A Spontaneous Complexation–Exfoliation Strategy for a Flexible Anode Towards Superior Durable and Ultrafast Lithium-Ion Batteries
Abstract
:1. Introduction
2. Results
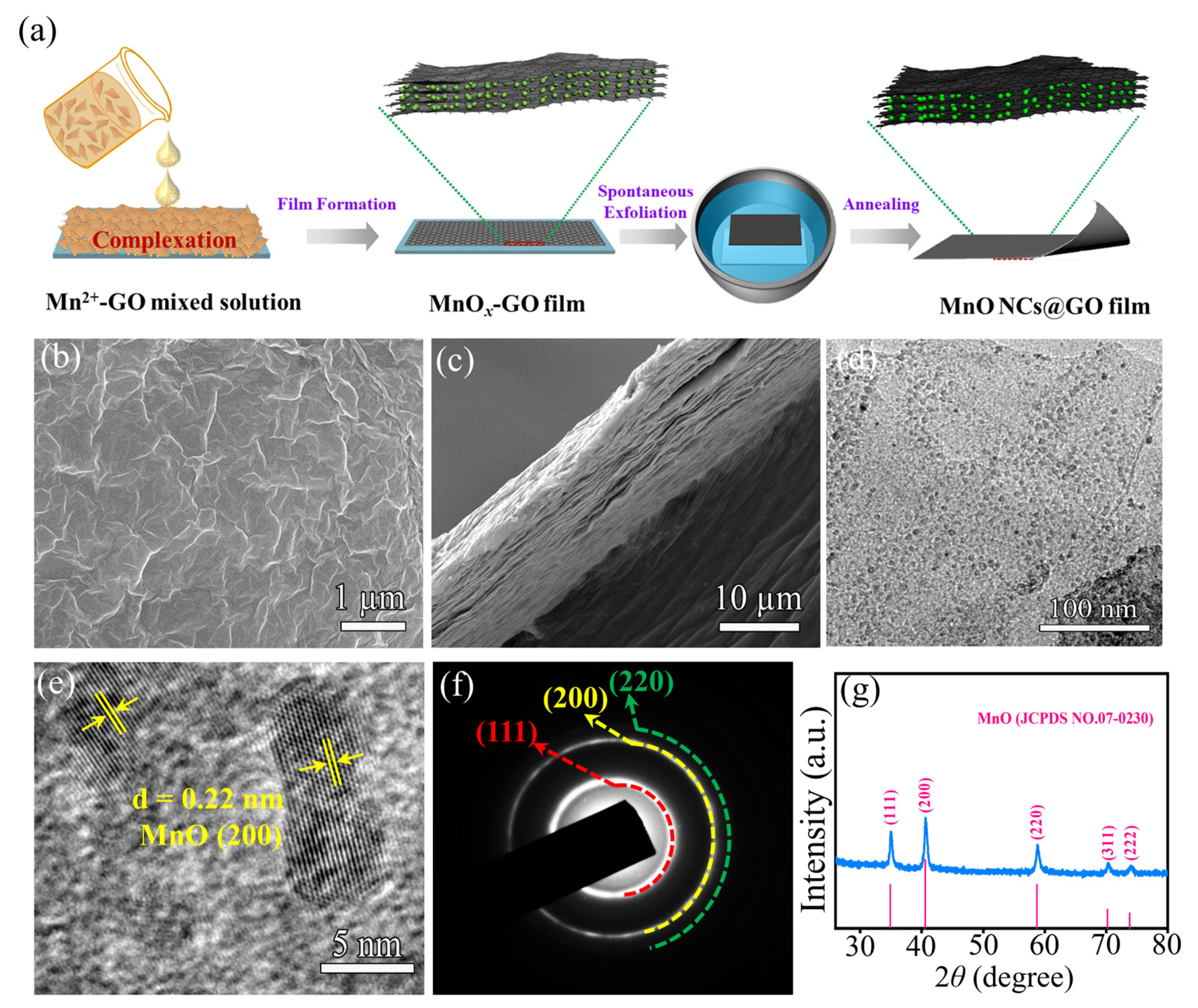
2.1. Synthesis and Structural Characterization
2.2. Electrochemical Properties
2.3. Electrochemical Reaction Mechanism
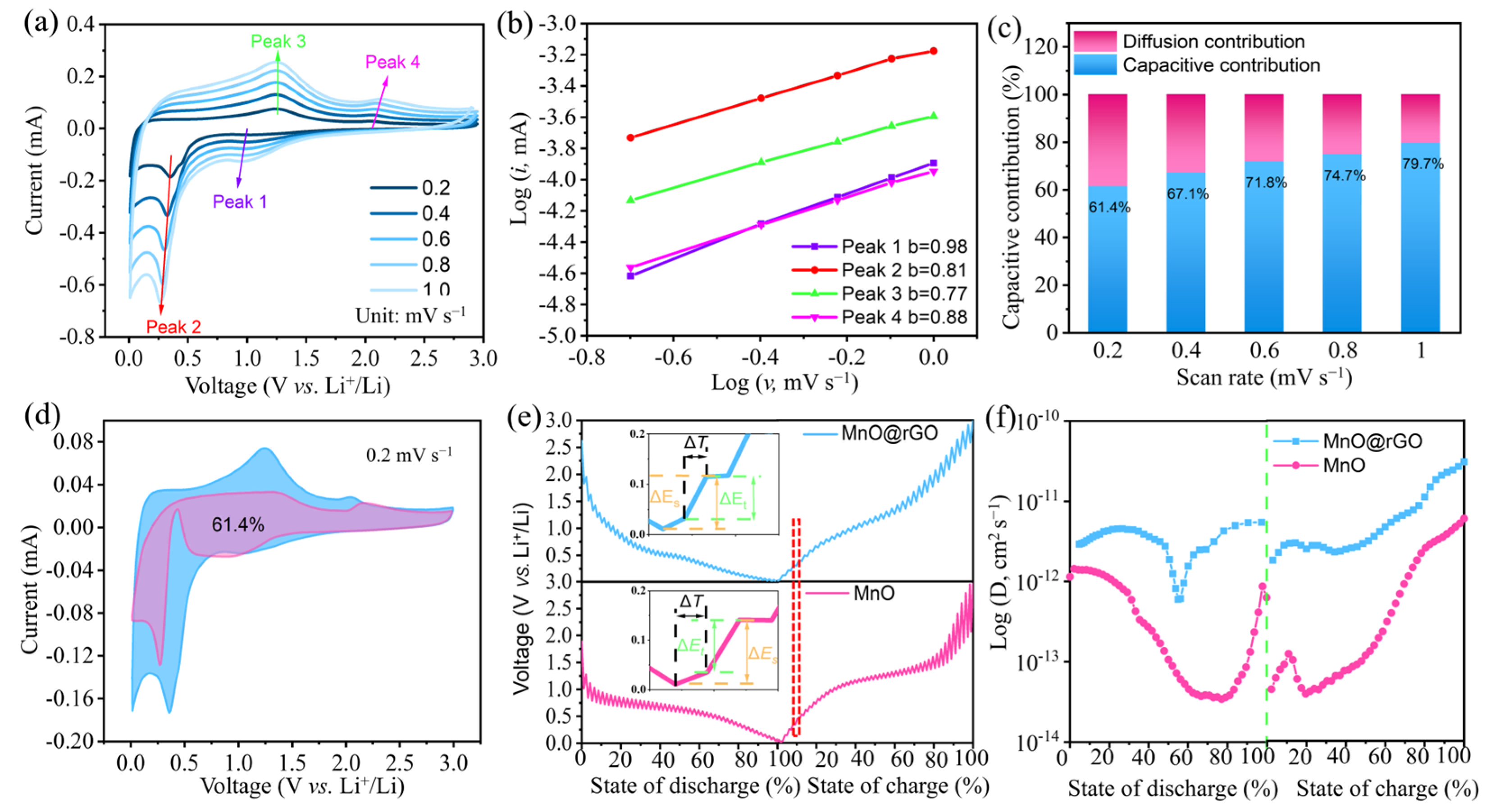
2.4. Kinetic Analysis
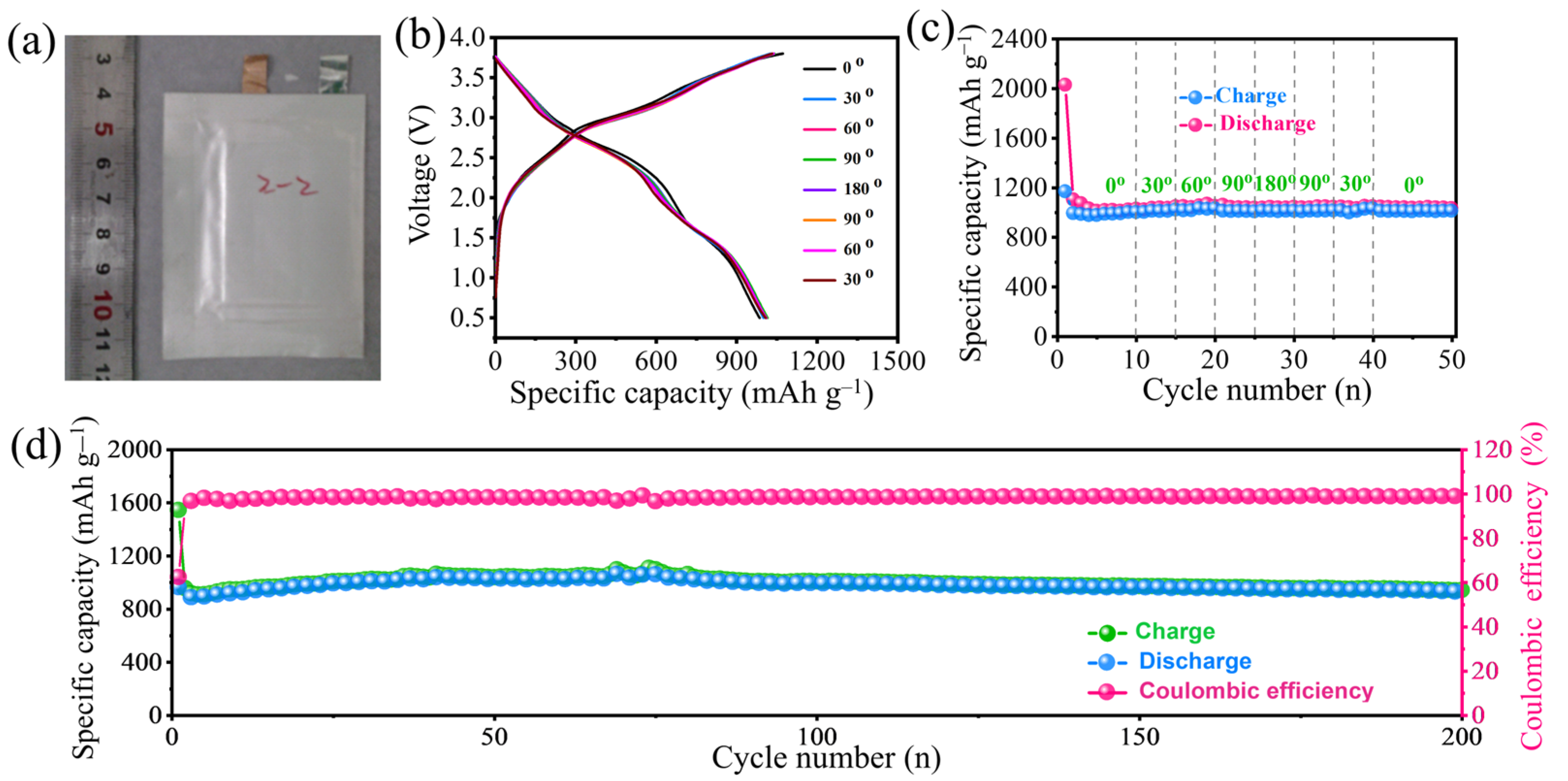
2.5. Flexible Full-Cell Assembly and Electrochemical Properties
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; He, K.E.; Chen, G.; Leow, W.R.; Chen, X. Nature-inspired structural materials for flexible electronic devices. Chem. Rev. 2017, 117, 12893–12941. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.H.Q.; Yang, J.L.; Wang, X.D.; Deng, Y.R.; Han, P.; Yuan, N.; Zhang, L.; Feng, M.; Wang, C.A.; Liu, R.P. Carbon-based flexible self-supporting cathode for lithium-sulfur batteries: Progress and perspective. Carbon Energy 2021, 3, 271–302. [Google Scholar] [CrossRef]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of Vital Signs with Flexible and Wearable Medical Devices. Adv. Mater. 2016, 28, 4373. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Ren, J.; Weng, W.; Peng, H. Advances in wearable fiber-shaped lithium-ion batteries. Adv. Mater. 2016, 28, 4524–4531. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.H.; Nguyen, Q.H.; So, S.; Hur, J. Efficient TiC-C hybrid conductive matrix for ZnTe anode in Lithium-ion storage. Appl. Surf. Sci. 2020, 534, 147679. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhang, X.; Xu, Y.N.; Wang, L.; Li, Z.; Li, C.; Wang, K.; Sun, X.Z.; An, Y.B.; Wu, Z.S.; et al. 2D Graphene/MnO Heterostructure with Strongly Stable Interface Enabling High-Performance Flexible Solid-state Lithium-Ion Capacitors. Adv. Funct. Mater. 2022, 32, 2202342. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, X.; Yang, Y.G.; Wang, X.; Yao, J.N. Electrospinning fabrication of flexible, foldable, and twistable Sb2S3/TiO2/C nanofiber anode for lithium ion batteries. Chem. Eng. J. 2021, 413, 127400. [Google Scholar] [CrossRef]
