Effects and Impact of Selenium on Human Health, A Review
Abstract
:1. Introduction
2. Distribution and Intake of Selenium
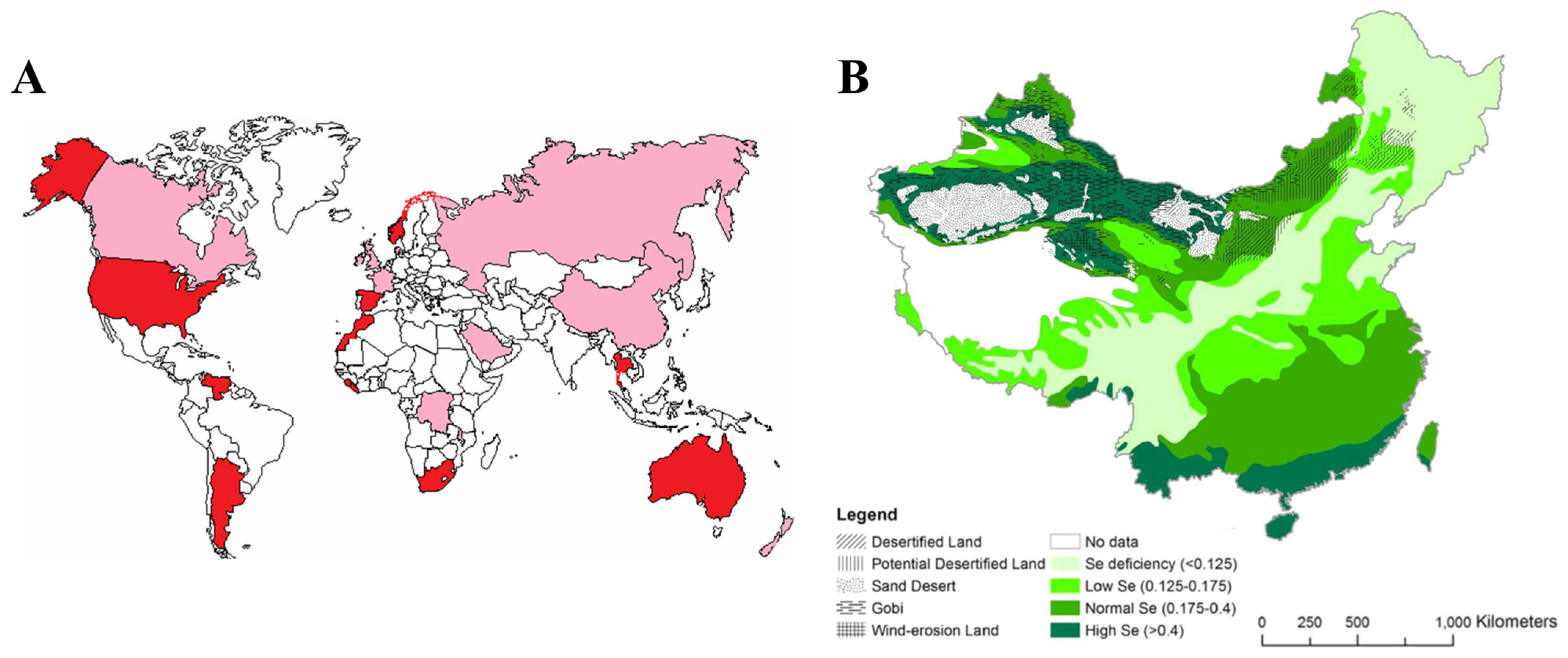
| Country (Region) | Sample Size | Content (mg/kg) | Remark | |
|---|---|---|---|---|
| Range | Mean | |||
| Global [37] | / | / | 0.4 | / |
| USA [38,39] | 910 | <0.1–4.32 | 0.31 | / |
| 1–10 | / | Selenium-rich area | ||
| Canada [40] | 173 | 0.03–2 | 0.26 | / |
| Japan [41] | 180 | 0.05–2.8 | 0.43 | Agricultural soil |
| 0.51 | ||||
| India [39] | / | 0.025–0.71 | / | Selenium-deficient area |
| / | 1–20 | / | Selenium-rich area | |
| Brazil [42] | 0–2.14 | / | / | |
| Spain [43] | 490 | 0.003–2.7 | 0.4 | Region of Murcia |
| Greece [39] | / | 0.05–0.10 | / | Selenium-deficient area |
| >0.2 | / | Selenium-sufficient area | ||
| Belgium [44] | 539 | 0.14–0.70 | / | Agricultural soil |
| UK [45] | 0.10–4 | / | / | |
| Netherlands [46] | 42 | 0.12–1.97 | 0.62 | Grassland |
| 41 | 0.20–1.20 | 0.53 | Cultivated land | |
| Scotland [47] | 661 | <0.06–19.2 | 1.04 | / |
| Sweden [47] | 5170 | <0.05–13.3 | 0.30 | / |
| New Zealand [39] | / | 0.1–4 | / | / |
| Scandinavian Peninsula [48] | / | 0.42–0.57 | / | / |
| Denmark [49] | / | 0.14–0.52 | / | / |
| Norway [37] | / | 3–6 | / | / |
| Pakistan [50] | / | 0.041 | / | / |
| Canada [51] | / | 0.30 | / | / |
| Iran [52] | / | 0.45 | / | / |
| Turkey [53] | / | 0.9 | / | / |
| Australia [54] | / | <0.2 | / | / |
| China [36] | / | 0.058 | / | / |
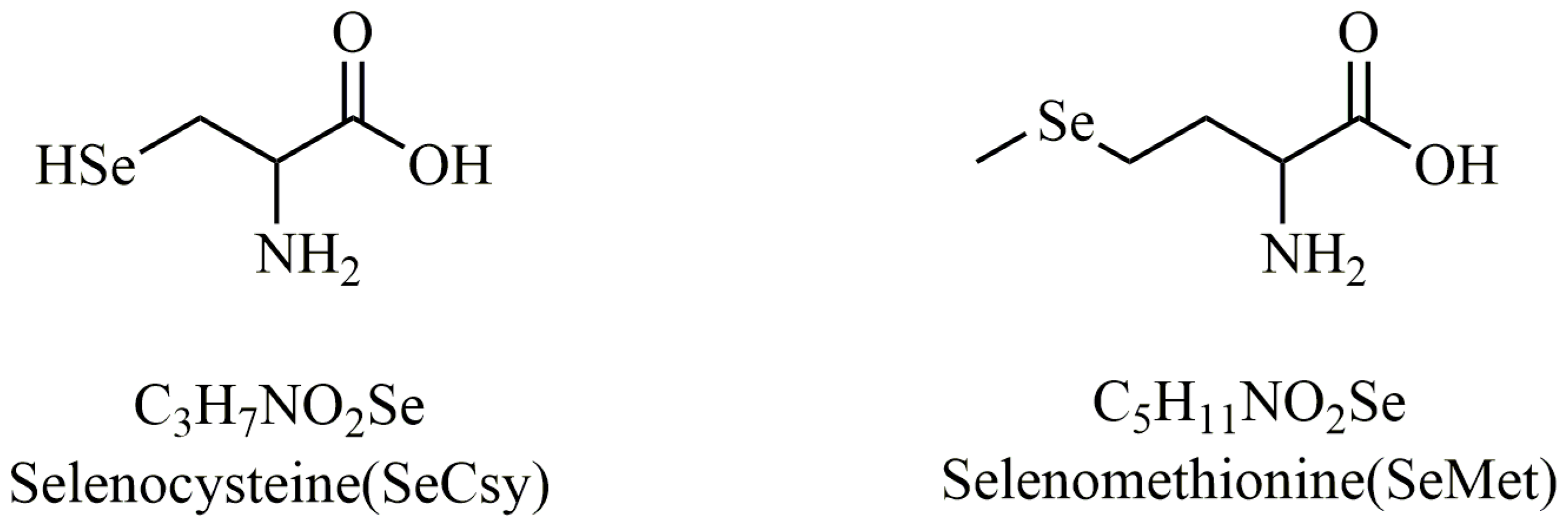
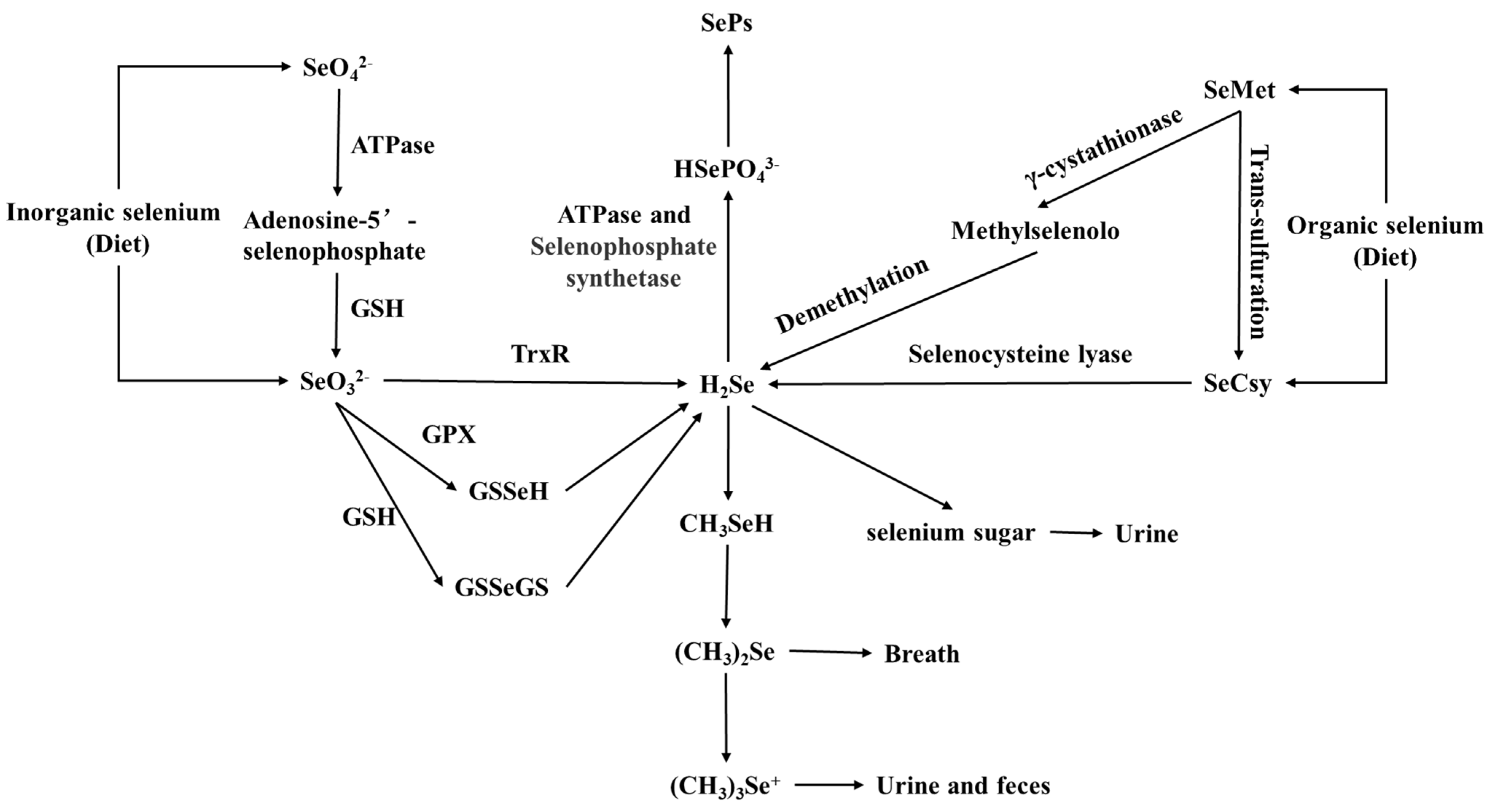
3. Absorption and Metabolism of Selenium in the Human Body
4. The Biological Functions of Selenium
| Selenoprotein | Abbreviation | Function | Sec Location in Protein [25] | Length of Protein [25] |
|---|---|---|---|---|
| Glutathione peroxidase 1 | GPX1 | Exists in the cytoplasm, reduces cellular H2O2 [86,87]. | 47 | 201 |
| Glutathione peroxidase 2 | GPX2 | Present in the gastrointestinal tract, reduces peroxide in gut [88,89]. | 40 | 190 |
| Glutathione Peroxidase 3 | GPX3 | Present in plasma, reduces peroxide in blood [90,91]. | 73 | 226 |
| Glutathione Peroxidase 4 | GPX4 | The enzyme, an anti-oxidative lipid repair enzyme, is localized to the cytosol, mitochondria, and nucleus. It reduces hydrogen peroxide radicals and lipid peroxides to water and lipid alcohols and prevents iron-induced cellular ferroptosis [92,93]. | 73 | 197 |
| Glutathione Peroxidase 5 | GPX5 | Present in epididymal tissue [25]. | Unknown | Unknown |
| Glutathione Peroxidase 6 | GPX6 | Present in olfactory epithelial cells and placental tissue [94]. | 73 | 221 |
| Thioredoxin reductase 1 | TXNRD1, TrxR1, TR1 | Localized to cytoplasm and nucleus and regenerates reduced thioredoxin [95]. | 498 | 499 |
| Thioredoxin reductase 2 | TXNRD2, TrxR2, TR3 | Localized to mitochondria and regenerates reduced thioredoxin [96]. | 655 | 656 |
| Thioredoxin reductase 3 | TXNRD3, TrxR3, TR2, TGR | Testes-specific expression, which regenerates reduced thioredoxin [97]. | 522 | 523 |
| Methionine-R-sulfoxide reductase B1 | MSRB1, SELR, SELX | Regulator of Factin repolymerization in macrophages during innate immune response, which works in concert with MICALs to reduce oxidated methionine (R)-sulfoxide (Met-RO) back to methionine [98,99]. | 95 | 116 |
| Selenophosphate synthetase 2 | SEPHS2, SPS2 | Involved in synthesis of all selenoproteins, including itself [100]. | 60 | 448 |
