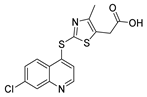
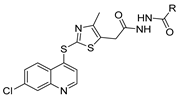
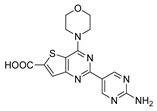
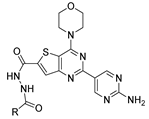
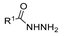
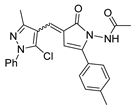
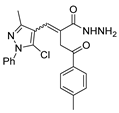
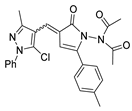
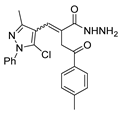
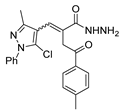
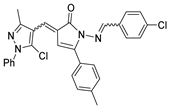
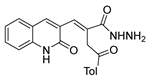
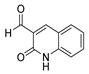
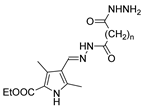
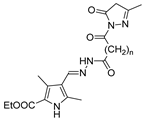
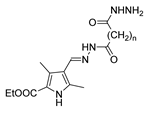
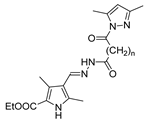
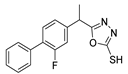
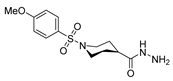
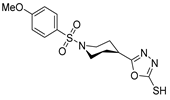
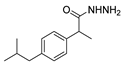
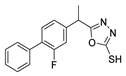
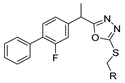
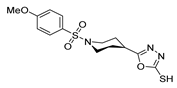
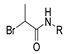
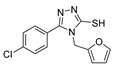
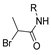
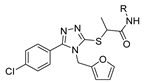
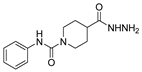
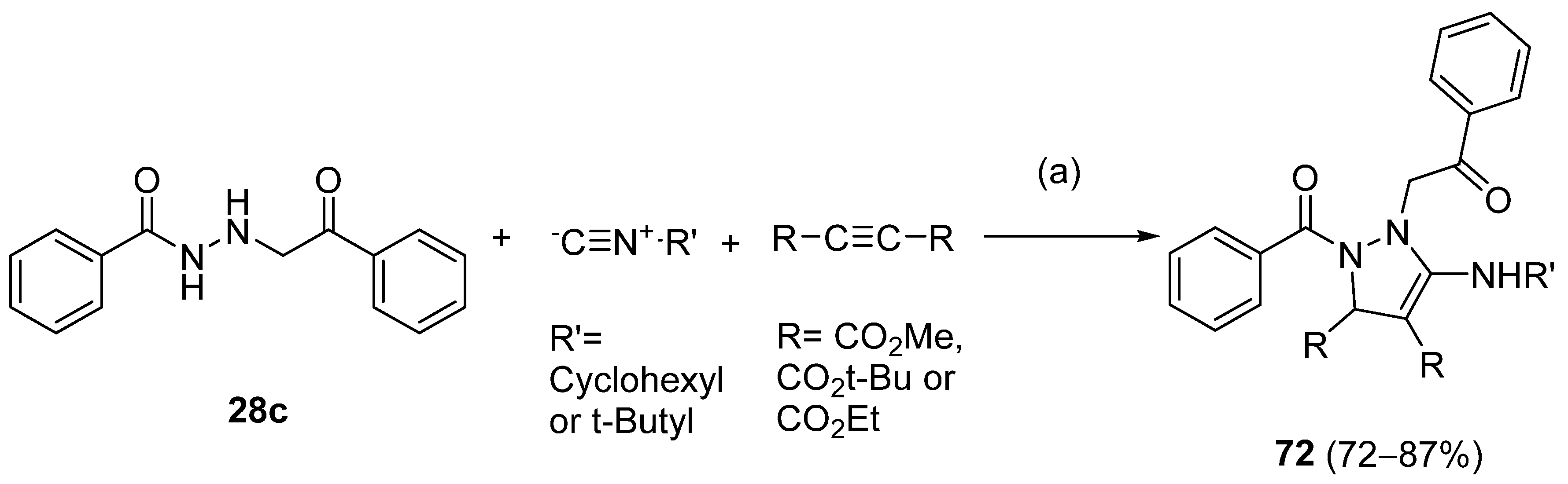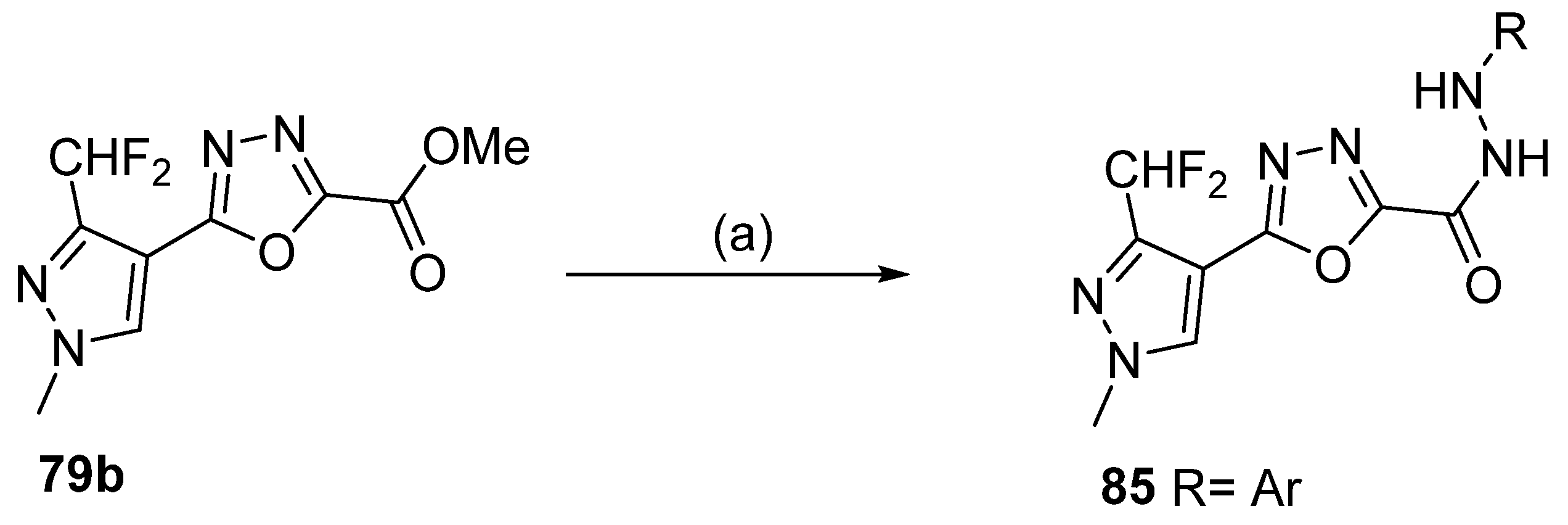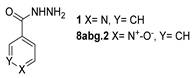Abstract
The increase in drug resistance and the high toxicity of current drugs have inspired the scientific community to develop new drugs for various diseases. Hydrazides have become an attractive functional group to easily obtain a plethora of novel compounds with a broad range of biological activities. This review, which contains studies in the literature from the previous five years, focuses on the synthesis methods and biological applications of hydrazides and their derivatives. Here, the details of the experimental reaction conditions used for the synthesis of hydrazides and their derivatives (hydrazide–hydrazones and heterocycle derivatives) are presented, as well as the purification methods and the biological activity of the synthesized compounds.
1. Introduction
According to the World Health Organization [1], diabetes, cardiovascular (ischemic heart disease and stroke), respiratory (chronic obstructive pulmonary disease and respiratory infections), cancers, and Alzheimer’s and dementia diseases are some of the leading causes of death worldwide. Besides these diseases, malaria, tuberculosis, HIV/AIDS, and cirrhosis of the liver are among the leading causes of death in low-income countries. The main obstacles to treating/eradicating these diseases include the increase in drug resistance to current drugs [2,3,4,5,6,7,8,9] and the high toxicity of the drugs used in the treatments [2,10,11,12].
Nowadays, many efforts have been made to develop novel and safer therapeutic alternatives. The scientific community has been searching for new compounds with reduced toxicity and improved biological efficacy, or even potential probes for bioimaging in disease diagnosis [13].
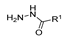
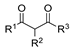
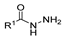
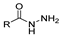
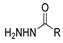
Hydrazides are a class of organic compounds with the functional group R-CON-R1N-R2R3 [14]. It is an extremely important group in organic chemistry, being an effective substrate in both domains of chemical reactions and medicinal chemistry [15,16].
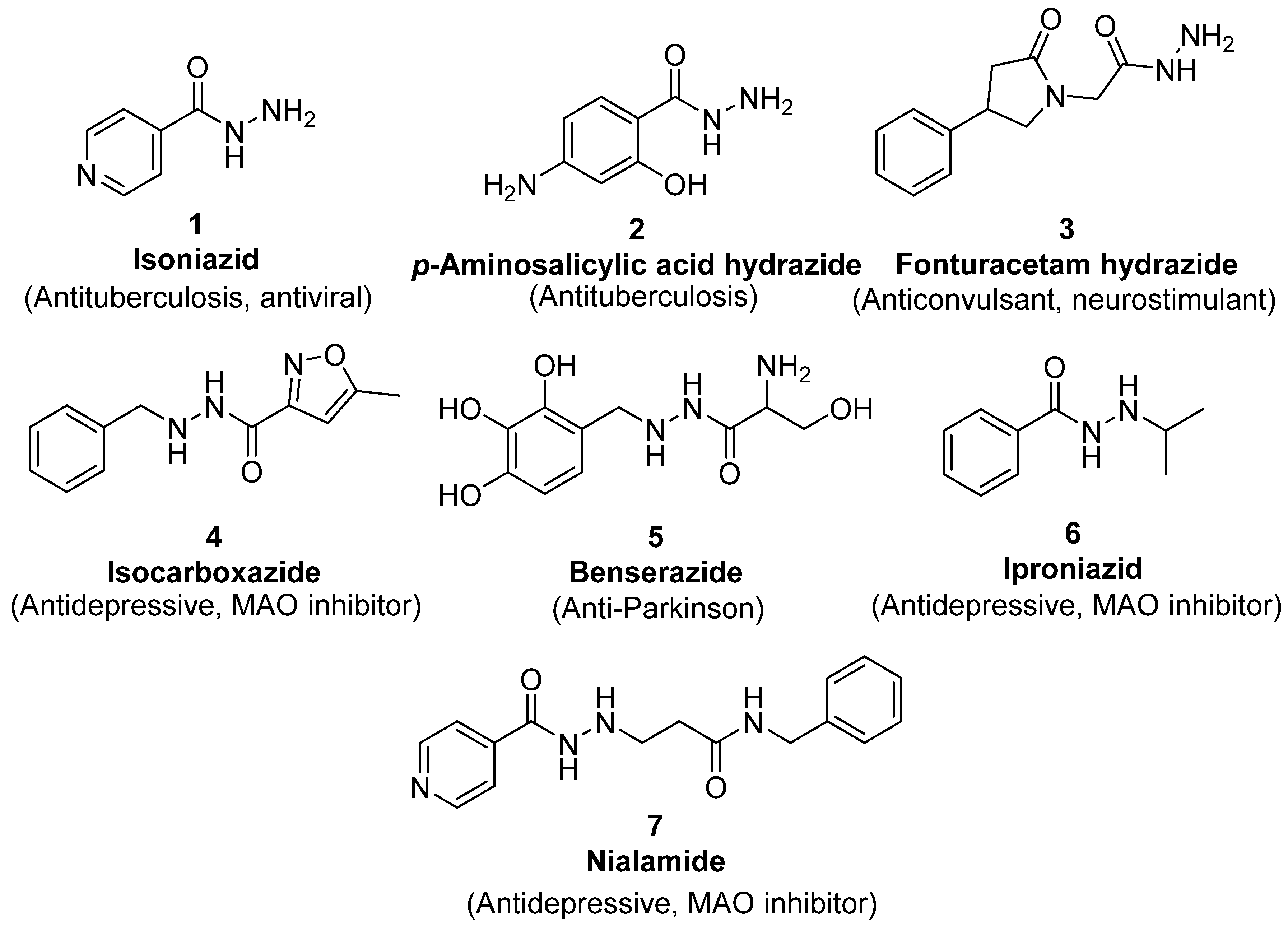
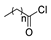
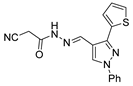
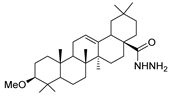
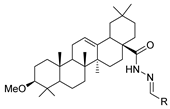
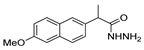
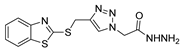
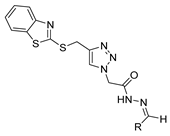
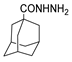
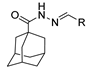
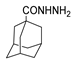
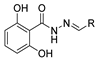
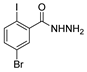
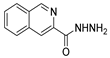
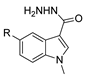
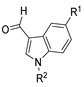
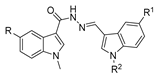
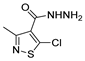
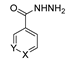
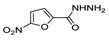
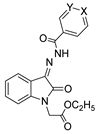
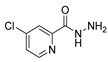
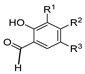
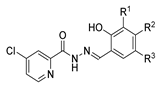
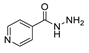
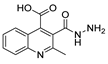
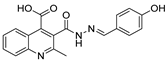
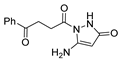
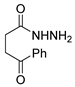
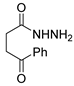
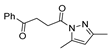
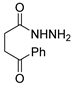
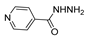
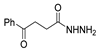
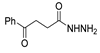
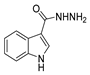
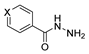
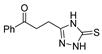
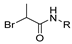
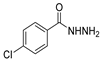
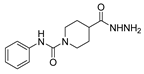
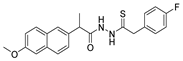
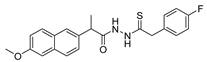
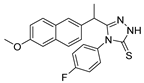
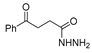
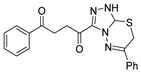
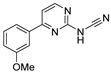
Since the 20th century, several hydrazides such as isoniazid 1 (isonicotinic acid hydrazide) [9,17,18], p-aminosalicylic acid hydrazide 2 [19], fonturacetam hydrazide 3 [20,21], isocarboxazide 4 [22], iproniazid 6 [23], nialamide 7 [11] and benserazide 5 [24] have been introduced for therapeutic purposes, as antituberculosis, antiviral, anticonvulsant, neurostimulator, antidepressive (monoamine oxidase inhibitor), and anti-Parkinson agents (Figure 1).
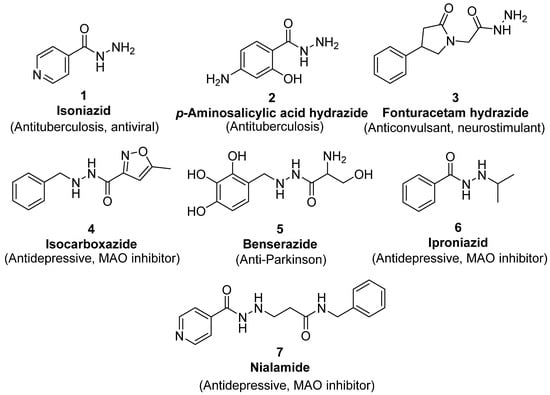
Figure 1.
Examples of hydrazides and their therapeutic applications.
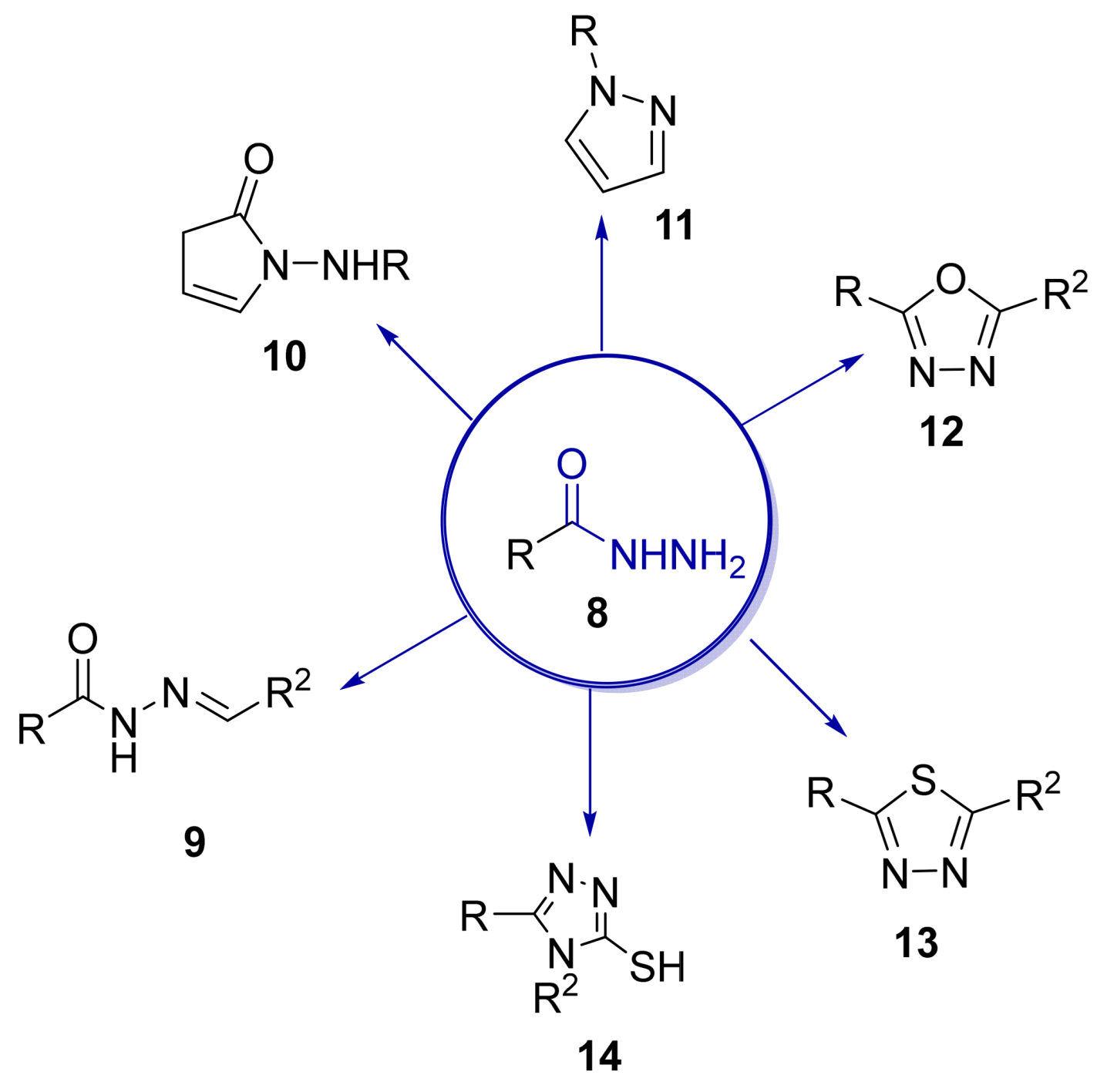
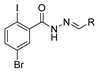
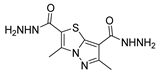
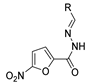
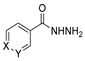
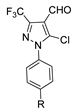
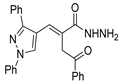
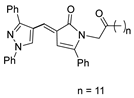
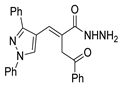
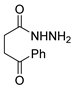
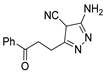
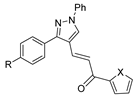
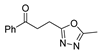
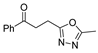
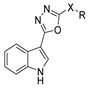
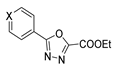
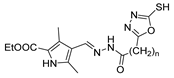
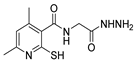
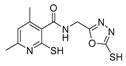
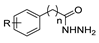
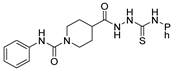
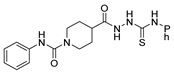
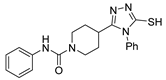
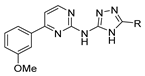
Over time, hydrazides 8 were found to be great precursors of other bioactive compounds such as hydrazine–hydrazones 9 [25,26]. Hydrazides 8 were also employed as building block synthons of different classes of heterocycles, like pyrrolones 10 [27,28], pyrazoles 11 [29,30], oxadiazoles 12 [31], thiadiazoles 13 [30,32], triazoles 14 [30], by cyclization or cycloaddition reactions with other reagents [28,33] (Figure 2). These hydrazide derivatives similarly revealed a wide range of biological activities, including antitumor [34,35,36,37], antimicrobial [38], antifungal [39], antimalarial [2], antileishmanial [40], anti-inflammatory [41], antidiabetic [42,43] and antioxidant [44] properties. They also showed herbicide activity or were used as dyes [45,46].
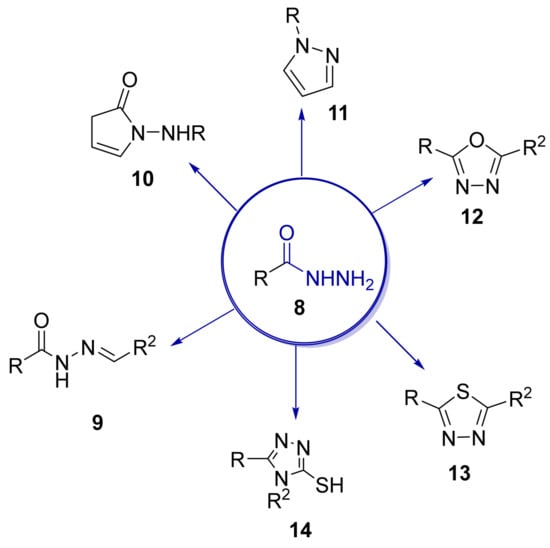
Figure 2.
Linear and heterocyclic compounds synthesized from hydrazides.
A review describing the synthesis of hydrazides and heterocyclic rings from hydrazides was published in 2014 by Majumdar et al. [47]. In 2018, Hosseini et al. [32] reported a compilation of the synthesis of heterocycles from cyanoacetohydrazides. Also, in 2021, Mali et al. [48] briefly reported the importance of the hydrazides and their derivatives (specifically hydrazide–hydrazones) as bioactive compounds over the years.
This review presents a comprehensive compilation and description of the methods used to synthesize hydrazides in the last 5 years, their use as precursors or synthons to generate new derivatives, and their main biological applications.
Moreover, throughout the review, tables will be provided summarizing the experimental conditions for the synthesis and purification of hydrazides and their derivatives.
2. Hydrazides
2.1. Synthesis of Hydrazides
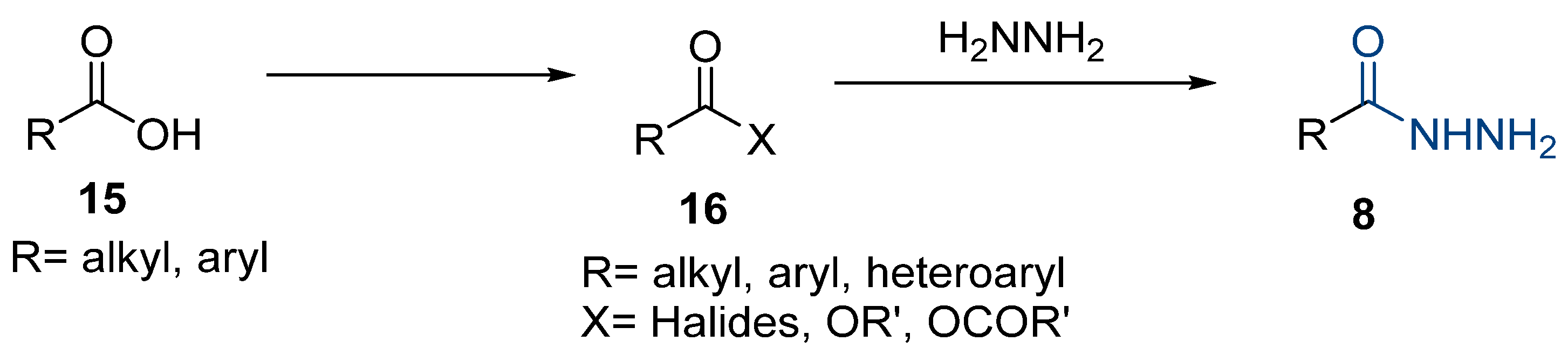
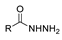
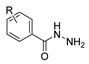
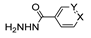
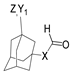
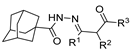
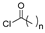
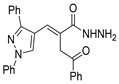
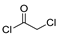
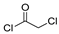
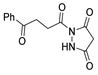
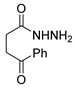
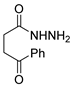
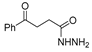
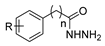
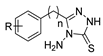
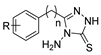
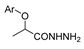
The first existing hydrazides, specifically formic and acetic acid hydrazides, were produced by Kurzius in 1895 [49]. Currently, many hydrazides with alkyl, aryl, and heteroaryl substituents are being synthesized to overcome drug resistance and toxicity. Hydrazides 8 (Scheme 1) are conventionally synthesized from compounds 16, such as esters [2,13,26,34,35,36,37,38,39,40,41,43,44,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95], anhydrides [96], and acyl chlorides [2,97,98], or others [99,100,101,102,103] that possess a good leaving group, and hydrazine. When it is not possible to have compounds 16 available, the leaving group is produced from the acid derivative 15 [82,85,88,96]. According to the reaction conditions outlined in Table 1, the experimental reaction conditions to generate hydrazide 8 from precursor 16 do not differ much, and usually, the reaction takes place in an alcohol solvent, at room temperature, or under reflux. The reactions did not last more than 24 h, and generally, product 8 was purified by recrystallization or by column chromatography. Hydrazides 8a to 8abb were obtained in low to excellent yields (26–98%) from esters (17a–17aaw), anhydrides (18), acyl chlorides (19a–c), or others (20–23).
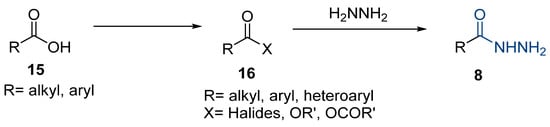
Scheme 1.
Representative scheme of hydrazide synthesis.
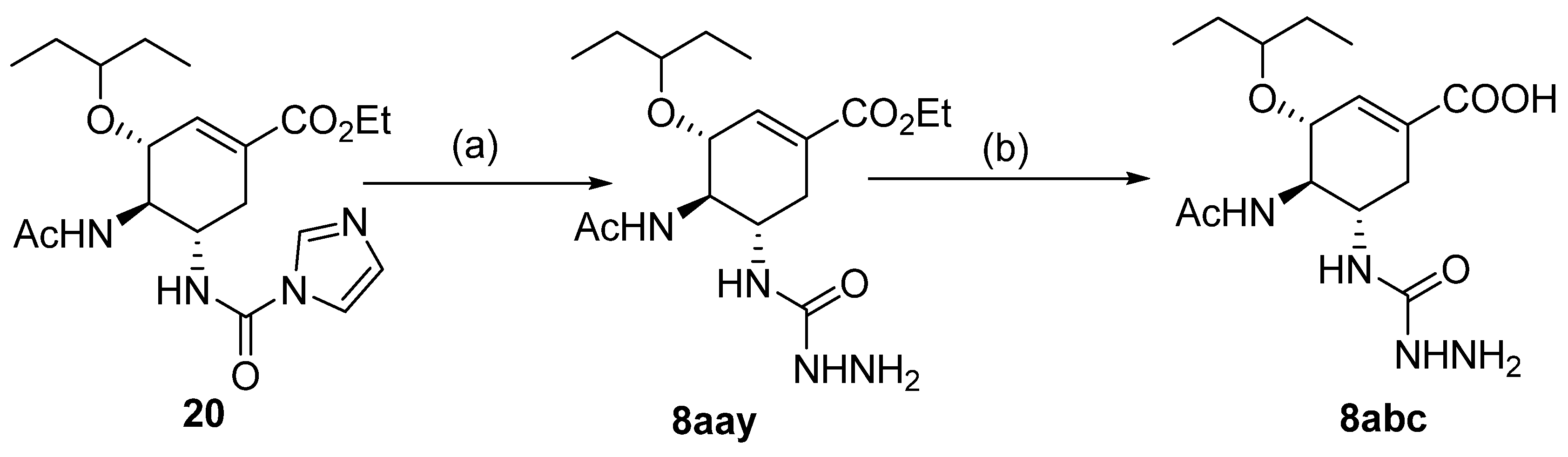
Besides the regular functional groups, Zhao et al. [99] obtained the hydrazide 8aay from activated intermediary 20 by reaction with hydrazine hydrate (Scheme 2). The acid derivative 8abc was obtained from 8aay by reaction with sodium hydroxide in aqueous methanol.
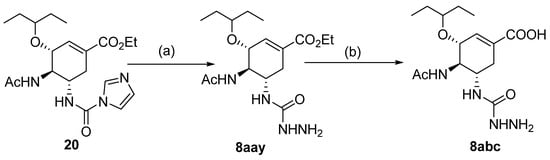
Scheme 2.
Synthesis of hydrazide 8abc. (a) H2NNH2·H2O, Et3N, Na2SO4, CHCl3, 35 °C; (b) NaOH, MeOH/H2O, r.t.
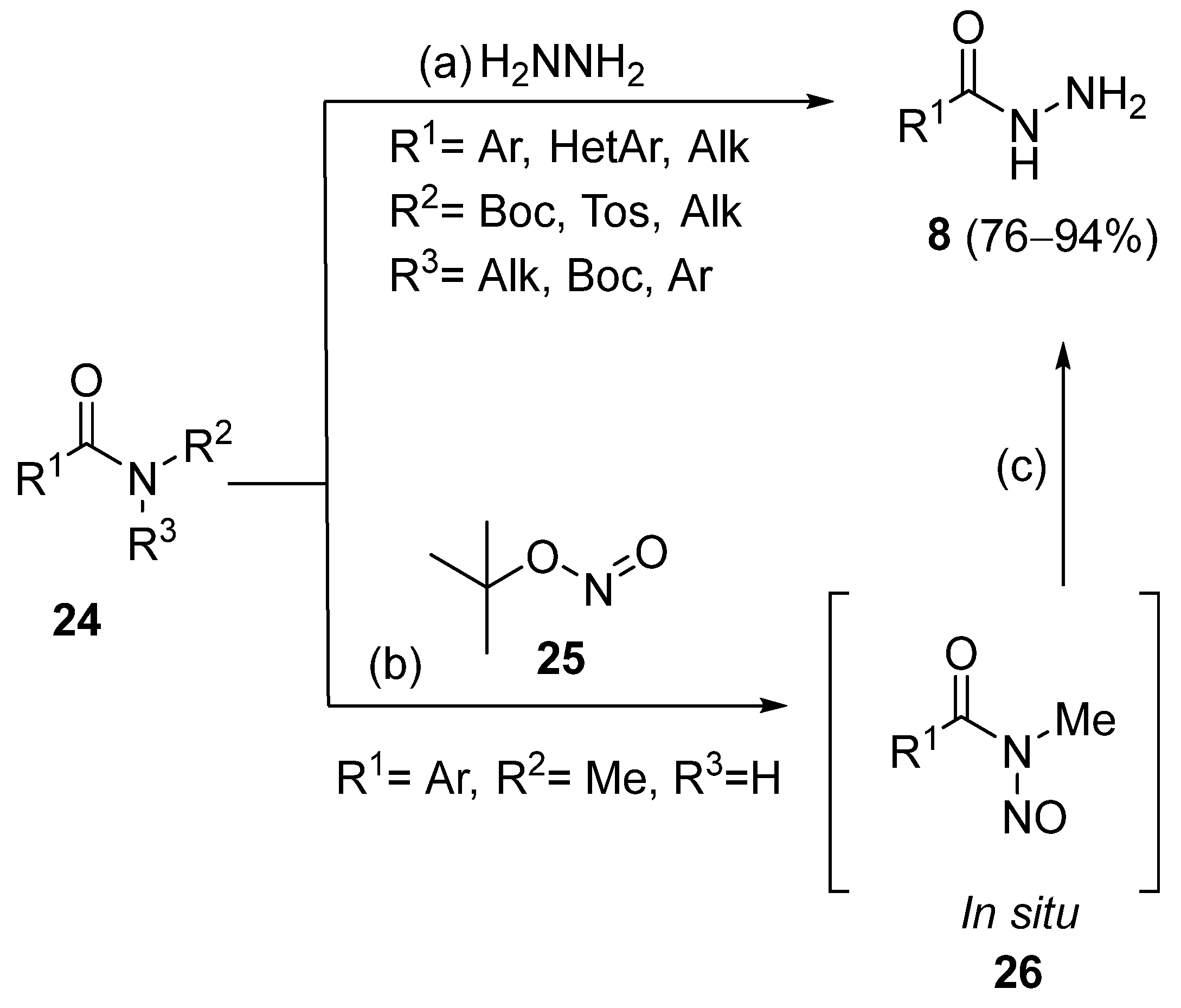
Singh et al. [104] obtained hydrazides via the transamidation of N-Boc, N-nitroso, and N-tosyl amides with hydrazine hydrate, at room temperature, in 76–94% yields (Scheme 3).
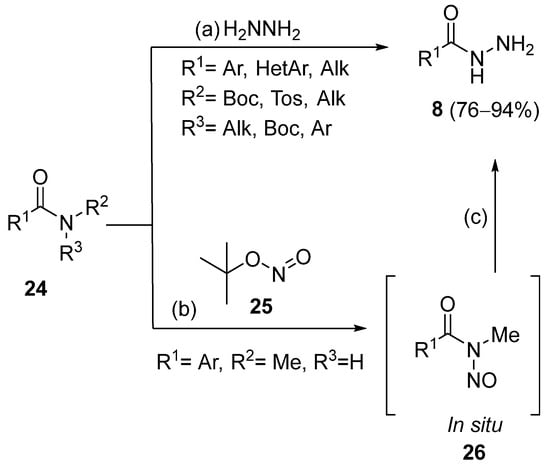
Scheme 3.
Synthesis of hydrazides 8 by transamidation; (a) 2HNNH2 (2-3 eq), DCM, r.t., 30 min–2 h; when R2 = Boc and R3 = Me, or R1 = Alk, DBU (1 eq) is also used; (b) 25 (3 eq), DCM, r.t., 1 h; (c) 2HNNH2 (3 eq), DCM, r.t., 1 h.

Table 1.
Reaction conditions for the synthesis and purification of hydrazides from esters, anhydrides, acyl chlorides, and others.
Table 1.
Reaction conditions for the synthesis and purification of hydrazides from esters, anhydrides, acyl chlorides, and others.
| Ref. | Starting Material | Experimental Conditions | Purification Process | Hydrazide Compounds (η%) |
|---|---|---|---|---|
| [50] |  17a | Hydrazine hydrate (1.2 eq) EtOH 75–80 °C 2 h | Silica gel column chromatography | 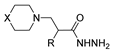 8a X = N, O; R = Alk (70–95%) |
| [51] | 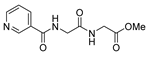 17b | Hydrazine hydrate (10 eq) MeOH Reflux 3 h | - |  8b |
| [26] | 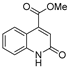 17c | Hydrazine hydrate (n.s.) EtOH or MeOH 85 °C 6 h | Recrystallization from aqueous ethanol or methanol | 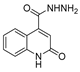 8c (88%) |
| [26] |  17d | Hydrazine hydrate (n.s.) EtOH or MeOH 85 °C 6 h | Recrystallization from aqueous ethanol or methanol | 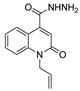 8d (54%) |
| [26] |  17e | Hydrazine hydrate (n.s.) EtOH or MeOH 85 °C 6 h | Recrystallization from aqueous ethanol or methanol | 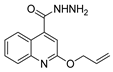 8e (75%) |
| [26] | 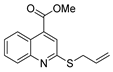 17f | Hydrazine hydrate (n.s.) EtOH or MeOH 85 °C 6 h | Recrystallization from aqueous ethanol or methanol | 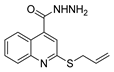 8f (83%) |
| [55] | 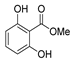 17g | Hydrazine hydrate (10 eq added dropwise) MeOH Reflux 2 h | - | 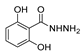 8g (80%) |
| [56] |  17g | Hydrazine hydrate (9 eq added dropwise) MeOH Reflux 2 h | Silica gel column chromatography | 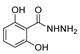 8g (55%) |
| [57] | 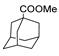 17h | Hydrazine hydrate (80%) (20.6 eq) Neat Reflux n.s. | - | 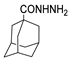 8h (96%) |
| [43] | 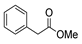 17i | Hydrazine 80% (n.s.) EtOH Reflux 6h | n.s. | 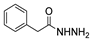 8i (50%) |
| [43] | 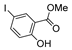 17j | Hydrazine 80% (n.s.) EtOH Reflux 6 h | n.s. | 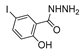 8j (57%) |
| [43] | 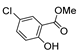 17k | Hydrazine 80% (n.s.) EtOH Reflux 6 h | n.s. |  8k (47%) |
| [13] | 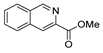 17l | Hydrazine hydrate (1.7 eq) MeOH Reflux 4.5 h | Recrystallization from methanol |  8l (63%) |
| [34] | 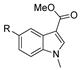 17m | Hydrazine hydrate (n.s.) EtOH Reflux 8–10 h | n.s. | 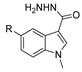 8m R = H, Br (n.s.) |
| [38] | 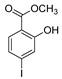 17n | Hydrazine hydrate (1.1 eq) EtOH Reflux 3 h | Recrystallization from ethanol | 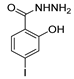 8n (75%) |
| [40] | 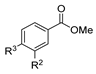 17o R2 = H, R3 = H 17p R2 = Cl, R3 = H 17q R2 = H, R3 = Cl | Hydrazine hydrate 80% (n.s.) EtOH Reflux 3 h | n.s. |  8o R2 = H, R3 = H 8p R2 = Cl, R3 = H 8q R2 = H, R3 = Cl (n.s.) |
| [53] | 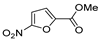 17r | Hydrazine hydrate (1 eq) Anhydrous EtOH ~0 °C 30 min | Recrystallization from ethanol |  8r (73%) |
| [54] | 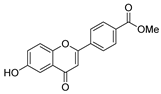 17s | Hydrazine hydrate (n.s.) MeOH Reflux 6 h | Recrystallization from ethanol |  8s (n.s.) |
| [39] | 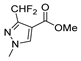 17t | Hydrazine monohydrate (8 eq) EtOH Ice bath 30 min | - | 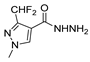 8t (n.s.) |
| [59] | 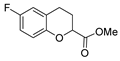 17u | Hydrazine hydrate (2 eq) EtOH Reflux 8 h | - | 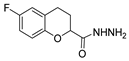 8u (91%) |
| [105] | 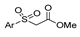 17v 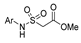 17w | Hydrazine hydrate (1.1 eq) MeOH Pyridine (cat.) Reflux 6–7 h | Recrystallization from methanol | 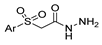 8v  8w (n.s) |
| [61] |  17x | Hydrazine hydrate (2 eq) EtOH Reflux 4 h | - | 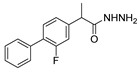 8x (n.s.) |
| [62] | 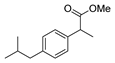 17y | Hydrazine hydrate 80% (~8 eq, dropwise) EtOH 95–100 °C 12 h | Recrystallization from ethanol |  8y (89%) |
| [36] | 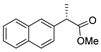 17z | Hydrazine hydrate (n.s.) EtOH 80–90 °C 8 h | - | 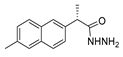 8z (n.s.) |
| [41] | 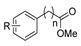 17aa | Hydrazine hydrate 85% (~6.5 eq) MeOH Reflux 8 h | - |  8aa R = Me, OMe (82–92%) |
| [63] | 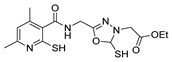 17ab | Hydrazine hydrate (~10 eq) MeOH Reflux 5 h | Recrystallization from methanol | 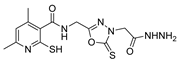 8ab (37%) |
| [64] | 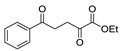 17ac | Hydrazine hydrate (n.s.) EtOH Reflux 6 h | - | 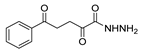 8ac (78%) |
| [35] | 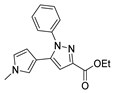 17ad | Hydrazine (4 eq) EtOH Reflux 5 h | Recrystallization from ethanol | 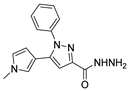 8ad (73%) |
| [65] |  17ae | Hydrazine hydrate (6 eq.) EtOH 30 °C 1 h | Recrystallization from ethanol | 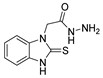 8ae (26%) |
| [66] | 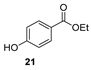 17af | Hydrazine hydrate 80% (31 eq) EtOH Reflux 8 h | Recrystallization from ethanol | 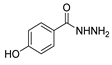 8af (n.s.) |
| [67] | 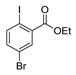 17ag | Hydrazide hydrate (4.6 eq) EtOH Reflux 3 h | Recrystallization from ethanol | 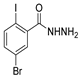 8ag (78%) |
| [68] | 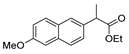 17ah | Hydrazine hydrate 80% (31 eq) EtOH Reflux 4 h | Recrystallization from ethanol | 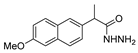 8ah (70%) |
| [43] | 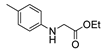 17ai | Hydrazine hydrate 80% (15 eq) EtOH Reflux 8 h | Recrystallization from ethanol | 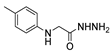 8ai (63%) |
| [43] | 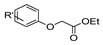 17aj | Hydrazine 80% (n.s.) EtOH Reflux 6 h | n.s. |  8aj R′ = Alk, Halide (39–54%) |
| [43] | 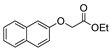 17ak | Hydrazine 80% (n.s.) EtOH Reflux 6 h | n.s. | 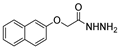 8ak (46%) |
| [43] | 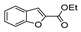 17al | Hydrazine 80% (n.s.) EtOH Reflux 6 h | n.s. | 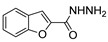 8al (57%) |
| [43] | 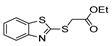 17am | Hydrazine 80% (n.s.) EtOH Reflux 6 h | n.s. | 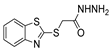 8am (56%) |
| [72] | 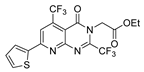 17ao | Hydrazine hydrate (~3 eq) EtOH Reflux 12 h | - | 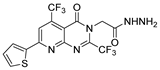 8ao (n.s.) |
| [73] | 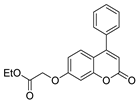 17ap | Hydrazine hydrate (2 eq) EtOH Reflux 8 h | - | 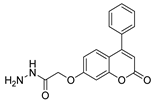 8ap (80%) |
| [75] | 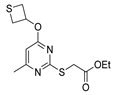 17aq | Hydrazine hydrate 85% (3 eq) EtOH r.t. 4 h | Recrystallization from isopropyl alcohol | 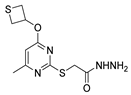 8aq (67%) |
| [76] | 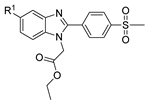 17ar | Hydrazine hydrate 99% (1 eq) EtOH Reflux 6 h | Recrystallization from ethanol | 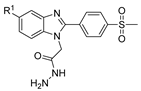 8ar R1 = H, Cl, CH3 (67–73%) |
| [77] | 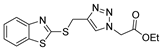 17as | Hydrazine hydrate (1.5 eq) EtOH Reflux 4 h | Recrystallization from ethanol | 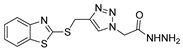 8as (90%) |
| [78] |  17at | Hydrazine hydrate (1.02 eq) EtOH Reflux 3 h | Recrystallization from ethanol or methanol | 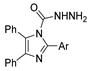 8at (89–97%) |
| [79] | 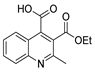 17au | Hydrazine hydrate (20 eq) EtOH Reflux 4 h | Recrystallization from ethanol | 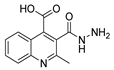 8au (61%) |
| [37] |  17av | Hydrazine hydrate 80% (3 eq) EtOH Reflux 3 h | Recrystallization from ethanol/DMF |  8v (90%) |
| [80] |  17ac | Hydrazine monohydrate (1 eq) EtOH Reflux 4 h | Recrystallization from dioxane |  8ac(90%) |
| [81] |  17w | Hydrazine hydrate (4 eq) EtOH Reflux 6 h | - |  8aw X = N, CH (48–55%) |
| [82] |  17x | Hydrazine (1 eq) EtOH Reflux 3 h | - |  8ax(94%) |
| [83] | 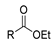 17ay R = 4-F-C6H4, 17az R = 4-CH3–C6H4 17aaa R = 2-Cl,4-Cl-C6H3 | Hydrazine hydrate (n.s.) EtOH Reflux n.s. | n.s. | 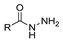 8ay R = 4-F-C6H4, 8az R = 4-CH3–C6H4 8aaaR = 2-Cl,4-Cl-C6H3 (n.s.) |
| [84] |  17aab | Hydrazine hydrate (10 eq) EtOH Reflux 7 h | - | 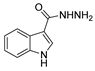 8aab (80%) |
| [2] | 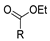 17aac R = 2-furyl 17aad R = 3,4,5-(MeO)3C6H2 17aai R = 3,4-(MeO)2C6H3 | Hydrazine hydrate (n.s.) - Reflux n.s. | - |  8aac R = 2-furyl 8aad R = 3,4,5-(MeO)3C6H2 8aai R = 3,4-(MeO)2C6H3 (n.s.) |
| [85] | 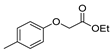 17al | Hydrazine hydrate (~11 eq) EtOH r.t. 3–4 h | - | 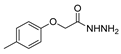 8al (98%) |
| [86] |  17aaj | Hydrazine hydrate (1.2 eq) EtOH r.t. 5–6 h | Recrystallization from ethanol | 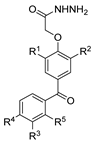 8aaj R1 = H, CH3, F; R2 = H, Cl; R3 = H, Cl; R4 = H, Cl, I; R5 = H, Cl (92% as an example) |
| [87] | 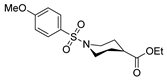 17aak | Hydrazine (1 eq) MeOH Reflux 2 h | - |  8aak (n.s.) |
| [42] | 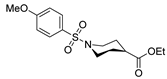 17aal | Hydrazine hydrate (n.s.) MeOH Reflux 4 h | - | 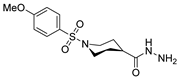 8aal (n.s.) |
| [88] | 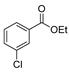 17aam | Hydrazine hydrate 80% (1 eq) Neat r.t. 4–5 h | - |  8p (96%) |
| [89] | 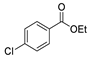 17aan | Hydrazine hydrate 80% (~11 eq) Neat r.t. 5–6 h | - | 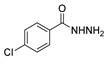 8q (96%) |
| [90] |  17aao | Hydrazine monohydrate (~34 eq) Neat r.t. 4–5 h | - | 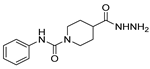 8aao (97%) |
| [91] | 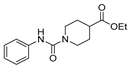 17aao | Hydrazine monohydrate 80% (~20 eq) Neat r.t. 4–5 h | - |  8aao (98%) |
| [92] |  17aap | Hydrazine hydrate (10 eq, dropwise) EtOH and chloroform r.t. 24 h | Recrystallization from ethanol/water (60:40) |  8aap R1 = Me, OMe, Br; R2 = Alk (72–80%) |
| [44] |  17aaq | Hydrazine hydrate (2 eq) EtOH Reflux 8 h | Recrystallization from ethanol |  8aaq (52%) |
| [93] |  17aar | Hydrazine hydrate (~4 eq) MeOH Reflux 6 h | Recrystallization from methanol |  8aar (92%) |
| [94] | 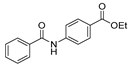 17aas | Hydrazine hydrate (n.s.) EtOH Reflux n.s. | n.s. | 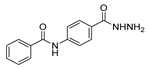 8aas (n.s.) |
| [95] | 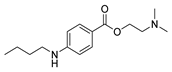 17aat | Hydrazine hydrate 80% (32.6 eq) EtOH Reflux n.s. | Recrystallization from ethanol | 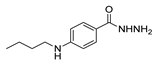 8aat (n.s.) |
| [96] | 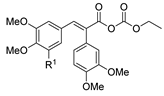 18 | Hydrazine hydrate (n.s.) THF r.t. 4 h | n.s. |  8aau R1 = Alk (80–95%) |
| [2] | 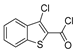 19a | Hydrazine hydrate (n.s.) - Reflux n.s. | - | 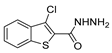 8aav (n.s.) |
| [97] |  19b | Hydrazine hydrate (2 eq) DCM 25 °C Overnight | Silica gel column chromatography |  8aaw (89%) |
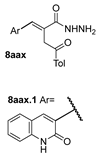
| [98] | 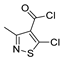 19c | 1. Acetone NaN3 (aq.) 8 °C, 30 min 2. Anhydrous hydrazine (4 eq) 2-propanol Reflux 45 min | - |  8aax (57%) |
| [99] |  20 | Hydrazine hydrate (2.2 eq) Et3N (1.5 eq), Na2SO4, CHCl3 1. r.t 2. 35 °C. 1. 30 min 2. Overnight | - | 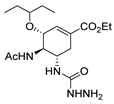 8aay (92%) |
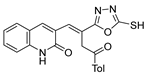
| [28] | 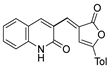 21 | Hydrazine hydrate (1 eq) [100] EtOH r.t. 1 h | Recrystallization from ethanol | 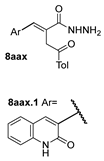 8aax.1 (81%) |
| [101] | 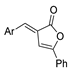 22 | Hydrazine hydrate (1.1 eq.) EtOH r.t. n.s. | - | 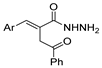 8aba (n.s.) |
| [103] |  23 | Hydrazine hydrate 85% (1 eq added dropwise) EtOH r.t. n.s. | Recrystallization from ethanol | 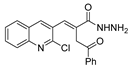 8abb.1 (78%) |
n.s.—not specified by the authors.