- Qian, G.Y.; Liao, X.B.; Zhu, Y.X.; Pan, F.; Chen, X.; Yang, Y. Designing Flexible Lithium-Ion Batteries by Structural Engineering. ACS Energy Lett. 2019, 4, 690–701. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Wu, Q.; Zhang, Q.; Chen, H.; Jing, H.; Wang, J.; Mi, S.-B.; Rogach, A.L.; Niu, C. Encapsulating silica/antimony into porous electrospun carbon nanofibers with robust structure stability for high-efficiency lithium storage. ACS Nano 2018, 12, 3406–3416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Bai, M.; Li, S.W.; Tang, X.Y.; Wu, W.W.; Sun, C.C.; Yin, X.K.; Zhou, J.; Yuan, S.; Ma, Y. Integrated Thin Film Battery Design for Flexible Lithium Ion Storage: Optimizing the Compatibility of the Current Collector-Free Electrodes. Adv. Funct. Mater. 2019, 29, 1903542. [Google Scholar] [CrossRef]
- Opra, D.P.; Sinebryukhov, S.L.; Modin, E.B.; Sokolov, A.A.; Podgorbunsky, A.B.; Ziatdinov, A.M.; Ustinov, A.Y.; Mayorov, V.Y.; Gnedenkov, S.V. Manganese, Fluorine, and Nitrogen Co-Doped Bronze Titanium Dioxide Nanotubes with Improved Lithium-Ion Storage Properties. Batteries 2023, 9, 229. [Google Scholar] [CrossRef]
- Toigo, C.; Arbizzani, C.; Pettinger, K.-H.; Biso, M. Study on Different Water-Based Binders for Li4Ti5O12 Electrodes. Molecules 2020, 25, 2443. [Google Scholar] [CrossRef]
- Opra, D.P.; Sokolov, A.A.; Sinebryukhov, S.L.; Tkachenko, I.A.; Ziatdinov, A.M.; Gnedenkov, S.V. Electronic Structure, Optical and Magnetic Properties of Oxygen-Deficient Gray TiO2–δ(B). Inorganics 2022, 10, 184. [Google Scholar] [CrossRef]
- Wang, S.H.; Teng, J.; Xie, Y.Y.; Wei, Z.W.; Fan, Y.A.; Jiang, J.J.; Wang, H.P.; Liu, H.G.; Wang, D.W.; Su, C.Y. Embedding CoO nanoparticles in a yolk–shell N-doped porous carbon support for ultrahigh and stable lithium storage. J. Mater. Chem. 2019, 7, 4036–4046. [Google Scholar] [CrossRef]