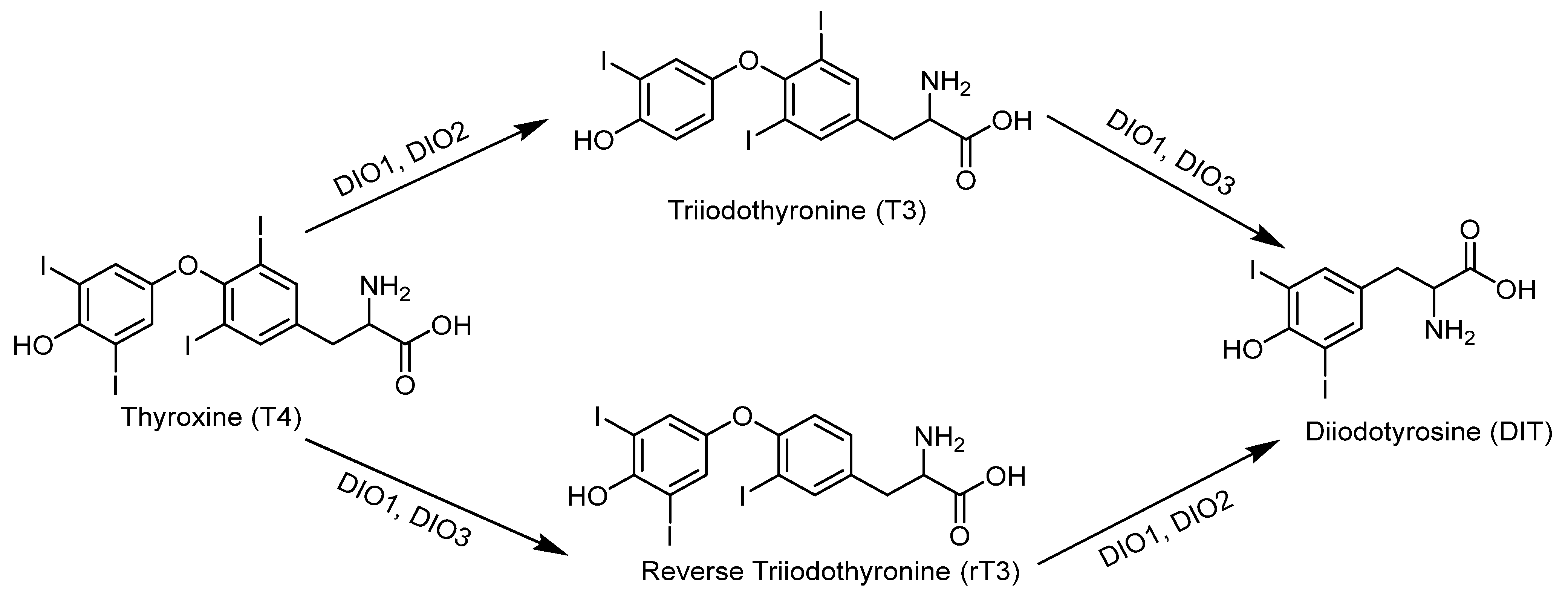
| Iodothyronine deiodinase 1 | DIO1, D1 | Important for systemic active thyroid hormone levels [101]. | 126 | 249 |
| Iodothyronine deiodinase 2 | DIO2, D2 | ER enzyme important for local active thyroid hormone levels [101]. | 133, 266 | 265 |
| Iodothyronine deiodinase 3 | DIO3, D3 | Inactivates thyroid hormone [101]. | 144 | 278 |
| Selenoprotein N | SELENON, SELN, SEPN1, SepN | Transmembrane protein localized to endoplasmic reticulum (ER). Mutations lead to multiminicore disease and other myopathies [102,103]. | 428 | 556 |
| Selenoprotein P | SELENOP, SEPP1, SEP, SELP, SEPP | Secreted into plasma for selenium transport to tissues [77,104]. | 59, 300, 318, 330, 345, 352, 367, 369, 376, 378 | 381 |
| Selenoprotein 15kDa | 15kDa, SEP15 | ER-resident thioredoxin-like oxidoreductase that complexes with uridine–guanosine–guanosine–thymodine (UGGT) and improves protein quality control by correcting misglycosylated/misfolded glycoproteins via the calnexin–calreticulinendoplasmic reticulum protein 57 (ERp57) axis and pH-dependent endoplasmic reticulum protein 44 (ERp44) system [105,106]. | 93 | 162 |
| Selenoprotein M | SELENOM, SELM, SEPM | Thioredoxin-like ER-resident protein that may be involved in the regulation of body weight and energy metabolism [107]. | 48 | 145 |
| Selenoprotein K | SELENOK, SELK | Transmembrane protein localized to the ER and involved in calcium flux in immune cells, as well as ER-associated degradation in cell lines [108,109]. | 92 | 94 |
| Selenoprotein S | SELENOS, SELS, SEPS1, VIMP | Transmembrane protein found in the ER and involved in ER-associated degradation [110,111]. | 188 | 189 |
| Selenoprotein O | SELENOO, SELO | Mitochondrial protein that contains a C-X-X-U motif (where C is cytosine, X is any nucleotide, and U is uridine), suggestive of redox function [112]. | 667 | 669 |
| Selenoprotein W | SELENOW, SELW, SEPW1 | Putative antioxidant role, which may be important in muscle growth [113]. | 13 | 87 |
| Selenoprotein T | SELENOT, SELT | Oxidoreductase localized to the Golgi complex and ER and manifests a thioredoxin-like fold and is involved in redox regulation and cell anchorage. Complexes with UDP-glucose: glycoprotein glucosyltransferases to improve process quality control. Deficiency leads to early embryonic lethality [114]. | 36 | 182 |
| Selenoprotein H | SELENOH, SELH, C11orf31 | Nuclear localization, which is involved in redox sensing and transcription [115,116]. | 44 | 122 |
| Selenoprotein V | SELENOV, SELV | Testes-specific expression [25]. | 273 | 346 |
| Selenoprotein I | SELENOI, SELI, SPT1 | Involved in phospholipid biosynthesis [117]. | 387 | 397 |
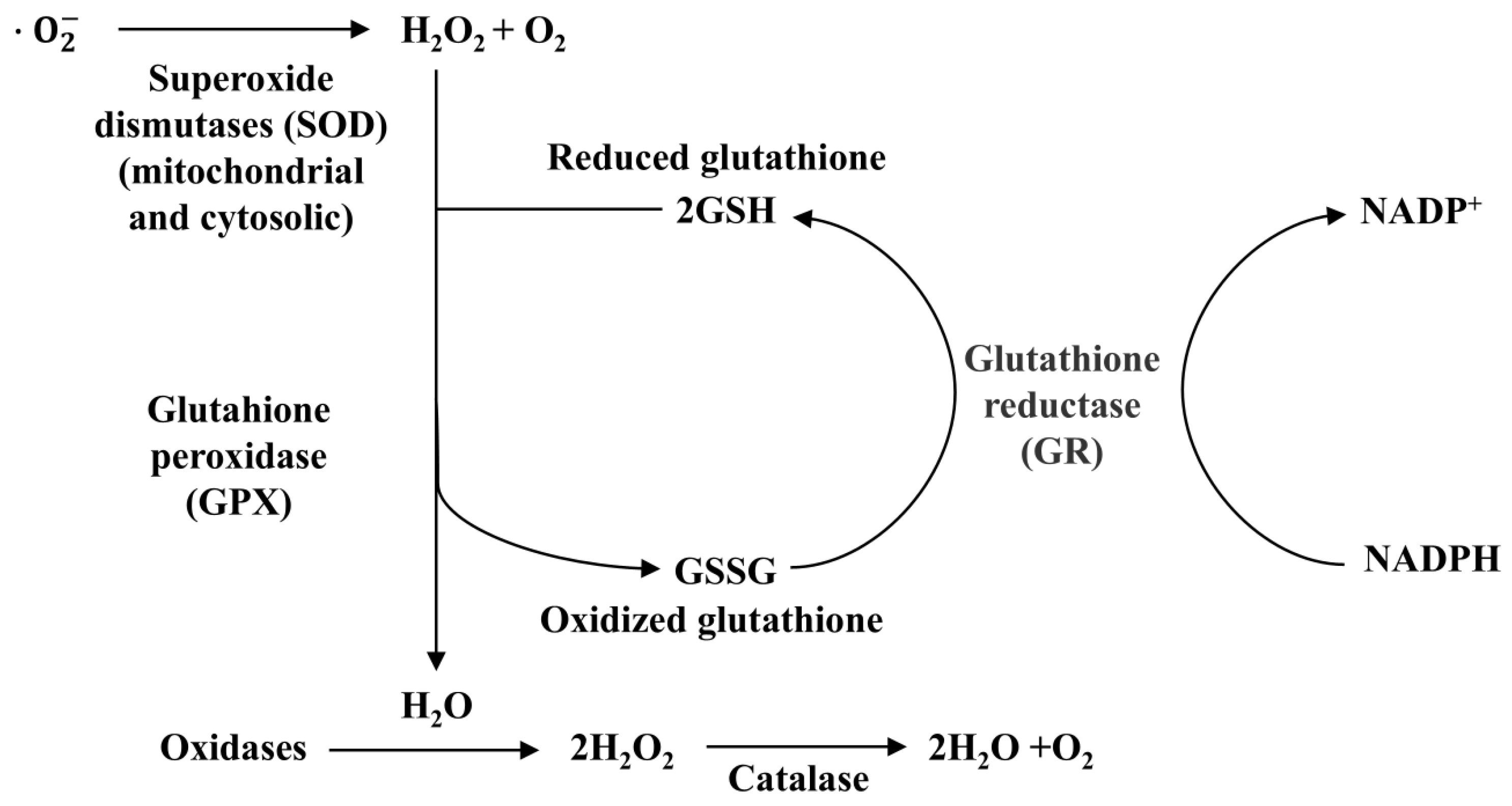
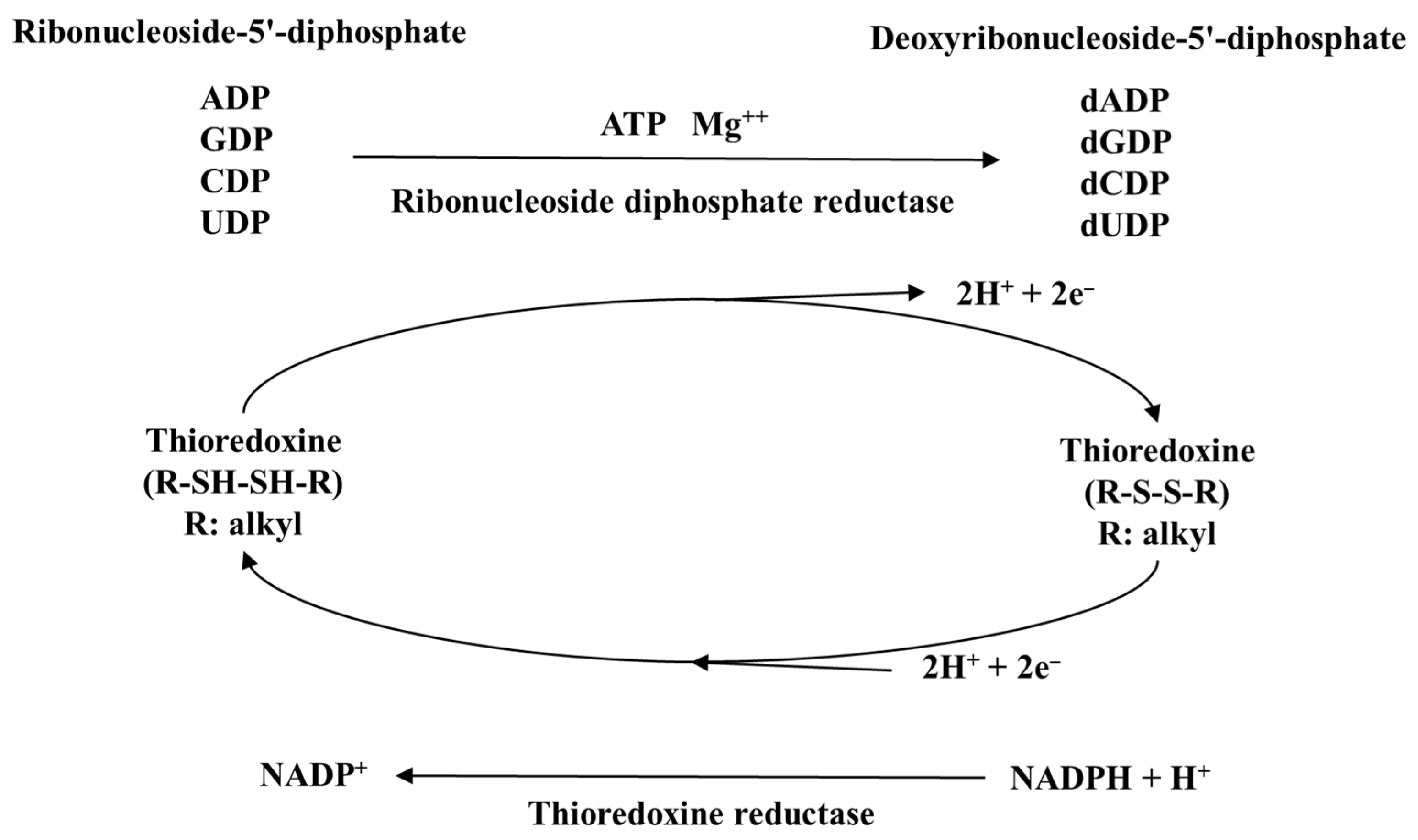
4.1. Antioxidant Properties of Selenium
4.2. Regulation of the Immune System by Selenium
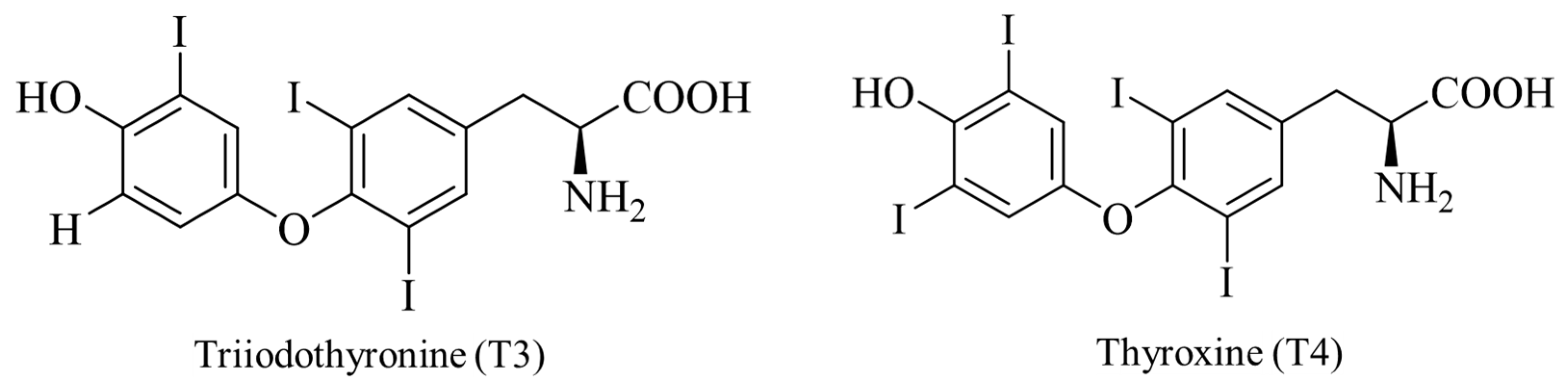
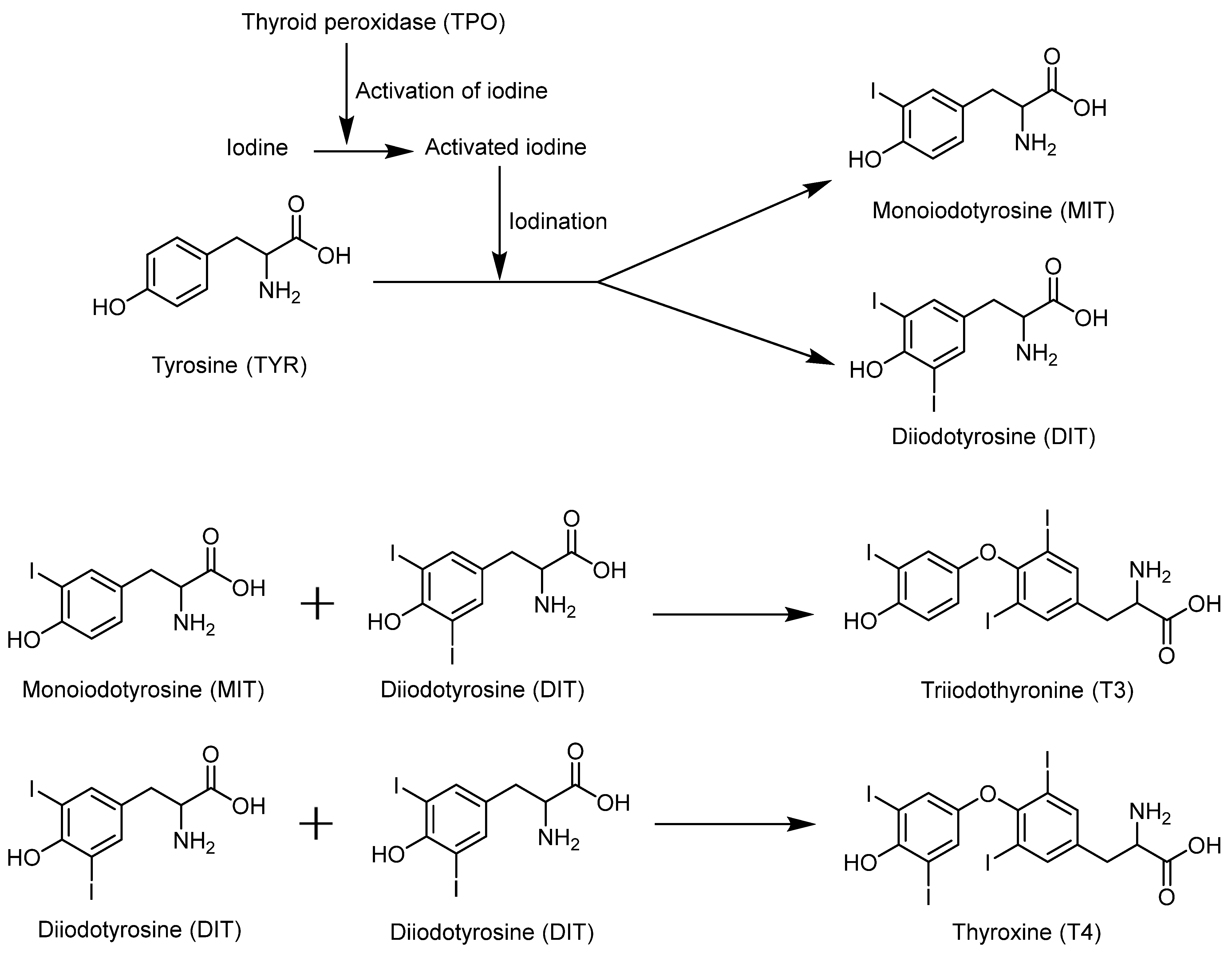
4.3. Selenium Promotes the Synthesis of Thyroid Hormones
4.4. Other Biological Functions of Selenium
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwarz, K.; Calvin, M.F. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kitts, D.; Giovannucci, E.L.; Sahye-Pudaruth, S.; Paquette, M.; Mejia, S.B.; Patel, D.; Kavanagh, M.; Tsirakis, T.; Kendall, C.W.; et al. Selenium, antioxidants, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 112, 1642–1652. [Google Scholar] [CrossRef]
