Efficient CO2 Electrocarboxylation Using Dye-Sensitized Photovoltaics
Abstract
1. Introduction
2. Results and Discussion
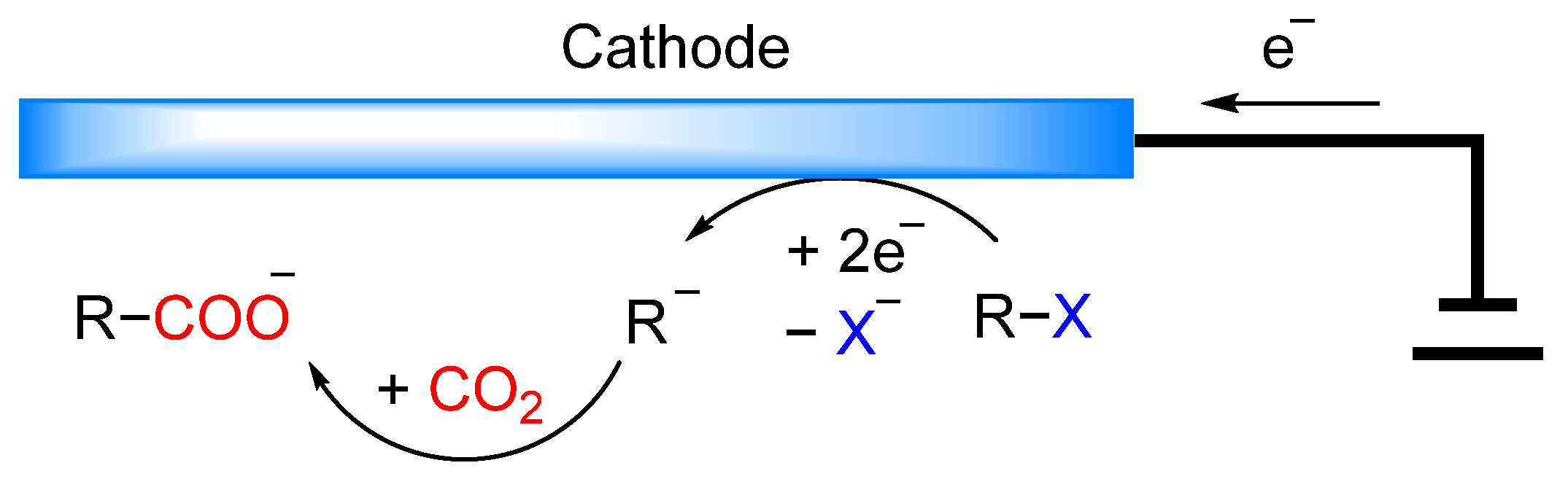
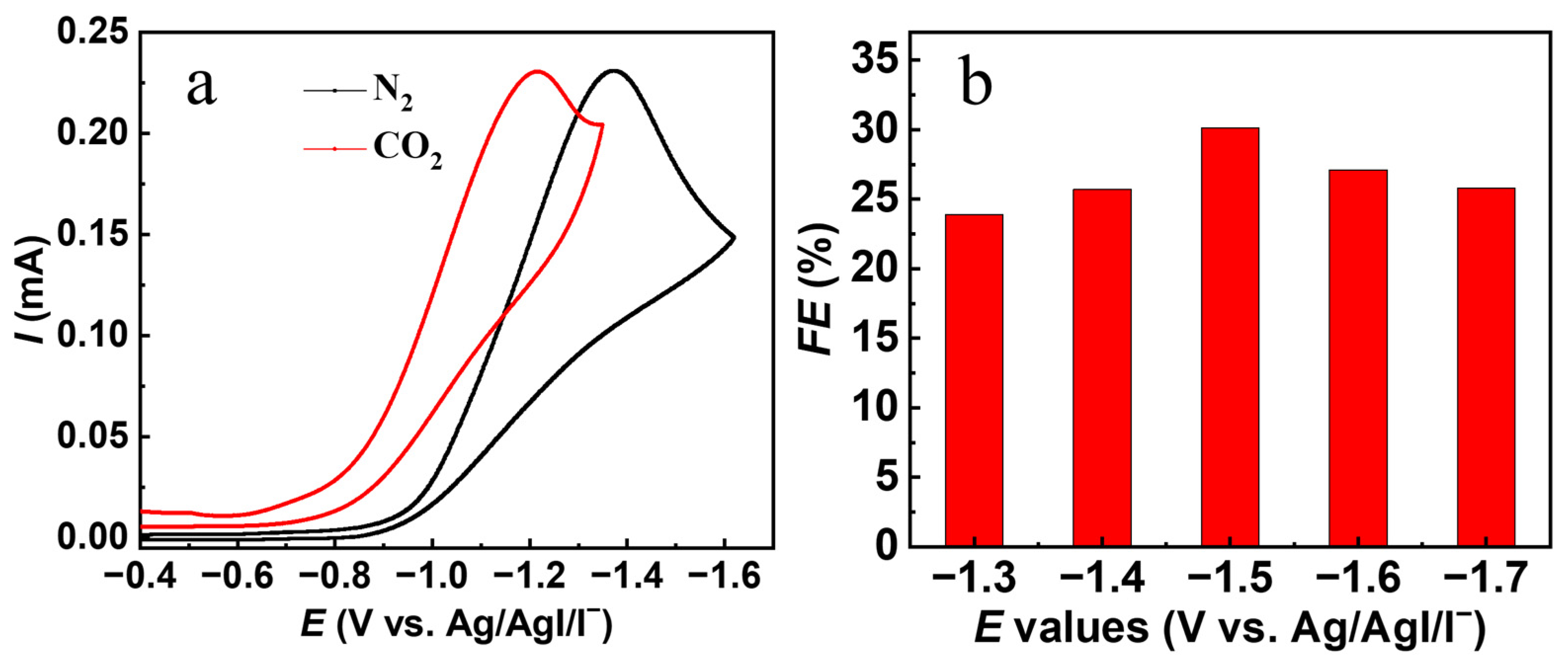
2.1. Cyclic Voltammetry (CV) Behavior for 2-BP and Controlled-Potential Electrolysis of 2-BP with CO2
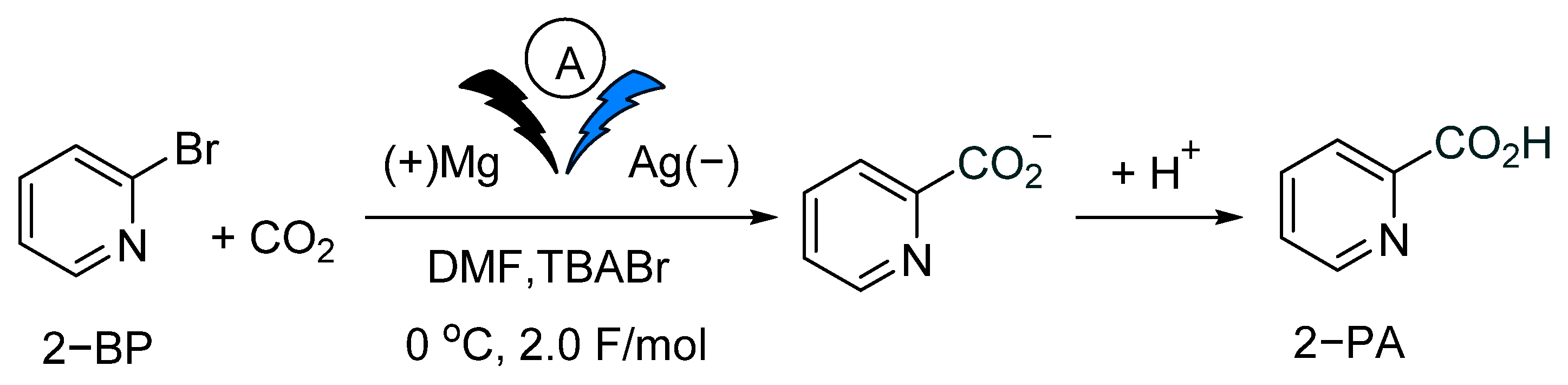
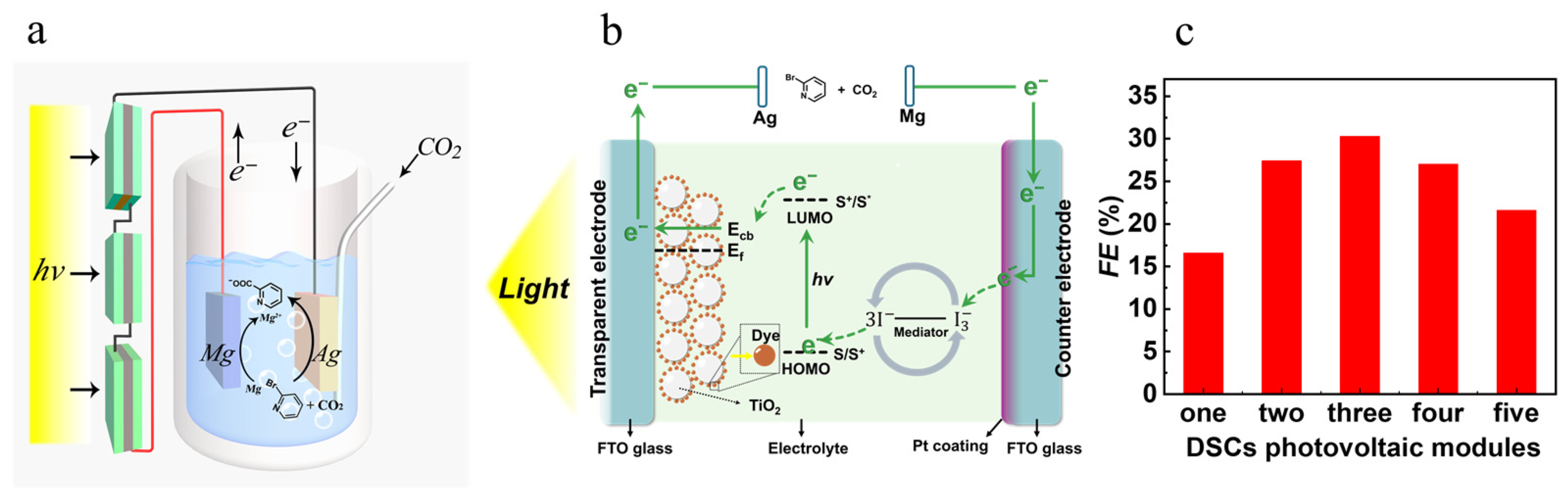
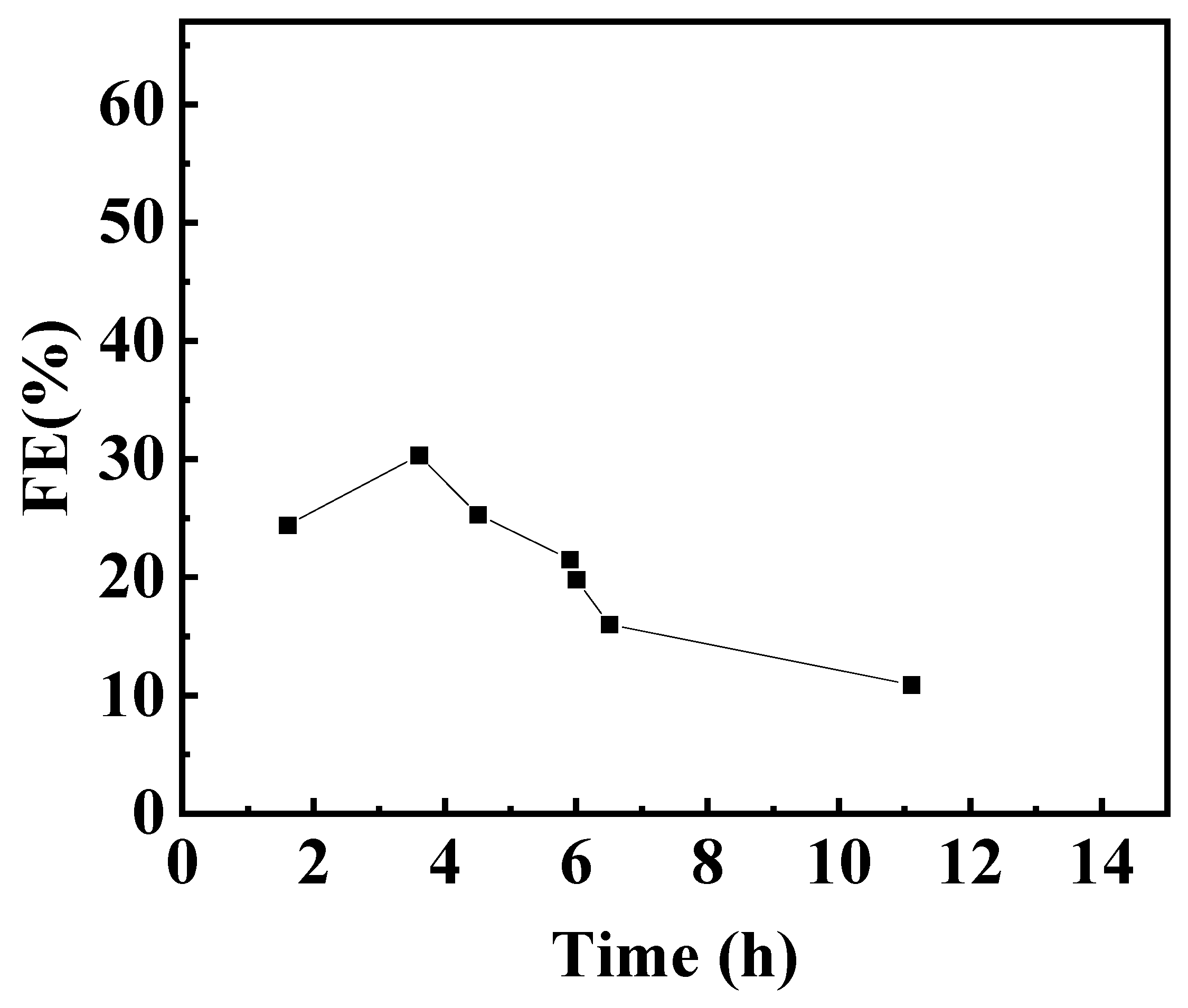
2.2. Artificial Photosynthesis for Electrocarboxylation of 2-BP with CO2 Using Dye-Sensitized Photovoltaics
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. A Typical Procedure for Cyclic Voltammogram and Controlled-Potential Electrolysis of 2-BP with CO2
3.3. A Typical Procedure for the Preparation and Assembling of DSCs and the Artificial Photosynthesis for Electrocarboxylation of 2-BP with CO2 Using Dye-Sensitized Photovoltaics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farhadian, A.; Semenov, M.E.; Mohammadi, A.; Mirzakimov, U.Z.; Son, E.R.; Varfolomeev, M.A. Efficient carbon dioxide capture using biodegradable surfactants in form of clathrate hydrate: New eco-friendly approach. J. Environ. Chem. Eng. 2024, 12, 113830. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.; Dong, X.; Ding, Y.; Hu, S.; Zhang, R.; Dai, Y.; Cui, P.; Xiao, Z.; Zhao, D.; et al. Selective CO2 electrolysis to CO using isolated antimony alloyed copper. Nat. Commun. 2023, 14, 340. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, X.; Wu, B.; Li, P.; Chen, S.; Lu, R.; Lai, W.; Shen, Y.; Zhuang, Z.; Zhu, J.; et al. Organic Molecule Functionalization Enables Selective Electrochemical Reduction of Dilute CO2 Feedstock. Angew. Chem. Int. Ed. 2024, e202417196. [Google Scholar] [CrossRef]