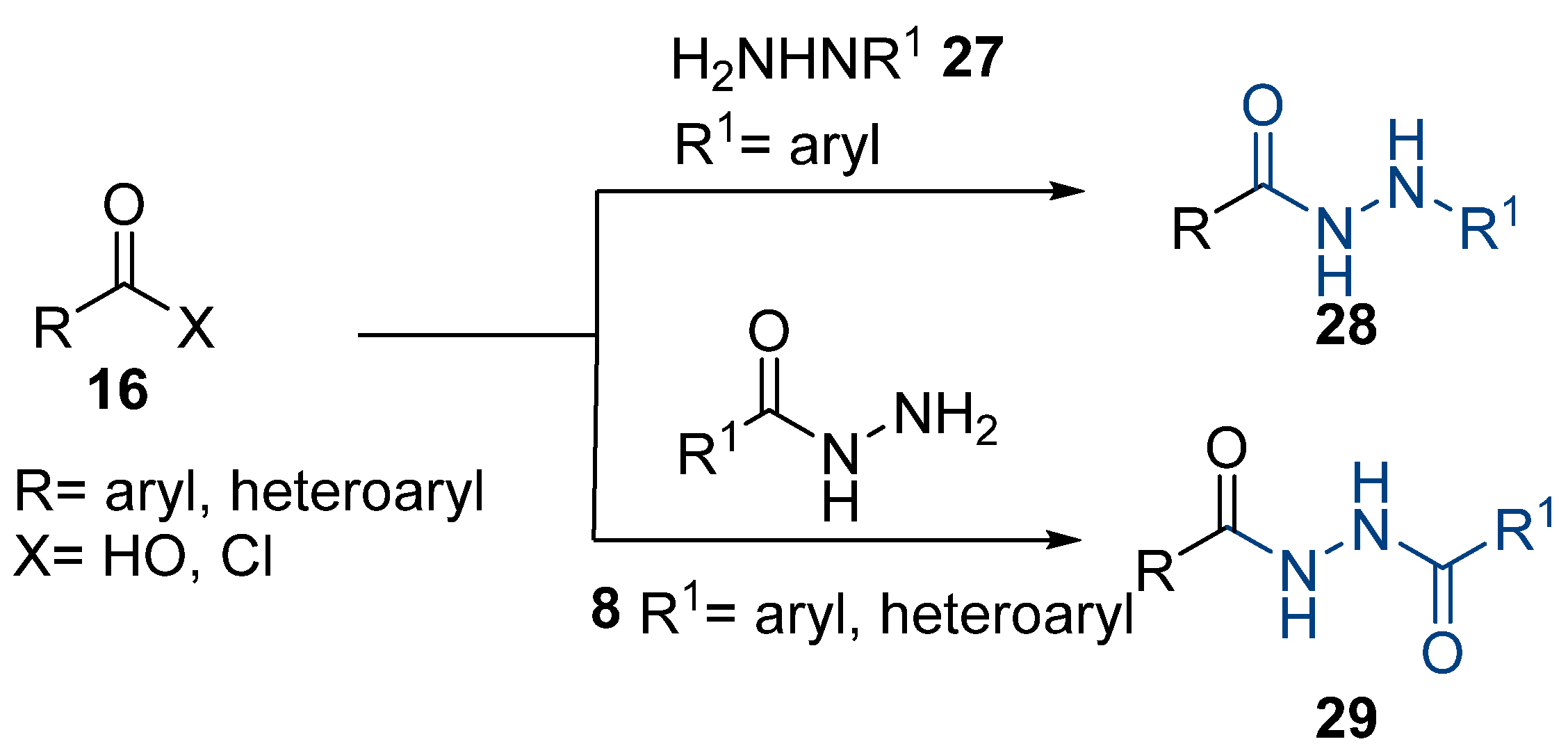
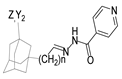
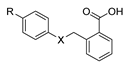
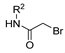
Aside from the synthesis of unsubstituted hydrazides, substituted ones can be synthesized as illustrated in Scheme 4 and outlined in Table 2. In these cases, acids [106,107,108,109] or acyl halides [92,110] 16 react with hydrazides 8 or hydrazines 27 to acylate the most nucleophilic amine unit. When an acid is used as the starting material, the reaction is performed in the presence of a coupling agent, such as 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) [106,107,109]. Generally, products 28 and 29 were obtained in low to good yields (35–97%).
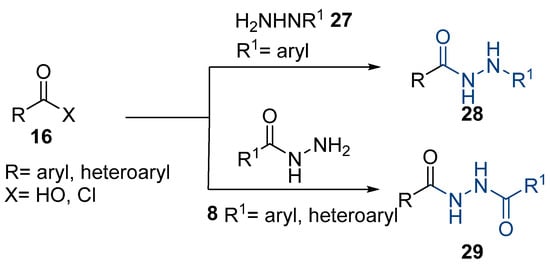
Scheme 4.
Representative scheme of substituted hydrazide synthesis.

Table 2.
Reaction conditions for the synthesis and purification of substituted hydrazides from acid or acyl chlorides.
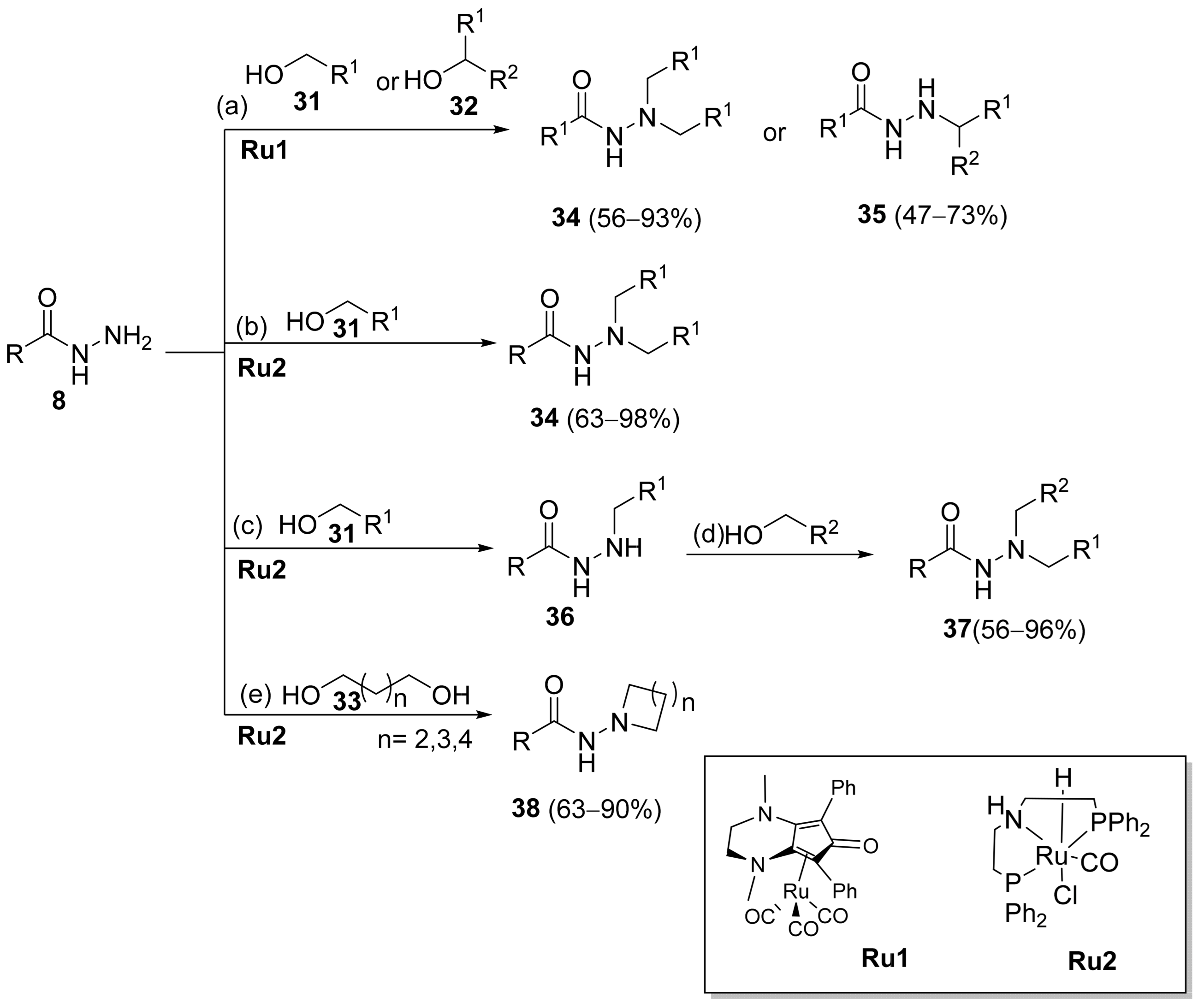
Substituted hydrazides were also obtained via the alkylation of hydrazides 8 by reaction with primary or secondary alcohols as alkylating agents, under ruthenium complex catalysis (Scheme 5). Joly et al. [111] reported the synthesis of dialkylated and monoalkylated hydrazides 34 or 35, respectively, in reasonable to excellent yields using diaminocyclopentadienone ruthenium tricarbonyl complex Ru1. On the other hand, Thiyagarajan et al. [112] reported the synthesis of both symmetrical and unsymmetrical N,N-disubstituted hydrazides 34 and 37. The use of diols led to the intramolecular cyclization of acylhydrazides to generate 38.
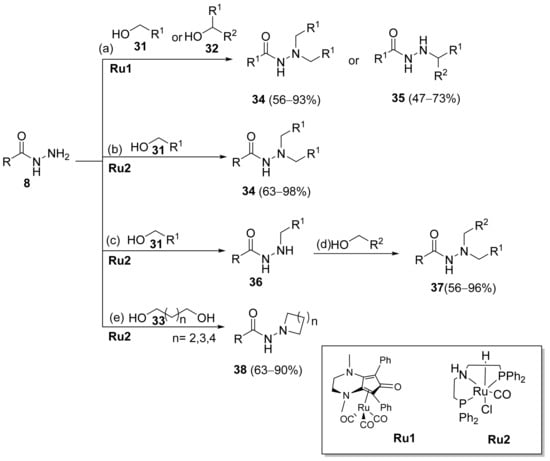
Scheme 5.
Synthesis of dialkylated and monoalkylated hydrazides; (a) alcohol (5 eq), Ru1 catalyst (1 mol%), Me3NO (2 mol%), NaOtBu (0.5 eq), t–BuOH (0.5 M), 130 °C, 24 h; (b) alcohol (2.2 eq), Ru2 catalyst (2 mol%), KOtBu (5 mol%), toluene, 135 °C, 24 h; (c) alcohol (1.1 eq), Ru2 catalyst (1 mol%), KOtBu (5 mol%), toluene, 135 °C,12 h; (d) alcohol (1.1 eq), Ru2 (1 mol%), KOtBu (5 mol%), toluene, 135 °C,12 h; (e) diol (2 eq), Ru2 catalyst, KOtBu (5 mol%), toluene, 135 °C, 24 h.
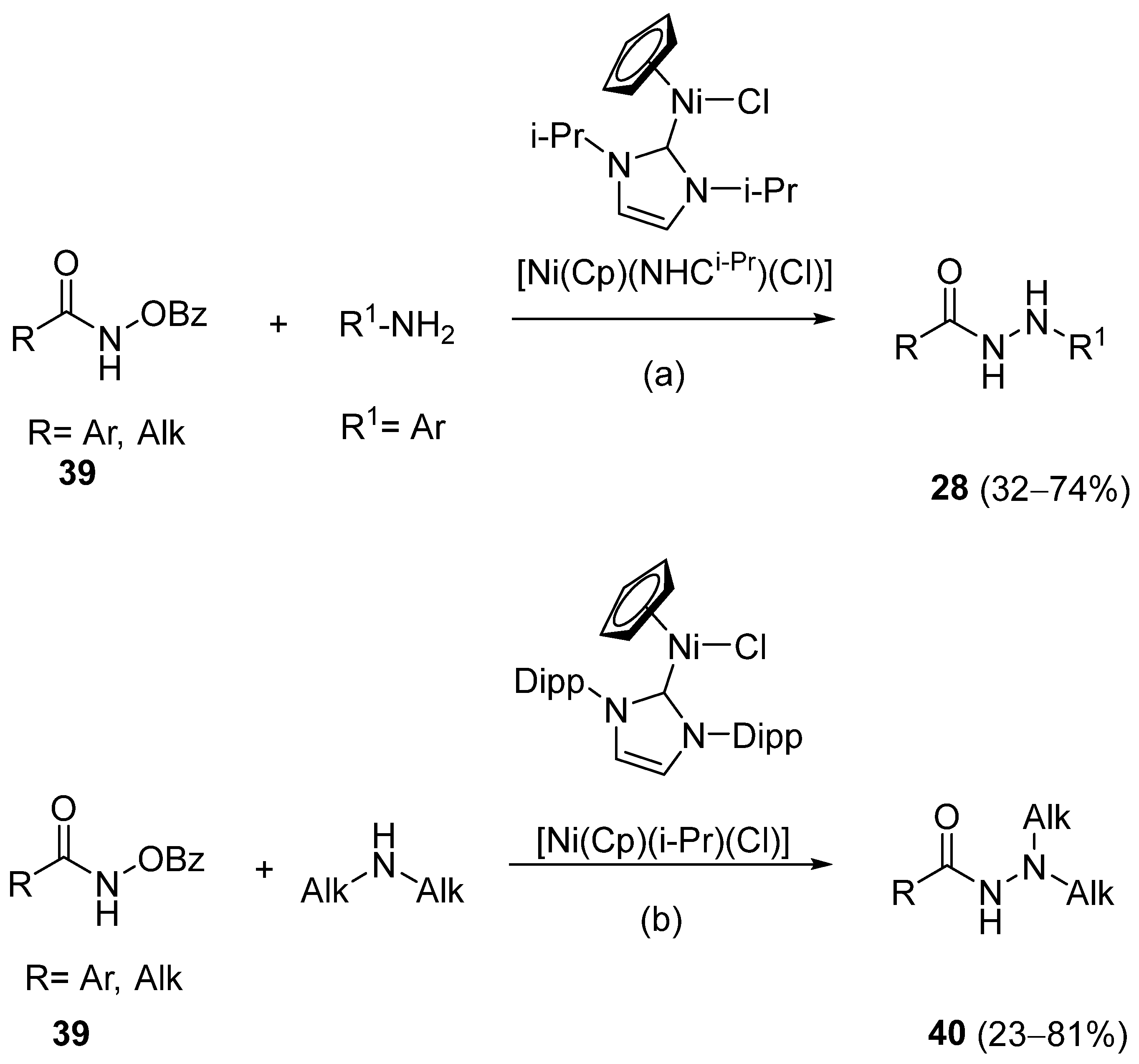
Barbor et al. [113] reported a novel nickel-catalyzed method for the synthesis of hydrazides 28 and 40 through N-N cross-coupling reactions from hydroxamates 39 in the presence of aromatic or aliphatic amines. These reactions occurred by catalysis with the nickel complex presented in Scheme 6. According to the authors, N-N coupling occurs efficiently when hydroxamates with electron-donating or -withdrawing para-substituents are used together with aniline derivatives. The aniline derivatives may have para-electron-donating or sterically hindered ortho substituents, such as halide (with no indication of protodehalogenation) or alkyl groups. Additionally, the reaction is also compatible with aniline derivatives bearing unprotected ketone and hydroxyl moieties. Primary, secondary, and tertiary aliphatic hydroxamates were also well tolerated. Moreover, in the presence of secondary aliphatic amines, in situ silylation allows N-N coupling in low to good yields [113].
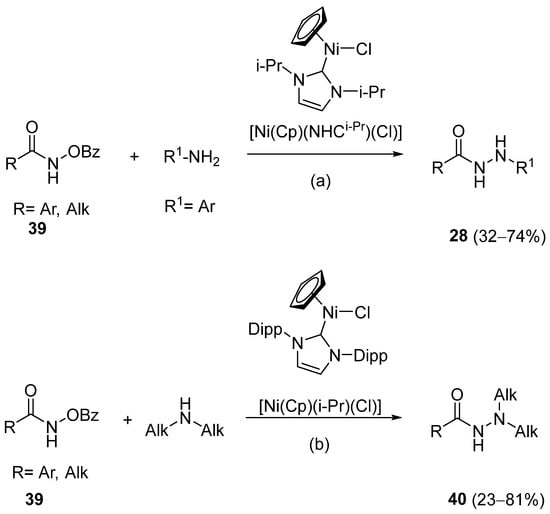
Scheme 6.
Synthesis of substituted hydrazides from hydroxamates. (a) Ni catalyst (10 mol%), PhSiH3 (1 eq), 1:4 CH2Cl2/THF (0.2 M), 30 °C, 24 h; (b) Ni catalyst (10 mol%), PhSiH3 (1 eq), MSTFA (1.5 eq), 1:4 CH2Cl2/THF (0.2 M), 30 °C, 24 h.
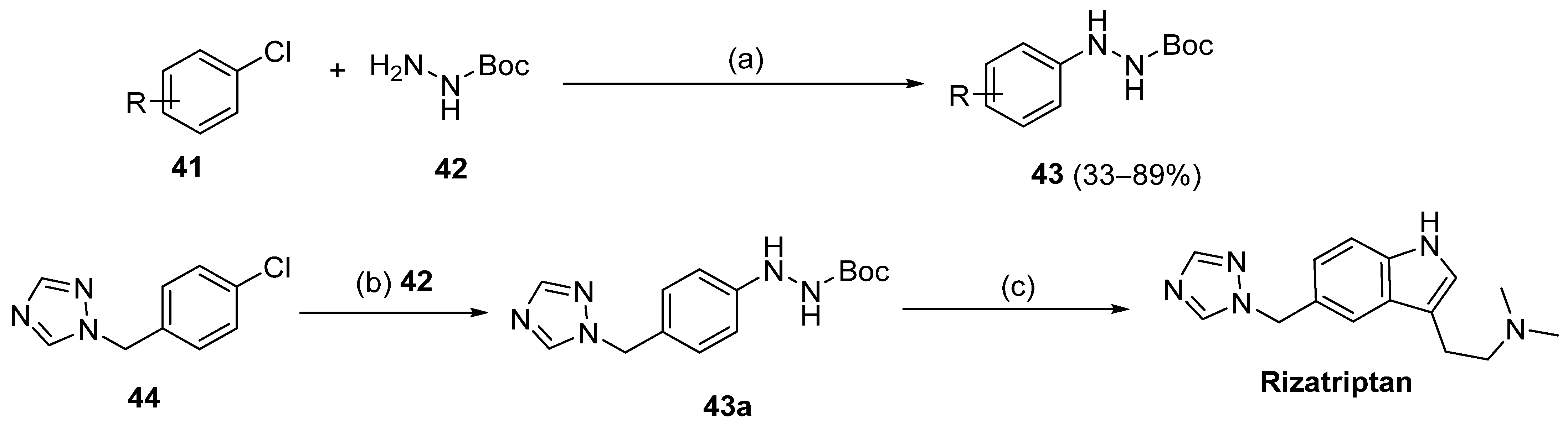
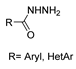
On the other hand, nickel-catalyzed photochemical C-N coupling reactions were performed by Li et al. [114] to react (hetero)aryl halides 41 with hydrazides/N-Boc hydrazine 42 (Scheme 7). This arylation reaction was catalyzed by a Ni(II)–bipyridine complex in the presence of purple LEDs. The reaction of N-Boc hydrazine with aryl chlorides 41 with electron-deficient and electron-neutral substituents at the para position yielded aryl hydrazides 43 with low to good yields (33–89%). Also, the synthesis of rizatriptan was conducted after applying this method for the synthesis of intermediate 43a [114].
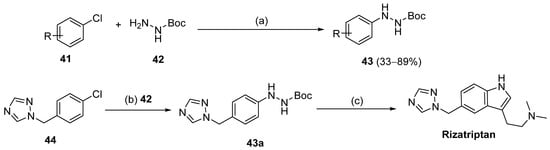
Scheme 7.
Synthesis of 43 and rizatriptan: (a) (NiBr2·glyme, d-Mebpy) (10 mol%) purple LEDs (390–395 nm), Cy2NMe, TBAI, CH3CN, 80 °C, Ar, 12 h; (b) 42 (NiBr2·glyme, d-Mebpy), purple LEDs (390–395 nm) Cy2NMe, TBAI, CH3CN, 80 °C, Ar, 24 h; (c) i. 4 M HCl, THF, r.t., 6 h; ii. Me2N(CH2)3CH(OEt)2, HCl, H2O, 70 °C, 2 h.
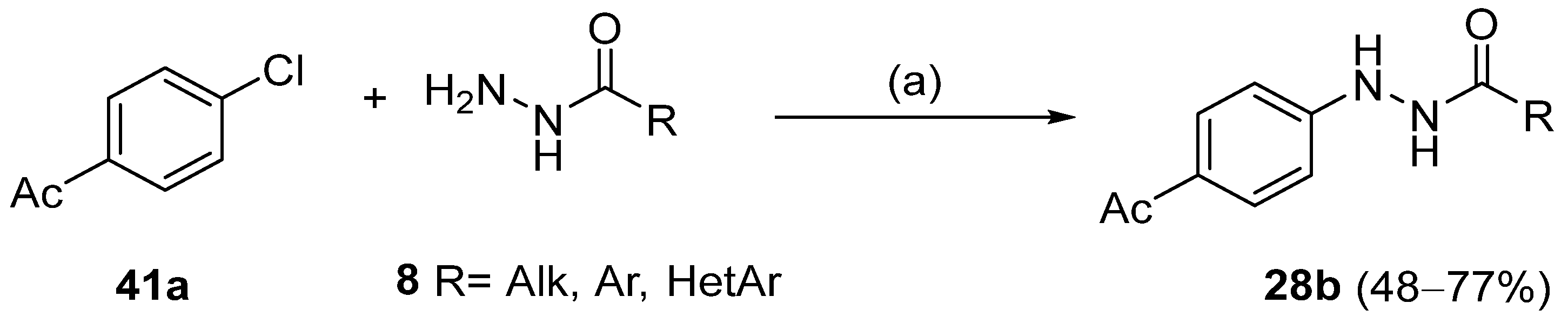
Moreover, many alkyl and aryl hydrazides 8 were coupled with 4-chloroacetophenone 41a, yielding hydrazides 28b generally in good yields (Scheme 8). Aryl hydrazides with electron-rich and electron-neutral substituents and heteroaryl hydrazides were well tolerated in this reaction (48–77%) [114].
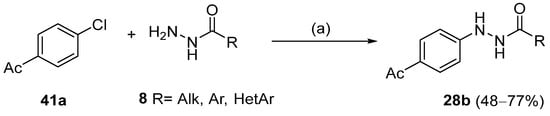
Scheme 8.
Synthesis of hydrazides 28b: (a) (NiBr2·glyme, d-Mebpy) (10 mol%) purple LEDs (390–395 nm), Cy2NMe, TBAI, CH3CN, 80 °C, Ar, 12 h.
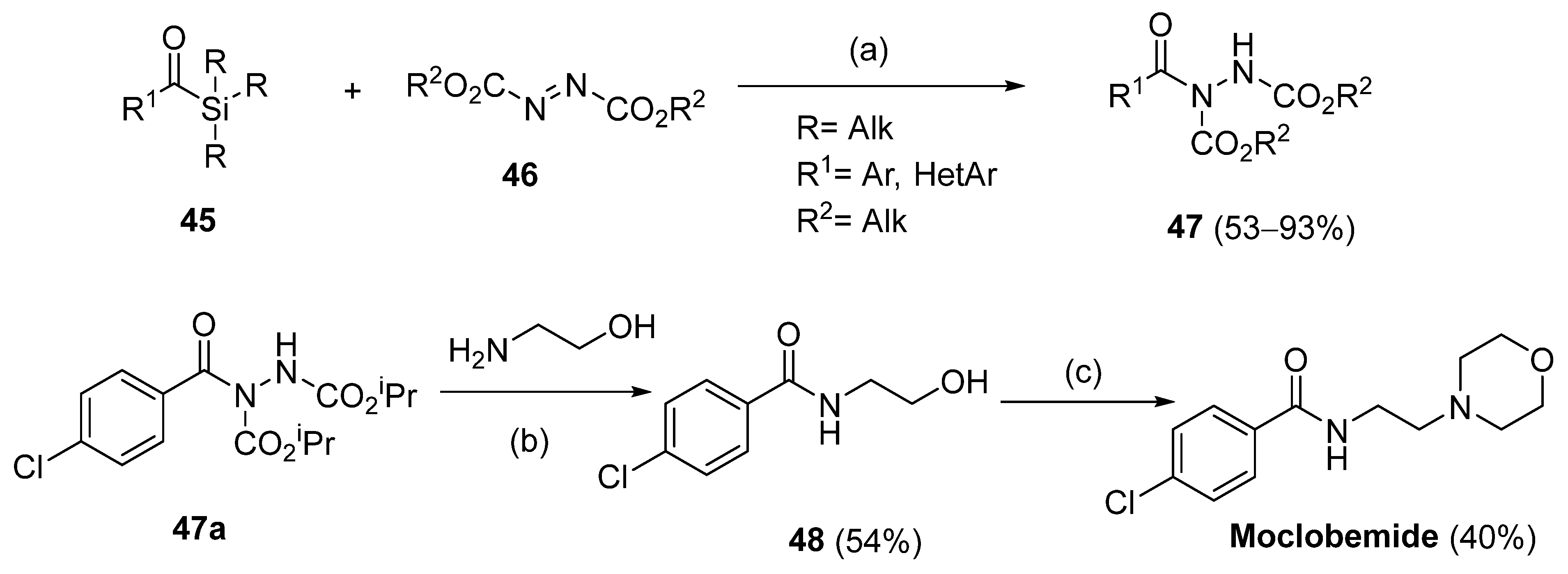
Hydrazides 47 were obtained from substituted azodicarboxylates 46 and arylic or heteroarylic acylsilanes 45 in the presence of visible light. This reaction afforded hydrazides 47 in good yields (53–93%). The drug moclobemide was obtained from the precursor 47a, which was synthesized by the previous method (Scheme 9) [115].
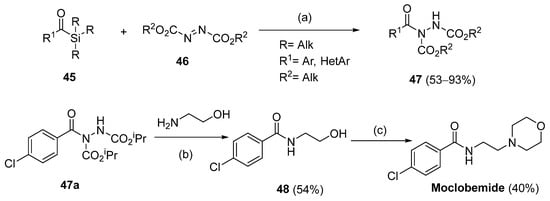
Scheme 9.
Synthesis of hydrazides 47 and moclobemide: (a) toluene, 456 nm, rt, 16 h; (b) DCM, r.t., 20 h; (c) i. MsCl, DCM, Et3N, DMAP, r.t., 24 h; ii. morpholine, 100 °C, 6 h.
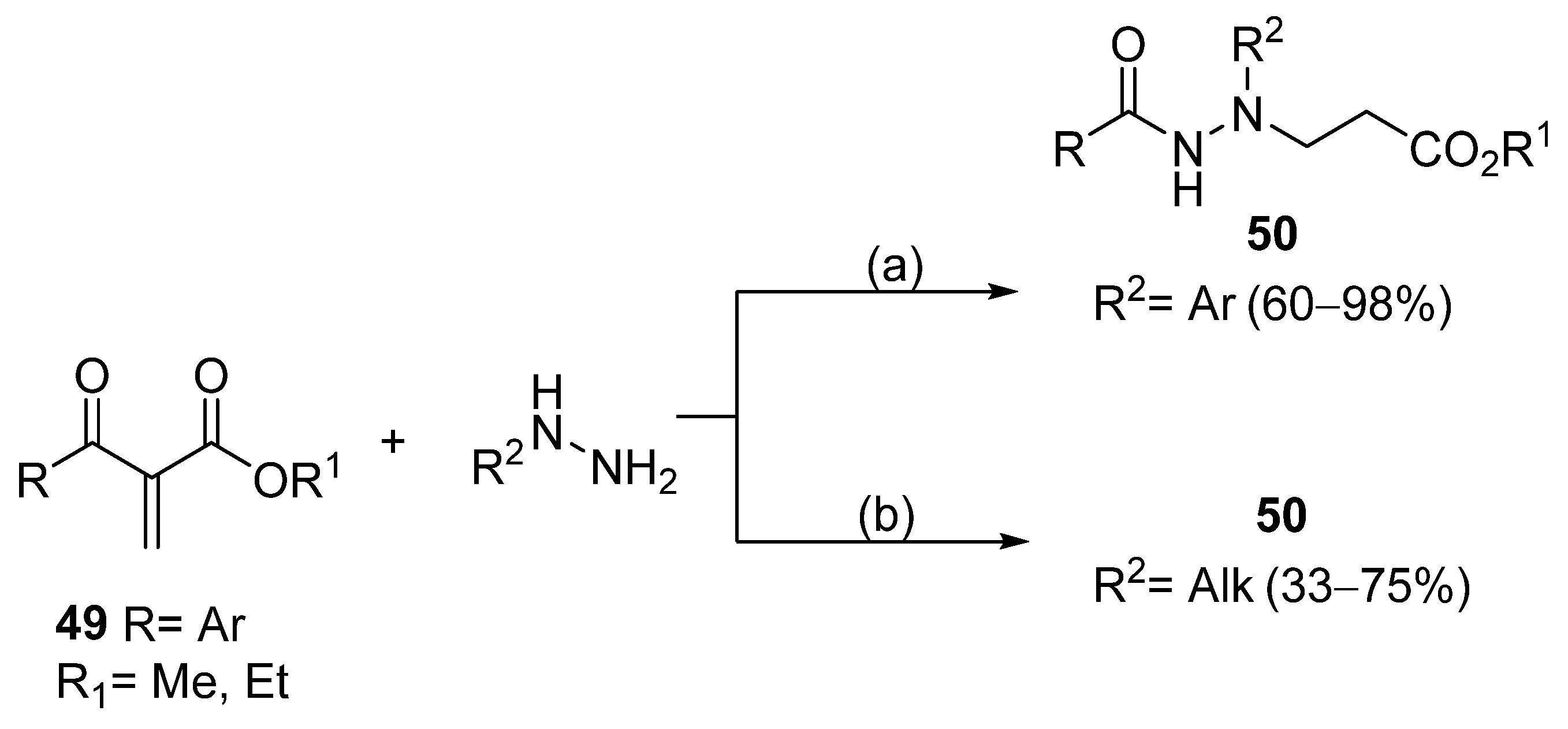
N,N′-disubstituted benzohydrazides 50 were obtained from benzoyl acrylates 49 via a hydrazine insertion into the β-position through an unexpected carbon–carbon bond cleavage (Scheme 10) [116].
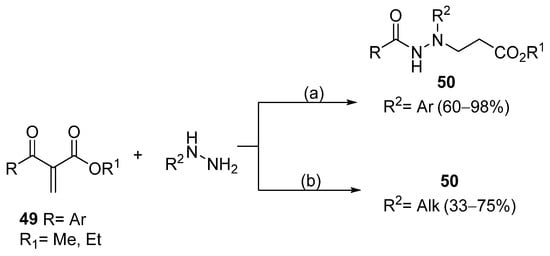
Scheme 10.
Synthesis of compounds 50; (a) Et3N (0.5–3.5 eq), CH3CN, r.t., 30 min–15 h; (b) Et3N (3.5 eq), H2O, CH3CN, r.t., 24 h.
2.2. Biological Activity of Hydrazides
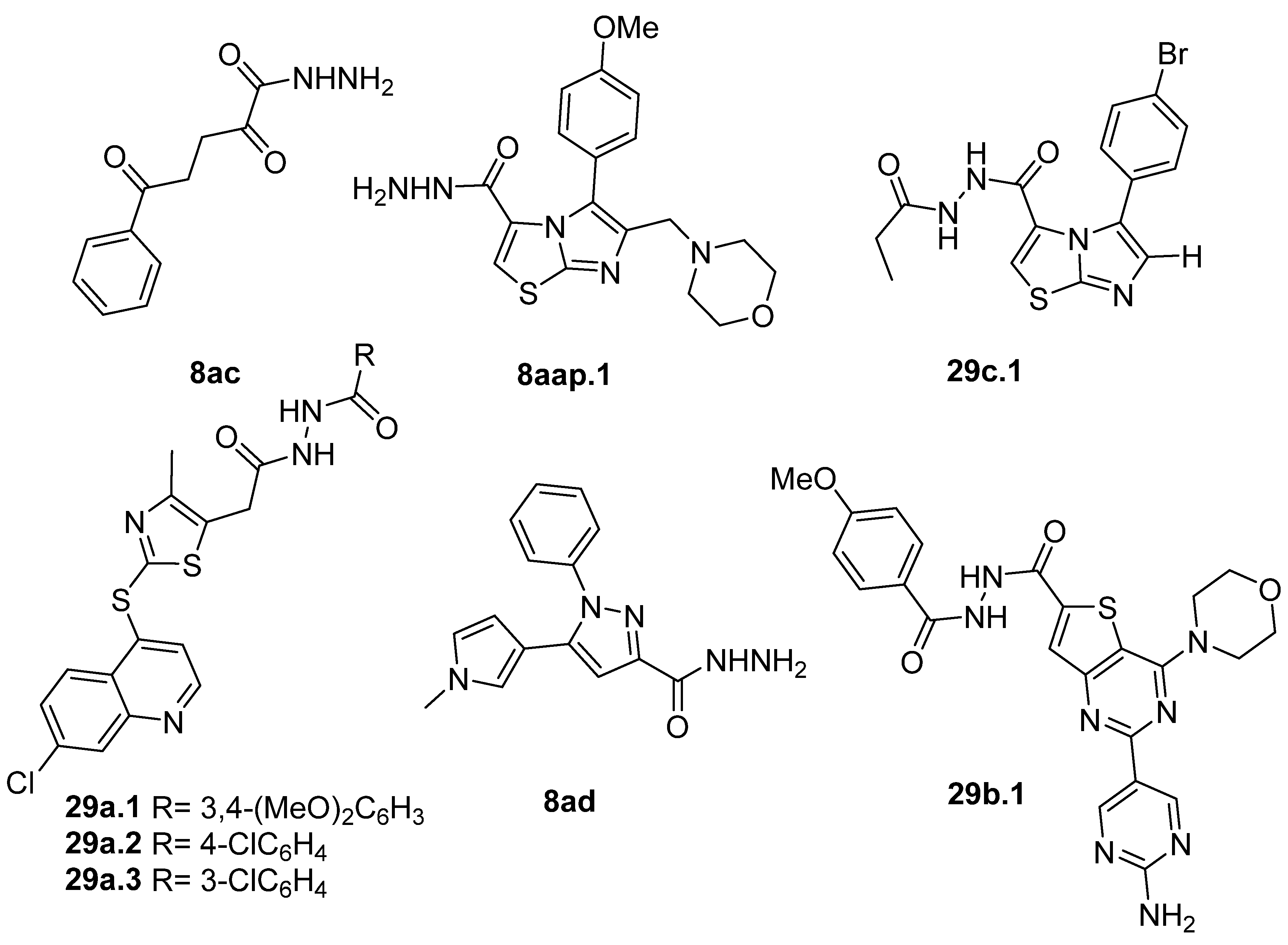
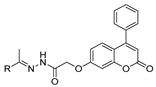
The compounds presented in Figure 3 showed anticancer activity against various cancer cell lines. Hydrazide 8ac showed good anticancer activity against MCF-7 breast cancer and HepG2 hepatocellular carcinoma cell lines with IC50 = 8.1 µM and IC50 = 28.6 µM, respectively [64]. Hydrazides 8aap.1 and 29c.1 also displayed anticancer activity towards the MCF-7 cancer cell line with IC50 values of 2.37 and 1.83 µM, respectively [92]. Besides that, Sabry et al. [92] reported that these hydrazides showed a strong dual inhibition activity of EGFR/HER2 kinase with IC50 values of 0.153 µM (EGFR) and 0.108 µM (HER2) for 29c.1 and 0.122 µM (EGFR) and 0.108 µM (HER2) for 8aap.1. In in vivo studies in Swiss albino mice mammary glands, compounds 8aap.1 and 29c.1 showed tumor volume reductions by 65.3 and 76.5%, respectively, at 10 mg/kg.
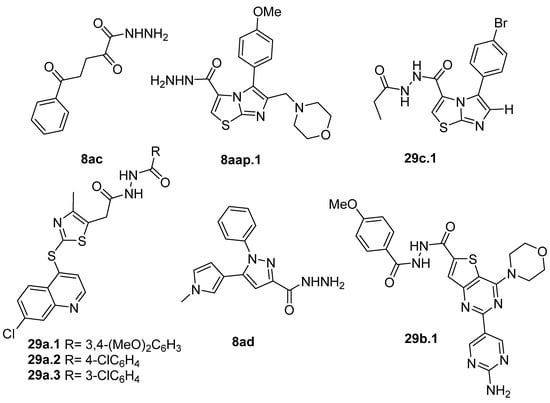
Figure 3.
Hydrazides with anticancer activity.
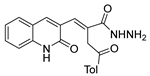
Derivatives 29a.1 and 29a.2, reported by Ramírez et al. [106], were also tested against the MCF-7 cancer cell line. Compounds 29a.1 and 29a.2 presented IC50 values of 15.41 and 12.99 µM, respectively. In addition, derivatives 29a.3 and 29a.2 were active against the A549 cell line (lung cancer) with IC50 values of 37.17 and 31.02 µM, respectively [106].
Hydrazides 8ad and 29b.1, reported by Abdelrehim et al. [35] and Han et al. [107], presented activity against the HCT-116 colorectal cancer cell line with IC50 values of 8.44 µg/mL and 2.02 µM (Figure 3). Compound 29b.1 also showed activity against PC-3 (prostatic adenocarcinoma), A549 (lung cancer), and MDA-MB-231 (triple-negative breast cancer) cancer cell lines, with IC50 values of 1.95, 1.62, and 1.55 µM. It also showed potent inhibitory activities against phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PI3Kα) with an IC50 = 0.46 nM and mammalian targeting of rapamycin (mTOR) with an IC50 = 12 nM [107].
