Synthesis, Physicochemical Properties, and Ion Recognition Ability of Azulene-Based Bis-(Thio)Semicarbazone
Abstract
:1. Introduction
2. Results and Discussion
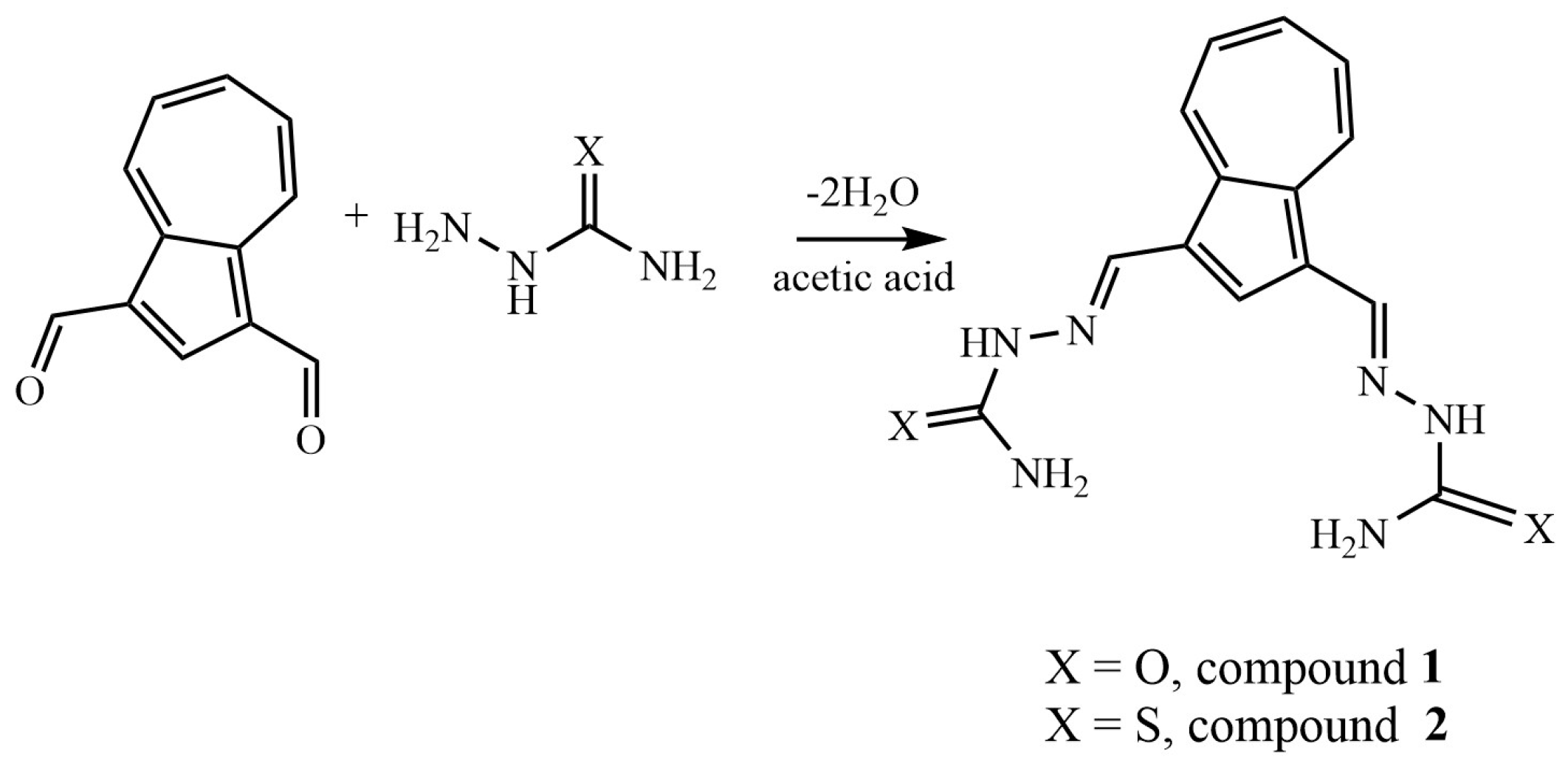
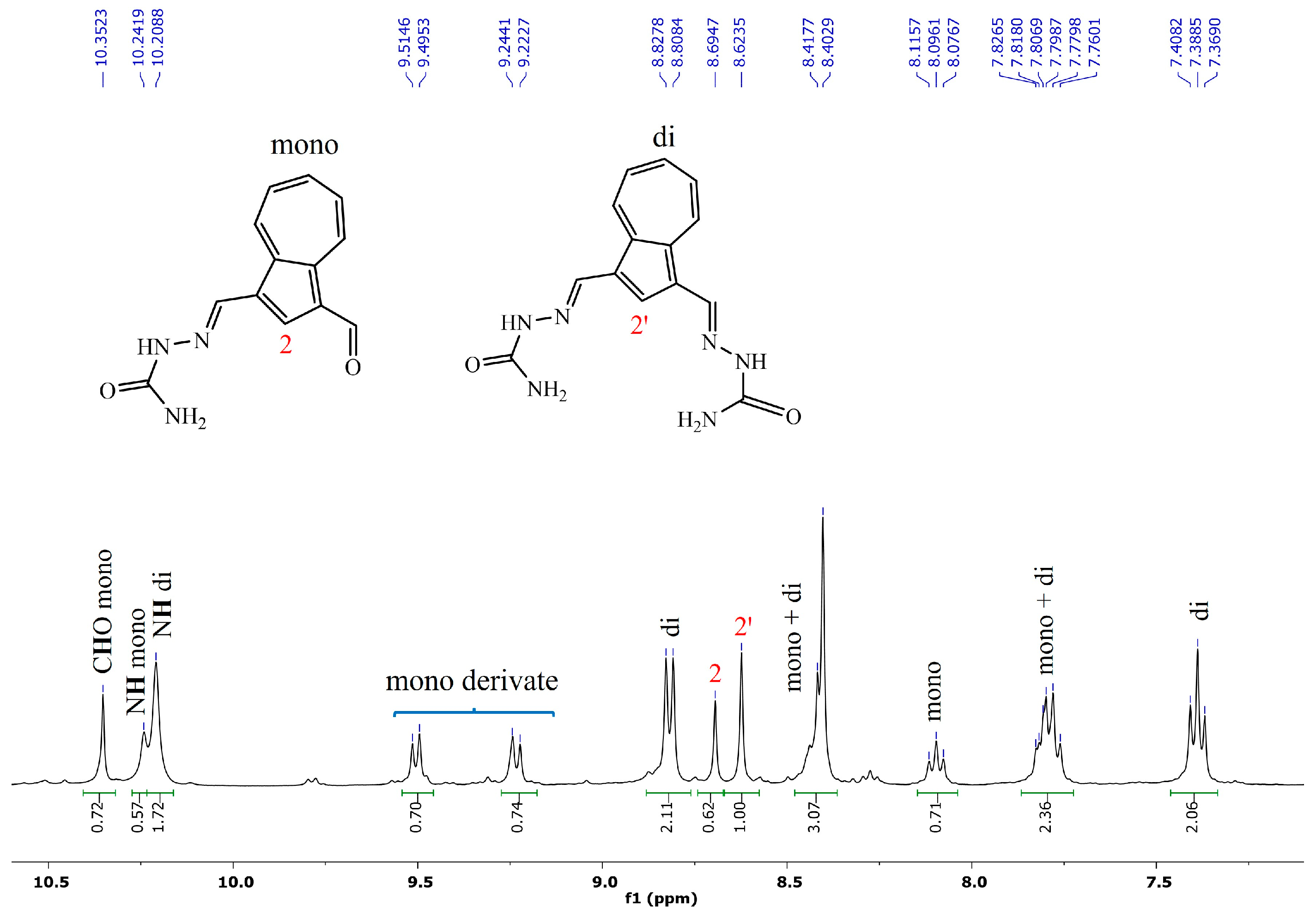
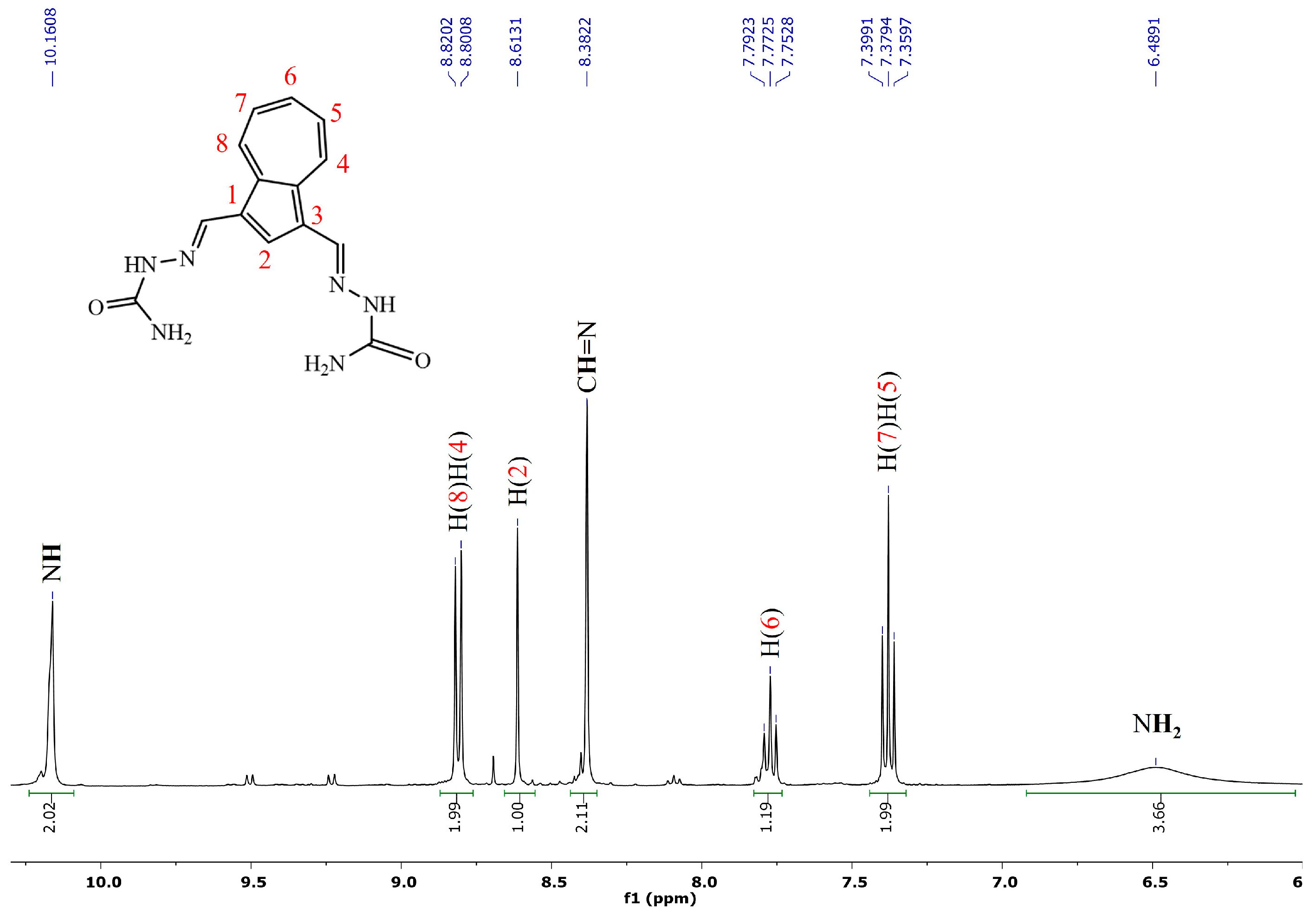
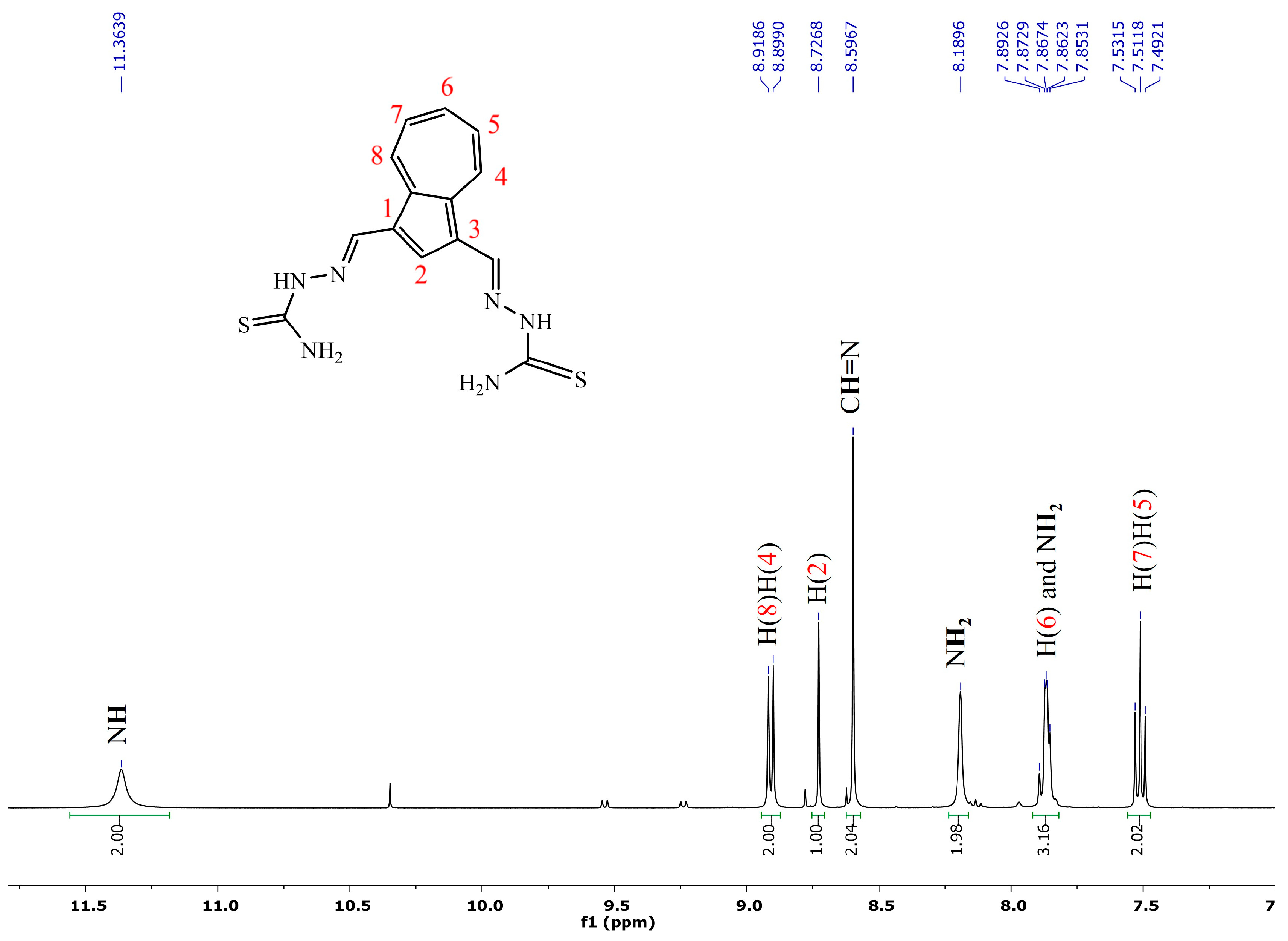
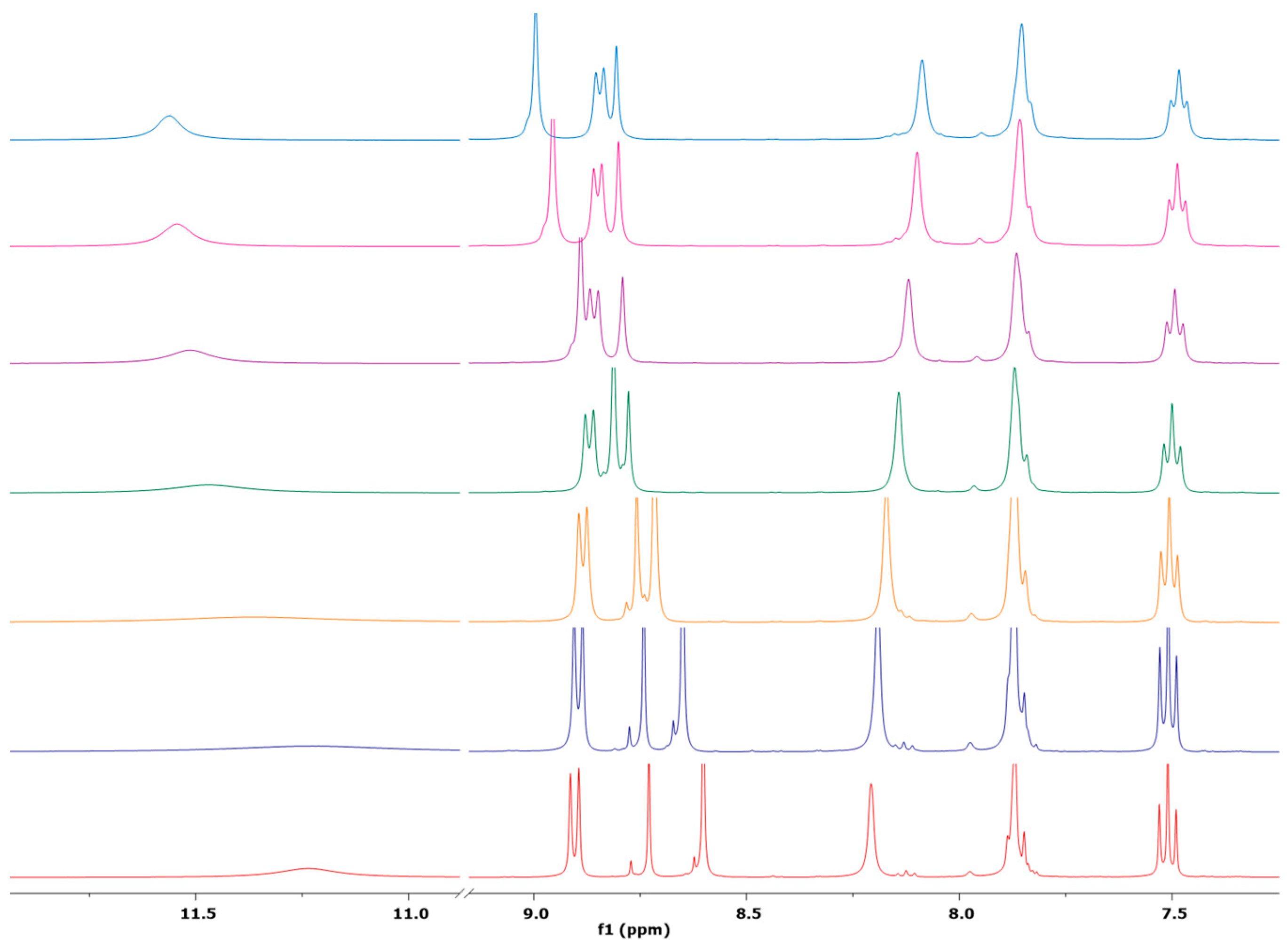
2.1. Synthesis and NMR Characterization
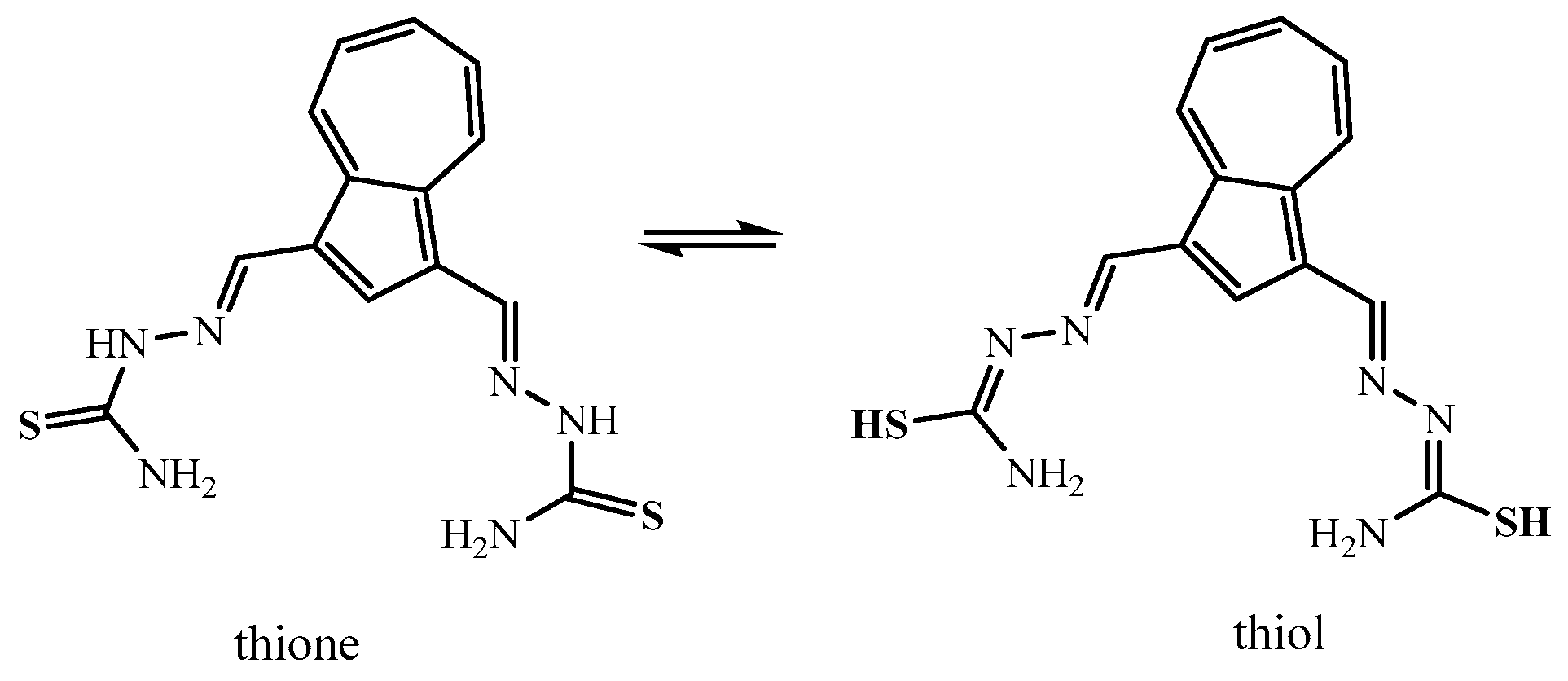
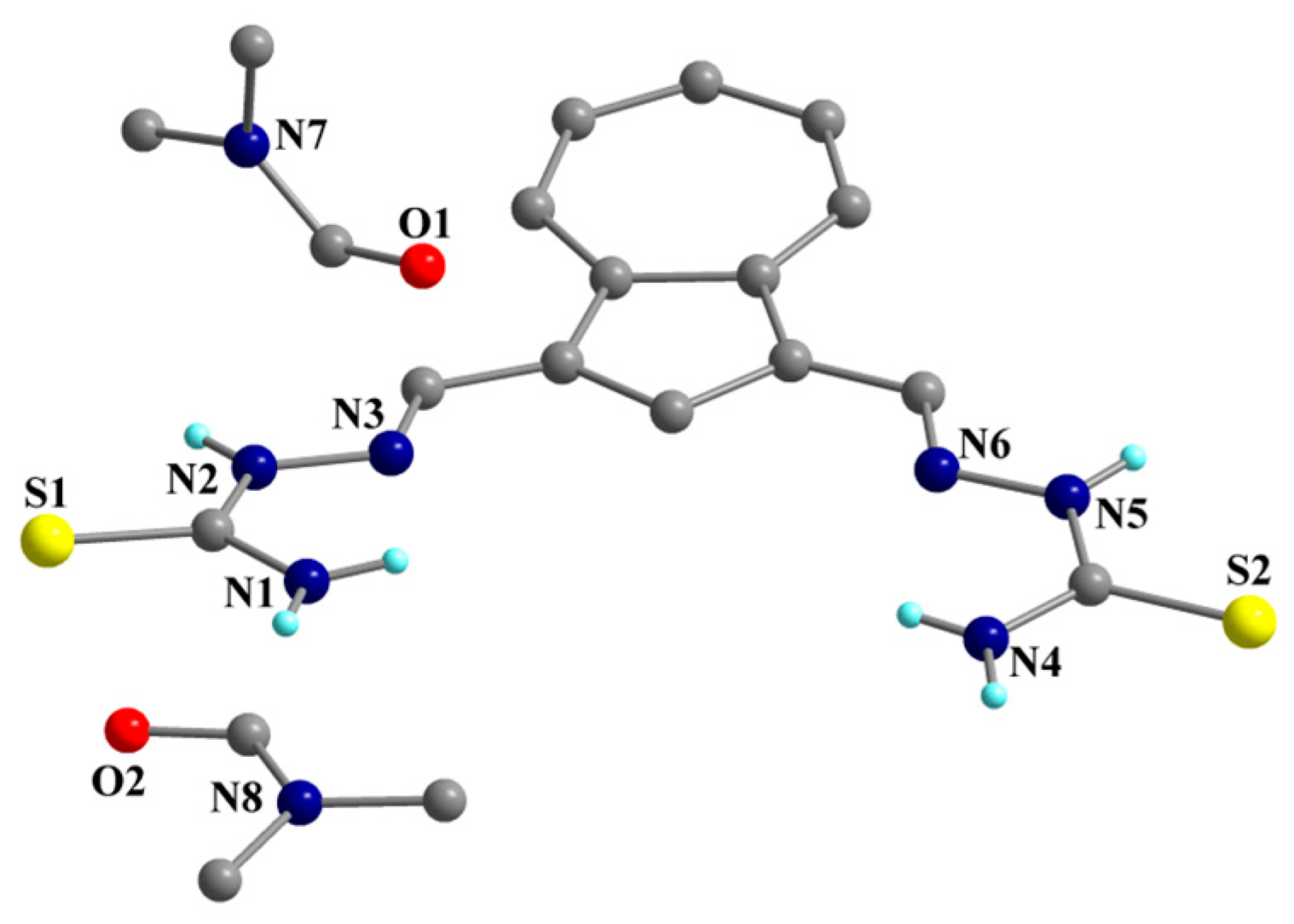
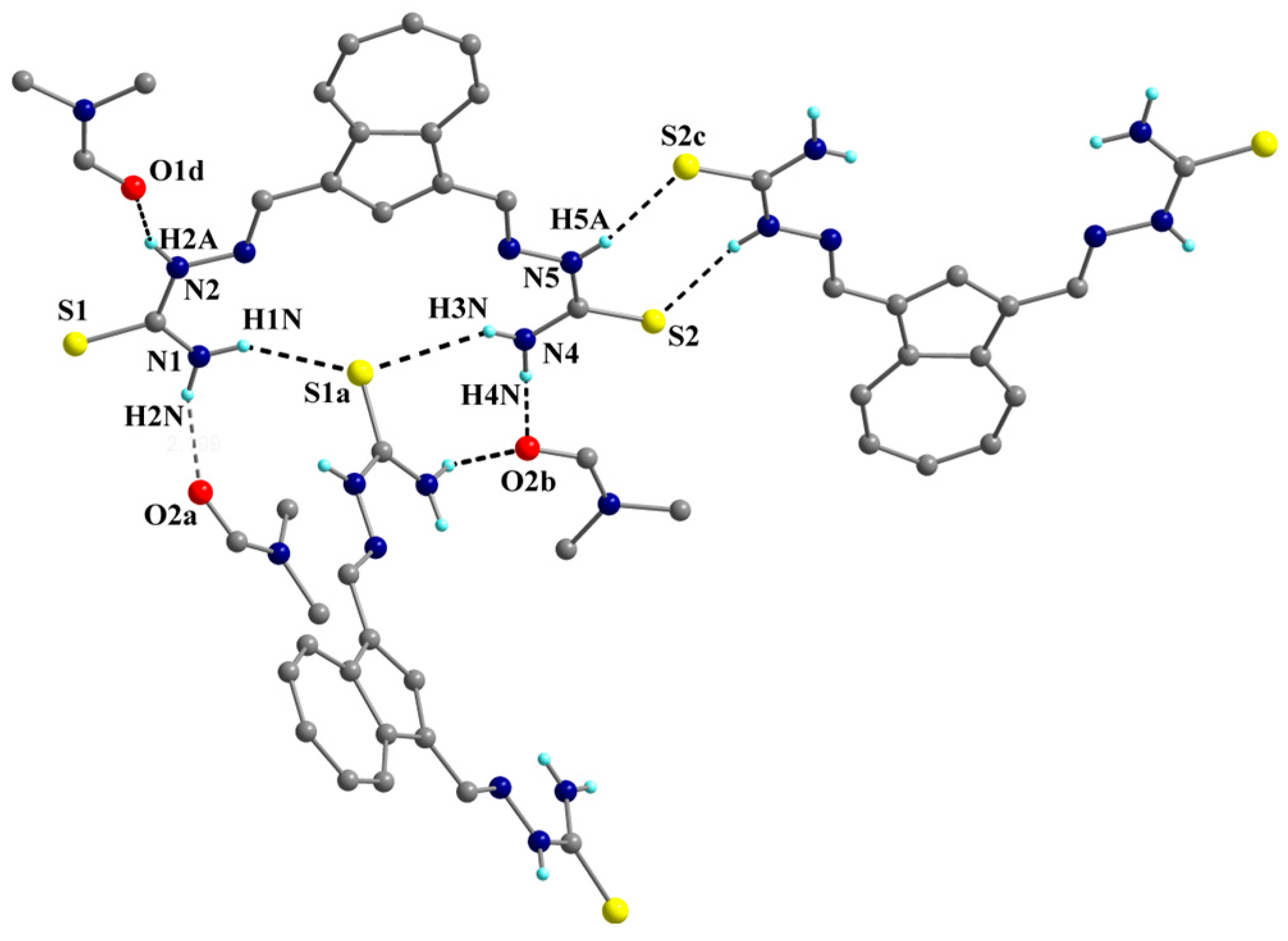