- Chang, X.Q.; Zhu, Q.Z.; Zhao, Q.; Zhang, P.; Sun, N.; Soomro, R.A.; Wang, X.X.; Xu, B. 3D Porous Co3O4/MXene Foam Fabricated via a Sulfur Template Strategy for Enhanced Li/K-Ion Storage. ACS Appl. Mater. Interfaces 2023, 15, 7999–8009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Lu, B.R.; Cao, F.H.; Yu, Z.L.; Cong, H.P.; Yu, S.H. Hierarchically structured Co3O4@carbon porous fibers derived from electrospun ZIF-67/PAN nanofibers as anodes for lithium ion batteries. J. Mater. Chem. A 2018, 6, 12962. [Google Scholar] [CrossRef]
- Zhang, S.L.; Guan, B.Y.; Wu, H.B.; Lou, X.W. Metal-Organic Framework-Assisted Synthesis of Compact Fe2O3 Nanotubes in Co3O4 Host with Enhanced Lithium Storage Properties. Nano-Micro Lett. 2018, 10, 44. [Google Scholar] [CrossRef]
- Guo, S.S.; Koketsu, T.; Hu, Z.W.; Zhou, J.; Kuo, C.Y.; Lin, H.J.; Chen, C.T.; Strasser, P.; Sui, L.J.; Xie, Y.; et al. Mo-Incorporated Magnetite Fe3O4 Featuring Cationic Vacancies Enabling Fast Lithium Intercalation for Batteries. Small 2022, 18, 2203835. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Shen, J.D.; Li, F.K.; Wang, Z.S.; Zhang, D.C.; Zuo, S.Y.; Liu, J. Fe3O4@C Nanotubes Grown on Carbon Fabric as a Free-Standing Anode for High-Performance Li-Ion Batteries. Chem. Eur. J. 2020, 26, 14708–14714. [Google Scholar] [CrossRef]
- Wang, J.K.; Wang, H.K.; Yao, T.H.; Liu, T.; Tian, Y.P.; Li, C.; Li, F.; Meng, L.J.; Cheng, Y.H. Porous N-doped carbon nanoflakes supported hybridized SnO2/Co3O4 nanocomposites as high-performance anode for lithium-ion batteries. J. Colloid Interf. Sci. 2020, 560, 546–554. [Google Scholar] [CrossRef]
- Zhan, G.H.; Liao, W.H.; Hu, Q.Q.; Wu, X.H.; Huang, X.Y. Rational Engineering of p-n Heterogeneous ZnS/SnO2 Quantum Dots with Fast Ion Kinetics for Superior Li/Na-Ion Battery. Small 2023, 19, 2300534. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.R.; Hamann, H.J.; Mitchell, G.M.; Ortalan, V.; Gribble, D.; Xiong, B.; Pol, V.G.; Ramachandran, P.V. Interconnected Sn@SnO2 Nanoparticles as an Anode Material for Lithium-Ion Batteries. ACS Appl. Nano Mater. 2023, 6, 11070–11076. [Google Scholar] [CrossRef]
