Sustainable and Eco-Friendly Single- and Multilayer Polyester Foils (Laminates) from Polylactide and Poly(Ethylene 2,5-Furandicarboxylate)
Abstract
:1. Introduction
2. Results and Discussion
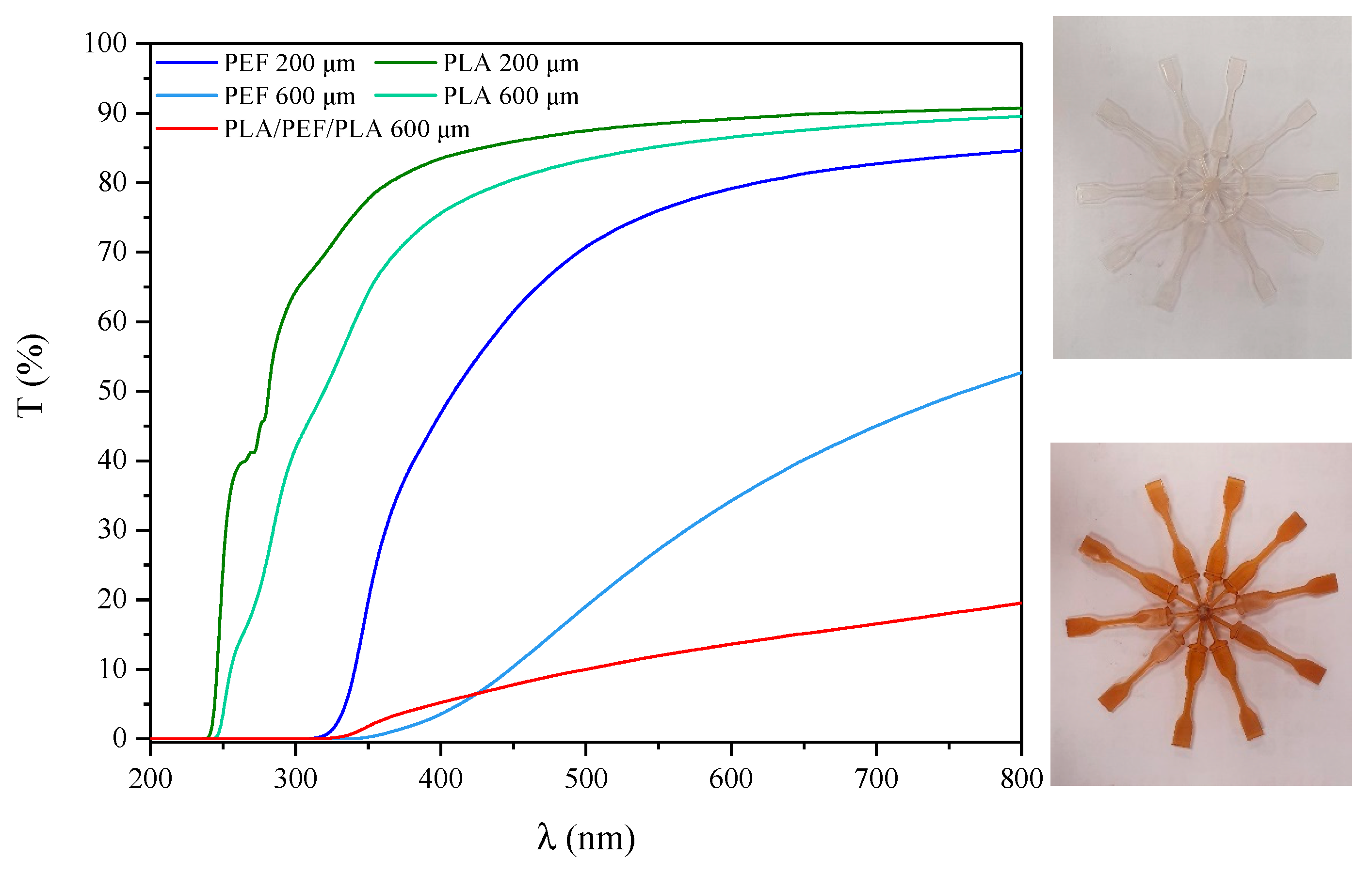