- Khosravi, M.; Poursaleh, A.; Ghasempour, G.; Farhad, S.; Najafi, M. The effects of oxidative stress on the development of atherosclerosis. Biol. Chem. 2019, 400, 711–732. [Google Scholar] [CrossRef]
- Schomburg, L.; Orho-Melander, M.; Struck, J.; Bergmann, A.; Melander, O. Selenoprotein-P deficiency predicts cardiovascular disease and death. Nutrients 2019, 11, 1852. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Chirumbolo, S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition 2017, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; Nasr, H.M. Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. J. Trace Elem. Med. Bio. 2014, 28, 89–93. [Google Scholar] [CrossRef]
- Yu, S.Y.; Zhu, Y.J.; Li, W.G. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol. Trace Elem. Res. 1997, 56, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W. Increased intakes of selenium-enriched foods may benefit human health. J. Sci. Food Agric. 2007, 87, 1620–1629. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef]
- Ellis, D.R.; Salt, D.E. Plants, selenium and human health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yu, T.; Yang, Z.; Zhao, W.; Zhang, M.; Wang, Q. Constraint on selenium bioavailability caused by its geochemical behavior in typical Kaschin–Beck disease areas in Aba, Sichuan Province of China. Sci. Total Environ. 2014, 493, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.C.; Ge, X.; Green, K.A.; Liu, X. Selenium distribution in the local environment of selected villages of the Keshan Disease belt, Zhangjiakou district, Hebei province, people’s republic of China. Appl. Geochem. 2000, 15, 385–401. [Google Scholar] [CrossRef]
- Tan, J.A.; Zhu, W.; Wang, W.; Li, R.; Hou, S.; Wang, D.; Yang, L. Selenium in soil and endemic diseases in China. Sci. Total Environ. 2002, 284, 227–235. [Google Scholar] [CrossRef]
- Pang, W. Determination of selenium in lentinusedodes in selenium. Guangdong Weiliang Yuanshu Kexue 2006, 13, 54. [Google Scholar]
- Ekermans, L.G.; Schneider, J.V. Selenium in livestock production: A review. J. S. Afr. Vet. Assoc. 1982, 53, 223–228. [Google Scholar]
- Lv, Q.; Liang, X.; Nong, K.; Gong, Z.; Qin, T.; Qin, X.; Wang, D.; Zhu, Y. Advances in research on the toxicological effects of selenium. Bull. Environ. Contam. Toxicol. 2021, 106, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Ji, M.; Li, X.; Li, Z.; Wu, G.; Fu, X.; Yang, X.; Gao, X. Relationship between higher serum selenium level and adverse blood lipid profile. Clin. Nutr. 2018, 37, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Truswell, S. Recommended Dietary Intakes for Use in Australia; Food Australia Official Journal of CAFTA and AIFST: Cherrybrook, Australia, 1989. [Google Scholar]
- National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Recommended Dietary Allowances, 10th ed.; National Academies Press (US): Washington, DC, USA, 1989. [Google Scholar]
- Hornig, D.H.; Walter, P. Risk assessment and risk management of vitamins and minerals. Int. J. Vitam. Nutr. Res. 2004, 74, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Jin, Y.; Unverzagt, F.W.; Liang, C.; Hall, K.S.; Cao, J.; Ma, F.; Murrell, J.R.; Cheng, Y.; Li, P.; et al. Selenium level and depressive symptoms in a rural elderly Chinese cohort. BMC Psychiatry 2012, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Filippini, T.; Chawla, R.; Chaudhary, R.; Cilloni, S.; Datt, C.; Singh, S.; Dhillon, K.S.; Vinceti, M. Exposure to a high selenium environment in Punjab, India: Effects on blood chemistry. Sci. Total Environ. 2020, 716, 135347. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar]