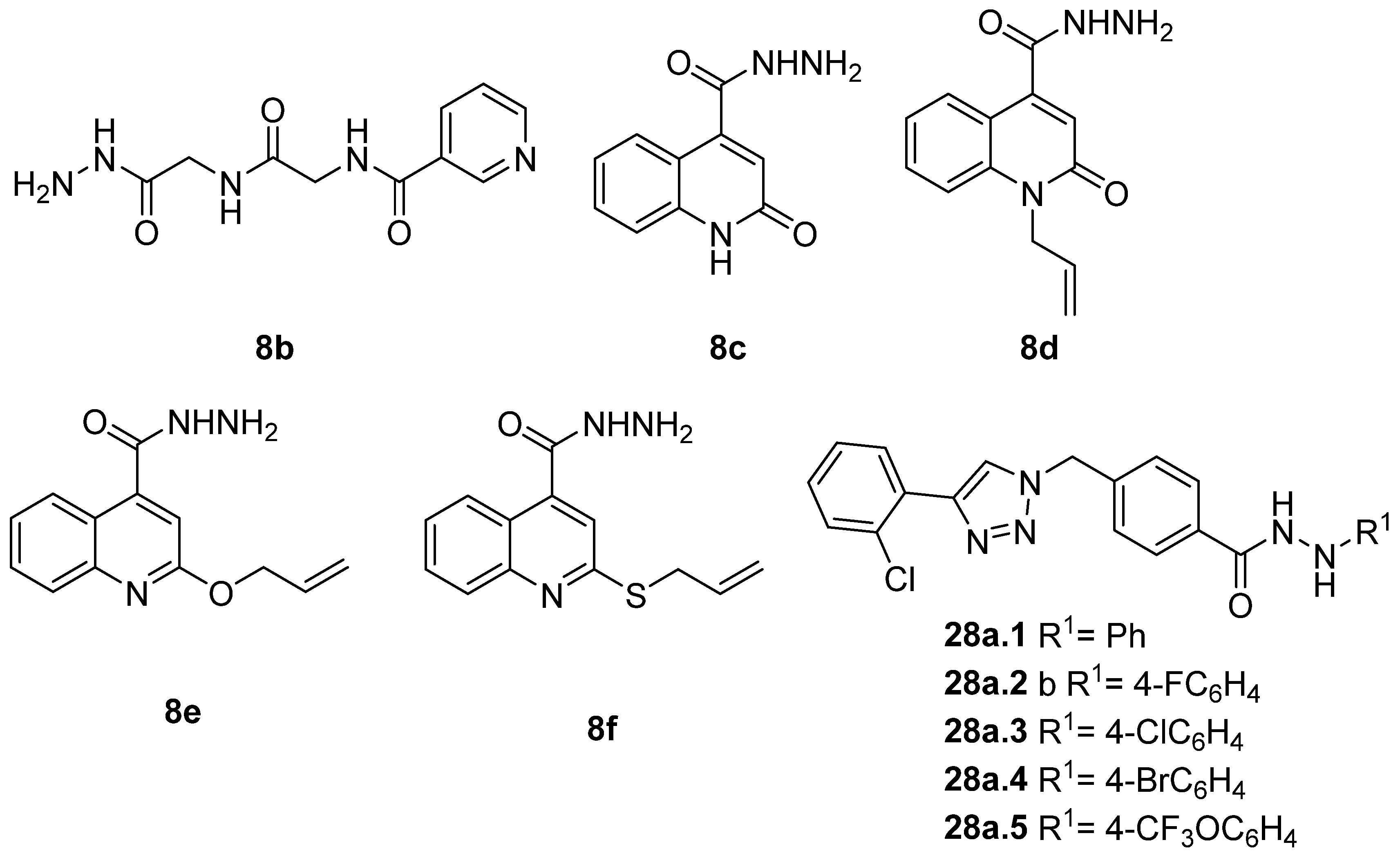
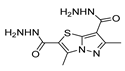
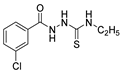
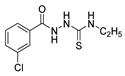
Hydrazide 8b (Figure 4) was evaluated for its antibacterial and antifungal activities. It showed a strong antibacterial and antifungal activity, with inhibition zones of 29, 30, 28 and 16 mm against Bacillus subtilis, Escherichia coli, Candida albicans and Aspergillus niger, respectively [51]. Compounds 8c–8f also exhibited activity against E. coli, B. subtils, and Asp. niger strains, presenting inhibition zones varying between 2 and 5 mm [26].
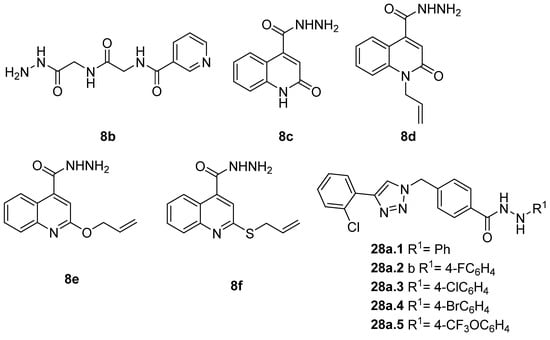
Figure 4.
Hydrazides with antibacterial and antifungal activity.
Compounds 28 (Figure 4) were evaluated as fungicides [109]. Compounds 28a.1–28a.5 exhibited growth inhibition activity against Botryosphaeria dothidea, Rhizoctonia solani, and Gibberella zeae with EC50 values within the 10.0–0.306 µg/mL range, which were higher activity than those of the commercial agrochemicals azoxystrobin, boscalid, and fluxapyroxad [109].
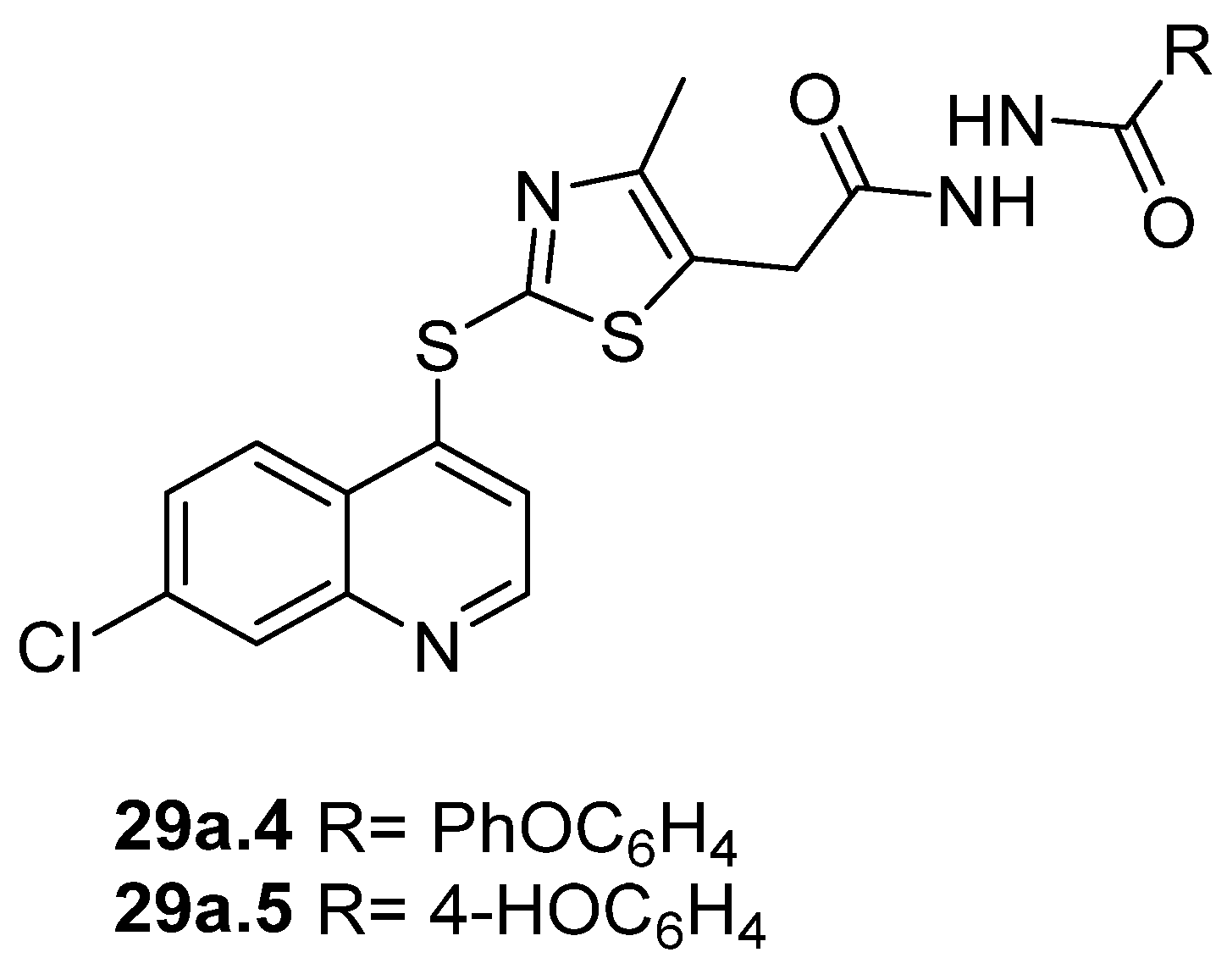
Disubstituted hydrazides 29 were tested as antimalarial agents. Compounds 29a.4 and 29a.5 (Figure 5) showed antimalarial activity with IC50 values of 0.65 and 0.64 µM, respectively [106].

Figure 5.
Hydrazides with antiparasitic activity.
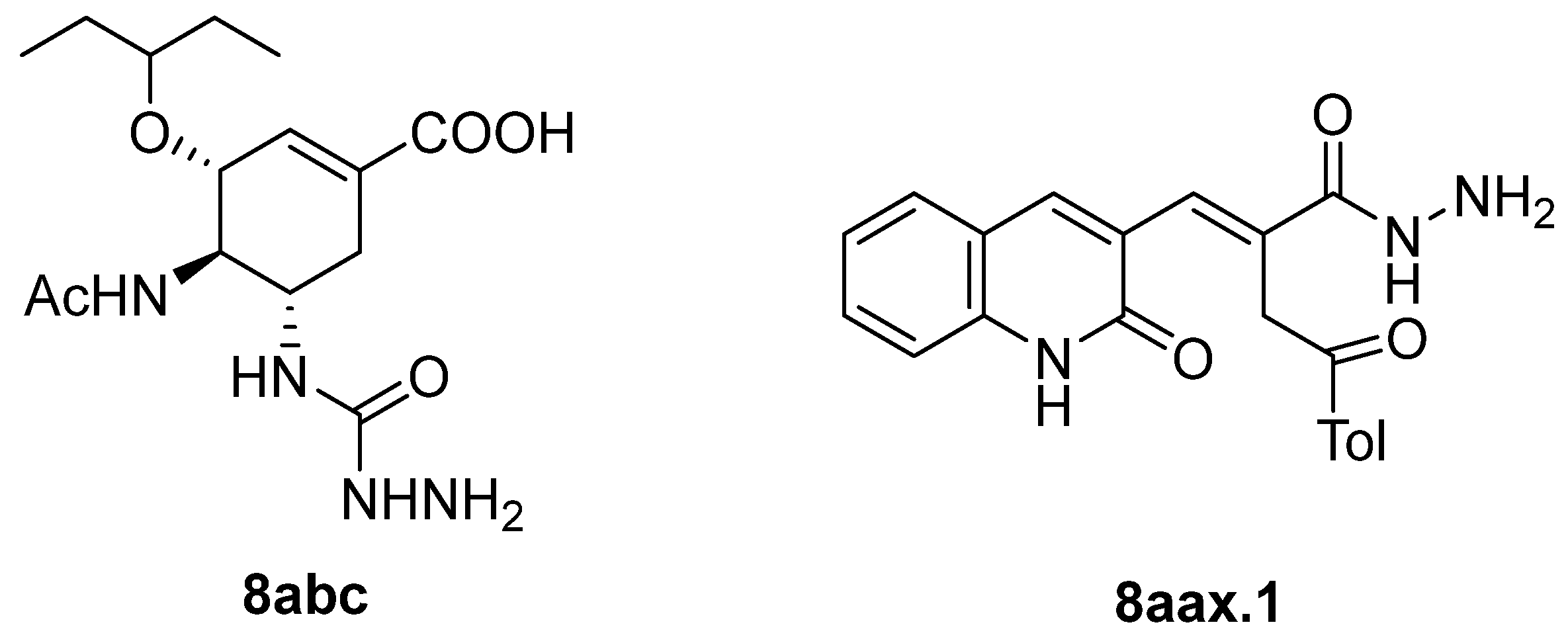
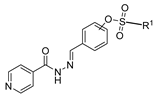
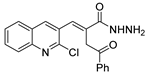
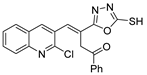
Hydrazides present in Figure 6 were evaluated as antivirals. Compound 8abc showed antiviral activity against influenza A as a Neuraminidase inhibitor against H5N1 and H1N1 subtypes with IC50 values of 26.8 nM and 11.9 nM, respectively [99]. Moreover, hydrazide 8aax.1 was presented as a great immunomodulator, presenting 80% protection against the highly pathogenic avian influenza virus (H5N8) [28].
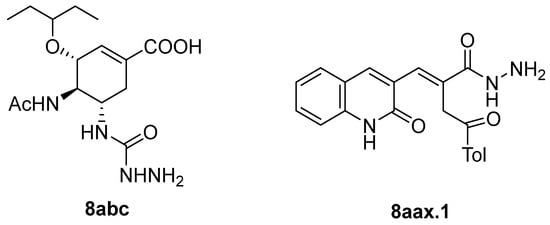
Figure 6.
Hydrazides with antiviral activity.
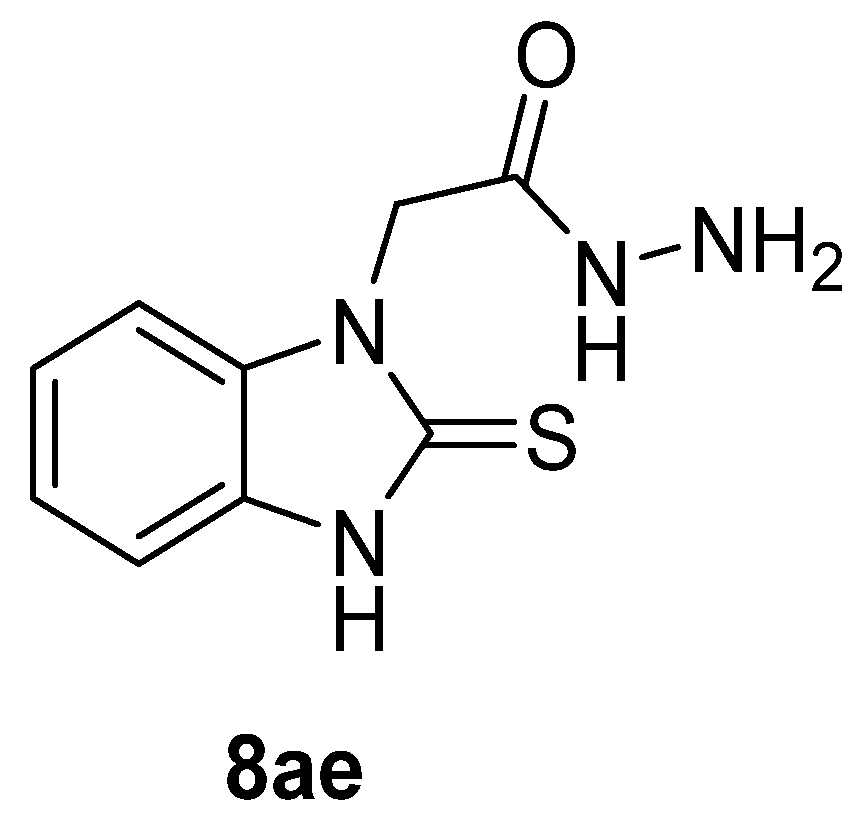
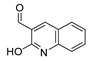
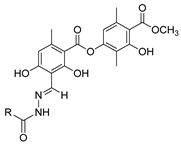
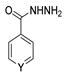
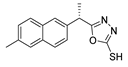
Myeloperoxidase plays a key role in the human antimicrobial system by oxidizing vital molecules of microorganisms in phagolysosomes through the production of hypochlorous acid. It has been associated with inflammatory diseases such as renal injury, multiple sclerosis, and cardiovascular and neurodegenerative diseases. Saylam et al. [65] reported compound 8ae (Figure 7) as an excellent myeloperoxidase inhibitor with an IC50 = 0.393 µM, which is comparable to the standard drug 4-aminobenzoic acid hydrazide.
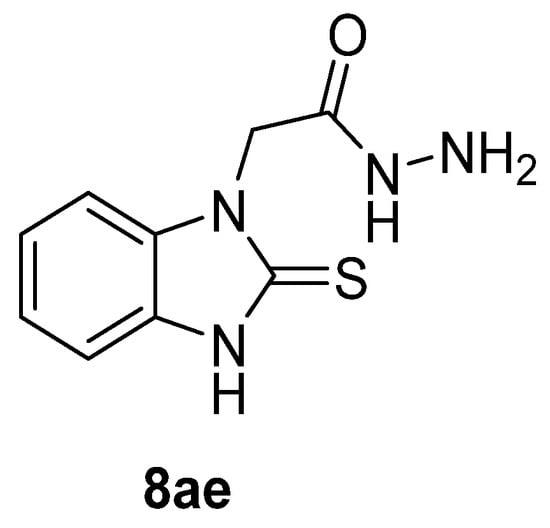
Figure 7.
Myeloperoxidase inhibitor.
3. Hydrazide Derivatives
3.1. Hydrazide–Hydrazones
3.1.1. Synthesis of Hydrazide–Hydrazones
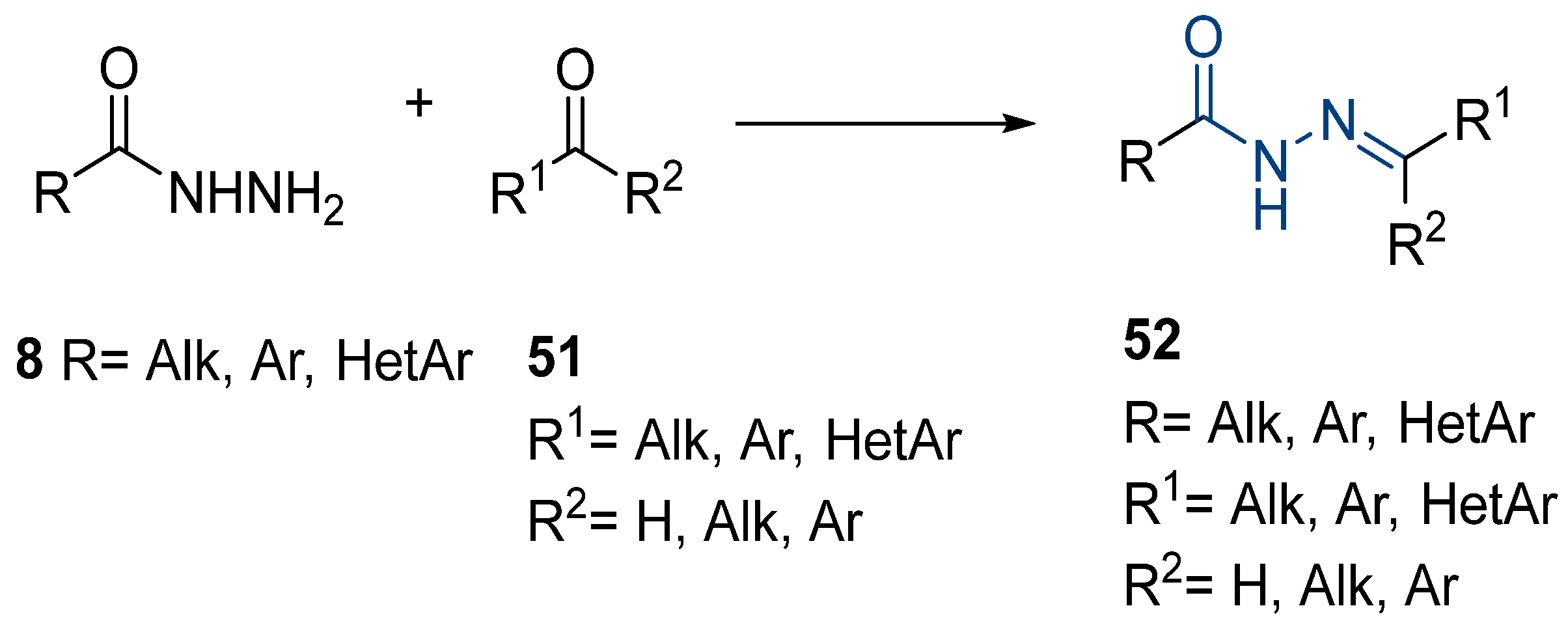
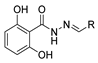
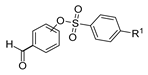
Hydrazide–hydrazone derivatives are among the most frequently synthesized and reported hydrazide derivatives in the literature. These compounds stand out in organic and medicinal chemistry since they have exhibited a wide range of biological activities and have been used as important intermediates in the synthesis of heterocycle rings from hydrazides. The hydrazide–hydrazone moiety contains the functional group -CO-NH-N=CR1R2, which is a combination of the hydrazide and imine groups. The imine group confers E/Z isomerism and photochromism in both solution and the solid state [55,97]. Moreover, the -NH- and C=O groups allow the compounds to have the capability of binding to anions/cations and biomolecules; the coexistence of imine and carbonyl groups allows them to establish metallo-assemblies [55].
In the past few years, hydrazides have been extensively used to synthesize several hydrazide–hydrazone derivatives, as potential aggregation-induced emission luminogens (AIEgens), probes, or anticancer, antimicrobial, antifungal, antituberculosis, antimalarial, antiviral, and antioxidant agents. Some derivatives exert their activity through the inhibition of specific enzymes such as acetylcholinesterase, butyrylcholinesterase, α-glucosidase, and others.
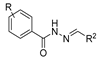
Hydrazide–hydrazones 52 are synthesized from the reaction between a hydrazide and an aldehyde/ketone [13,34,37,38,43,53,54,56,57,66,67,68,71,72,73,75,76,77,78,79,95,96,97,98,101,117,118,119,120,121,122,123,124,125,126,127] (Scheme 11). According to the studies in this review, these reactions, in general, occur in alcohols (ethanol or methanol) and at high temperatures (Table 3). The reactions occurred without or with acid catalysis, such as acetic acid [34,43,54,66,71,73,76,77,79,95,96,117,119,121,123,127] or p-TsOH [55,56], and in these cases, the reactions may occur at room [55,56,79,117] or high temperatures [34,38,43,54,66,68,71,72,73,76,77,95,96,119,121,123,127]. Hydrazide–hydrazone derivatives were obtained in low to excellent yields.
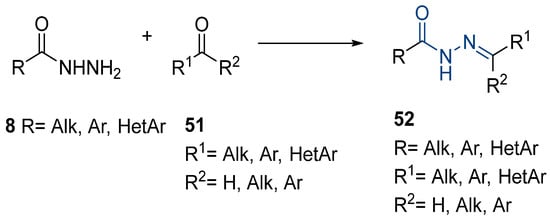
Scheme 11.
Representative scheme of hydrazide–hydrazones synthesis.

Table 3.
Reaction conditions for the synthesis and purification of hydrazide–hydrazones.
As mentioned earlier, some hydrazides are commercially available. However, others are synthesized by the scientific community to originate the required compounds. Here, hydrazide–hydrazones synthesized from alkyl, aryl, or heteroaryl hydrazides as starting materials, produced or not by the authors, will be presented. The biological activity of the generated compounds will also be reviewed.
3.1.2. Biological Activity of Hydrazide–Hydrazones
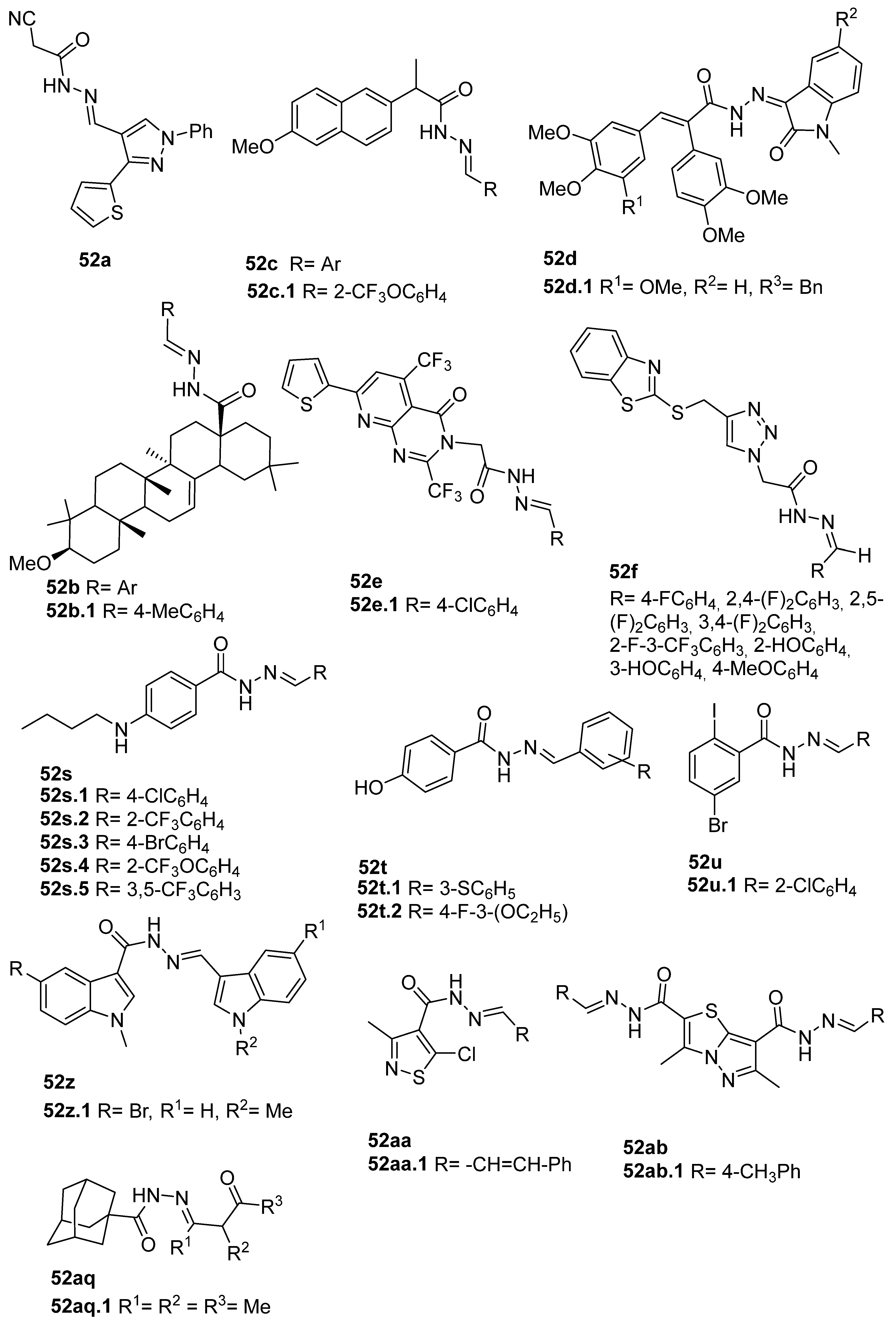
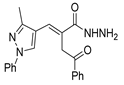
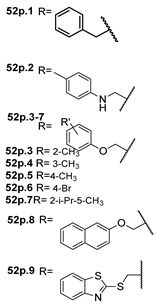
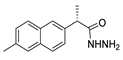
The new hydrazide–hydrazone derivatives represented in Figure 8 were evaluated as anticancer agents. Compounds 52a, 52c.1, 52d.1, and 52e.1 showed good activity against human breast cancer cell lines, specifically against the MCF7 line, with IC50 values of 7.38, 59.81, 3.49, and 14.6 µM, respectively [68,72,96,124]. Moreover, compound 52c.1 also showed promising anticancer activity, with IC50 = 22.42 µM, against the human breast cancer cell line MDA-MB-231. Compound 52c.1 was tested in vivo and decreased the tumor volume in both low (60 mg/kg) and high (120 mg/kg) doses in mice [68]. Besides the activity against breast cancer, compound 52a showed activity against the HepG2 cancer cell line with IC50 = 8.79 µM [124]. In addition, derivatives 52d were also tested against HCT-116 and SK-MEL-28 (melanoma) cancer cell lines. Compound 52d.1 displayed the highest activity with IC50 values of 6.82 and 10.39 µM, respectively, with no relevant toxicity on non-malignant HaCaT (human keratinocyte) cells [96].
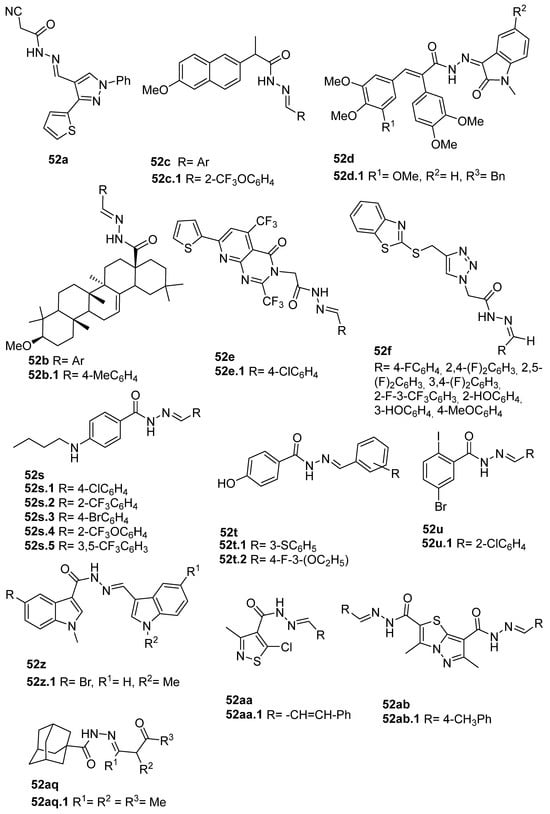
Figure 8.
Hydrazide–hydrazones with anticancer activity.
According to Halil et al. [97], natural compounds with bioactive properties, when combined with hydrazides, can lead to new active compounds with increased activity. Hence, Halil et al. [97] synthesized molecules with structure 52b (Figure 8) starting from the natural product oleanolic acid. The in vitro anticancer activity was studied on the A549 (adenocarcinomic human alveolar basal epithelial) cell line. Of the thirteen compounds synthesized, compound 52b.1 showed the best activity with IC50 = 0.08 µM and low cytotoxicity on the BEAS-2B cells (human non-tumorigenic lung epithelial cells).
The anticancer activity of combretastatin–oxindole 52d, pyrimidine derivatives 52e, and triazoles 52f (Figure 8) was also evaluated against the A549 cell line. Compounds 52d.1 and 52e.1 were promising anticancer agents with IC50 values of 1.26 and 11.3 µM, respectively [72,77,96]. Furthermore, Abba et al. [72] identified the derivative 52e.1 as a potent compound against DU145 (prostate cancer) using HeLa (cervical cancer) cell lines with IC50 values of 13.4 and 9.1 µM, respectively [72].
According to Almehmadi et al. [77], molecules 52f revealed an anticancer capacity, presenting a growth inhibition ranging from 55 to 90% at 400 µg/mL against the A549 cell line.
Han et al. [95] described derivatives 52s.1 and 52s.2 with high anticancer activity against the human colorectal adenocarcinoma (Colo-205) cell line (IC50 = 50.0 and 20.5 µM, respectively). On the other hand, compounds 52s.3, 52s.4, 52s.5, 52t.1, and 52t.2 displayed the great anticancer activity against the liver hepatocellular carcinoma HepG2 cell line with IC50 = 30.5, 35.9, 20.8, 42.4, and 37.4 µM, respectively [66]. Derivatives 52t.1 and 52t.2, reported by Han et al. [66] (Figure 8) exhibited lower activity than derivatives 52s. Among the thirteen different hydrazones 52u, described by Popiołek et al. [67], compound 52u.1 exhibited the best cytotoxicity with IC50 = 33.45 and 11.94 µM against hepatocellular carcinoma (HepG2) and renal adenocarcinoma (769-P) cell lines, respectively, and additionally showed high selectivity, with low cytotoxicity against the normal Vero cell line, with IC50 = 320.54 µM.
Among indole derivatives 52z [34], compound 52z.1 was the most active against the A549 lung adenocarcinoma cell line with IC50 = 0.793 µM. This compound also showed great activity against cervical HeLa and breast MCF-7 cancer cells with IC50 = 1.69 and 1.19 µM. The authors studied the mechanisms of action of compound 52z.1 regarding different signaling pathways triggered in HeLa and MCF-7 cells, and it was verified that this compound induced cell apoptosis through the generation of reactive oxygen species and activation of many signal transduction pathways [34].
Thiazole derivatives 52aa (Figure 8) were screened towards various cancer cell lines, and 52aa.1 exhibited the highest antiproliferative activities with IC50 = 14, 25, 34.2, 39.3, and 68.6 µM against human leukemia MV4-11 cells, colon LoVo and LoVo/DX, and breast MCF-7 and MCF-10A cancer cell lines, respectively [98].
Alsayari et al. [37] reported compounds 52ab (Figure 8), from which 52ab.1 presented the highest activity towards HepG-2 and HCT-116 cell lines with IC50 = 30.5 and 86.9 µg/mL, respectively.
Adamantane-1-carbohydrazone derivatives 52aq were tested as anticancer agents for breast, liver, and lung cancers. Derivative 52aq.1 stood out with IC50 = 8.35, 7.82 and 4.39 µM, for MCF-7, HepG-2 and A549 cell lines, respectively [127].
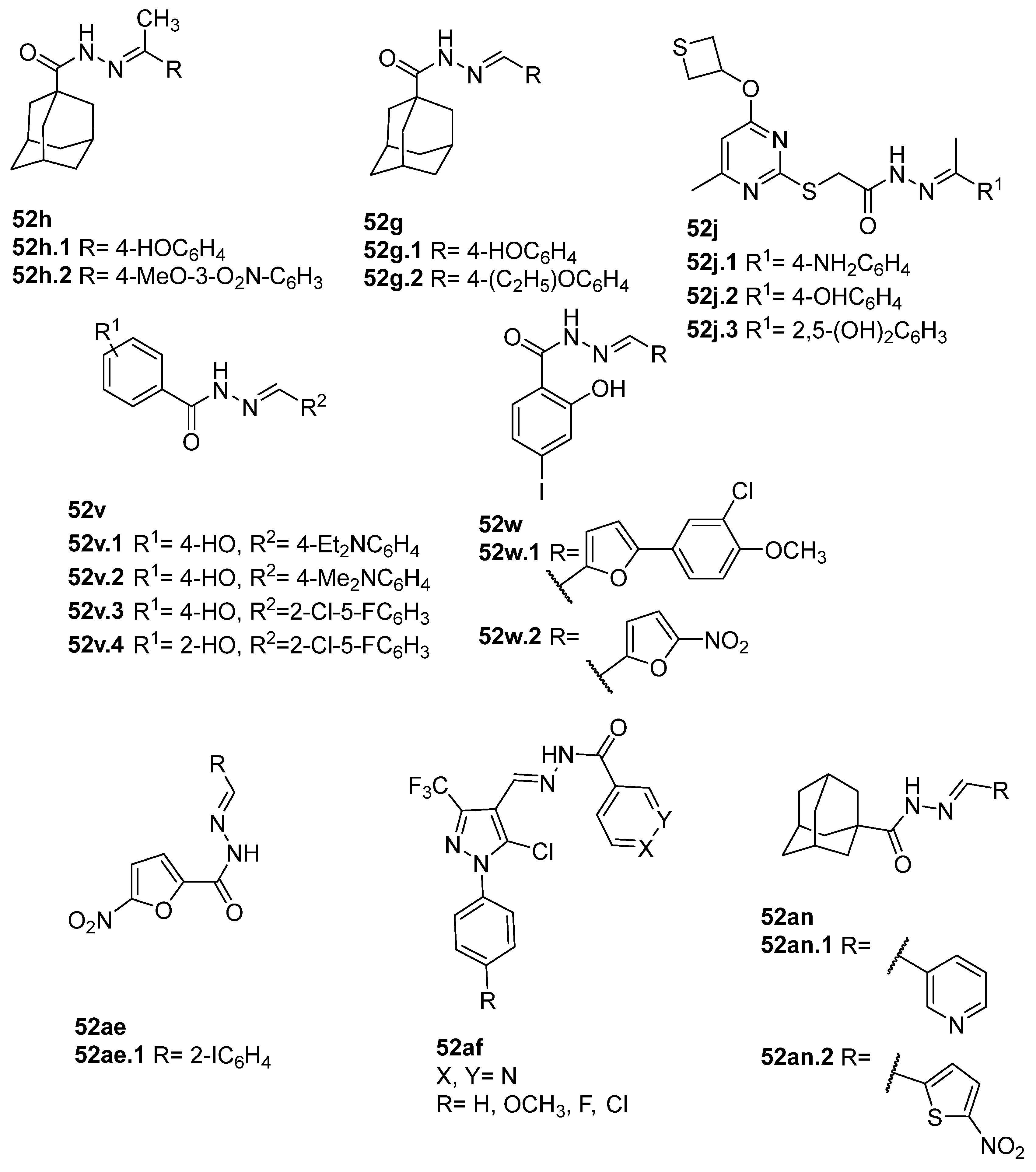
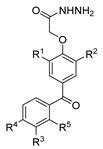
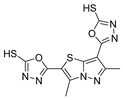
Compounds 52h and 52g (Figure 9) were evaluated for their antimicrobial activity against Enterococcus faecalis, Staphylococcus aureus, Bacillus cereus, and Candida albicans. Compounds 52h.1 presented an MIC of 12.5 µM for the four strains, and 52h.2 presented IC50 values of 6.35 µM, 6.77 µM, 6.12 µM, and 6.37 µM against the same strains. Compounds 52g.1 and 52g.2 showed good inhibitory activity with IC50 values of 3.56 and 6.73 µM against Enterococcus faecalis and 6.77 and 6.66 µM against Candida albicans, respectively [57].
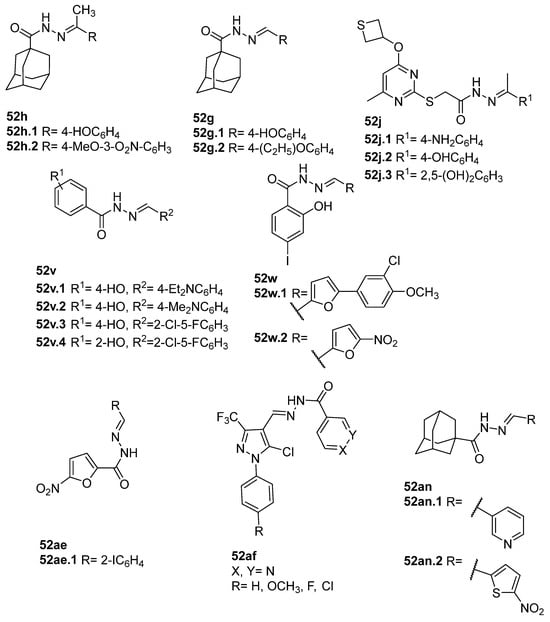
Figure 9.
Hydrazide–hydrazones with antibacterial, antifungal, or antiparasitary activity.
Pyrimidine derivatives 52j.1–52j.3 (Figure 9) also exhibited high antimicrobial activities with MIC = 0.05 µg/mL against St. aureus, Str. Pyogenes, P. vulgaris, K. pneumoniae, Ent. Aerogenes, P.S aeruginosa, and C. Albican [75].
Compounds 52v and 52w (Figure 9) showed antibacterial potential against S. aureus, E. Coli, and B. subtilis. Specifically, compound 52v.1 was the best antibacterial agent against S. aureus with an MIC value of 0.625 µg/mL. Compounds 52v.1–52v.4 had higher activity against E. coli with MIC = 0.625 µg/mL. Moreover, compounds 52v.3 and 52v.4 displayed the best activities against B. subtilis with MIC = 0.312 µg/mL [118]. Popiołek et al. [38] reported compounds 52w.1 and 52w.2 with MIC = 7.81 µg/mL towards B. subtilis (ATCC 6633).
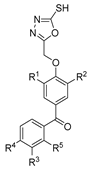
In 2020, furyl hydrazide–hydrazones 52ae were also tested for their potential antimicrobial activity [53]. The assays of antibacterial and antifungal activity revealed that several of the synthesized compounds 52ae (Figure 9) presented very strong bioactivity with MIC < 10 µg/mL. Some compounds showed higher activity than the standard drugs (nitrofurantoin, cefuroxime, and ampicillin). For example, compound 52ae.1 was 130 times more active towards Bacillus subtilis ATCC 6633 (MIC = 0.48 µg/mL) than ampicillin [53].
The novel derivatives 52af (Figure 9) were evaluated for their inhibitory effects in several bacterial and fungal strains [120]. These compounds exhibited the maximum zone of inhibition ranging from 7 to 20 mm against B. licheniformis and S. aureus, as well as good antifungal activity against both Asp. niger and C. albicans with maximum zones of inhibition of 8–14 and 6–14 mm, respectively [120].
Adamantane hydrazide–hydrazone derivatives 52an.1 and 52an.2 presented by Al-Wahaibi et al. [125] also showed good antibacterial activity. Compound 52an.2 showed broad-spectrum antibacterial and antifungal activity with MIC = 1.5 µM against St. aureus and B. subtilis, MIC = 3 µM against M. luteus, and MIC = 6 µM against E. coli and P.S aeruginosa. Compound 52an.1 only showed potent activity against the tested Gram-positive bacterial strains, with MIC = 3.53 uM against St. aureus and B. subtilis and MIC = 7.06 uM for M. luteus [125].
