Rare-Earth Pretreatment Improves Performance of Reactive Dye Argazol Navy Blue on Banana-Fiber Fabric
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Laboratory Equipment
2.3. Pretreatment Process
2.4. Rare-Earth Pretreatment
2.5. Dyeing Process
2.6. Percentage of Depletion
2.7. Fixation Rate
2.8. Color Fastness Test
2.9. Color Difference Test
3. Discussion
3.1. Factors Affecting Dyeing Performance on Banana-Fiber Fabrics
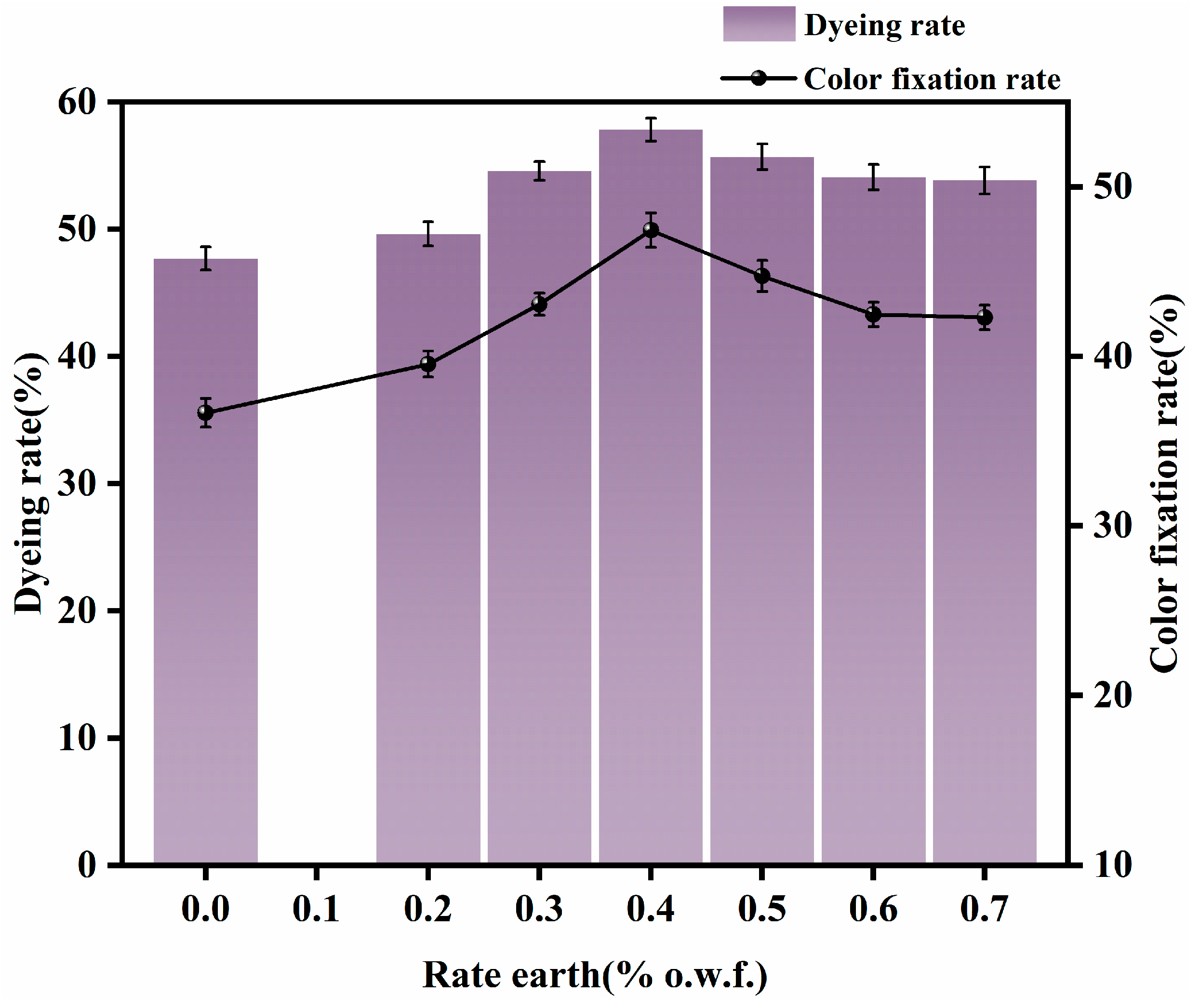
3.1.1. Appropriate Amount of Rare Earth
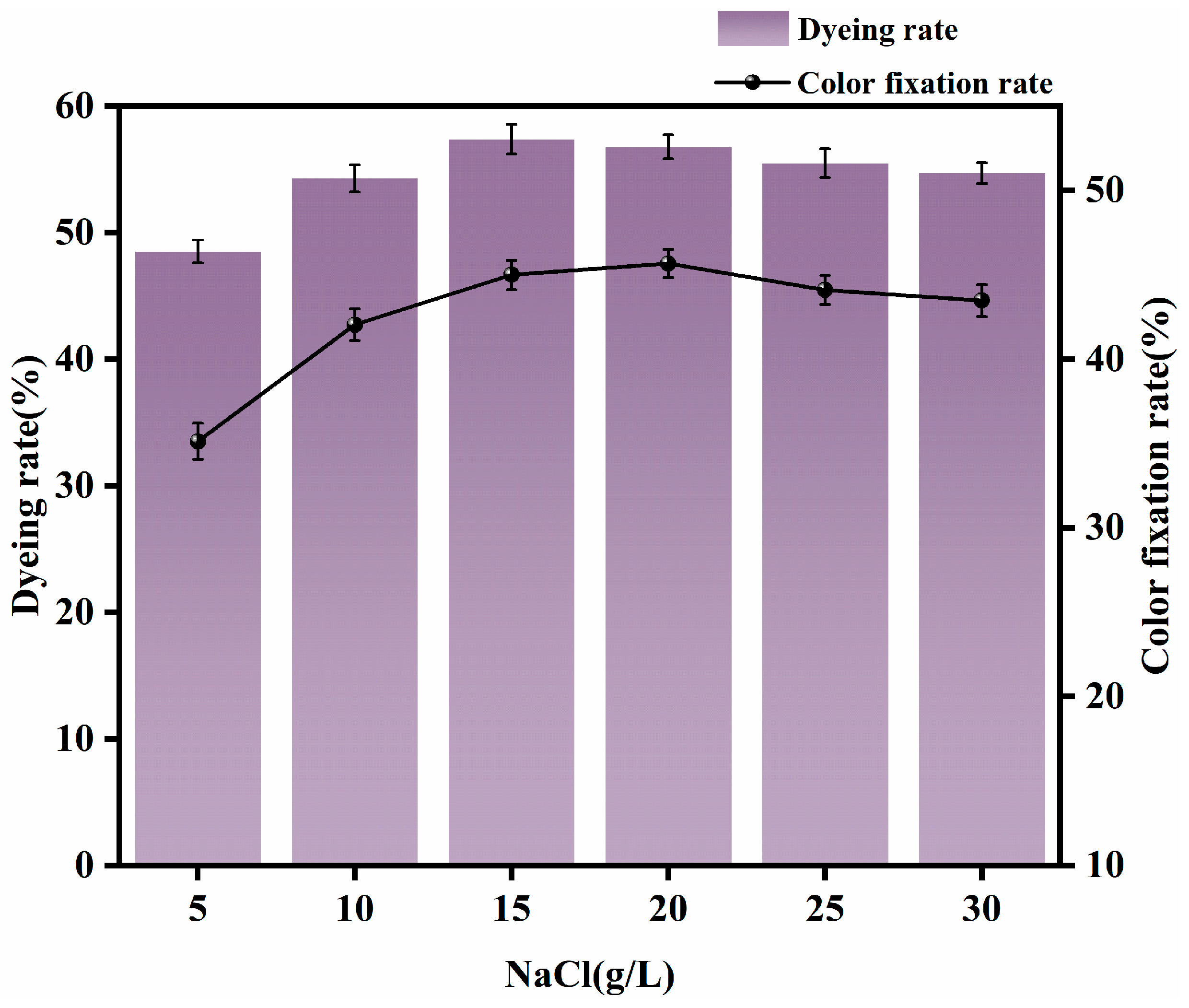
3.1.2. Appropriate Amount of NaCl
3.1.3. Appropriate Amount of Na2CO3
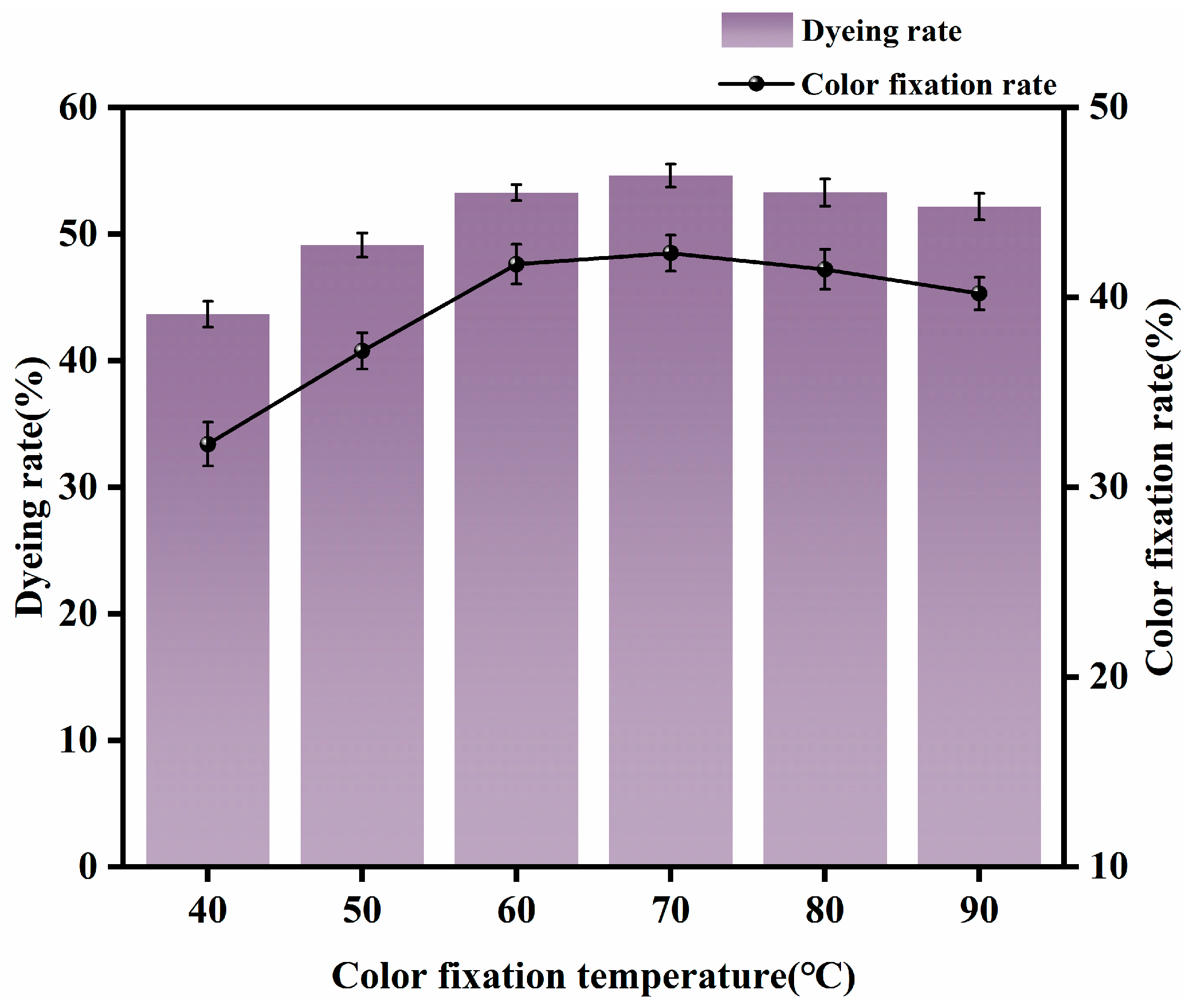
3.1.4. Appropriate Fixing Temperature
3.1.5. Appropriate Fixation Time
3.1.6. Orthogonal Optimization of Dyeing Process for Banana-Fiber Fabric Using Argazol Navy Blue
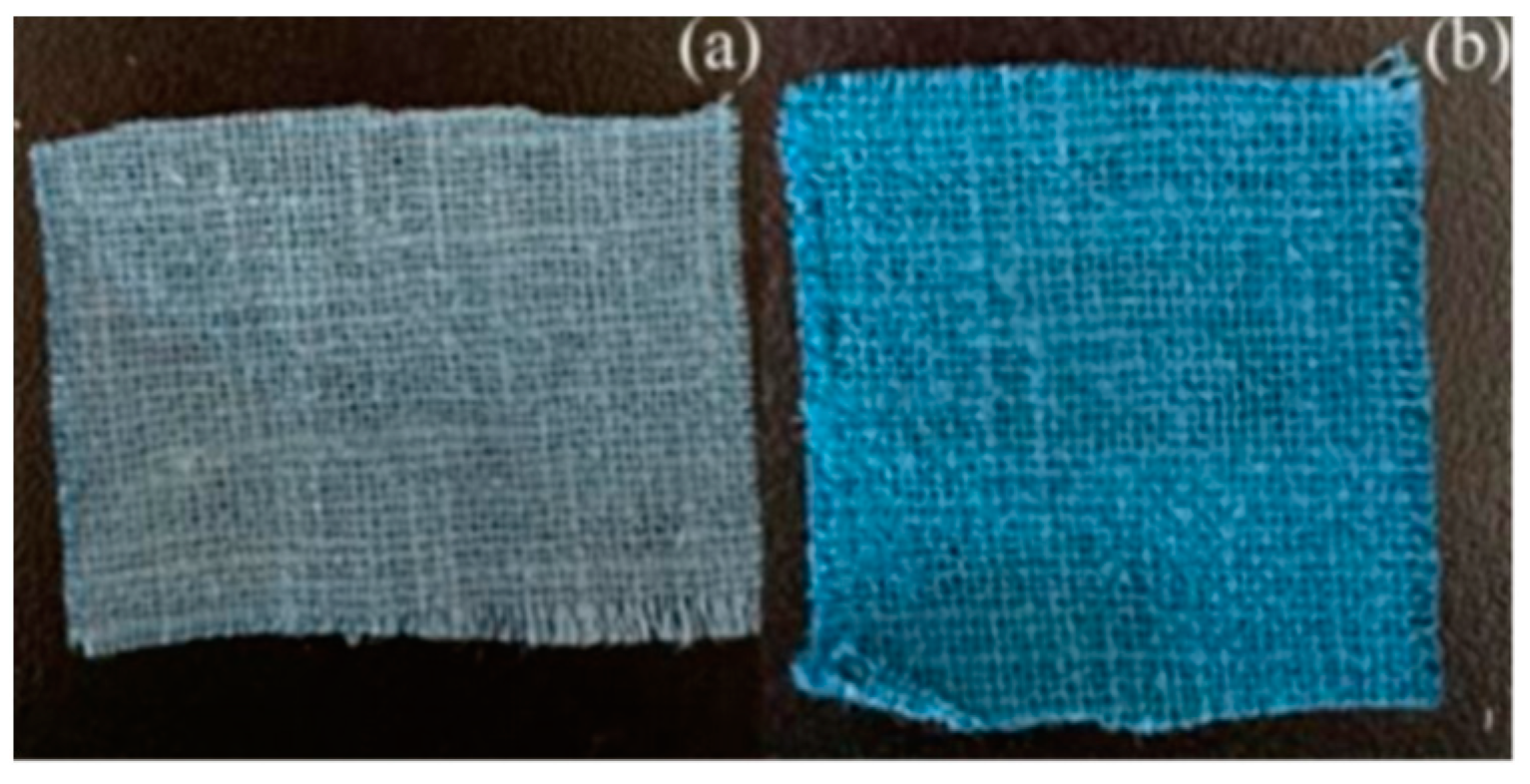
3.2. Comparison of Dyeing Properties of Banana-Fiber Fabric Dyed with Argazol Navy Blue
3.2.1. Color Fastness
3.2.2. Chromatic Aberration
3.2.3. ATR-FTIR Spectroscopy
3.2.4. Scanning Electron Microscope Analysis