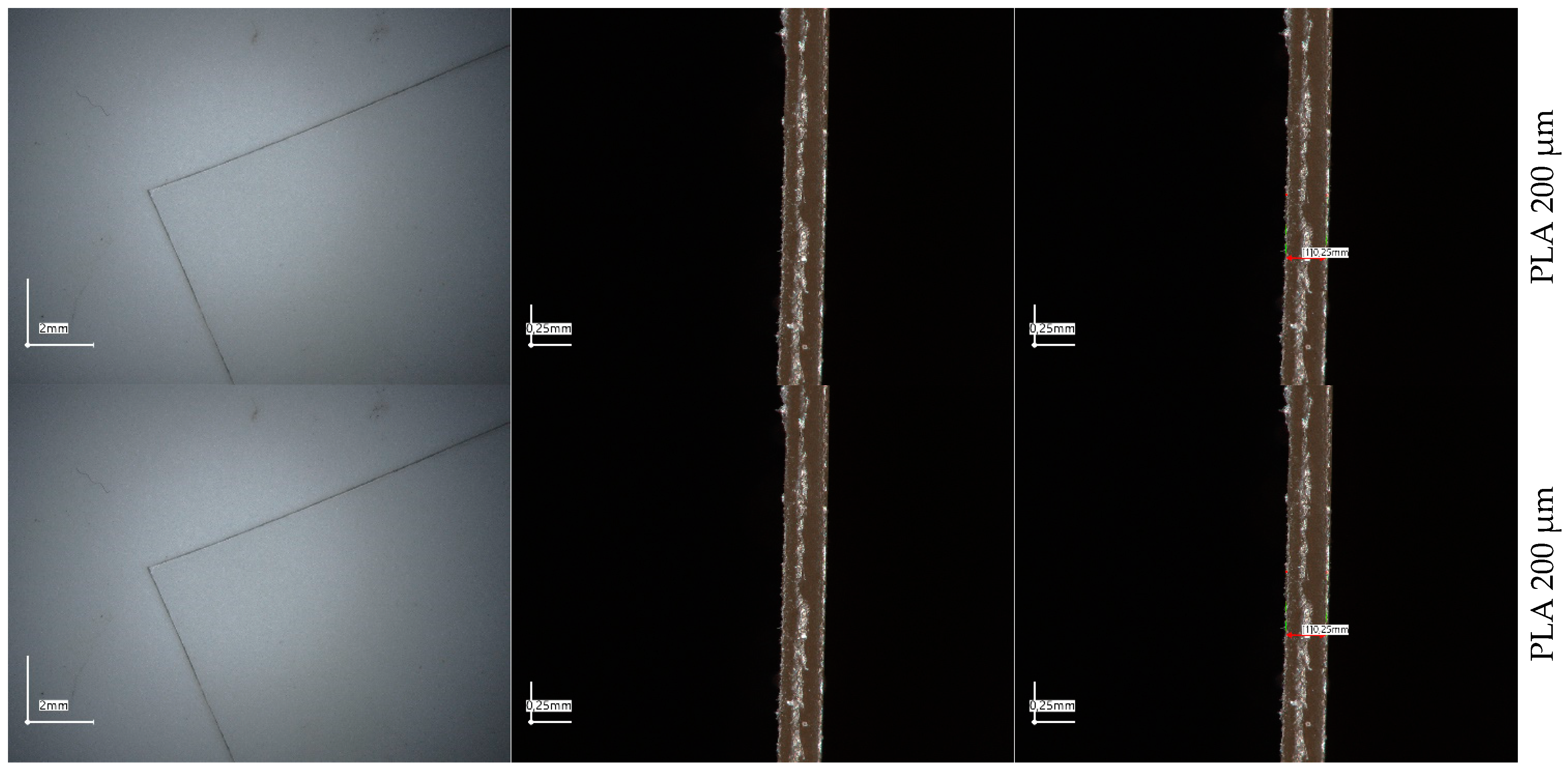
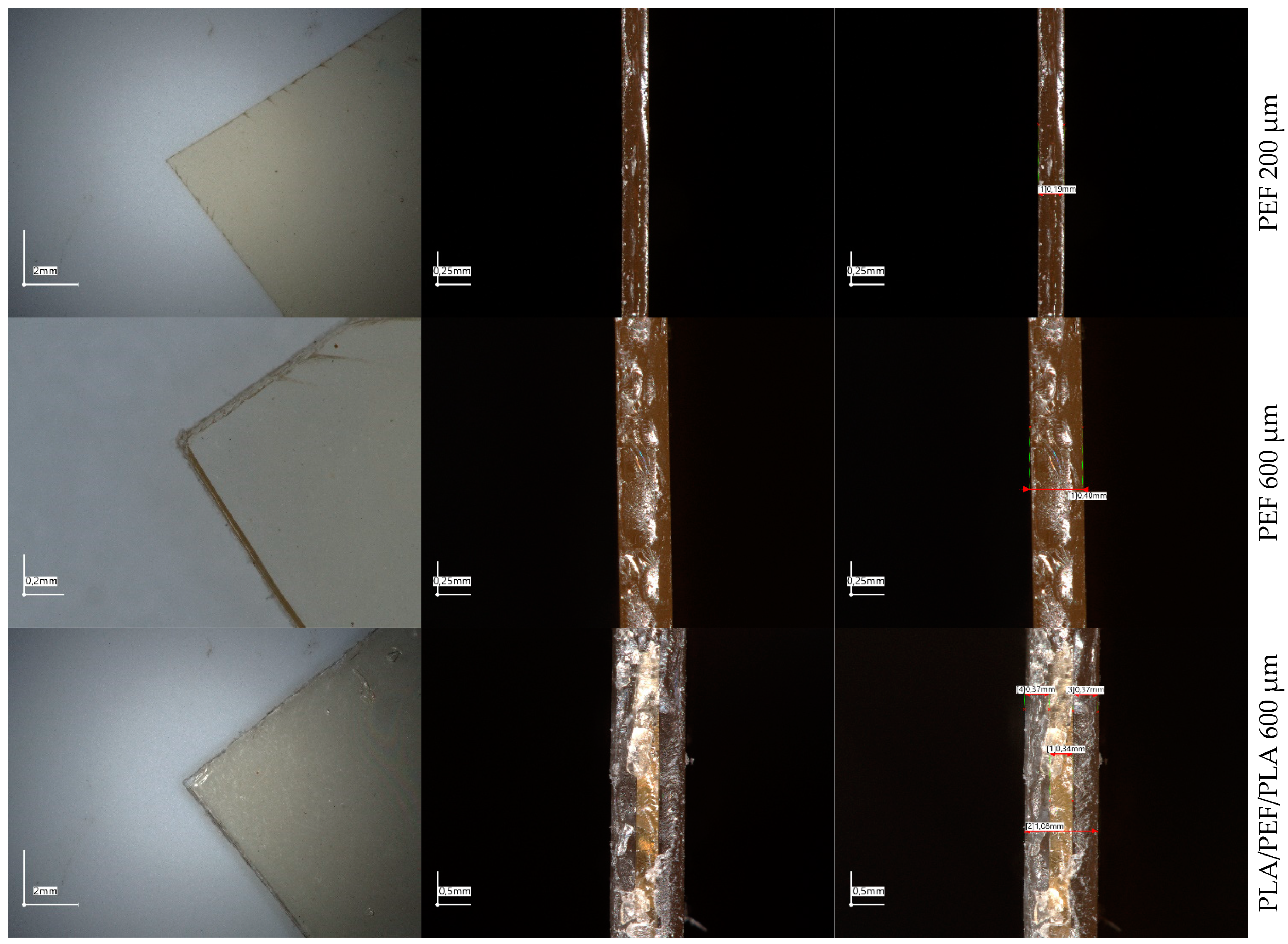
2.1. UV-Vis Spectrophotometry and Microscopic Evaluation of Polyesters’ Foils
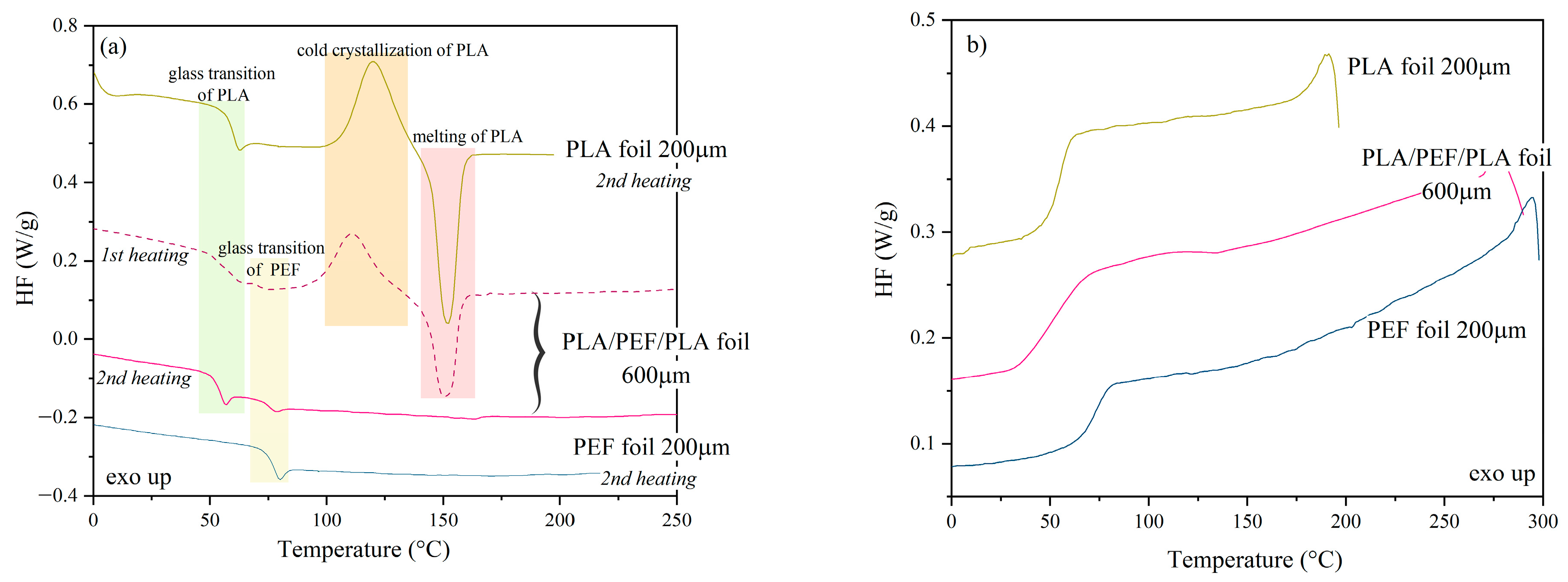
2.2. DSC Analysis
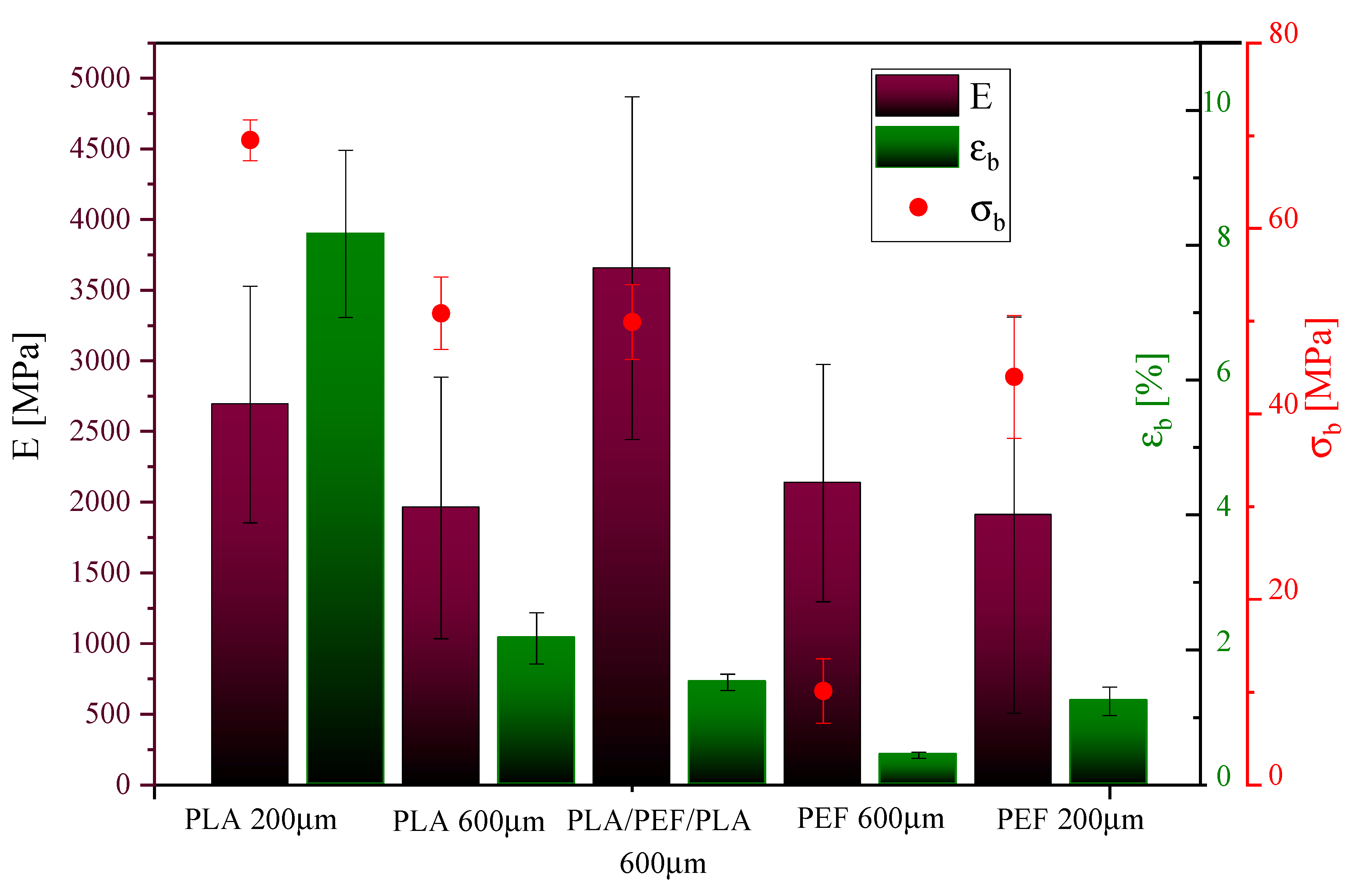
