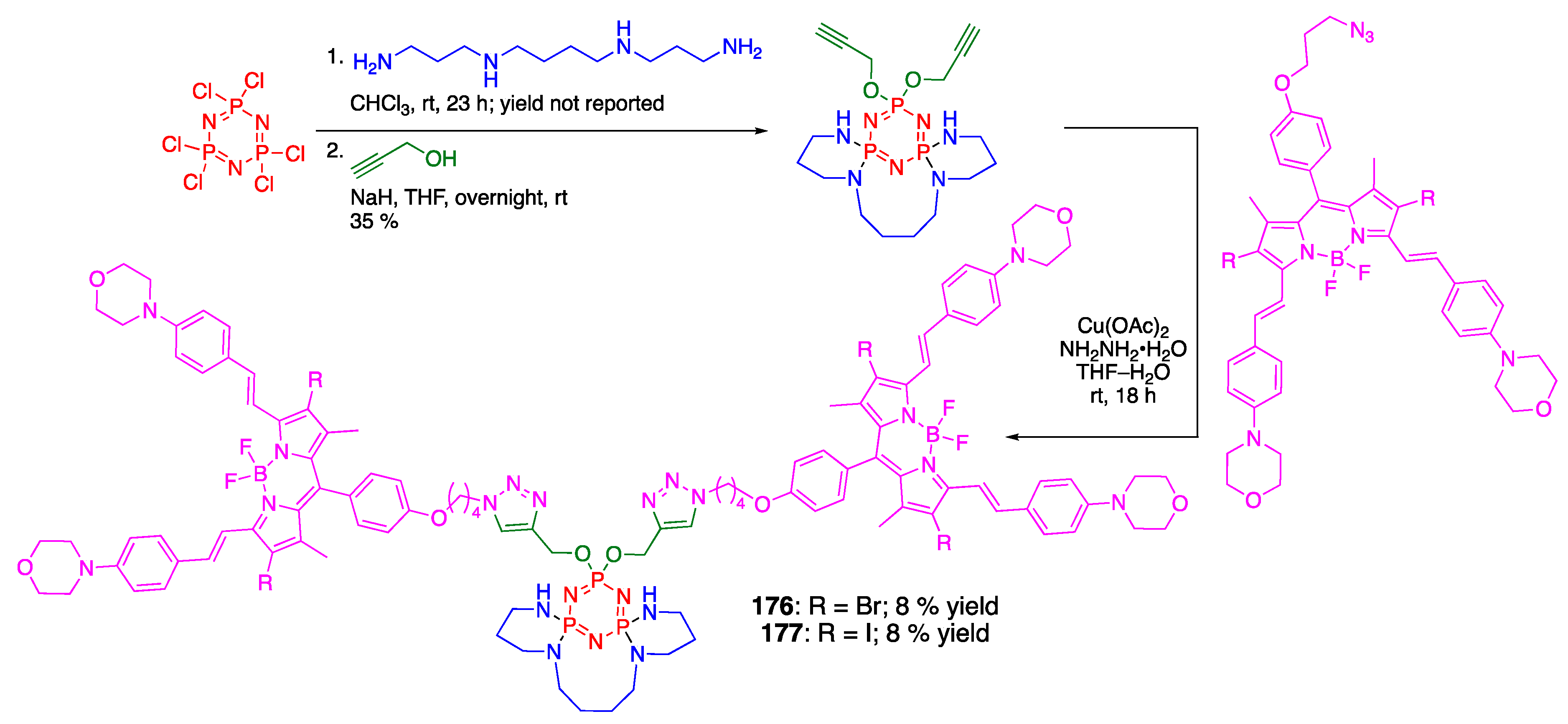
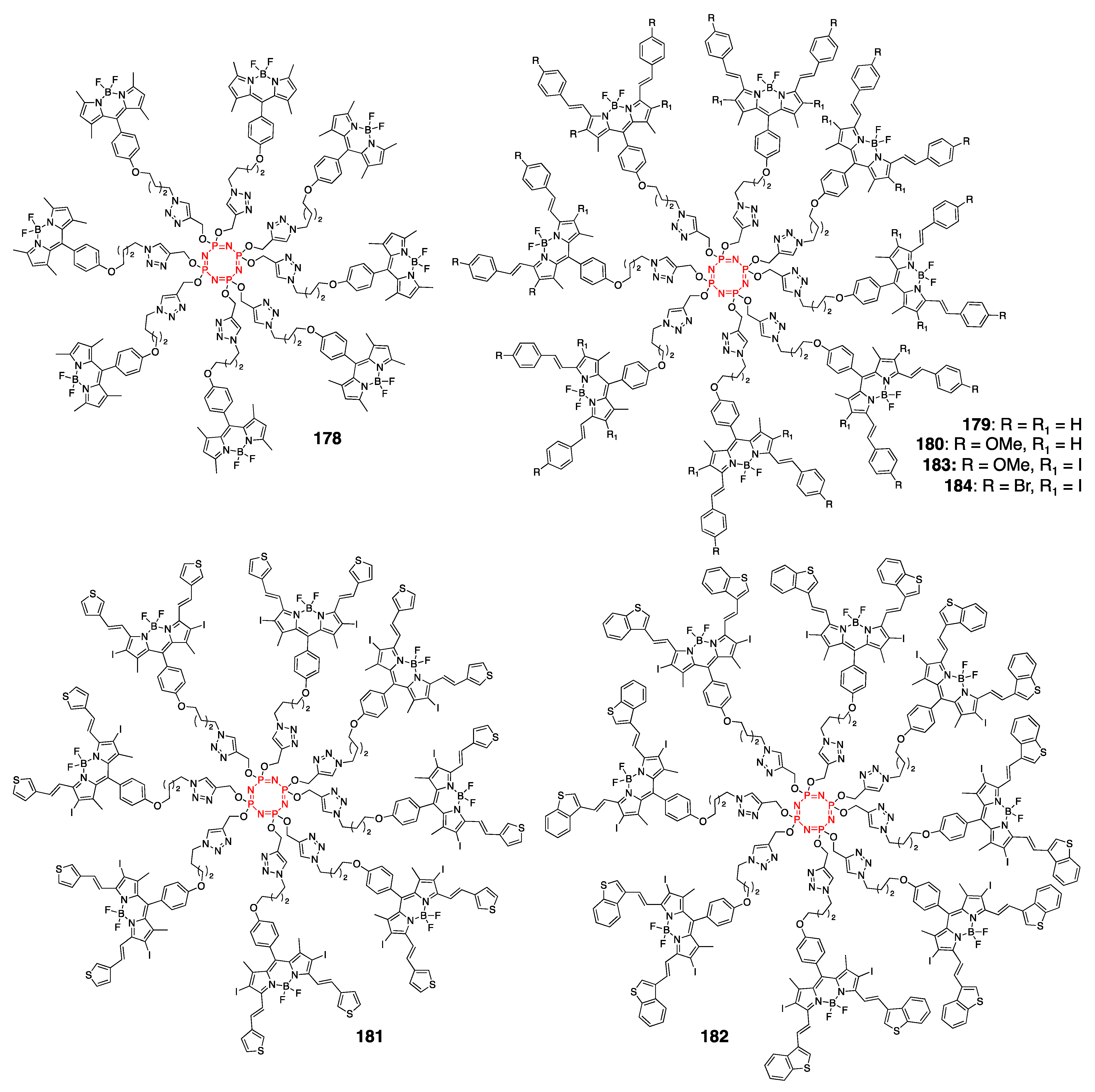
4.4. Cyclotriphosphazene- and Cyclotetraphosphazene-Containing BODIPY Dyes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, I.; Sivaramakrishna, A. Phosphorus-based polymeric flame retardant–recent advances and perspectives. ChemistrySelect 2024, 9, e202401485. [Google Scholar] [CrossRef]
- Pathiraja, G.; Bonner, C.D.J.; Obare, S.O. Recent advances of enzyme-free electrochemical sensors for flexible electronics in the detection of organophosphorus compounds: A review. Sensors 2023, 23, 1226. [Google Scholar] [CrossRef]
- Lidman Olsson, E.O.; Giarborg, P.; Dam-Johansen, K.; Wu, H. Review of phosphorus chemistry in the thermal conversion of biomass: Progress and perspectives. Energy Fuels 2023, 37, 6907–6998. [Google Scholar] [CrossRef]
- Asok, N.; Gaffen, J.R.; Baumgartner, T. Unique phosphorus-based avenues for the tuning of functional materials. Acc. Chem. Res. 2023, 56, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Ung, S.P.-M.; Li, C.-J. From rocks to bioactive compounds: A journey through the global P(V) organophosphorus industry and its sustainability. RSC Sustain. 2023, 1, 11–37. [Google Scholar] [CrossRef]
- Kolodiazhnyi, O.I. Phosphorus compounds of natural origin: Prebiotic, stereochemistry, application. Symmetry 2021, 13, 889. [Google Scholar] [CrossRef]
- Xie, C.; Shaligo, A.J.; Song, X.-R.; Kwon, O. Phosphorus-based catalysis. ACS Cent. Sci. 2021, 7, 536–558. [Google Scholar] [CrossRef] [PubMed]
- Sgcharbin, D.; Bryszewska, M.; Mignani, S.; Shi, X.; Majoral, J.-P. Phosphorus dendrimers as powerful nanoplatforms for drug delivery, as fluorescent probes and for liposome interaction studies: A concise overview. Eur. J. Med. Chem. 2020, 208, 112788. [Google Scholar] [CrossRef] [PubMed]
- Glueck, D.S. Metal-catalyzed P–C bond formation via P–H oxidative addition: Fundamentals and recent advances. J. Org. Chem. 2020, 85, 14276–14285. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.B.; Gallo-Rodriguez, C. The role of the phosphorus atom in drug design. ChemMedChem 2019, 14, 190–216. [Google Scholar] [CrossRef]
- Shameem, M.A.; Orthaber, A. Organophosphorus compounds in organic electronics. Chem. Eur. J. 2016, 22, 10718–10735. [Google Scholar] [CrossRef] [PubMed]
- Montchamp, J.-L. Phosphinate chemistry in the 21st century: A viable alternative to the use of phosphorus trichloride in organophosphorus synthesis. Acc. Chem. Res. 2014, 47, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, A.; Chou, P.-T.; Koshevoy, I.O. Cationic organophosphorus chromophores: A diamond in the rough among ionic dyes. Chem. Eur. J. 2021, 27, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Thakur, D.; Sushmita; Meena, S.A.; Verma, A.K. Advancement in synthetic strategies of phosphorus heterocycles: Recent progress from synthesis to emerging class of optoelectronic materials. Chem. Rec. 2024, 24, e202400058. [Google Scholar] [CrossRef]
- Reus, C.; Baumgartner, T. Stimuli-responsive chromism in organophosphorus chemistry. Dalton Trans. 2016, 45, 1850–1855. [Google Scholar] [CrossRef] [PubMed]
- Matano, Y. Synthesis and structure-property relationships of phosphole-based π-systems and their applications in organic solar cells. Chem. Rec. 2015, 15, 636–650. [Google Scholar] [CrossRef]
- Hissler, M.; Lescop, C.; Reau, R. Coordination chemistry of phosphole ligands: From supramolecular assemblies to OLEDs. Compt. Rend. Chem. 2008, 11, 628–640. [Google Scholar] [CrossRef]
- Louder, A.; Burgess, K. BODIPY dyes and their derivatives: Synthesis and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4931. [Google Scholar]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, V.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Yadav, I.S.; Misra, R. Design, synthesis and functionalization of BODIPY dyes: Application in dye-sensitized solar cells (DSSCs) and photodynamic therapy (PDT). J. Mater. Chem. C 2023, 11, 8688–8723. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z. Functional supramolecular aggregates based on BODIPY and aza-BODIPY dyes: Control over the pathway complexity. Org. Chem. Front. 2023, 10, 2581–2602. [Google Scholar] [CrossRef]
- Sreejith, S.; Carol, P.; Chithra, P.; Ajayaghosh, A. Squaraine dyes: A mine of molecular materials. J. Mater. Chem. 2008, 18, 264–274. [Google Scholar] [CrossRef]
- He, J.; Jo, Y.J.; Sun, X.; Qiao, W.; Ok, J.; Kim, T.-i.; Li, Z. Squaraine dyes for photovoltaic and biomedical applications. Adv. Funct. Mater. 2021, 31, 2008201. [Google Scholar] [CrossRef]
- Illina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine dyes: Molecular design for different applications and remaining challenges. Bioconjugate Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Wang, H. Squaraine dyes: The hierarchical synthesis and its application in optical detection. J. Photochem. Photobiol. C 2017, 31, 84–113. [Google Scholar] [CrossRef]