3.2.5. X-Ray Energy Spectrum Analysis (EDS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monzón, M.D.; Paz, R.; Verdaguer, M.; Suárez, L.; Badalló, P.; Ortega, Z.; Diaz, N. Experimental Analysis and Simulation of Novel Technical Textile Reinforced Composite of Banana Fibre. Materials 2019, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.N.; Nayab-Ul-Hossain, A.K.M.; Hasan, N.; Rahman, M.I.; Mominul Alam, S.M. Physico-mechanical Properties of Naturally Dyed Jute-banana Hybrid Fabrics. J. Nat. Fibers 2021, 19, 8616–8627. [Google Scholar] [CrossRef]
- Essien, J.; Akpan, E.; Essien, E. Studies on mould growth and biomass production using waste banana peel. Bioresour. Technol. 2005, 96, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Z.; Morón, M.; Monzón, M.D.; Badalló, P.; Paz, R. Production of Banana Fiber Yarns for Technical Textile Reinforced Composites. Materials 2016, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.O.B.; de Araújo Nunes, M.A. Acoustic performance of the banana pseudostem fiber. Appl. Acoust. 2022, 191, 108657. [Google Scholar] [CrossRef]
- Cruz, J.; Fangueiro, R. Surface Modification of Natural Fibers: A Review. Procedia Eng. 2016, 155, 285–288. [Google Scholar] [CrossRef]
- Paramasivam, S.K.; Panneerselvam, D.; Sundaram, D.; Shiva, K.N.; Subbaraya, U. Extraction, Characterization and Enzymatic Degumming of Banana Fiber. J. Nat. Fibers 2020, 19, 1333–1342. [Google Scholar] [CrossRef]