- Xu, L.Q.Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Li, L. P-Doped Ni/NiO Heterostructured Yolk-Shell Nanospheres Encapsulated in Graphite for Enhanced Lithium Storage. Small 2022, 18, 2105897. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zong, L.S.; Zhang, Z.J.; Li, J.X.; Wu, H.Z.; Cui, Z.Y.; Kang, J.L. Oxygen vacancies activated porous MnO/graphene submicron needle arrays for high-capacity lithium-ion batteries. Carbon 2022, 190, 402–411. [Google Scholar] [CrossRef]
- Lin, J.; Zeng, C.H.; Lin, X.M.; Xu, C.; Xu, X.; Luo, Y.F. Metal-Organic Framework-Derived Hierarchical MnO/Co with Oxygen Vacancies toward Elevated-Temperature Li-Ion Battery. ACS Nano 2021, 15, 4594–4607. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Dong, A.Q.; Guo, Z.X.; Ye, L.; Cui, Y.H.; Guo, M.Y.; Zhao, L.J.; Jiang, Q. MnO/Mn2O3 Nanowires Coated by Porous N-Doped Carbon for Long-Cycle and High-Rate Lithium-Ion Batteries. ACS Appl. Nano Mater. 2020, 3, 5612–5624. [Google Scholar] [CrossRef]
- Zhong, M.; Li, L.L.; Zhao, K.; Peng, H.; Xu, S.X.; Su, B.T.; Wang, D.H. Metal–organic framework-engaged synthesis of core–shell MoO2/ZnSe@N-C nanorods as anodes in high-performance lithium-ion batteries. New J. Chem. 2021, 45, 12064–12070. [Google Scholar] [CrossRef]
- Lu, X.; Wang, P.; Liu, K.; Niu, C.M.; Wang, H.K. Encapsulating nanoparticulate Sb/MoOx into porous carbon nanofibers via electrospinning for efficient lithium storage. Chem. Eng. J. 2018, 336, 701–709. [Google Scholar] [CrossRef]
- Li, Q.H.; Zhang, W.; Peng, J.; Zhang, W.; Liang, Z.X.; Wu, J.W.; Feng, J.J.; Li, H.; Huang, S.M. Metalorganic framework derived ultrafine Sb@Porous carbon octahedron via in situ substitution for high-performance sodium-ion batteries. ACS Nano 2021, 15, 15104–15113. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.H.; Peng, J.; Zhang, W.; Wang, L.X.; Liang, Z.X.; Wang, G.; Wu, J.W.; Fan, W.B.; Li, H.; Wang, J.L.; et al. Manipulating the polytellurides of metallic telluride for ultra-stable potassium-ion storage: A case study of carbon-confined CoTe2 nanofibers. Adv. Energy Mater. 2023, 16, 2300150. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.W.; Wong, K.W.; Zhang, W.; Chen, T.; Zhao, W.; Huang, S.M. Rational design of embedded CoTe2 nanoparticles in freestanding N-doped multichannel carbon fibers for sodium-ion batteries with ultralong cycle lifespan. ACS Appl. Mater. Interfaces 2021, 13, 34134–34144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.T.; Chen, C.J.; Li, Z.; Huang, Y.H.; Hu, X.L. Flexible and Binder-Free Electrodes of Sb/rGO and Na3V2(PO4)3/rGO Nanocomposites for Sodium-Ion Batteries. Small 2015, 11, 3822–3829. [Google Scholar] [CrossRef]
- Chen, M.H.; Li, T.Y.; Li, Y.; Liang, X.Q.; Sun, W.F.; Chen, Q.G. Rational Design of a MnO Nanoparticle-Embedded Carbon Nanofiber Interlayer for Advanced Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 10793–10801. [Google Scholar] [CrossRef]
- Li, Q.H.; Zhang, W.; Peng, J.; Yu, D.D.; Liang, Z.X.; Zhang, W.; Wang, G.; Wu, J.W.; Li, H.; Huang, S.M. Nanodot-in-nanofiber structured carbon-confined Sb2Se3 crystallites for fast and durable sodium storage. Adv. Funct. Mater. 2022, 32, 2112776. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Zhang, W.; Peng, J.; Li, Q.H.; Liang, Z.X.; Wang, G.Y.; Wang, J.L.; Wang, G.; Huang, Z.J.; Huang, S.M. Synergistic Regulation of Polyselenide Dissolution and Na-Ion Diffusion of Se-Vacancy-Rich Bismuth Selenide toward Ultrafast and Durable Sodium-Ion Batteries. Adv. Energy Mater. 2024, 14, 2402110. [Google Scholar] [CrossRef]
- Wang, J.L.; Huang, Z.J.; Zhang, W.; Li, Q.H.; Liang, Z.X.; Lu, J.J.; Lin, Z.Y.; Wang, G.; Wu, J.X.; Huang, S.M. Balancing Graphitic Nanodomains and Heteroatom Doping in Hard Carbons Toward High Capacity and Durable Potassium-Ion Battery Anodes. Adv. Funct. Mater. 2024, 34, 2409937. [Google Scholar] [CrossRef]