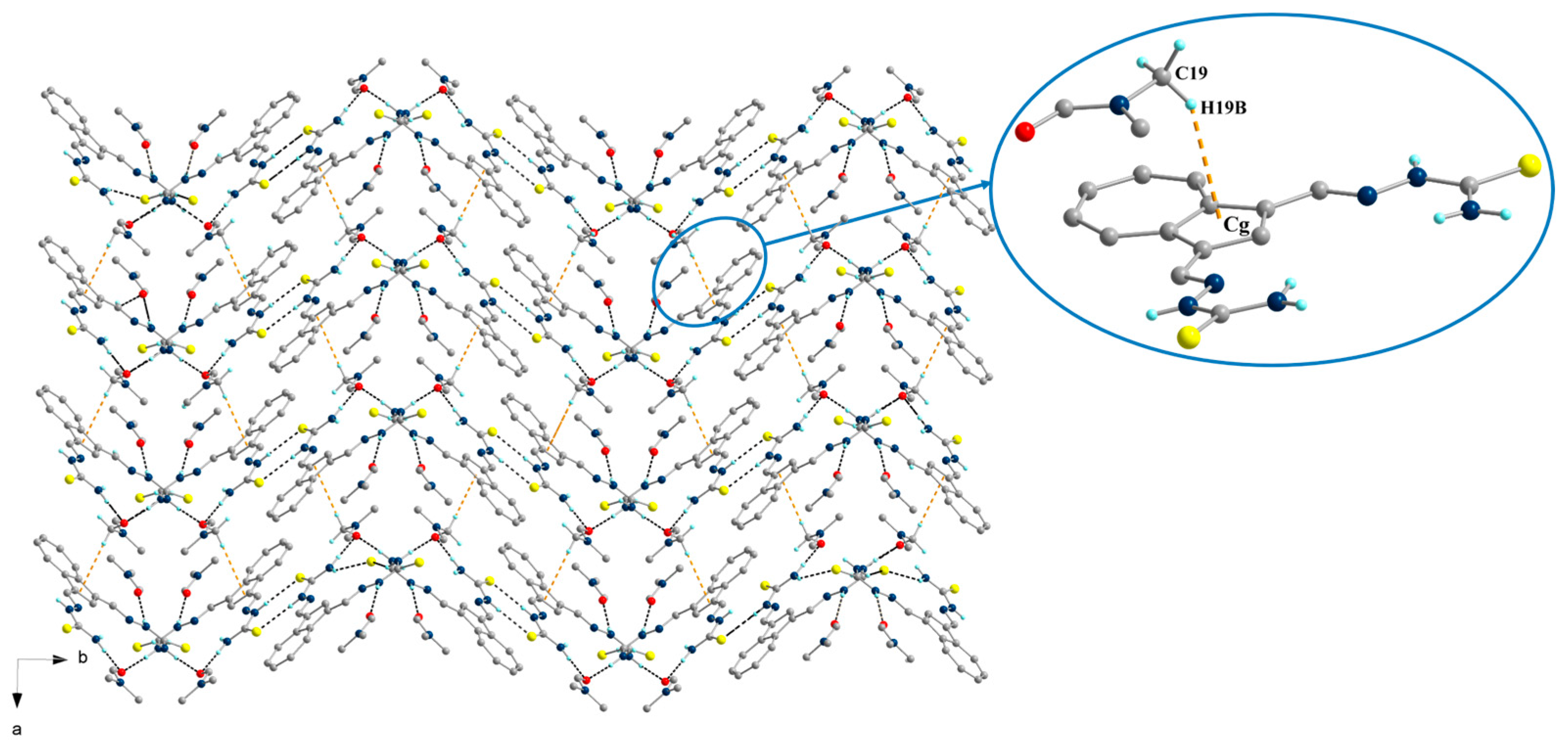
2.2. Single-Crystal X-Ray Structure Determination and IR Spectroscopy
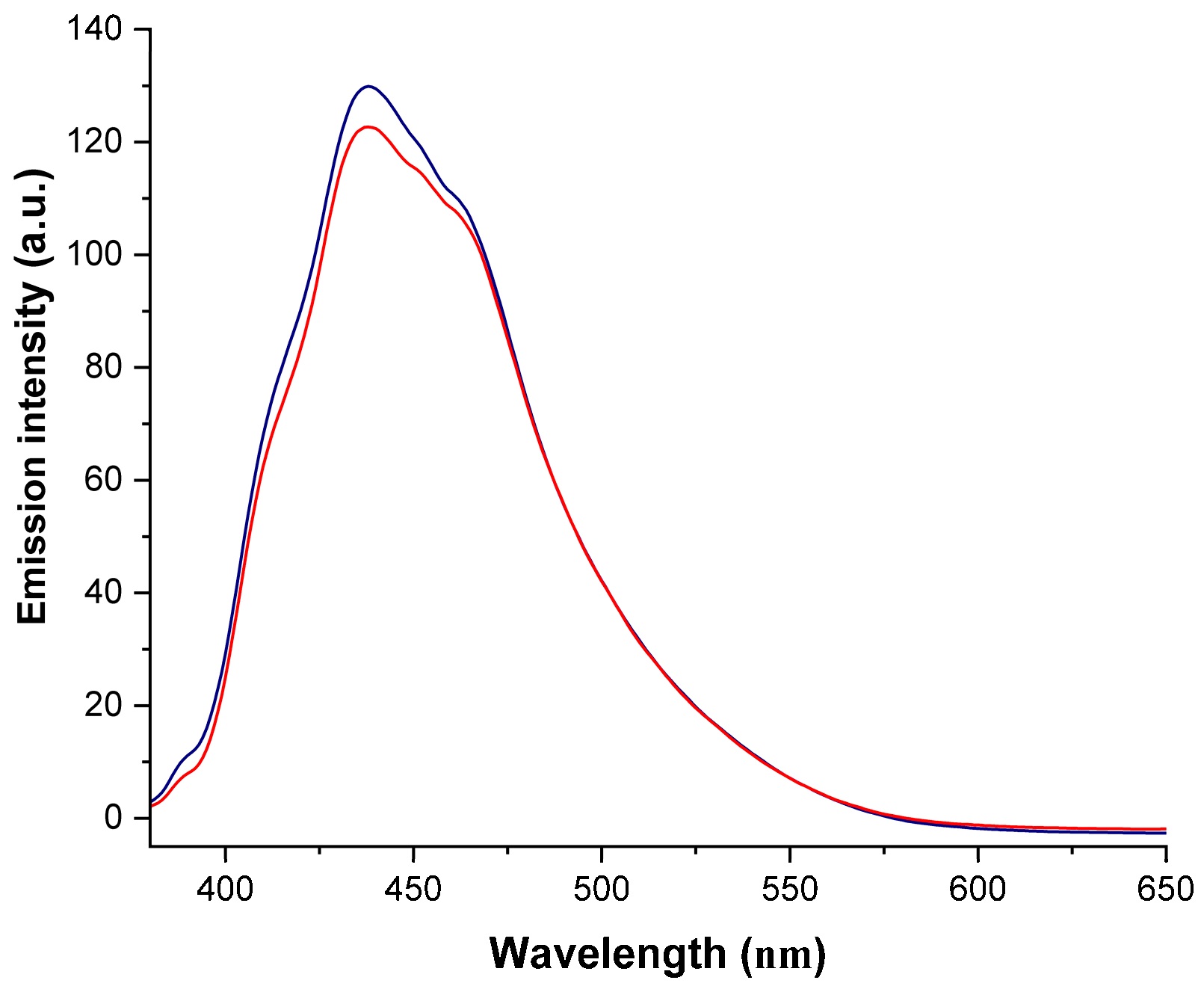
2.3. Electronic Absorption Spectroscopy and Fluorescence
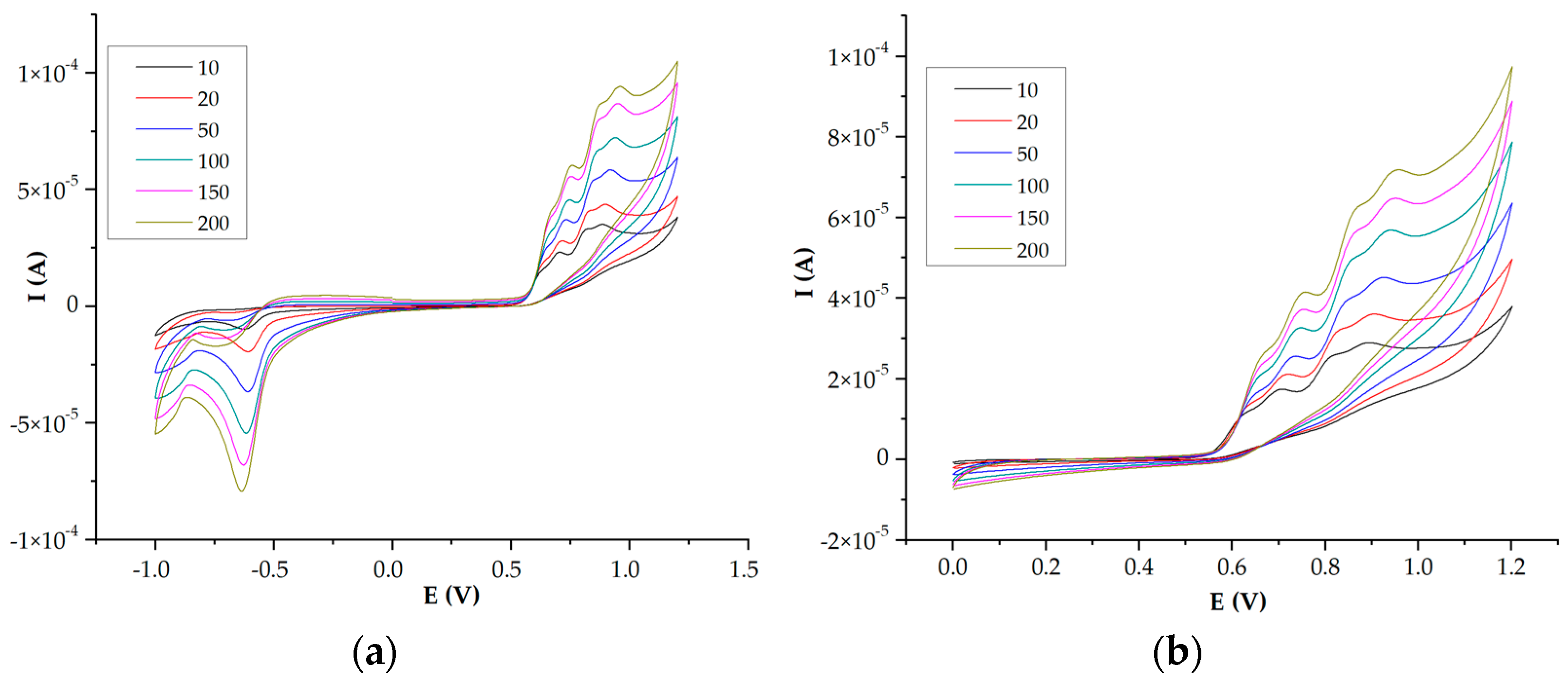
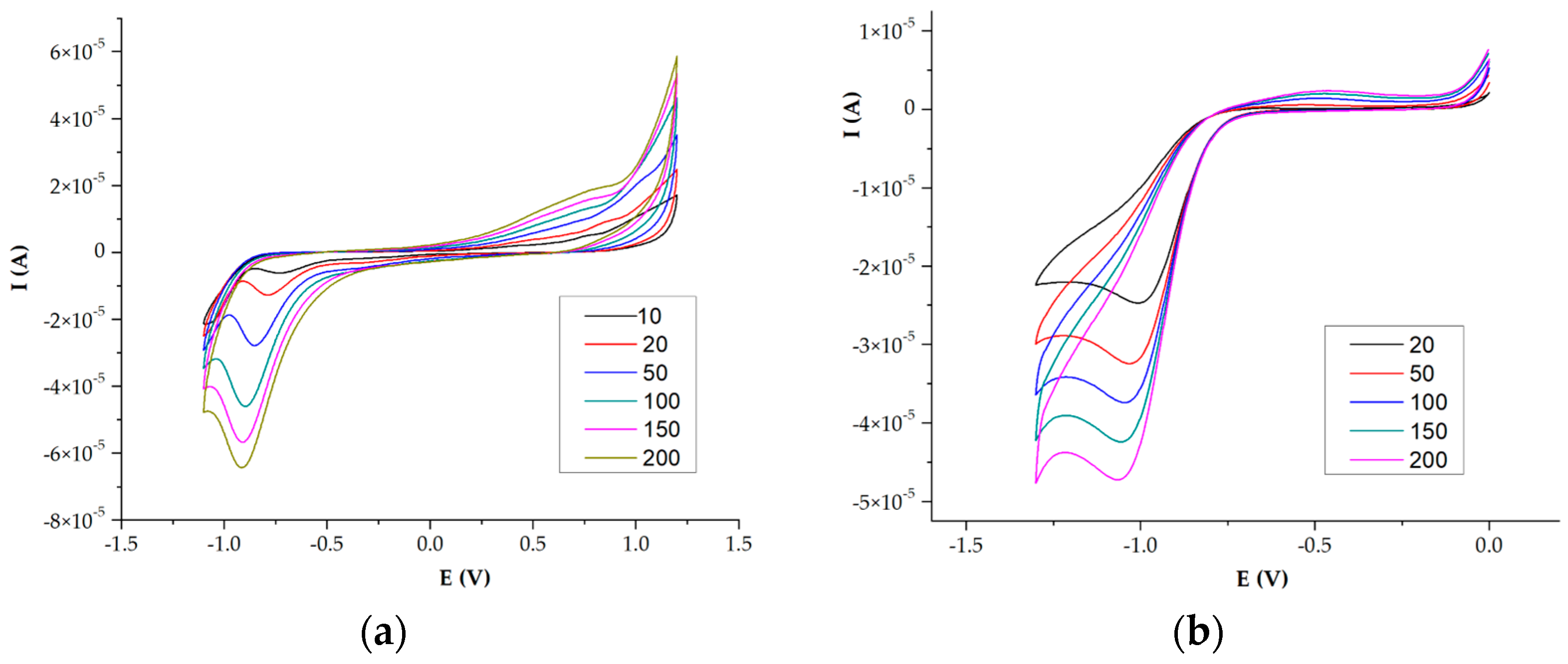
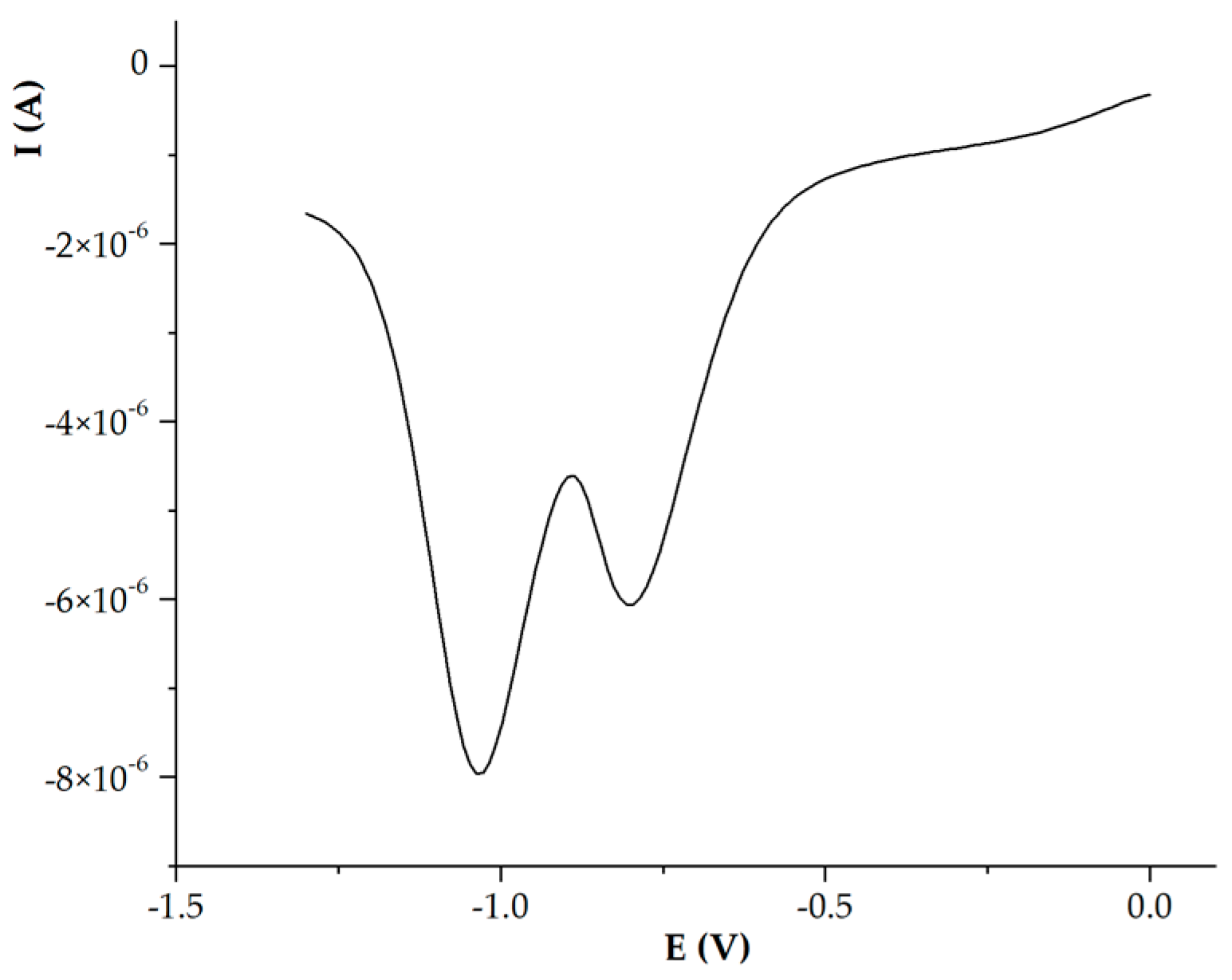
2.4. Electrochemistry
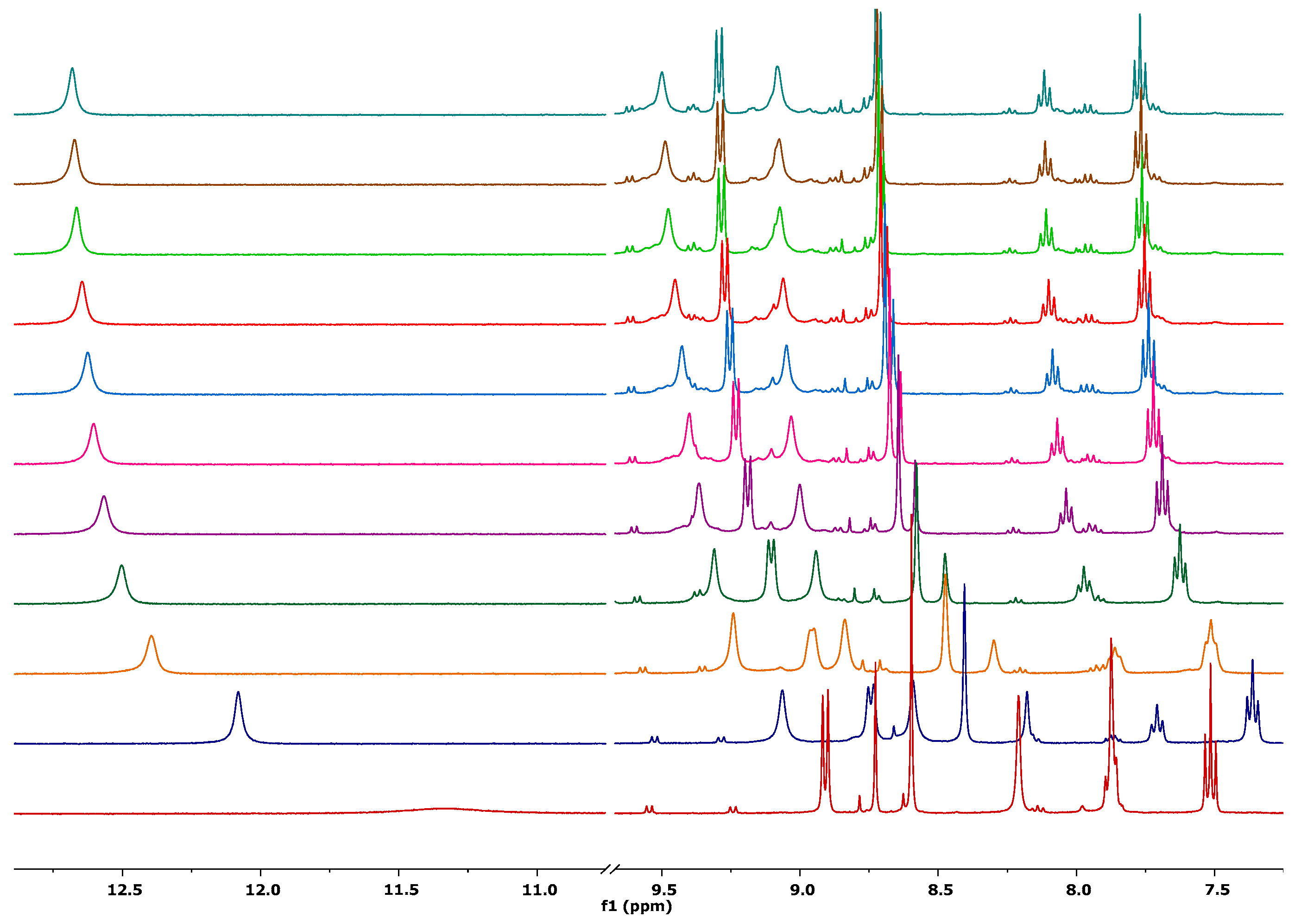
2.5. Receptor Ability
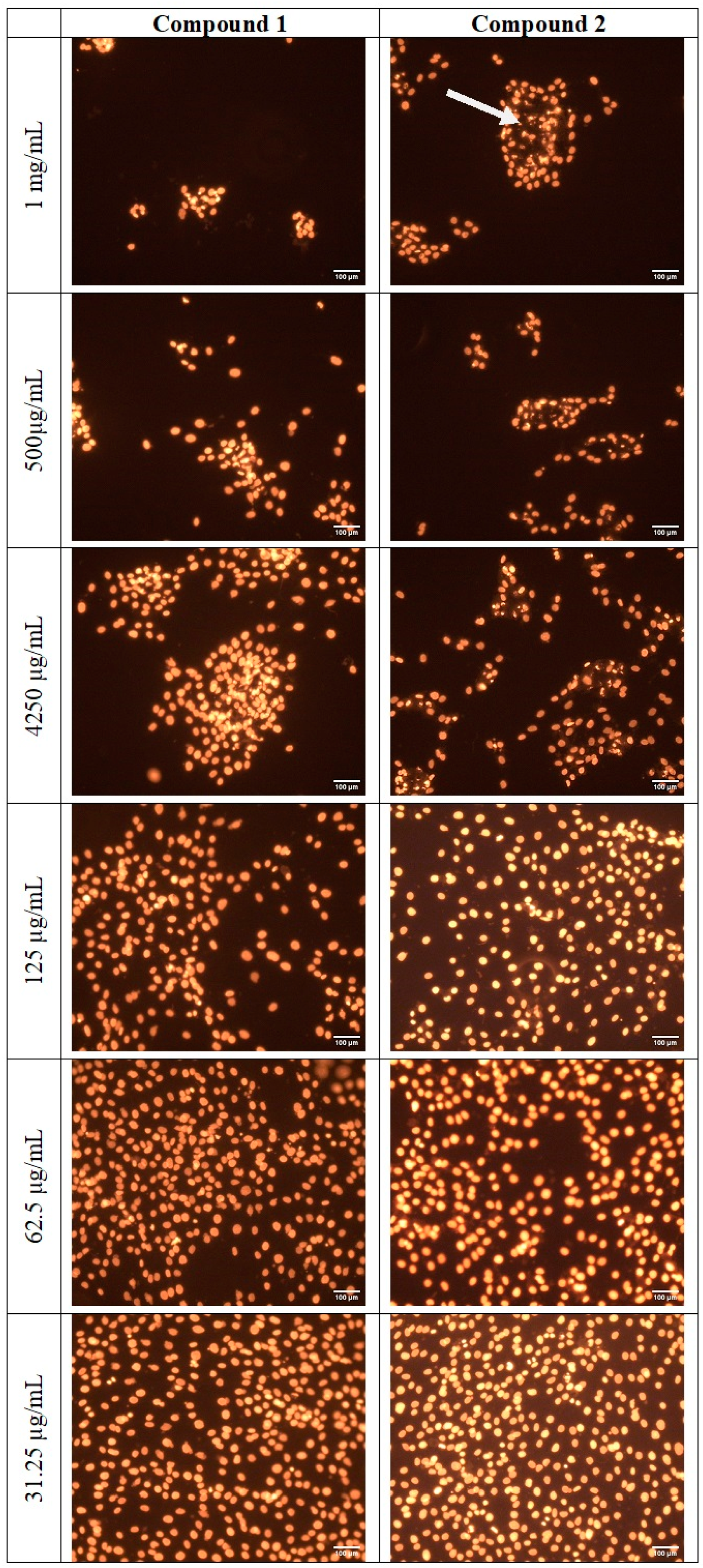
2.6. Cytotoxicity
3. Experimental