- Chattopadhyay, S.K.; Khandal, R.K.; Uppaluri, R.; Ghoshal, A.K. Mechanical, thermal, and morphological properties of maleic anhydride-g-polypropylene compatibilized and chemically modified banana-fiber-reinforced polypropylene composites. J. Appl. Polym. Sci. 2010, 117, 1731–1740. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Plant fibre based bio-composites: Sustainable and renewable green materials. Renew. Sustain. Energy Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Mukhopadhyay, S. Banana Fibers—Variability and Fracture Behaviour.pdf. J. Eng. Fibers Fabr. 2008, 3. [Google Scholar] [CrossRef]
- Milani, M.D.Y.; Samarawickrama, D.S.; Dharmasiri, G.P.C.A.; Kottegoda, I.R.M. Study the Structure, Morphology, and Thermal Behavior of Banana Fiber and Its Charcoal Derivative from Selected Banana Varieties. J. Nat. Fibers 2016, 13, 332–342. [Google Scholar] [CrossRef]
- Idicula, M.; Neelakantan, N.R.; Oommen, Z.; Joseph, K.; Thomas, S. A study of the mechanical properties of randomly oriented short banana and sisal hybrid fiber reinforced polyester composites. J. Appl. Polym. Sci. 2005, 96, 1699–1709. [Google Scholar] [CrossRef]
- Jayaprabha, J.S.; Brahmakumar, M.; Manilal, V.B. Banana Pseudostem Characterization and Its Fiber Property Evaluation on Physical and Bioextraction. J. Nat. Fibers 2011, 8, 149–160. [Google Scholar] [CrossRef]