2.3. Mechanical Properties
2.4. Water Sorption and Oxygen Transmission Rate
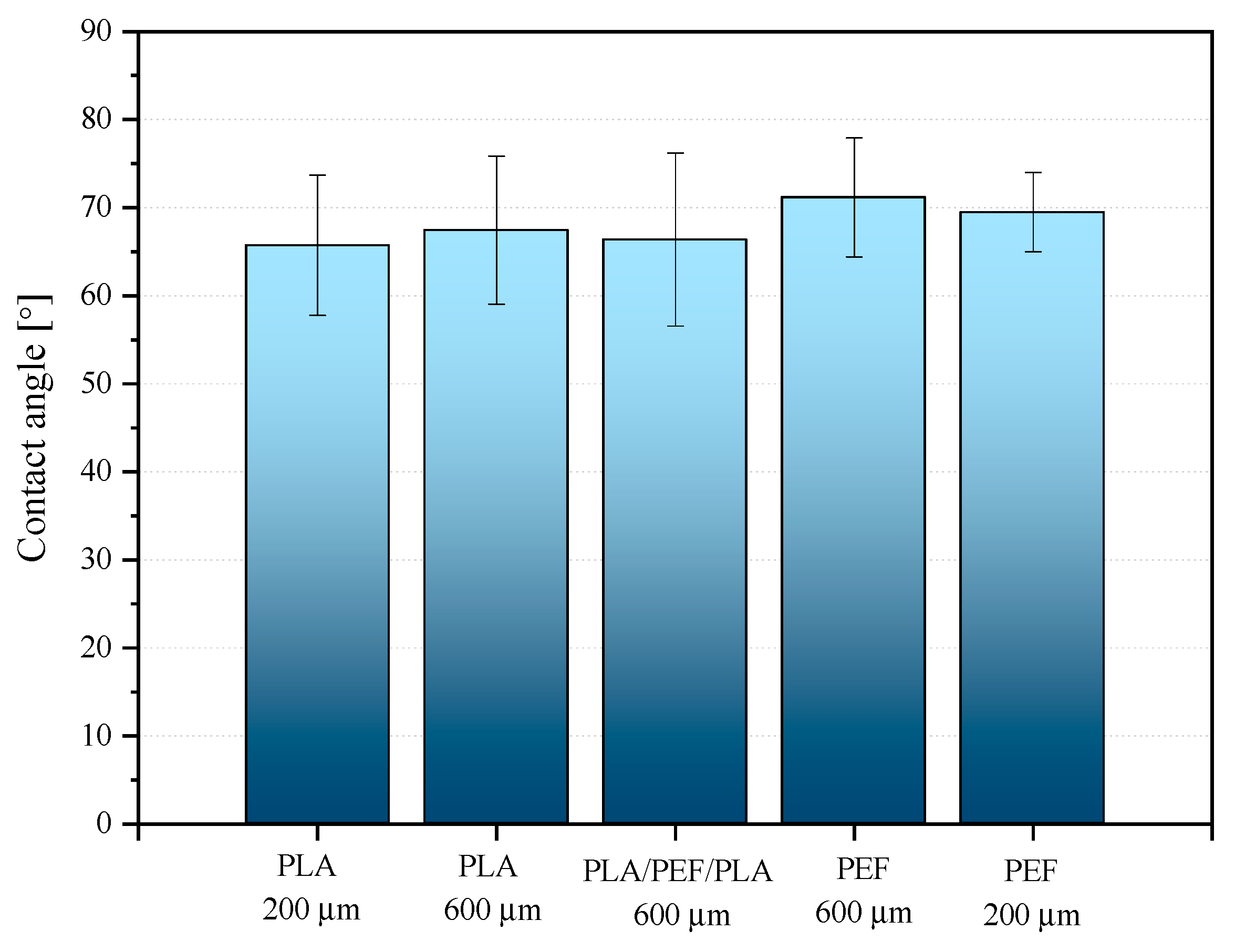
2.5. Surface Properties
2.6. Preliminary Study of Biofilm Formation on Surface of Polymer Foils
- (−)—strain not forming the biofilm (corresponded to the lack of cells);
- (+)—strain weakly forming the biofilm (corresponded to 103–104 CFU/mL);
- (++)—strain strongly forming the biofilm (corresponded to 105–106 CFU/mL);
- (+++)—strain strongly forming the biofilm (corresponded to 107–108 CFU/mL).