- Ta, D.D.; Dzyuba, S.V. Squaraine-based optical sensors: Designer toolbox for exploring ionic and molecular recognitions. Chemosensors 2021, 9, 302. [Google Scholar] [CrossRef]
- Treibs, A.; Jacob, K. Cyclotrimethine dyes derived from quadratic acid [1,2-hydroxycylobutenedione]. Angew. Chem. 1965, 77, 680–681. [Google Scholar] [CrossRef]
- Treibs, A.A.; Kreuzer, F.-H. Difluroboryl complexes of di- and tripyrrylmethenes. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- Mahanta, C.S.; Ravichandiran, V.; Swain, S.P. Recent developments in the design of new water-soluble boron dipyrromethenes and their applications: An updated review. ACS Appl. Bio Mater. 2023, 6, 2995–3018. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Yang, L.; Chen, Z. Water-soluble BODIPY and aza-BODIPY dyes: Synthetic progress and applications. Front. Chem. Sci. Eng. 2014, 8, 405–417. [Google Scholar] [CrossRef]
- Song, R.; Dong, Y.; Zhing, Z.; Zhao, Q.; Hu, Y.; Lei, M.; Lei, P.; Jiang, Z.; Qian, K.; Shi, C.; et al. Systematic structural modification of squaraine dye for near-infrared window one and two multiplexed in vivo imaging and photothermal therapy. J. Med. Chem. 2024, 67, 10275–10292. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Meador, W.E.; Pramanik, A.; Ray, P.; Delcamp, J.H.; Zhao, Y. An indolizine squaraine-based water soluble NIR dye for fluorescence imaging of multidrug-resistant bacteria and antibacterial/antibiofilm activity using the photothermal effect. J. Photochem. Photobiol. B 2023, 240, 112652. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.S.D.; Ferreira, J.C.C.; Boto, R.E.F.; Almeida, P.; Fernandes, J.R.; Sousa, M.J.; Goncalves, M.S.T.; Reis, L.V. Squaraine dyes derived from indolenine and benzo[e]indole as potential fluorescent probes for HSA detection and antifungal agents. Photochem. Photobiol. 2022, 98, 1402–1417. [Google Scholar] [CrossRef] [PubMed]
- Hovor, I.V.; Kosolova, O.S.; Obukhova, O.M.; Tatarets, A.L.; Pantsenker, L.D. Comparison of water-soluble squaraine and norsquaraine as fluorescent material for biomedical applications. Funct. Mater. 2020, 27, 836–845. [Google Scholar]
- Bura, T.; Ziessel, R. Water-soluble phosphonate-substituted BODIPY derivatives with tunable emission channels. Org. Lett. 2011, 13, 3072–3075. [Google Scholar] [CrossRef]
- Romieu, A.; Massif, C.; Rihn, S.; Ulrich, G.; Ziessel, R.; Renard, R.-Y. The first comparative study of the ability of different hydrophilic groups to water-solubilise fluorescent BODIPY dyes. New J. Chem. 2013, 37, 1016–1027. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Haefele, A. A general synthetic route of 3,5-substituted boron dipyrromethenes: Applications and properties. J. Org. Chem. 2012, 77, 4298–4311. [Google Scholar] [CrossRef]
- Lv, F.; Li, H.; Wu, Q.; Zhang, H.; Yu, C.; Jiao, L.; Hao, E. Silver-mediated, direct phosphorylation of BODIPY dyes at the 3- or 3,5-positions with H-phosphonates. Chem. Commun. 2022, 58, 3937–3940. [Google Scholar] [CrossRef]
- Tasgin, D.I. The design, synthesis and spectroscopic/photophysical characterization of phosphonated-substituted BODIPY. Hacet. J. Biol. Chem. 2020, 48, 197–201. [Google Scholar] [CrossRef]
- Tasgin, D.I.; Sirin, P.S. A theoretical investigation: Effect of structural modifications on the molecular, electronic, and optical properties of phosphonate substituted BODIPY dyes. ChemistrySelect 2021, 6, 4677–4683. [Google Scholar] [CrossRef]
- Reddington, M.V. Synthesis and properties of phosphonic acid containing cyanine and squaraine dyes for use as fluorescent labels. Bioconjugate Chem. 2007, 18, 2178–2190. [Google Scholar] [CrossRef]
- Davies, L.H.; Stewart, B.; Harrington, R.W.; Clegg, W.; Higham, L.J. Air-stable, highly fluorescent primary phosphanes. Angew. Chem. Int. Ed. 2012, 51, 4921–4924. [Google Scholar] [CrossRef]
- Davies, L.H.; Stewart, B.; Higham, L.J. Air-stable, fluorescent primary phosphines. Organomet. Chem. 2014, 39, 51–71. [Google Scholar]
- Bange, C.A.; Mucha, N.T.; Cousins, M.E.; Gehsmann, A.C.; Singer, A.; Truax, T.; Higham, L.J.; Waterman, R. Zirconium-catalyzed alkene hydrophosphination and dehydrocoupling with an air-stable, fluorescent primary phosphine. Inorganics 2016, 4, 26. [Google Scholar] [CrossRef]
- Davies, L.H.; Wallis, J.F.; Harrington, R.W.; Waddell, P.G.; Higham, L.J. Air-stable fluorescent phosphine complexes of molybdenum and tungsten. J. Coord. Chem. 2016, 69, 2069–2080. [Google Scholar] [CrossRef]
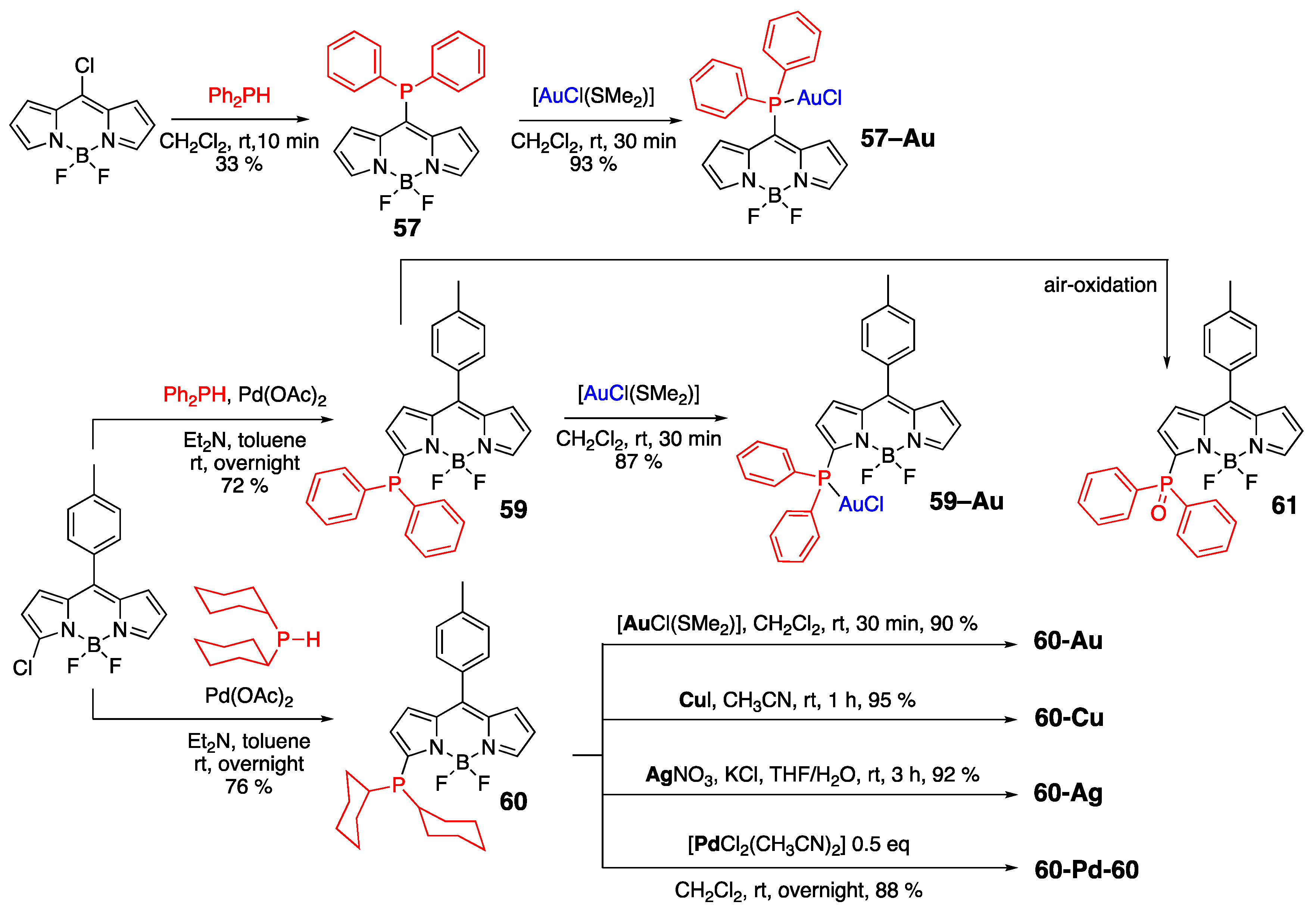
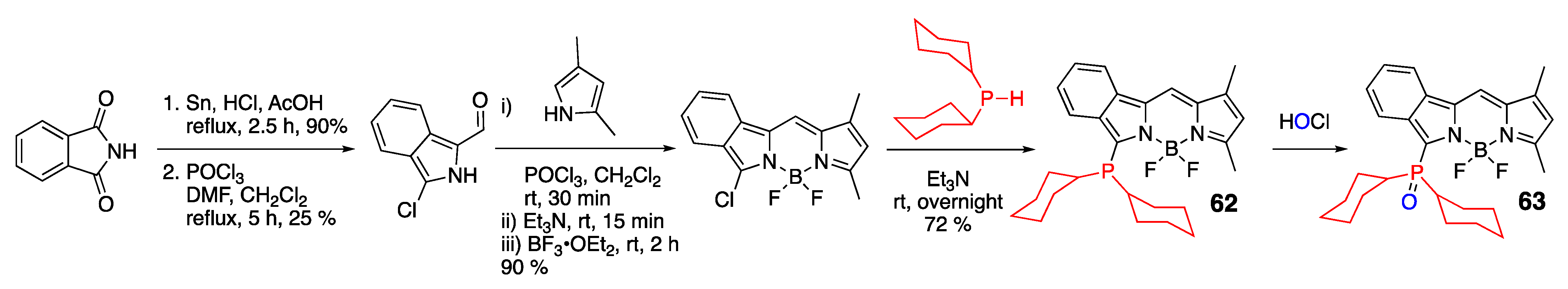
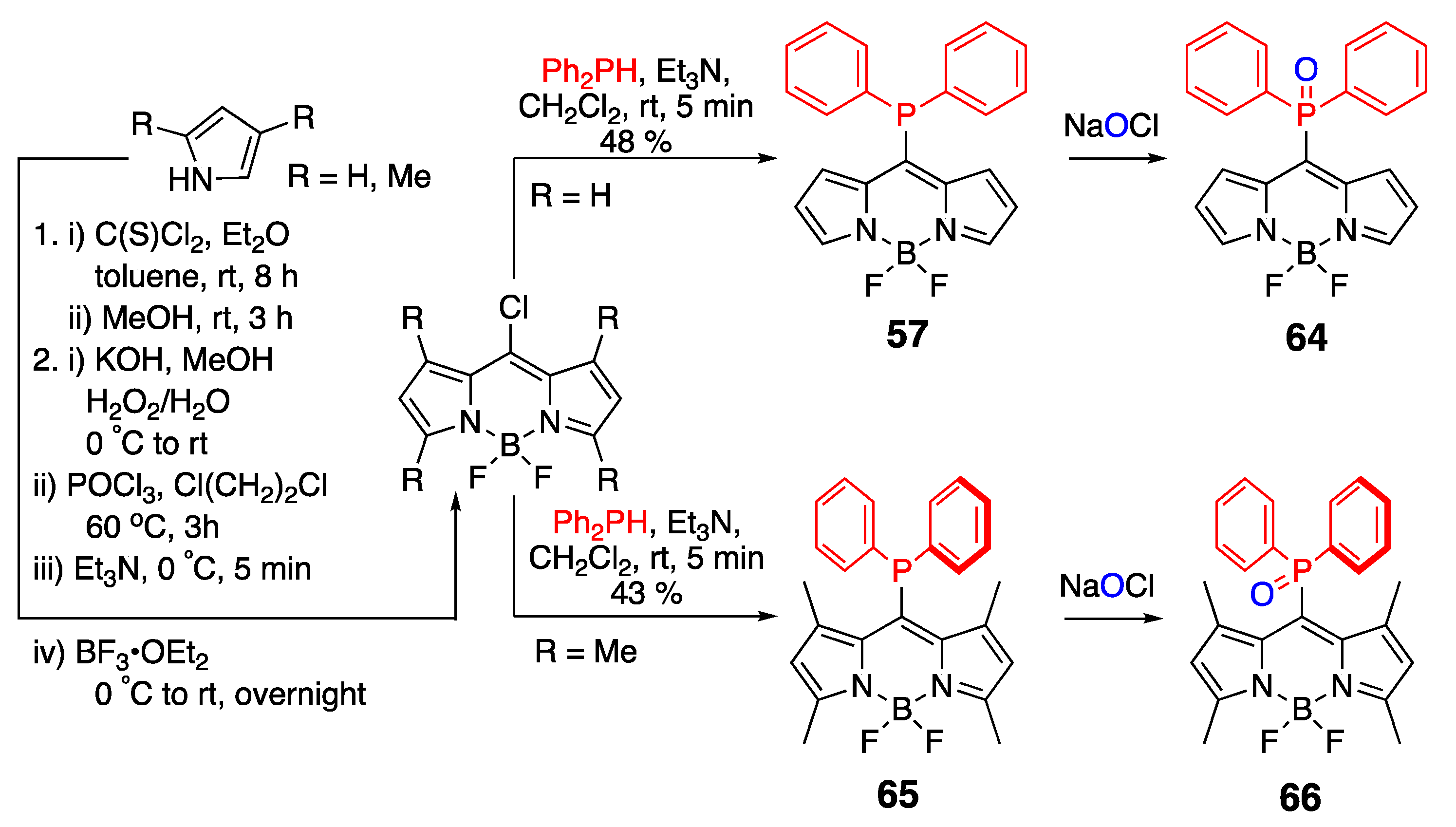
