Interrelation of Natural Polyphenol and Fibrosis in Diabetic Nephropathy
Abstract
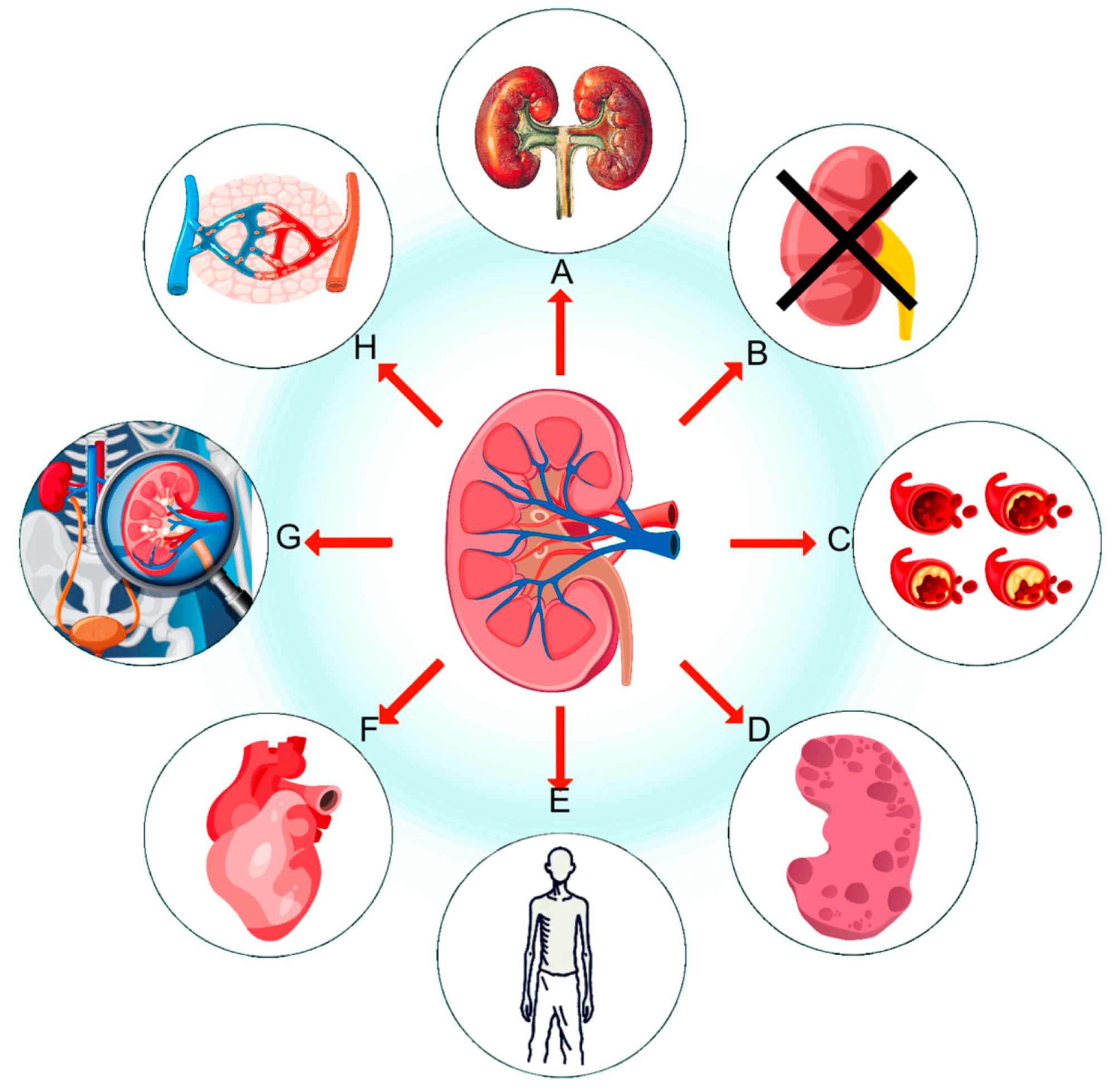
:1. Introduction
2. Kidney Fibrosis in Diabetes
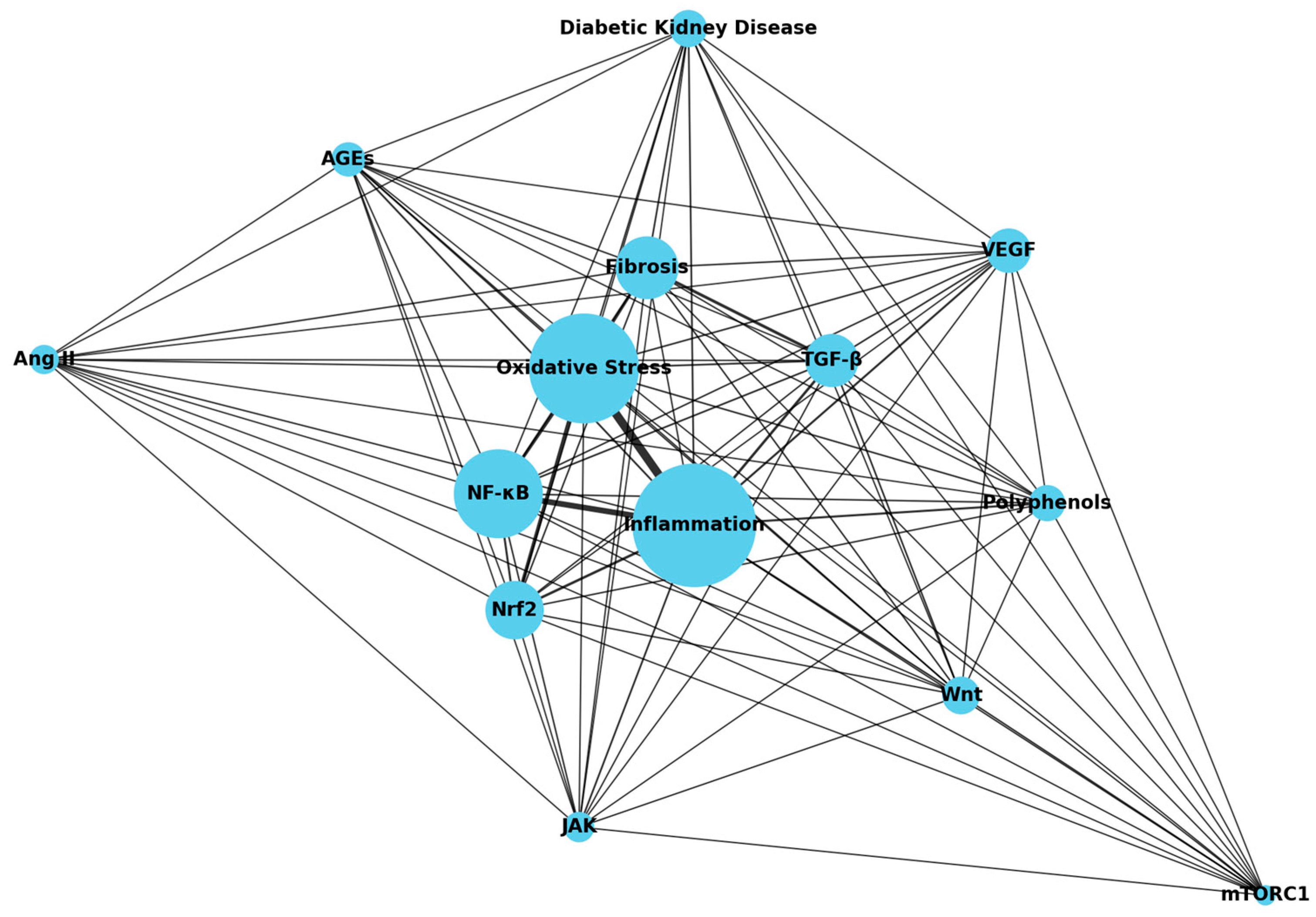
2.1. Signaling Pathways Related to Kidney Fibrosis
2.1.1. TGF-β/SMAD
2.1.2. mTORC1/p70S6K
2.1.3. JAK/STAT/SOCS
2.1.4. Wnt/β-Catenin
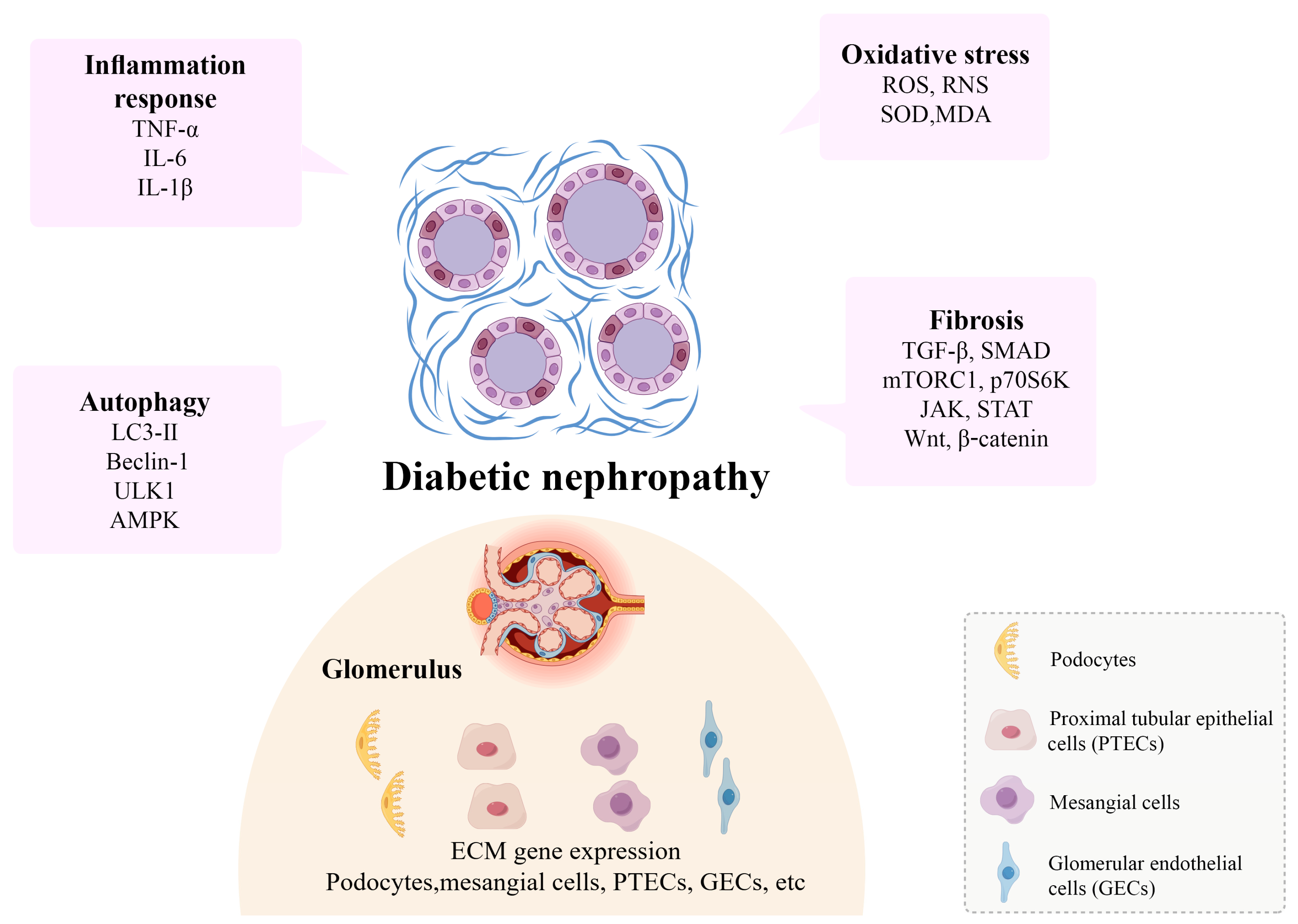
2.2. Potential Mechanisms of Kidney Fibrosis
2.2.1. Fibrosis and Autophagy
2.2.2. Fibrosis and Oxidative Stress
2.2.3. Fibrosis and Inflammation
2.3. Fibrotic Niche in Kidney Fibrosis
2.3.1. Podocytes
2.3.2. Proximal Tubular Epithelial Cells
2.3.3. Mesangial Cells
2.3.4. Glomerular Endothelial Cells
3. Polyphenols and Their Applications
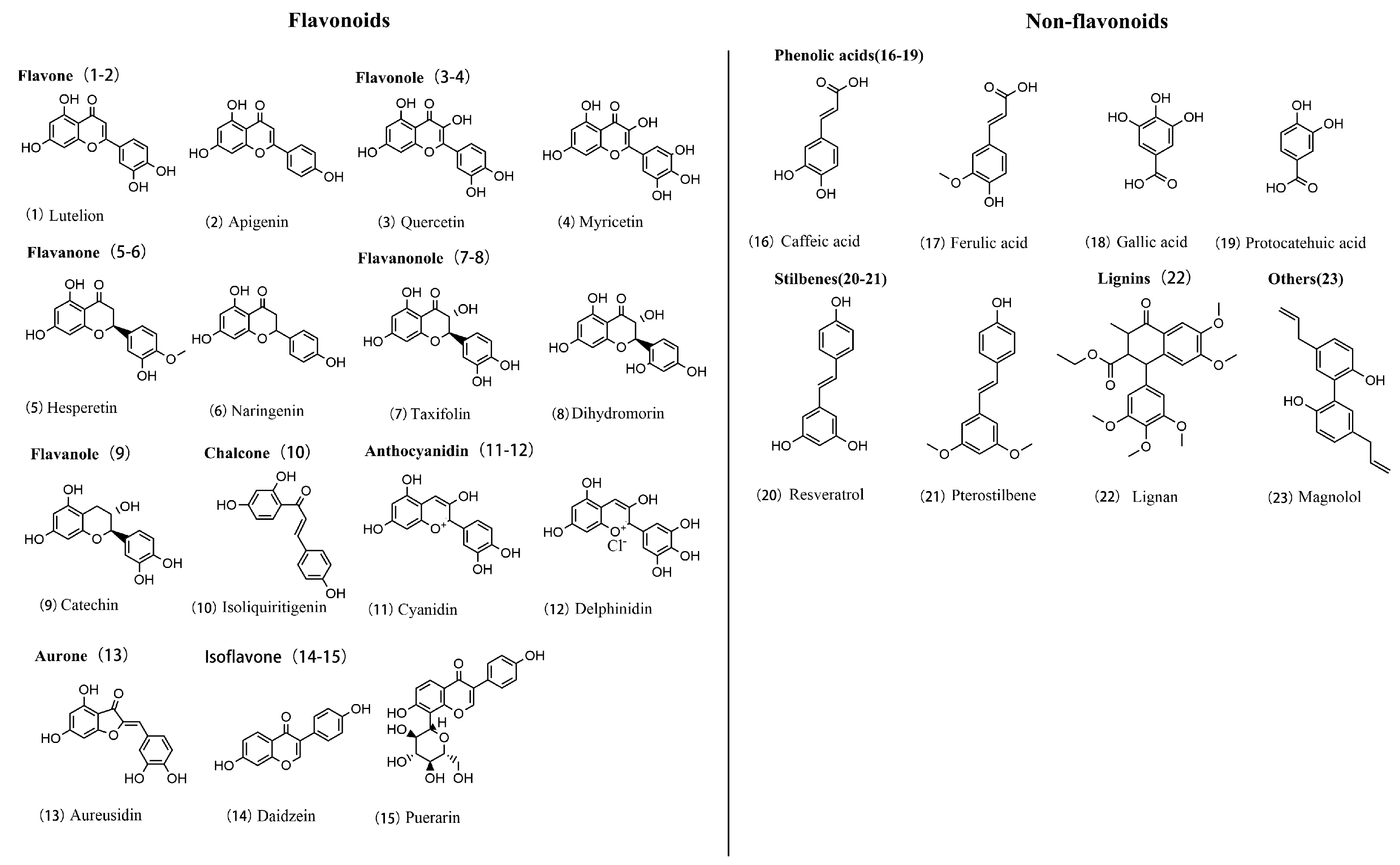
3.1. Polyphenol Chemistry
3.2. Pharmacological Effects of Polyphenols
| Polyphenol | Mechanism | Dosage | Experimental Parameters | Efficacy | Reference |
|---|---|---|---|---|---|
| Resveratrol | TGF-β/SMAD, NF-κB | 20 mg/kg | Model: bleomycin-induced mouse model duration: 21 days administration: intraperitoneal, daily | anti-inflammatory, antioxidant | [109] |
| Curcumin | TGF-β/SMAD, NF-κB, PI3K/Akt | 100 mg/kg | Model: high-fat diet-induced obese mice duration: 8 weeks administration: oral, daily | anti-inflammatory, anticancer | [110] |
| Proanthocyanidins | TGF-β/SMAD, MAPK | 100 mg/kg | Model: MRI images from various disease groups duration: data collection and analysis phase administration: different levels of grey-level discretization on MRI images | antioxidant, improves cardiovascular health | [111] |
| EGCG | TGF-β/SMAD, NF-κB | 5 mg/kg | Model: male Wistar rats duration: 5 weeks A administration: T treadmill exercise training | anticancer, lowers blood lipids | [112] |
| Gallic acid | TGF-β/SMAD, AMPK | 100 mg/kg | Model: rabbit ear hypertrophic scar model duration: 28 days administration: topical application of gallic acid ointment (varied concentrations) | anti-inflammatory, antibacterial | [113] |
| Protocatechuic acid | NF-κB, PI3K/Akt | 20 mg/kg | Model: review article (no specific experimental model) duration: N/A administration: discusses various antioxidant therapies and mechanisms | anti-inflammatory, antioxidant | [114] |
| Ginkgolide | Wnt/β-catenin, TGF-β/SMAD | 60 mg/kg | Model: elderly patients with heart valve disease duration: surgical procedure and assessment phase administration: evaluated risk and benefit through clinical assessments | improves blood circulation, anti-inflammatory | [115] |
| Luteolin | NF-κB, PI3K/Akt | 50 mg/kg | Model: in vitro and in vivo models duration: varies across studies administration: various concentrations and methods | antiallergic, antioxidant | [116] |
| Baicalin | TGF-β/SMAD, Wnt/β-catenin | 100 mg/kg | Model: review article (no specific model) duration: N/A administration: N/A | antioxidant, antidepressant | [117] |
| Ginsenoside Rg1 | TGF-β/SMAD, NF-κB | 20 mg/kg | Model: MRI images from various disease groups duration: data collection and analysis phase administration: different levels of grey-level discretization on MRI images | boosts immune system, anti-fatigue | [118] |
| Tripterygium glycosides | JAK/STAT, TGF-β/SMAD | 50 mg/kg | Model: clinical case study duration: clinical observation administration: not specified | immunosuppressive, anticancer | [119] |
| Naringin | NF-κB, TGF-β/SMAD | 80 mg/kg | Model: rats with DMN-induced liver fibrosis duration: not specified administration: Ganshuang granules in varying dosages | antioxidant, anti-inflammatory | [120] |
| Hesperidin | PI3K/Akt, NF-κB | 100 mg/kg | Model: review article duration: N/A administration: N/A | anti-inflammatory, antiviral, improves microcirculation | [121] |
| Emodin | TGF-β/SMAD, JAK/STAT | 40 mg/kg | Model: not specified duration: not specified Administration: dapansutrile | anti-inflammatory, antibacterial | [122] |
| Apigenin | NF-κB, TGF-β/SMAD | 25 mg/kg | Model: review article (no specific model) duration: N/A administration: N/A | anticancer, anti-anxiety | [123] |
3.3. The Therapeutic Potential of Polyphenols Targeting Fibrosis in the Treatment of DN