3. Experimental Section
3.1. Materials
3.2. Sample Preparation
3.2.1. Testing Sample Preparation
3.2.2. Preparation of Single- and Multilayer Foils
3.3. Characterization Methods
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cleo, G.; Isenring, E.; Thomas, R.; Glasziou, P. Could habits hold the key to weight loss maintenance? A narrative review. J. Hum. Nutr. Diet. 2017, 30, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Alejandra, N.M.; Paula, C.M.; Patricia, A.M. Study of Nutrition Habits in Primary School Students. J. Clin. Nutr. Diet. 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, H. Biodegradable Polylactic Acid Polymer with Nisin for Use in Antimicrobial Food Packaging. J. Food Sci. 2008, 73, M127–M134. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 29, 1008. [Google Scholar] [CrossRef] [PubMed]
- Lagaron, J.M.; Lopez-Rubio, A. Nanotechnology for bioplastics: Opportunities, challenges and strategies. Trends Food Sci. Technol. 2011, 22, 611–617. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3511. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Burgos, N.; Peltzer, M.A.; López, J.; Peponi, L. Nanocellulose-Based Polymeric Blends for Food Packaging Applications. In Multifunctional Polymeric Nanocomposites Based on Cellulosic Reinforcements; William Andrew: Norwich, NY, USA, 2016; pp. 205–252. [Google Scholar] [CrossRef]
- Piergiovanni, L.; Limbo, S. Plastic packaging materials. In Food Packaging Materials; Springer International Publishing: Cham, Switzerland, 2016; pp. 33–49. [Google Scholar]
- Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 13 November 2024).
- Briassoulis, D.; Dejean, C. Critical review of norms and standards for biodegradable agricultural plastics part I. Biodegradation in soil. J. Polym. Environ. 2010, 18, 384–400. [Google Scholar] [CrossRef]
- Avérous, L. Biodegradable multiphase systems based on plasticized starch: A review. J. Macromol. Sci.—Polym. Rev. 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Genovese, L.; Lotti, N.; Siracusa, V.; Munari, A. Poly(neopentyl glycol furanoate): A member of the furan-based polyester family with smart barrier performances for sustainable food packaging applications. Materials 2017, 10, 1028. [Google Scholar] [CrossRef]
- Dorgan, J.R.; Lehermeier, H.; Mang, M. Thermal and Rheological Properties of Commercial-Grade Poly(Lactic Acid)s. J. Polym. Environ. 2000, 8, 1–9. [Google Scholar] [CrossRef]
- Patel, M.K.; Shen, L.; Worrell, E. Modeling and Analysis Comparing life cycle energy and GHG emissions of bio-based PET, recycled PET, PLA, and man-made cellulosics. Biofuels Bioprod. Biorefin. 2012, 6, 625–639. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Bioplastics and petroleum-based plastics: Strengths and weaknesses. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 1949–1959. [Google Scholar] [CrossRef]
- Ingrao, C.; Gigli, M.; Siracusa, V. An attributional Life Cycle Assessment application experience to highlight environmental hotspots in the production of foamy polylactic acid trays for fresh-food packaging usage. J. Clean. Prod. 2017, 150, 93–103. [Google Scholar] [CrossRef]
- Bałdowska-Witos, P.; Kruszelnicka, W.; Kasner, R.; Tomporowski, A.; Flizikowski, J.; Kłos, Z.; Piotrowska, K.; Markowska, K. Application of LCA Method for Assessment of Environmental Impacts of a Polylactide (PLA) Bottle Shaping. Polymers 2020, 12, 388. [Google Scholar] [CrossRef] [PubMed]
- Madival, S.; Auras, R.; Singh, S.P.; Narayan, R. Assessment of the environmental profile of PLA, PET and PS clamshell containers using LCA methodology. J. Clean. Prod. 2009, 17, 1183–1194. [Google Scholar] [CrossRef]
- Papong, S.; Malakul, P.; Trungkavashirakun, R.; Wenunun, P.; Chom-In, T.; Nithitanakul, M.; Sarobol, E. Comparative assessment of the environmental profile of PLA and PET drinking water bottles from a life cycle perspective. J. Clean. Prod. 2014, 65, 539–550. [Google Scholar] [CrossRef]
- Changwichan, K.; Silalertruksa, T.; Gheewala, S.H. Eco-Efficiency Assessment of Bioplastics Production Systems and End-of-Life Options. Sustainability 2018, 10, 952. [Google Scholar] [CrossRef]
- Sousa, A.F.; Patrício, R.; Terzopoulou, Z.; Bikiaris, D.N.; Stern, T.; Wenger, J.; Loos, K.; Lotti, N.; Siracusa, V.; Szymczyk, A.; et al. Recommendations for replacing PET on packaging, fiber, and film materials with biobased counterparts. Green Chem. 2021, 23, 8795–8820. [Google Scholar] [CrossRef]