- Davies, L.H.; Harrington, R.W.; Clegg, W.; Higham, L.J. BRBodPR2: Highly fluorescent alternatives to PPh3 and PhPCy2. Dalton Trans. 2014, 43, 13485–13499. [Google Scholar] [CrossRef] [PubMed]
- Lifschitz, A.M.; Shade, C.M.; Spokoyny, A.M.; Mendez-Arroyo, J.; Stern, C.L.; Sarjeant, A.A.; Mirkin, C.A. Boron-dipyrromethene-functionalized hemilabile ligands as “turn-on” fluorescent probes for coordination changes in weak-link approach complexes. Inorg. Chem. 2013, 52, 5484–5492. [Google Scholar] [CrossRef] [PubMed]
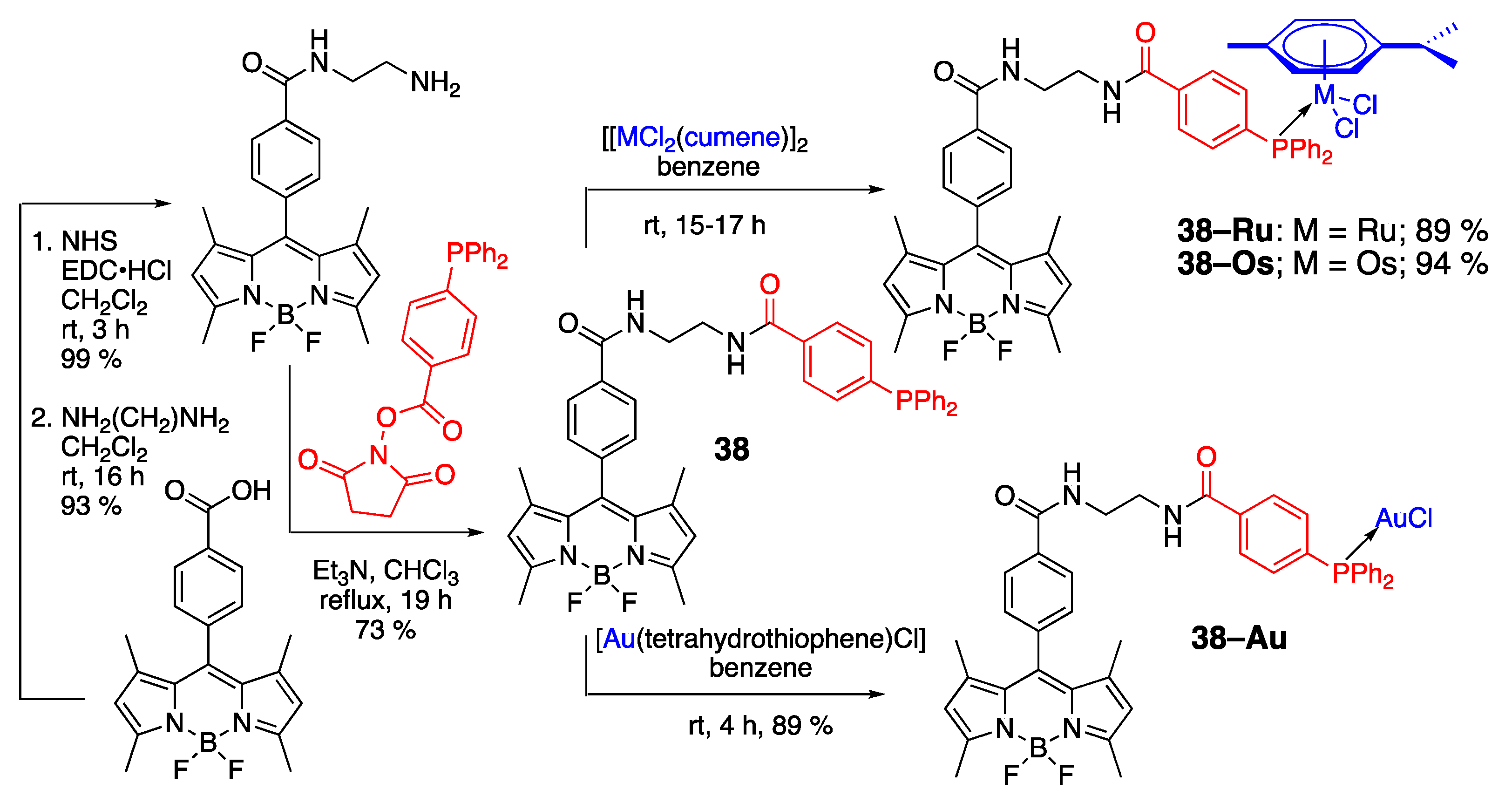
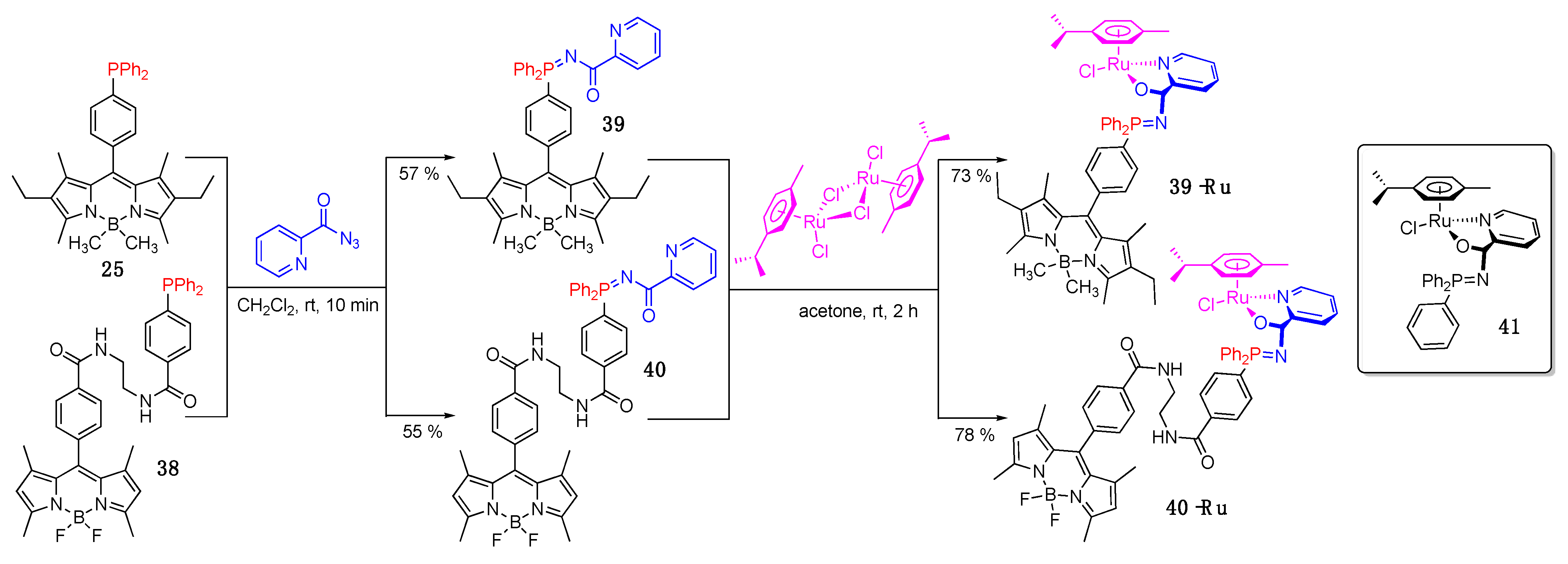
- Tasan, S.; Zava, O.; Bertrand, B.; Bernhard, C.; Goze, C.; Picquet, M.; Le Gendre, P.; Harvey, P.; Denat, F.; Casini, A.; et al. BODIPY–phosphane as a versatile tool for easy access to new metal-based theranostics. Dalton Trans. 2013, 42, 6102–6109. [Google Scholar] [CrossRef]
- Miachin, K.; Del Solar, V.; El Khoury, E.; Nayeem, N.; Khrystenko, A.; Appelt, P.; Neary, M.C.; Bucella, D.; Contel, M. Intracellular localization studies of the luminescent analogue of an anticancer ruthenium iminophosphorane with high efficacy in a triple-negative breast cancer mouse model. Inorg. Chem. 2021, 60, 19152–19164. [Google Scholar] [CrossRef] [PubMed]
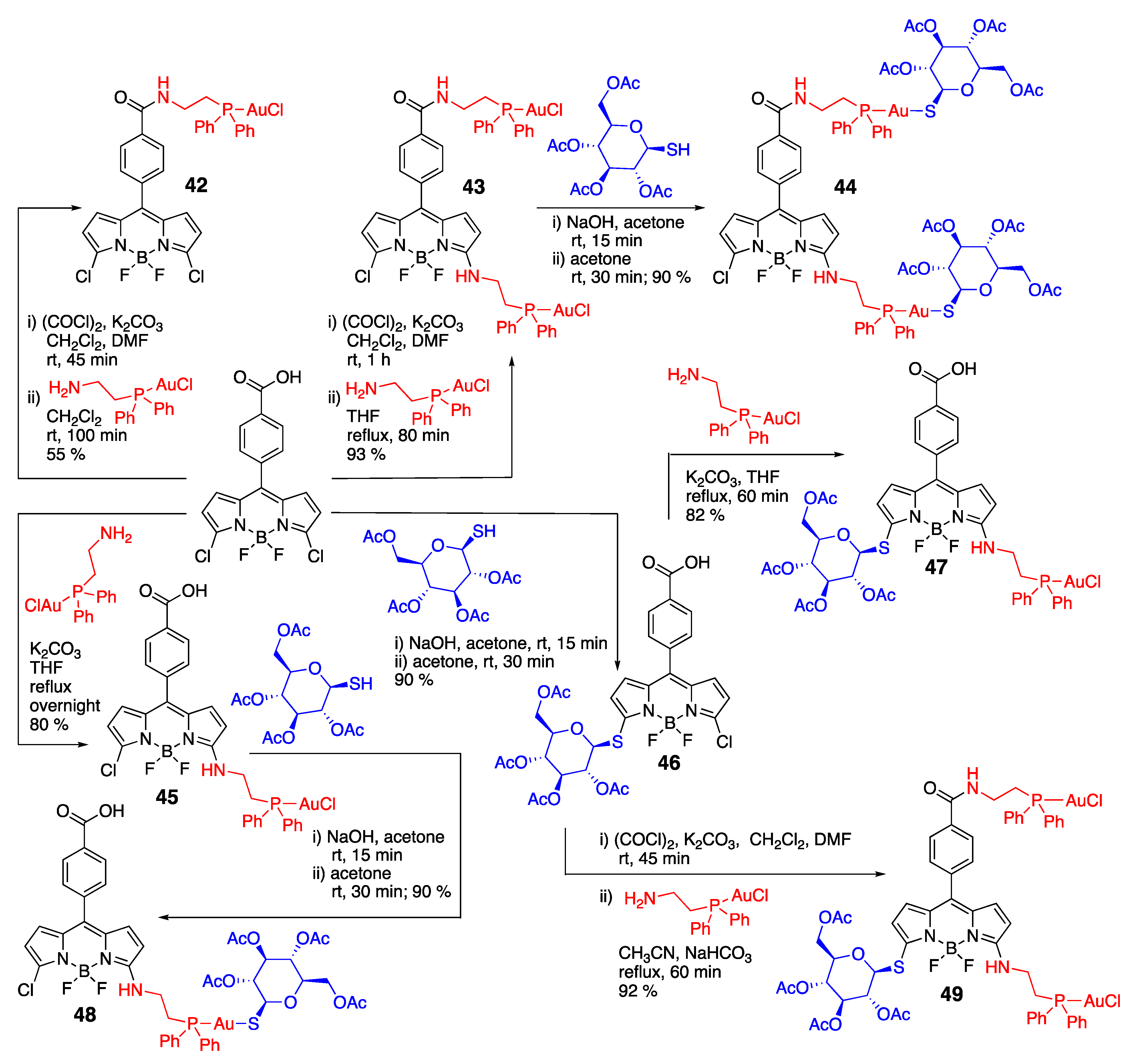
- Pliquett, J.; Amor, S.; Ponce-Vargas, M.; Laly, M.; Racoeur, C.; Rousselin, Y.; Denat, F.; Bettaïeb, A.; Fleurat-Lessard, P.; Paul, C.; et al. Design of a multifunctionalizable BODIPY platform for the facile elaboration of a large series of gold(I)-based optical theranostics. Dalton Trans. 2018, 47, 11203–11218. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, Q.; Li, Y.; Silva, D.S.; Ruiz, M.E.L.; Ouyang, R.; Liu, B.; Miao, Y. Recent development of rhenium-based materials in the application of diagnosis and tumor therapy. Molecules 2023, 28, 2733. [Google Scholar] [CrossRef]
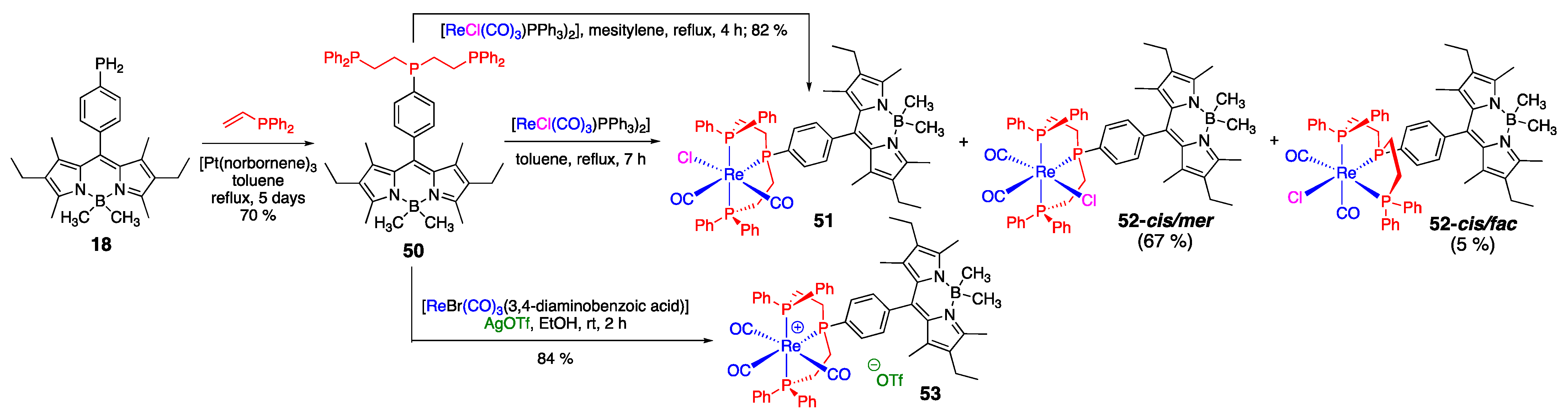
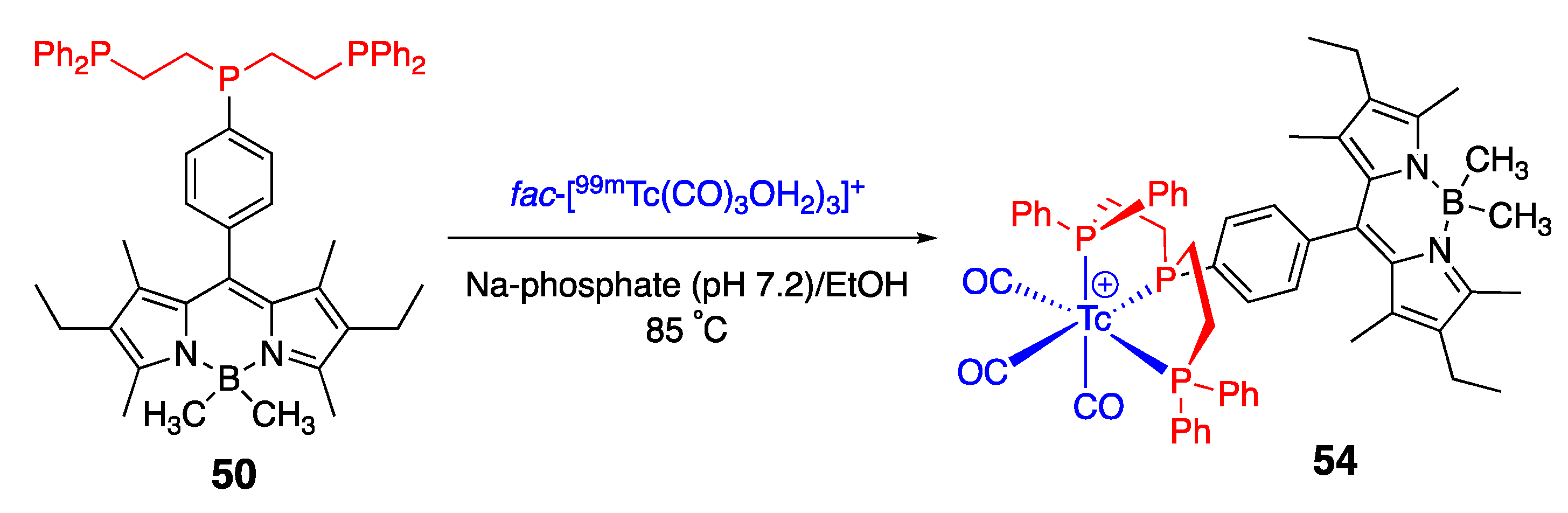
- Davies, L.H.; Kasten, B.B.; Benny, P.D.; Arrowsmith, R.L.; Ge, H.; Pascu, S.I.; Botchway, S.W.; Clegg, W.; Harrington, R.W.; Higham, L.J. Re and 99mTc complexes of BodP3–multi modality imaging probes. Chem. Commun. 2014, 50, 15503–15505. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.L.; Simons, C.R.; Heiden, Z.M. Redox switchable catalysis utilizing a fluorescent dye. Chem. Commun. 2019, 55, 11430–11433. [Google Scholar] [CrossRef] [PubMed]
- Esnal, I.; Valois-Escamilla, I.; Gómez-Durán, C.F.A.; Urías-Benavides, A.; Berancourt-Mendiola, M.L.; López-Arbeloa, I.; Bañuelos, J.; Carcía-Moreno, I.; Costela, A.; Peña-Cabrera, E. Blue-to-orange color-tunable laser emission from tailored boron-dipyrromethene dyes. ChemPhysChem 2013, 14, 4134–4142. [Google Scholar] [CrossRef] [PubMed]
- Vasiuta, R.; Plenio, H. Observing initial steps in gold-catalyzed alkyne transformations by utilizing bodipy-tagged phosphine-gold complexes. Chem. Eur. J. 2016, 22, 6353–6360. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Yang, T.; An, K.; Wang, S.; Han, Y. A phosphine-based fluorescent probe for fluorescent imaging of hypochlorous acid in living cells and zebrafish. New J. Chem. 2023, 47, 11912–11918. [Google Scholar] [CrossRef]
- Ma, C.; Hou, S.; Zhou, X.; Wang, Z.; Yoon, J. Rational design of meso-phosphino-substituted BODIPY probes for imaging hypochlorite in living cells and mice. Anal. Chem. 2021, 93, 9640–9646. [Google Scholar] [CrossRef] [PubMed]
- Bacher, E.P.; Lepore, A.J.; Pena-Romero, D.; Smith, B.D.; Ashfeld, B.L. Nucleophilic addition of phosphorus(III) derivatives to squaraines: Colorimetric detection of transition meta-mediated or thermal reversion. Chem. Commun. 2019, 55, 3286–3289. [Google Scholar] [CrossRef]
- Bacher, E.P.; Koh, K.J.; Lepore, A.J.; Oliver, A.G.; Wiest, O.; Ashfeld, B.L. A phosphine-mediated skeletal rearrangement of dianiline squaraine dyes. Org. Lett. 2021, 23, 2853–2857. [Google Scholar] [CrossRef]
- Twiringiyimana, R.; Ashfeld, B.L. Pseudoaromaticity-driven, transition metal detection by squaraine-derived enol phosphoniumylide chemodosimeters. Chem. Commun. 2024, 60, 5638–5641. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.; O’Neill, L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Hoye, A.T.; Davoren, J.E.; Wipf, P.; Fink, M.P.; Kagan, V.E. Targeting mitochondria. Acc. Chem. Res. 2008, 41, 87–97. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, L. Fluorescent probes for the selective detection of chemical species inside mitochondria. Chem. Commun. 2016, 52, 1094–1119. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-targeted triphenylphosphonium-based compounds: Synthesis, mechanisms of action, and therapeutic and diagnostic applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef] [PubMed]