- Santos, D.S.; Almeida, C.V.S.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Selectivity and catalytic performance of Pdx@Pty/C nanoparticles for methanol electrooxidation. Electrochim. Acta 2023, 467, 143018. [Google Scholar] [CrossRef]
- Bastos, T.L.; Gelamo, R.V.; Colmati, F. Carbon-graphene hybrid supporting platinum–tin electrocatalyst to enhance ethanol oxidation reaction. J. Appl. Electrochem. 2023, 54, 1225–1237. [Google Scholar] [CrossRef]
- Chen, B.; Liu, Q.; Wang, H.; Lu, J. Recent Advances in the Electrocarboxylation of CO2 with Ketones, Aldehydes, and Imines. Curr. Org. Chem. 2023, 27, 734–740. [Google Scholar] [CrossRef]
- Liu, H.; Bai, Y.; Wu, M.; Yang, Y.; Wang, Y.; Li, L.; Hao, J.; Yan, W.; Shi, W. A Regenerable Bi-Based Catalyst for Efficient and Stable Electrochemical CO2 Reduction to Formate at Industrial Current Densities. Angew. Chem. Int. Ed. 2024, 63, e202411575. [Google Scholar] [CrossRef]
- Zeng, W.; Qiu, Y. Electrochemical conversion of organic compounds and inorganic small molecules. Sci. China Chem. 2024, 67, 3223–3246. [Google Scholar] [CrossRef]
- Sun, G.; Liao, L.; Ran, C.; Ye, J.; Yu, D. Recent Advances in Electrochemical Carboxylation with CO2. Acc. Chem. Res. 2024, 57, 2728–2745. [Google Scholar] [CrossRef]
- Kannan, N.; Vakeesan, D. Solar energy for future world: A review. Renew. Sustain. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- Li, B.; Yang, X.; Li, S.; Yuan, J. Stable block copolymer single-material organic solar cells: Progress and perspective. Energy Environ. Sci. 2023, 16, 723–744. [Google Scholar] [CrossRef]
- Lewis, N.S. Research opportunities to advance solar energy utilization. Science 2016, 351, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, Z.; Wu, Y.; Wang, P.; Liu, R.; Zhang, L. Review on photovoltaic with battery energy storage system for power supply to buildings: Challenges and opportunities. J. Energy Storage 2023, 61, 106763. [Google Scholar] [CrossRef]