- Benavides, P.T.; Lee, U.; Zarè-Mehrjerdi, O. Life cycle greenhouse gas emissions and energy use of polylactic acid, bio-derived polyethylene, and fossil-derived polyethylene. J. Clean. Prod. 2020, 277, 124010. [Google Scholar] [CrossRef]
- Conn, R.E.; Kolstad, J.J.; Borzelleca, J.F.; Dixler, D.S.; Filer, L.J.; Ladu, B.N.; Pariza, M.W. Safety Assessment of Polylactide (PLA) for Use as a Food-contact Polymer. Food Chem. Toxicol. 1995, 33, 273–283. [Google Scholar] [CrossRef]
- Bogaert, J.-C.; Coszach, P. Poly(lactic acids): A potential solution to plastic waste dilemma. Macromol. Symp. 2000, 153, 287–303. [Google Scholar] [CrossRef]
- Singha, S.; Hedenqvist, M.S. A review on barrier properties of poly(lactic Acid)/clay nanocomposites. Polymers 2020, 12, 1095. [Google Scholar] [CrossRef]
- Fairclough, A.; Vayshbeyn, L.I.; Mastalygina, E.E.; Olkhov, A.A.; Podzorova, M.V. Poly(lactic acid)-Based Blends: A Comprehensive Review. Appl. Sci. 2023, 13, 5148. [Google Scholar] [CrossRef]
- Huang, H.D.; Ren, P.G.; Zhong, G.J.; Olah, A.; Li, Z.M.; Baer, E.; Zhu, L. Promising strategies and new opportunities for high barrier polymer packaging films. Prog. Polym. Sci. 2023, 144, 101722. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain mobility; thermal, and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Burgess, S.K.; Karvan, O.; Johnson, J.R.; Kriegel, R.M.; Koros, W.J. Oxygen sorption and transport in amorphous poly(ethylene furanoate). Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon dioxide sorption and transport in amorphous poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.S.; Yu, D.B.; Kim, D.J.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly(ethylene furanoate) compared to poly(ethylene terephthalate). Part 1: Equilibrium sorption. Polymer 2014, 55, 6861–6869. [Google Scholar] [CrossRef]
- Eerhart, A.J.J.E.; Faaij, A.P.C.; Patel, M.K. Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance. Energy Environ. Sci. 2012, 5, 6407–6422. [Google Scholar] [CrossRef]
- van Berkel, J.G.; Kolstad, J.J. Multilayer Container Comprising a Polyethylene Furanoate Layer. U.S. Patent 11,305,910, 19 April 2020. [Google Scholar]
- Duncan, A.J.; Fagan, P. Enhanced Barrier Performance via Blends of Poly(ethylene furandicarboxylate) and Poly(ethylene terephthalate). P. 2016200653A1, 15 December 2016. [Google Scholar]
- Available online: www.coreychem.com (accessed on 13 November 2024).
- Paszkiewicz, S.; Irska, I.; Piesowicz, E. Environmentally friendly polymer blends based on post-consumer glycol-modified poly(ethylene terephthalate) (PET-G) foils and poly(Ethylene 2,5-Furanoate) (PEF): Preparation and characterization. Materials 2020, 13, 2673. [Google Scholar] [CrossRef]
- Chen, S.; Zou, R.; Li, L.; Shang, J.; Lin, S.; Lan, J. Preparation of biobased poly(propylene 2,5-furandicarboxylate) fibers: Mechanical, thermal and hydrolytic degradation properties. J. Appl. Polym. Sci. 2021, 138, app50345. [Google Scholar] [CrossRef]
- Siracusa, C.; Quartinello, F.; Soccio, M.; Manfroni, M.; Lotti, N.; Dorigato, A.; Guebitz, G.M.; Pellis, A. On the Selective Enzymatic Recycling of Poly(pentamethylene 2,5-furanoate)/Poly(lactic acid) Blends and Multiblock Copolymers. ACS Sustain. Chem. Eng. 2023, 11, 9751–9760. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Nikzad, M.; Sbarski, I. Permeability control in polymeric systems: A review. J. Polym. Res. 2018, 25, 232. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of polymer-based multilayer packaging: A review. Recycling 2018, 3, 1. [Google Scholar] [CrossRef]
- Dziadowiec, D.; Matykiewicz, D.; Szostak, M.; Andrzejewski, J. Overview of the Cast Polyolefin Film Extrusion Technology for Multi-Layer Packaging Applications. Materials 2023, 16, 1071. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.I.; Morris, B.A. PE-based multilayer film structures. In Multilayer Flexible Packaging; Wagner, J.R., Ed.; Elsevier Inc.: San Diego, CA, USA, 2016; pp. 281–310. [Google Scholar]
- Wagner, J.R. Multilayer Flexible Packaging; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Ramos, M.; Valdés, A.; Mellinas, A.C.; Garrigós, M.C. New trends in beverage packaging systems: A review. Beverages 2015, 1, 248–272. [Google Scholar] [CrossRef]
- Available online: https://www.m-chemical.co.jp/en/news/mpi/201202150477.html (accessed on 11 November 2024).