3.1. Materials and Methods
3.2. General Procedure for the Synthesis of Compounds 1 and 2
- (2E,2′E)-2,2′-(azulene-1,3-diylbis(methaneylylidene))bis(hydrazine-1-carboxamide), 1, was synthesized following the general procedure using semicarbazide (188 mg, 2.5 mmol). After removal of the solvent, the obtained dark green solid was washed with ethanol and diethyl ether. There were isolated 230 mg (0.77 mmol) green powder, 77% yield; m.p > 300 °C with decomposition; a transition phase was observed at 223–225 °C; 1H NMR: (DMSO-d6, 500 MHz): δ 10.17 (s, 2H, NH), 8.81 (d, 2H, J = 9.8 Hz, H-8 and H-4), 8.61 (s, 1H, H-2), 8.38 (s, 2H, CH=N), 7.77 (t, 1H, J = 9.8 Hz, H-6), 7.37 (t, 1H, J = 9.8 Hz, H-5 and H-7), and 6.49 (bs, 4H, NH2) ppm. 13C-NMR (DMSO-d6, 125 MHz): δ 156.89 (C=O), 140.36 (CH-6), 138.36 (Cq-9/10), 136.10 (CH-4/8), 135.95 (CH-2), 135.55 (CH=N), 126.54 (CH-5/7), 123.46 (Cq-1/3) ppm. UV-Vis (DMSO):296 (lgɛ = 4.63), 330 (lg ɛ = 4.79), 410 (lg ɛ = 4.10). Selected IR: 3470, 2884, 2827, 1676, 1577, 1433, 1400, 1111, 1045, 746 cm−1. MS: 299.12532 [M + H]+ (calcd. for C14H14N6O2 299.12510).