- Xu, Z.N.; Lin, Z.Q.; Zhao, G.S.; Guo, Y.B. Biogeochemical behavior of selenium in soil-air-water environment and its effects on human health. Int. J. Environ. Sci. Technol. 2024, 21, 1159–1180. [Google Scholar]
- Rosenfeld, I.; Beath, O.A. Selenium: Geobotany, Biochemistry, Toxicity, and Nutrition; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Wang, Z.J.; Gao, Y.X. Biogeochemical cycling of selenium in Chinese environments. Appl. Geochem. 2001, 16, 1345–1351. [Google Scholar]
- Fordyce, F.M. Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology: Revised Edition; Springer: Berlin/Heidelberg, Germany, 2012; pp. 375–416. [Google Scholar]
- Barker, A.V.; Pilbeam, J.D. Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2015; pp. 165–198. [Google Scholar]
- Fleming, G.A. Selenium in Irish soils and plants. Soil Sci. 1962, 94, 28–35. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [PubMed]
- Liu, Y.; Li, F.; Yin, X.B.; Lin, Z.Q. Plant-based biofortification: From phytoremediation to Se-enriched agriculture products. In Green Chemistry for Environmental Sustainability; Routledge: London, UK, 2011; pp. 341–356. [Google Scholar]
- Li, J.; Du Laing, G.; Ferrer Martí, I.; Lens, P. Selenium biofortification for human and animal nutrition. In Environmental Technologies to Treat Selenium Pollution; IWA Publishing: London, UK, 2021; pp. 265–285. [Google Scholar]
- Mombo, S.; Schreck, E.; Dumat, C.; Laplanche, C.; Pierart, A.; Longchamp, M.; Besson, P.; Castrec-Rouelle, M. Bioaccessibility of selenium after human ingestion in relation to its chemical species and compartmentalization in maize. Environ. Geochem. Health 2016, 38, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.X.; Meharg, A.A.; Li, G.; Chen, Z.; Yang, L.; Chen, S.C.; Zhu, Y.G. Distribution of soil selenium in China is potentially controlled by deposition and volatilization? Sci. Rep. 2016, 6, 20953. [Google Scholar]
- Fordyce, F. Selenium geochemistry and health. Ambio 2007, 36, 94–97. [Google Scholar] [CrossRef]
- Shacklette, H.T.; Boerngen, J.G. Element Concentrations in Soils and Other Surficial Materials of the Conterminous United States: An Account of the Concentrations of 50 Chemical Elements in Samples of Soils and Other Regoliths; US Government Printing Office: Washington, DC, USA, 1984; p. 1270. [Google Scholar]
- Plant, J.A.; Bone, J.; Voulvoulis, N.; Kinniburgh, D.G.; Smedley, P.L.; Fordyce, F.M.; Klinck, B.A. Arsenic and selenium. Environ. Geochem. 2014, 11, 13–57. [Google Scholar]
- Mckeague, J.A.; Wolynetz, M.S. Background levels of minor elements in some Canadian soils. Geoderma 1980, 24, 299–307. [Google Scholar] [CrossRef]
- Yamada, H.; Kamada, A.; Usuki, M.; Yanai, J. Total selenium content of agricultural soils in Japan. Soil Sci. Plant Nutr. 2009, 55, 616–622. [Google Scholar]
- Matos, R.P.; Lima, V.M.; Windmöller, C.C.; Nascentes, C.C. Correlation between the natural levels of selenium and soil physicochemical characteristics from the Jequitinhonha Valley (MG), Brazil. J. Geochem. Explor. 2017, 172, 195–202. [Google Scholar]
- Pérez-Sirvent, C.; Martínez-Sánchez, M.J.; García-Lorenzo, M.L.; Molina, J.; Tudela, M.L.; Mantilla, W.; Bech, J. Selenium content in soils from Murcia Region (SE, Spain). J. Geochem. Explor. 2010, 107, 100–109. [Google Scholar]