- Parida, B.; Iniyan, S.; Goic, R. A review of solar photovoltaic technologies. Renew. Sustain. Energy Rev. 2011, 15, 1625–1636. [Google Scholar] [CrossRef]
- Schreier, M.; Curvat, L.; Giordano, F.; Steier, L.; Abate, A.; Zakeeruddin, S.M.; Luo, J.; Mayer, M.T.; Gratzel, M. Efficient photosynthesis of carbon monoxide from CO2 using perovskite photovoltaics. Nat. Commun. 2015, 6, 7326. [Google Scholar] [CrossRef]
- Gao, J.; Sahli, F.; Liu, C.; Ren, D.; Guo, X.; Werner, J.; Jeangros, Q.; Zakeeruddin, S.M.; Ballif, C.; Grätzel, M.; et al. Solar Water Splitting with Perovskite/Silicon Tandem Cell and TiC-Supported Pt Nanocluster Electrocatalyst. Joule 2019, 3, 2930–2941. [Google Scholar] [CrossRef]
- Luo, J.; Im, J.-H.; Mayer, M.T.; Schreier, M.; Nazeeruddin, M.K.; Park, N.-G.; Tilley, S.D.; Fan, H.J.; Grätzel, M. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 2014, 345, 1593–1596. [Google Scholar] [CrossRef]
- Marshall, J. Springtime for the artificial leaf. Nature 2014, 510, 22–24. [Google Scholar] [CrossRef]
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef]
- Han, G.H.; Bang, J.; Park, G.; Choe, S.; Jang, Y.J.; Jang, H.W.; Kim, S.Y.; Ahn, S.H. Recent Advances in Electrochemical, Photochemical, and Photoelectrochemical Reduction of CO2 to C2+ Products. Small 2023, 19, 2205765. [Google Scholar] [CrossRef]
- Li, Y.; Cui, F.; Ross, M.B.; Kim, D.; Sun, Y.; Yang, P. Structure-Sensitive CO2 Electroreduction to Hydrocarbons on Ultrathin 5-fold Twinned Copper Nanowires. Nano Lett. 2017, 17, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.T.H.; Verma, S.; Ma, S.; Fister, T.T.; Timoshenko, J.; Frenkel, A.I.; Kenis, P.J.A.; Gewirth, A.A. Nanoporous Copper-Silver Alloys by Additive-Controlled Electrodeposition for the Selective Electroreduction of CO2 to Ethylene and Ethanol. J. Am. Chem. Soc. 2018, 140, 5791–5797. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Chen, P.; Jaroniec, M.; Qiao, S.Z. Molecular Scaffolding Strategy with Synergistic Active Centers To Facilitate Electrocatalytic CO2 Reduction to Hydrocarbon/Alcohol. J. Am. Chem. Soc. 2017, 139, 18093–18100. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R.; et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016, 353, 467–470. [Google Scholar] [CrossRef]
- Tian, K.; Chen, R.; Xu, J.; Yang, G.; Xu, X.; Zhang, Y. Understanding the Photo- and Electro-Carboxylation of o-Methylbenzophenone with Carbon Dioxide. Catalysts 2020, 10, 664. [Google Scholar] [CrossRef]
- Chen, R.; Tian, K.; He, D.; Gao, T.; Yang, G.; Xu, J.; Chen, H.; Wang, D.; Zhang, Y. Carboxylation of α,β-Unsaturated Ketones by CO2 Fixation through Photoelectro-chemistry. ACS Appl. Energy Mater. 2020, 3, 5813–5818. [Google Scholar] [CrossRef]
- Zhong, B.; He, D.; Chen, R.; Gao, T.; Wang, Y.; Chen, H.; Zhang, Y.; Wang, D. Understanding photoelectrochemical kinetics in a model CO2 fixation reaction. Phys. Chem. Chem. Phys. 2019, 21, 17517–17520. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, C.; Ren, H.; Luo, P.; Wan, Q.; Zhou, H.; Chen, B.; Zhang, X. Efficient Photosynthesis of Value-Added Chemicals by Electrocarboxylation of Bromobenzene with CO2 Using a Solar Energy Conversion Device. Int. J. Mol. Sci. 2024, 25, 10608. [Google Scholar] [CrossRef]
- Lee, K.M.; Jang, J.H.; Balamurugan, M.; Kim, J.E.; Jo, Y.I.; Nam, K.T. Redox-neutral electrochemical conversion of CO2 to dimethyl carbonate. Nat. Energy 2021, 6, 733–741. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, D.; Zhang, K.; Zhang, G.; Luo, Y.; Lu, J. Electrochemical activation of CO2 in ionic liquid (BMIMBF4): Synthesis of organic carbonates under mild conditions. Green Chem. 2008, 10, 202–206. [Google Scholar] [CrossRef]