- Subramanya, R.; Satyanarayana, K.G.; Shetty Pilar, B. Evaluation of Structural, Tensile and Thermal Properties of Banana Fibers. J. Nat. Fibers. 2016, 14, 485–497. [Google Scholar]
- Padam, B.S.; Tin, H.S.; Chye, F.Y.; Abdullah, M.I. Banana by-products: An under-utilized renewable food biomass with great potential. J. Food Sci. Technol. 2012, 51, 3527–3545. [Google Scholar] [CrossRef] [PubMed]
- Balda, S.; Sharma, A.; Capalash, N.; Sharma, P. Banana fibre: A natural and sustainable bioresource for eco-friendly applications. Clean Technol. Environ. Policy 2021, 23, 1389–1401. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, W.; Lv, C. Dyeing of soybean protein/flax blended yarns with reactive dyes and subsequent dye-fixation. Sci. Rep. 2022, 12, 1506. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Wickramasinghe, G.D.; Wijayapala, U.S. Study on dyeing behavior of banana fiber with reactive dyes. J. Eng. Fibers Fabr. 2019, 14. [Google Scholar] [CrossRef]
- Sheng, D.; Deng, B.; Wang, Y.; Liu, L.; Chen, X.; Xia, H.; Cao, G.; Xu, W. Isotherms and kinetics of dyeing poly-m-phenyleneisophthal-amide with Basic Red 46 based on the macro-cation dyeing mechanism. Text. Res. J. 2020, 91, 297–305. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Q.; Wang, Y.; Chen, R.; Xia, Y.; Wang, B.; Jin, W.; Wu, S.; Chen, Z.; Iqbal, A.; et al. Solvent-free mechanochemical synthesis of azo dyes. RSC Mechanochem. 2024, 1, 447–451. [Google Scholar] [CrossRef]
- Knudsen, H.H.; Wenzel, H. Environmentally friendly method in reactive dyeing of cotton. Water Sci. Technol. 1996, 33, 17–27. [Google Scholar] [CrossRef]
- Khatri, A.; Peerzada, M.H.; Mohsin, M.; White, M. A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J. Clean. Prod. 2015, 87, 50–57. [Google Scholar] [CrossRef]
- Zheng, G.H.; Bin Fu, H.; Liu, G.P. Application of rare earth as mordant for the dyeing of ramie fabrics with natural dyes. Korean J. Chem. Eng. 2011, 28, 2148–2155. [Google Scholar] [CrossRef]
- GB/T 3921.1-1997; Textiles—Tests for Colour Fastness—Part 1: General Principles. Standardization Administration of China: Beijing, China, 1997.
- GB/T 3920-2008; Textiles—Tests for Colour Fastness—Test Method for Colour Fastness to Washing. Standardization Administration of China: Beijing, China, 2008.
- GB/T 8426-1998; Textiles—Tests for Colour Fastness—Test Method for Colour Fastness to Laundering. Standardization Administration of China: Beijing, China, 1998.