- De Temmerman, L.; Waegeneers, N.; Thiry, C.; Du Laing, G.; Tack, F.; Ruttens, A. Selenium content of Belgian cultivated soils and its uptake by field crops and vegetables. Sci. Total Environ. 2014, 468, 77–82. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Bryson, R.J.; Meacham, M.C.; Bowen, H.C.; Johnson, S.E.; Hawkesford, M.J.; McGrath, S.P.; Zhao, F.J.; Breward, N.; et al. Biofortification of UK food crops with selenium. Proc. Nutr. Soc. 2006, 65, 169–181. [Google Scholar] [PubMed]
- Supriatin, S.; Weng, L.; Comans, R.N.J. Selenium speciation and extractability in Dutch agricultural soils. Sci. Total. Environ. 2015, 532, 368–382. [Google Scholar]
- Shand, C.A.; Eriksson, J.; Dahlin, A.S.; Lumsdon, D.G. Selenium concentrations in national inventory soils from Scotland and Sweden and their relationship with geochemical factors. J. Geochem. Explor. 2012, 121, 4–14. [Google Scholar]
- Gupta, U.C.; Gupta, S.C. Selenium in soils and crops, its deficiencies in livestock and humans: Implications for management, Commun. Soil Sci. Plan. 2000, 31, 1791–1807. [Google Scholar] [CrossRef]
- Bitterli, C.; Bañuelos, G.S.; Schulin, R. Use of transfer factors to characterize uptake of selenium by plants. J. Geochem. Explor. 2010, 107, 206–216. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ashraf, M.; Danish, M.; Ahmad, K.; Valeem, E.E. Assessment of selenium content in pasture and ewes in Punjab, Pakistan. Pak. J. Bot. 2008, 40, 1159–1162. [Google Scholar]
- B’Hymer, C.; Caruso, J.A. Canadian soil quality guidelines selenium environmental and human health effects. Can. J. Anal. Sci. Spectrosc. 2001, 46. [Google Scholar]
- Nazemi, L.; Nazmara, S.; Eshraghyan, M.R.; Younesian, M.; Sereshti, H.; Moameni, A.; Shahtaheri, J.; Nasseri, S. Selenium concentration in soil of Iran. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Sasmaz, M.; Akgül, B.; Sasmaz, A. Distribution and accumulation of selenium in wild plants growing naturally in the Gumuskoy (Kutahya) Mining Area, Turkey. B Environ. Contam. Tox. 2015, 94, 598–603. [Google Scholar] [CrossRef]
- Lyons, G.H.; Judson, G.J.; Ortiz-Monasterio, I.; Genc, Y.; Stangoulis, J.C.; Graham, R.D. Selenium in Australia: Selenium status and biofortification of wheat for better health. J. Trace Elem. Med. Bio. 2015, 19, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Sign. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yamashita, M.; Iida, H. Selenium content in seafood in Japan. Nutrients 2013, 5, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Haratake, M.; Fuchigami, T.; Nakayama, M. Selenium in seafood materials. J. Health Sci. 2011, 57, 215–224. [Google Scholar] [CrossRef]
- Xia, X.J.; Zhang, X.L.; Liu, M.C.; Duan, M.Y.; Zhang, S.S.; Wei, X.B.; Liu, X.Y. Toward improved human health: Efficacy of dietary selenium on immunity at the cellular level. Food Func. 2021, 12, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhao, C.; Zhang, T. Selenium transformation and selenium-rich foods. Food Biosci. 2021, 40, 100875. [Google Scholar] [CrossRef]
- Han, M.; Liu, K. Selenium and selenoproteins: Their function and development of selenium-rich foods. Int. J. Food Sci. Technol. 2022, 57, 7026–7037. [Google Scholar] [CrossRef]
- GB 13105-1991; Hygienic Standard for the Limit of Selenium in Food. Standardization Administration of China: Beijing, China, 1991.