- You, Y.; Kanna, W.; Takano, H.; Hayashi, H.; Maeda, S.; Mita, T. Electrochemical Dearomative Dicarboxylation of Heterocycles with Highly Negative Reduction Potentials. J. Am. Chem. Soc. 2022, 144, 3685–3695. [Google Scholar] [CrossRef] [PubMed]
- Sheta, A.M.; Alkayal, A.; Mashaly, M.A.; Said, S.B.; Elmorsy, S.S.; Malkov, A.V.; Buckley, B.R. Selective Electrosynthetic Hydrocarboxylation of α,β-Unsaturated Esters with Carbon Dioxide. Angew. Chem. Int. Ed. 2021, 60, 21832–21837. [Google Scholar] [CrossRef] [PubMed]
- Maret, C.; David, N.; Pierrot, D.; Leonel, E.; Levacher, V.; Briere, J.F.; Oudeyer, S. Synthesis of α-Chloroarylacetic Acid via Electrochemical Carboxylation of α,α-Dichloroarylmethane Derivatives. Molecules 2023, 28, 6704. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, Y.; Chen, B.; Yu, S.; Zhou, H.; Qu, K.; Kong, Y.; Huang, X.; Zhang, X.; Lu, J. Electrocarboxylation of Dichlorobenzenes on a Silver Electrode in DMF. Catalysts 2017, 7, 274. [Google Scholar] [CrossRef]
- Li, L.; Yan, Z.; Ran, C.; Liu, Y.; Zhang, S.; Gao, T.; Dai, L.; Liao, L.; Ye, J.; Yu, D. Electro-reductive carboxylation of C—Cl bonds in unactivated alkyl chlorides and polyvinyl chloride with CO2. Chin. Chem. Lett. 2024, 35, 110104. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.F.; Zhang, W.Z.; Ren, W.M.; Lu, X.B. Electrocarboxylation of N-Acylimines with Carbon Dioxide: Access to Substituted α-Amino Acids. Org. Lett. 2022, 24, 3565–3569. [Google Scholar] [CrossRef]
- Guan, A.; Quan, Y.; Chen, Y.; Liu, Z.; Zhang, J.; Kan, M.; Zhang, Q.; Huang, H.; Qian, L.; Zhang, L.; et al. Efficient CO2 fixation with acetophenone on Ag-CeO2 electrocatalyst by a double activation strategy. Chin. J. Catal. 2022, 43, 3134–3141. [Google Scholar] [CrossRef]