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; De Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic Kidney Disease: A Report from an ADA Consensus Conference. Diabetes Care 2014, 37, 2864–2883. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative Stress and Inflammation in Diabetic Nephropathy: Role of Polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef]
- Kourtidou, C.; Stangou, M.; Marinaki, S.; Tziomalos, K. Novel Cardiovascular Risk Factors in Patients with Diabetic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 11196. [Google Scholar] [CrossRef] [PubMed]
- Pelle, M.C.; Provenzano, M.; Busutti, M.; Porcu, C.V.; Zaffina, I.; Stanga, L.; Arturi, F. Up-Date on Diabetic Nephropathy. Life 2022, 12, 1202. [Google Scholar] [CrossRef] [PubMed]
- Mazzieri, A.; Porcellati, F.; Timio, F.; Reboldi, G. Molecular Targets of Novel Therapeutics for Diabetic Kidney Disease: A New Era of Nephroprotection. Int. J. Mol. Sci. 2024, 25, 3969. [Google Scholar] [CrossRef]
- Park, C.H.; Yoo, T.-H. TGF-β Inhibitors for Therapeutic Management of Kidney Fibrosis. Pharmaceuticals 2022, 15, 1485. [Google Scholar] [CrossRef] [PubMed]
- Calle, P.; Hotter, G. Macrophage Phenotype and Fibrosis in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 2806. [Google Scholar] [CrossRef] [PubMed]
- Gauffin, E.; Chisalita, S.I.; Engvall, J.; Nyström, F.H.; Östgren, C.J. Plasma Mid-Regional pro-Atrial Natriuretic Peptide Predicts Cardiovascular Events in Patients with Type 2 Diabetes Independently of Subclinical Organ Damage. Diabetes Res. Clin. Pract. 2021, 182, 109095. [Google Scholar] [CrossRef] [PubMed]
- Sawinski, D.; Locke, J.E. Evaluation of Kidney Donors: Core Curriculum 2018. Am. J. Kidney Dis. 2018, 71, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jin, Q.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Li, P.; Zhan, Y. Regulation of Autophagy by Natural Polyphenols in the Treatment of Diabetic Kidney Disease: Therapeutic Potential and Mechanism. Front. Endocrinol. 2023, 14, 1142276. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.; Li, S.; Morales, G.; Daw, C.C.; Chupp, D.P.; Fisher, A.D.; Zan, H. Epigenetic Modulation of Class-Switch DNA Recombination to IgA by miR-146a through Downregulation of Smad2, Smad3 and Smad4. Front. Immunol. 2021, 12, 761450. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Miao, N.; Xu, J.; Gan, X.; Xu, D.; Zhou, L.; Xue, H.; Zhang, W.; Lu, L. Metformin Prevents Renal Fibrosis in Mice with Unilateral Ureteral Obstruction and Inhibits Ang II-Induced ECM Production in Renal Fibroblasts. Int. J. Mol. Sci. 2016, 17, 146. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, Y.; Liu, F. Transforming Growth Factor-Beta1 in Diabetic Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-M.; Tang, P.M.-K.; Li, J.; Lan, H.Y. TGF-β/Smad Signaling in Renal Fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, F.; Wu, G.; Wang, D.; Liu, W.; Chen, J.; Qi, Y.; Wang, B.; Chen, Y. SMAD Proteins in TGF-β Signalling Pathway in Cancer: Regulatory Mechanisms and Clinical Applications. Diagnostics 2023, 13, 2769. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, Y.; Zhang, N.; Wang, D.; Cheng, X.; Li, K.; Huang, R.; Lu, Y.; Wang, H.; Han, D.; et al. The Phosphorylation of the Smad2/3 Linker Region by Nemo-like Kinase Regulates TGF-β Signaling. J. Biol. Chem. 2021, 296, 100512. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Bartenstein, M.; Zhao, W.; Ho, W.-T.; Zhang, L.; Rapaport, F.; Levine, R.L.; Shao, Z.; Zhao, Z.J.; Verma, A.; et al. Preclinical Efficacy of TGF-Beta Receptor I Kinase Inhibitor, Galunisertib, in Myelofibrosis. Blood 2015, 126, 603. [Google Scholar] [CrossRef]
- He, H.; Huang, X.; Ma, R.C.; Lan, H. 579-P: Dyslipidemia in Type 2 Diabetic Mice Is Improved by Targeting Smad3 with a Smad3 Inhibitor SIS3. Diabetes 2020, 69, 579-P. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Guan, K.-L. mTOR as a Central Hub of Nutrient Signalling and Cell Growth. Nat. Cell Biol. 2019, 21, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, X.; Shi, W.; Yao, L.; Gao, M.; Yang, Y.; Hao, A. Zdhhc15b Regulates Differentiation of Diencephalic Dopaminergic Neurons in Zebrafish. J. Cell. Biochem. 2015, 116, 2980–2991. [Google Scholar] [CrossRef]