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Amiard, J.C.; Berthet, B.; Boutaghou, S. Seasonal selenium variations in mussels and oysters from a French marine farm. J. Food Compos. Anal. 1993, 6, 370–380. [Google Scholar] [CrossRef]
- Foster, L.H.; Sumar, S. Selenium in health and disease: A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 211–228. [Google Scholar] [CrossRef]
- Oster, O.; Prellwitz, W. Selenium and cardiovascular disease. Biol. Trace Elem. Res. 1990, 24, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Q.; Wang, S.Z.; Zhou, R.H.; Sun, S.Z. Endemic selenium intoxication of humans in China. Am. J. Clin. Nutr. 1983, 37, 872–881. [Google Scholar] [CrossRef]
- Meyers, L.D.; Hellwig, J.P.; Otten, J.J. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Nutrient, S. Energy intakes for the European community. In Report of the Scientific Committee for Food, 31st ed.; DG Industry: San Francisco, CA, USA, 1993. [Google Scholar]
- Monsen, E.R. Dietary reference iintakes for the antioxidantnutrients: Vitamin C, vitamin E, selenium, and carotenoids. J. Am. Dietetic Assoc. 2000, 100, 637–640. [Google Scholar] [CrossRef]
- Department of Health Committee on the Medical Aspects of Food Policy. Dietary Reference Values, Dietary Reference Values for Food Energy and Nutrients for the United Kingdom: Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy; HM Stationery Office: London, UK, 1991; p. 41. [Google Scholar]
- Thomson, C.; Paterson, E. Australian and New Zealand nutrient reference values for selenium. In Report to the Ministry of Health Department of Human Nutrition; University of Otago: Dunedin, New Zealand, 2001. [Google Scholar]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.E.; Leschik-Bonnet, E.; Heseker, H. German Nutrition Society, Revised reference values for selenium intake. J. Trace Elem. Med. Bio. 2015, 32, 195–199. [Google Scholar] [CrossRef]
- Nève, J. Methods in determination of selenium states. J. Trace Elem. Electrolytes Health Dis. 1991, 5, 1–17. [Google Scholar] [PubMed]
- Joint FAO, WHO. Vitamin and mineral requirements in human nutrition; FAO, WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Combs , G.F., Jr. Selenium in global food systems. Brit. J. Nutr. 2001, 85, 517–547. [Google Scholar]
- Kato, T.; Read, R.; Rozga, J.; Burk, R.F. Evidence for intestinal release of absorbed selenium in a form with high hepatic extraction. Am. J. Physiol. 1992, 262, G854–G858. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Regulation of selenium metabolism and transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef]
- Ganther, H.E. Pathways of selenium metabolism including respiratory excretory products. J. Am. Coll. Toxicol. 1986, 5, 1–5. [Google Scholar] [CrossRef]
- Lu, J.; Berndt, C.; Holmgren, A. Metabolism of selenium compounds catalyzed by the mammalian selenoprotein thioredoxin reductase. Biochim. Biophys. Acta 2009, 1790, 1513–1519. [Google Scholar] [CrossRef]
- Esaki, N.; Nakamura, T.; Tanaka, H.; Suzuki, T.; Morino, Y.; Soda, K. Enzymic synthesis of selenocysteine in rat liver. Biochemistry 1981, 20, 4492–4496. [Google Scholar] [CrossRef] [PubMed]
- Tobe, R.; Mihara, H. Delivery of selenium to selenophosphate synthetase for selenoprotein biosynthesis. Biochim. Biophys. Acta 2018, 1862, 2433–2440. [Google Scholar] [CrossRef]
- Mistry, H.D.; Pipkin, F.B.; Redman, C.W.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef] [PubMed]
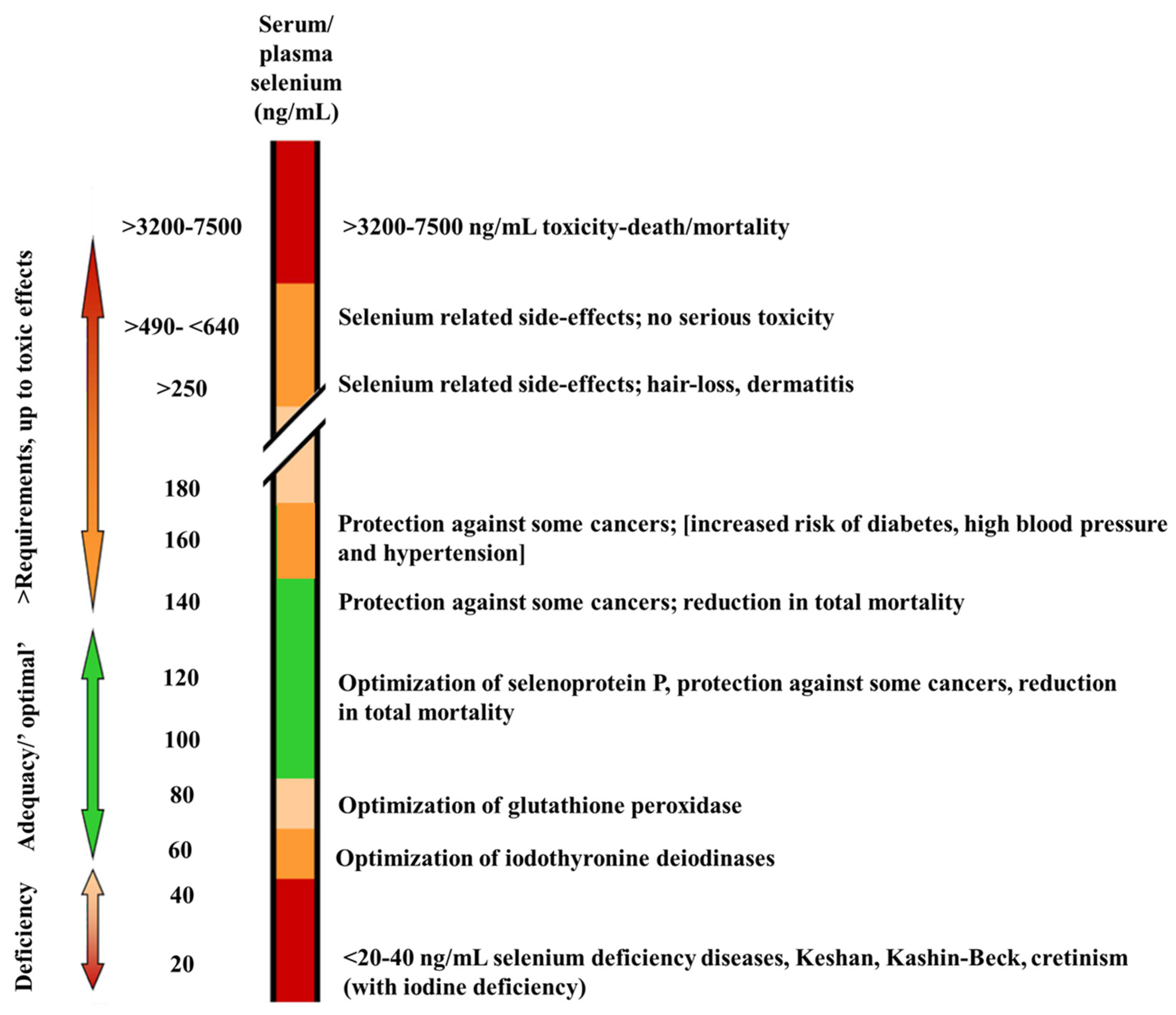
- Sun, Y.; Wang, Z.; Gong, P.; Yao, W.; Ba, Q.; Wang, H. Review on the health-promoting effect of adequate selenium status. Front. nutr. 2023, 10, 1136458. [Google Scholar] [CrossRef] [PubMed]
- Benhar, M. Roles of mammalian glutathione peroxidase and thioredoxin reductase enzymes in the cellular response to nitrosative stress. Free Radical Biol. Med. 2018, 127, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.C.; Zoidis, E.; Surai, P.F.; Zervas, G. Selenoproteins and maternal nutrition. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 361–372. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Lei, X.G.; Cheng, W.H.; McClung, J.P. Metabolic regulation and function of glutathione peroxidase-1. Annu. Rev. Nutr. 2007, 27, 41–61. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Kipp, A. Glutathione peroxidases in different stages of carcinogenesis. Biochim. Biophys. Acta 2009, 1790, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Wingler, K.; Brigelius-Flohe, R. Gastrointestinal glutathione peroxidase. Biofactors 1999, 10, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Omura, K.; Ejima, A.; Kasanuma, Y.; Watanabe, C.; Satoh, H. Separation of selenium-containing proteins in human and mouse plasma using tandem high-performance liquid chromatography columns coupled with inductively coupled plasma-mass spectrometry. Anal. Biochem. 1999, 267, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.F.; Esworthy, R.S.; Doroshow, J.H.; Doan, K.; Liu, X.F. Expression of plasma glutathione peroxidase in human liver in addition to kidney, heart, lung, and breast in humans and rodents. Blood 1992, 79, 3233–3238. [Google Scholar] [CrossRef]
- Conrad, M.; Schneider, M.; Seiler, A.; Bornkamm, G.W. Physiological role of phospholipid hydroperoxide glutathione peroxidase in mammals. Biol. Chem. 2007, 388, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Freitas, F.P.; Seibt, T.; et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 2018, 172, 409–422. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R. Glutathione peroxidases and redox-regulated transcription factors. Biol. Chem. 2006, 387, 1329–1335. [Google Scholar] [CrossRef]
- Crosley, L.K.; Meplan, C.; Nicol, F.; Rundlof, A.K.; Arner, E.S.; Hesketh, J.E.; Arthur, J.R. Differential regulation of expression of cytosolic and mitochondrial thioredoxin reductase in rat liver and kidney. Arch. Biochem. Biophys. 2007, 459, 178–188. [Google Scholar] [CrossRef]
- Conrad, M.; Jakupoglu, C.; Moreno, S.G.; Lippl, S.; Banjac, A.; Schneider, M.; Beck, H.; Hatzopoulos, A.K.; Just, U.; Sinowatz, F.; et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol. Cell. Biol. 2004, 24, 9414–9423. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Novoselov, S.V.; Sun, Q.A.; Moustafa, M.E.; Zhou, Y.; Oko, R.; Hatfield, D.L.; Gladyshev, V.N. Mammalian selenoprotein thioredoxin-glutathione reductase. Roles in disulfide bond formation and spermmaturation. J. Biol. Chem. 2005, 280, 26491–26498. [Google Scholar] [CrossRef]
- Lee, B.C.; Lee, S.G.; Choo, M.K.; Kim, J.H.; Lee, H.M.; Kim, S.; Fomenko, D.E.; Kim, H.Y.; Park, J.M.; Gladyshev, V.N. Selenoprotein MsrB1 promotes anti-inflammatory cytokine gene expression in macrophagesand controls immune response in vivo. Sci. Rep. 2017, 7, 5119. [Google Scholar]
- Fomenko, D.E.; Novoselov, S.V.; Natarajan, S.K.; Lee, B.C.; Koc, A.; Carlson, B.A.; Lee, T.H.; Kim, H.Y.; Hatfield, D.L.; Gladyshev, V.N. MsrB1 (methionine-R-sulfoxide reductase 1) knock-out mice: Roles of MsrB1 in redox regulation and identification of a novel selenoprotein form. J. Biol. Chem. 2009, 284, 5986–5993. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Carlson, B.A.; Irons, R.; Mix, H.; Zhong, N.; Gladyshev, V.N.; Hatfield, D.L. Selenophosphate synthetase 2 is essential for selenoprotein biosynthesis. Biochem. J. 2007, 404, 115–120. [Google Scholar] [CrossRef]