- Zhao, S.; Horne, M.; Bond, A.M.; Zhang, J. Electrocarboxylation of acetophenone in ionic liquids: The influence of proton availability on product distribution. Green Chem. 2014, 16, 2242–2251. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, S.; Luo, P.; Xu, S.; Zhang, X.; Zhou, H.; Du, J.; Yang, J.; Xin, N.; Kong, Y.; et al. Fixation of CO2 along with bromopyridines on a silver electrode. R. Soc. Open Sci. 2018, 5, 180897. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yin, J.; Nie, Z.; Yang, Z.; Li, D.; Wang, J.; Liu, X.; Jin, C.; Zhang, X.; Ma, T. Earth-abundant and nano-micro composite catalysts of Fe3O4@reduced graphene oxide for green and economical mesoscopic photovoltaic devices with high efficiencies up to 9%. J. Mater. Chem. A 2016, 4, 67–73. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, K.; Tao, L.; Lu, X.; Zhang, W. Recent advances in electrochemical carboxylation reactions using carbon dioxide. Green Chem. Eng. 2022, 3, 125–137. [Google Scholar] [CrossRef]
- Wang, S.; Feng, T.; Wang, Y.; Qiu, Y. Recent Advances in Electrocarboxylation with CO2. Chem. Asian J. 2022, 17, e202200543. [Google Scholar] [CrossRef]
- Niu, D.; Zhang, J.; Zhang, K.; Xue, T.; Lu, J. Electrocatalytic Carboxylation of Benzyl Chloride at Silver Cathode in Ionic Liquid BMIMBF4. Chin. J. Chem. 2009, 27, 1041–1044. [Google Scholar] [CrossRef]
- Niu, D.; Xiao, L.; Zhang, A.; Zhang, G.; Tan, Q.; Lu, J. Electrocatalytic carboxylation of aliphatic halides at silver cathode in acetonitrile. Tetrahedron 2008, 64, 10517–10520. [Google Scholar] [CrossRef]
- Isse, A.A.; Ferlin, M.G.; Gennaro, A. Electrocatalytic reduction of arylethyl chlorides at silver cathodes in the presence of carbon dioxide: Synthesis of 2-arylpropanoic acids. J. Electroanal. Chem. 2005, 581, 38–45. [Google Scholar] [CrossRef]