- Repon, R.; Islam, M.T.; Al Mamun, A. Ecological risk assessment and health safety speculation during color fastness properties enhancement of natural dyed cotton through metallic mordants. Fash. Text. 2017, 4, 24. [Google Scholar] [CrossRef]



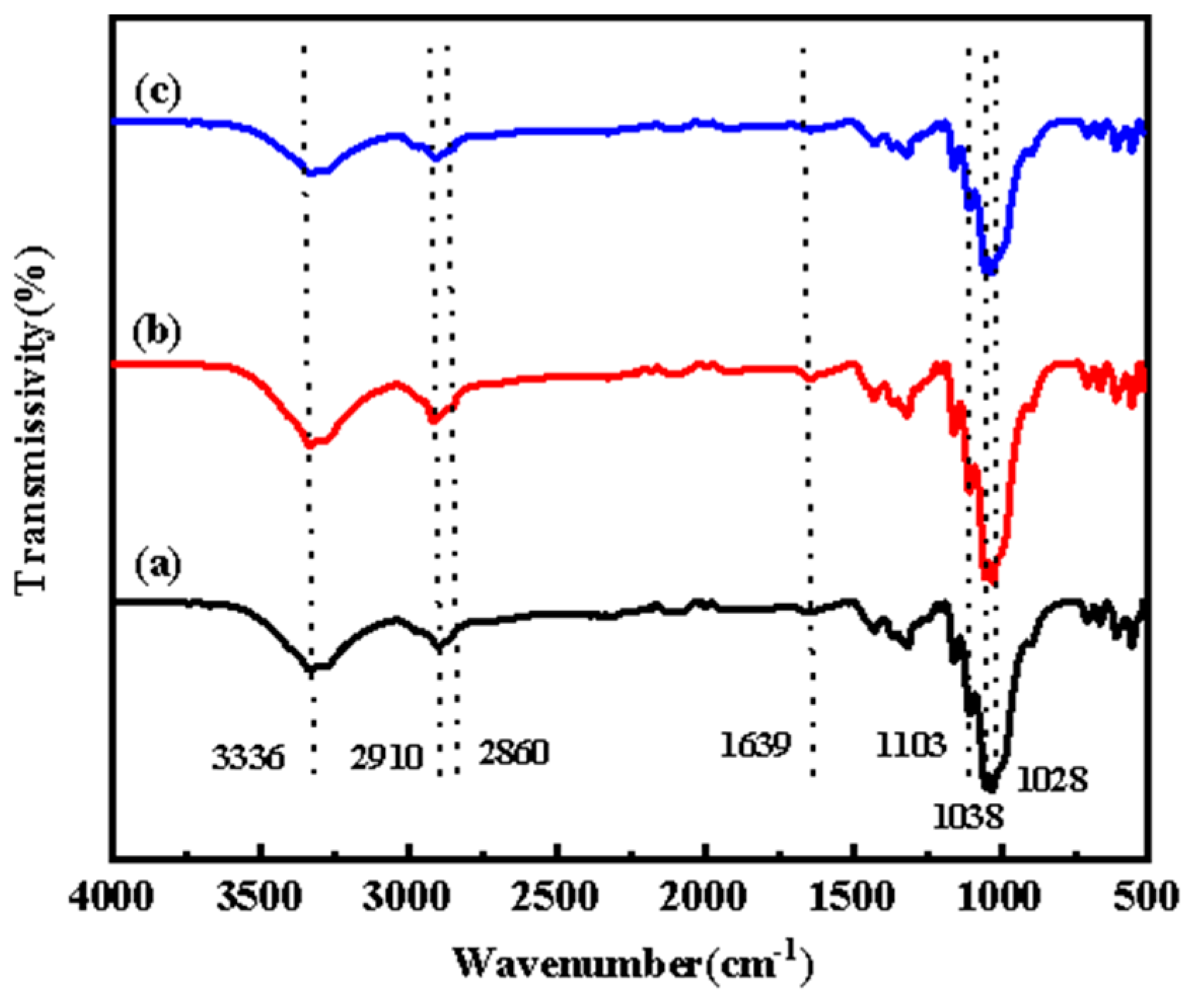
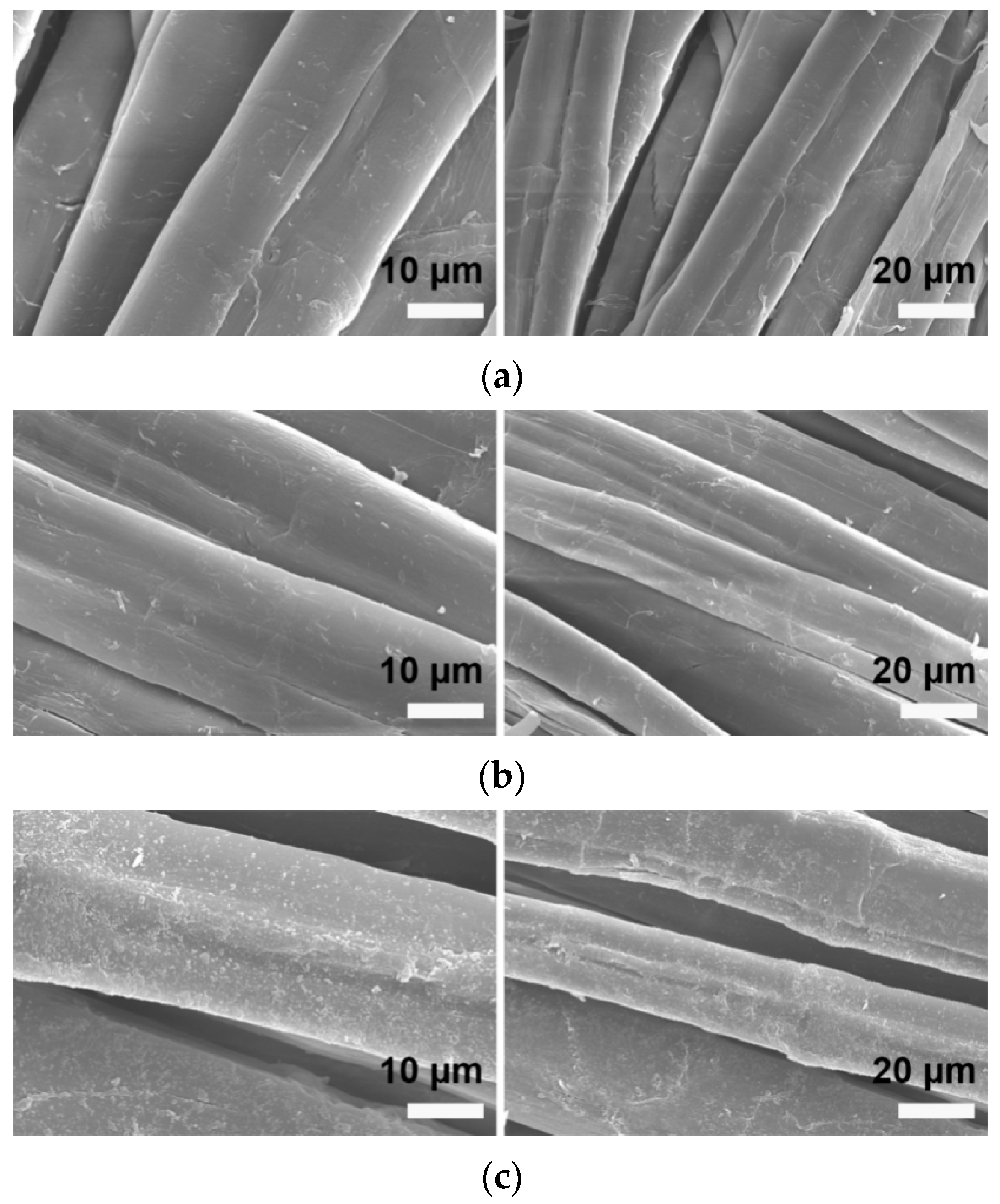
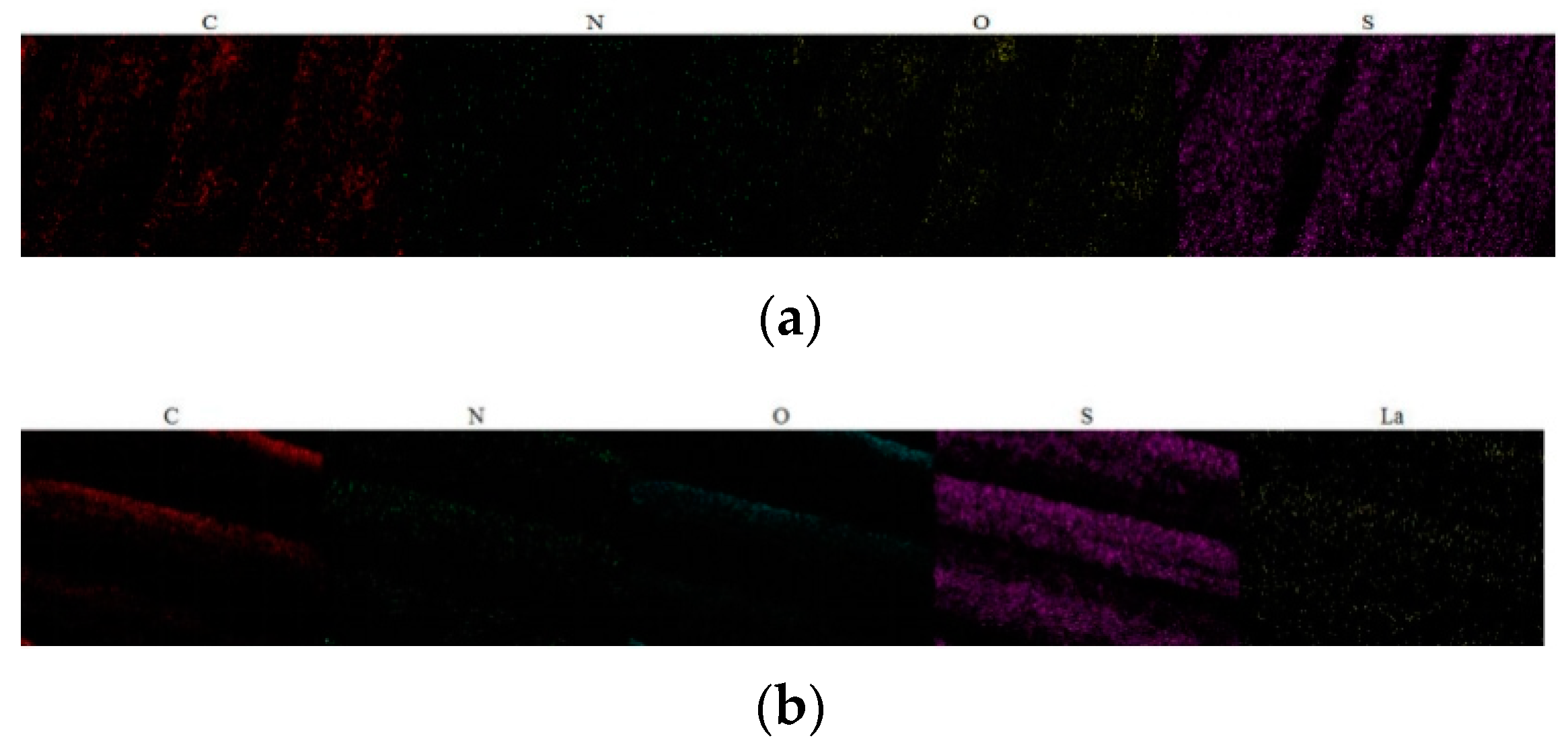
| Chromatic Aberration | Color Difference Size | Color Difference Feeling Degree | Chromatic Aberration | Color Difference Size | Color Difference Feeling Degree |
|---|---|---|---|---|---|
| 0~0.5 | Very small | Trace | 3.0~6.0 | Large | Identifiability |
| 0.5~1.5 | Small | Mild | 6.0~12.0 | Large | Magnum |
| 1.5~3.0 | Smaller | Perceptible | 12.0 and up | Very large | Very large |
| Number | A Rare Earth (%o.w.f.) | B NaCl (g/L) | C Temperature (°C) | D Time (min) | Dyeing Rate (%) | Fixation Rate (%) |
|---|---|---|---|---|---|---|
| 1 | 0.35 | 10 | 65 | 35 | 47.44 | 34.25 |
| 2 | 0.35 | 15 | 75 | 45 | 52.24 | 38.63 |
| 3 | 0.35 | 20 | 85 | 55 | 50.20 | 38.25 |