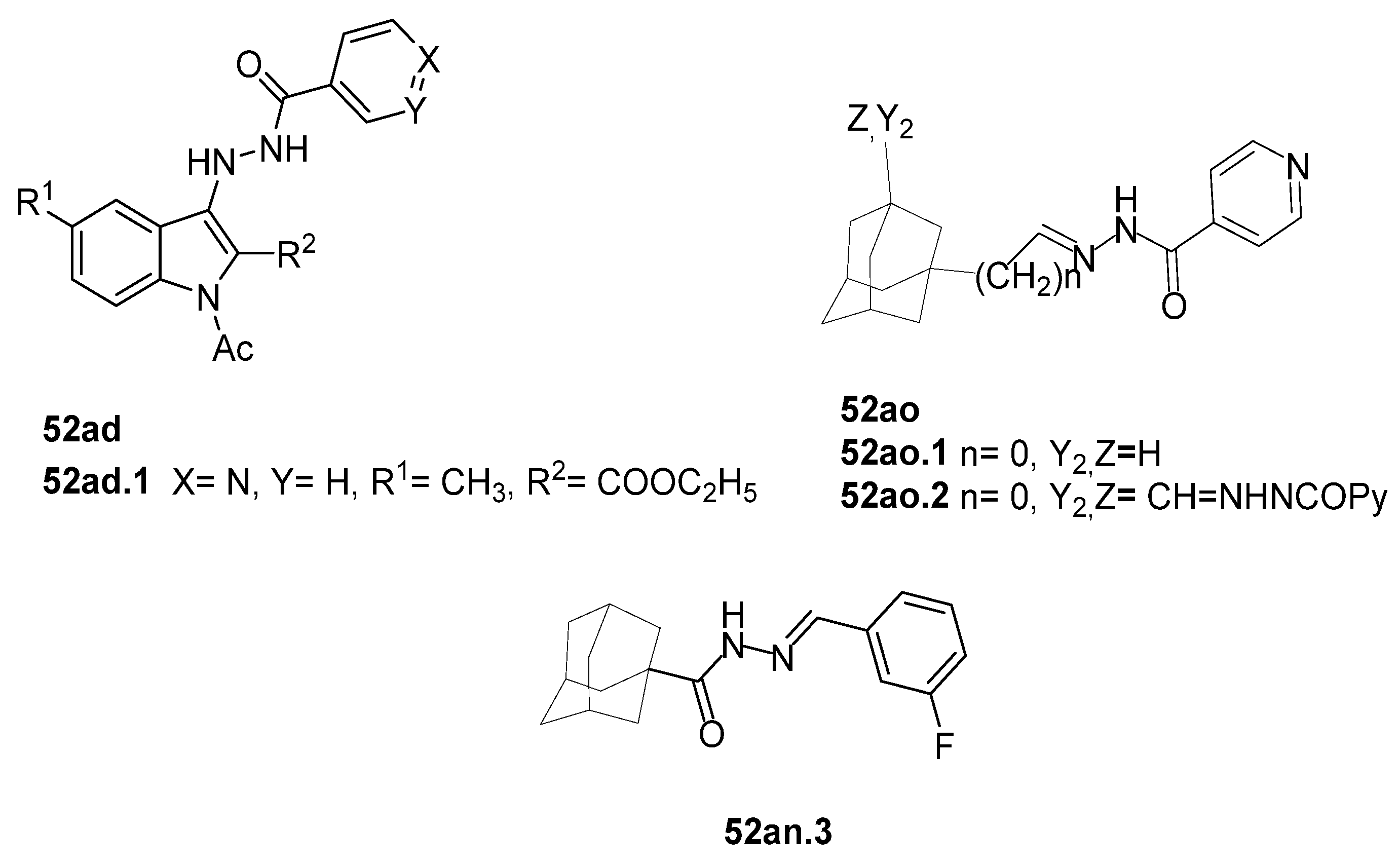
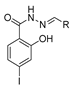
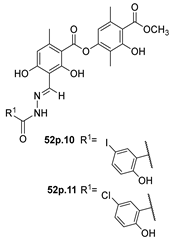
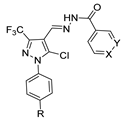
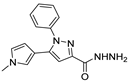
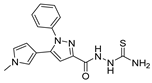
In the literature, simpler heteroaryl hydrazides, like isonicotinic hydrazide, or similar ones, have been employed to obtain compounds with a wide range of biological activities (Figure 10) [119,121,123,128]. A series of derivatives 52ad that combine pyridine and indole moieties were reported, and the in vitro antimycobacterial activity was studied against strain M. tuberculosis H37Rv and against a clinical isolate of isoniazid-resistant M. tuberculosis strain, designated as CN-40 [119]. The hydrazide hybrid 52ad.1 (Figure 10) was the most promising compound with MIC = 0.05 µg/mL and with a high selectivity index (SI = 300). Compared to isoniazid 1, the new compound 52ad.1 had similar activity against M. tuberculosis H37Rv; still, the new compound showed higher activity than isoniazid against the isoniazid-resistant M. tuberculosis CN-40 strain [119]. Papageorgiou et al. [126] reported new isoniazid-based adamantane derivatives, 52ao.1 and 52ao.2, with activity against M. tuberculosis H37Rv, MIC = 0.14 and 0.09 nM, respectively, and very low cytotoxicity against HepG2 (selectivity index ≥ 2500).
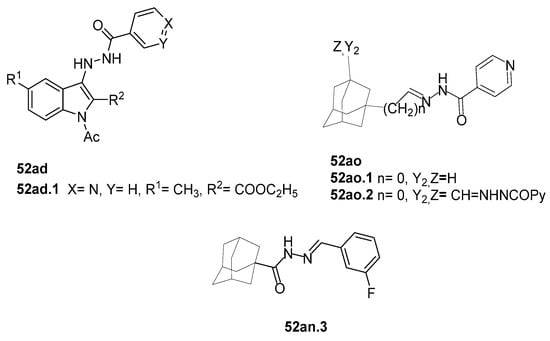
Figure 10.
Hydrazide–hydrazones with antimycobacterial activity.
On the other hand, adamantane hydrazide–hydrazone derivative 52an.3 was also reported as an antituberculosis agent exhibiting MIC values of 0.2, 0.3, 1.5, 12.5, and 12.5 µg/mL for M. tuberculosis, M. bovis BCG, M. smegmatis, M. abscessus, and M. marinum, respectively [129].
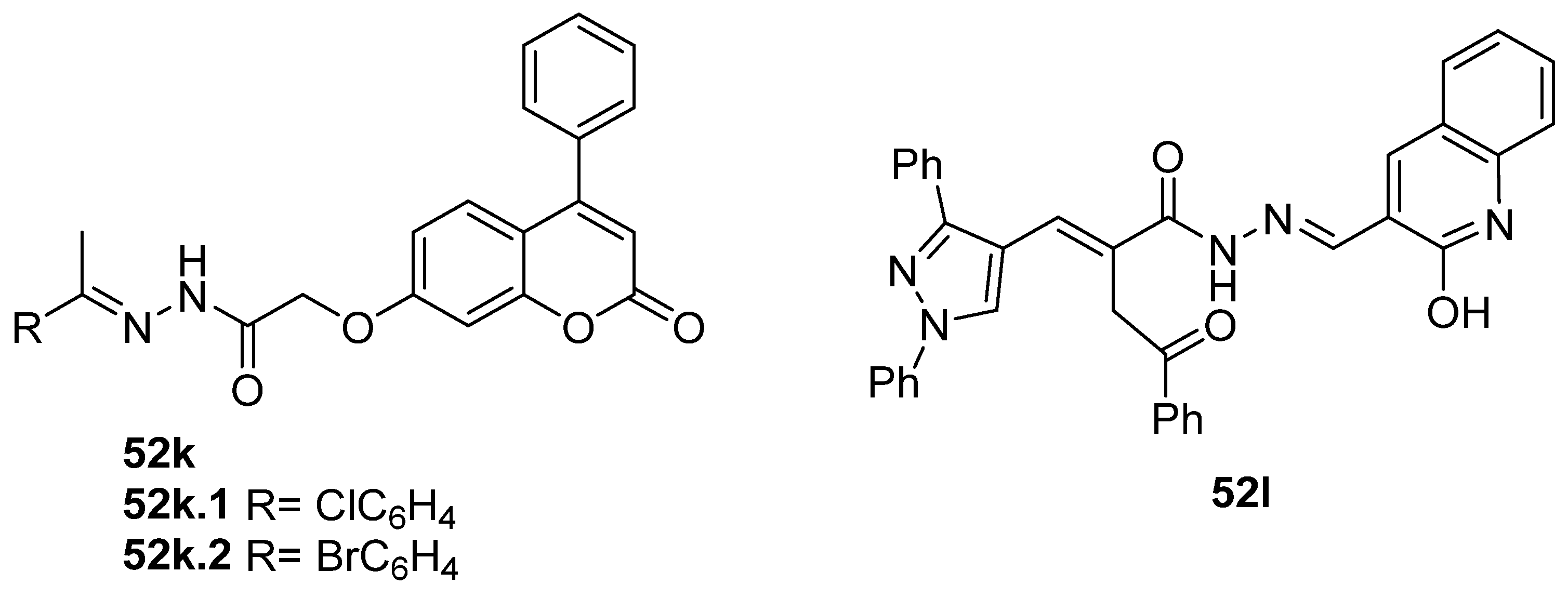
Antiviral activity was detected for hydrazide derivatives represented in Figure 11. Kassem et al. [73] obtained the 4-phenylcoumarin derivatives 52k, and these proved to be potential inhibitors of the 3C protease of the hepatitis A virus. Compounds 52k.2 and 52k.1 presented the highest effects against viral adsorption and replication with IC50 = 8.5 and 10.7 µg/mL, respectively. Morsy et al. [101] reported derivative 52l that showed 100% protection against Newcastle disease virus.
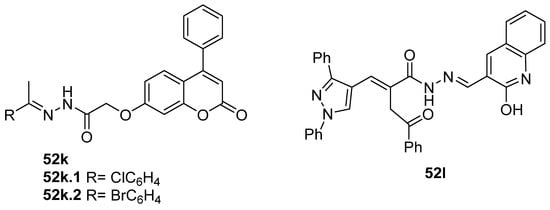
Figure 11.
Hydrazide–hydrazones with antiviral activity.
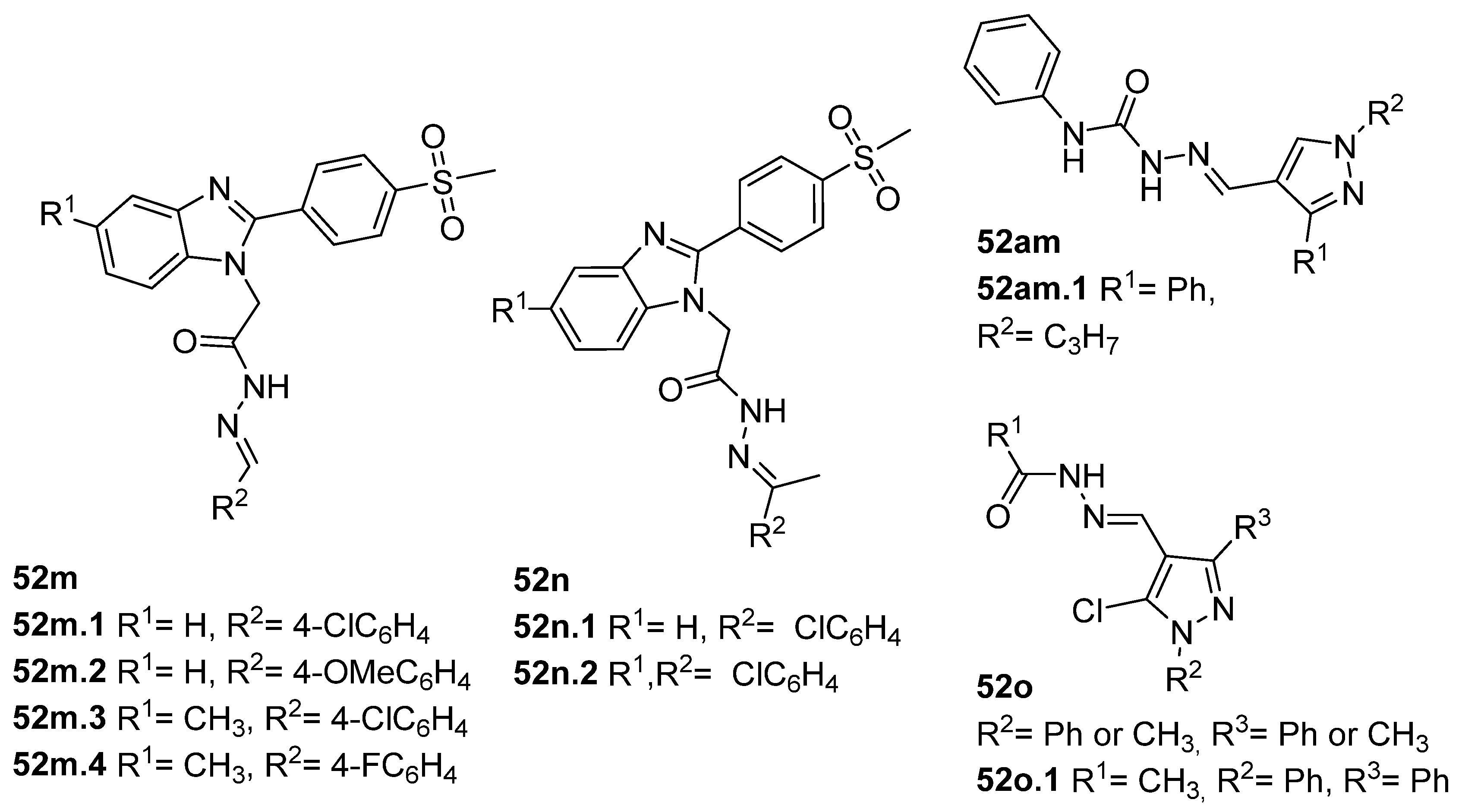
A series of benzimidazole derivatives were synthesized and evaluated as cyclooxygenase-1(COX-1)/cyclooxygenase-2 (COX-2) inhibitors [76]. The results of the cyclooxygenase (COX) inhibition assay generally showed that the compounds 52m and 52n.1 (Figure 12) exhibited low selectivity towards the COX-1 isozyme compared to those of reference drugs (indomethacin and celecoxib). Compounds 52m.1, 52m.4, 52n.1, and 52n.2 showed selective inhibition of the COX-2 isozyme. Among all, compound 52m.1 exhibited the highest COX-2 inhibitory activity with an IC50 = 0.10 µM and selectivity index SI = 134 [76]. Compounds 52m.1–4, 52n.1, and 52n.2 also exhibited good anti-inflammatory activity, reducing inflammation by more than 93%. Regarding the ulcerogenic liability, compound 52m.1 was the safest one with an Ulcer Index (UI) = 0.83 and a lower ulcerogenic effect than the reference drugs celecoxib (UI = 3.5) and indomethacin (UI = 13) [76].
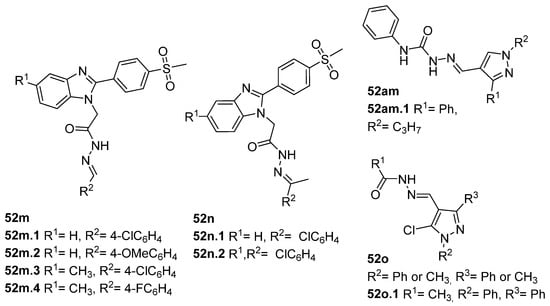
Figure 12.
Hydrazide–hydrazones with anti-inflammatory activity.
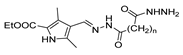
Tumor Necrosis Factor alpha (TNF-α) is a pro-inflammatory cytokine that may trigger and amplify inflammatory signals via multiple signaling pathways [130]. The dysregulation of inflammation, particularly the dysregulation of TNF-α, has been associated with various diseases such as arthritis, atherosclerosis, neurodegenerative diseases, diabetes, and cancer [131]. Therefore, inhibition of TNF-α leads to higher control and better treatment of inflammatory diseases [117]. In 2019, Liang et al. [132] synthesized pyrazole–hydrazone 52am.1, which showed excellent TNF-α inhibitory activity, and some displayed comparable anti-inflammatory activity to dexamethasone (reference drug) in vivo. In continuation of this work, Song et al. [117] discovered new pyrazole–hydrazone derivatives 52o (Figure 12), particularly compound 52o.1, which inhibited TNF-α in a dose-dependent manner with IC50 = 5.56 µM. The authors also presented molecular docking results for 52o.1, concluding that the compounds’ phenyl and hydrazide groups play an important role in binding to the target site [117].
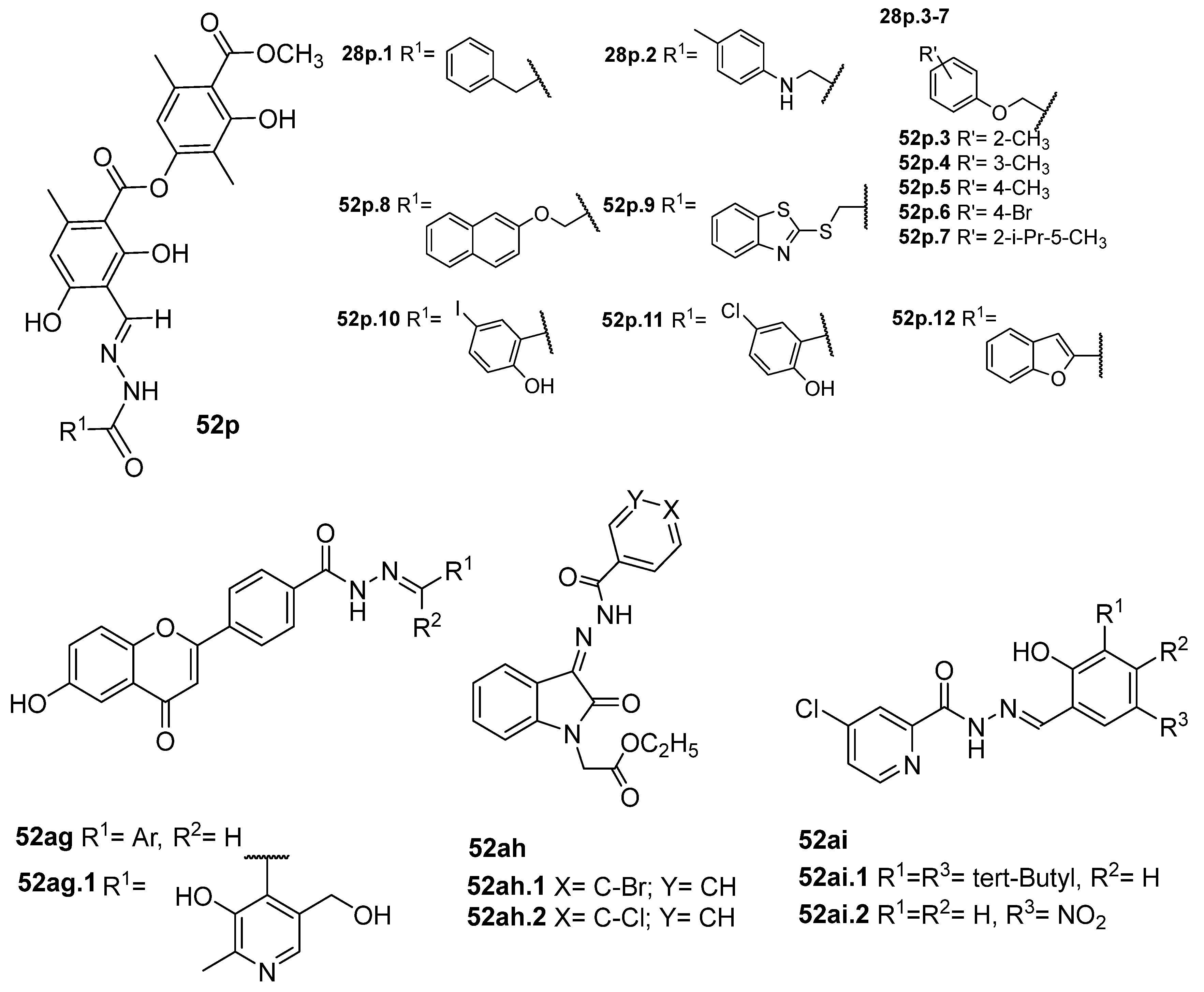
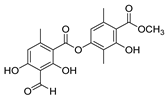
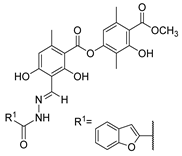
Duong et al. [43] obtained the compounds 52p (Figure 13) by reacting atranorin (natural product) with several hydrazides. The compounds were tested for the inhibition of α-glucosidase. Compounds 52p1–12 exhibited stronger activities than the natural product and acarbose, with IC50 values ranging from 6.67 to 54.7 µM. Compound 52p.1 exhibited the best activity, with IC50 = 6.67 µM. Additionally, the cytotoxicity of these compounds against the normal cell line HEK293 was evaluated, and they exhibited weak to no cytotoxicity [43].
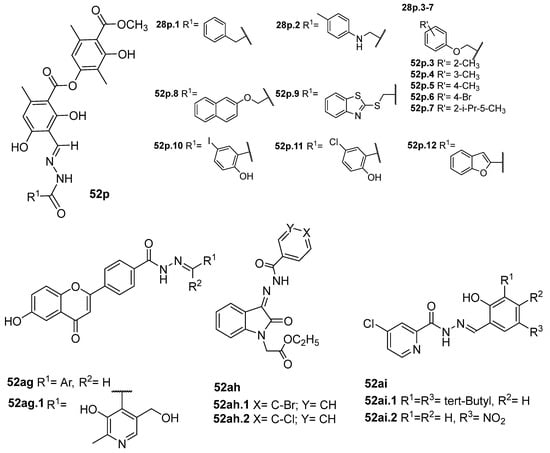
Figure 13.
α-glucosidase, α-amylase, acetylcholinesterase, and butyrylcholinesterase inhibitors.
New flavone derivatives 52ag and indolone derivatives 52ah also exhibited inhibitory activity against α-glucosidase (Figure 13). Compounds 52ag.1 (IC50 = 1.02 µM), 52ah.1 (IC50 = 14.8 µg/mL), and 52ah.2 (IC50 = 14.5 µg/mL) showed higher activity than the standard drug acarbose [54,121].
Additionally, Abbasi et al. [121] reported compounds 52ah as inhibitors of the α-amylase enzyme. Compounds 52ah.1 and 52ah.2 were the most potent against the α-amylase enzyme with IC50 = 19.6 and 18.3 µg/mL, respectively.
In addition, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) are other critical enzymes in health care, and their inhibitors are used in the clinical management of Alzheimer’s disease. AChE inhibitors include galantamine, donepezil, rivastigmine, and tacrine; however, these drugs have numerous side effects. So, Güngör [122] reported the synthesis of eleven new derivatives 52ai (Figure 13) as potential inhibitors of AChE enzymes. Among these, compound 52ai.1 showed the best inhibitory activity with IC50 = 2.01 µM against AChE, comparable to the control Galantamine (IC50 = 2.60 µM). In addition, compound 52ai.2 showed the best inhibitory effect with IC50 = 2.83 µM against BChE, which was lower than the IC50 of control galantamine (IC50 = 3.70 µM) [122].
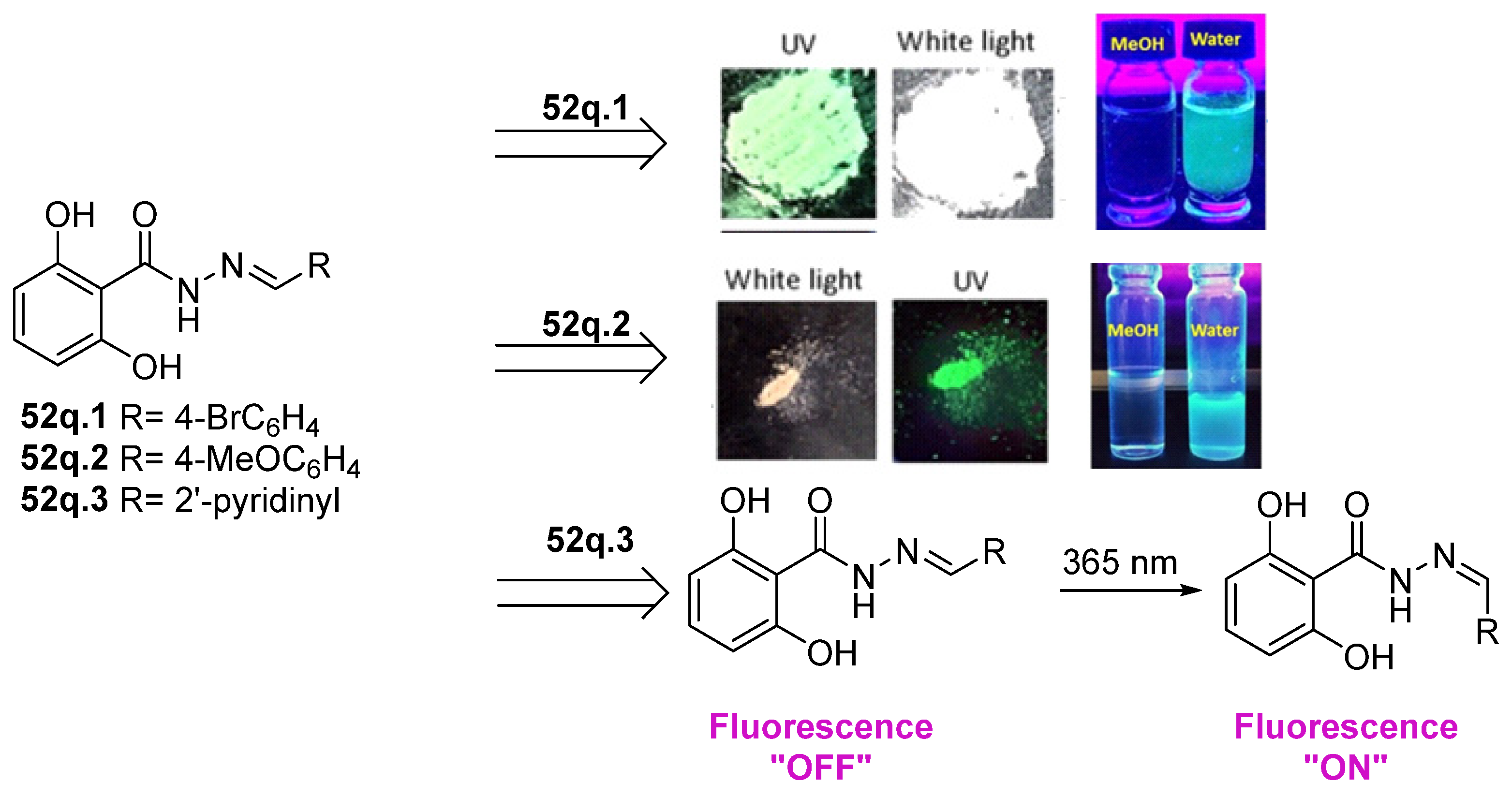
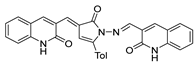
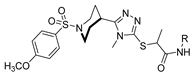
Hydrazides with aryl-substituted groups have also been used to obtain hydrazide–hydrazones with aggregation-induced emission (AIE) properties. Patil et al. [55] reported novel hydrazide–hydrazone 52q with remarkable AIE properties. The study showed that compounds 52q.1 and 52q.2 behaved as aggregate-induced emission luminogens to illuminate and record images of subcellular organelles and targets in cancer cells (Figure 14) [55]. Moreover, the internalization of these compounds into the HeLa cervical cancer cells without showing any cytotoxicity was observed. On the other hand, Wu et al. [56] reported that compound 52q.3 (the E isomer) exhibited photoisomerization to the Z isomer after light irradiation at 365 nm (Figure 14).
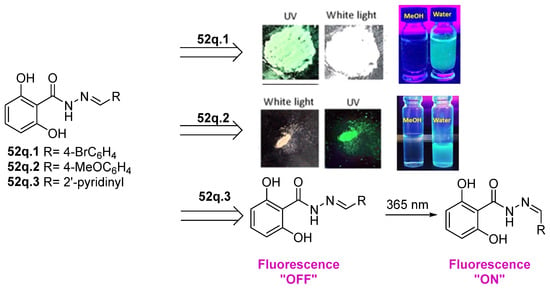
Figure 14.
Hydrazide–hydrazones with aggregate-induced emission properties. Adapted with permission from Ref. [55] Copyright 2019 John Wiley & Sons, Inc., and Ref. [56] Copyright 2021 Royal Society of Chemistry.
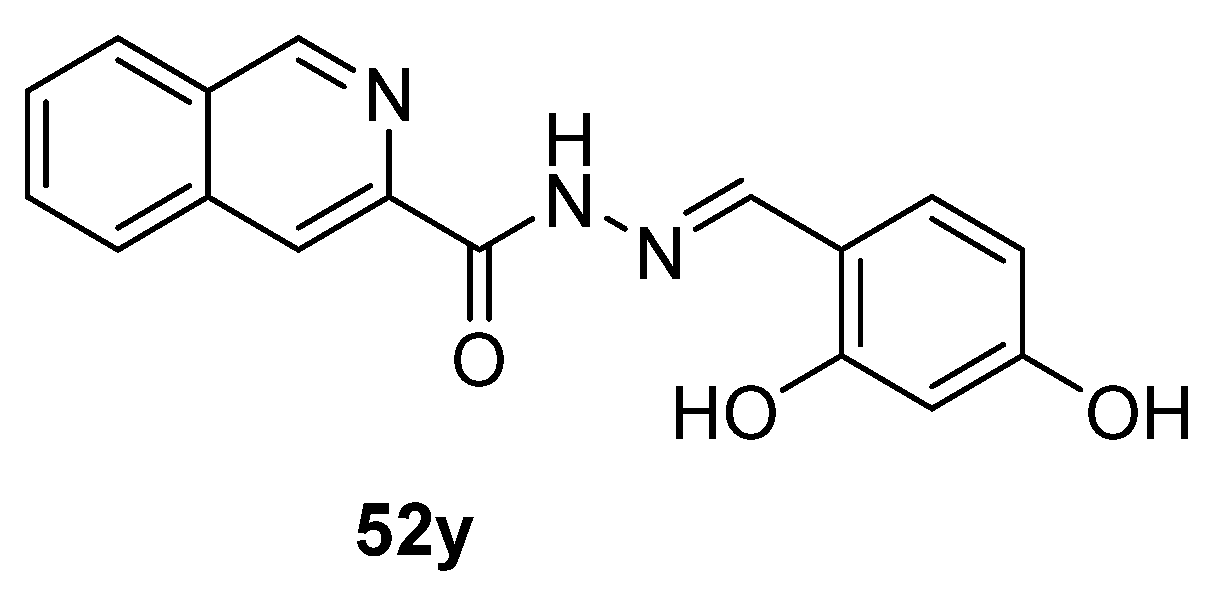
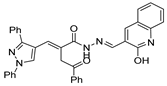
Recently, the research of Fan et al. [13] highlighted a new fluorescent probe 52y (Figure 15) capable of self-assembling into nanospheres in aqueous solution, and then, when placed in the presence of human serum albumin (HSA), they disassemble and display an evident fluorescence signal.
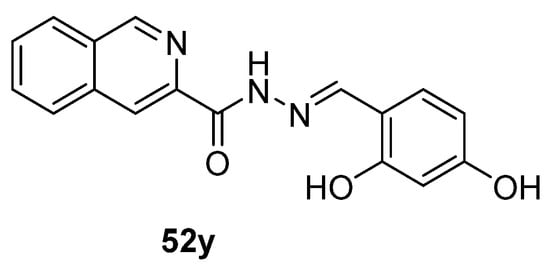
Figure 15.
Hydrazide–hydrazone as potential probes.
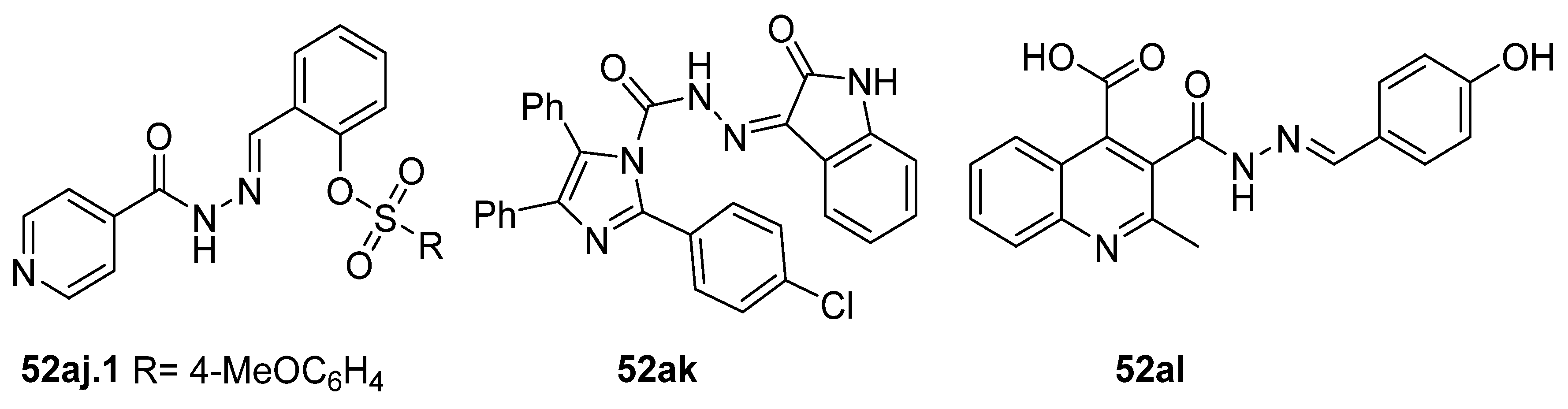
Compounds 52aj, 52ak, and 52al (Figure 16) were tested as potential antioxidants [78,79,123]. Among the twelve derivatives 52aj tested in vitro, compound 52aj.1 showed the best activity according to the DPPH method (SC50 = 0.03 mg/mL) [123]. However, compound 52ak showed antioxidant activity in vivo in rats according to Abdelhamid et al. [78]. Amongst the quinoline hydrazide–hydrazone derivatives synthesized by Cahyana et al. [79], compound 52al showed the best antioxidant activity by DPPH assay with IC50 = 843.52 ppm, yet this was weak compared to ascorbic acid with IC50 = 11 ppm.
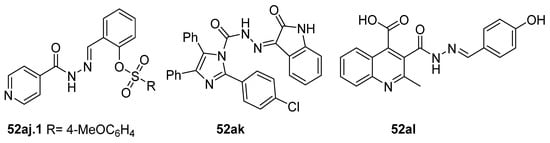
Figure 16.
Hydrazide–hydrazone with antioxidant activity.
Apart from their biological importance, hydrazide–hydrazone compounds are occasionally mentioned in the following points as useful intermediate synthons for the synthesis of some heterocyclic rings [40,47].
3.2. Heterocycles from Hydrazides
Hydrazides are widely used as synthons in the synthesis of a variety of heterocycles via electrophilic reactions. After the cyclization process, the different heterocycles can also be further modified or not to obtain compounds with biological activity. Within the heterocycles generated from hydrazides, it has become possible to identify the synthesis of pyrrolones, pyrazoles, oxadiazoles, thiadiazoles, triazoles, and triazepinones in the recent literature, which will be discussed in the following sections. The biological activity of the synthesized compounds will also be presented.
3.2.1. Pyrrolones
Synthesis of Pyrrolones
Pyrrolones are five-membered heterocyclic lactams recognized as important scaffolds whose origin may be natural or synthetic, with a wide variety of pharmacological activities [133,134]. These compounds can present anticancer [27,135,136,137], antimalarial [138], anti-inflammatory [139], antiviral [28], and antioxidant activities [140]. In 2015, Pelkey et al. [141] reported different methods, including one-component intramolecular or two-component intermolecular cyclization approaches for pyrrolone synthesis that were reported through the end of 2014.
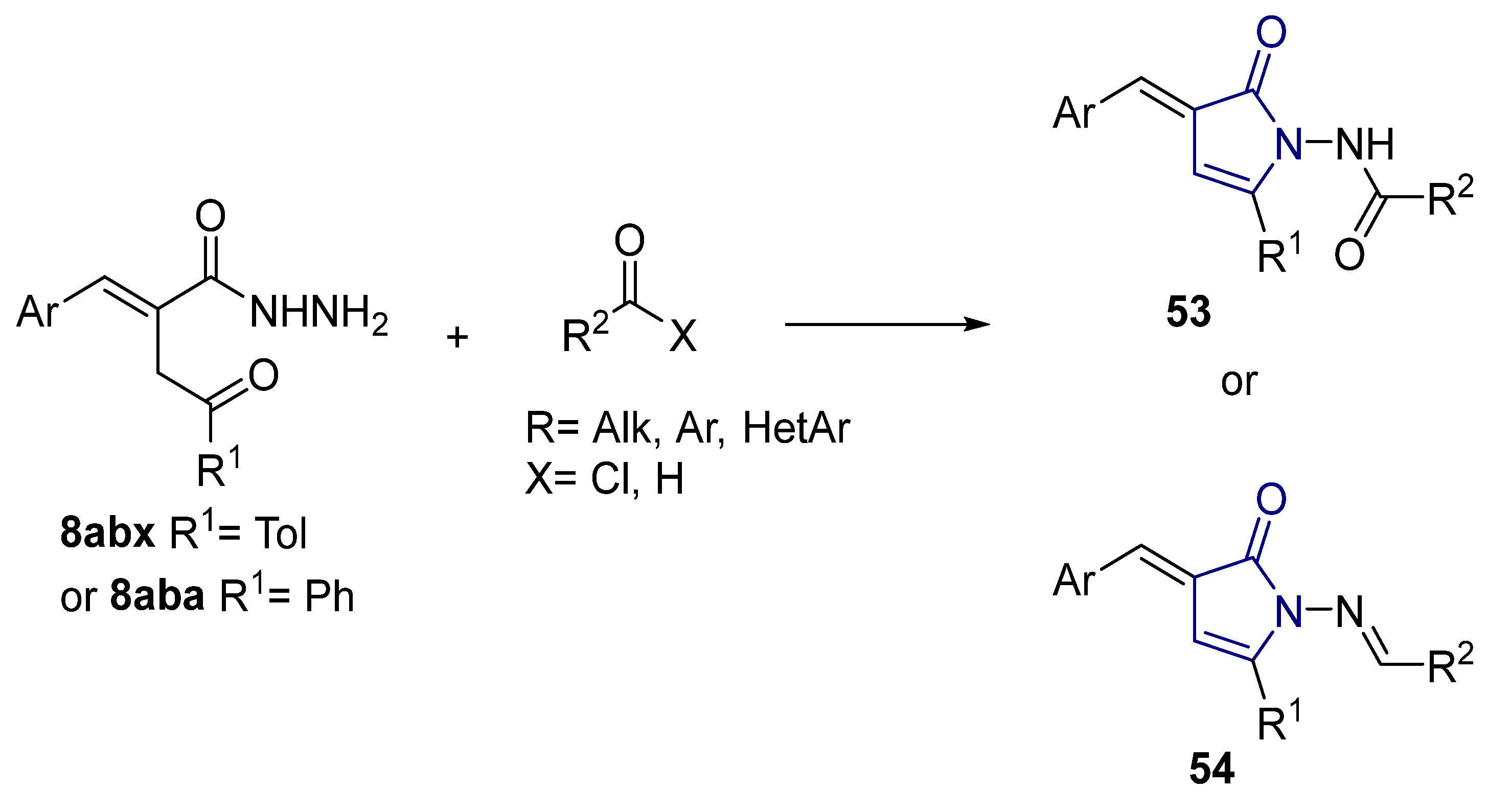
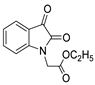
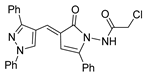
According to the literature mentioned in Table 4, from 2019 to 2024, [27,28,101,137,140], pyrrolones 53 and 54 can be formed from the reaction of hydrazides (compounds 8aax or 8aba) and electrophiles (e.g., acyl chlorides or aldehydes) (Scheme 12) [27,28,101,137,140]. The reactions with acyl chlorides occurred under reflux [27,28,140] or at room temperature [28,137], and in some cases, a base [28] was used. When the reaction occurred with aldehydes [28,101,140], the reactions were performed via the catalysis of acetic acid, in ethanol, under reflux conditions. The products were generally obtained in good yields.

Table 4.
Reaction conditions for the synthesis and purification of pyrrolones from hydrazides.
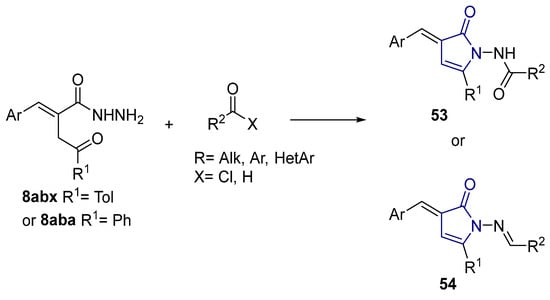
Scheme 12.
Representative scheme of pyrrolone synthesis from hydrazides.
Biological Activity of Pyrrolone Derivatives
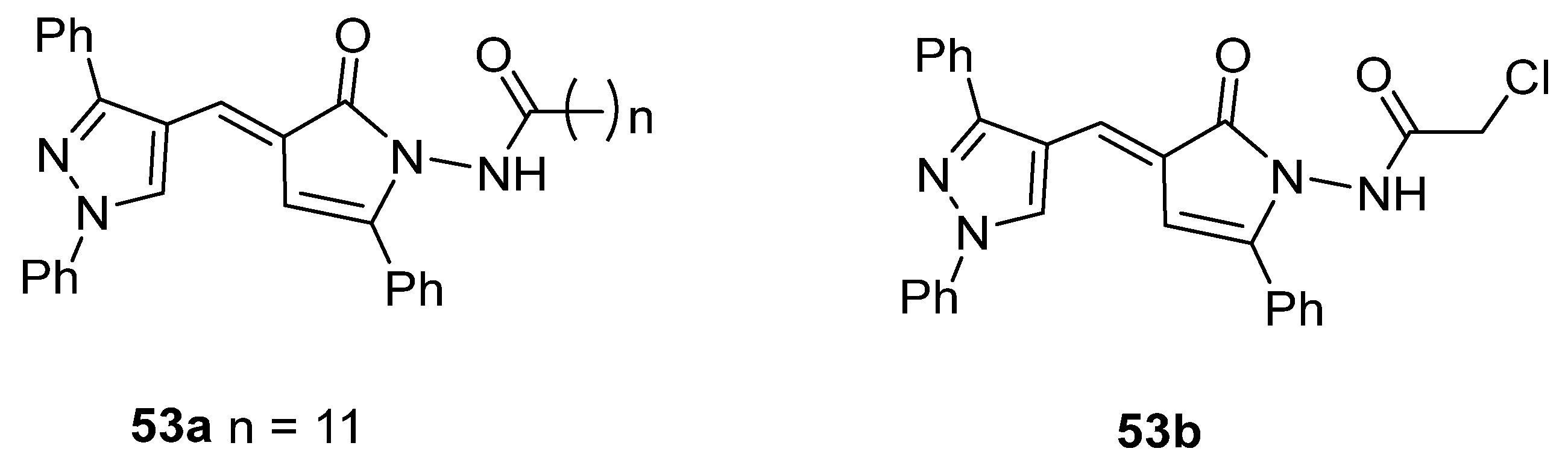
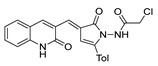
The pyrrolone derivative 53a exhibited great in vitro anticancer activity against HCT-116 and MCF-7 cell lines, with IC50 = 7.49 and 8.51 µM [27], respectively (Figure 17). Also, compound 53b showed IC50 = 46.3 µg/mL against HePG2 cell lines [137].
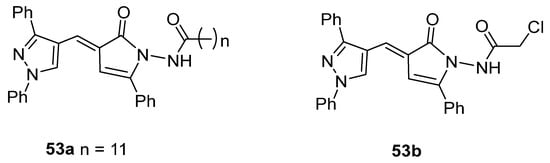
Figure 17.
Pyrrolones with anticancer activity.
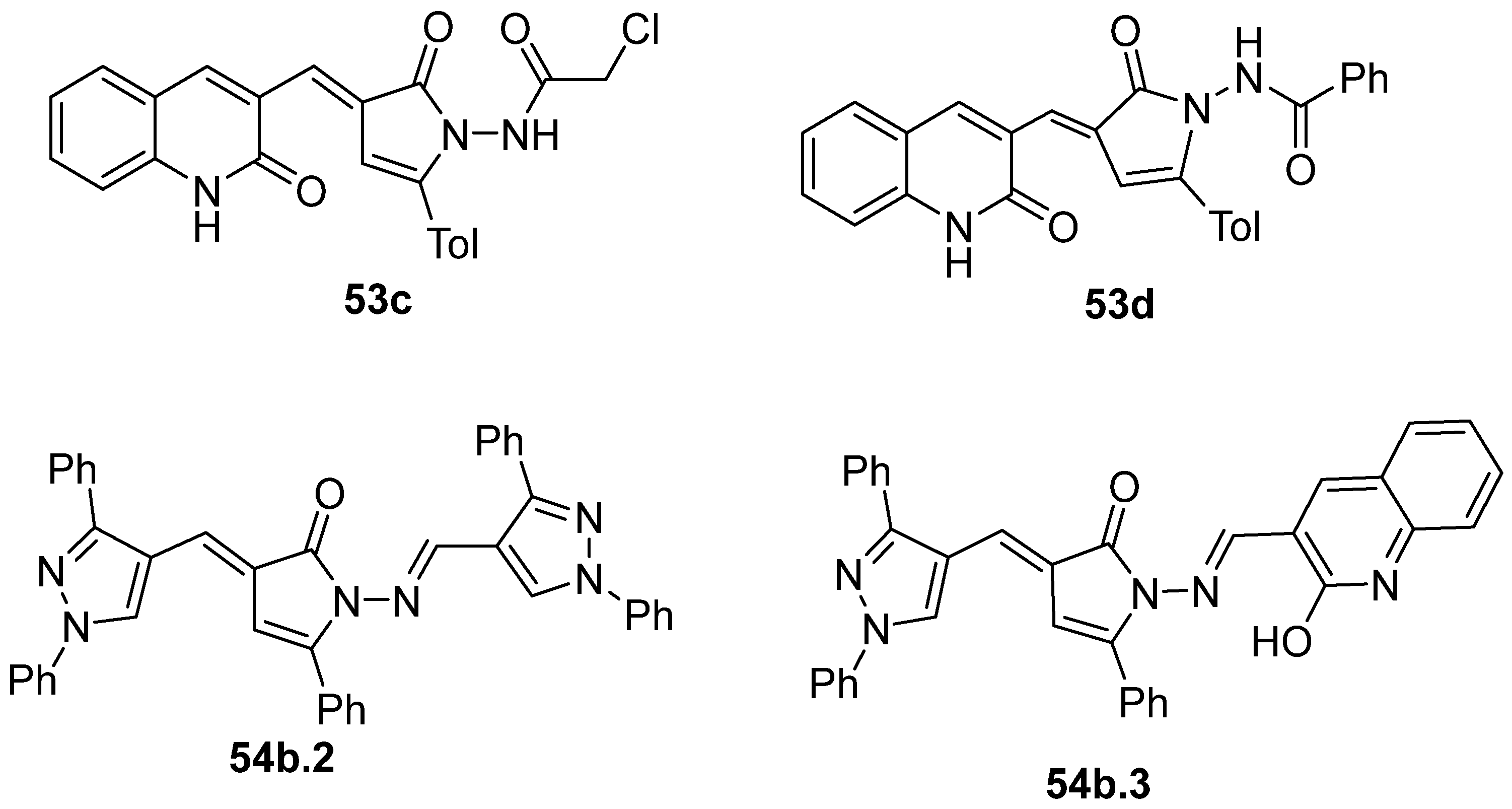
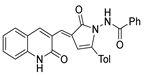
Compounds 53c and 53d, reported by El-Helw et al. [28], showed a high percentage of protection against the pathogenic avian influenza virus (H5N8) [28], higher than 80% of immunomodulators. Morsy et al. [101] reported on compounds 54b.2 and 54b.3, which exhibited antiviral activity with 100% protection against Newcastle disease virus (Figure 18).