- Harnchana, V.; Chaiyachad, S.; Pimanpang, S.; Saiyasombat, C.; Srepusharawoot, P.; Amornkitbamrung, V. Hierarchical Fe3O4-reduced graphene oxide nanocomposite grown on NaCl crystals for triiodide reduction in dye-sensitized solar cells. Sci. Rep. 2019, 9, 1494. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.; Wang, Y.; Zhang, H.; Zhou, H.; Wei, Y.; Tao, S.; Ma, T. Composite catalyst of rosin carbon/Fe3O4: Highly efficient counter electrode for dye-sensitized solar cells. Chem. Commun. 2014, 50, 1701–1703. [Google Scholar] [CrossRef]
- Isse, A.A.; Gennaro, A. Electrocatalytic carboxylation of benzyl chlorides at silver cathodes in acetonitrile. Chem. Commun. 2002, 2798–2799. [Google Scholar] [CrossRef]
- Isse, A.A.; Gottardello, S.; Maccato, C.; Gennaro, A. Silver nanoparticles deposited on glassy carbon. Electrocatalytic activity for reduction of benzyl chloride. Electrochem. Commun. 2006, 8, 1707–1712. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ren, H.; Zhou, H.; Luo, P.; Wan, Q.; Zhang, X.; Wang, B.; Chen, B.; Zhang, B. Efficient CO2 Electrocarboxylation Using Dye-Sensitized Photovoltaics. Molecules 2025, 30, 40. https://doi.org/10.3390/molecules30010040
Zhang Y, Ren H, Zhou H, Luo P, Wan Q, Zhang X, Wang B, Chen B, Zhang B. Efficient CO2 Electrocarboxylation Using Dye-Sensitized Photovoltaics. Molecules. 2025; 30(1):40. https://doi.org/10.3390/molecules30010040
Chicago/Turabian StyleZhang, Yingtian, Huaiyan Ren, Huawei Zhou, Peipei Luo, Qi Wan, Xianxi Zhang, Bo Wang, Baoli Chen, and Bo Zhang. 2025. "Efficient CO2 Electrocarboxylation Using Dye-Sensitized Photovoltaics" Molecules 30, no. 1: 40. https://doi.org/10.3390/molecules30010040
APA StyleZhang, Y., Ren, H., Zhou, H., Luo, P., Wan, Q., Zhang, X., Wang, B., Chen, B., & Zhang, B. (2025). Efficient CO2 Electrocarboxylation Using Dye-Sensitized Photovoltaics. Molecules, 30(1), 40. https://doi.org/10.3390/molecules30010040