- (2E,2′E)-2,2′-(azulene-1,3-diylbis(methaneylylidene))bis(hydrazine-1-carbothioamide), 2, was synthesized following the general procedure using thiosemicarbazone (230 mg, 2.5 mmol). After removal of the solvent, the green solid was washed with ethanol (3 × 10 mL) and diethyl ether for drying. There were isolated 238 mg (72 mmol) light green powder, 72% yield; m.p = 268–269 °C; 1H NMR: (DMSO-d6, 500 MHz): δ 11.36 (s, 2H, NH), 8.91 (d, 2H, J = 9.8 Hz, H-8 and H-4), 8.72 (s, 1H, H-2), 8.60 (s, 2H, CH=N), 8.19 (bs, 2H, NH2), 7.89–7.85 (m, 3H, NH2 and H-6), and 7.51 (t, 1H, J = 9.8 Hz, H-5 and H-7) ppm. 13C-NMR (DMSO-d6, 125 MHz): δ 177.13 (C=S), 140.83 (CH-6), 139.64 (Cq-9/10), 138.52 (CH=N), 137.72 (CH-2), 136.70 (CH-4/8), 127.87 (CH-5/7), 122.90 (Cq-1/3) ppm. UV-Vis (DMSO):360 (lg ɛ = 4.90), 410 (lg ɛ = 4.22). Selected IR: 3440, 3262, 3151, 3033, 2991, 2885, 2814, 1591, 1537, 1388, 831 cm−1. MS: 331.07944 [M + H]+ (calcd. for C14H14N6S2 331.07941).