- Dittrich, E.; Schmaldienst, S.; Soleiman, A.; Horl, W.H.; Pohanka, E. Rapamycin-Associated Post-Transplantation Glomerulonephritis and Its Remission after Reintroduction of Calcineurin-Inhibitor Therapy. Transpl. Int. 2004, 17, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Li, Y.; Lu, L.; Peng, Y.; Zhang, S.; Xia, A. Metformin Attenuates Renal Interstitial Fibrosis through Upregulation of Deptor in Unilateral Ureteral Obstruction in Rats. Exp. Ther. Med. 2020, 20, 17. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Holland, S.M.; Staudt, L.M. JAKs and STATs in Immunity, Immunodeficiency, and Cancer. N. Engl. J. Med. 2013, 368, 161–170. [Google Scholar] [CrossRef]
- Villarino, A.V.; Kanno, Y.; Ferdinand, J.R.; O’Shea, J.J. Mechanisms of Jak/STAT Signaling in Immunity and Disease. J. Immunol. 2015, 194, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Dodington, D.W.; Desai, H.R.; Woo, M. JAK/STAT—Emerging Players in Metabolism. Trends Endocrinol. Metab. 2018, 29, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Lin, Y.; Xie, J.; Zhang, Y.; Wang, H.; Zheng, D. MiR-27b-3p Inhibits the Progression of Renal Fibrosis via Suppressing STAT1. Hum. Cell 2021, 34, 383–393. [Google Scholar] [CrossRef]
- Chakraborty, D.; Šumová, B.; Mallano, T.; Chen, C.-W.; Distler, A.; Bergmann, C.; Ludolph, I.; Horch, R.E.; Gelse, K.; Ramming, A.; et al. Activation of STAT3 Integrates Common Profibrotic Pathways to Promote Fibroblast Activation and Tissue Fibrosis. Nat. Commun. 2017, 8, 1130. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Liau, N.P.D.; Laktyushin, A.; Lucet, I.S.; Murphy, J.M.; Yao, S.; Whitlock, E.; Callaghan, K.; Nicola, N.A.; Kershaw, N.J.; Babon, J.J. The Molecular Basis of JAK/STAT Inhibition by SOCS1. Nat. Commun. 2018, 9, 1558. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J. Reaching Consensus on the Important Outcomes for Peritoneal Dialysis Patients. Kidney Int. 2019, 96, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Huffstater, T.; Merryman, W.D.; Gewin, L.S. Wnt/β-Catenin in Acute Kidney Injury and Progression to Chronic Kidney Disease. Semin. Nephrol. 2020, 40, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Liu, Y. New Insights into the Role and Mechanism of Wnt/Β-catenin Signalling in Kidney Fibrosis. Nephrology 2018, 23, 38–43. [Google Scholar] [CrossRef]
- Lee, C.; Pratap, K.; Zhang, L.; Chen, H.D.; Gautam, S.; Arnaoutova, I.; Raghavankutty, M.; Starost, M.F.; Kahn, M.; Mansfield, B.C.; et al. Inhibition of Wnt/β-Catenin Signaling Reduces Renal Fibrosis in Murine Glycogen Storage Disease Type Ia. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2024, 1870, 166874. [Google Scholar] [CrossRef] [PubMed]
- Lunge, A.; Gupta, R.; Choudhary, E.; Agarwal, N. The Unfoldase ClpC1 of Mycobacterium Tuberculosis Regulates the Expression of a Distinct Subset of Proteins Having Intrinsically Disordered Termini. J. Biol. Chem. 2020, 295, 9455–9473. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Sun, Q.; Hua, M.-R.; Suo, P.; Chen, J.-R.; Yu, X.-Y.; Zhao, Y.-Y. Targeting the Wnt/β-Catenin Signaling Pathway as a Potential Therapeutic Strategy in Renal Tubulointerstitial Fibrosis. Front. Pharmacol. 2021, 12, 719880. [Google Scholar] [CrossRef]
- Niu, C.; Meng, X.; Wang, T. Identification of Ferroptosis-Inflammation Related Hub Genes and the Disease Subtypes in Idiopathic Pulmonary Fibrosis via System Biology Approaches. Mol. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takabatake, Y.; Kimura, T.; Takahashi, A.; Namba, T.; Matsuda, J.; Minami, S.; Kaimori, J.; Matsui, I.; Kitamura, H.; et al. Time-Dependent Dysregulation of Autophagy: Implications in Aging and Mitochondrial Homeostasis in the Kidney Proximal Tubule. Autophagy 2016, 12, 801–813. [Google Scholar] [CrossRef]
- Lopez-Soler, R.I.; Nikouee, A.; Kim, M.; Khan, S.; Sivaraman, L.; Ding, X.; Zang, Q.S. Beclin-1 Dependent Autophagy Improves Renal Outcomes Following Unilateral Ureteral Obstruction (UUO) Injury. Front. Immunol. 2023, 14, 1104652. [Google Scholar] [CrossRef]
- Burnier, M.; Wuerzner, G. Correction: Chronic Kidney Disease: Should Sodium Intake Be Restricted in Patients with CKD? Nat. Rev. Nephrol. 2016, 12, 666. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, J.; Li, B.; Ren, D.; Chen, X.; Yu, J.; Zhang, Q. Multiscale Construction of Bifunctional Electrocatalysts for Long-lifespan Rechargeable Zinc–Air Batteries. Adv. Funct. Mater. 2020, 30, 2003619. [Google Scholar] [CrossRef]