| 4 | 0.40 | 10 | 75 | 55 | 52.87 | 39.49 |
| 5 | 0.40 | 15 | 85 | 35 | 55.38 | 44.83 |
| 6 | 0.40 | 20 | 65 | 45 | 53.43 | 42.58 |
| 7 | 0.45 | 10 | 85 | 45 | 53.37 | 42.85 |
| 8 | 0.45 | 15 | 65 | 55 | 50.20 | 40.72 |
| 9 | 0.45 | 20 | 75 | 35 | 52.74 | 42.66 |
| K1 | 49.96 | 51.23 | 50.36 | 51.85 | ||
| K2 | 53.89 | 52.61 | 52.61 | 53.01 | ||
| K3 | 52.11 | 52.12 | 52.98 | 51.09 | ||
| R | 3.93 | 1.38 | 2.63 | 1.92 | ||
| k1 | 37.04 | 38.86 | 39.18 | 40.58 | ||
| k2 | 42.30 | 41.40 | 40.26 | 41.35 | ||
| k3 | 42.08 | 41.16 | 41.97 | 39.48 | ||
| r | 5.26 | 2.53 | 2.79 | 1.87 |
| Dyeing Process | Friction Fastness (Grade) | Soap Fastness (Grade) | Fastness to Sunlight (Grade) | |||
|---|---|---|---|---|---|---|
| Trunk | Humidity | Transfer of Color | Discoloration | Transfer of Color | Discoloration | |
| Conventional dyeing | 4 | 3–4 | 4 | 3–4 | 4 | 4 |
| Dyeing after rare-earth pretreatment | 4 | 4–5 | 4 | 4–5 | 4 | 4 |
| L* | a* | b* | c* | h* | |
|---|---|---|---|---|---|
| Conventional dyeing | 43.65 | 9.78 | −18.83 | 21.22 | 242.56 |