- Available online: http://www.quantum-packaging.com/q-top-biopeel.html (accessed on 13 November 2024).
- Kasmi, N.; Papageorgiou, G.; Achilias, D.; Bikiaris, D. Solid-State Polymerization of Poly(Ethylene Furanoate) Biobased Polyester, II: An Efficient and Facile Method to Synthesize High Molecular Weight Polyester Appropriate for Food Packaging Applications. Polymers 2018, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Codou, A.; Moncel, M.; Van Berkel, J.G.; Guigo, N.; Sbirrazzuoli, N. Glass transition dynamics and cooperativity length of poly(ethylene 2,5-furandicarboxylate) compared to poly(ethylene terephthalate). Phys. Chem. Chem. Phys. 2016, 18, 16647–16658. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A.; Dussin, A.; Xanthopoulou, E.; Bikiaris, D.N.; Botta, L.; Fiore, V.; Pegoretti, A. Compatibilization of Polylactide/Poly(ethylene 2,5-furanoate) (PLA/PEF) Blends for Sustainable and Bioderived Packaging. Molecules 2022, 27, 6371. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Mysiukiewicz, O.; Barczewski, M.; Skórczewska, K.; Matykiewicz, D. Correlation between processing parameters and degradation of different polylactide grades during twin-screw extrusion. Polymers 2020, 12, 1333. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Martín-Alfonso, J.E. Thermal, thermo-oxidative and thermomechanical degradation of PLA: A comparative study based on rheological, chemical and thermal properties. Polym. Degrad. Stab. 2018, 150, 37–45. [Google Scholar] [CrossRef]
- Pantani, R.; De Santis, F.; Sorrentino, A.; De Maio, F.; Titomanlio, G. Crystallization kinetics of virgin and processed poly(lactic acid). Polym. Degrad. Stab. 2010, 95, 1148–1159. [Google Scholar] [CrossRef]
- Martínez-Tong, D.E.; Soccio, M.; Sanz, A.; García, C.; Ezquerra, T.A.; Nogales, A. Chain arrangement and glass transition temperature variations in polymer nanoparticles under 3D-confinement. Macromolecules 2013, 46, 4698–4705. [Google Scholar] [CrossRef]
- Long, T.E. Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Wawrzyniak, P. Mechanical properties of PET material—Literature review. Tech. Issues 2015, 1, 54–67. [Google Scholar]
- Paszkiewicz, S.; Walkowiak, K.; Irska, I.; Mechowska, S.; Stankiewicz, K.; Zubkiewicz, A.; Piesowicz, E.; Miadlicki, P. Influence of the Multiple Injection Moulding and Composting Time on the Properties of Selected Packaging and Furan-Based Polyesters. J. Polym. Environ. 2023, 31, 722–742. [Google Scholar] [CrossRef]
- Karmaker, A.C. Effect of water absorption on dimensional stability and impact energy of jute fibre reinforced polypropylene. J. Mater. Sci. Lett. 1997, 16, 462–464. [Google Scholar] [CrossRef]
- Gaudichet-Maurin, E.; Thominette, F.; Verdu, J. Water sorption characteristics in moderately hydrophilic polymers, part 1: Effect of polar groups concentration and temperature in water sorption in aromatic polysulfones. J. Appl. Polym. Sci. 2008, 109, 3279–3285. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L.; Polymers, B. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Yew, G.H.; Yusof, A.M.M.; Ishak, Z.A.M.; Ishiaku, U.S. Water absorption and enzymatic degradation of poly(lactic acid)/rice starch composites. Polym. Degrad. Stab. 2005, 90, 488–500. [Google Scholar] [CrossRef]
- Taib, R.M.; Ramarad, S.; Ishak, Z.A.M.; Todo, M. Properties of kenaf fiber/polylactic acid biocomposites plasticized with polyethylene glycol. Polym. Compos. 2010, 31, 1213–1222. [Google Scholar] [CrossRef]
- Sangroniz, A.; Sangroniz, L.; Gonzalez, A.; Santamaria, A.; del Rio, J.; Iriarte, M.; Etxeberria, A. Improving the barrier properties of a biodegradable polyester for packaging applications. Eur. Polym. J. 2019, 115, 76–85. [Google Scholar] [CrossRef]
- ASTM D-3985; Standard test method for oxygen gas transmission rate through plastic film and sheeting using coulometric sensor. In Annual Book of ASTM Standards. ASTM: West Conshohocken, PA, USA, 1995.