- Mai, D.K.; Badon, I.W.; Lim, J.M.; Vales, T.P.; Kim, C.; Yang, J.; Lee, J.; Kim, H.-J. Triphenylphosphonium-functionalized BODIPY derivatives for mitochondria-targeted cell imaging and fluorescence turn-on sensing with protein selectivity. Dyes Pigments 2023, 208, 110856. [Google Scholar] [CrossRef]
- Wang, S.; Gai, L.; Chen, Y.; Ji, X.; Lu, H.; Guo, Z. Mitochondria-targeted BODIPY dyes for small molecule recognition, bio-imaging and photodynamic therapy. Chem. Soc. Rev. 2024, 53, 3976–4019. [Google Scholar] [CrossRef]
- Gao, T.; He, H.; Huang, R.; Zheng, M.; Wang, F.-F.; Hu, Y.-J.; Jiang, F.-L.; Liu, Y. BODIPY-based fluorescent probes for mitochondria-targeted cell imaging with superior brightness, low cytotoxicity and high photostability. Dyes Pigments 2017, 141, 530–535. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, J.; Dong, X.; Zhao, W.; Chen, Z. Visualizing nitric oxide in mitochondria and lysosomes of living cells with N-nitrosation of BODIPY-based fluorescent probes. Anal. Chim. Acta 2019, 1067, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Fan, J.; Sun, W.; Sui, K.; Jin, X.; Wang, J.; Peng, X. A highly specific BODIPY-based probe localized in mitochondria for HClO imaging. Analyst 2013, 138, 6091–6096. [Google Scholar] [CrossRef]
- Yang, Z.; Kang, D.H.; Lee, H.; Shin, J.; Yan, W.; Rathore, B.; Kim, H.-R.; Kim, S.J.; Singh, H.; Liu, L.; et al. A fluorescent probe for stimulated emission depletion super-resolution imaging of vicinal-dithiol-proteins on mitochondrial membrane. Bioconjugate Chem. 2018, 29, 1446–1453. [Google Scholar] [CrossRef]
- Xu, C.; Qian, Y. The α, β-unsaturated pyrazolone-based fluorescent sensor with red emission and its application for real-life monitoring hypochlorite in cancer cells and zebrafish. Dyes Pigments 2019, 161, 303–312. [Google Scholar] [CrossRef]
- Gao, C.; Lin, L.; Sun, W.; Tan, Z.-L.; Huang, J.-R.; He, L.; Lu, Z.-L. Dihydropyridine-derived BODIPY probe for detecting exogenous and endogenous nitric oxide in mitochondria. Talanta 2018, 176, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Sui, B.; Tang, S.; Woodward, A.W.; Kim, B.; Belfield, K.D. A BODIPY-based water-soluble fluorescent probe for mitochondria targeting. Eur. J. Org. Chem. 2016, 2016, 2851–2857. [Google Scholar] [CrossRef]
- Krumova, K.; Greene, L.E.; Cosa, G. Fluorogenic α-tocopherol analogue for monitoring the antioxidant status within the inner mitochondrial membrane of live cells. J. Am. Chem. Soc. 2013, 135, 17135–17143. [Google Scholar] [CrossRef]
- Kand, D.; Pizarro, L.; Angel, I.; Avni, A.; Friedmann-Morvinski, D.; Weinstain, R. Organelle-targeted BODIPY photocages: Visible-light-mediated subcellular photorelease. Angew. Chem. Int. Ed. 2019, 58, 4659–4663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, Y.; Qi, J.; Qu, J.; Kim, B.; Yue, X.; Belfield, K.D. Long-wavelength, photostable, two-photon excitable BODIPY fluorophores readily modifiable for molecular probes. J. Org. Chem. 2013, 78, 9153–9160. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Zhang, L.; Gao, L.-X.; Chen, J.-L.; Zhou, T.; Liu, Y.; Jiang, F.-L. A bright, red-emitting water-soluble BODIPY fluorophore as an alternative to the commercial Mito Tracker Red for high-resolution mitochondrial imaging. J. Mater. Chem. B 2021, 9, 8639–8645. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Huo, Y.; Yao, G.; Feng, Y.; Weng, J.; Zhao, W.; Guo, W. Heavy atom-free, mitochondria-targeted, and activatable photosensitizers for photodynamic therapy with real-time in-situ therapeutic monitoring. Angew. Chem. Int. Ed. 2022, 61, e202201815. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Bai, S.; He, N.; Wang, R.; Xing, Y.; Lv, C.; Yu, F. Real-time evaluation of hydrogen peroxide injuries in pulmonary fibrosis mice models with a mitochondria-targeted near-infrared fluorescent probe. ACS Sens. 2021, 6, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, R.; Yu, F.; Chen, L. Evaluation of sulfane sulfur bioeffects via a mitochondria-targeting selenium-containing near-infrared fluorescent probe. Biomaterials 2018, 160, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Su, F.; Zhang, L.; Yaron, J.; Lee, F.; Shi, Z.; Tian, Y.; Meldrum, D.R. A highly selective mitochondria-targeting fluorescent K+ sensor. Angew. Chem. Int. Ed. 2015, 54, 12053–12057. [Google Scholar] [CrossRef]