3.3. Determination of Cell Viability by the CellTiter Assay
3.4. Determination of Cell Viability Using the Incucyte System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, L.; Liu, J.; Li, P.; Tang, B.; James, T.D. Two-photon small-molecule fluorescence-based agents for sensing, imaging, and therapy within biological systems. Chem. Soc. Rev. 2021, 50, 702–734. [Google Scholar] [CrossRef] [PubMed]
- Cowper, P.; Pockett, A.; Kociok-Köhn, G.; Cameron, P.J.; Lewis, S.E. Azulene—Thiophene—Cyanoacrylic acid dyes with donor-π-acceptor structures. Synthesis, characterisation and evaluation in dye-sensitized solar cells. Tetrahedron 2018, 74, 2775–2786. [Google Scholar] [CrossRef]
- Xin, H.; Hou, B.; Gao, X. Azulene-based π-functional materials: Design, synthesis, and applications. Acc. Chem. Res. 2021, 54, 1737–1753. [Google Scholar] [CrossRef] [PubMed]
- Dragu, E.A.; Ion, A.E.; Shova, S.; Bala, D.; Mihailciuc, C.; Voicescu, M.; Ionescu, S.; Nica, S. Visible-light triggered photoswitching systems based on fluorescent azulenyl-substituted dithienylcyclopentenes. RSC Adv. 2015, 5, 63282–63286. [Google Scholar] [CrossRef]
- Hou, C.-Y.; Berger, F.; Narita, A.; Müllen, K.; Hecht, S. Proton-gated ring-closure of a negative photochromic azulene-based diarylethene. Angew. Chem. Int. Ed. 2020, 59, 18532–18536. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Ge, C.; Jiao, X.; Jiao, X.; Yang, X.; Rundel, K.; McNeill, C.R.; Gao, X. Incorporation of 2,6-connected azulene units into the backbone of conjugated polymers: Towards high-performance organic optoelectronic materials. Angew. Chem. Int. Ed. 2018, 57, 1322–1326. [Google Scholar] [CrossRef]
- Dong, J.-X.; Zhang, H.-L. Azulene-based organic functional molecules for optoelectronics. Chin. Chem. Lett. 2016, 27, 1097–1104. [Google Scholar] [CrossRef]
- Xin, H.; Gao, X. Application of azulene in constructing organic optoelectronic materials: New tricks for an old dog. ChemPlusChem 2017, 82, 945–956. [Google Scholar] [CrossRef]
- Anderson, A.G., Jr.; Steckler, B.M. Azulene. VIII. A study of the visible absorption spectra and dipole moments of some 1- and 1,3-substituted azulenes. J. Am. Chem. Soc. 1959, 81, 4941–4946. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Hamano, T.; Inoue, M.; Nakamura, T.; Wakamiya, A.; Mazaki, Y. Intense absorption of azulene realized by molecular orbital inversion. Chem. Commun. 2023, 59, 10604–10607. [Google Scholar] [CrossRef] [PubMed]
- Dragu, A.E.; Nica, S.; Tecuceanu, V.; Bala, D.; Mihailciuc, C.; Hanganu, A.; Razus, A.C. Synthesis and electrochemical properties of carbocyclic and heterocyclic diazulenylethenes. Eur. J. Org. Chem. 2013, 2013, 6601–6610. [Google Scholar] [CrossRef]
- Shoji, T.; Ito, S. Azulene-based donor–acceptor systems: Synthesis, optical, and electrochemical properties. Chem. Eur. J. 2017, 23, 16696–16709. [Google Scholar] [CrossRef]
- Bakun, P.; Czarczynska-Goslinska, B.; Goslinski, T.; Lijewski, S. In vitro and in vivo biological activities of azulene derivatives with potential applications in medicine. Med. Chem. Res. 2021, 30, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Longuet-Higgins, H.C. Anomalous light emission of azulene. J. Chem. Phys. 1955, 23, 1390–1391. [Google Scholar] [CrossRef]
- Murfin, C.L.; Lewis, S.E. Azulene—A bright core for sensing and imaging. Molecules 2021, 26, 353. [Google Scholar] [CrossRef]
- Teratani, M.; Nakamura, S.; Sakagami, H.; Fujise, M.; Hashimoto, M.; Okudaira, N.; Bandow, K.; Iijima, Y.; Nagai, J.; Uesawa, Y.; et al. Antitumor effects and tumor-specificity of guaiazulene-3-carboxylate derivatives against oral squamous cell carcinoma in vitro. Anticancer Res. 2020, 40, 4885–4894. [Google Scholar] [CrossRef]
- Peet, J.; Selyutina, A.; Bredihhin, A. Antiretroviral (HIV-1) activity of azulene derivatives. Bioorg. Med. Chem. 2016, 24, 1653–1657. [Google Scholar] [CrossRef]
- Ma, D.; He, J.; He, D. Chamazulene reverses osteoarthritic inflammation through regulation of matrix metalloproteinases (MMPs) and NF-kβ pathway in in-vitro and in-vivo models. Biosci. Biotechnol. Biochem. 2020, 84, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Nihei, S.; Sato, J.; Komatsu, H.; Ishida, K.; Kimura, T.; Tomita, T.; Kudo, K. The efficacy of sodium azulene sulfonate L-glutamine for managing chemotherapy-induced oral mucositis in cancer patients: A prospective comparative study. J. Pharm. Health Care Sci. 2018, 4, 20. [Google Scholar] [CrossRef]
- NIH National Center of Advancing Translational Sciences. Egualen Sodium. 2020. Available online: https://drugs.ncats.io/substance/3368L0W034 (accessed on 20 October 2024).
- Zieliński, T.; Kędziorek, M.; Jurczak, J. The azulene moiety as a chromogenic building block for anion receptors. Tetrahedron Lett. 2005, 46, 6231–6234. [Google Scholar] [CrossRef]
- López-Alled, C.M.; Park, S.J.; Lee, D.J.; Murfin, L.C.; Kociok-Köhn, G.; Hann, J.L.; Wenk, J.; James, T.D.; Kim, H.M.; Lewis, S.E. Azulene-based fluorescent chemosensor for adenosine diphosphate. Chem. Commun. 2021, 57, 10608–10611. [Google Scholar] [CrossRef]
- Hu, J.; Hu, Z.; Liu, S.; Zhang, Q.; Gao, H.W.; Uvdal, K. A new ratiometric fluorescent chemodosimeter based on an ICT modulation for the detection of Hg2+. Sens. Actuators B Chem. 2016, 230, 639–644. [Google Scholar] [CrossRef]
- Salman, H.; Abraham, Y.; Tal, S.; Meltzman, S.; Kapon, M.; Tessler, N.; Speiser, S.; Eichen, Y. 1,3-Di(2-pyrrolyl)azulene: An efficient luminescent probe for fluoride. Eur. J. Org. Chem. 2005, 2005, 2207–2212. [Google Scholar] [CrossRef]
- Lopez-Alled, C.M.; Sanchez-Fernandez, A.; Edler, K.J.; Sedgwick, A.C.; Bull, S.D.; McMullin, C.L.; Kociok-Kohn, G.; James, T.D.; Wenk, J.; Lewis, S.E. Azulene–boronate esters: Colorimetric indicators for fluoride in drinking water. Chem. Commun. 2017, 53, 12580–12583. [Google Scholar] [CrossRef] [PubMed]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff bases—Structure, importance and classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, A.; Nica, S.; Debel, R.; Fenn, A.; Breitzke, H.; Buntkowsky, G.; Plass, W. Vanadium complexes with side chain functionalized N-salicylidene hydrazides: Hydrogen-bonding relays as structural directive for supramolecular interactions. Inorg. Chim. Acta 2014, 420, 166–176. [Google Scholar] [CrossRef]
- Cheng, C.; Sun, J.; Wang, C.; Zhang, Y.; Wei, S.; Jiang, F.; Wu, Y. Protonated N′-benzyl-N′-prolyl proline hydrazide as highly enantioselective catalyst for direct asymmetric aldol reaction. Chem. Commun. 2006, 37, 215–217. [Google Scholar] [CrossRef]
- Xiong, X.; Jiang, Y.; Ma, D. Assembly of N,N-Disubstituted hydrazines and 1-aryl-1H-indazoles via copper-catalyzed coupling reactions. Org. Lett. 2012, 14, 2552–2555. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, U. Synthetic methodology for alkyl substituted hydrazines. Chem. Soc. Rev. 2001, 30, 205–213. [Google Scholar] [CrossRef]
- Słomiak, K.; Łazarenkow, A.; Checinska, L.; Kusz, J.; Ochocki, J.; Nawrot-Modranka, J. Synthesis, spectroscopic analysis and assessment of the biological activity of new hydrazine and hydrazide derivatives of 3-formylchromone. Molecules 2018, 23, 2067. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Kuman, M.M.; Palvai, S.; Sengupta, P.; Basu, S. Impairing powerhouse in colon cancer cells by hydrazide-hydrazone-based small molecule. ACS Omega 2018, 3, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Boudissa, R.; Benmohammed, A.; Chafai, N.; Boudechicha, A.; Rekiba, N.; Lagraa, H.; Achour, M.; Khoumeri, O.; Djafri, A.; Terme, T.; et al. Synthesis, characterization, DFT, antibacterial, ADME-T properties, and molecular docking of new N-functionalized thiazolidinones. J. Mol. Struct. 2024, 1307, 138004. [Google Scholar] [CrossRef]