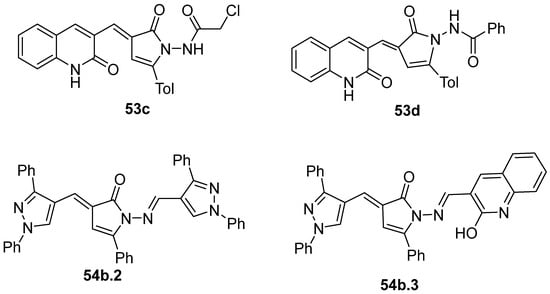
Figure 18.
Pyrrolones with antiviral activity.
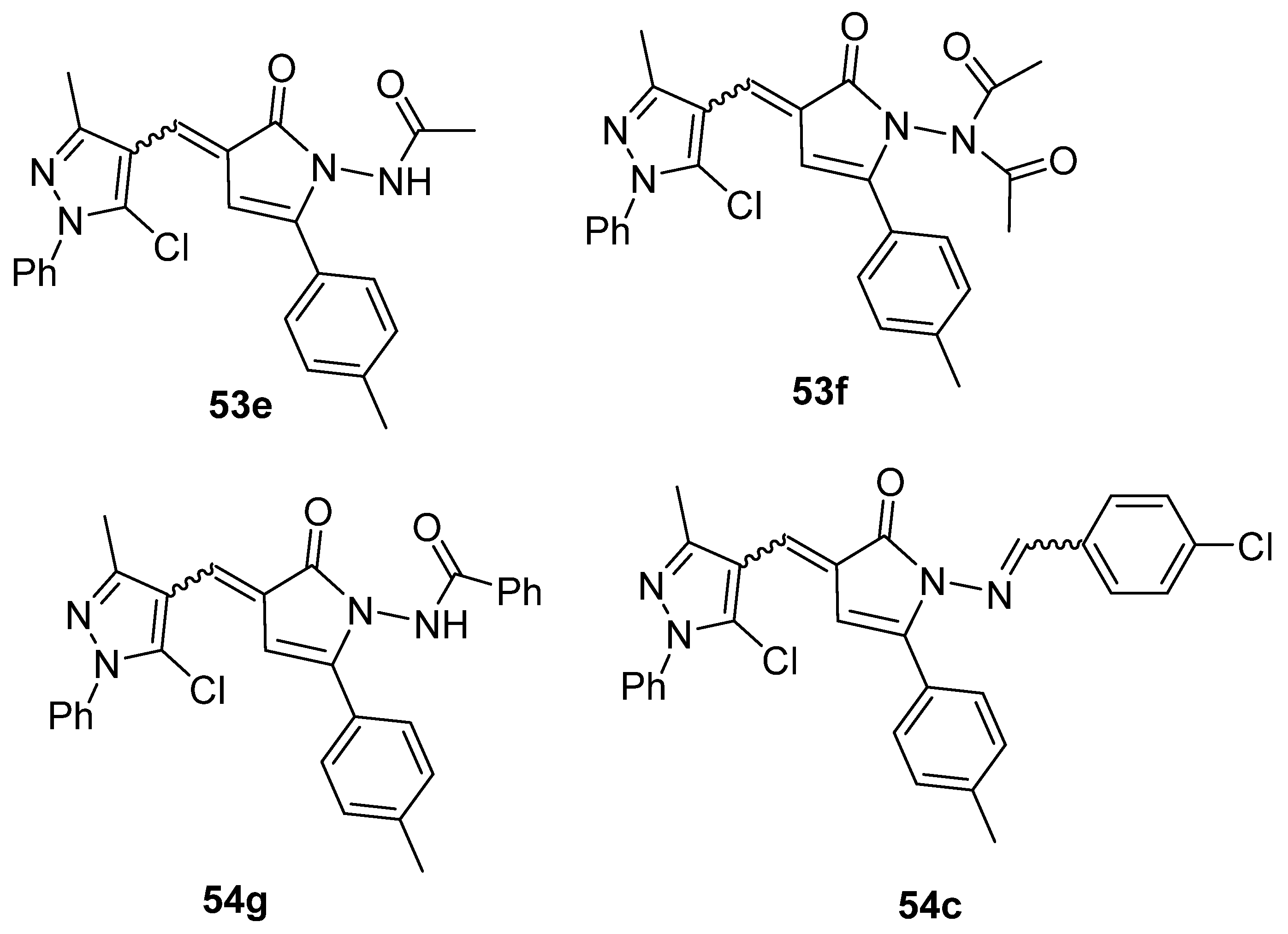
Moreover, Youssef et al. [140] used the phosphomolybdenum method to determine the antioxidant capacity of compounds 53e–g and 54c (Figure 19). The compounds showed good to moderate antioxidant capacity, presenting 163.0 to 262.27 mg of acid ascorbic equivalents per gram (AEE/g) of dry compound.
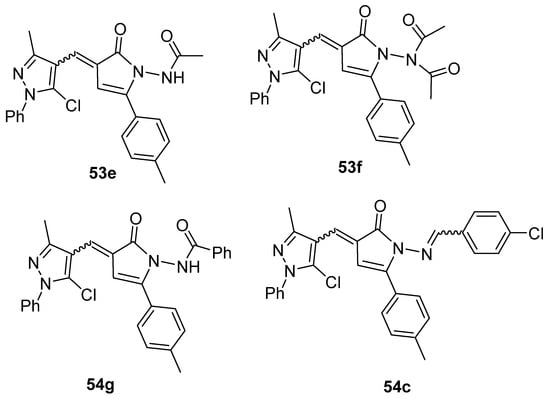
Figure 19.
Pyrrolones with antioxidant activity.
3.2.2. Pyrazoles
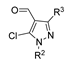
Synthesis of Pyrazoles
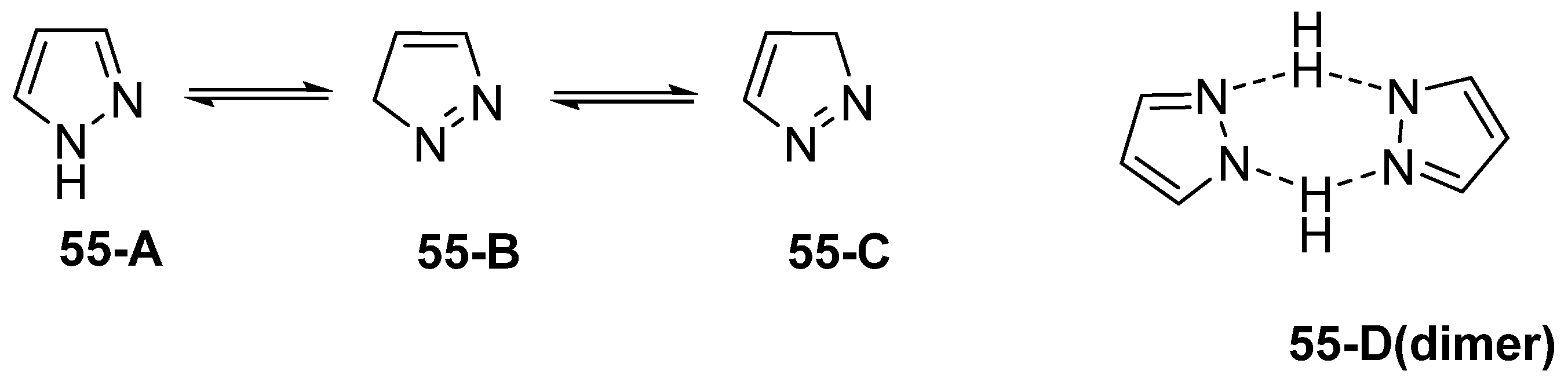
Pyrazole derivatives are five-membered N-heterocycle compounds with two adjacent nitrogen atoms (1,2-positions) [29]. Unsubstituted pyrazole is a planar structure with three possible tautomeric forms (55-A, 55-B, and 55-C), as represented in Figure 20. However, it can also exist as a dimer (55-D), in concentrated solution, via hydrogen bonding [142].
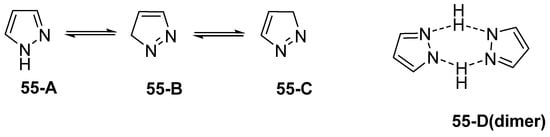
Figure 20.
Tautomeric structures and dimer of pyrazole.
In the azole family, pyrazole derivatives are one of the most studied compounds, with a wide range of chemical and biological properties [29,143,144]. In clinical use, rimonabant, sildenafil, fomepizole, celecoxib, and ruxolitinib are some of the pyrazole-based drugs [142]. In the literature, pyrazoles have been described as antimicrobial [128,145,146], anti-inflammatory [147,148], and anticancer agents [149,150,151].
Hassani et al. [29] and Ríos et al. [143] compiled the works reporting the synthesis of pyrazole derivatives between 2013 and 2023 and between 2017 and 2022, respectively. Pyrazoles were obtained from the reaction between hydrazine and a carbon unit, such as 1,3-dicarbonyl, α,β-unsaturated carbonyl compounds, acetylenic ketones, or β-enaminones or similar compounds.
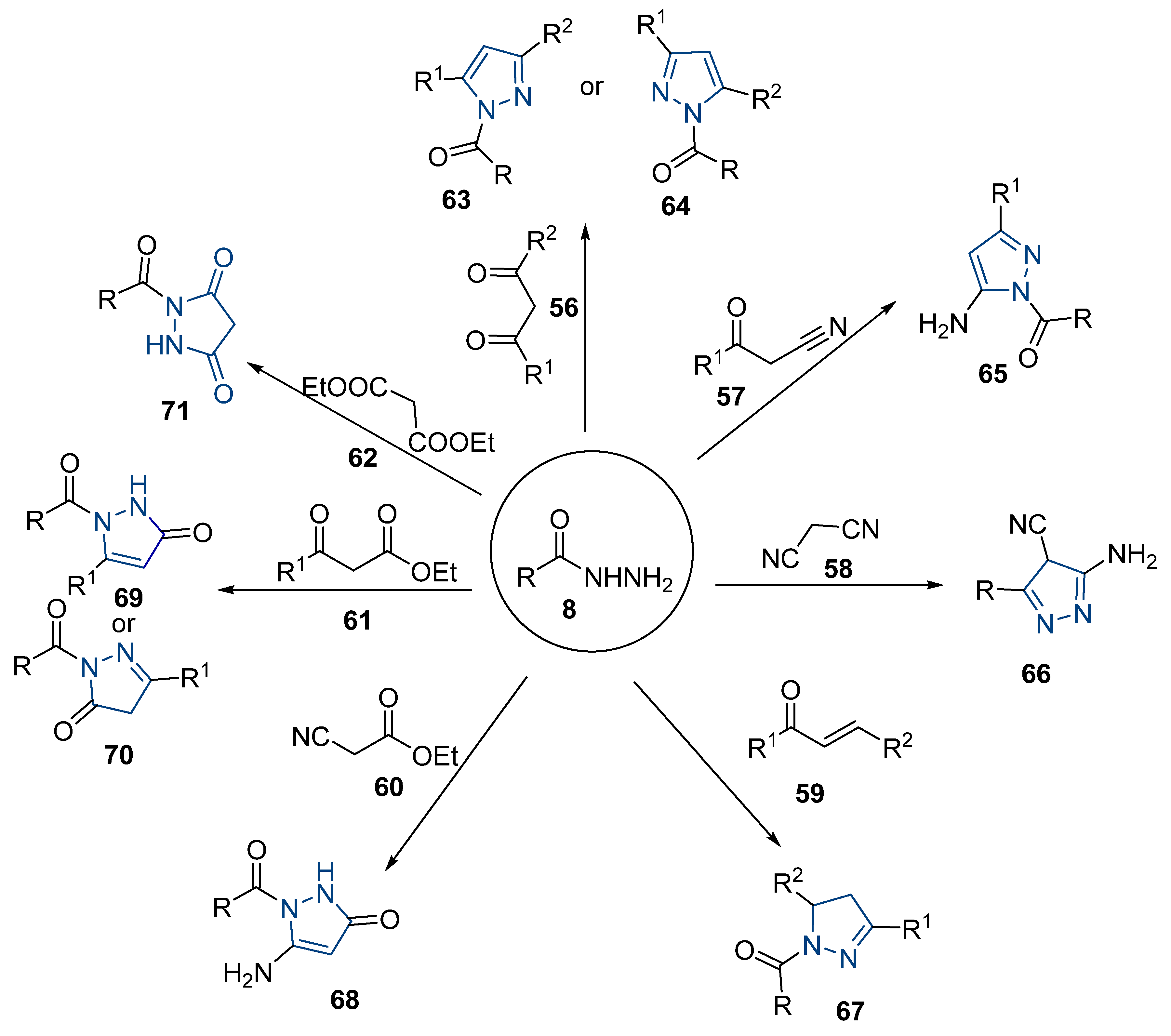
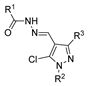
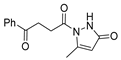
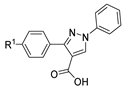
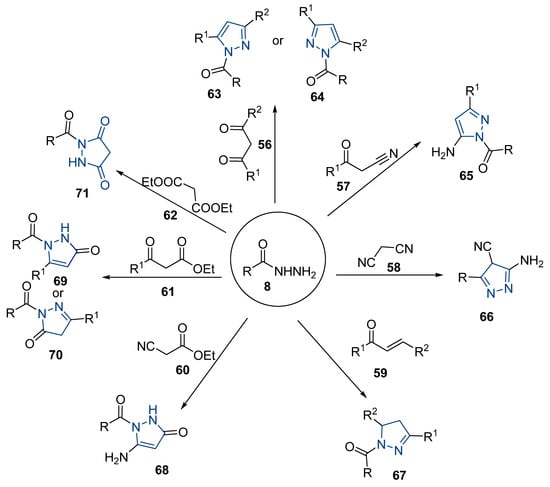
Although pyrazoles are usually obtained from hydrazine, in this review, we present hydrazides as precursors of pyrazoles, dihydropyrazoles, or pyrazolidine-diones (Table 5). The synthesis of these compounds occurred between hydrazides 8 and several carbonyl/nitrile compounds as represented in Scheme 13. Pyrazoles 63 to 66 were obtained in ethanol, under reflux, in the presence or not of an organic base [80,146]. Dihydropyrazoles 67–70 [80,128,146] or pyrazolidine-diones 71 [80] were generated from hydrazides and carbonyl/nitrile compounds in the presence of a strong inorganic base, in ethanol or DMF, at room temperature or under reflux. The products were usually obtained with good yields [80].

Table 5.
Reaction conditions for the synthesis and purification of pyrazoles, dihydropyrazoles, and pyrazolidine-dione from hydrazides.
Scheme 13.
Representative scheme of pyrazoles, dihydropyrazoles, and pyrazolidine-dione synthesis from hydrazides.
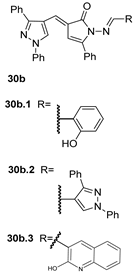
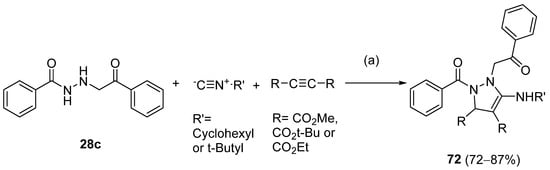
Also, recently, Ardakani et al. [145] reported the synthesis of dihydropyrazole 72 by the reaction of substituted hydrazide 28c with alkyl isocyanides and dialkyl acetylenedicarboxylates at room temperature, in 72–84% yields (Scheme 14).
Scheme 14.
Synthesis of dihydropyrazole derivatives 48: (a) acetone, –5 °C, 10 min, r.t., 24 h.
Biological Activity of Dihydropyrazole and Pyrazole Derivatives
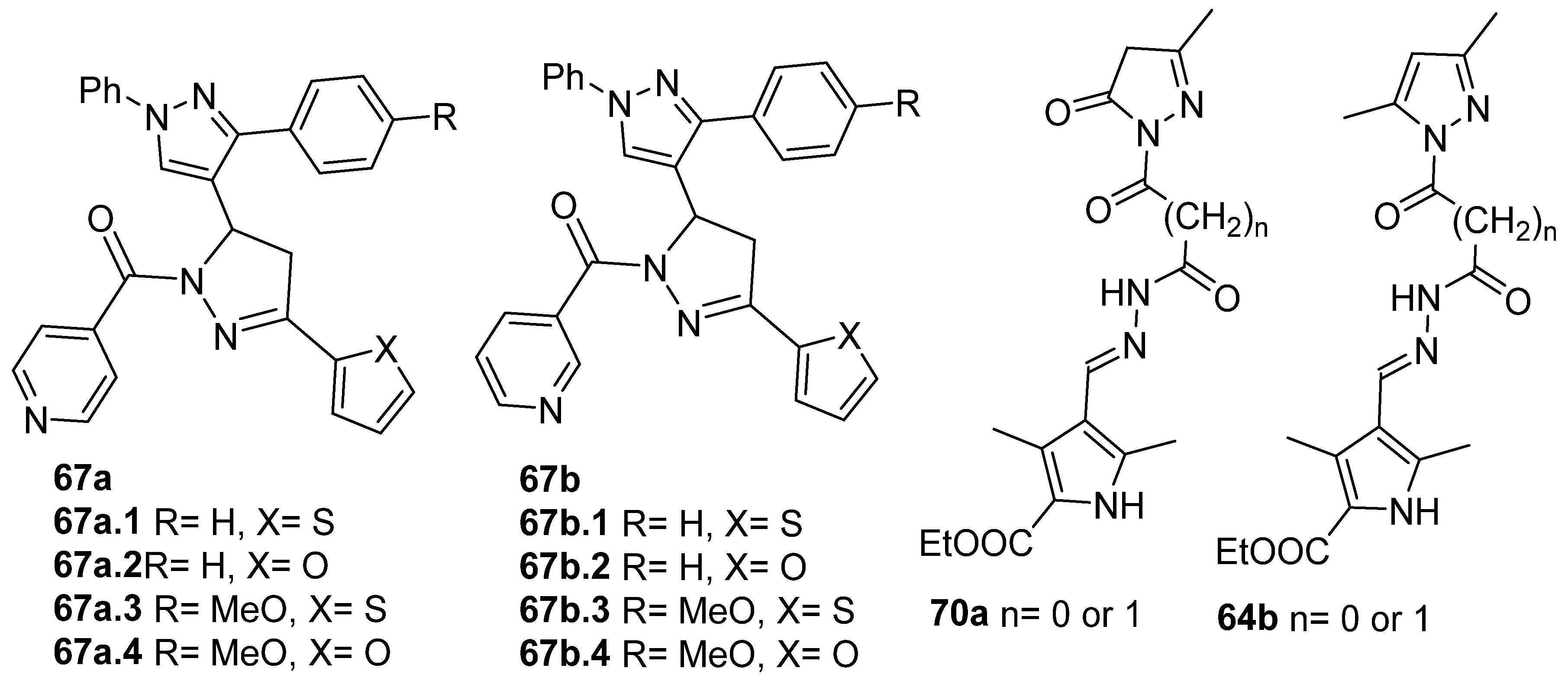
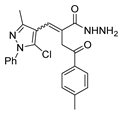
Compounds 67a,b (Figure 21) were screened for their in vitro antibacterial and antifungal activities. Compound 67a.2, with an MIC = 100 µg/mL against Gram-positive B. subtilis and a stronger MIC = 50 µg/mL against C. tetani, was equipotent or more potent than the reference drugs ampicillin (MIC 250 µg/mL) and ciprofloxacin (MIC 100 µg/mL). Compound 67a.4 was more potent than ampicillin against S. aureus (MIC 62.5 µg/mL). In general, compounds 67a, with isoniazid moieties, were more effective against all microorganisms than those with nicotinic hydrazide derivatives 67b (Figure 21) [128].
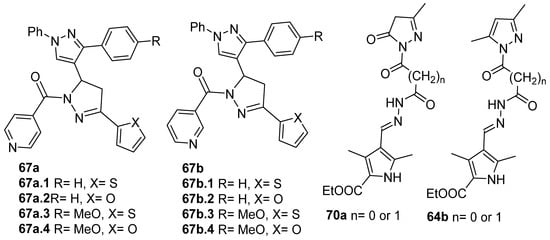
Figure 21.
Pyrazole derivatives with antibacterial, antifungal, or anticancer activities.
Compounds 70a and 64b (Figure 21) demonstrated effective antibacterial activity against Staphylococcus aureus, Bacillus subtilis, E. coli, and Pseudomonas aeruginosa, with MIC values ranging from 8 to 16 µg/mL, and good cytotoxicity in vitro against two human cancer cells, HCT-116 (colon) and HL-60 (leukemia), though it was less than the standard 5-fluorouracil [146].
3.2.3. Oxadiazoles
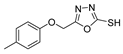
Synthesis of Oxadiazole Derivatives
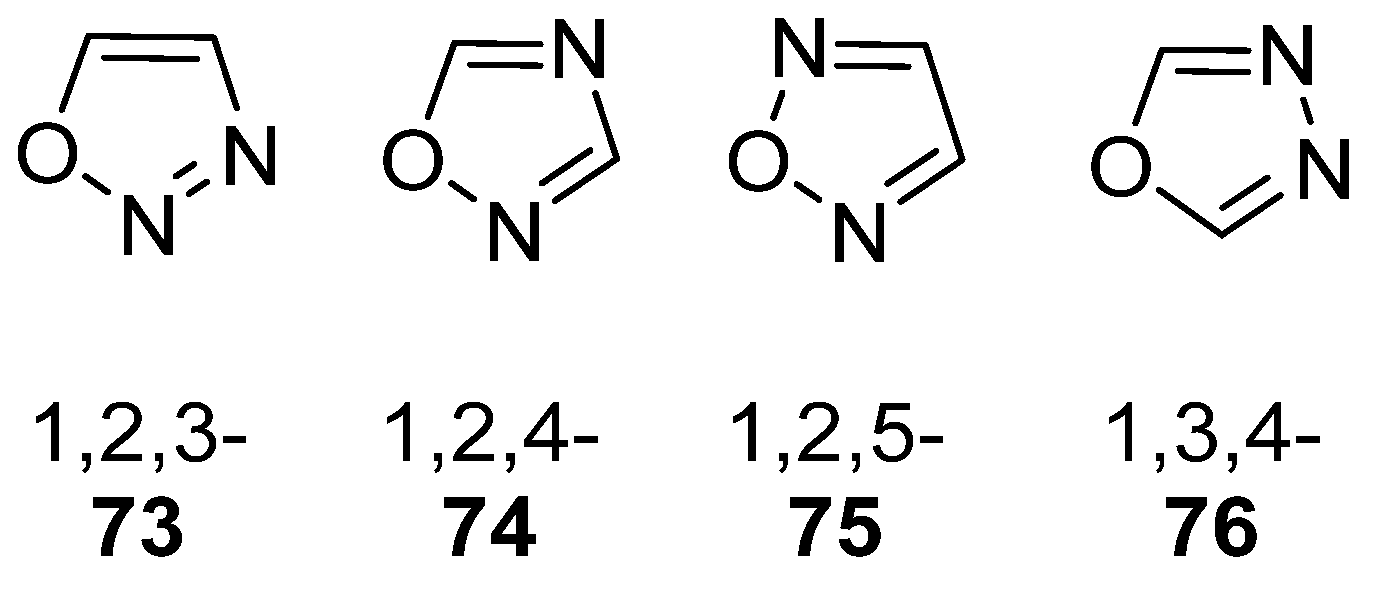
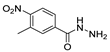
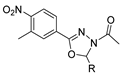
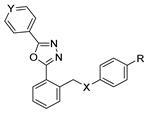
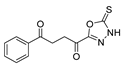
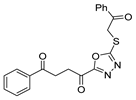
Oxadiazoles are one of the most valuable five-membered heterocycles, holding one oxygen and two nitrogen atoms, with an extensive spectrum of applications [31]. From the oxadiazole isomers of 1,2,3-oxadiazole 73, 1,2,4-oxadiazole 74, 1,2,5-oxadiazole 75, and 1,3,4-oxadiazole 76, presented in Figure 22, 1,3,4-oxadiazole 76 stands among the most studied and used, due to its broad activity spectrum [152,153]. This isomer appears in some available drugs, such as Zibotentan, Furamizole, Raltegravir, and Nesapidil [83], but recently, new derivatives have been shown to have biological activities, including anticancer [82], antibacterial, antifungal [154], antimalarial [2], antileishmanial [40], antitubercular [81], antiviral [28], anti-inflammatory [41,85], antioxidant [105], and insecticidal activities [103].
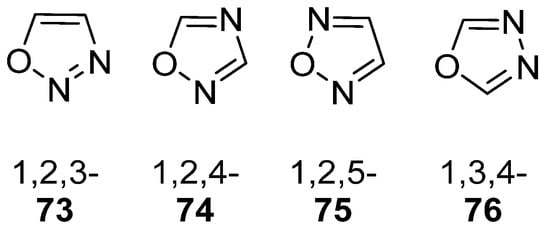
Figure 22.
Structures of oxadiazole isomers.
Sharma et al. [31] collected and discussed the synthesis of 1,3,4-oxadiazoles in the past 15 years. The authors discussed dehydrogenative cyclization of 1,2-diacylhydrazines with phosphorus oxychloride (POCl3), phosphoric acid (H3PO4), and thionyl chloride (SOCl2); oxidative cyclization of hydrazide–hydrazones; and decarboxylative cyclization.
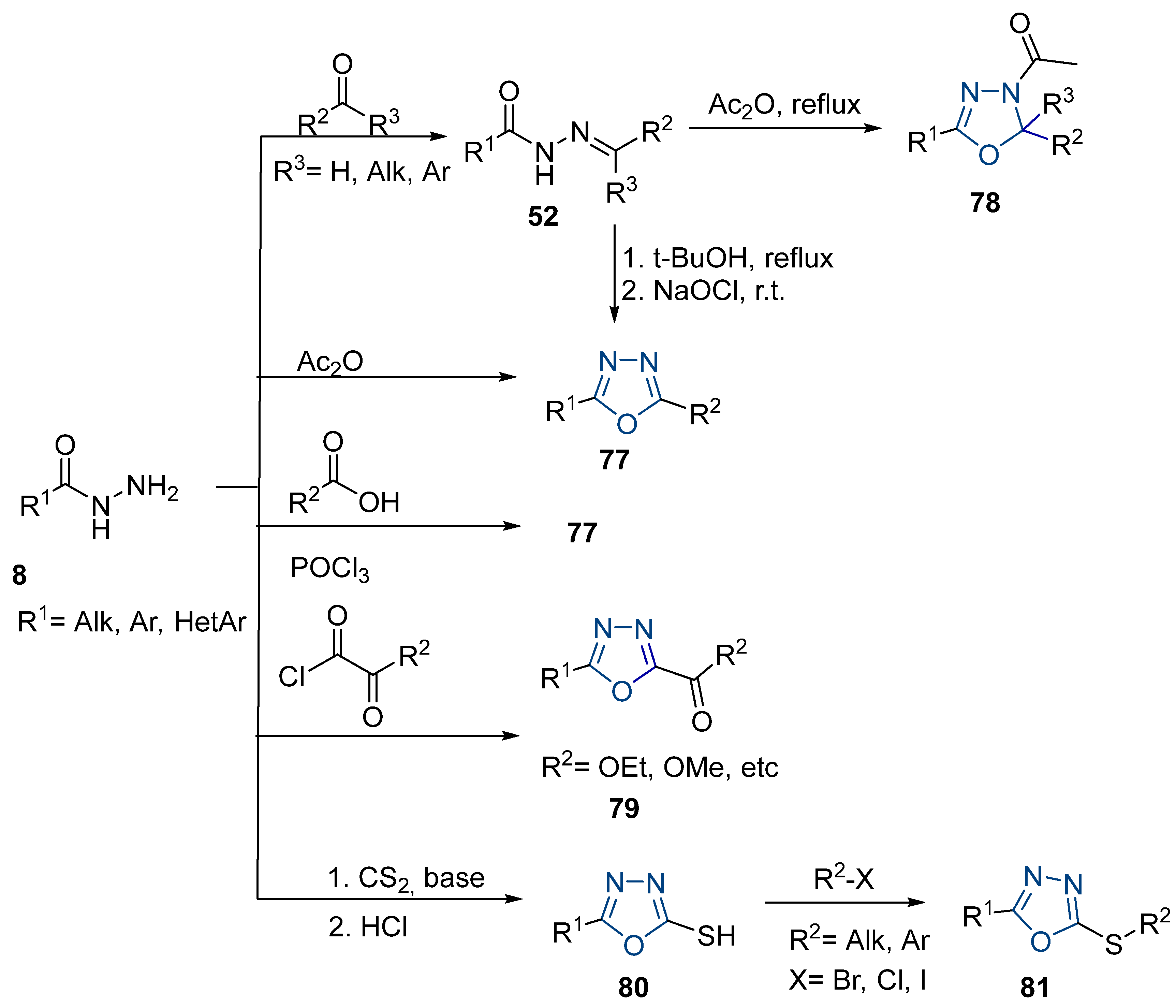
Here, we report the use of hydrazides in the synthesis of 1,3,4-oxadiazoles. According to Table 6, 1,3,4-oxadiazoles can be synthesized from the reaction between a hydrazide and carbon electrophilic reagents such as aldehydes, oxalyl chlorides, carboxylic acids, or carbon disulfide, as represented in Scheme 15. Some 2,3-dihydro-1,3,4-oxadiazol-2-yl derivatives 78 have been synthesized from hydrazides 8, with hydrazide–hydrazones 52 as intermediates [40,82,154]. This method starts with the reaction of hydrazide and an aldehyde, and then the reaction follows in the presence of acetic anhydride under reflux. The oxadiazoles 78 obtained by this method were generally obtained in low to excellent yields. On the other hand, Paidi et al. [153] reported the synthesis of 2,5-disubstituted 1,3,4-oxadiazoles 77 via one-pot NaOCl-mediated oxidative cyclization from hydrazide–hydrazones 52, generated in situ from hydrazides 8 and aldehydes (Scheme 15). The best conditions reported by Paidi et al. [153] included hydrazide 8 in the presence of aldehydes and t-BuOH, under reflux, followed by a reaction with 10–12% aqueous NaOCl at room temperature. These reaction conditions were applied to hydrazides and aldehydes with both electron-donating and electron-withdrawing groups, and the desired products 77 were obtained in moderate to excellent yields. Compounds 77 were also generated directly from 8 by reaction with acetic anhydride [80].

Table 6.
Reaction conditions for the synthesis and purification of 1,3,4-oxadiazoles and their derivatives.
Scheme 15.
Representative scheme of 1,3,4-oxadiazole synthesis from hydrazides.
The reaction of hydrazides with carboxylic acids is one of the most widely used processes to produce 2,5-substituted-1,3,4-oxadiazoles 77. The reaction normally occurs in the presence of POCl3 under high temperatures or reflux, yielding the products in reasonable to excellent yields [2,59,83,84,105,152].
The reactions between hydrazides and oxalyl chlorides occur at room temperature in the presence of a base or at high temperatures in the presence of POCl3 to produce compounds 79 [39].
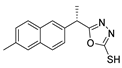
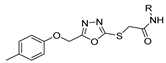
Additionally, hydrazide derivatives 8 in the presence of carbon disulfide and a base (KOH, pyridine, or NaOH) are cyclized to produce different 1,3,4-oxadiazole-2-thiol derivatives 80 (Scheme 15) [28,36,37,41,61,62,63,64,86,87,103,146]. These reactions occurred generally under reflux, and the product 80 is isolated in good yield after neutralization of the reaction mixture with HCl (Table 6). Some of the compounds 80 were then alkylated with various alkyl/aryl halides to generate the new derivatives 81 (Table 6) [36,61,62,64,85,86,87].
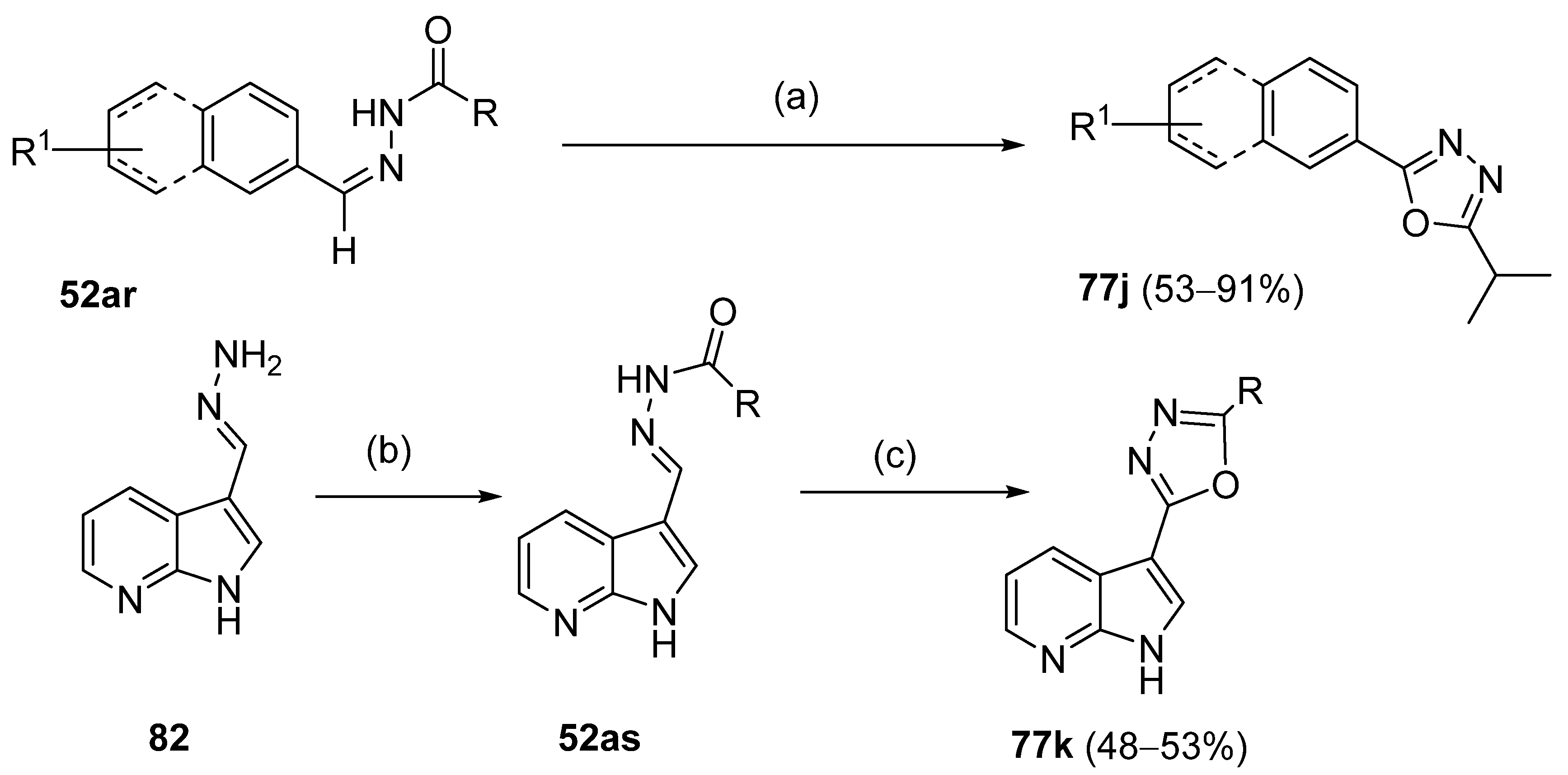
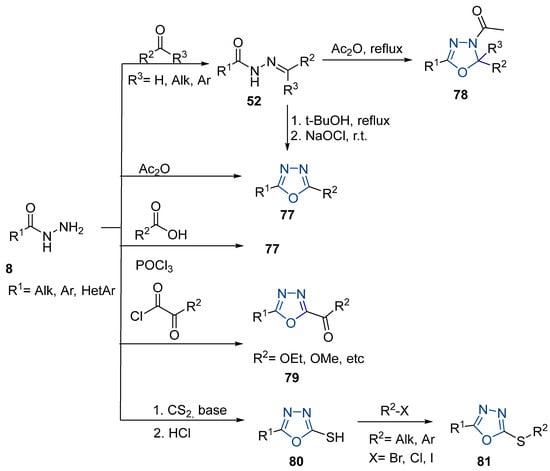
On the other hand, 1,3,4-oxadiazoles 77 can also be obtained in reasonable to excellent yields by the iodine-mediated synthesis approach [155,156]. Chauhan et al. [155] reported the synthesis of oxadiazoles 77j from 52ar in the presence of isobutyraldehyde and p-anisolyl iodide under auto-oxidation conditions in the presence of molecular oxygen. The 2,5-disubstituted 1,3,4-oxadiazole 77k was obtained from substituted carbohydrazides 52as by the iodine-mediated synthesis approach represented in Scheme 16 [156].
Scheme 16.
Synthesis of oxadiazole derivatives: (a) Me2CHCHO, p-anisolyliodide (0.1 eq), O2, acetone, 35 °C, 6–8 h; (b) RCOCl, Et3N, CH3CN, r.t.; (c) DMSO, I2, K2CO3, 110 °C, 24 h.
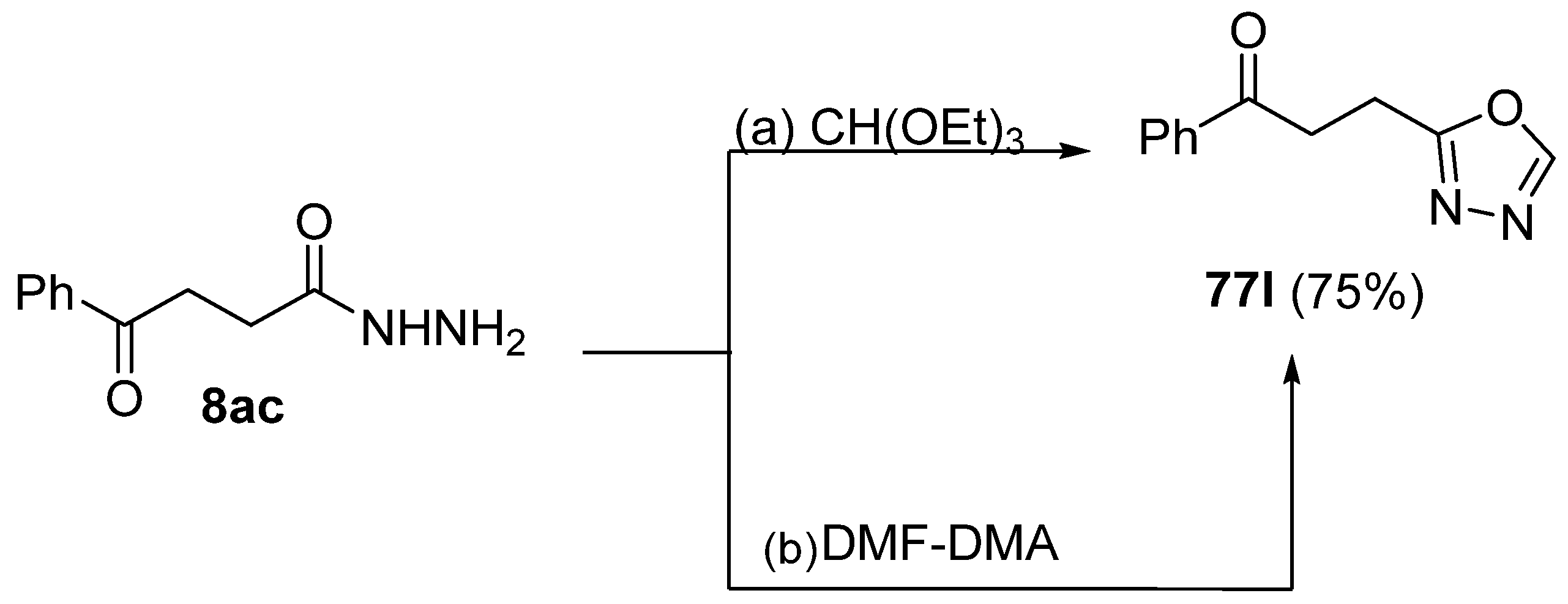
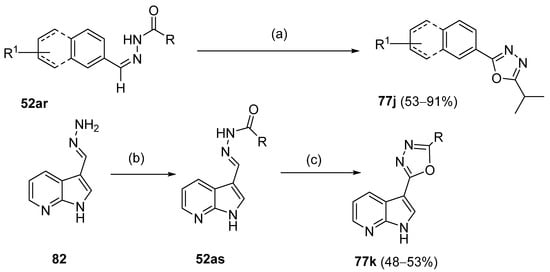
Moreover, Abu-Hashem et al. [80] reported the synthesis of 1,3,4-oxadiazoles 77l (Scheme 17) starting from hydrazide 8ac in the presence of triethyl orthoformate or dimethylformamide dimethyl acetal [80].
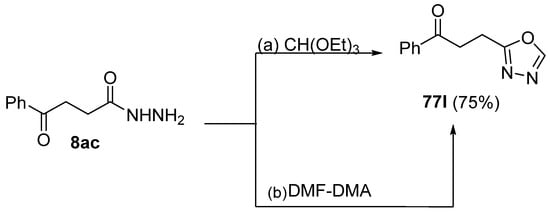
Scheme 17.
Synthesis of 2-substituted 1,3,4-oxadiazole 77l: (a) CH(OEt)3, reflux, 15–18 h; (b) DMF–DMA.