- Viana, S.D.; Reis, F.; Alves, R. Therapeutic Use of mTOR Inhibitors in Renal Diseases: Advances, Drawbacks, and Challenges. Oxidative Med. Cell. Longev. 2018, 2018, 3693625. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Liu, S.; Hu, L.; He, Q.; Shi, W.; Yan, D.; Cao, Y.; Zhang, G.; Wang, Z.; Wu, J.; et al. Single-cell RNA Sequencing Identify SDCBP in ACE2-positive Bronchial Epithelial Cells Negatively Correlates with COVID-19 Severity. J. Cell. Mol. Med. 2021, 25, 7001–7012. [Google Scholar] [CrossRef]
- Guillot, A.; Debarnot, U. Benefits of Motor Imagery for Human Space Flight: A Brief Review of Current Knowledge and Future Applications. Front. Physiol. 2019, 10, 396. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, Y.; Wang, S.; Su, Y.; Liu, Y.; Li, C.; Jin, L.; Wan, Q.; Sang, X.; Wang, Z. Therapeutic Effects and Perspective of Stem Cell Extracellular Vesicles in Aging and Cancer. J. Cell. Physiol. 2021, 236, 4783–4796. [Google Scholar] [CrossRef]
- Ekpenyong-Akiba, A.E.; Poblocka, M.; Althubiti, M.; Rada, M.; Jurk, D.; Germano, S.; Kocsis-Fodor, G.; Shi, Y.; Canales, J.J.; Macip, S. Amelioration of Age-related Brain Function Decline by Bruton’s Tyrosine Kinase Inhibition. Aging Cell 2020, 19, e13079. [Google Scholar] [CrossRef]
- Ishimura, N.; Shiotsu, Y.; Masuzawa, N.; Sawai, S.; Sunahara, Y.; Urata, N.; Kanehisa, F.; Tamagaki, K. Renal Granulomatous Arteritis Induced by Immune Checkpoint Inhibitors. Kidney Int. 2020, 98, 793. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, C.M.; Teodoro, J.S.; Amorim, J.A.; Steegborn, C.; Sinclair, D.A.; Rolo, A.P. Mitohormesis and Metabolic Health: The Interplay between ROS, cAMP and Sirtuins. Free Radic. Biol. Med. 2019, 141, 483–491. [Google Scholar] [CrossRef]
- Wang, L.; Hauenstein, A.V. The NLRP3 Inflammasome: Mechanism of Action, Role in Disease and Therapies. Mol. Asp. Med. 2020, 76, 100889. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Chen, Y.; Guo, Y.; Bock, C.E.; Hagan, J.P.; Kim, D.H.; Xu, Z. Cover Image, Volume 235, Number 5, May 2020. J. Cell. Physiol. 2020, 235, iii. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, L.; Zhou, Y.; Chen, Y.; Chen, P.; Xiao, W. BMSC Transplantation Aggravates Inflammation, Oxidative Stress, and Fibrosis and Impairs Skeletal Muscle Regeneration. Front. Physiol. 2019, 10, 87. [Google Scholar] [CrossRef]
- Harris, A.N.; Weiner, I.D. Sex Differences in Renal Ammonia Metabolism. Am. J. Physiol.-Ren. Physiol. 2021, 320, F55–F60. [Google Scholar] [CrossRef] [PubMed]
- Pua, L.J.W.; Mai, C.-W.; Chung, F.F.-L.; Khoo, A.S.-B.; Leong, C.-O.; Lim, W.-M.; Hii, L.-W. Functional Roles of JNK and P38 MAPK Signaling in Nasopharyngeal Carcinoma. Int. J. Mol. Sci. 2022, 23, 1108. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.M.; Ibrahim, I.M.; Abd-alhameed, E.K.; Sharawi, Z.W.; Jaber, F.A.; Althagafy, H.S. Nrf2/HO-1 as a Therapeutic Target in Renal Fibrosis. Life Sci. 2023, 334, 122209. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, H.; Huang, L.; Zhu, X.; Sha, J.; Li, G.; Ma, G.; Zhang, W.; Gu, M.; Guo, Y. Nrf2 Signaling Attenuates Epithelial-to-Mesenchymal Transition and Renal Interstitial Fibrosis via PI3K/Akt Signaling Pathways. Exp. Mol. Pathol. 2019, 111, 104296. [Google Scholar] [CrossRef]
- Huang, R.; Fu, P.; Ma, L. Kidney Fibrosis: From Mechanisms to Therapeutic Medicines. Signal Transduct. Target. Ther. 2023, 8, 129. [Google Scholar] [CrossRef]
- Yiu, W.H.; Lin, M.; Tang, S.C.W. Toll-like Receptor Activation: From Renal Inflammation to Fibrosis. Kidney Int. Suppl. 2014, 4, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, F.; Qiu, M.; He, L. Expression and Cellular Distribution of TLR4, MyD88, and NF-κB in Diabetic Renal Tubulointerstitial Fibrosis, in Vitro and in Vivo. Diabetes Res. Clin. Pract. 2014, 105, 206–216. [Google Scholar] [CrossRef]