- Dodani, S.C.; Leary, S.C.; Cobine, P.A.; Winge, D.R.; Chang, C.J. A targetable fluorescent sensor reveals that copper-deficient SCO1 and SCO2 patient cells prioritize mitochondrial copper homeostasis. J. Am. Chem. Soc. 2011, 133, 8606–8616. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-L.; Niu, L.-Y.; Chen, Y.-Z.; Zheng, M.-L.; Yang, Y.; Yang, Q.-Z. A mitochondria-targeting fluorescent probe for the selective detection of glutathione in living cells. Org. Biomol. Chem. 2017, 15, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Prime, T.A.; Forkink, M.; Logan, A.; Finichiu, P.G.; McLachlan, J.; Li Pun, P.B.; Koopman, W.J.H.; Larsen, L.; Latter, M.J.; Smith, R.A.J.; et al. A ratiometric fluorescent probe for assessing mitochondrial phospholipid peroxidation within living cells. Free Radic. Biol. Med. 2012, 53, 544–553. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Madhu, S.; Gao, Q.; Lian, Q.; Raghavan, S.S.; Geng, J. BODIPY based realtime, reversible and targeted fluorescent probes for biothiol imaging in living cells. Chem. Commun. 2020, 56, 14717–14720. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Myers, C.R.; Wang, Y.; You, M. Mitochondria as a novel target for cancer chemoprevention: Emergence of mitochondrial-targeting agents. Cancer Prev. Res. 2021, 14, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Mai, D.K.; Lee, J.; Jo, J.; Kim, S.; Badon, I.W.; Lim, J.M.; Kim, H.-J.; Yang, J. Triphenylphosphonium-functionalized dimeric BODIPY-based nanoparticles for mitochondria-targeting photodynamic therapy. Nanoscale 2024, 16, 9462–9475. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Cho, H.; Chen, H.H.; Panagia, M.; Sosnovik, D.E.; Josephson, L. Fluorescent and radiolabeled triphenylphosphonium probes for imaging mitochondria. Chem. Commun. 2013, 49, 10361–10363. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.; Burke, B.P.; Davies, L.H.; Domarkas, J.; Wallis, J.F.; Waddell, P.G.; Waby, J.S.; Benoit, D.M.; Seymour, A.-M.; Cawthorne, C.; et al. Structurally optimized BODIPY derivatives for imaging of mitochondrial dysfunction in cancer and heart cells. Chem. Commun. 2016, 52, 7114–7117. [Google Scholar] [CrossRef]
- Walter, E.R.H.; Lee, L.C.-C.; Leung, P.K.-K.; Lo, K.K.-W.; Long, N.J. Mitochondria-targeting biocompatible fluorescent BODIPY probes. Chem. Sci. 2024, 15, 4846–4852. [Google Scholar] [CrossRef] [PubMed]
- Das, R.S.; Saha, P.C.; Sepay, N.; Mukherjee, A.; Chatterjee, S.; Guha, S. Design and synthesis of near-infrared mechanically interlocked molecules for specific targeting of mitochondria. Org. Lett. 2020, 22, 5839–5843. [Google Scholar] [CrossRef] [PubMed]
- Kommidi, S.S.R.; Atkinson, K.M.; Smith, B.D. Steric protection of near-infrared fluorescent dyes for enhanced bioimaging. J. Mater. Chem. B 2024, 12, 8310–8320. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-H.; Smith, B.D. Molecular recognition using tetralactam macrocycles with parallel aromatic sidewalls. Beilstein J. Org. Chem. 2019, 15, 1086–1095. [Google Scholar] [CrossRef]
- Gassensmith, J.J.; Baumes, J.M.; Smith, B.D. Discovery and early development of squaraine rotaxanes. Chem. Commun. 2009, 6329–6338. [Google Scholar] [CrossRef]
- Das, R.S.; Maiti, D.; Kar, S.; Bera, T.; Mucherjee, A.; Saha, R.C.; Mondal, A.; Guha, S. Design of water-soluble rotaxane-capped superparamagnetic, ultrasmall Fe3O4 nanoparticles for targeted NIR fluorescence imaging in combination with magnetic resonance imaging. J. Am. Chem. Soc. 2023, 145, 20451–20461. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, B.; Zhang, X.; Guan, D.; Sun, K.; Zhang, Y.; Liu, Q. Thiolation for enhancing photostability of fluorophores at the single-molecule level. Angew. Chem. Int. Ed. 2024, 63, e202316192. [Google Scholar] [CrossRef]
- Jacob, M.; Schmitt, A.; Jung, G. Disabling photoinduced electron transfer in 4,4-difluoro-8(-4’-hydroxyphenyl)-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacene by phosphorylation. J. Fluoresc. 2008, 18, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, D.; Yoon, J. Recent advances in the development of chromophore-based chemosensors for nerve agents and phosgene. ACS Sens. 2018, 3, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.-Q.; Sedgwick, A.C.; Kwon, N.; Sun, M.; Xiao, K.; He, X.-P.; Anslyn, E.V.; James, T.D.; Yoon, J. Fluorescent probes for the detection of chemical warfare agents. Chem. Soc. Rev. 2023, 52, 601–662. [Google Scholar] [CrossRef]
- Kim, T.I.; Maity, S.B.; Bouffard, J.; Kim, Y. Molecular rotors for the detection of chemical warfare agent simulants. Anal. Chem. 2016, 88, 9259–9263. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Fan, W.; Shi, X.; Black, C.A.; Fan, C.; Wang, F. A highly specific BODIPY-based fluorescent probe for the detection of nerve-agent simulants. Sens. Actuators B 2018, 255, 176–182. [Google Scholar] [CrossRef]
- Paez-Perez, M.; Kuimova, M.K. Molecular rotors: Fluorescent sensors for microviscosity and conformation of biomolecules. Angew. Chem. Int. Ed. 2024, 63, e202311233. [Google Scholar] [CrossRef]