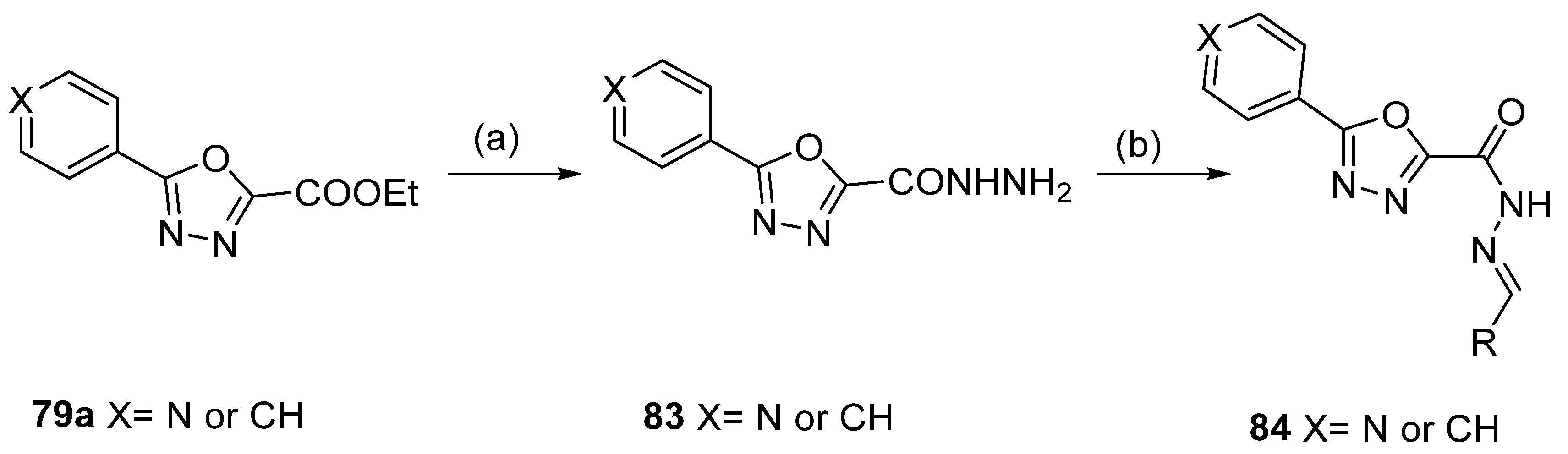
1,3,4-oxadiazoles 79a were used to generate new derivatives 83 and 84. Compounds 79a reacted with hydrazine to generate the corresponding hydrazide 83, which was converted to 84 by reaction with an aldehyde under acid catalysis [81] (Scheme 18).
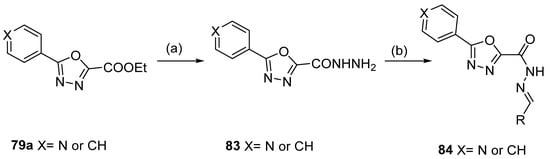
Scheme 18.
Synthesis of 2,5-disubstituted 1,3,4-oxadiazole derivatives: (a) H2NNH2, EtOH, reflux; (b) RCOH (R = Ar, HetAr), EtOH, CH3COOH (cat.), reflux.
Moreover, 1,3,4-oxadiazole derivatives 85 were obtained by reacting derivative 79b with aryl hydrazines in an ionic liquid at 100 °C (Scheme 19) [39].
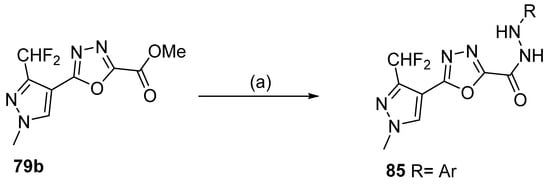
Scheme 19.
Synthesis of 1,3,4-oxadiazole derivative 85: (a) 1-butyl-3-methyl imidazolium chloride, RNHNH2, Ar, 100 °C.
Biological Activity of Oxadiazole Derivatives
Hamdy et al. [84] reported the new series of 2-(1H-indol-3-yl)-5-substituted-1,3,4-oxadiazoles 77c as inhibitors of the Bcl-2 anti-apoptotic gatekeeper protein. Compound 77c.1 (Figure 23) exhibited potent anticancer activity with IC50 = 0.52, 0.88, and 0.73 µM against MDA-MB-231, HeLa 2, and KG1a3 (Bcl-2-expressing) cell lines, respectively. Moreover, it showed no inhibitory effects in the Bcl-2-negative Jurkat cell line.
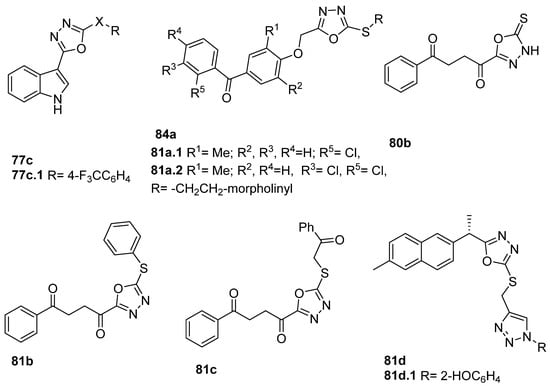
Figure 23.
1,3,4-oxadiazoles with anticancer activity.
Derivatives 81a.1 and 81a.2 (Figure 23) showed excellent activities against the MCF-7 cell line (breast cancer), with IC50 = 127.0 and 126.7 µg/mL, respectively, and against KB (oral cancer) cell lines, with IC50 = 113.8 and 112.6 µg/mL. Furthermore, these compounds revealed no toxicity against the normal cell line L929 at higher concentrations with IC50 > 201 μg/mL [86].
Oxadiazole derivatives 80b, 81b, and 81c (Figure 23) presented antiproliferative activities against MCF-7 breast cancer cells with IC50 values between 8.2 and 8.8 µM, which were lower compared to doxorubicin (10.3 µM), and against hepatocellular HepG2 cells with IC50 values between 26.9 and 29.8 µM, which were quite similar compared to doxorubicin (28.5 µM) [64].
Among the derivatives 81d, oxadiazole 81d.1 showed the highest activity against MCF-7 and HepG2 cancer cell lines with IC50 = 2.13 and 1.63 µg/mL, respectively. In addition, compound 81d.1 inhibited EGFR kinase with IC50 = 0.41 µM [36].
Shankara et al. [82] evaluated the anticancer activity of compounds 78b (Figure 24), and among them, compound 78b.1 was the most potent with an IC50 = 8.14 µM against the LN229 Glioblastoma cell line. Also, derivative 80d (Figure 24) showed good activities for HepG2 and HCT-116, with IC50 = 6.9 and 13.6 µg/mL, respectively [37]. Rawat et al. [146] reported on compounds 80e.1 and 80e.2 (Figure 24), showing good cytotoxicity against HCT-116 and HL-60 (leukemia) cell lines, with the growth inhibition percentage being superior to 70% at 5 µg/mL, but less than the standard 5-fluorouracil (a growth inhibition percentage superior to 85% at the same concentration). Compounds 80e also showed good antibacterial activity with MICs between 2 and 8 µg/mL against Gram-positive bacteria (Staphylococcus aureus, Bacillus subtilis) and Gram-negative bacteria (E. coli, Pseudomonas aeruginosa) [146].
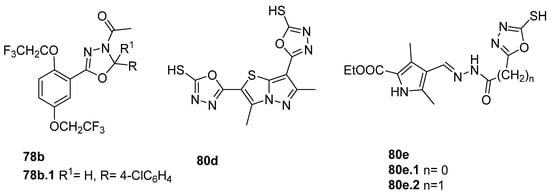
Figure 24.
1,3,4-oxadiazole-2-thiol derivatives with anticancer and/or antibacterial activities.
Compound 78a.1 (Figure 25) showed promising activity against Staphylococcus epidermidis with an MIC = 0.48 µg/mL, as well as low cytotoxicity against the L929 normal cell line [154].
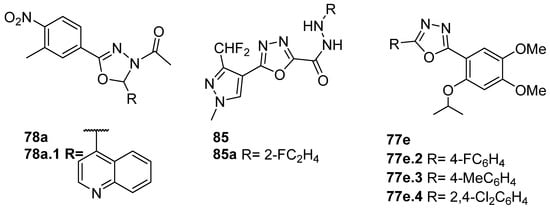
Figure 25.
1,3,4-oxadiazoles as antibacterial and antifungal agents.
Long et al. [39] designed, synthesized, and evaluated oxadiazole derivatives 85 (Figure 25) for their antifungal, antioomycete, and antibacterial activities. Compound 85a showed the best in vitro antifungal activity against Gibberella zeae and antioomycete activity against Phytophora infestins, with EC50 = 0.47 µg/mL and 3.92 µg/mL, respectively. In the in vivo study against corn scab, compound 60a showed protective and curative activities of 90.2 and 86.3% at 200 µg/mL, which were comparable to those of fungicides boscalid and fluopyram. These 1,3,4-oxadiazole-tailored pyrazole compounds with hydrazide functions in the middle as a linker are potential agricultural fungicides for controlling fungal diseases.
The 2,5-disubstituted 1,3,4-oxadiazoles 77e (Figure 25) were evaluated for their in vitro antibacterial activity. Compound 77e.2 exhibited the best broad-spectrum antibacterial and antifungal activity, with MIC = 15.62, 7.81, 3.9, and 31.25, 62.5 µg/mL against E. coli, S. typhi, B. subtilis, B. megaterium, and A. niger, respectively [83].
Derivatives 84 (Figure 26) were evaluated for their in vitro antimycobacterial activity against the M. tuberculosis H37Ra-attenuated strain, H37Rv virulent strain, and several resistant strains. From the 5-phenyl-substituted oxadiazole subseries, derivatives 84a and 84b presented an MIC = 4 µM against pyrazinamide-resistant strains. Moreover, these compounds exhibited selectivity for mycobacteria and low cytotoxicity against human SH-SY5Y cells (CC50 = 50 and 100 µM for 84a and 84b, respectively) [81].
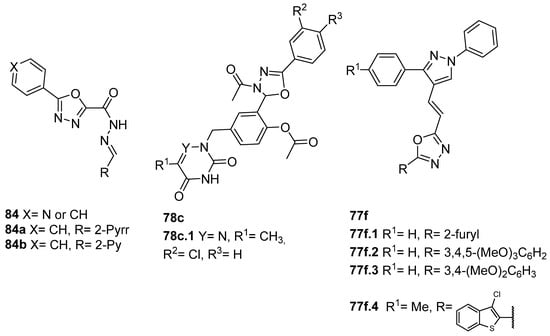
Figure 26.
1,3,4-oxadiazoles with antimycobacterial and antiparasitic activities.
Also, N3-acetyl-1,3,4-oxadiazoline derivatives 78c (Figure 26) were screened against Leishmania donovani, and compound 78c.1 exhibited an antileishmanial activity with IC50 = 8.98 µM on L. donovani intramacrophage amastigotes [40].
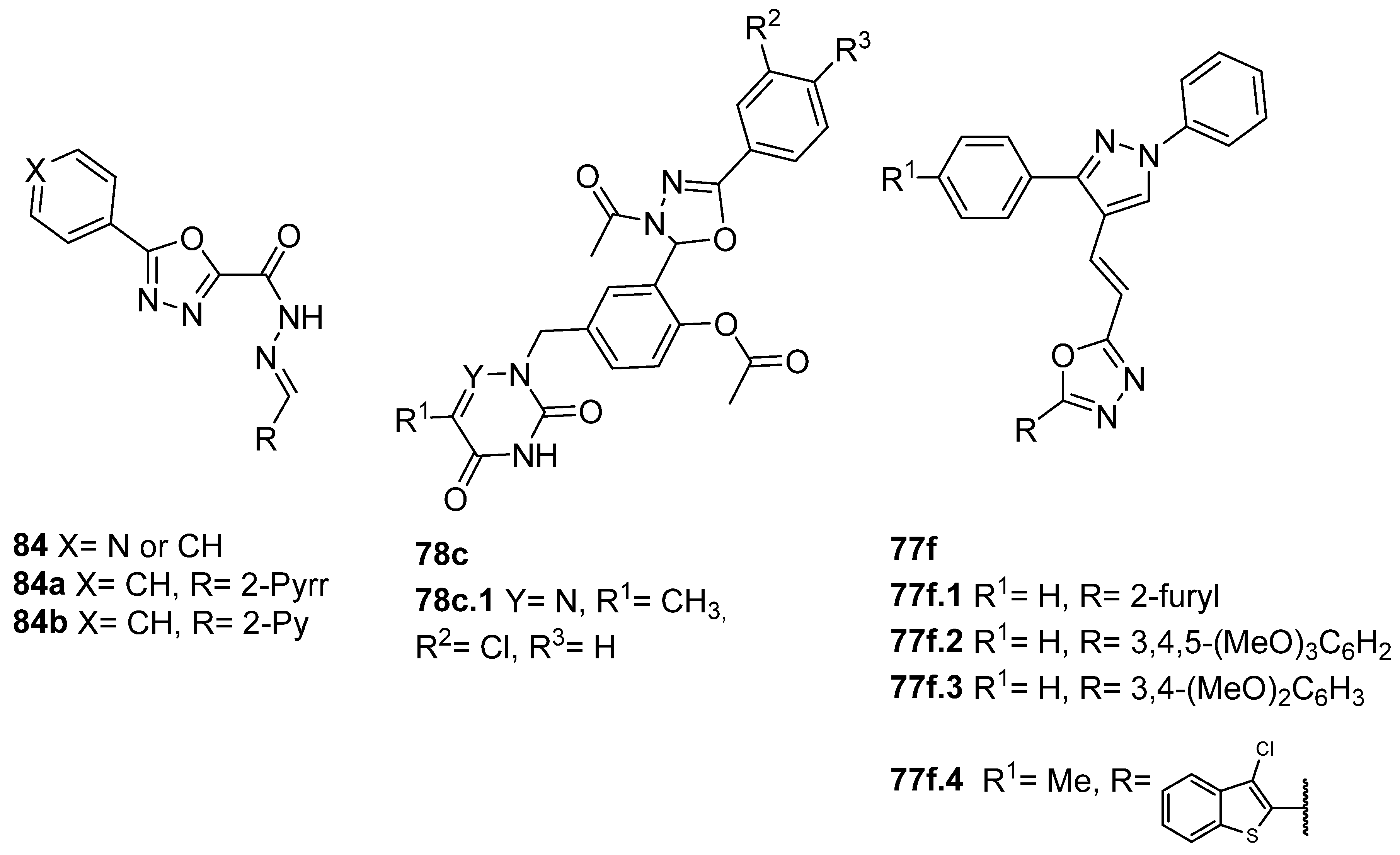
Moreover, Verma et al. [2] synthesized the hybrid compounds 77f and evaluated their activity against P. falciparum 3D7 (chloroquine-sensitive) and RKL 9 (chloroquine-resistant) strains. Among the evaluated compounds 77f (Figure 26), compound 77f.1 exhibited the best activity with an IC50 = 0.25 µg/mL against the 3D7 (chloroquine-sensitive) strain and 0.86 µg/mL against the RKL 9 (chloroquine-resistant) strain of P. falciparum. Moreover, the antileishmanial activity of compounds 77f against L. donovani promastigotes was also evaluated. Compounds 77f.2, 77f.3, and 77f.4 exhibited IC50 = 33.3, 40.1, and 19.0 µg/mL, respectively. The same compounds (77f.2, 77f.3, and 77f.4) also had effects on amastigote infectivity with IC50 = 44.2, 66.8, and 73.1 µg/mL, respectively. Among the tested compounds, the most promising were 77f.1 and 77f.4 for their good antimalarial and antileishmanial activity, respectively; hence, their cytotoxicity was studied, as well as their safety profile.
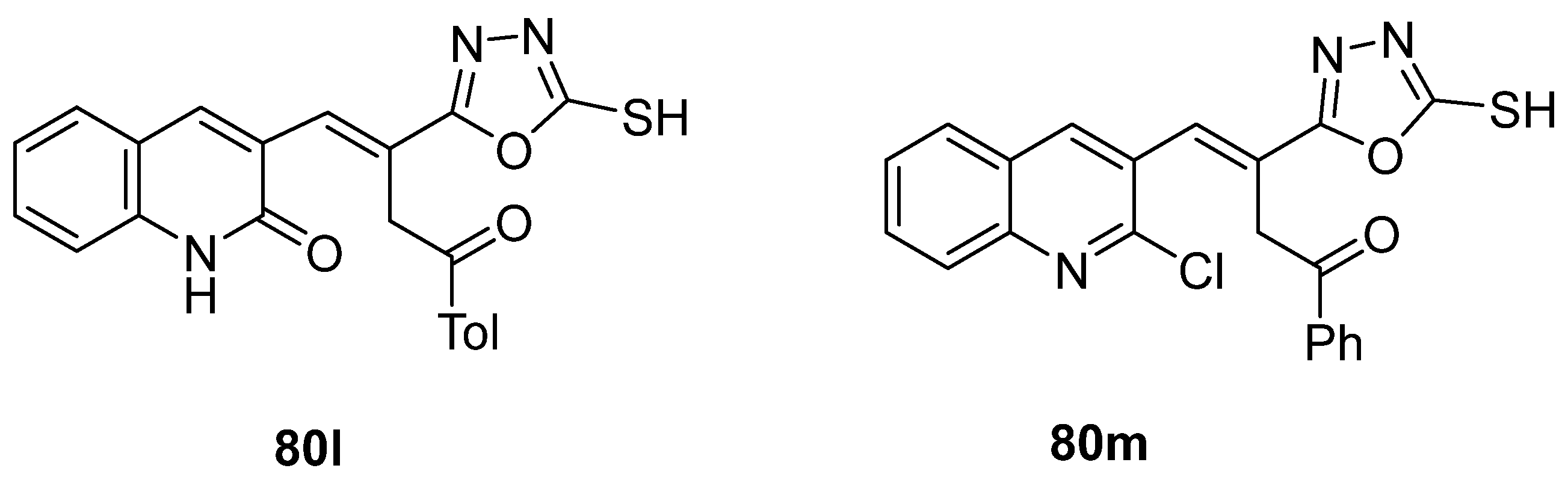
El-Helw et al. [28] reported compound 80l (Figure 27) as an immunomodulator against the highly pathogenic avian influenza virus (H5N8), with the high potency of 100% protection, and Ramadan et al. [103] reported compound 80m (Figure 27) as an insecticide with low LC50 = 9.67 and 1.07 mg/mL against lab and field strains of the third larval instar of Culex pipiens.
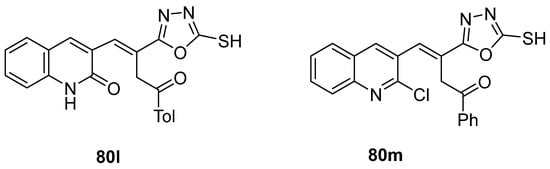
Figure 27.
1,3,4-oxadiazole-2-thiol derivatives with antiviral (80l) or insecticidal activity (80m).
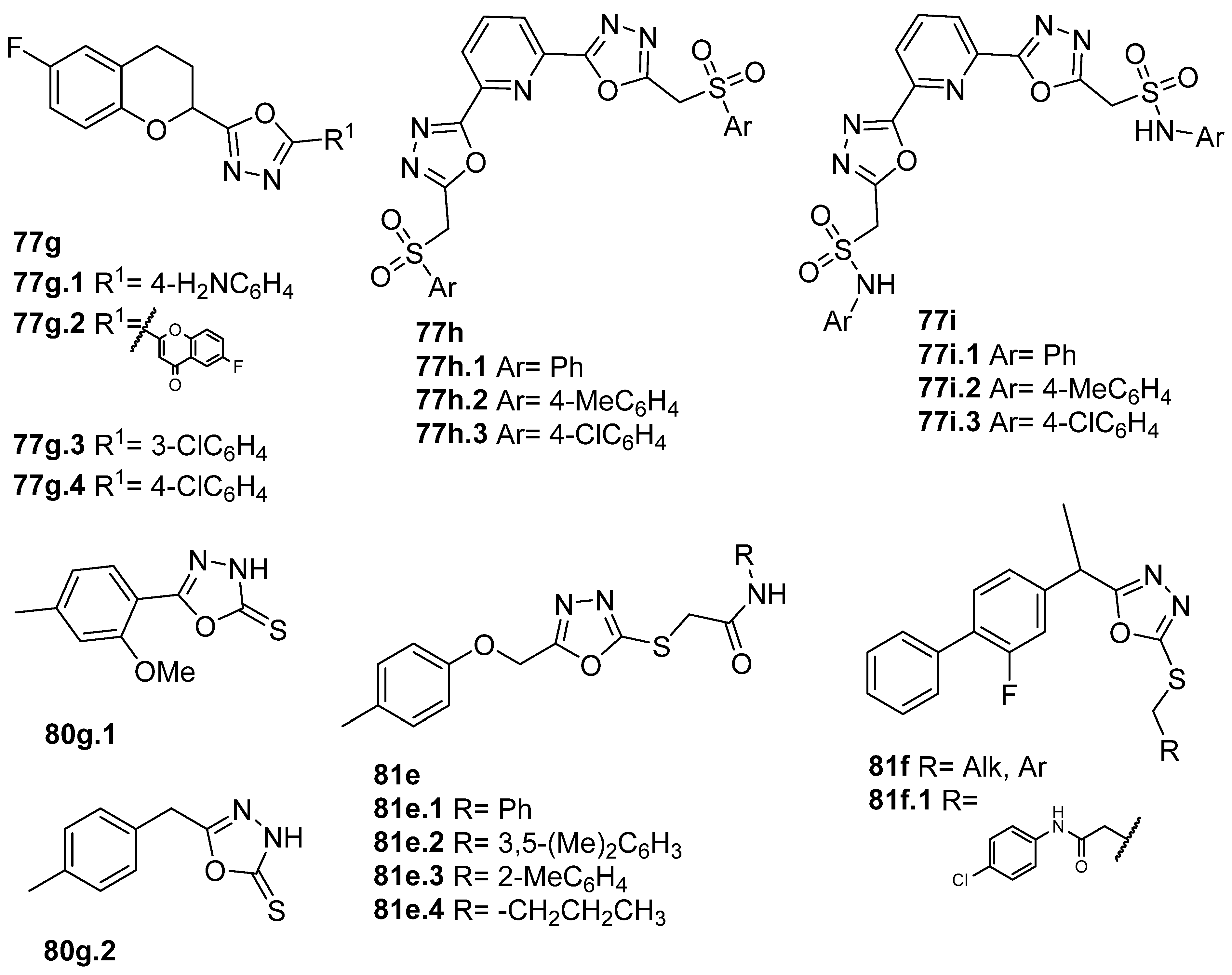
New derivatives of novel 2,5-disubstituted 1,3,4-oxadiazole (Figure 28) were also synthesized as potential anti-inflammatory and antioxidant agents. Kashid et al. [59] reported compounds 77g (Figure 28) with great anti-inflammatory and antioxidant activities, of which compounds 77g.1, 77g.2, and 77g.3 showed better anti-inflammatory activities with IC50 = 45.69, 58.54, and 56.70 µM, respectively, compared to the standard drug diclofenac sodium that presents an IC50 = 90.21 µM. According to the DPPH assay, compound 77g.4 exhibited good antioxidant activity with IC50 = 17.15 µM, which was better than the reference antioxidant ascorbic acid (IC50 = 44.18 µM). Also, a molecular docking study showed that these compounds can recognize the active site and accomplish significant bonded and non-bonded interactions with main residues in the anti-inflammatory target cyclooxygenase-2 (COX-2) [59]. Gunthanakkala et al. [105] reported compounds 77h and 77i (Figure 28) as potential antioxidants with IC50 values between 32.95 and 121.12, 29.90 and 117.73, and 31.34 and 106.42 µg/mL, for DPPH, NO, and H2O2 assays, respectively. Among them, compound 77h.2 stood out with IC50 values = 32.95, 31.64, and 32.42 μg/mL, and compound 77i.2 with IC50 values = 32.01, 29.90, and 31.34 µg/mL for DPPH, NO, and H2O2 assays, respectively.
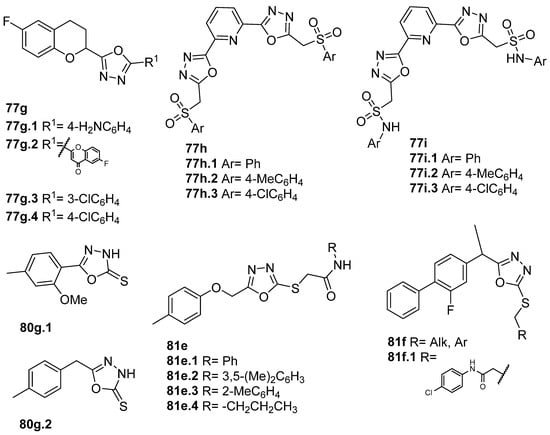
Figure 28.
1,3,4-oxadiazole derivatives as potential anti-inflammatory and antioxidant agents.
Some oxadiazole-2-thiol derivatives 80g (Figure 28) also presented anti-inflammatory activity as inhibitors of COX or lipoxygenase (LOX) enzymes [41,85]. Munir et al. [41] identified derivatives 80g with good in vitro cyclooxygenase inhibition activity, with IC50 values ranging from 31.5 to 39.5 µM for COX-2 and from 43.91 to 27.55 µM for COX-1. On the other hand, Bashir et al. [85] identified oxadiazoles 81e.1–4, which showed good LOX inhibitory activities with IC50 values of 21.5, 29.1, 31.3, and 24.3 µM, respectively.
Rana et al. [61] reported new derivatives 81f (Figure 28) incorporating the flurbiprofen moiety. Compound 81f.1 showed the highest anti-inflammatory activity of the series, displaying 74.16% activity at 200 µg/mL, which is slightly lower than standard ibuprofen (84.31% activity). The same compound also showed antioxidant activity in the DPPH assay, with an IC50 = 25.35 µg/mL, while for ascorbic acid, the IC50 value was 6.13 µg/mL.
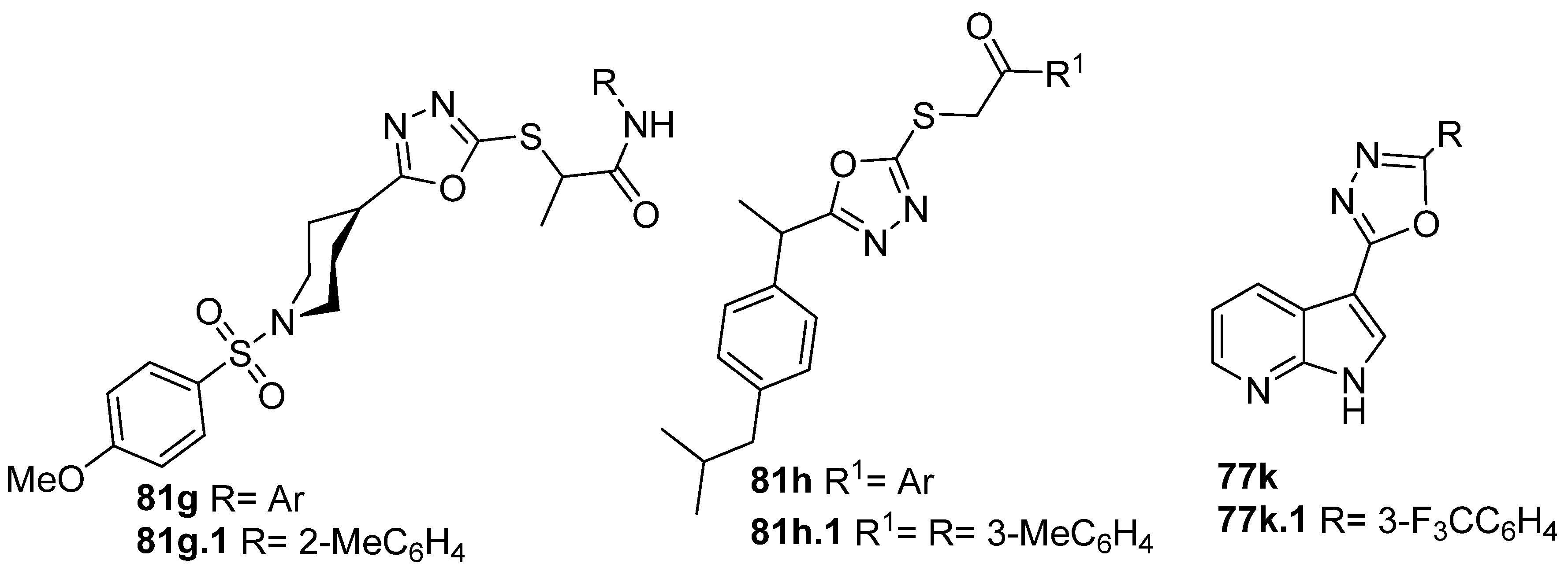
Furthermore, both derivatives 81g and 81h (Figure 29) showed good inhibition against α-glucosidase. Compound 81g.1 had the inhibition potential of 72.13% at 500 µM, which was higher than that of the standard drug acarbose (65.73% at 500 µM) [87]. Additionally, Daud et al. [62] identified compound 81h.1 with IC50 = 56.01 µM as more active than acarbose, the standard drug, which presents an IC50 = 375.82 µM, in the same assay. Compound 77k.1 also showed good α-glucosidase inhibition activity with an IC50 = 460 µM [156].
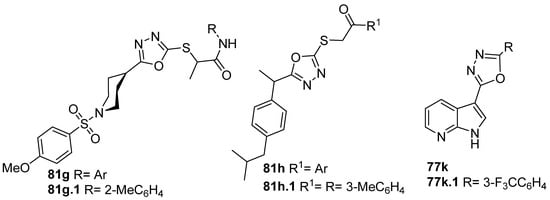
Figure 29.
Oxadiazole derivatives as α-glucosidase inhibitors.
3.2.4. Thiazoles and Thiadiazoles
Synthesis of Thiazole and Thiadiazole Derivatives
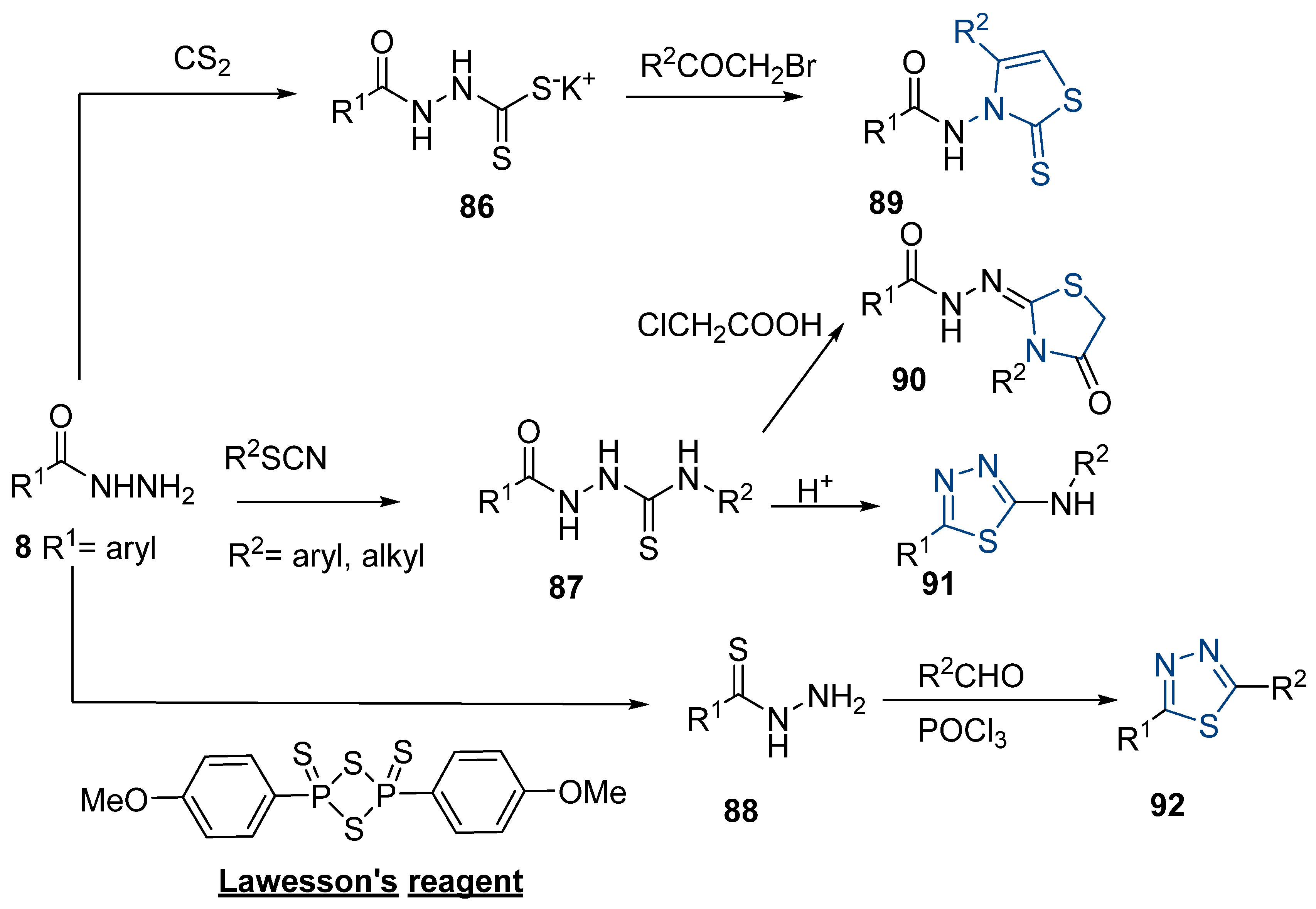
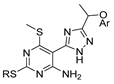
Thiazole and thiadiazole are five-membered N,S- and N,N,S-heterocycles with important biological applications that foster the search for new derivatives. Recently, Babalola et al. [157] and Ahmad et al. [158] collected and discussed recent synthetic methodologies and the biological activity of thiadiazoles. Babalola et al. [157] discussed the synthesis of thiadiazoles over the last 10 years using heterogeneous catalysts, microwave-assisted synthesis, ultrasound-aided techniques, solvent-free synthesis, or complex catalyzed reactions; Ahmad et al. [158] discussed the synthesis of thiadiazoles, since 2008, from hydrazides, thiosemicarbazide, acylhydrazines, thioacylhydrazone, dithiocarbazates, and isothiocyanate. Scheme 20 presents the general approaches to obtain these heterocycles from hydrazides 8, and Table 7 describes the reaction conditions to obtain the different derivatives from the reactions between hydrazides and carbon disulfide, isothiocyanate reagents, or Lawesson’s reagent.
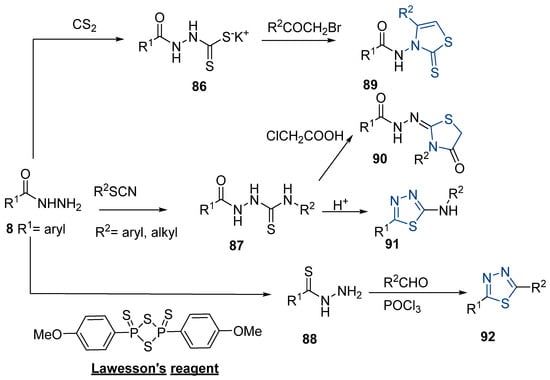
Scheme 20.
General approaches to 1,3-thiazole and 1,3,4-thiadiazole synthesis from hydrazides.

Table 7.
Reaction conditions for the synthesis and purification of 1,3-thiazole and 1,3,4-thiadiazole derivatives from hydrazides.
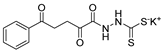
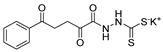
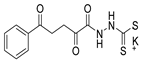
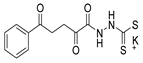
Tolan et al. [64] reported the synthesis of compounds 89 in a two-step approach (Scheme 20). The reaction of hydrazide 8 with carbon disulfide produced intermediary 86, which reacted with an acyl bromide reagent in ethanol and was refluxed to generate the thiazole ring of derivative 89. Abumelha et al. [44] synthesized thiazole derivatives 90 (Scheme 20). The synthetic approach involved the conversion of hydrazide 8 into intermediate 87, by reaction of 8 with isothiocyanate, under heating. The reaction of intermediate 87 with chloroacetic acid promoted the formation of thiazole ring 90. Moreover, intermediary 87 was converted to 1,3,4-thiadiazoles 91 by treatment with sulfuric acid under reflux [94,159].
Hydrazides 8, in the presence of Lawesson’s reagent, generated the corresponding thio-derivatives 88, which generated 1,3,4-thiadiazole derivatives 92 in the presence of phosphoryl chloride and an aldehyde, under heating (Scheme 20) [93].
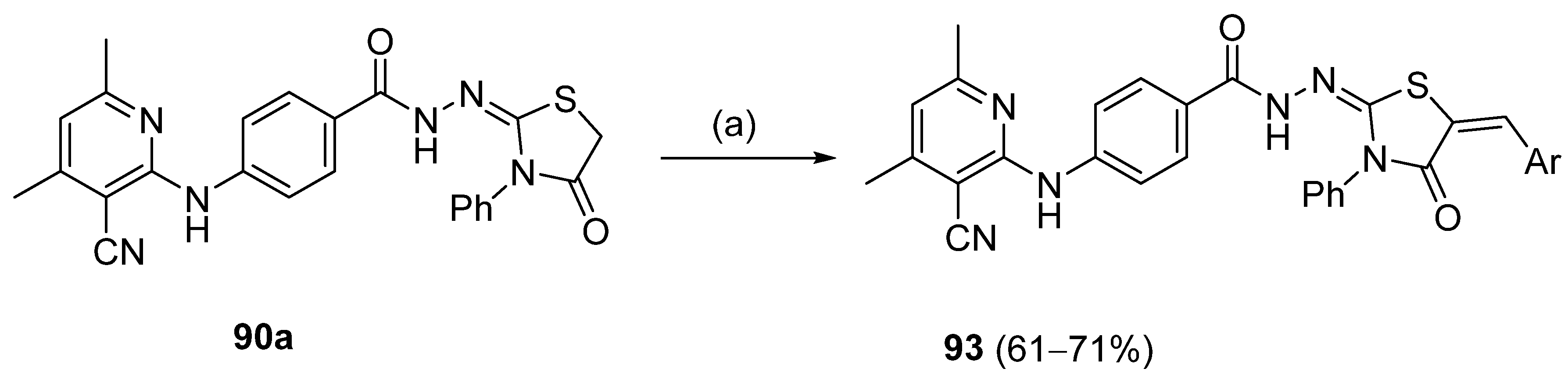
Abumelha et al. [44] synthesized thiazole derivatives 93 as precursors of antioxidant agents. Compound 90a was converted to the hybrids 93 by reaction with aldehydes under reflux, in an acidic medium. Products 93 were obtained in good to moderate yields (Scheme 21).
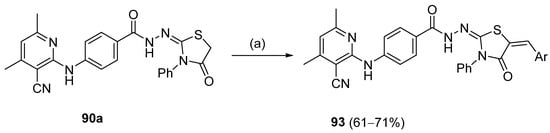
Scheme 21.
Synthesis of thiazole derivative 93: (a) ArCHO, CH3COOH/AcONa, reflux, 4 h.
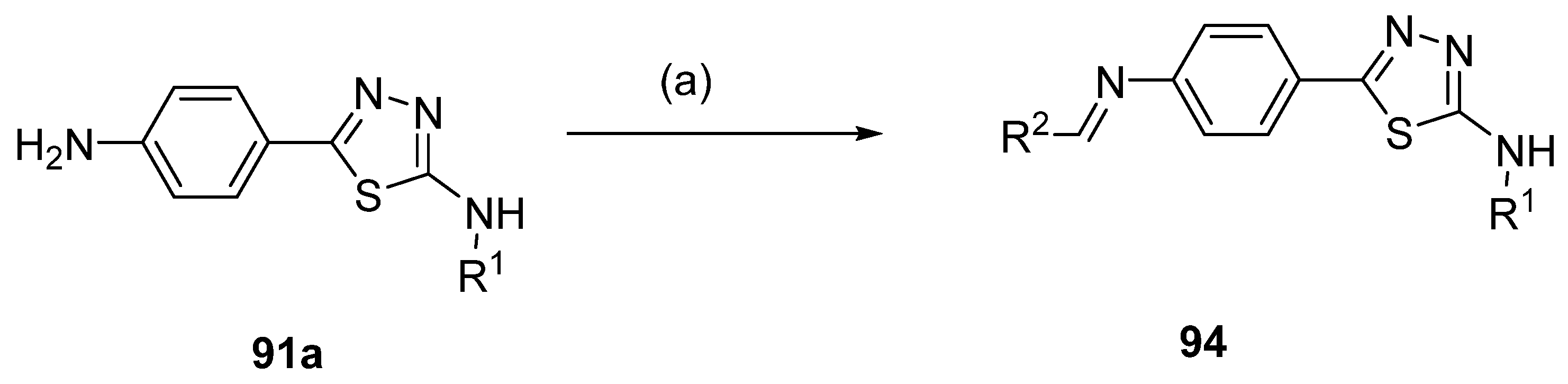
Compounds 94 (Scheme 22) were yielded from thiadiazole derivative 91a and aldehydes under reflux conditions, in methanol [94].
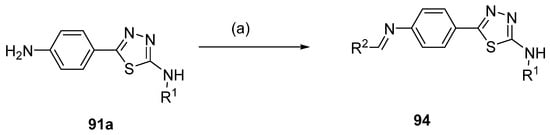
Scheme 22.
Synthesis of thiadiazole derivative 94: (a) R2CHO, MeOH, reflux.
Biological Activity of Thiazole and Thiadiazole Derivatives
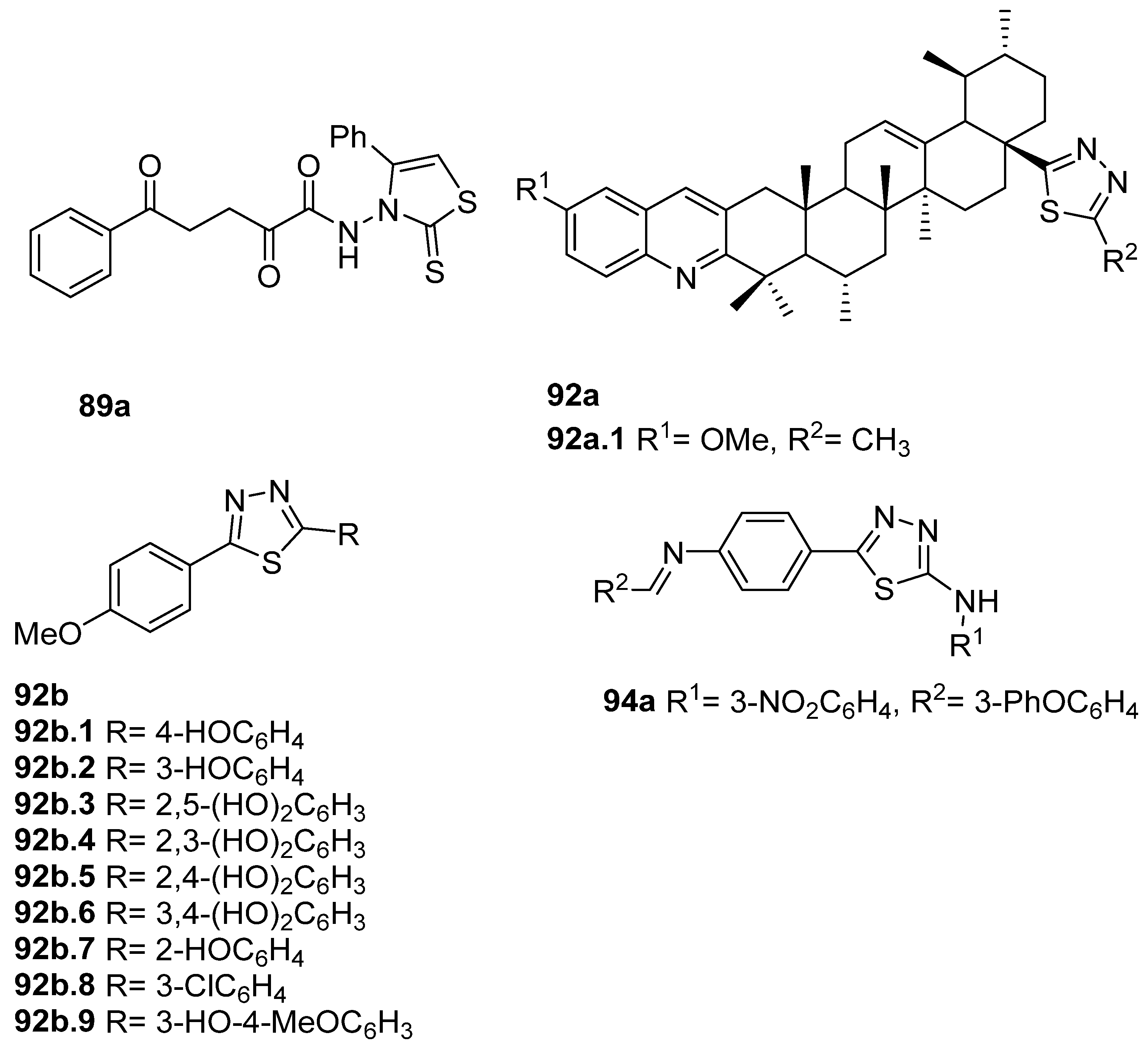
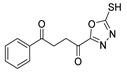
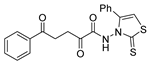
Compound 89a (Figure 30) was evaluated for anticancer activity and showed good antiproliferative activities against MCF-7 breast cancer cells and against hepatocellular HepG2 cells with IC50 = 8.0 and 28.2 µM, respectively. These IC50 values are better or similar to those of doxorubicin, which has IC50 = 10.3 and 28.5 µM, for the same cell lines [64].