- Yu, J.; Luo, J.D.; Zhang, H.; Zhang, Z.; Wei, J.C.; Yang, H.Y. Renewable agaric-based hierarchically porous cocoon-like MnO/Carbon composites enable high-energy and high-rate Li-ion batteries. Electrochim. Acta 2019, 322, 134757. [Google Scholar] [CrossRef]
- Yang, C.; Yao, Y.; Lian, Y.B.; Chen, Y.J.; Shah, A.; Zhao, X.H.; Chen, M.Z.; Peng, Y.; Deng, Z. A Double-Buffering Strategy to Boost the Lithium Storage of Botryoid MnOx/C Anodes. Small 2019, 15, 1900015. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Li, H.P.; Xu, G.B.; Liu, X.; Zhang, Q.; Yang, L.W.; Cao, J.X.; Wei, X.L. Porous N-doped carbon sheets wrapped MnO in 3D carbon networks as high-performance anode for Li-ion batteries. Electrochim. Acta 2020, 342, 136115. [Google Scholar] [CrossRef]
- Hou, C.X.; Wang, J.; Zhang, W.B.; Li, J.J.; Zhang, R.H.; Zhou, J.J.; Fan, Y.Q.; Li, D.J.; Dang, F.; Liu, J.Q.; et al. Interfacial Superassembly of Grape-Like MnO-Ni@C Frameworks for Superior Lithium Storage. ACS Appl. Mater. Interfaces 2020, 12, 13770–13780. [Google Scholar] [CrossRef]
- Chen, P.H.; Zhou, W.Y.; Xiao, Z.J.; Li, S.Q.; Chen, H.L.; Wang, Y.C.; Wang, Z.B.; Xi, W.; Xia, X.G.; Xie, S.S. In situ anchoring MnO nanoparticles on self-supported 3D interconnected graphene scroll framework: A fast kinetics boosted ultrahigh-rate anode for Li-ion capacitors. Energy Storage Mater. 2020, 33, 298–308. [Google Scholar] [CrossRef]
- Liang, Z.X.; Li, Q.H.; Zhang, W.; Yu, D.D.; Zhang, W.; Wu, J.W.; Wang, G.Y.; Fan, W.B.; Wang, J.L.; Huang, S.M. Pomegranate-inspired porous SnSe/ZnSe@C anode: A stress-buffer nanostructure for fast and ultrastable sodium-ion storage. J. Energy Chem. 2020, 75, 369–377. [Google Scholar] [CrossRef]
- Zhao, W.M.; Zhang, W.; Lei, Y.X.; Wang, L.X.; Wang, G.Y.; Wu, J.W.; Fan, W.B.; Huang, S.M. Dual-Type Carbon Confinement Strategy: Improving the Stability of CoTe2 Nanocrystals for Sodium-Ion Batteries with a Long Lifespan. ACS Appl. Mater. Interfaces 2022, 14, 6801–6809. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.H.; Yu, D.D.; Peng, J.; Zhang, W.; Huang, J.L.; Liang, Z.X.; Wang, G.Y.; Li, H.X.; Xiong, S.Y.; Wang, J.Z.; et al. Efficient Polytelluride Anchoring for Ultralong-Life Potassium Storage: Combined Physical Barrier and Chemisorption in Nanogrid-in-Nanofiber. Nano-Micro Lett. 2024, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Xu, S.; Lv, Z.; Zhang, S.; Gao, Y.; Lin, T.; Huang, F. Toward Extremely Fast Charging Through Boosting Intercalative Redox Pseudocapacitance: A SbCrSe3 Anode for Large and Fast Sodium Storage. Adv. Energy Mater. 2022, 13, 2203187. [Google Scholar] [CrossRef]
- Dong, C.; Shao, H.; Zhou, Y.; Du, W.; Li, L.; Sun, J.; Yan, Z.; Hu, Z.; Chou, S.; Jiang, F. Construction of ZnS/Sb2S3 Heterojunction as an Ion-Transport Booster toward High Performance Sodium Storage. Adv. Funct. Mater. 2022, 33, 2211864. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, H.; Zhang, J.; Zhao, P.; Li, Y.; Liu, Z.; Zhang, H. A Spontaneous Complexation–Exfoliation Strategy for a Flexible Anode Towards Superior Durable and Ultrafast Lithium-Ion Batteries. Molecules 2025, 30, 133. https://doi.org/10.3390/molecules30010133
Chu H, Zhang J, Zhao P, Li Y, Liu Z, Zhang H. A Spontaneous Complexation–Exfoliation Strategy for a Flexible Anode Towards Superior Durable and Ultrafast Lithium-Ion Batteries. Molecules. 2025; 30(1):133. https://doi.org/10.3390/molecules30010133
Chicago/Turabian StyleChu, Heying, Jingchuan Zhang, Pengsen Zhao, Yong Li, Zhaoxia Liu, and Hongzhou Zhang. 2025. "A Spontaneous Complexation–Exfoliation Strategy for a Flexible Anode Towards Superior Durable and Ultrafast Lithium-Ion Batteries" Molecules 30, no. 1: 133. https://doi.org/10.3390/molecules30010133
APA StyleChu, H., Zhang, J., Zhao, P., Li, Y., Liu, Z., & Zhang, H. (2025). A Spontaneous Complexation–Exfoliation Strategy for a Flexible Anode Towards Superior Durable and Ultrafast Lithium-Ion Batteries. Molecules, 30(1), 133. https://doi.org/10.3390/molecules30010133