- Boaru, S.G.; Borkham-Kamphorst, E.; Van De Leur, E.; Lehnen, E.; Liedtke, C.; Weiskirchen, R. NLRP3 Inflammasome Expression Is Driven by NF-κB in Cultured Hepatocytes. Biochem. Biophys. Res. Commun. 2015, 458, 700–706. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, J.; Zeng, A.; Song, L. Pharmacological Functions of Salidroside in Renal Diseases: Facts and Perspectives. Front. Pharmacol. 2024, 14, 1309598. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, G.; Darisipudi, M.N.; Anders, H.-J. Canonical and Non-Canonical Effects of the NLRP3 Inflammasome in Kidney Inflammation and Fibrosis. Nephrol. Dial. Transplant. 2014, 29, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, X.; Lei, W.; Hou, Y.; Zhang, Y.; Tang, R.; Yang, Z.; Tian, Y.; Zhu, Y.; Wang, C.; et al. The NLRP3 Inflammasome in Fibrosis and Aging: The Known Unknowns. Ageing Res. Rev. 2022, 79, 101638. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-C.; Wang, G.-H.; Lu, J.; Chen, P.-P.; Zhang, Y.; Hu, Z.-B.; Ma, K.-L. Role of Podocyte Injury in Glomerulosclerosis. In Renal Fibrosis: Mechanisms and Therapies; Liu, B.-C., Lan, H.-Y., Lv, L.-L., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1165, pp. 195–232. ISBN 9789811388705. [Google Scholar]
- Armelloni, S.; Corbelli, A.; Giardino, L.; Li, M.; Ikehata, M.; Mattinzoli, D.; Messa, P.; Pignatari, C.; Watanabe, S.; Rastaldi, M.P. Podocytes: Recent Biomolecular Developments. Biomol. Concepts 2014, 5, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Balkawade, R.S.; Chen, C.; Crowley, M.R.; Crossman, D.K.; Clapp, W.L.; Verlander, J.W.; Marshall, C.B. Podocyte-Specific Expression of Cre Recombinase Promotes Glomerular Basement Membrane Thickening. Am. J. Physiol.-Ren. Physiol. 2019, 316, F1026–F1040. [Google Scholar] [CrossRef]
- Bruno, V.; Mühlig, A.K.; Oh, J.; Licht, C. New Insights into the Immune Functions of Podocytes: The Role of Complement. Mol. Cell. Pediatr. 2023, 10, 3. [Google Scholar] [CrossRef]
- Meng, X.; Chen, Y.; Macip, S.; Leppard, K. PML-II Regulates ERK and AKT Signal Activation and IFNα-Induced Cell Death. Cell Commun. Signal. 2021, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kumar, G.S.; Hansen, U.; Zoccheddu, M.; Sacchetti, C.; Holmes, Z.J.; Lee, M.C.; Beckmann, D.; Wen, Y.; Mikulski, Z.; et al. Oxidative Stress Promotes Fibrosis in Systemic Sclerosis through Stabilization of a Kinase-Phosphatase Complex. JCI Insight 2022, 7, e155761. [Google Scholar] [CrossRef]
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 2017, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Gu, C.; Tian, F.; Jia, Z.; Yang, J. NDRG2 Knockdown Promotes Fibrosis in Renal Tubular Epithelial Cells through TGF-Β1/Smad3 Pathway. Cell Tissue Res. 2017, 369, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Piera-Velazquez, S.; Jimenez, S.A. Oxidative Stress Induced by Reactive Oxygen Species (ROS) and NADPH Oxidase 4 (NOX4) in the Pathogenesis of the Fibrotic Process in Systemic Sclerosis: A Promising Therapeutic Target. J. Clin. Med. 2021, 10, 4791. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Li, S.; Zhang, Y.; Tie, X.; Feng, R.; Guo, X.; Qiao, X.; Wang, L. Overexpression of Corin Ameliorates Kidney Fibrosis through Inhibition of Wnt/β-Catenin Signaling in Mice. Am. J. Pathol. 2024, 194, 101–120. [Google Scholar] [CrossRef]
- Balakumar, P.; Alqahtani, A.; Khan, N.A.; Mahadevan, N.; Dhanaraj, S.A. Mechanistic Insights into Hyperuricemia-Associated Renal Abnormalities with Special Emphasis on Epithelial-to-Mesenchymal Transition: Pathologic Implications and Putative Pharmacologic Targets. Pharmacol. Res. 2020, 161, 105209. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, R.N.; Sallam, N.; El-Abhar, H.S. Activated ROCK/Akt/eNOS and ET-1/ERK Pathways in 5-Fluorouracil-Induced Cardiotoxicity: Modulation by Simvastatin. Sci. Rep. 2020, 10, 14693. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-H. Mesangial Cells and Renal Fibrosis. In Renal Fibrosis: Mechanisms and Therapies; Liu, B.-C., Lan, H.-Y., Lv, L.-L., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1165, pp. 165–194. ISBN 9789811388705. [Google Scholar]
- Zhang, Y.; Jin, D.; Kang, X.; Zhou, R.; Sun, Y.; Lian, F.; Tong, X. Signaling Pathways Involved in Diabetic Renal Fibrosis. Front. Cell Dev. Biol. 2021, 9, 696542. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bager, C.L.; Karsdal, M.A.; Chondros, D.; Taverna, D.; Willumsen, N. Blood-Based Extracellular Matrix Biomarkers as Predictors of Survival in Patients with Metastatic Pancreatic Ductal Adenocarcinoma Receiving Pegvorhyaluronidase Alfa. J. Transl. Med. 2021, 19, 39. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Q.; Huang, J.; Gong, W.; Zou, Y.; Zhang, L.; Liu, P.; Huang, H. CK2α Promotes Advanced Glycation End Products-Induced Expressions of Fibronectin and Intercellular Adhesion Molecule-1 via Activating MRTF-a in Glomerular Mesangial Cells. Biochem. Pharmacol. 2018, 148, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Moon, J.-Y. The Role of Inflammation in Diabetic Kidney Disease. Korean J. Intern. Med. 2021, 36, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Zhao, X. USP9X Prevents AGEs-Induced Upregulation of FN and TGF-Β1 through Activating Nrf2-ARE Pathway in Rat Glomerular Mesangial Cells. Exp. Cell Res. 2020, 393, 112100. [Google Scholar] [CrossRef]