- Alcaraz, R.; Muñiz, P.; Cavia, M.; Palacios, O.; Samper, K.G.; Gil-Garcia, R.; Jiménez-Pérez, A.; García-Tojal, J.; García-Girón, C. Thiosemicarbazone-metal complexes exhibiting cytotoxicity in colon cancer cell lines through oxidative stress. J. Inorg. Biochem. 2020, 206, 110993. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, K.; Buchanan, R.M.; Grapperhaus, C.A. Antifungal activity of thiosemicarbazones, bis(thiosemicarbazones), and their metal complexes. J. Inorg. Biochem. 2021, 225, 111620. [Google Scholar] [CrossRef] [PubMed]
- Savir, S.; Wei, Z.J.; Liew, J.W.K.; Vythilingam, I.; Lim, Y.A.L.; Saad, H.M.; Sim, K.S.; Tan, K.W. Synthesis, cytotoxicity and antimalarial activities of thiosemicarbazones and their nickel (II) complexes. J. Mol. Struct. 2020, 1211, 128090. [Google Scholar] [CrossRef]
- Jiménez-Pérez, A.; Marcos-Gómez, S.; Madariaga, G.; Zapico, M.; Vitoria, P.; Tercero, J.; Torres, M.B.; Lezama, L.; Cuevas, J.V.; Etxebarria, I.; et al. Thiosemicarbazonecopper/halido systems: Structure and DFT analysis of the magnetic coupling. Inorganics 2023, 11, 31. [Google Scholar] [CrossRef]
- Arefyeva, N.; Sandleben, A.; Krest, A.; Baumann, U.; Schäfer, M.; Kempf, M.; Klein, A. [2 × 2] molecular grids of Ni(II) and Zn(II) with redox-active 1,4-pyrazine-bis(thiosemicarbazone) ligands. Inorganics 2018, 6, 51. [Google Scholar] [CrossRef]
- Lobana, T.S.; Sharma, R.; Bawa, G.; Khanna, S. Bonding and structure trends of thiosemicarbazone derivatives of metals—An overview. Coord. Chem. Rev. 2009, 253, 977–1055. [Google Scholar] [CrossRef]
- Udhayakumari, D.; Suganya, S.; Velmathi, S. Thiosemicabazone based fluorescent chemosensor for transition metal ions in aqueous medium. J. Lumin. 2013, 141, 48–52. [Google Scholar] [CrossRef]
- Rhoufal, F.; Guesmi, S.; Jouffret, L.; Ketatni, E.M.; Sergent, N.; Hlil, E.K.; Obbade, S.; Bentiss, F. Novel copper(II) and nickel(II) coordination complexes of 2,4-pentanedione bis-thiosemicarbazone: Synthesis, structural characterization, computational studies, and magnetic properties. Inorg. Chem. Commun. 2014, 133, 568–578. [Google Scholar] [CrossRef]
- Ain, Q.U.; Sharma, R. Structure and bonding trends of bisthiosemicarbazones: An overview. Appl. Organomet. Chem. 2023, 37, e7100. [Google Scholar] [CrossRef]
- Parrilha, G.L.; dos Santos, R.G.; Beraldo, H. Applications of radiocomplexes with thiosemicarbazones and bis(thiosemicarbazones) in diagnostic and therapeutic nuclear medicine. Coord. Chem. Rev. 2022, 458, 214418. [Google Scholar] [CrossRef]
- Paterson, B.M.; Donnelly, P.S. Copper complexes of bis(thiosemicarbazones): From chemotherapeutics to diagnostic and therapeutic radiopharmaceuticals. Chem. Soc. Rev. 2011, 40, 3005–3018. [Google Scholar] [CrossRef] [PubMed]
- Lobana, T.S.; Bhatia, P.K.; Tiekink, E.R.T. Synthesis and X-ray crystal structure of chloro [2(1H)-pyridinethione-S]-bis(triphenylphosphine)copper(I). J. Chem. Soc. Dalton Trans. 1989, 749–751. [Google Scholar] [CrossRef]
- Velo-Heleno, I.; Fernández-Fariña, S.; Barreiro-Sisto, U.; Rodríguez-Silva, L.; Pedrido, R. Design, synthesis and characterization of a bicompartmental bisthiosemicarbazone ligand. Chem. Proc. 2023, 14, 39. [Google Scholar] [CrossRef]
- Nöll, G.; Lambert, C.; Lynch, M.; Porsch, M.; Daub, J. Electronic structure and properties of poly- and oligoazulenes. J. Phys. Chem. C 2008, 112, 2156–2164. [Google Scholar] [CrossRef]
- Hibbert, D.B.; Thordarson, P. The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef] [PubMed]
- Ion, A.E.; Cristian, L.; Voicescu, M.; Bangesh, M.; Madalan, A.M.; Bala, D.; Mihailciuc, C.; Nica, S. Synthesis and properties of fluorescent 4′-azulenyl-functionalized 2,2′:6′,2″-terpyridines. Beilstein J. Org. Chem. 2016, 12, 1812–1825. [Google Scholar] [CrossRef]
- Basu, A.; Das, G. Zn(II) and Hg(II) complexes of naphthalene based thiosemicarbazone: Structure and spectroscopic studies. Inorg. Chim. Acta 2011, 372, 394–399. [Google Scholar] [CrossRef]
- Murugappan, S.; Dastari, S.; Jungare, K.; Barve, M.N.; Shankaraiah, N. Hydrazide-hydrazone/hydrazone as enabling linkers in anti-cancer drug discovery: A comprehensive review. J. Mol. Struct. 2024, 1307, 138012. [Google Scholar] [CrossRef]
- Zeller, K.-P.; Weyl, H. Methoden der Organischen Chemie; Kropf, H., Ed.; Georg Thieme Verlag: Stuttgart, Germany, 1985; Volume 5, pp. 127–418. [Google Scholar]
- Melhuish, W.H. Quantum efficiencies of fluorescence of organic substrate: Effect of solvent and concentration of the fluorescence solute. J. Phys. Chem. 1961, 65, 229–235. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]




| Chemical formula | C18H29N3OS |
| Molar mass (g mol−1) | 335.50 |
| T (K) | 100 |
| Crystal system | monoclinic |
| Space group | P21/c |
| a (Å) | 18.4754(14) |
| b (Å) | 9.2040(7) |
| c (Å) | 11.5714(9) |
| α (°) | 90 |
| β (°) | 95.517(2) |
| γ (°) | 90 |
| V (Å3) | 1958.6(3) |
| Z | 4 |
| ρcalc (g cm−3) | 1.138 |
| μ (mm−1) | 0.173 |
| F(000) | 728.0 |
| Crystal size (mm) | 0.500 × 0.200 × 0.150 |
| Radiation (λ/Å) | Mo-Kα (λ = 0.71073) |
| 2θ range (°) | 4.43–57.282 |
| Reflections collected | 37828 |
| Rint | 0.0661 |
| GOF on F2 | 1.051 |
| R1, wR2 (I ≥ 2σ (I)) | 0.0403, 0.0909 |
| Selected bond lengths (Å) for compound 2 | ||||||
| C1-S1 = 1.691(3) | C1-N2 = 1.341(4) | C14-S2 = 1.688(3) | ||||
| C1-N1 = 1.339(4) | C2-N3 = 1.284(4) | C14-N4 = 1.320(4) | ||||
| N3-N2 = 1.391(3) | N6-N5 = 1.386(3) | C14-N5 = 1.342(4) | ||||
| C13-N6 = 1.284(4) | ||||||
| Hydrogen bond metrics for compound 2 | ||||||
| D-H···A | d(DH)/Å | H···A/Å | D···A/Å | (D-H···A)/° | ||
| N4-H3N···S1a | 0.82 | 2.76 | 3.470 | 144.6 | ||
| N1-H1N···S1a | 0.92 | 2.65 | 3.433 | 142.1 | ||
| N5-H5A···S2c | 0.86 | 2.44 | 3.287 | 168.5 | ||
| N2-H2A···O1d | 0.86 | 2.14 | 2.902 | 148.6 | ||
| N4-H4N···O2b | 0.96 | 2.19 | 2.879 | 178.9 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanganu, A.; Maxim, C.; Dogaru, A.; Ion, A.E.; Bleotu, C.; Madalan, A.M.; Bala, D.; Nica, S. Synthesis, Physicochemical Properties, and Ion Recognition Ability of Azulene-Based Bis-(Thio)Semicarbazone. Molecules 2025, 30, 83. https://doi.org/10.3390/molecules30010083
Hanganu A, Maxim C, Dogaru A, Ion AE, Bleotu C, Madalan AM, Bala D, Nica S. Synthesis, Physicochemical Properties, and Ion Recognition Ability of Azulene-Based Bis-(Thio)Semicarbazone. Molecules. 2025; 30(1):83. https://doi.org/10.3390/molecules30010083
Chicago/Turabian StyleHanganu, Anamaria, Catalin Maxim, Andreea Dogaru, Adrian E. Ion, Coralia Bleotu, Augustin M. Madalan, Daniela Bala, and Simona Nica. 2025. "Synthesis, Physicochemical Properties, and Ion Recognition Ability of Azulene-Based Bis-(Thio)Semicarbazone" Molecules 30, no. 1: 83. https://doi.org/10.3390/molecules30010083
APA StyleHanganu, A., Maxim, C., Dogaru, A., Ion, A. E., Bleotu, C., Madalan, A. M., Bala, D., & Nica, S. (2025). Synthesis, Physicochemical Properties, and Ion Recognition Ability of Azulene-Based Bis-(Thio)Semicarbazone. Molecules, 30(1), 83. https://doi.org/10.3390/molecules30010083