Figure 30.
Thiazole and 1,3,4-thiadiazole derivatives with anticancer and antimycobacterial activity.
Compounds with structure 92a (Figure 30) were screened against cancer cell lines MDA-MB-231 and HeLa. Compound 92a.1 (Figure 30) exhibited good anticancer activity with IC50 = 15.75 and 12.82 µM against cancer cell lines MDA-MB-231 and HeLa, respectively, although it had a lower activity than the positive control etoposide [108]. Taha et al. [93] reported the 2,5-disubstituted thiadiazoles 92b (Figure 30) as potent β-glucuronidase inhibitors presenting IC50 values between 6.74 and 52.36 µM, revealing higher or equivalent activity to the standard D-saccharic acid-1,4-lactone (IC50 = 48.4 µM). Among these, compound 92b.5 was the most potent, with IC50 = 6.74 μM.
On the other hand, thiazoles 94 (Figure 30) were screened against Mycobacterium tuberculosis H37Rv, and compound 94a was the most potent with an inhibitory activity of 80% at 6.25 µg/mL [94].
3.2.5. Triazoles
Synthesis of Triazole Derivatives
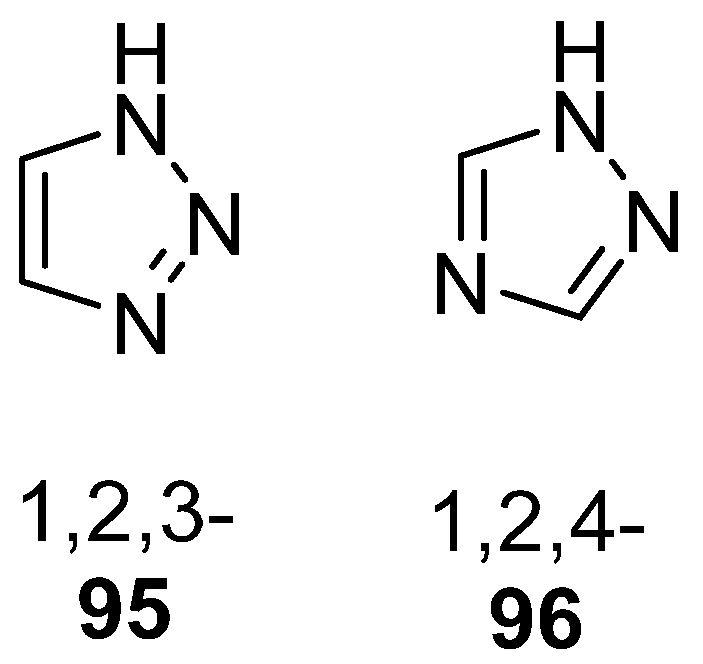
Triazole, also known as pyrrodiazole, is a five-membered heterocyclic ring system containing three nitrogen atoms, existing in two isomeric forms, 1,2,3- 95 or 1,2,4-triazoles 96 (Figure 31) [160]. Both isomers present a wide range of pharmacological activities.
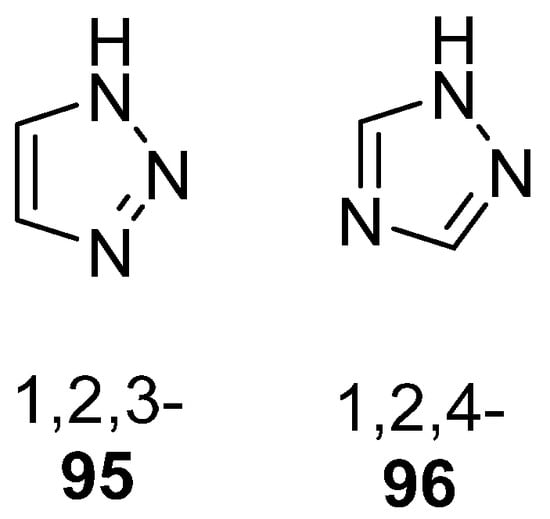
Figure 31.
Isomeric forms of the triazole ring.
In a recent review, Hassani et al. [161] reported the advances in the synthesis of triazole derivatives. The authors presented multiple methods to obtain 1,2,3- or 1,2,4-triazoles, including metal-free and metal-catalyzed reactions. Among them are the cycloaddition of azides and terminal alkynes; the reaction between two nitriles and hydroxylamine hydrochloride; the reaction of formamide reagents and hydrazide; the reaction of acylhydrazines with carbon disulfide, followed by the reaction with hydrazine monohydrate; and others [161]. Ren et al. [162] recently reported a different approach for the synthesis of 1,2,3-triazoles, involving an iodine-mediated condensation–cyclization reaction from α-azido acetophenones and p-toluenesulfonyl hydrazide. Moreover, Clark et al. [163] developed the synthesis of substituted 1,2,3-triazoles from α-ketoacetals, tosyl hydrazide, and a primary amine. On the other hand, Patterson et al. [164] presented the synthesis of 1,2,3-triazoles from tosylhydrazide, aldehydes, and a primary amine, as an alternative to azides.
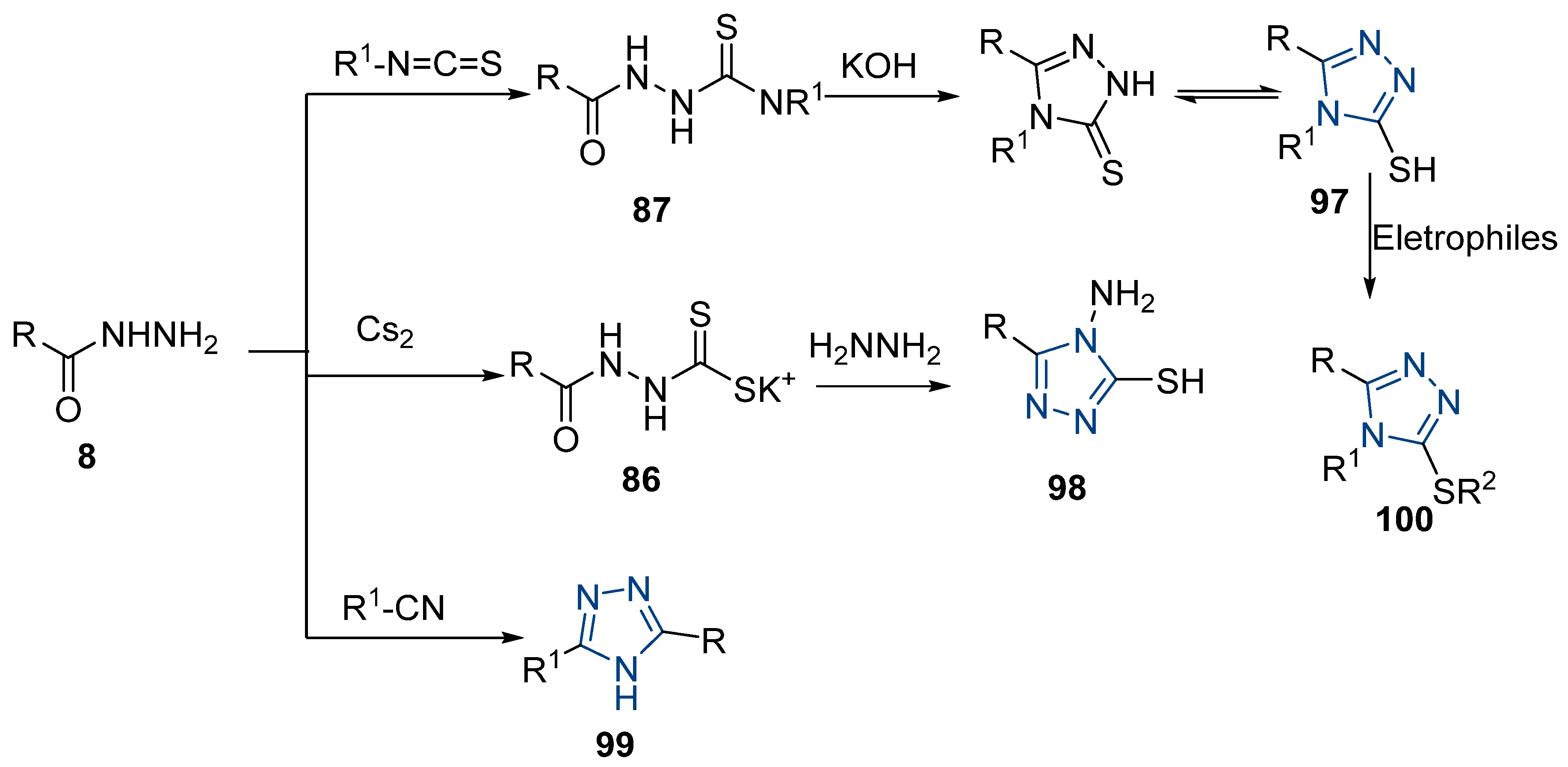
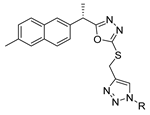
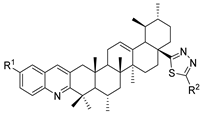
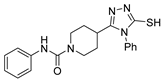
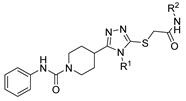
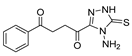
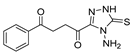
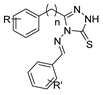
Here, in this review, we present the synthesis of 1,2,4-triazole (Table 8), in which hydrazides are often combined with thiocyanate or isothiocyanates, carbon disulfide, or nitrile derivatives (Scheme 23). Hydrazides 8, in the presence of isothiocyanates and under reflux conditions, generate the intermediates 87 (in a neutral or acidic medium), which cyclize in a basic medium under reflux, to give 97 [35,42,80,88,89,91,165]. Several derivatives of 100 were obtained by the condensation of 97 with electrophiles [42,88,89,90,91]. The reaction with carbon disulfide took place in a basic medium with reflux, followed by cyclization with hydrazine hydrate to obtain compounds 98 [41]. Reflux or high temperatures are also used when nitrile derivatives are used as reagents to obtain compounds 99 [166,167]. The products 97, 98, and 99 were typically isolated in good to excellent yields. The experimental conditions for the synthesis of 1,2,4-triazole-3-thione derivatives are presented in Table 8. The 1,2,4-triazole-3-thione compounds were sometimes just intermediates to obtain the compounds 100 or others with potential biological activity [110].

Table 8.
Reaction conditions for the synthesis and purification of 1,2,4-triazole derivatives from hydrazides and their derivatives.
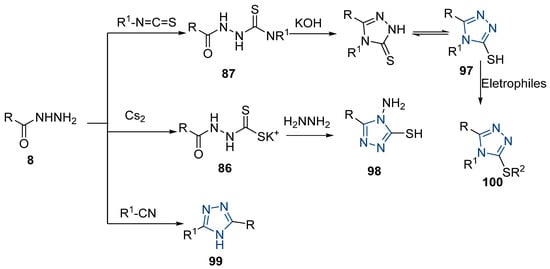
Scheme 23.
Representative scheme of 1,2,4-triazoles from hydrazides.
Biological Activity of Triazole Derivatives
According to the literature [35,41,42,64,80,88,89,90,91], 1,2,4-triazole derivatives showed enzymatic inhibition (α-glucosidase, 15-lipooxigenase, acetylcholinesterase, and butyrylcholinesterase enzymes), as well as anticancer or anti-inflammatory activity.
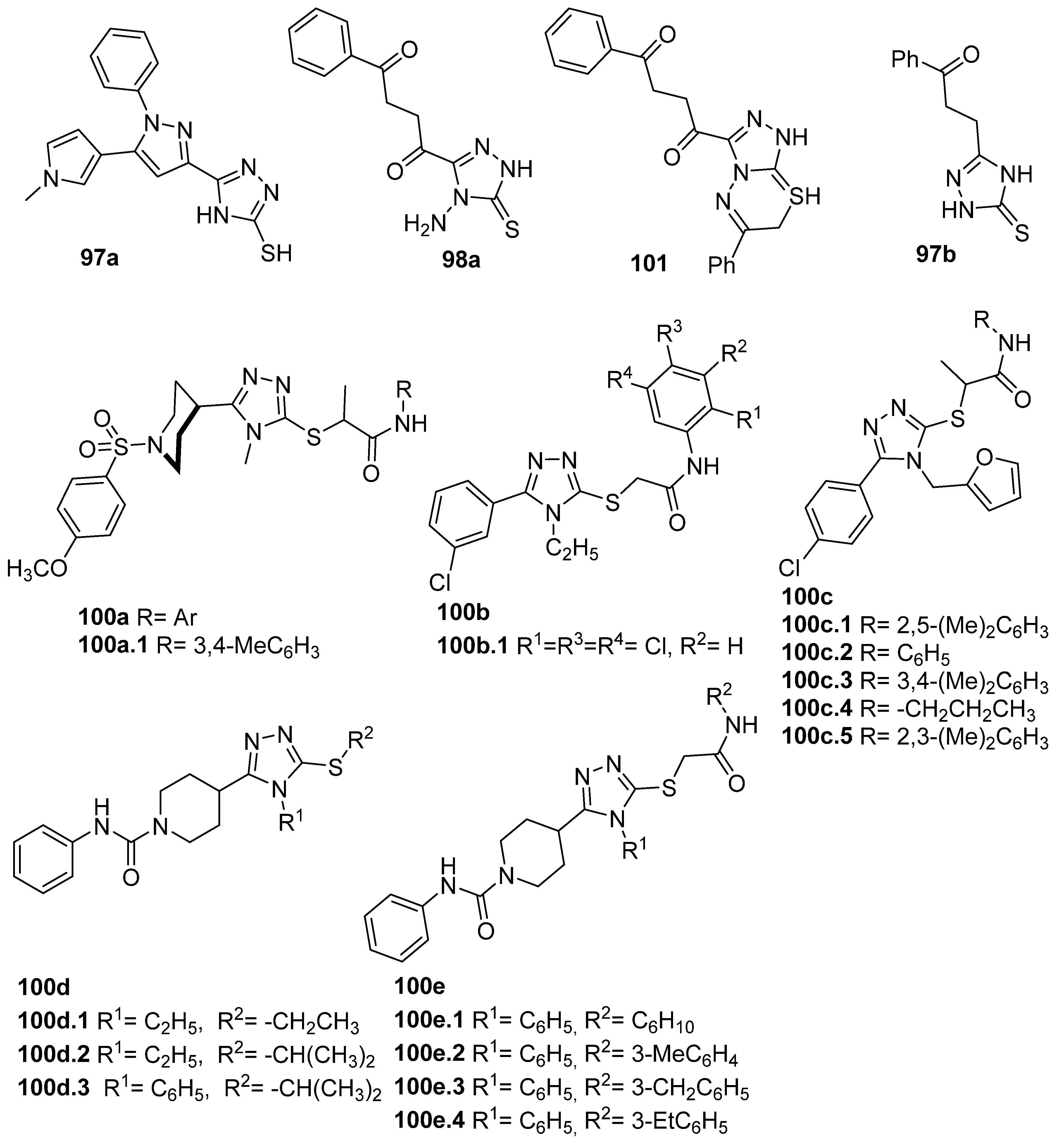
Derivative 97a (Figure 32) was tested against the human colon carcinoma cancer cell line HCT-116 and showed moderate cytotoxic effects with IC50 = 12.05 µg/mL [35]. Both compounds 98a and 101 (Figure 32) presented antiproliferative activities against MCF-7 breast cancer cells with IC50 values of 8.2 and 9.2 µM, respectively, which were lower than the IC50 of the reference drug doxorubicin (10.3 µM). They were also active against hepatocellular HepG2 cells with IC50 values of 33.7 and 30.8 µM, which were quite similar compared to doxorubicin (28.5 µM) [64]. Moreover, Abu-Hashem et al. [80] evaluated compound 97b against human gastric carcinoma (MGC-803), nasopharyngeal carcinoma (CNE2), oral carcinoma (KB), and breast adenocarcinoma (MCF-7) cell lines. The compound showed IC50 values in the range of 12.8 to 14.2 µM.

Figure 32.
1,2,4-triazole derivatives with anticancer and enzymatic (α-glucosidase, acetylcholinesterase, butyrylcholinesterase, and 15-lipoxygenase) inhibition activity.
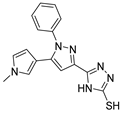
Virk et al. [42] and Riaz et al. [88] evaluated the biological potential of compounds 100a (Figure 32) against AChE. Derivative 100a.1 showed good inhibition against α-glucosidase (IC50 = 27.52 mM) compared to acarbose (IC50 = 375.82 mM) and lower inhibition against AChE (IC50 = 407.24 mM) in comparison with the standard drug eserine (IC50 = 0.19 mM) [42]. On the other hand, compound 100b.1 showed activity against AChE and BChE with IC50 values of 5.41 and 7.52 µM, respectively [88]. Compounds 100c–e (Figure 32) were tested as potential lipoxygenase inhibitors. Among these [89], compounds 100c.1–5 demonstrated good activity as inhibitors of 15-lipoxygenase with IC50 values of 17.43, 19.35, 23.59, 26.35, and 27.53 µM, respectively. The 1,2,4-triazole thioethers 100d.1–3 also showed very good inhibitory profiles against the same enzyme, with IC50 values ranging from 12.52 to 35.64 µM [90].
Muzaffar et al. [91] reported on derivatives 100e.1–4 (Figure 32), which displayed inhibitory potential against the 15-lipoxygenase enzyme with IC50 values between 9.25 and 21.82 µM.
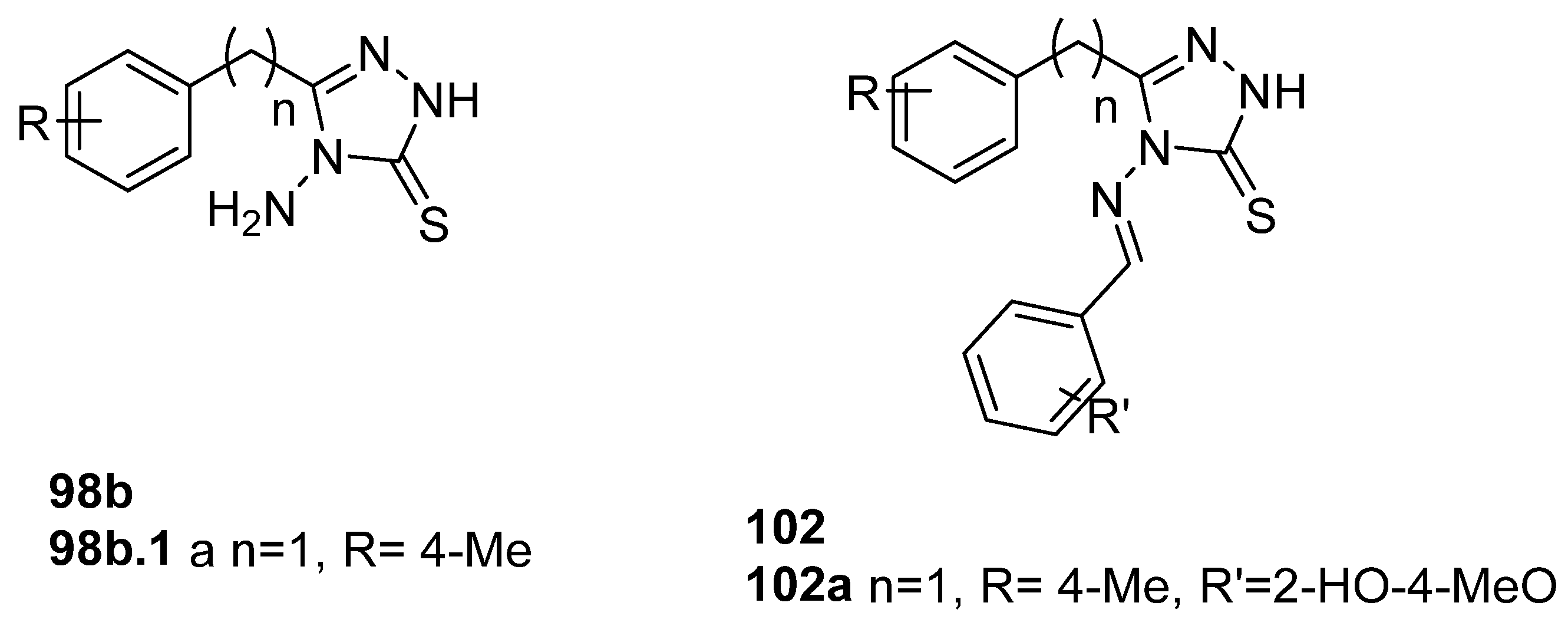
Munir et al. [41] obtained 1,2,4-triazole derivatives 98b and 102 (Figure 33), and compounds 98b.1 and 102a displayed excellent and good activity for the COX-2 isozyme with IC50 values of 1.76 and 23.47 µM, respectively. Other compounds 102 of this series showed COX-2 inhibition in the range of 12.56–26.58 µM. In vivo anti-inflammatory studies, by using the carrageenan-induced paw edema test, showed that after 5 h, the maximum percentage inhibition was 29.4% for compound 98b.1 and 17.6% for compound 102a [41].
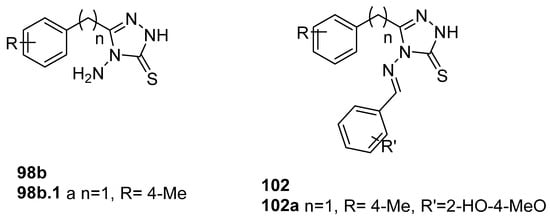
Figure 33.
1,2,4-triazole derivatives with anti-inflammatory activity.
4. Miscellany
In the literature, hydrazides are also mentioned as reagents to generate triazine or triazepine rings [80], coating agents of nanoparticles [168], or even ligands for complexes [169,170].
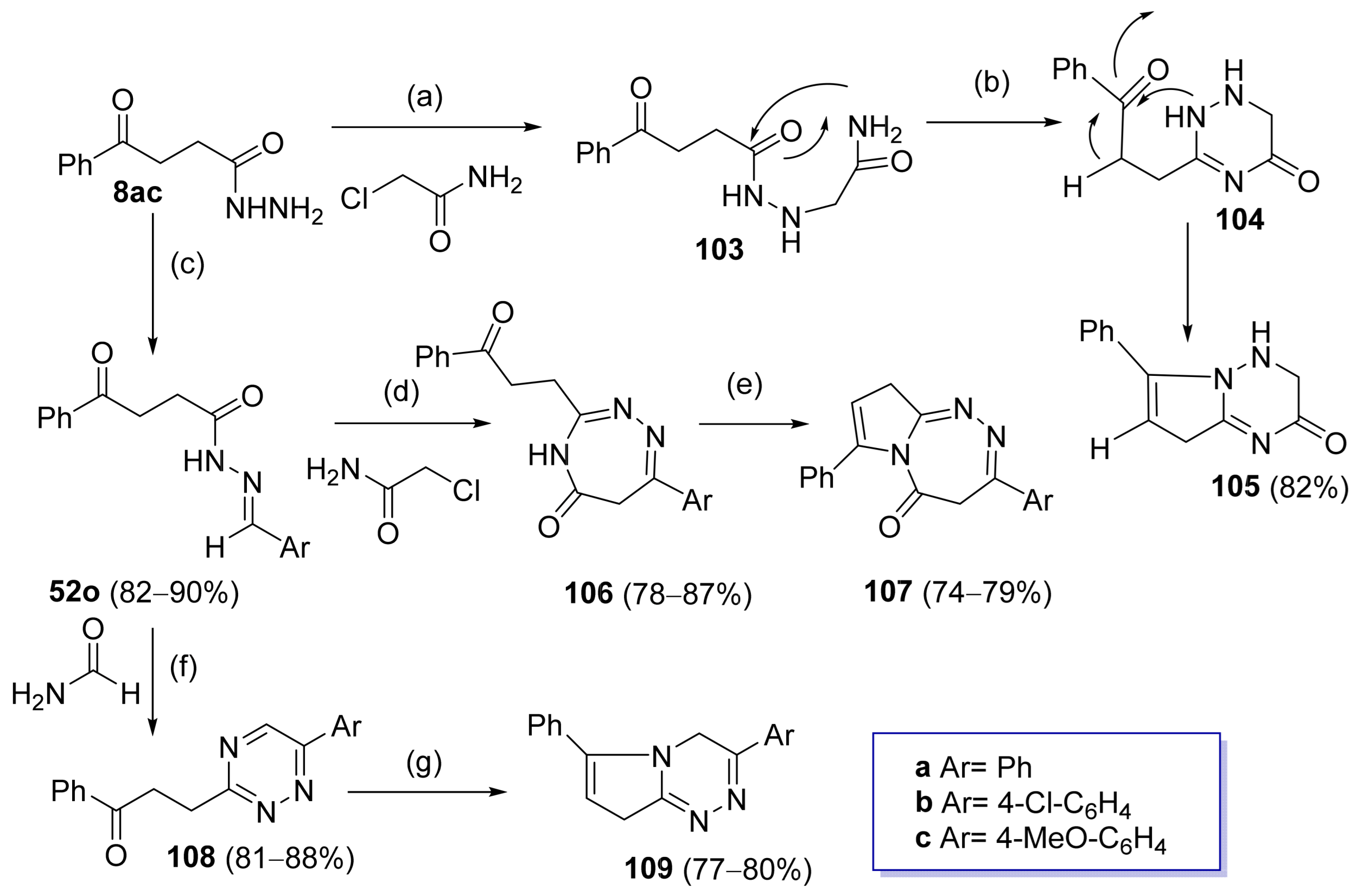
Abu-Hashem et al. [80] synthesized triazine or triazepine derivatives from hydrazide 8ac (Scheme 24). Intermediate 103 was synthesized from hydrazide 8ac and chloroacetamide under reflux. It was converted to 104 by reflux under basic medium. The latter further suffered an intramolecular cyclization, generating pyrrolotriazinones 105. This hydrazide was also the starting material of hydrazide–hydrazone derivatives 52o, which were used to obtain new compounds such as 1,2,4-triazepinones 106, pyrrolotriazepinones 107, 1,2,4-triazines 108, and pyrrolotriazines 109. Derivatives 106 were obtained from intermediate 52o in the presence of chloroacetamide, which can undergo a posterior cyclization in the presence of a base and reflux to form compound 107. Compound 52o in the presence of formamide and reflux generated 1,2,4-triazines 108, which under reflux led to the formation of pyrrolotriazines 109. Compounds 107a–c, 106a–c, 109a–c, and 105 displayed activities against human gastric carcinoma (MGC-803), nasopharyngeal carcinoma (CNE2), oral carcinoma (KB), and breast adenocarcinoma (MCF-7) lines with IC50 values ranging from 11.1 to 14.2 µM.
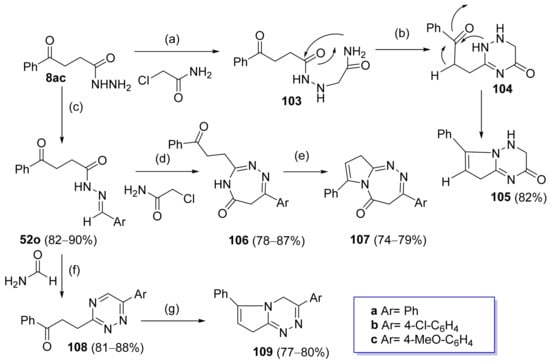
Scheme 24.
Synthesis of other heterocyclic rings from hydrazides: (a) ClCH2CONH2, DMF, reflux, 24–48 h; (b) DMF, K2CO3, reflux, 13–16 h; (c) ArCHO, EtOH, piperidine (cat.), reflux, 5–8 h; (d) H2NCOCH2Cl, DMF; (e) DMF, K2CO3, reflux, 17–20 h; (f) H2NCOH, DMF, reflux, 9–24 h; (g) DMF, K2CO3, reflux, 15–18 h.
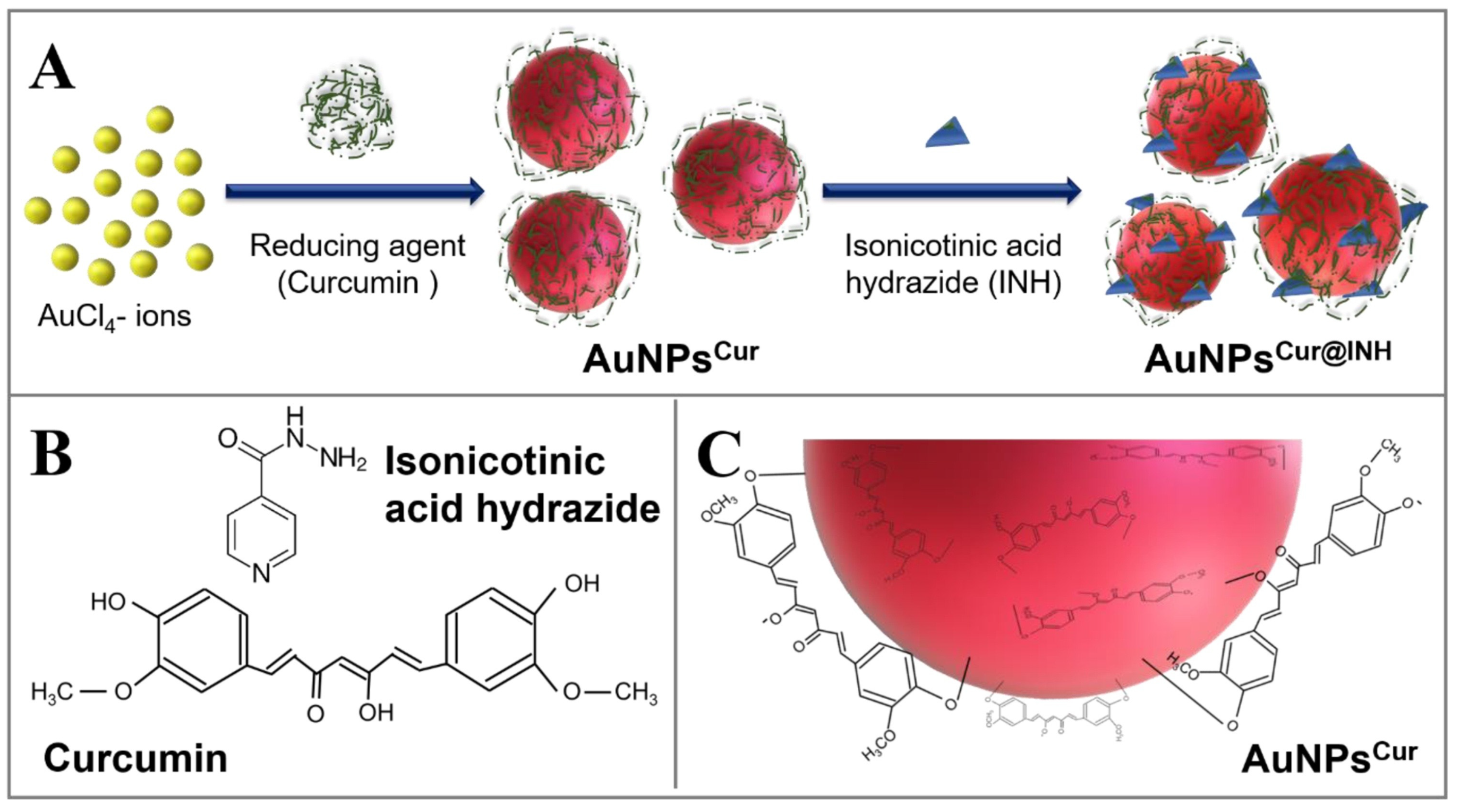
Umapathi et al. [168] prepared gold nanoparticles with a surface corona of curcumin and isonicotinic acid hydrazide for improved anticancer activity, since gold nanoparticles can carry and stabilize these molecules. The isonicotinic acid hydrazide was used due to its biological importance. The resulting nanoparticles with the isonicotinic hydrazide at a 5 ppm concentration showed good anticancer activity towards human lung squamous carcinoma (LK-2) through ROS generation (Figure 34).
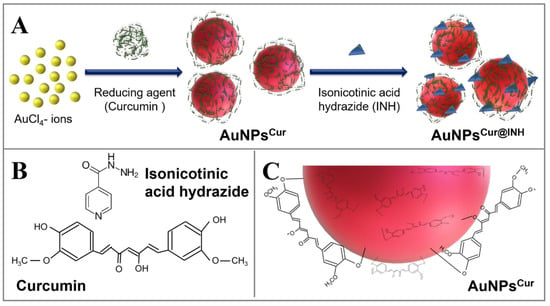
Figure 34.
Representation of the synthesis of curcumin-coated gold nanoparticles with surface modification with isonicotinic acid hydrazide. (A) Schematic representation of the formation of curcumin (Cur) coated AuNPsCur, and their sequential surface modification with isonicotinic acid hydrazide (INH) to develop AuNPsCur@INH; (B) The chemical structures of curcumin and isonicotinic acid hydrazide (INH); (C) The phenolic and enolic hydroxide groups of curcumin are involved in the reduction of AuCl4− ions, and orientation of oxidized moieties of curcumin on the surface of AuNPs. Reprinted with permission from Ref. [168] Copyright 2020 Elsevier.
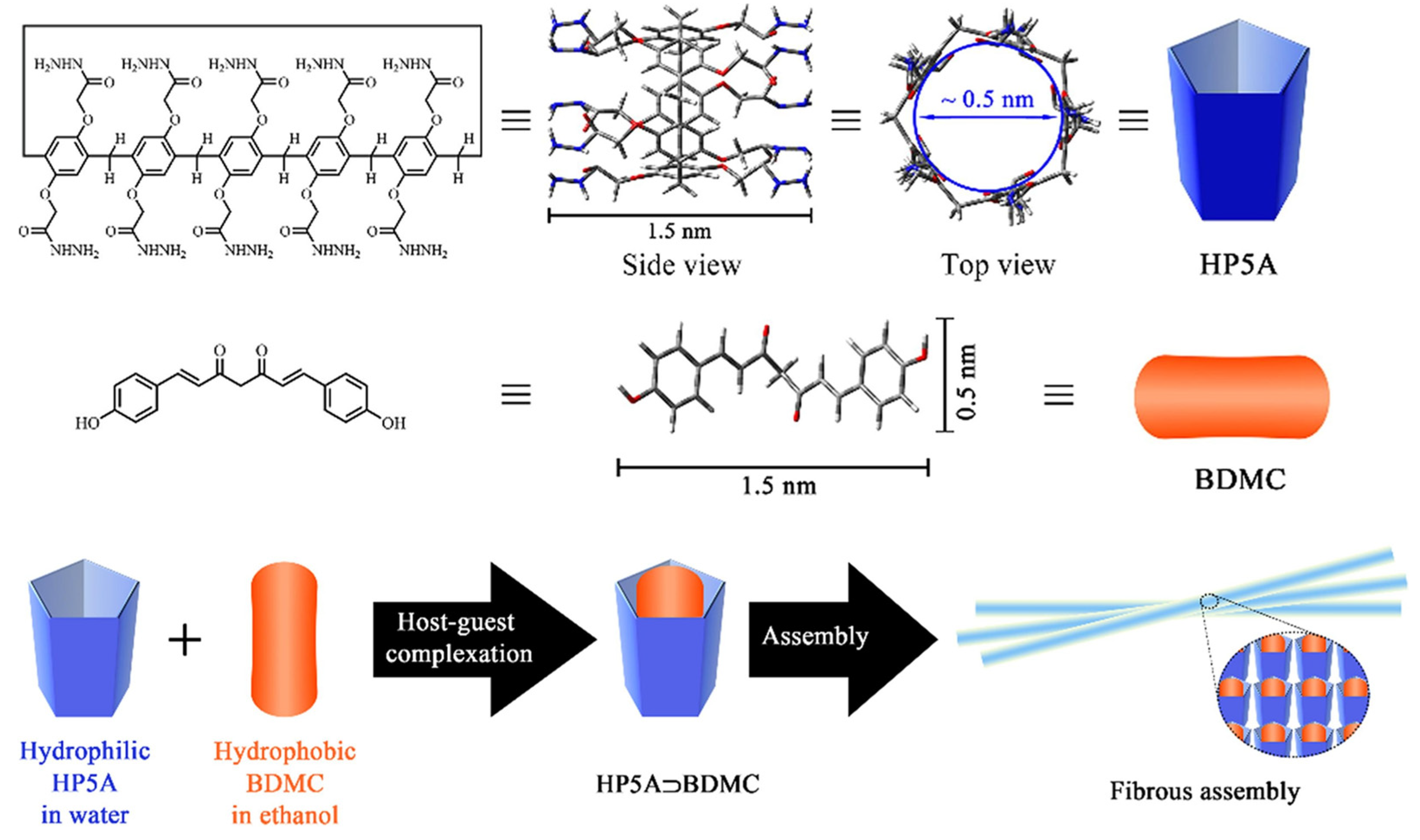
To improve the water solubility and stability of bisdemethoxycurcumin (BDMC), Guo et al. [169] obtained pillar[5]arene complexes of this compound (Figure 35). They synthesized hydrazide–pillar[5]arene (HP5A) and the complex of the two (BDMC and HP5A) self-assembled into fibers. Regarding the IC50 of free BDMC (IC50 = 50.6 µg/mL) and in complex (IC50 = 32.4 µg/mL), this complex showed greater antiproliferative activity in vitro against hepatocellular carcinoma HepG2 cells and, at the same time, reduced the undesirable side effects on normal cells.
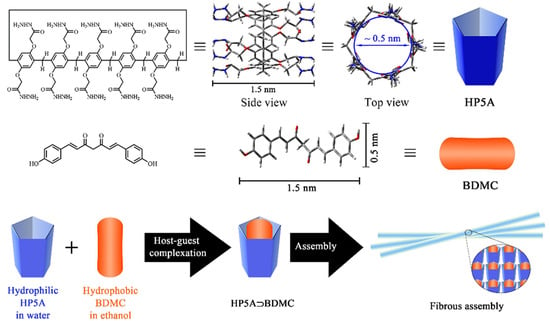
Figure 35.
Representation of hydrazide–pillar[5]arene (HP5A), the complex of these with bisdemethoxycurcumin (BDMC), and the self-assembly into fibers. Reprinted with permission from Ref. [169] Copyright 2021 Elsevier.
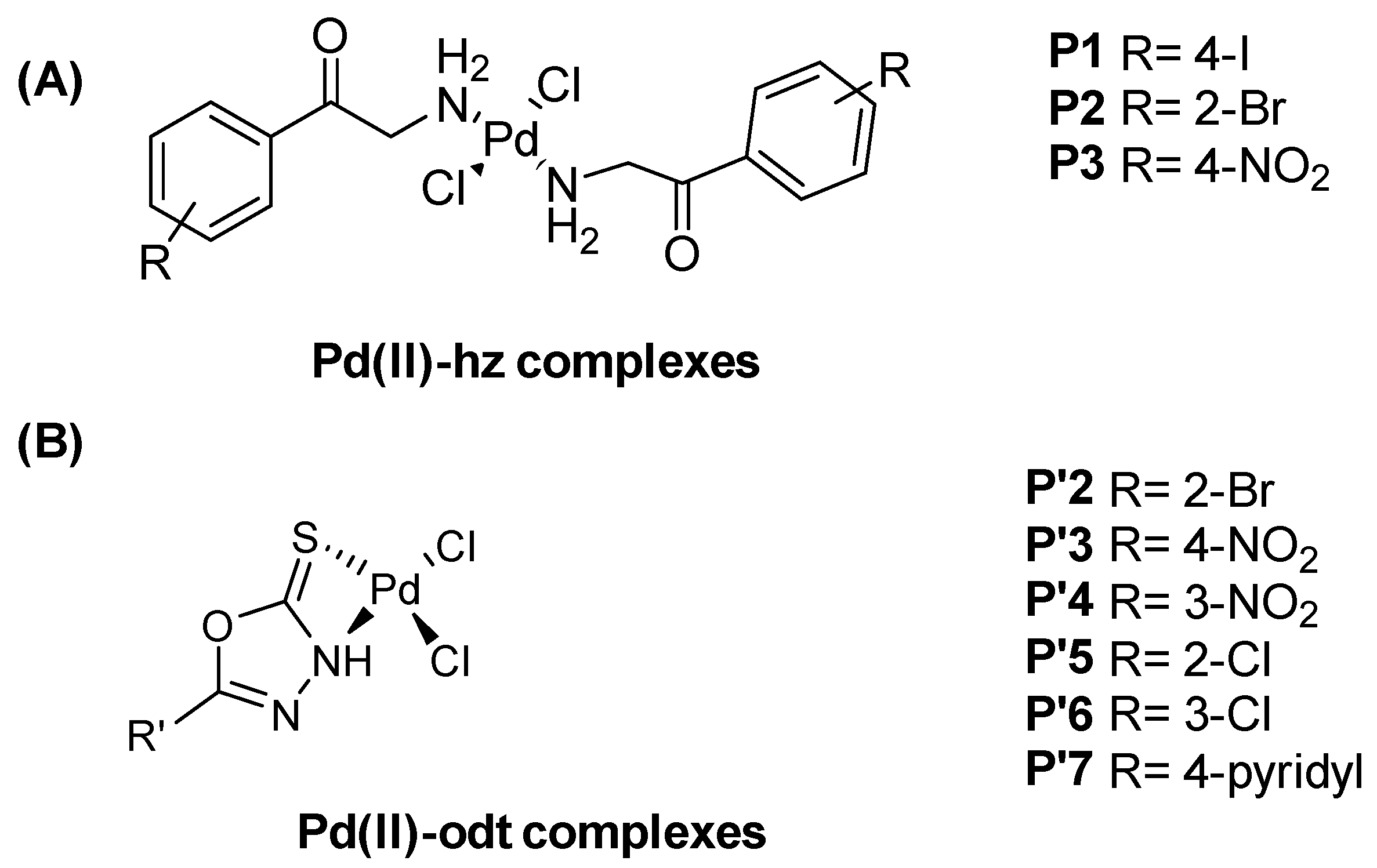
In 2022, new 1,3,4-oxadiazole (odt) derivatives and hydrazides (hz) were used as ligands for Pd(II) complexes, aiming to generate inhibitors of lipoxygenase (LOX) and butyrylcholinesterase (BChE). The Pd(II)-hz complexes (P1–P3) and six new Pd(II)-odt complexes (P′2–P′7), shown in Figure 36, were obtained via the reaction of the ligands with Pd(II) in a 1:2 metal/ligand molar ratio in acetonitrile or ethanol at room temperature [170].

Figure 36.
(A) New Pd(II)–hydrazide (Pd(II)-hz) complexes (P1–P3) and (B) new Pd(II)–oxadiazole (Pd(II)-odt) complexes (P′2–P′7).
All compounds synthesized exhibited moderate BChE inhibition with IC50 values ranging between 21.5 and 95.6 μM, which were lower than the IC50 of the reference drug eserine (IC50 = 7.3 µM). The most active complexes were P1 and P’2 with IC50 values of 21.5 and 23.4 µM, respectively [170].