- Agarwal, A. Single-Nephron Glomerular Filtration Rate in Healthy Adults. N. Engl. J. Med. 2017, 377, 1202–1204. [Google Scholar] [CrossRef]
- Huang, Z.; Liao, Y.; Zheng, Y.; Ye, S.; Zhang, Q.; Yu, X.; Liu, X.; Li, N. Zinc Deficiency Causes Glomerulosclerosis and Renal Interstitial Fibrosis through Oxidative Stress and Increased Lactate Metabolism in Rats. Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Siani, A.; Infante-Teixeira, L.; d’Arcy, R.; Roberts, I.V.; El Mohtadi, F.; Donno, R.; Tirelli, N. Polysulfide Nanoparticles Inhibit Fibroblast-to-Myofibroblast Transition via Extracellular ROS Scavenging and Have Potential Anti-Fibrotic Properties. Biomater. Adv. 2023, 153, 213537. [Google Scholar] [CrossRef] [PubMed]
- Sulijaya, B.; Takahashi, N.; Yamada, M.; Yokoji, M.; Sato, K.; Aoki-Nonaka, Y.; Nakajima, T.; Kishino, S.; Ogawa, J.; Yamazaki, K. The Anti-inflammatory Effect of 10-oxo- Trans -11-octadecenoic Acid (KetoC) on RAW 264.7 Cells Stimulated with Porphyromonas Gingivalis Lipopolysaccharide. J. Periodontal Res. 2018, 53, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Pförringer, D.; Breu, M.; Crönlein, M.; Kolisch, R.; Kanz, K.-G. Closure Simulation for Reduction of Emergency Patient Diversion: A Discrete Agent-Based Simulation Approach to Minimizing Ambulance Diversion. Eur. J. Med. Res. 2018, 23, 32. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Song, Y.; Liu, Z.; Zhang, L.; Yang, L.; Li, J. Receptor for Advanced Glycation End Products (RAGE): A Pivotal Hub in Immune Diseases. Molecules 2022, 27, 4922. [Google Scholar] [CrossRef]
- Prajapati, R.; Park, S.E.; Seong, S.H.; Paudel, P.; Fauzi, F.M.; Jung, H.A.; Choi, J.S. Monoamine Oxidase Inhibition by Major Tanshinones from Salvia Miltiorrhiza and Selective Muscarinic Acetylcholine M4 Receptor Antagonism by Tanshinone I. Biomolecules 2021, 11, 1001. [Google Scholar] [CrossRef] [PubMed]
- The Committee on the Proper Use of SGLT2 Inhibitors. Recommendations on the Proper Use of SGLT2 Inhibitors. J. Diabetes Investig. 2020, 11, 257–261. [Google Scholar] [CrossRef]
- Tziastoudi, M.; Theoharides, T.C.; Nikolaou, E.; Efthymiadi, M.; Eleftheriadis, T.; Stefanidis, I. Key Genetic Components of Fibrosis in Diabetic Nephropathy: An Updated Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 15331. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Vivarelli, S.; Costa, C.; Teodoro, M.; Giambò, F.; Tsatsakis, A.M.; Fenga, C. Polyphenols: A Route from Bioavailability to Bioactivity Addressing Potential Health Benefits to Tackle Human Chronic Diseases. Arch. Toxicol. 2023, 97, 3–38. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Slavova-Kazakova, A.; Janiak, M.A.; Sulewska, K.; Kancheva, V.D.; Karamać, M. Synergistic, Additive, and Antagonistic Antioxidant Effects in the Mixtures of Curcumin with (−)-Epicatechin and with a Green Tea Fraction Containing (−)-Epicatechin. Food Chem. 2021, 360, 129994. [Google Scholar] [CrossRef] [PubMed]
- Rafii, F. The Role of Colonic Bacteria in the Metabolism of the Natural Isoflavone Daidzin to Equol. Metabolites 2015, 5, 56–73. [Google Scholar] [CrossRef]
- Kim, C.-K.; Yu, J.; Le, D.; Han, S.; Yu, S.; Lee, M. Anti-Inflammatory Activity of Caffeic Acid Derivatives from Ilex Rotunda. Int. Immunopharmacol. 2023, 115, 109610. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, Y.; Wu, W.; Li, H.; Ren, S.; Li, J. Polycyclic Aromatics Observed in Enzymatic Lignin by Spectral Characterization and Ruthenium Ion-Catalyzed Oxidation. J. Agric. Food Chem. 2021, 69, 12148–12155. [Google Scholar] [CrossRef]