- Darras, V.M.; Van Herck, S.L. Iodothyronine deiodinase structure and function: From ascidians to humans. J. Endocrinol. 2012, 215, 189–206. [Google Scholar] [PubMed]
- Lescure, A.; Rederstorff, M.; Krol, A.; Guicheney, P.; Allamand, V. Selenoprotein function and muscle disease. Biochim. Biophys. Acta 2009, 1790, 1569–1574. [Google Scholar] [PubMed]
- Castets, P.; Lescure, A.; Guicheney, P.; Allamand, V. Selenoprotein N in skeletal muscle: From diseases to function. J. Mol. Med. (Berl.) 2012, 90, 1095–1107. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Selenoprotein P-expression, functions, and roles in mammals. Biochim. Biophys. Acta 2009, 1790, 1441–1447. [Google Scholar] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. The Sep15 protein family: Roles in disulfide bond formation and quality control in the endoplasmic reticulum. IUBMB Life 2007, 59, 1–5. [Google Scholar] [CrossRef]
- Yim, S.H.; Everley, R.A.; Schildberg, F.A.; Lee, S.G.; Orsi, A.; Barbati, Z.R.; Karatepe, K.; Fomenko, D.E.; Tsuji, P.A.; Luo, H.R.; et al. Role of Selenof as a gatekeeper of secreted disulfide-rich glycoproteins. Cell. Rep. 2018, 23, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Pitts, M.W.; Reeves, M.A.; Hashimoto, A.C.; Ogawa, A.; Kremer, P.; Seale, L.A.; Berry, M.J. Deletion of selenoprotein M leads to obesity without cognitive deficits. J. Biol. Chem. 2013, 288, 26121–26134. [Google Scholar] [CrossRef]
- Verma, S.; Hoffmann, F.W.; Kumar, M.; Huang, Z.; Roe, K.; Nguyen-Wu, E.; Hashimoto, A.S.; Hoffmann, P.R. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immuneresponses. J. Immunol. 2011, 186, 2127–2137. [Google Scholar] [CrossRef]
- Fredericks, G.J.; Hoffmann, P.R. Selenoprotein K and protein palmitoylation. Antioxid. Redox Signal. 2015, 23, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Shibata, Y.; Yun, C.; Ron, D.; Rapoport, T.A. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 2004, 429, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Turanov, A.A.; Shchedrina, V.A.; Everley, R.A.; Lobanov, A.V.; Yim, S.M.; Marino, S.H.; Gygi, S.P.; Hatfield, D.L.; Gladyshev, V.N. Selenoprotein S is involved in maintenance and transport of multiproteincomplexes. Biochem. J. 2014, 462, 555–565. [Google Scholar] [CrossRef]
- Han, S.J.; Lee, B.C.; Yim, S.H.; Gladyshev, V.N.; Lee, S.R. Characterization of mammalian selenoprotein O: A redox-active mitochondrial protein. PLoS ONE 2014, 9, e95518. [Google Scholar] [CrossRef]
- Jeon, Y.H.; Park, Y.H.; Lee, J.H.; Hong, J.H.; Kim, I.Y. Selenoprotein W enhances skeletal muscle differentiation by inhibiting TAZ binding to 14-3-3 protein. Biochim. Biophys. Acta 2014, 1843, 1356–1364. [Google Scholar] [CrossRef]
- Boukhzar, L.; Hamieh, A.; Cartier, D.; Tanguy, Y.; Alsharif, I.; Castex, M.; Arabo, A.; Hajji, S.E.; Bonnet, J.J.; Errami, M.; et al. Selenoprotein T exerts an essential oxidoreductase activity that protects dopaminergic neurons in mouse models of Parkinson’s disease. Antioxid. Redox Signal. 2016, 24, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Panee, J.; Stoytcheva, Z.R.; Liu, W.; Berry, M.J. Selenoprotein H is a redox-sensing high mobility group family DNA-binding protein that up-regulates genes involved in glutathione synthesis and phase II detoxification. J. Biol. Chem. 2007, 282, 23759–23765. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, S.V.; Kryukov, G.V.; Xu, X.M.; Carlson, B.A.; Hatfield, D.L.; Gladyshev, V.N. Selenoprotein H is a nucleolar thioredoxin-like protein with a unique expression pattern. J. Biol. Chem. 2007, 282, 11960–11968. [Google Scholar] [CrossRef] [PubMed]
- Horibata, Y.; Hirabayashi, Y. Identification and characterization of human ethanolaminephosphotransferase1. J. Lipid Res. 2007, 48, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 2003, 57, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Sign. 2007, 9, 775–806. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, V.N.; Jeang, K.T.; Stadtman, T.C. Selenocysteine, identified as the penultimate C-terminal residue in human T-cell thioredoxin reductase, corresponds to TGA in the human placental gene. Proc. Natl. Acad. Sci. USA 1996, 93, 6146–6151. [Google Scholar] [CrossRef]
- Saccoccia, F.; Angelucci, F.; Boumis, G.; Carotti, D.; Desiato, G.E.; Miele, A.; Bellelli, A. Thioredoxin reductase and its inhibitors. Curr. Protein Pept. Sci. 2014, 15, 621–646. [Google Scholar] [CrossRef]
- Lu, J.M.; Wei, N.N.; Zhou, J.L. Research progress on structure and function of thioredoxin reductase. Progr. Anim. Med. 2019, 40, 79–83. (In Chinese) [Google Scholar]
- Giammanco, M.; Di Liegro, C.M.; Schiera, G.; Di Liegro, I. Genomic and non-genomic mechanisms of action of thyroid hormones and their catabolite 3, 5-diiodo-l-thyronine in mammals. Int. J. Mol. Sci. 2020, 21, 4140. [Google Scholar] [CrossRef] [PubMed]
- Marsan, E.S.; Bayse, C.A. A halogen bonding perspective on iodothyronine deiodinase activity. Molecules 2020, 25, 1328. [Google Scholar] [CrossRef] [PubMed]
- Persson Moschos, M. Selenoprotein P. Cell. Mol. Life Sci. 2000, 57, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G. Preparation and immunological activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 112, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Górska, S.; Maksymiuk, A.; Turło, J. Selenium-containing polysaccharides—Structural diversity, biosynthesis, chemical modifications and biological activity. Appl. Sci. 2021, 11, 3717. [Google Scholar] [CrossRef]
- Zhuang, T.; Xu, H.; Hao, S.; Ren, F.; Chen, X.; Pan, C.; Huang, K. Effects of selenium on proliferation, interleukin-2 production and selenoprotein mRNA expression of normal and dexamethasone-treated porcine splenocytes. Res. Vet. Sci. 2015, 98, 59–65. [Google Scholar] [CrossRef]
- Hu, L.Q.; Qian, B.; Bing, K.J.; Mei, L.; Qu, X.C. Distribution of selenium in China and the relationship between selenium and thyroid disease. Saf. Environ. Eng. 2022, 29, 13–21. [Google Scholar]
- Winther, K.H.; Rayman, M.P.; Bonnema, S.J.; Hegedüs, L. Selenium in thyroid disorders-essential knowledge for clinicians. Nat. Rev. Endocrinol. 2020, 16, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. Selenoproteins. J. Biol. Chem. 2009, 284, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Verloop, H.; Dekkers, O.M.; Peeters, R.P.; Schoones, J.W.; Smit, J.W. Genetics in endocrinology: Genetic variation in deiodinases: A systematic review of potential clinical effects in humans. Eur. J. Endocrinol. 2014, 171, R123–R135. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological activity of selenium and its impact on human health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Kester, M.H.; Peeters, R.P.; Visser, T.J. Biochemical mechanisms of thyroid hormone deiodination. Thyroid 2005, 15, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Kohrle, J.; Jakob, F.; Contempreé, B.; Dumont, J.E. Selenium, the thyroid, and the endocrine system. Endocr. Rev. 2005, 26, 944–984. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L.; Schweizer, U.; Kohrle, J. Selenium and selenoproteins in mammals: Extraordinary, essential, enigmatic. Cell. Mol. Life. Sci. 2004, 61, 1988–1995. [Google Scholar] [CrossRef]
- Davis, C.D. Selenium supplementation and cancer prevention. Curr. Nutr. Rep. 2012, 1, 16–23. [Google Scholar] [CrossRef]