- Lee, S.C.; Heo, J.; Woo, C.; Lee, J.-A.; Seo, Y.H.; Lee, C.-L.; Kim, S.; Kwon, O.-P. Fluorescent molecular rotors for viscosity sensors. Chem. Eur. J. 2018, 24, 13706–13718. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Tsay, O.G.; Murale, D.P.; Jeong, J.A.; Segev, A.; Churchill, D.G. Novel and selective detection of Tabun mimics. Chem. Commun. 2014, 50, 7531–7534. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Zheng, Y.-C.; Zhu, X.-M.; Wang, H.-B.; Liang, L.-H.; Wang, X.-Z.; Yuan, L.; Zhang, F.-H.; Zheng, H.; Zhao, C.-L. A novel BODIPY-based fluorescent probe for sensitive and selective detection of nerve agent simulants through base-assisted photo-induced electron transfer process. Sens. Actuators B 2021, 337, 129804. [Google Scholar] [CrossRef]
- Begera, K.C.; Mahanty, R.; Ravikanth, M. 3-Pyrrolyl BODIPY based Schiff base fluorophores: A selective chemodosimetric and optical sensor for diethyl chlorophosphate. ChemPlusChem 2023, 88, e202300132. [Google Scholar]
- Dagnaw, F.W.; Cai, Y.-P.; Song, Q.-H. Rapid and sensitive detection of nerve agent mimics by meso-substituted BODIPY piperazines as fluorescent chemosensors. Dyes Pigment. 2021, 189, 109257. [Google Scholar] [CrossRef]
- Anitha, T.; Mrinalini, M.; Vani, D.; Prasanthkumar, S.; Reddy, K.R.; Giribabu, L. Synthesis and opto-electronic properties of BODIPY o-OPhos systems. Photochem. Photobiol. 2020, 96, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, C.; Zhang, M.; Zhao, Y.; Yuan, B.; Yang, B.; Li, H. Solvent-driven micro/nanostructures of squaraine and croconane dyes based on quinoxalinone for visual detection of nerve agent simulants. Dyes Pigments 2022, 208, 110824. [Google Scholar] [CrossRef]
- Pyuol, M.; Encinas, C.; Rivera, L.; Miltsov, S.; Alonso, J. Characterization of new norcyanine dyes and their application as pH chromoionophores in optical sensors. Dyes Pigments 2007, 73, 383–389. [Google Scholar] [CrossRef]
- Zhao, J.; Qin, M.; You, J.; Liu, K.; Ding, L.; Liu, T.; Kong, J.; Fang, Y. Rapid and colorimetric evaluation of G-series nerve agents and simulants using the squaraine-ethanolamine adducts. Dyes Pigments 2022, 197, 109870. [Google Scholar] [CrossRef]
- Simon, P.; Tichotova, M.; Garcia Gallardo, M.; Prochazkova, E.; Baszcynzki, O. Phosphate-based self-immolative linkers for tunable double cargo release. Chem. Eur. J. 2021, 27, 12763–12775. [Google Scholar] [CrossRef] [PubMed]
- Djud, M.; Tichotova, M.; Prochazkova, E.; Baszczynski, O. Phosphate-based self-immolative linkers for the delivery of amine-containing drugs. Molecules 2021, 26, 5160. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Jiao, L.; Hoa, E. BODIPY-base photocages: Rational design and their medical application. Chem. Soc. Rev. 2024, 60, 5770–5789. [Google Scholar]
- Singh, P.K.; Majumdar, P.; Singh, S.P. Advances in BODIPY photocleavable protecting groups. Coord. Chem. Rev. 2021, 449, 214193. [Google Scholar] [CrossRef]
- Shrestha, P.; Mukhopadhya, A.; Dissanayake, K.C.; Winter, A.H. Efficiency of functional groups caging with second-generation green- and red-light labile BODIPY photoremovable protecting groups. J. Org. Chem. 2022, 87, 14334–14341. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, J.; Müller, P.; Andreadou, E.; Heckel, A. Green-light activatable BODIPY and coumarin 5’-caps for oligonucleotide photocaging. Chem. Eur. J. 2022, 28, e202200477. [Google Scholar] [CrossRef]
- Benaim, G.; Paniz-Mondolfi, A. Umasking the mechanism behind miltefosine: Revealing the disruption of intracellular Ca2+ homeostasis as a rational therapeutic target in Leishmaniasis and Chagas disease. Biomolecules 2024, 14, 406. [Google Scholar] [CrossRef] [PubMed]
- de Castro Spadari, C.; Borba-Santos, L.P.; Rozental, S.; Ishida, K. Miltefosine repositioning: A review of potential alternative antifungal therapy. J. Med. Mycol. 2023, 33, 101436. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.; Balasegaram, M.; Beijnen, J.H.; de Vries, J. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef] [PubMed]
- Hornillos, V.; Carrillo, E.; Amat-Guerri, F.; Acuña, A.U. Synthesis of BODIPY-labeled alkylphosphocholines with leishmanicidal activity, as fluorescent analogues of miltefosine. Bioorg. Med. Chem. Lett. 2008, 18, 6336–6339. [Google Scholar] [CrossRef] [PubMed]
- Marcos, S.; Requeji-Isidro, J.; Merayo-Lloves, J.; Acuna, A.U.; Hornillos, V.; Carrilo, E.; Pérez-Merino, P.; del Olmo-Aguado, S.; del Aguila, C.; Amat-Guerri, F.; et al. Fluorescent labeling of Acanthamoeba assessed in situ from corneal sectioned microscopy. Biomed. Opt. Exp. 2012, 3, 2489–2499. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, B.G.; Hornillos, V.; Luque-Ortega, J.R.; Abengózar, M.A.; Amat-Guerri, F.; Acuna, A.U.; Rivas, L.; Andreu, B. A BODIPY-embedding miltefosine analog linked to cell penetrating Tat(48-60) peptide factors intracellular delivery and visualization of the antiparasitic drug. Amino Acids 2014, 46, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Courrier, E.; Maret, C.; Charaoui-Boukerzaza, S.; Lambert, V.; De Nicola, A.; Muzuzu, W.; Ulrich, G.; Rabrin, H.; Flori, P.; Moine, B.; et al. Synthesis of fluorescent BODIPY-labeled analogue of staining of Acathamoeba. ChemistrySelect 2018, 3, 7674–7679. [Google Scholar] [CrossRef]