- Yang, X.; Tomás-Barberán, F.A. Tea Is a Significant Dietary Source of Ellagitannins and Ellagic Acid. J. Agric. Food Chem. 2019, 67, 5394–5404. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Nishimura, S.; Hatano, M.; Igarashi, M.; Kakeya, H. Total Synthesis and Antimicrobial Activity of Chlorocatechelin a. J. Org. Chem. 2015, 80, 6076–6082. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Soares, C.; Tenreiro Machado, J.A.; Lopes, A.M.; Vieira, E.; Delerue-Matos, C. Electrochemical Impedance Spectroscopy Characterization of Beverages. Food Chem. 2020, 302, 125345. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent Advances on the Improvement of Quercetin Bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Murakami, N.; Mulvaney, P.; Danesh, M.; Abudayyeh, A.; Diab, A.; Abdel-Wahab, N.; Abdelrahim, M.; Khairallah, P.; Shirazian, S.; Kukla, A.; et al. A Multi-Center Study on Safety and Efficacy of Immune Checkpoint Inhibitors in Cancer Patients with Kidney Transplant. Kidney Int. 2021, 100, 196–205. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Muchová, J.; Országhová, Z.; Žitnanová, I.; Trebatický, B.; Breza, J.; Duracková, Z. The Effect of Natural Polyphenols on the Oxidative Stress Markers in Patients with Diabetic Nephropathy. Free Radic. Biol. Med. 2014, 75, S42. [Google Scholar] [CrossRef]
- Sahakyan, G.; Vejux, A.; Sahakyan, N. The Role of Oxidative Stress-Mediated Inflammation in the Development of T2DM-Induced Diabetic Nephropathy: Possible Preventive Action of Tannins and Other Oligomeric Polyphenols. Molecules 2022, 27, 9035. [Google Scholar] [CrossRef]
- Han, G.; Wang, Y.; Liu, T.; Gao, J.; Duan, F.; Chen, M.; Yang, Y.; Wu, C. Salvianolic Acid B Acts against Non-small Cell Lung Cancer A549 Cells via Inactivation of the MAPK and Smad2/3 Signaling Pathways. Mol. Med. Rep. 2022, 25, 184. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J. Autophagy and Polyphenol Intervention Strategy in Aging. Trends Food Sci. Technol. 2023, 132, 1–10. [Google Scholar] [CrossRef]
- Huo, R.; Huang, X.; Yang, Y.; Yang, Y.; Lin, J. Potential of Resveratrol in the Treatment of Interstitial Lung Disease. Front. Pharmacol. 2023, 14, 1139460. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, Toxicity, and Clinical Applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Veres, G.; Vas, N.F.; Lyngby Lassen, M.; Béresová, M.; Krizsan, A.K.; Forgács, A.; Berényi, E.; Balkay, L. Effect of Grey-Level Discretization on Texture Feature on Different Weighted MRI Images of Diverse Disease Groups. PLoS ONE 2021, 16, e0253419. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Nascimento, A.; Bolea, G.; Meyer, G.; Gayrard, S.; Lacampagne, A.; Cazorla, O.; Reboul, C. Key Role of Endothelium in the eNOS-Dependent Cardioprotection with Exercise Training. J. Mol. Cell. Cardiol. 2017, 102, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lyu, C.; Chen, D.; Cai, W.; Kou, F.; Li, Q.; Wei, H.; Zhang, H. Gallic Acid Treats Hypertrophic Scar in Rabbit Ears via the TGF-β/Smad and TRPC3 Signaling Pathways. Pharmaceuticals 2023, 16, 1514. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Taramasso, M.; Pozzoli, A.; Buzzatti, N.; Alfieri, O. Assessing Operative Risk and Benefit in Elderly Patients with Heart Valve Disease. Eur. Heart J. 2013, 34, 2788–2791. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy, K.; Kaczor, J.; Sołtyk, A.; Szymańska, N.; Stecko, J.; Sleziak, J.; Kulbacka, J.; Baczyńska, D. Application of Luteolin in Neoplasms and Nonneoplastic Diseases. Int. J. Mol. Sci. 2023, 24, 15995. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Sun, Q.; Zhang, Y.-M.; Zou, L.; Zhao, Y.-Y. TGF-β/Smad Signaling Pathway in Tubulointerstitial Fibrosis. Front. Pharmacol. 2022, 13, 860588. [Google Scholar] [CrossRef]
- Hou, J.; Ma, R.; Zhu, S.; Wang, Y. Revealing the Therapeutic Targets and Mechanism of Ginsenoside Rg1 for Liver Damage Related to Anti-Oxidative Stress Using Proteomic Analysis. Int. J. Mol. Sci. 2022, 23, 10045. [Google Scholar] [CrossRef] [PubMed]
- Jicman, D.; Niculet, E.; Lungu, M.; Onisor, C.; Rebegea, L.; Bobeica, C.; Elisei, A.; Anghel, L.; Tatu, A. Rare Case of Metachronous Tumor: Nasopharyngeal and Colorectal Carcinoma. Exp. Ther. Med. 2021, 22, 1417. [Google Scholar] [CrossRef]