- Abdellatief, A.; Welt, B.A. Comparison of new dynamic accumulation method for measuring oxygen transmission rate of packaging against the steady-state method described by ASTM D3985. Packag. Technol. Sci. 2013, 26, 281–288. [Google Scholar] [CrossRef]
- Siracusa, V. Food packaging permeability behaviour: A report. Int. J. Polym. Sci. 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Weiss, C.K.; Pellis, A.; Paszkiewicz, S.; Guigo, N.; Ajd, S.; Loos, K.; Zhang, R.; Pereira, I.; Agostinho, B.; Hu, H.; et al. A Perspective on PEF Synthesis, Properties, and End-Life. Front. Chem. 2020, 8, 585. [Google Scholar] [CrossRef]
- Ji, P.; Lu, D.; Zhang, S.; Zhang, W.; Wang, C.; Wang, H. Modification of poly(ethylene,5-furandicarboxylate) with poly(ethylene glycol) for biodegradable copolyesters with good mechanical properties and spinnability. Polymers 2019, 11, 2105. [Google Scholar] [CrossRef]
- Zhu, C.; Yin, J.; Zhang, Z.; Shi, F. Bio-based poly(ethylene furanoate)/ZnO transparent thin films with improved water vapor barrier and antibacterial properties for food packaging application. Mater. Res. Express 2022, 9, 115304. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Zamboulis, A.; Papadopoulos, L.; Grigora, M.E.; Tsongas, K.; Tzetzis, D.; Bikiaris, D.N.; Papageorgiou, G.Z. Blending PLA with Polyesters Based on 2,5-Furan Dicarboxylic Acid: Evaluation of Physicochemical and Nanomechanical Properties. Polymers 2022, 14, 4725. [Google Scholar] [CrossRef] [PubMed]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New weapons to fight old enemies: Novel strategies for the (bio)control of bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef] [PubMed]
- Grigore-Gurgu, L.; Bucur, F.I.; Borda, D.; Alexa, E.-A.; Neagu, C.; Nicolau, A.I. Biofilms Formed by Pathogens in Food and Food Processing Environments. In Bacterial Biofilms; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Vert, M.; Li, S.M.; Guerin, P. Bioresorbability and biocompatibility of aliphatic polyesters. J. Mater. Sci. Mater. Med. 1992, 3, 432–446. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Nair, N.R.; Sekhar, V.C.; Nampoothiri, K.M. Augmentation of a Microbial Consortium for Enhanced Polylactide (PLA) Degradation. Indian J. Microbiol. 2016, 56, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Oktay, B.; Eroğlu, G.Ö.; Demir, S.; Kuruca, S.E.; Apohan, N.K. Poly(lactic acid) nanofibers containing phosphorylcholine grafts for transdermal drug delivery systems. Mater. Today Sustain. 2022, 18, 100132. [Google Scholar] [CrossRef]
- Backes, E.H.; Pires, L.d.N.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Analysis of the Degradation During Melt Processing of PLA/Biosilicate® Composites. J. Compos. Sci. 2019, 3, 52. [Google Scholar] [CrossRef]
- ISO 527; Plastics—Determination of Tensile properties—Part 1: General Principles. ISO: Geneva, Switzerland, 2019.
- Tsuji, H. Poly(lactide) stereocomplexes: Formation; structure; properties; degradation; applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef]
- Fosse, C.; Bourdet, A.; Ernault, E.; Esposito, A.; Delpouve, N.; Delbreilh, L.; Thiyagarajan, S.; Knoop, R.J.I.; Dargent, E. Determination of the equilibrium enthalpy of melting of two-phase semi-crystalline polymers by fast scanning calorimetry. Thermochim. Acta 2019, 677, 67–78. [Google Scholar] [CrossRef]
- ISO 178; Plastics—Determination of Flexural Properties. ISO: Geneva, Switzerland, 2019.
- ASTM D3985-05; Standard Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM: West Conshohocken, PA, USA, 2010.
- Paszkiewicz, S.; Lesiak, P.; Walkowiak, K.; Irska, I.; Miądlicki, K.; Królikowski, M.; Piesowicz, E.; Figiel, P. The Mechanical, Thermal, and Biological Properties of Materials Intended for Dental Implants: A Comparison of Three Types of Poly(aryl-ether-ketones) (PEEK and PEKK). Polymers 2023, 15, 3706. [Google Scholar] [CrossRef] [PubMed]





| Material | Strain Used in Biofilm Analysis | |
|---|---|---|
| S. aureus | E. coli | |
| PLA, 200 μm | ++ | + |
| PLA, 600 μm | ++ | + |
| PLA/PEF/PLA, 600 μm | +++ | + |
| PEF, 600 μm | + | + |
| PEF, 200 μm | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paszkiewicz, S.; Irska, I.; Walkowiak, K.; Włodarczyk, F.; Zdanowicz, M.; Piesowicz, E.; Barczewski, M. Sustainable and Eco-Friendly Single- and Multilayer Polyester Foils (Laminates) from Polylactide and Poly(Ethylene 2,5-Furandicarboxylate). Molecules 2025, 30, 178. https://doi.org/10.3390/molecules30010178
Paszkiewicz S, Irska I, Walkowiak K, Włodarczyk F, Zdanowicz M, Piesowicz E, Barczewski M. Sustainable and Eco-Friendly Single- and Multilayer Polyester Foils (Laminates) from Polylactide and Poly(Ethylene 2,5-Furandicarboxylate). Molecules. 2025; 30(1):178. https://doi.org/10.3390/molecules30010178
Chicago/Turabian StylePaszkiewicz, Sandra, Izabela Irska, Konrad Walkowiak, Filip Włodarczyk, Magdalena Zdanowicz, Elżbieta Piesowicz, and Mateusz Barczewski. 2025. "Sustainable and Eco-Friendly Single- and Multilayer Polyester Foils (Laminates) from Polylactide and Poly(Ethylene 2,5-Furandicarboxylate)" Molecules 30, no. 1: 178. https://doi.org/10.3390/molecules30010178
APA StylePaszkiewicz, S., Irska, I., Walkowiak, K., Włodarczyk, F., Zdanowicz, M., Piesowicz, E., & Barczewski, M. (2025). Sustainable and Eco-Friendly Single- and Multilayer Polyester Foils (Laminates) from Polylactide and Poly(Ethylene 2,5-Furandicarboxylate). Molecules, 30(1), 178. https://doi.org/10.3390/molecules30010178