- Dehmchi, D.A.; Bouchareb, F.; Berredjem, M. Recent advances in the synthesis of phosphoramidate derivatives: A comprehensive review and analysis. Synth. Commun. 2024, 54, 1909–1939. [Google Scholar] [CrossRef]
- Itumoh, E.J.; Data, S.; Leitao, E.M. Opening up the toolbox: Synthesis and mechanisms of phosphoramidates. Molecules 2020, 25, 3684. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.M.; Barbosa, L.C.A.; Ismail, F.M.D. The diverse pharmacology and medicinal chemistry of phosphoramidates–A review. RSC Adv. 2014, 4, 18998–19012. [Google Scholar] [CrossRef]
- Machado, L.A.; de Souza, M.C.; da Silva, C.M.; Yineda, J.; de Rezende, L.C.D.; Emery, F.S.; de Simobe, C.A.; da Silva Júnior, E.N.; Pedrosa, L.F. On the synthesis, optical and computational studies of novel BODIPY-based phosphoramidate fluorescent dyes. J. Fluor. Chem. 2019, 220, 9–15. [Google Scholar] [CrossRef]
- da Silva Marques, B.; Nascimento de Andrade, K.; Pereira Peixoto, B.; dos Santos, F.M., Jr.; Ferreira Pedrosa, L.; Costa de Souza, M. Sequential nucleophilic aromatic substitutions on cyanuric chloride: Synthesis of BODIPY derivatives and mechanistic insights. Org. Biomol. Chem. 2024, 22, 5987–5998. [Google Scholar] [CrossRef]
- Teeuwen, P.C.; Melissari, Z.; Senge, M.O.; Williams, R.M. Metal coordination effects on the photophysics of dipyrrinato photosensitizers. Molecules 2022, 27, 6967. [Google Scholar] [CrossRef]
- Baudron, S.A. Dipyrrin based metal complexes: Reactivity and catalysis. Dalton Trans. 2020, 49, 6161–6175. [Google Scholar] [CrossRef]
- Jiang, X.-D.; Zhao, J.; Xi, D.; Yu, H.; Guan, J.; Li, S.; Sun, C.-L.; Xiao, L.-J. A new water-soluble phosphorus-dipyrromethene and phosphorus-azadipyrromethene dye: PODIPY/aza-PODIPY. Chem. Eur. J. 2015, 21, 6079–6082. [Google Scholar] [CrossRef]
- Jiang, X.-D.; Yu, H.-F.; Zhao, J.-J.; Sun, C.-L.; Xiao, L.-J. A colorimetric chemosensor based on new water-soluble BODIPY dye for Hg2+ detection. Chin. Chem. Lett. 2015, 26, 1241–1245. [Google Scholar] [CrossRef]
- Marfin, Y.S.; Vodyanova, O.S.; Usoltsev, S.D.; Kazak, A.V.; Rumyantsev, E.V. Oxophosphoryl complexes of dipyrrin: Spectral and aggregation characteristics of solutions and thin films. Crystallogr. Rep. 2019, 64, 644–648. [Google Scholar] [CrossRef]
- Fihey, A.; Favennec, A.; Le Guennic, B.; Jacquemin, D. Investigating the properties of PODIPYs (phosphorus-dipyrromethene) with ab initio tools. Phys. Chem. Chem. Phys. 2016, 18, 9358–9366. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.-X.; Shi, X.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P. Cyclotripohosphazene core-based dendrimers for biomedical applications: An update on recent advances. J. Mater. Chem. B 2018, 6, 884–895. [Google Scholar] [CrossRef]
- Usri, S.N.K.; Jamain, Z.; Makmud, M.Z.H. A review of synthesis, structural, flame retardancy and dielectric properties of hexasubstituted cyclotriphosphazene. Polymers 2021, 13, 2916. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Su, X.; Xire, J.-H.; Ming, L.-J. Specific recognitions of multivalent cyclotriphosphazene derivatives in sensing, imaging, theranostics, and biomimetic catalysis. Coord. Chem. Rev. 2022, 454, 214326. [Google Scholar] [CrossRef]
- Liu, P.; Wang, L.; Yang, Y.; Qu, Y.; Ming, L.-J. Recent advances of cyclotriphosphazene derivatives as fluorescent dyes. Dyes Pigment. 2021, 188, 109214. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Hameau, A.; Moineau-Chane Ching, K.; Turrin, C.-O. Fluorescent groups at the core of phosphorus dentrons and their properties. Helv. Chim. Acta. 2023, 106, e202300048. [Google Scholar] [CrossRef]
- Rao, M.R.; Bolligaria, R.; Butcher, R.J.; Ravikanth, M. Hexa boron-dipyrromethene cyclotriphosphazenes: Synthesis, crystal structure, and photophysical properties. Inorg. Chem. 2010, 49, 10606–10616. [Google Scholar] [CrossRef] [PubMed]
- Eserci, H.; Öztürk, E.; Okutan, E. Novel BODIPY-bridged cyclotriphosphazenes. Turk. J. Chem. 2020, 44, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Çosut, B. Highly efficient energy transfer in BODIPY-pyrene decorated cyclotriphosphazene. Dyes Pigment. 2014, 100, 11–16. [Google Scholar] [CrossRef]
- Senkuyutu, E.; Cebesoy, Z.; Yenilmez Çiftçi, G.; Tanrıverdi Eçik, E. Study on the synthesis, photophysical properties and singlet oxygen generation behavior of BODIPY-functionalized cyclotriphosphazenes. J. Fluoresc. 2017, 27, 595–601. [Google Scholar] [CrossRef]