| Dyeing after rare-earth pretreatment | 29.54 | 32.07 | −21.38 | 38.54 | 326.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, A.; Zheng, Y.; Jiang, W.; Liu, J. Rare-Earth Pretreatment Improves Performance of Reactive Dye Argazol Navy Blue on Banana-Fiber Fabric. Molecules 2025, 30, 176. https://doi.org/10.3390/molecules30010176
Du A, Zheng Y, Jiang W, Liu J. Rare-Earth Pretreatment Improves Performance of Reactive Dye Argazol Navy Blue on Banana-Fiber Fabric. Molecules. 2025; 30(1):176. https://doi.org/10.3390/molecules30010176
Chicago/Turabian StyleDu, Ao, Yongjie Zheng, Wenqi Jiang, and Jie Liu. 2025. "Rare-Earth Pretreatment Improves Performance of Reactive Dye Argazol Navy Blue on Banana-Fiber Fabric" Molecules 30, no. 1: 176. https://doi.org/10.3390/molecules30010176
APA StyleDu, A., Zheng, Y., Jiang, W., & Liu, J. (2025). Rare-Earth Pretreatment Improves Performance of Reactive Dye Argazol Navy Blue on Banana-Fiber Fabric. Molecules, 30(1), 176. https://doi.org/10.3390/molecules30010176