- Jonklaas, J.; Danielsen, M.; Wang, H. A pilot study of serum selenium, vitamin D, and thyrotropin concentrations in patients with thyroid cancer. Thyroid 2013, 23, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.Y.; Li, H.; Guo, Y.; Jin, Y.P.; Lin, D.G. Sodium selenite inhibits the expression of VEGF, TGFβ1 and IL-6 induced by LPS in human PC3 cells via TLR4-NF-KB signaling blockage. Int. Immunopharmacol. 2010, 10, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Sinha, I.; Null, K.; Wolter, W.; Suckow, M.A.; King, T.; Pinto, J.T.; Sinha, R. Methylseleninic acid downregulates hypoxia-inducible factor-1α in invasive prostate cancer. Int. J. Cancer 2012, 130, 1430–1439. [Google Scholar] [CrossRef]
- Song, H.; Kim, J.; Lee, H.K.; Park, H.J.; Nam, J.; Park, G.B.; Kim, Y.S.; Cho, D.; Hur, D.Y. Selenium inhibits migration of murine melanoma cells via down-modulation of IL-18 expression. Int. Immunopharmacol. 2011, 11, 2208–2213. [Google Scholar] [CrossRef]
- Shargorodsky, M.; Debby, O.; Matas, Z.; Zimlichman, R. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr. Metab. 2010, 7, 1–8. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Ju, W.; Wu, G.; Yang, X.; Fu, X.; Gao, X. High serum selenium levels are associated with impaired fasting glucose and elevated fasting serum glucose in Linyi, China. J. Trace Elem. Med. Bio. 2018, 45, 64–69. [Google Scholar] [CrossRef]
- Jia, L. To study the effect of selenium content on the growth and development of fetuses in pregnant women with gestational diabetes, Guide of China. Medicine 2021, 19, 39–40. [Google Scholar]
- Solovyev, N.D. Importance of selenium and selenoprotein for brain function: From antioxidant protection to neuronal signalling. J. Inorg. Biochem. 2015, 153, 1–12. [Google Scholar] [CrossRef]
- Kryscio, R.J.; Abner, E.L.; Schmitt, F.A.; Goodman, P.J.; Mendiondo, M.; Caban-Holt, A.; Dennis, B.C.; Mathews, M.; Klein, E.A.; Crowley, J.J. A randomized controlled Alzheimer’s disease prevention trial’s evolution into an exposure trial: The PREADViSE Trial. J. Nutr. Health Aging 2013, 17, 72–75. [Google Scholar] [CrossRef]
- Chen, J.; Berry, M.J. Selenium and selenoproteins in the brain and brain diseases. J. Neurochem. 2003, 86, 1–12. [Google Scholar] [CrossRef]
- Vinceti, M.; Solovyev, N.; Mandrioli, J.; Crespi, C.M.; Bonvicini, F.; Arcolin, E.; Georgoulopoulou, E.; Michalke, B. Cerebrospinal fluid of newly diagnosed amyotrophic lateral sclerosis patients exhibits abnormal levels of selenium species including elevated selenite. Neurotoxicology 2013, 38, 25–32. [Google Scholar] [CrossRef]
- Foresta, C.; Flohé, L.; Garolla, A.; Roveri, A.; Ursini, F.; Maiorino, M. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol. Reprod. 2002, 67, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Kommisrud, E.; Østerås, O.; Vatn, T. Blood selenium associated with health and fertility in Norwegian dairy herds. Acta Vet. Scand. 2005, 46, 1–12. [Google Scholar]
- Meseguer, M.; Antonio Martinez-Conejero, J.; Muriel, L.; Pellicer, A.; Remohí, J.; Garrido, N. The human sperm glutathione system: A key role in male fertility and successful cryopreservation. Drug Metab. Lett. 2007, 1, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, M.; Hou, J.; Jiang, C.; Li, S.; Wang, T. The prevalence of Keshan disease in China. Int. J. Cardiol. 2013, 168, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Allander, E. Kashin-Beck disease. An analysis of research and public health activities based on a bibliography 1849-1992. Scand. J. Rheumatol. 1994, 23, 1–36. [Google Scholar] [CrossRef]
| Food Categories | Selenium Content (mg/kg) |
|---|---|
| Grains and grain products | 0.01–0.55 |
| Meat, fish, eggs | 0.01–0.36 |
| Milk and milk products | <0.001–0.17 |
| Vegetables and fruits | <0.001–0.022 |
| Bovine kidney | 0.78–1.45 |
| Brazil nuts | 0.83–53 |
| Cabbage | <0.001–0.46 |
| Asparagus | 0.01–1.40 |
| Country | Intake (μg/day) | Serum (μg/L) | Breast Milk (μg/L) | Urine (μg/L) | Soil (mg/kg) |
|---|---|---|---|---|---|
| Belgium | 28–61 | 73–110 | 9.7–153 | 13–30 | 0.11 |
| Brazil | 60 | / | 14.1 | / | / |
| China (Enshi Province) | 3200–6690 | 1300–7500 | 94.8–120.5 | 2680 | 10–40 |
| China (Keshan region) | 3–11 | 23.9 | 3.0 | 7 | 0.17 |
| Finland | 125 | 77–134 | 6–14.3 | / | 0.15–0.72 |
| France | 47 | 84.7 | / | 12.3 | 0.18 |
| Germany | 47 | 63–106 | 9.9–59 | 16–23 | 6.6 |
| Italy | 49 | 76–94 | 13.3 | 7.4 | / |
| Japan | 133 | / | 11.2–40.3 | 36–288 | 0.7–1.0 |
| Spain | 60 | 74–84 | 11.4–21.7 | / | 0.07–0.39 |
| Sweden | 38 | 105 | 13.1 | 36 | 0.39 |
| Switzerland | 70 | 96–113 | / | / | / |
| Turkey | 30 | 58–113 | 11.2–48.6 | / | 0.03 |
| Netherlands | 67 | 93.6 | / | / | / |
| UK | 41 | 60–81 | 8.3 | 5 | 0.18–29.70 |
| USA | 98 | 95–320 | 7–105 | 19.2–118 | 0.11–18.36 |
| Age | EAR (μg/d) | RNI (μg/d) | UL (μg/d) |
|---|---|---|---|
| 0 to under 6 months | / | 15 (AI) | 55 |
| 6 to under 12 months | / | 20 (AI) | 80 |
| 1 to under 4 years | 20 | 25 | 100 |
| 4 to under 7 years | 25 | 30 | 150 |
| 7 to under 11 years | 35 | 40 | 200 |
| 11 to under 14 years | 45 | 55 | 300 |
| 14 to under 18 years | 50 | 60 | 350 |
| 18 to under 50 years | 50 | 60 | 400 |
| 50 years and older | 50 | 60 | 400 |
| Pregnant women | 54 | 65 | 400 |
| Lactating women | 65 | 78 | 400 |
| Age | USA [67] | EU [68] | Canada [69] | UK [70] | New Zealand [71] | Germany [72] | Austria [72] | Switzerland [72] | Australian [73] | WHO [74] |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 to under 4 months | / | / | / | / | / | 10 | 10 | 10 | 10 | 6 |
| 4 to under 12 months | / | / | / | / | / | 15 | 15 | 15 | 15 | 10 |
| 1 to under 4 years | 20 | / | / | / | / | 15 | 15 | 15 | 25 | 17 |
| 4 to under 7 years | 30 | / | / | / | / | 20 | 20 | 20 | 30 | 22 |
| 7 to under 10 years | 30 | / | / | / | / | 30 | 30 | 30 | 50 | 21 |
| 10 to under 13 years (male) | 40 | / | / | / | / | 45 | 45 | 45 | 50 | 32 |
| 10 to under 13 years (female) | 555 | / | / | / | / | 45 | 45 | 45 | 50 | 26 |
| 13 to under 15 years (male) | 55 | / | / | / | / | 60 | 60 | 60 | 85 | 34 |
| 13 to under 15 years (female) | 55 | / | / | / | / | 60 | 60 | 60 | 85 | 26 |
| 15 to under 19 years (male) | 55 | / | / | / | / | 70 | 70 | 70 | 85 | 34 |
| 15 to under 19 years (female) | 55 | / | / | / | / | 60 | 60 | 60 | 85 | 26 |
| 19 to under 65 years (male) | 55 | 55 | 55 | 75 | 60 | 70 | 70 | 70 | 60 | 34 |
| 19 to under 65 years (female) | 55 | 55 | 55 | 60 | 55 | 60 | 60 | 60 | 55 | 26 |
| 65 years and older (male) | 55 | / | / | / | / | 70 | 70 | 70 | / | 33 |
| 65 years and older (female) | 55 | / | / | / | / | 60 | 60 | 60 | / | 30 |
| Pregnant women | 49 | / | / | / | / | 60 | 60 | 60 | 80 | 29 |
| Lactating women | 59 | / | / | / | / | 75 | 75 | 75 | 85 | 39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Feng, S. Effects and Impact of Selenium on Human Health, A Review. Molecules 2025, 30, 50. https://doi.org/10.3390/molecules30010050
Bai S, Zhang M, Tang S, Li M, Wu R, Wan S, Chen L, Wei X, Feng S. Effects and Impact of Selenium on Human Health, A Review. Molecules. 2025; 30(1):50. https://doi.org/10.3390/molecules30010050
Chicago/Turabian StyleBai, Song, Miaohe Zhang, Shouying Tang, Miao Li, Rong Wu, Suran Wan, Lijun Chen, Xian Wei, and Shuang Feng. 2025. "Effects and Impact of Selenium on Human Health, A Review" Molecules 30, no. 1: 50. https://doi.org/10.3390/molecules30010050
APA StyleBai, S., Zhang, M., Tang, S., Li, M., Wu, R., Wan, S., Chen, L., Wei, X., & Feng, S. (2025). Effects and Impact of Selenium on Human Health, A Review. Molecules, 30(1), 50. https://doi.org/10.3390/molecules30010050