- Wang, F.; Gan, J.; Li, R.; Yang, R.; Mao, X.; Liu, S.; Chen, Y.; Duan, Z.; Li, J. Naringin from Ganshuang Granule Inhibits Inflammatory to Relieve Liver Fibrosis through TGF-β-Smad Signaling Pathway. PLoS ONE 2024, 19, e0304185. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Babiker, A.Y.; Anwar, S. Hesperidin, a Bioflavonoid in Cancer Therapy: A Review for a Mechanism of Action through the Modulation of Cell Signaling Pathways. Molecules 2023, 28, 5152. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Rakib, A.; Singh, U.P. The NLRP3 Inflammasome Inhibitor Dapansutrile Attenuates Cyclophosphamide-Induced Interstitial Cystitis. Front. Immunol. 2022, 13, 903834. [Google Scholar] [CrossRef] [PubMed]
- Allemailem, K.S.; Almatroudi, A.; Alharbi, H.O.A.; AlSuhaymi, N.; Alsugoor, M.H.; Aldakheel, F.M.; Khan, A.A.; Rahmani, A.H. Apigenin: A Bioflavonoid with a Promising Role in Disease Prevention and Treatment. Biomedicines 2024, 12, 1353. [Google Scholar] [CrossRef]
- Sapian, S.; Budin, S.B.; Taib, I.S.; Mariappan, V.; Zainalabidin, S.; Chin, K.Y. Role of Polyphenol in Regulating Oxidative Stress, Inflammation, Fibrosis, and Apoptosis in Diabetic Nephropathy. Endocr. Metab. Immune Disord.-Drug Targets 2022, 22, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Au, E.H.; Francis, A.; Bernier-Jean, A.; Teixeira-Pinto, A. Prediction Modeling—Part 1: Regression Modeling. Kidney Int. 2020, 97, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Runa, F.; Ortiz-Soto, G.; De Barros, N.R.; Kelber, J.A. Targeting SMAD-Dependent Signaling: Considerations in Epithelial and Mesenchymal Solid Tumors. Pharmaceuticals 2024, 17, 326. [Google Scholar] [CrossRef]
- Islam, F.; Roy, S.; Zehravi, M.; Paul, S.; Sutradhar, H.; Yaidikar, L.; Kumar, B.R.; Dogiparthi, L.K.; Prema, S.; Nainu, F.; et al. Polyphenols Targeting MAP Kinase Signaling Pathway in Neurological Diseases: Understanding Molecular Mechanisms and Therapeutic Targets. Mol. Neurobiol. 2024, 61, 2686–2706. [Google Scholar] [CrossRef]
- Reed, E.B.; Ard, S.; La, J.; Park, C.Y.; Culligan, L.; Fredberg, J.J.; Smolyaninova, L.V.; Orlov, S.N.; Chen, B.; Guzy, R.; et al. Anti-Fibrotic Effects of Tannic Acid through Regulation of a Sustained TGF-Beta Receptor Signaling. Respir. Res. 2019, 20, 168. [Google Scholar] [CrossRef]
- Moliterno, P.; Donangelo, C.M.; Borgarello, L.; Pécora, M.; Olascoaga, A.; Noboa, O.; Boggia, J. Association of Dietary Patterns with Cardiovascular and Kidney Phenotypes in an Uruguayan Population Cohort. Nutrients 2021, 13, 2213. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of Oxidative Stress, Cellular Communication and Signaling Pathways in Cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Miao, Z.; Jiang, Y.; Xia, W.; Wang, X.; Shi, Y.; Ni, L.; Li, S.; Xiao, J.; Sheng, S.; et al. Chrysophanol Prevents IL-1β-Induced Inflammation and ECM Degradation in Osteoarthritis via the Sirt6/NF-κB and Nrf2/NF-κB Axis. Biochem. Pharmacol. 2023, 208, 115402. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef] [PubMed]
- Allamreddy, S.; Arora, M.; Ganugula, R.; Friend, R.; Basu, R.; Kumar, M.N.V.R. Prospects for the Convergence of Polyphenols with Pharmaceutical Drugs in Type 2 Diabetes: Challenges, Risks, and Strategies. Pharmacol. Rev. 2024, 9, 100003. [Google Scholar] [CrossRef]
- Macena, M.d.L.; Nunes, L.F.d.S.; da Silva, A.F.; Pureza, I.R.O.M.; Praxedes, D.R.S.; Santos, J.C.d.F.; Bueno, N.B. Effects of Dietary Polyphenols in the Glycemic, Renal, Inflammatory, and Oxidative Stress Biomarkers in Diabetic Nephropathy: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2022, 80, 2237–2259. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wang, J.; Fan, J.; Jia, H.; Li, J. Interrelation of Natural Polyphenol and Fibrosis in Diabetic Nephropathy. Molecules 2025, 30, 20. https://doi.org/10.3390/molecules30010020
Ma Y, Wang J, Fan J, Jia H, Li J. Interrelation of Natural Polyphenol and Fibrosis in Diabetic Nephropathy. Molecules. 2025; 30(1):20. https://doi.org/10.3390/molecules30010020
Chicago/Turabian StyleMa, Ye, Jiakun Wang, Juyue Fan, Huiyang Jia, and Jinyao Li. 2025. "Interrelation of Natural Polyphenol and Fibrosis in Diabetic Nephropathy" Molecules 30, no. 1: 20. https://doi.org/10.3390/molecules30010020
APA StyleMa, Y., Wang, J., Fan, J., Jia, H., & Li, J. (2025). Interrelation of Natural Polyphenol and Fibrosis in Diabetic Nephropathy. Molecules, 30(1), 20. https://doi.org/10.3390/molecules30010020