- Çetindere, S.; Okutan, E.; Oguz Tümay, S.; Yesilot, S.; Kiliç, A. Novel water-soluble cyclotriphosphazene-bodipy conjugates: Synthesis, characterization and photophysical properties. J. Fluoresc. 2019, 29, 1143. [Google Scholar] [CrossRef] [PubMed]
- Tanrıverdi Eçik, E.; Senkuytu, E.; Cebesoy, Z.; Ynilmez Çiftçi, G. BODIPY decorated dendrimeric cyclotriphosphazene photosensitizers: Synthesis and efficient singlet oxygen generators. RSC Adv. 2016, 6, 47600–47606. [Google Scholar] [CrossRef]
- Yıldız Gül, E.; Tanrıverdi Eçik, E. Cyclophosphazene-based photocatalysts containing orthogonal BODIPY moieties for chemical transformations. ChemPhotoChem 2024, 8, e202300314. [Google Scholar]
- Cetindere, S.; Yesilot, S.; Kiliç, A. Pyrene-BODIPY-substituted novel water-soluble cyclotriphosphazenes: Synthesis, characterization, and photophysical properties. Turk. J. Chem. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Senkuytu, E.; Öztürk, E.; Aydinogly, F.; Tanrıverdi Eçik, E.; Okutan, E. Cyclotriphosphazene cored naphthalimide-BODIPY dendrimeric systems: Synthesis, photophysical and antimicrobial properties. Inorg. Chim. Acta 2020, 502, 119386. [Google Scholar] [CrossRef]
- Sarikaya, S.Y.; Yesilot, S.; Kiliç, A.; Okutan, E. Visible light harvesting BODIPY-cyclotriphosphazene-fullerene assemblies: Photophysical properties and solvent effect on photosensitized generation of singlet oxygen. Dyes Pigments 2019, 162, 734–740. [Google Scholar] [CrossRef]
- Çetindere, S.; Oguz Tümay, S.; Kiliç, A.; Durmus, M.; Yesilot, S. Synthesis and physico-chemical properties of cyclotriphosphazene-BODIPY conjugates. Dyes Pigments 2017, 139, 517–523. [Google Scholar] [CrossRef]
- Kwon, N.; Kim, K.H.; Park, S.; Cho, Y.; Park, E.-Y.; Lim, J.; Çetindere, S.; Oguz Tümay, S.; Kim, W.J.; Li, X.; et al. Hexa-BODIPY-cyclotriphosphazene based nanoparticle for NIR fluorescence/photoacoustic dual-modal imaging and photothermal cancer therapy. Biosens. Bioelectron. 2022, 216, 114612. [Google Scholar] [CrossRef] [PubMed]
- Yıldız Gül, E.; Aydın Karataş, E.; Aydın Doğan, H.; Yenilmez Çiftçi, G.; Tanrıverdi Eçik, E. BODIPY precursors and their cyclotriphosphazene derivatives: Synthesis, photochemical properties and their application in PDT. Spectrochim. Acta. A 2024, 311, 124006. [Google Scholar] [CrossRef]
- Senkuytu, E.; Tanrıverdi Eçik, E. Octa-BODIPY derivative dendrimeric cyclotetraphosphazenes: Photophysical properties and fluorescent chemosensor for Co2+ ions. Spectrochim. Acta A 2017, 173, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Tanrıverdi Eçik, E.; Senkuyutu, E.; Okutan, E.; Çiftçi, G.Y. Synthesis of BODIPY-cyclotetraphosphazene triad systems and their sensing behaviors towards Co(II) and Cu(II). Inorg. Chim. Acta 2019, 495, 119009. [Google Scholar] [CrossRef]
- Senkuytu, E.; Eçik, E.T. Novel fully-BODIPY functionalized cyclotetraphosphazene photosensitizers having high singlet oxygen quantum yields. Spectrochim. Acta A 2017, 182, 26–31. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: New vistas at the aggregate level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Peng, Q.; Shuai, Z. Molecular mechanism of aggregation-induced emission. Aggregate 2021, 2, e91. [Google Scholar] [CrossRef]
- Antunes, P.; Cruz, A.; Barbosa, J.; Bonifácio, V.D.B.; Pinto, S.N. Lipid droplets in cancer: From composition and role to imaging and therapeutics. Molecules 2022, 27, 991. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Q.; Lv, Y.; Liu, Z.; Lee, K.; Kim, D.; Won, M.; Shen, J.; Kim, J.S. Fluorescence imaging-guided lipid droplets-localized photodynamic therapy. Aggregate 2024, e665. [Google Scholar] [CrossRef]









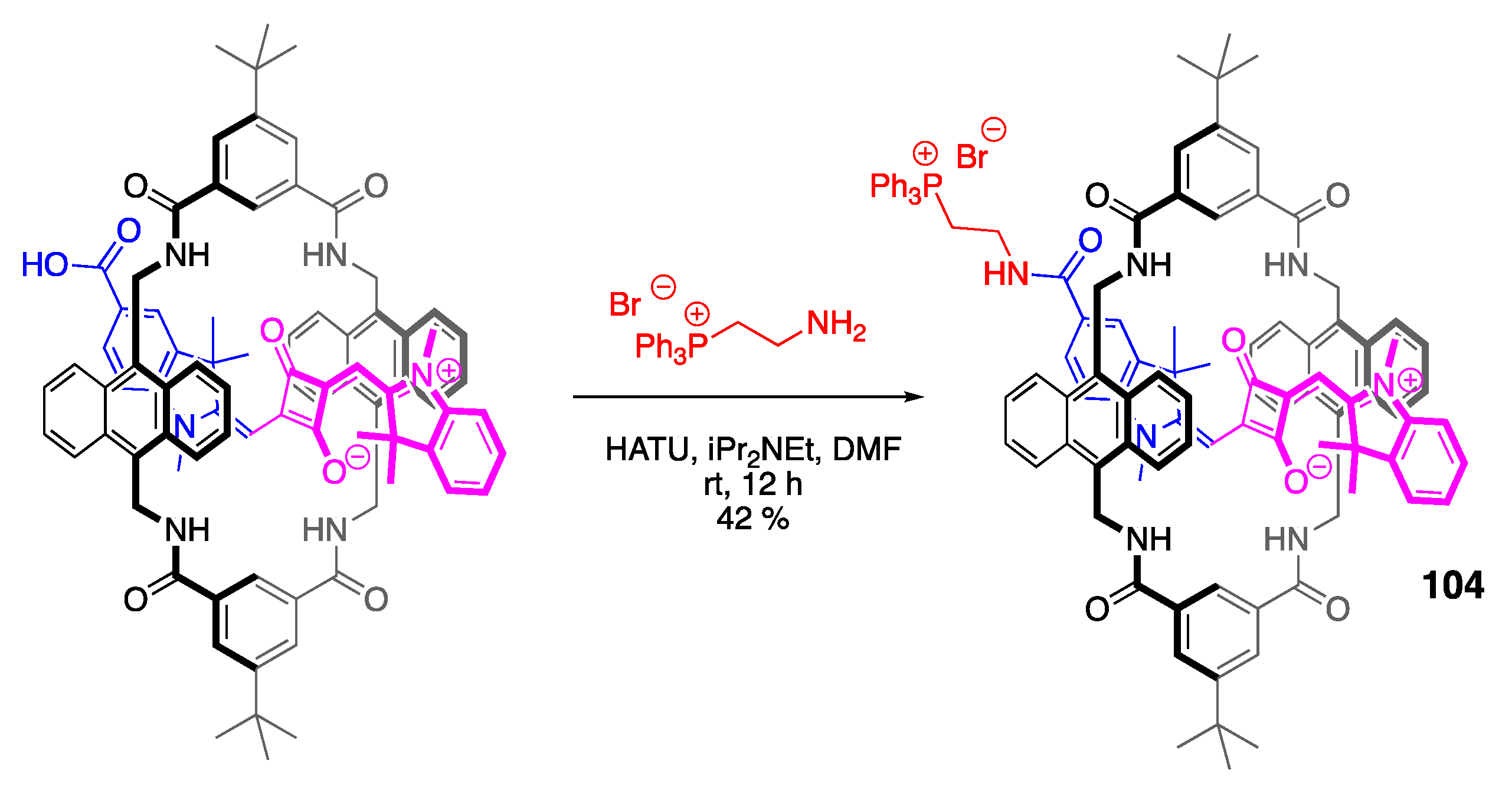
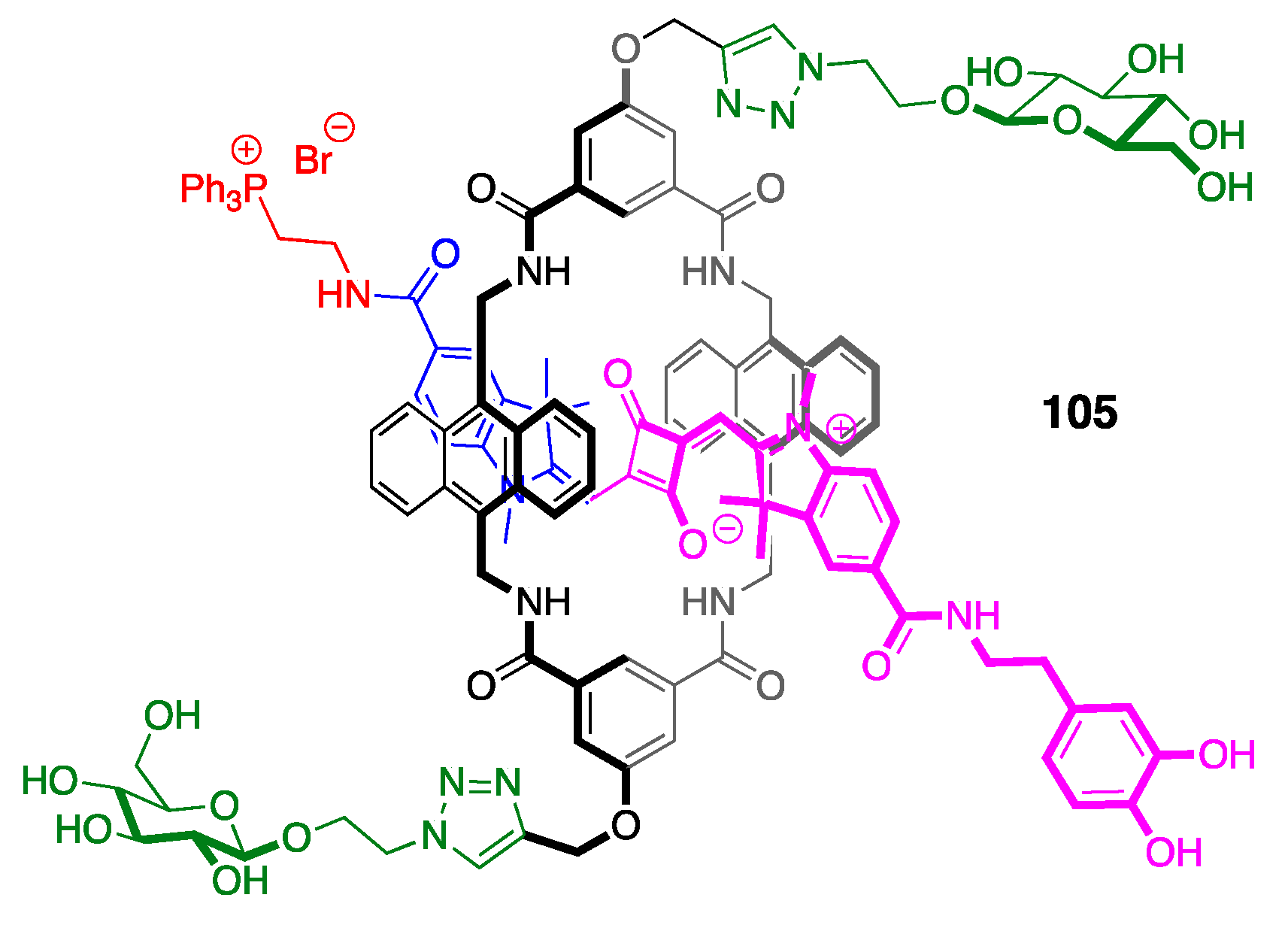
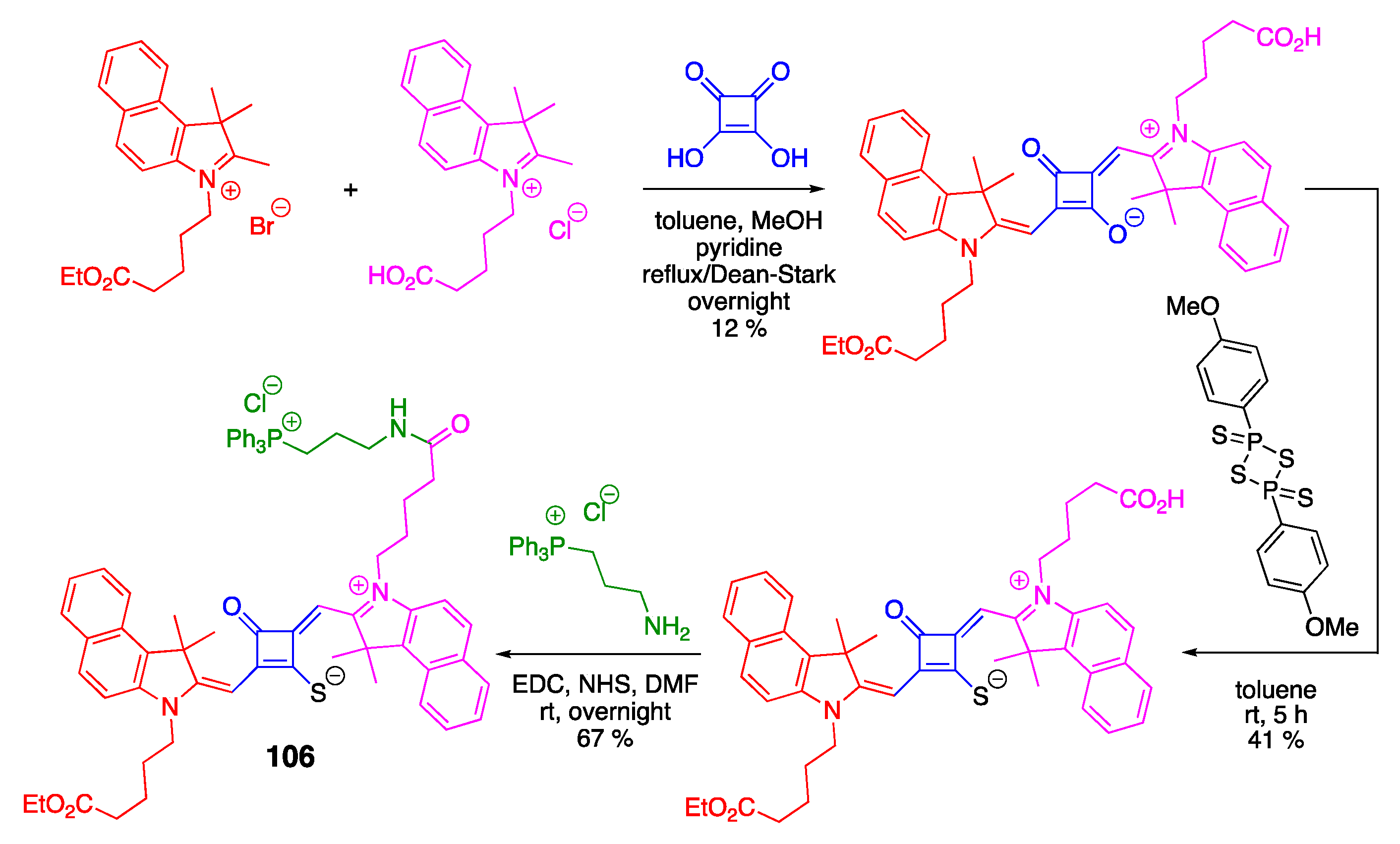
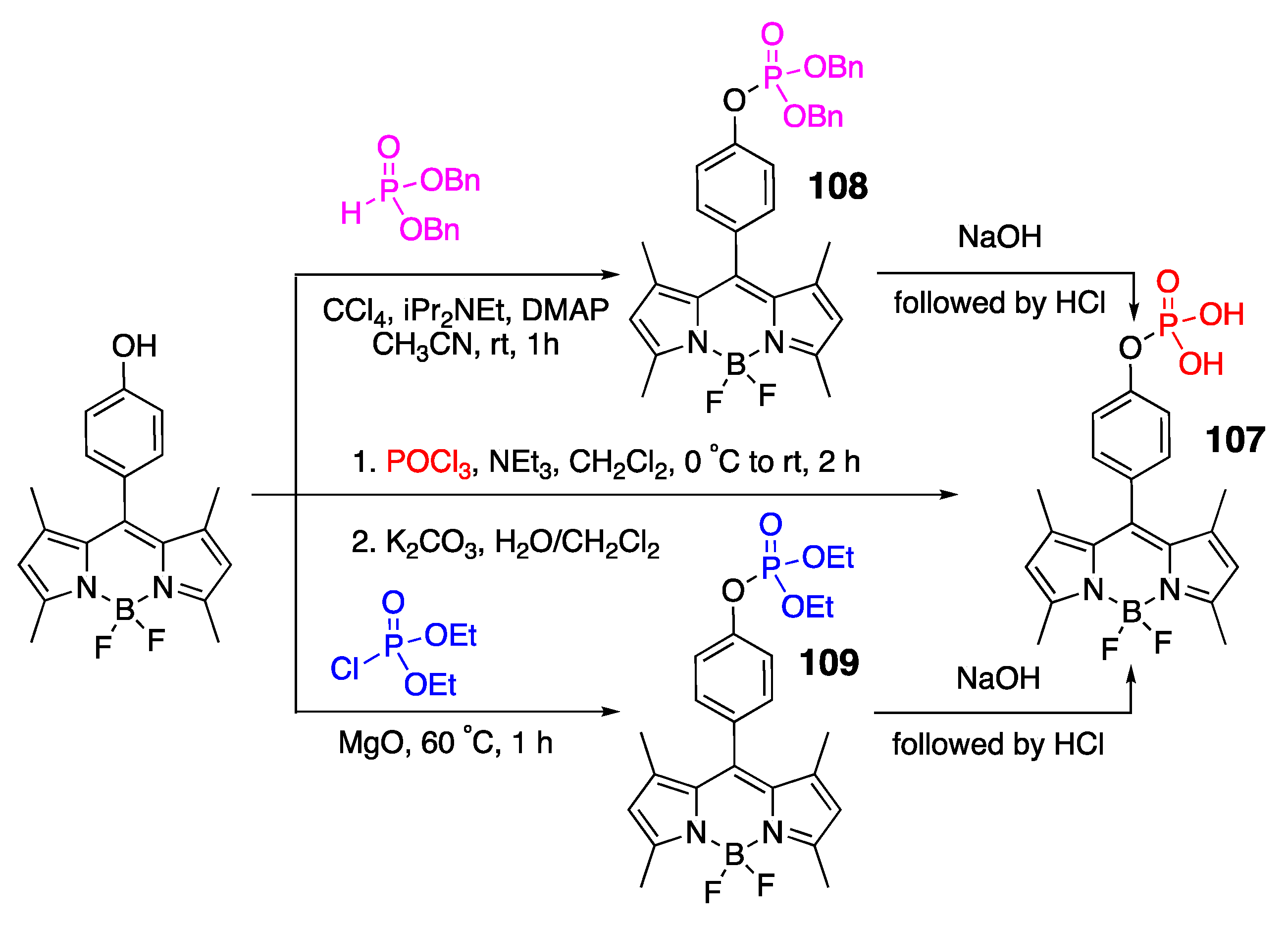






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favret, J.M.; Dzyuba, S.V. Synthetic Approaches Toward Phosphorus-Containing BODIPY and Squaraine Dyes: Enhancing Versatility of Small-Molecule Fluorophores. Molecules 2025, 30, 116. https://doi.org/10.3390/molecules30010116
Favret JM, Dzyuba SV. Synthetic Approaches Toward Phosphorus-Containing BODIPY and Squaraine Dyes: Enhancing Versatility of Small-Molecule Fluorophores. Molecules. 2025; 30(1):116. https://doi.org/10.3390/molecules30010116
Chicago/Turabian StyleFavret, Jeanne M., and Sergei V. Dzyuba. 2025. "Synthetic Approaches Toward Phosphorus-Containing BODIPY and Squaraine Dyes: Enhancing Versatility of Small-Molecule Fluorophores" Molecules 30, no. 1: 116. https://doi.org/10.3390/molecules30010116
APA StyleFavret, J. M., & Dzyuba, S. V. (2025). Synthetic Approaches Toward Phosphorus-Containing BODIPY and Squaraine Dyes: Enhancing Versatility of Small-Molecule Fluorophores. Molecules, 30(1), 116. https://doi.org/10.3390/molecules30010116