5. Conclusions
Many diseases (infections, cancers, diabetes, parasitic diseases, Alzheimer’s disease, dementia, etc.) have concerned the scientific community due to the high mortality, high toxicity, and lack of effective drugs, as well as the presence of drug resistance to the available treatments. Aiming for new therapeutic solutions, the scientific community has synthesized the simplest to the most complex hydrazides to obtain new compounds with different biological activities. Most of the reported hydrazides in the last five years have been synthesized from acids or acid derivatives by reactions with hydrazine. A few new methods were also reported to synthesize hydrazides, such as the transamidation of N-substituted amides with hydrazine, the alkylation of hydrazides promoted by ruthenium catalysts, N-N cross-coupling reactions between hydroxamates and amines catalyzed by nickel complexes, nickel-catalyzed photochemical C-N coupling reactions between hetero(aryl) halides and hydrazides, photochemical reactions between azodicarboxylates and aryl or heteroaryl acylsilanes, and via hydrazine insertion into the β-position of benzoyl acrylates. The newly developed methods mostly generated substituted hydrazides, except for transamidation, which allowed the synthesis of non-substituted hydrazides in high yield. A wide variety of hydrazides have been used as synthons and precursors to hydrazide–hydrazones or heterocyclic derivatives. From hydrazides, hydrazide–hydrazone derivatives, oxadiazoles, and pyrazoles are among the most synthesized compounds. In addition, pyrrolones, thiazoles, thiadiazoles, and triazoles were also synthesized. Overall, the synthesis methods allow for obtaining these compounds in reasonable to excellent yields from hydrazides. The purification methods include silica gel column chromatography and recrystallization, with the latter being the most common. Hydrazides and their derivatives showed many biological activities, such as anticancer, antidiabetic (as alpha-glucosidase inhibitors), antibacterial, antifungal, antiparasitic, antiviral, anti-inflammatory (as COX inhibitors), and cholinesterase inhibition activity (neurodegenerative diseases), among others. This review represents a convenient tool for those aiming to explore the synthesis of new hydrazides or their derivatives to generate different scaffolds with biological activity.
Author Contributions
S.T.: writing—original draft. E.M.S.C.: supervision and writing—review and editing. M.A.C.: supervision and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Fundação para a Ciência e a Tecnologia (FCT—Portugal) in the framework of the Strategic Funding of CF-UM-UP (UIDB/04650/2020) and CQUM (UIDB/00686/2020) and by funds from FEDER/FCT through the project PTDC/MED-ONC/31354/2017 and PTDC/SAU-PAR/2766/2021. S. Teixeira acknowledges FCT and FSE (Fundo Social Europeu) through “Programa Operacional Regional Norte” for funding the PhD grant 2020.04975.BD.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data was used for the research described in the article. Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO—The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 19 May 2024).
- Verma, G.; Khan, M.F.; Mohan Nainwal, L.; Ishaq, M.; Akhter, M.; Bakht, A.; Anwer, T.; Afrin, F.; Islamuddin, M.; Husain, I.; et al. Targeting malaria and leishmaniasis: Synthesis and pharmacological evaluation of novel pyrazole-1,3,4-oxadiazole hybrids. Part II. Bioorg. Chem. 2019, 89, 102986. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed Saeed, S.; Alomari, B.A.; Al-Hakimi, A.N.; Abd El-Hady, M.M.; Alnawmasi, J.S.; Elganzory, H.H.; El-Sayed, W.A. Pyrimidine hydrazide ligand and its metal complexes: Synthesis, characterization, and antimicrobial activities. Egypt. J. Chem. 2023, 66, 315–329. [Google Scholar] [CrossRef]
- Verma, S.; Lal, S.; Narang, R.; Sudhakar, K. Quinoline Hydrazide/Hydrazone Derivatives: Recent Insights on Antibacterial Activity and Mechanism of Action. ChemMedChem 2023, 18, e202200571. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Parmanik, A.; Das, S.; Kar, B.; Bose, A.; Dwivedi, G.R.; Pandey, M.M. Current Treatment Strategies Against Multidrug-Resistant Bacteria: A Review. Curr. Microbiol. 2022, 79, 388. [Google Scholar] [CrossRef]
- Lohrasbi, V.; Talebi, M.; Bialvaei, A.Z.; Fattorini, L.; Drancourt, M.; Heidary, M.; Darban-Sarokhalil, D. Trends in the discovery of new drugs for Mycobacterium tuberculosis therapy with a glance at resistance. Tuberculosis 2018, 109, 17–27. [Google Scholar] [CrossRef]
- Phillips, M.A.; Burrows, J.N.; Manyando, C.; Van Huijsduijnen, R.H.; Van Voorhis, W.C.; Wells, T.N.C. Malaria. Nat. Rev. Dis. Prim. 2017, 3, 17050. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rhee, K.Y. Tuberculosis Drug Development: History and Evolution of the Mechanism-Based Paradig. Cold Spring Harb. Perspect. Med. 2015, 5, a021147. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2018, 54, 407–419. [Google Scholar] [CrossRef]
- Blackburn, T.; Wasley, J. Affective Disorders: Depression and Bipolar Disorders. In Comprehensive Medicinal Chemistry II; Taylor, J.B., Triggle, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 6, pp. 45–83. ISBN 9780080450445. [Google Scholar]
- Goldman, A.L.; Braman, S.S. Isoniazid: A Review with Emphasis on Adverse Effects. Chest 1972, 62, 71–77. [Google Scholar] [CrossRef]
- Fan, J.; Li, Z.; Zhao, Y.R.; Wang, H.C.; Yan, X.J.; Shi, S.H.; Liu, H.B.; Xie, C.Z.; Xu, J.Y. A self-assembled nanoprobe for detecting HSA based on hydrazide Schiff base: Its applications in diseases diagnosis and lysosome targeting imaging. Dye. Pigment. 2023, 216, 111330. [Google Scholar] [CrossRef]
- Moss, G.P.; Smith, P.A.S.; Tavernier, D. Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 1307–1375. [Google Scholar] [CrossRef]
- Paulsen, H.; Stoye, D. The chemistry of hydrazides. In The Chemistry of Amide; Zabicky, J., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1970; pp. 515–600. [Google Scholar]
- Narang, R.; Narasimhan, B.; Sharma, S. A Review on Biological Activities and Chemical Synthesis of Hydrazide Derivatives. Curr. Med. Chem. 2012, 19, 569–612. [Google Scholar] [CrossRef]
- Coates, E.O.; Meade, G.M.; Steenken, W.; Wolinsky, E.; Brinkman, G.L. The Clinical Significance of the Emergence of Drug-Resistant Organisms during the Therapy of Chronic Pulmonary Tuberculosis with Hydrazides of Isonicotinic Acid. N. Engl. J. Med. 1953, 248, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Bhilare, N.V.; Dhaneshwar, S.S.; Mahadik, K.R. Amelioration of hepatotoxicity by biocleavable aminothiol chimeras of isoniazid: Design, synthesis, kinetics and pharmacological evaluation. World J. Hepatol. 2018, 10, 496–508. [Google Scholar] [CrossRef]
- Baghini, L. Clinical study of the effects of p-aminosalicylic acid hydrazide (pasdrazide) in some forms of pulmonary and extra-pulmonary tuberculosis. Gazz. Med. Ital. 1955, 114, 112–113. [Google Scholar]
- El-Kawy, O.A.; Shweeta, H.A.; Sallam, K.M. Radiolabeling and evaluation of fonturacetam hydrazide as a radiotracer for visualization of brain function. J. Radioanal. Nucl. Chem. 2023, 332, 3273–3283. [Google Scholar] [CrossRef]
- Malykh, A.G.; Sadaie, M.R. Piracetam and Piracetam-Like Drugs. Drugs 2010, 70, 287–312. [Google Scholar] [CrossRef]
- Larsen, J.K.; Rafaelsen, O.J. Long-term treatment of depression with isocarboxazide. Acta Psychiatr. Scand. 1980, 62, 456–463. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Baldwin, D.S. Monoamine Oxidase Inhibitors (MAOIs) in Psychiatric Practice: How to Use them Safely and Effectively. CNS Drugs 2021, 35, 703–716. [Google Scholar] [CrossRef]
- Baba, Y.; Futamura, A.; Kinno, R.; Nomoto, S.; Takahashi, S.; Yasumoto, T.; Osakabe, Y.; Shoji, D.; Nabeshima, Y. The relationship between the distinct ratios of benserazide and carbidopa to levodopa and motor complications in Parkinson’s disease: A retrospective cohort study. J. Neurol. Sci. 2022, 437, 120263. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.A.I.; El-Sakka, S.S.A.; Soliman, M.H.A.; Mohamed, O.E.A. In silico, In Vitro and docking applications for some novel complexes derived from new quinoline derivatives. J. Mol. Struct. 2019, 1196, 8–32. [Google Scholar] [CrossRef]
- Ramadan, S.K.; Shaban, S.S.; Hashem, A.I. Facile and expedient synthesis and anti-proliferative activity of diversely pyrrolones bearing 1,3-diphenylpyrazole moiety. Synth. Commun. 2020, 50, 185–196. [Google Scholar] [CrossRef]
- El-Helw, E.A.E.; Morsy, A.R.I.; Hashem, A.I. Evaluation of some new heterocycles bearing 2-oxoquinolyl moiety as immunomodulator against highly pathogenic avian influenza virus (H5N8). J. Heterocycl. Chem. 2021, 58, 1003–1014. [Google Scholar] [CrossRef]
- Hassani, I.A.E.; Rouzi, K.; Assila, H.; Karrouchi, K.; Ansar, M. Recent Advances in the Synthesis of Pyrazole Derivatives: A Review. Reactions 2023, 4, 478–504. [Google Scholar] [CrossRef]
- Baashen, M.A. Synthesis of N,N′-Diacylhydrazines and their Use in Various Synthetic Transformations. Curr. Org. Chem. 2021, 25, 1394–1403. [Google Scholar] [CrossRef]
- Sharma, D.; Om, H.; Sharma, A.K. Potential Synthetic Routes and Metal-Ion Sensing Applications of 1,3,4-Oxadiazoles: An Integrative Review. Crit. Rev. Anal. Chem. 2024, 54, 416–436. [Google Scholar] [CrossRef]
- Hosseini, H.; Bayat, M. Cyanoacetohydrazides in Synthesis of Heterocyclic Compounds. Top. Curr. Chem. 2018, 376, 40. [Google Scholar] [CrossRef]
- Algohary, A.M.; Hassan, A.M.A.; Alzahrani, A.Y.; Rizk, S.A. Microwave-ultrasonic assisted synthesis, and characterization of novel 3′-(amino, hydrazino and hydrazide)-6′-bromo-spiro(isobenzofuran-1,2′-quinazoline)-3,4′-dione derivatives as antimicrobial agents. J. Heterocycl. Chem. 2023, 60, 1014–1026. [Google Scholar] [CrossRef]
- Sreenivasulu, R.; Reddy, K.T.; Sujitha, P.; Kumar, C.G.; Raju, R.R. Synthesis, antiproliferative and apoptosis induction potential activities of novel bis(indolyl)hydrazide-hydrazone derivatives. Bioorg. Med. Chem. 2019, 27, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Abdelrehim, E.S.M. Synthesis and Screening of New [1,3,4]Oxadiazole, [1,2,4]Triazole, and [1,2,4]Triazolo[4,3- b][1,2,4]triazole Derivatives as Potential Antitumor Agents on the Colon Carcinoma Cell Line (HCT-116). ACS Omega 2021, 6, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Nazreen, S.; Almalki, A.S.A.; Elhenawy, A.A.; Alsenani, N.I.; Elbehairi, S.E.I.; Malebari, A.M.; Alfaifi, M.Y.M.; Alsharif, M.A.; Alfaifi, S.Y.M. Naproxen Based 1,3,4-Oxadiazole Derivatives as EGFR Inhibitors: Design, Synthesis, Anticancer, and Computational Studies. Pharmaceuticals 2021, 14, 870. [Google Scholar] [CrossRef]
- Alsayari, A.; Muhsinah, A.B.; Asiri, Y.I.; Al-aizari, F.A.; Kheder, N.A.; Almarhoon, Z.M.; Ghabbour, H.A.; Mabkhot, Y.N. Synthesis, Characterization, and Biological Evaluation of Some Novel Pyrazolo [5,1-b]thiazole Derivatives as Potential Antimicrobial and Anticancer Agents. Molecules 2021, 26, 5383. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Tuszyńska, K.; Biernasiuk, A. Searching for novel antimicrobial agents among hydrazide-hydrazones of 4-iodosalicylic acid. Biomed. Pharmacother. 2022, 153, 113302. [Google Scholar] [CrossRef]
- Long, Z.-Q.; Yang, L.-L.; Zhang, J.-R.; Liu, S.-T.; Xie, J.; Wang, P.-Y.; Zhu, J.-J.; Shao, W.-B.; Liu, L.-W.; Yang, S. Fabrication of Versatile Pyrazole Hydrazide Derivatives Bearing a 1,3,4-Oxadiazole Core as Multipurpose Agricultural Chemicals against Plant Fungal, Oomycete, and Bacterial Diseases. J. Agric. Food Chem. 2021, 69, 8380–8393. [Google Scholar] [CrossRef]
- Lachhab, S.; El Mansouri, A.; Mehdi, A.; Dennemont, I.; Neyts, J.; Jochmans, D.; Andrei, G.; Snoeck, R.; Sanghvi, Y.S.; Ait Ali, M.; et al. Synthesis of new 3-acetyl-1,3,4-oxadiazolines combined with pyrimidines as antileishmanial and antiviral agents. Mol. Divers. 2023, 27, 2147–2159. [Google Scholar] [CrossRef]
- Munir, A.; Khushal, A.; Saeed, K.; Sadiq, A.; Ullah, R.; Ali, G.; Ashraf, Z.; Ullah Mughal, E.; Saeed Jan, M.; Rashid, U.; et al. Synthesis, in-vitro, in-vivo anti-inflammatory activities and molecular docking studies of acyl and salicylic acid hydrazide derivatives. Bioorg. Chem. 2020, 104, 104168. [Google Scholar] [CrossRef]
- Virk, N.A.; Rehman, A.U.; Abbasi, M.A.; Siddiqui, S.Z.; Iqbal, J.; Rasool, S.; Khan, S.U.; Htar, T.T.; Khalid, H.; Laulloo, S.J.; et al. Microwave-assisted synthesis of triazole derivatives conjugated with piperidine as new anti-enzymatic agents. J. Heterocycl. Chem. 2020, 57, 1387–1402. [Google Scholar] [CrossRef]
- Duong, T.-H.; Paramita Devi, A.; Tran, N.-M.-A.; Phan, H.-V.-T.; Huynh, N.-V.; Sichaem, J.; Tran, H.-D.; Alam, M.; Nguyen, T.-P.; Nguyen, H.-H.; et al. Synthesis, α-glucosidase inhibition, and molecular docking studies of novel N-substituted hydrazide derivatives of atranorin as antidiabetic agents. Bioorg. Med. Chem. Lett. 2020, 30, 127359. [Google Scholar] [CrossRef]
- Abumelha, H.M.A. Synthesis and antioxidant assay of new nicotinonitrile analogues clubbed thiazole, pyrazole and/or pyridine ring systems. J. Heterocycl. Chem. 2020, 57, 1011–1022. [Google Scholar] [CrossRef]
- Chang, J.; Liu, Y.; Zhang, T.; Chen, Z.; Fang, H.; Hua, X. A Comprehensive Investigation of Hydrazide and Its Derived Structures in the Agricultural Fungicidal Field. J. Agric. Food Chem. 2023, 71, 8297–8316. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.-A.; Yaqoob, S.; Ali, S.; Tanveer, N.; Wang, Y.; Ashraf, S.; Hasan, K.A.; Khalifa, S.A.M.; Shou, Q.; Ul-Haq, Z.; et al. Designing Functionally Substituted Pyridine-Carbohydrazides for Potent Antibacterial and Devouring Antifungal Effect on Multidrug Resistant (MDR) Strains. Molecules 2022, 28, 212. [Google Scholar] [CrossRef]
- Majumdar, P.; Pati, A.; Patra, M.; Behera, R.K.; Behera, A.K. Acid hydrazides, potent reagents for synthesis of oxygen-, nitrogen-, and/or sulfur-containing heterocyclic rings. Chem. Rev. 2014, 114, 2942–2977. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.N.; Thorat, B.R.; Gupta, D.R.; Pandey, A. Mini-Review of the Importance of Hydrazides and Their Derivatives—Synthesis and Biological Activity. Engeering Proc. 2021, 11, 21. [Google Scholar] [CrossRef]
- Smith, P.A.S. Organic Reactions; Foreign Literature Publishers: Moscow, Russia, 1951. [Google Scholar]
- Berillo, D.A.; Dyusebaeva, M.A. Synthesis of hydrazides of heterocyclic amines and their antimicrobial and spasmolytic activity. Saudi Pharm. J. 2022, 30, 1036–1043. [Google Scholar] [CrossRef]
- Khalaf, H.S.; Naglah, A.M.; Al-Omar, M.A.; Moustafa, G.O.; Awad, H.M.; Bakheit, A.H. Synthesis, Docking, Computational Studies, and Antimicrobial Evaluations of New Dipeptide Derivatives Based on Nicotinoylglycylglycine Hydrazide. Molecules 2020, 25, 3589. [Google Scholar] [CrossRef]
- Moustafa, G.; Khalaf, H.; Naglah, A.; Al-Wasidi, A.; Al-Jafshar, N.; Awad, H. Synthesis, Molecular Docking Studies, In Vitro Antimicrobial and Antifungal Activities of Novel Dipeptide Derivatives Based on N-(2-(2-Hydrazinyl-2-oxoethylamino)-2-oxoethyl)-Nicotinamide. Molecules 2018, 23, 761. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Rysz, B.; Biernasiuk, A.; Wujec, M. Synthesis of promising antimicrobial agents: Hydrazide-hydrazones of 5-nitrofuran-2-carboxylic acid. Chem. Biol. Drug Des. 2020, 95, 260–269. [Google Scholar] [CrossRef]
- Jamil, W.; Shaikh, J.; Yousuf, M.; Taha, M.; Khan, K.M.; Shah, S.A.A. Synthesis, anti-diabetic and in silico QSAR analysis of flavone hydrazide Schiff base derivatives. J. Biomol. Struct. Dyn. 2022, 40, 12723–12738. [Google Scholar] [CrossRef]
- Patil, S.; Pandey, S.; Singh, A.; Radhakrishna, M.; Basu, S. Hydrazide–Hydrazone Small Molecules as AIEgens: Illuminating Mitochondria in Cancer Cells. Chem. Eur. J. 2019, 25, 8229–8235. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.-X.; Chang, H.-Y.; Liao, Y.-S.; Yeh, M.-Y. Synthesis, photochemical isomerization and photophysical properties of hydrazide–hydrazone derivatives. New J. Chem. 2021, 45, 1651–1657. [Google Scholar] [CrossRef]
- Pham, V.H.; Phan, T.P.D.; Phan, D.C.; Vu, B.D. Synthesis and Bioactivity of Hydrazide-Hydrazones with the 1-Adamantyl-Carbonyl Moiety. Molecules 2019, 24, 4000. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, H.; Liu, P.; Hu, Q.; Wang, Y.; Liu, C.; Hu, J. A highly selective and sensitive turn-on probe for aluminum(III) based on quinoline Schiff’s base and its cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 104–110. [Google Scholar] [CrossRef]
- Kashid, B.B.; Salunkhe, P.H.; Dongare, B.B.; More, K.R.; Khedkar, V.M.; Ghanwat, A.A. Synthesis of novel of 2, 5-disubstituted 1, 3, 4- oxadiazole derivatives and their in vitro anti-inflammatory, anti-oxidant evaluation, and molecular docking study. Bioorg. Med. Chem. Lett. 2020, 30, 127136. [Google Scholar] [CrossRef]
- Padmavathi, V.; Sudhakar Reddy, G.; Padmaja, A.; Kondaiah, P. Ali-Shazia Synthesis, antimicrobial and cytotoxic activities of 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. Eur. J. Med. Chem. 2009, 44, 2106–2112. [Google Scholar] [CrossRef]
- Rana, S.M.; Islam, M.; Saeed, H.; Rafique, H.; Majid, M.; Aqeel, M.T.; Imtiaz, F.; Ashraf, Z. Synthesis, Computational Studies, Antioxidant and Anti-Inflammatory Bio-Evaluation of 2,5-Disubstituted-1,3,4-Oxadiazole Derivatives. Pharmaceuticals 2023, 16, 1045. [Google Scholar] [CrossRef]
- Daud, S.; Abid, O.-R.; Sardar, A.; Shah, B.A.; Rafiq, M.; Wadood, A.; Ghufran, M.; Rehman, W.; Zain-ul-Wahab; Iftikhar, F.; et al. Design, synthesis, in vitro evaluation, and docking studies on ibuprofen derived 1,3,4-oxadiazole derivatives as dual α-glucosidase and urease inhibitors. Med. Chem. Res. 2022, 31, 316–336. [Google Scholar] [CrossRef]
- Świątek, P.; Glomb, T.; Dobosz, A.; Gębarowski, T.; Wojtkowiak, K.; Jezierska, A.; Panek, J.J.; Świątek, M.; Strzelecka, M. Biological Evaluation and Molecular Docking Studies of Novel 1,3,4-Oxadiazole Derivatives of 4,6-Dimethyl-2-sulfanylpyridine-3-carboxamide. Int. J. Mol. Sci. 2022, 23, 549. [Google Scholar] [CrossRef]
- Tolan, H.E.M.; Fahim, A.M.; Ismael, E.H.I. Synthesis, biological activities, molecular docking, theoretical calculations of some 1,3,4-oxadiazoles, 1,2,4-triazoles, and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines derivatives. J. Mol. Struct. 2023, 1283, 135238. [Google Scholar] [CrossRef]
- Saylam, M.; Aydın Köse, F.; Pabuccuoglu, A.; Barut Celepci, D.; Aygün, M.; Pabuccuoglu, V. Design, synthesis, and biological activity studies on benzimidazole derivatives targeting myeloperoxidase. Eur. J. Med. Chem. 2023, 248, 115083. [Google Scholar] [CrossRef] [PubMed]
- Han, M.İ.; Atalay, P.; İmamoğlu, N.; Küçükgüzel, G. Synthesis, characterization and anticancer activity of novel hydrazide-hydrazones derived from ethyl paraben. J. Res. Pharm. 2020, 24, 341–349. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Patrejko, P.; Gawrońska-Grzywacz, M.; Biernasiuk, A.; Berecka-Rycerz, A.; Natorska-Chomicka, D.; Piątkowska-Chmiel, I.; Gumieniczek, A.; Dudka, J.; Wujec, M. Synthesis and in vitro bioactivity study of new hydrazide-hydrazones of 5-bromo-2-iodobenzoic acid. Biomed. Pharmacother. 2020, 130, 110526. [Google Scholar] [CrossRef] [PubMed]
- Han, M.İ.; Atalay, P.; Tunç, C.Ü.; Ünal, G.; Dayan, S.; Aydın, Ö.; Küçükgüzel, Ş.G. Design and synthesis of novel (S)-Naproxen hydrazide-hydrazones as potent VEGFR-2 inhibitors and their evaluation in vitro/in vivo breast cancer models. Bioorg. Med. Chem. 2021, 37, 116097. [Google Scholar] [CrossRef]
- Han, M.İ.; Bekçi, H.; Cumaoğlu, A.; Küçükgüzel, Ş. Synthesis and characterization of 1,2,4-triazole containing hydrazide-hydrazones derived from (S)-naproxen as anticancer agents. Marmara Pharm. J. 2018, 22, 229–239. [Google Scholar] [CrossRef]
- Rawat, B.S.; Shukla, S.K.; Gangwar, N.; Tandon, R.; Mehra, S.C. Synthesis Characterization and Anti-Inflammatory Activities of Substituted Aniline Oxadiazolyl Derivatives. Int. J. Sci. Res. Sci. Eng. Technol. 2017, 3, 290–295. [Google Scholar]
- Kumar, P.; Kadyan, K.; Duhan, M.; Sindhu, J.; Singh, V.; Saharan, B.S. Design, synthesis, conformational and molecular docking study of some novel acyl hydrazone based molecular hybrids as antimalarial and antimicrobial agents. Chem. Cent. J. 2017, 11, 115. [Google Scholar] [CrossRef]
- Abba, C.; Puram, N.; Betala, S. Synthesis of Novel Amide Functionalized Pyrido[2,3-d]pyrimidine Derivatives and their Anticancer Activity. Asian J. Chem. 2021, 33, 1579–1584. [Google Scholar] [CrossRef]
- Kassem, A.F.; Batran, R.Z.; Abbas, E.M.H.; Elseginy, S.A.; Shaheen, M.N.F.; Elmahdy, E.M. New 4-phenylcoumarin derivatives as potent 3C protease inhibitors: Design, synthesis, anti-HAV effect and molecular modeling. Eur. J. Med. Chem. 2019, 168, 447–460. [Google Scholar] [CrossRef]
- Halawa, A.H.; Hassan, A.A.E.-H.; El-Nassag, M.A.; Abd El-All, M.M.; Abd El-Jaleel, G.E.-R.; Eliwa, E.M.; Bedair, A.H. Synthesis, Reactions, Antioxidant and Anticancer Evaluation of Some Novel Coumarin Derivatives Using Ethyl 2-(2-Oxo-4-Phenyl-2H-Chromen-7-Yloxy) Acetate As a Starting Material. Eur. J. Chem. 2014, 5, 111–121. [Google Scholar] [CrossRef]
- Meshcheryakova, S.; Shumadalova, A.; Beylerli, O.; Gareev, I. Synthesis and biological activity of 2-[6-methyl-4-(thietan-3-yloxy)pyrimidin-2-ylthio]acetohydrazide derivatives. ADMET DMPK 2021, 9, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.A.S.; Abdelall, E.K.A.; El-Nahass, E.S.; Abdellatif, K.R.A.; Abdel-Rahman, H.M. Design, synthesis, biological assessment and: In silico ADME prediction of new 2-(4-(methylsulfonyl) phenyl) benzimidazoles as selective cyclooxygenase-2 inhibitors. RSC Adv. 2021, 11, 27659–27673. [Google Scholar] [CrossRef] [PubMed]
- Almehmadi, M.A.; Aljuhani, A.; Alraqa, S.Y.; Ali, I.; Rezki, N.; Aouad, M.R.; Hagar, M. Design, synthesis, DNA binding, modeling, anticancer studies and DFT calculations of Schiff bases tethering benzothiazole-1,2,3-triazole conjugates. J. Mol. Struct. 2021, 1225, 129148. [Google Scholar] [CrossRef]
- Abdelhamid, A.A.; Salah, H.A.; Marzouk, A.A. Synthesis of imidazole derivatives: Ester and hydrazide compounds with antioxidant activity using ionic liquid as an efficient catalyst. J. Heterocycl. Chem. 2020, 57, 676–685. [Google Scholar] [CrossRef]
- Cahyana, A.; Halim, D.; Amaliyah, L. Synthesis of antioxidant and antimicrobial bioactive compounds based on the quinoline-hydrazone and benzimidazole structure. J. Adv. Pharm. Technol. Res. 2023, 14, 125. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A. Synthesis of new pyrazoles, oxadiazoles, triazoles, pyrrolotriazines, and pyrrolotriazepines as potential cytotoxic agents. J. Heterocycl. Chem. 2021, 58, 805–821. [Google Scholar] [CrossRef]
- Zampieri, D.; Fortuna, S.; Romano, M.; De Logu, A.; Cabiddu, G.; Sanna, A.; Mamolo, M.G. Synthesis, Biological Evaluation and Computational Studies of New Hydrazide Derivatives Containing 1,3,4-Oxadiazole as Antitubercular Agents. Int. J. Mol. Sci. 2022, 23, 15295. [Google Scholar] [CrossRef]
- Shankara, S.D.; Isloor, A.M.; Kudva, A.K.; Raghu, S.V.; Jayaswamy, P.K.; Venugopal, P.P.; Shetty, P.; Chakraborty, D. 2,5-Bis(2,2,2-trifluoroethoxy)phenyl-tethered 1,3,4-Oxadiazoles Derivatives: Synthesis, In Silico Studies, and Biological Assessment as Potential Candidates for Anti-Cancer and Anti-Diabetic Agent. Molecules 2022, 27, 8694. [Google Scholar] [CrossRef]
- Dhonnar, S.L.; More, R.A.; Adole, V.A.; Jagdale, B.S.; Sadgir, N.V.; Chobe, S.S. Synthesis, spectral analysis, antibacterial, antifungal, antioxidant and hemolytic activity studies of some new 2,5-disubstituted-1,3,4-oxadiazoles. J. Mol. Struct. 2022, 1253, 132216. [Google Scholar] [CrossRef]
- Hamdy, R.; Elseginy, S.A.; Ziedan, N.I.; El-Sadek, M.; Lashin, E.; Jones, A.T.; Westwell, A.D. Design, Synthesis and Evaluation of New Bioactive Oxadiazole Derivatives as Anticancer Agents Targeting Bcl-2. Int. J. Mol. Sci. 2020, 21, 8980. [Google Scholar] [CrossRef]
- Bashir, B.; Riaz, N.; Abida Ejaz, S.; Saleem, M.; Ashraf, M.; Iqbal, A.; Muzaffar, S.; Ejaz, S.; Aziz-ur-Rehman; Mohammad Kashif Mahmood, H.; et al. Assessing p-tolyloxy-1,3,4-oxadiazole acetamides as lipoxygenase inhibitors assisted by in vitro and in silico studies. Bioorg. Chem. 2022, 129, 106144. [Google Scholar] [CrossRef] [PubMed]
- Mamatha, S.V.; Belagali, S.L.; Bhat, M. Synthesis, characterisation and evaluation of oxadiazole as promising anticancer agent. SN Appl. Sci. 2020, 2, 882. [Google Scholar] [CrossRef]
- Javid, J.; Aziz-ur-Rehman; Abbasi, M.A.; Siddiqui, S.Z.; Iqbal, J.; Virk, N.A.; Rasool, S.; Ali, H.A.; Ashraf, M.; Shahid, W.; et al. Comparative conventional and microwave assisted synthesis of heterocyclic oxadiazole analogues having enzymatic inhibition potential. J. Heterocycl. Chem. 2021, 58, 93–110. [Google Scholar] [CrossRef]
- Riaz, N.; Iftikhar, M.; Saleem, M.; Aziz-ur-Rehman; Hussain, S.; Rehmat, F.; Afzal, Z.; Khawar, S.; Ashraf, M.; Al-Rashida, M. New synthetic 1,2,4-triazole derivatives: Cholinesterase inhibition and molecular docking studies. Results Chem. 2020, 2, 100041. [Google Scholar] [CrossRef]
- Yasin, M.; Shahid, W.; Ashraf, M.; Saleem, M.; Muzaffar, S.; Aziz-ur-Rehman; Ejaz, S.A.; Saeed, A.; Majer, T.; Bhattarai, K.; et al. 4-Chlorophenyl-N-furfuryl-1,2,4-triazole Methylacetamides as Significant 15-Lipoxygenase Inhibitors: An Efficient Approach for Finding Lead Anti-inflammatory Compounds. ACS Omega 2022, 7, 19721–19734. [Google Scholar] [CrossRef]
- Shahid, W.; Ashraf, M.; Saleem, M.; Bashir, B.; Muzaffar, S.; Ali, M.; Kaleem, A.; Aziz-ur-Rehman; Amjad, H.; Bhattarai, K.; et al. Exploring phenylcarbamoylazinane-1,2,4-triazole thioethers as lipoxygenase inhibitors supported with in vitro, in silico and cytotoxic studies. Bioorg. Chem. 2021, 115, 105261. [Google Scholar] [CrossRef]
- Muzaffar, S.; Shahid, W.; Riaz, N.; Saleem, M.; Ashraf, M.; Aziz-ur-Rehman; Bashir, B.; Kaleem, A.; Al-Rashida, M.; Baral, B.; et al. Probing phenylcarbamoylazinane-1,2,4-triazole amides derivatives as lipoxygenase inhibitors along with cytotoxic, ADME and molecular docking studies. Bioorg. Chem. 2021, 107, 104525. [Google Scholar] [CrossRef]
- Sabry, M.A.; Ghaly, M.A.; Maarouf, A.R.; El-Subbagh, H.I. New thiazole-based derivatives as EGFR/HER2 and DHFR inhibitors: Synthesis, molecular modeling simulations and anticancer activity. Eur. J. Med. Chem. 2022, 241, 114661. [Google Scholar] [CrossRef]
- Taha, M.; Barak Almandil, N.; Rashid, U.; Ali, M.; Ibrahim, M.; Gollapalli, M.; Mosaddik, A.; Mohammed Khan, K. 2,5-Disubstituted thiadiazoles as potent β-glucuronidase inhibitors; Synthesis, in vitro and in silico studies. Bioorg. Chem. 2019, 91, 103126. [Google Scholar] [CrossRef]
- Türk, S.; Karakuş, S.; Maryam, A.; Oruç-Emre, E.E. Synthesis, characterization, antituberculosis activity and computational studies on novel schiff bases of 1,3,4-thiadiazole derivatives. J. Res. Pharm. 2020, 24, 793–800. [Google Scholar] [CrossRef]
- Han, M.İ.; İmamoğlu, N. Design, Synthesis, and Anticancer Evaluation of Novel Tetracaine Hydrazide-Hydrazones. ACS Omega 2023, 8, 9198–9211. [Google Scholar] [CrossRef] [PubMed]
- Bora, D.; Sharma, A.; John, S.E.; Shankaraiah, N. Development of hydrazide hydrazone-tethered combretastatin-oxindole derivatives as antimitotic agents. J. Mol. Struct. 2023, 1275, 134675. [Google Scholar] [CrossRef]
- Halil, Ş.; Berre, M.; Rabia Büşra, Ş.; Halil Burak, K.; Ebru, H. Synthesis of oleanolic acid hydrazide-hydrazone hybrid derivatives and investigation of their cytotoxic effects on A549 human lung cancer cells. Results Chem. 2022, 4, 100317. [Google Scholar] [CrossRef]
- Jęśkowiak, I.; Ryng, S.; Świtalska, M.; Wietrzyk, J.; Bryndal, I.; Lis, T.; Mączyński, M. The N′-Substituted Derivatives of 5-Chloro-3-Methylisothiazole-4-Carboxylic Acid Hydrazide with Antiproliferative Activity. Molecules 2020, 25, 88. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, S.; Ye, Z.; Zhu, H.; Hu, B.; Meng, P.; Hu, Y.; Zhang, H.; Wang, K.; Wang, J.; et al. Discovery of hydrazide-containing oseltamivir analogues as potent inhibitors of influenza A neuraminidase. Eur. J. Med. Chem. 2021, 221, 113567. [Google Scholar] [CrossRef]
- El-Helw, E.A.E.; Hashem, A.I. Synthesis and antitumor activity evaluation of some pyrrolone and pyridazinone heterocycles derived from 3-((2-oxo-5-(p-tolyl)furan-3(2H)-ylidene)methyl)quinolin-2(1H)-one. Synth. Commun. 2020, 50, 1046–1055. [Google Scholar] [CrossRef]
- Morsy, A.R.I.; Ramadan, S.K.; Elsafty, M.M. Synthesis and antiviral activity of some pyrrolonyl substituted heterocycles as additives to enhance inactivated Newcastle disease vaccine. Med. Chem. Res. 2020, 29, 979–988. [Google Scholar] [CrossRef]
- Hashem, A.I.; Youssef, A.S.A.; Kandeel, K.A.; Abou-Elmagd, W.S.I. Conversion of some 2(3H)-furanones bearing a pyrazolyl group into other heterocyclic systems with a study of their antiviral activity. Eur. J. Med. Chem. 2007, 42, 934–939. [Google Scholar] [CrossRef]
- Ramadan, S.K.; Abdel Haleem, D.R.; Abd-Rabboh, H.S.M.; Gad, N.M.; Abou-Elmagd, W.S.I.; Haneen, D.S.A. Synthesis, SAR studies, and insecticidal activities of certain N-heterocycles derived from 3-((2-chloroquinolin-3-yl)methylene)-5-phenylfuran-2(3 H)-one against Culex pipiens L. larvae. RSC Adv. 2022, 12, 13628–13638. [Google Scholar] [CrossRef]
- Singh, S.; Kandasamy, J. Synthesis of Acyl Hydrazides from Carboxamides and Hydrazine Hydrate Under Metal-Free Conditions at Room Temperature. Asian J. Org. Chem. 2023, 12, 10–15. [Google Scholar] [CrossRef]
- Gunthanakkala, A.K.; Mangali, M.S.; Venkatapuram, P.; Adivireddy, P. Synthesis, characterization and antioxidant activity of bis (arylsulfonylmethyl/arylaminosulfonylmethylazolyl) pyridines. J. Heterocycl. Chem. 2020, 57, 4164–4174. [Google Scholar] [CrossRef]
- Ramírez, H.; Fernandez, E.; Rodrigues, J.; Mayora, S.; Martínez, G.; Celis, C.; De Sanctis, J.B.; Mijares, M.; Charris, J. Synthesis and antimalarial and anticancer evaluation of 7-chlorquinoline-4-thiazoleacetic derivatives containing aryl hydrazide moieties. Arch. Pharm. 2021, 354, e2100002. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tian, Y.; Wang, R.; Fu, S.; Jiang, J.; Dong, J.; Qin, M.; Hou, Y.; Zhao, Y. Design, synthesis and biological evaluation of thieno[3,2-d]pyrimidine derivatives containing aroyl hydrazone or aryl hydrazide moieties for PI3K and mTOR dual inhibition. Bioorg. Chem. 2020, 104, 104197. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.Y.; Chen, H.; Li, D.D.; Li, A.L.; Wang, W.Y.; Gu, W. Design, synthesis, and anticancer evaluation of novel quinoline derivatives of ursolic acid with hydrazide, oxadiazole, and thiadiazole moieties as potent MEK inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 955–972. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, L.; Yang, S.; Li, Z.; Wang, P.Y. Synthesis, Antimicrobial Activity, and Molecular Docking of Benzoic Hydrazide or Amide Derivatives Containing a 1,2,3-Triazole Group as Potential SDH Inhibitors. Chin. J. Chem. 2021, 39, 1319–1330. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Z.C.; Chen, Y.F.; Cao, L.L.; Yan, W.; Li, S.K.; Wang, J.X.; Zhang, Z.G.; Ye, Y.H. Synthesis of 1,2,3-triazole hydrazide derivatives exhibiting anti-phytopathogenic activity. Eur. J. Med. Chem. 2017, 126, 171–182. [Google Scholar] [CrossRef]
- Joly, N.; Bettoni, L.; Gaillard, S.; Poater, A.; Renaud, J.L. Phosphine-free ruthenium complex-catalyzed synthesis of mono- Or dialkylated acyl hydrazides via the borrowing hydrogen strategy. J. Org. Chem. 2021, 86, 6813–6825. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Gunanathan, C. Direct Catalytic Symmetrical, Unsymmetrical N,N-Dialkylation and Cyclization of Acylhydrazides Using Alcohols. Org. Lett. 2020, 22, 6617–6622. [Google Scholar] [CrossRef]
- Barbor, J.P.; Nair, V.N.; Sharp, K.R.; Lohrey, T.D.; Dibrell, S.E.; Shah, T.K.; Walsh, M.J.; Reisman, S.E.; Stoltz, B.M. Development of a Nickel-Catalyzed N-N Coupling for the Synthesis of Hydrazides. J. Am. Chem. Soc. 2023, 145, 15071–15077. [Google Scholar] [CrossRef]
- Li, F.; Xiong, W.; Song, G.; Yan, Y.; Li, G.; Wang, C.; Xiao, J.; Xue, D. Light-Promoted Ni-Catalyzed Cross-Coupling of Aryl Chlorides with Hydrazides: Application to the Synthesis of Rizatriptan. Org. Lett. 2023, 25, 3287–3292. [Google Scholar] [CrossRef]
- Saleem, M.; Ratwan, A.; Yamini, P.; Yadagiri, D. Visible-Light-Induced Siloxycarbene Addition to N═N of Azodicarboxylates: Synthesis of Acyl Hydrazides from Acylsilanes. Org. Lett. 2024, 26, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Kumari, R.; Easwar, S. A Hydrazine Insertion Route to N′-Alkyl Benzohydrazides by an Unexpected Carbon-Carbon Bond Cleavage. Org. Lett. 2019, 21, 8191–8195. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, B.; Yu, S.; He, S.; Liang, Y.; Li, S.; Chen, Q.; Deng, X. New Hydrazone Derivatives of Pyrazole-4-carboxaldehydes Exhibited Anti-inflammatory Properties. Lett. Drug Des. Discov. 2020, 17, 502–511. [Google Scholar] [CrossRef]
- Nurkenov, O.A.; Fazylov, S.D.; Satpaeva, Z.B.; Seilkhanov, T.M.; Turdybekov, D.M.; Mendibayeva, A.Z.; Akhmetova, S.B.; Shulgau, Z.T.; Alkhimova, L.E.; Kulakov, I.V. Synthesis, structure and biological activity of hydrazones derived from 2- and 4-hydroxybenzoic acid hydrazides. Chem. Data Collect. 2023, 48, 101089. [Google Scholar] [CrossRef]
- Velezheva, V.; Brennan, P.; Ivanov, P.; Kornienko, A.; Lyubimov, S.; Kazarian, K.; Nikonenko, B.; Majorov, K.; Apt, A. Synthesis and antituberculosis activity of indole-pyridine derived hydrazides, hydrazide-hydrazones, and thiosemicarbazones. Bioorg. Med. Chem. Lett. 2016, 26, 978–985. [Google Scholar] [CrossRef]
- Bhavanarushi, S.; Luo, Z.; Bharath, G.; Rani, J.; Khan, I.; Xu, Y.; Liu, B.; Xie, J. F(1 H -Pyrazol-4-yl)methylene-Hydrazide derivatives: Synthesis and antimicrobial activity. J. Heterocycl. Chem. 2020, 57, 751–760. [Google Scholar] [CrossRef]
- Abbasi, I.; Nadeem, H.; Saeed, A.; Kharl, H.A.A.; Tahir, M.N.; Naseer, M.M. Isatin-hydrazide conjugates as potent α-amylase and α-glucosidase inhibitors: Synthesis, structure and in vitro evaluations. Bioorg. Chem. 2021, 116, 105385. [Google Scholar] [CrossRef]
- Güngör, S.A. Synthesis, in silico and in vitro studies of hydrazide-hydrazone imine derivatives as potential cholinesterase inhibitors. Chem. Biol. Drug Des. 2023, 102, 676–691. [Google Scholar] [CrossRef]
- Aslanhan, Ö.; Kalay, E.; Tokalı, F.S.; Can, Z.; Şahin, E. Design, synthesis, antioxidant and anticholinesterase activities of novel isonicotinic hydrazide-hydrazone derivatives. J. Mol. Struct. 2023, 1279, 135037. [Google Scholar] [CrossRef]
- El-Helw, E.A.E.; El-Badawy, A.A. Synthesis of chromenone, pyrimidinone, thiazoline, and quinolone derivatives as prospective antitumor agents. J. Heterocycl. Chem. 2020, 57, 2354–2364. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Alvarez, N.; Blacque, O.; Veiga, N.; Al-Mutairi, A.A.; El-Emam, A.A. Synthesis and Structure Insights of Two Novel Broad-Spectrum Antibacterial Candidates Based on (E)-N′-[(Heteroaryl)methylene]adamantane-1-carbohydrazides. Molecules 2020, 25, 1934. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, A.; Foscolos, A.S.; Papanastasiou, I.P.; Vlachou, M.; Siamidi, A.; Vocat, A.; Cole, S.T.; Kellici, T.F.; Mavromoustakos, T.; Tsotinis, A. Synthesis, biology, computational studies and in vitro controlled release of new isoniazid-based adamantane derivatives. Future Med. Chem. 2019, 11, 2779–2802. [Google Scholar] [CrossRef]
- Wassel, M.M.S.; Ragab, A.; Elhag Ali, G.A.M.; Mehany, A.B.M.; Ammar, Y.A. Novel adamantane-pyrazole and hydrazone hybridized: Design, synthesis, cytotoxic evaluation, SAR study and molecular docking simulation as carbonic anhydrase inhibitors. J. Mol. Struct. 2020, 1223, 128966. [Google Scholar] [CrossRef]
- Zala, M.; Vora, J.J.; Patel, H.B. Synthesis, Characterization, and Comparative Study of Some Heterocyclic Compounds Containing Isoniazid and Nicotinic Acid Hydrazide Moieties. Russ. J. Org. Chem. 2020, 56, 1795–1800. [Google Scholar] [CrossRef]
- Briffotaux, J.; Xu, Y.; Huang, W.; Hui, Z.; Wang, X.; Gicquel, B.; Liu, S. A Hydrazine–Hydrazone Adamantine Compound Shows Antimycobacterial Activity and Is a Probable Inhibitor of MmpL3. Molecules 2022, 27, 7130. [Google Scholar] [CrossRef]
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Puimège, L.; Libert, C.; Van Hauwermeiren, F. Regulation and dysregulation of tumor necrosis factor receptor-1. Cytokine Growth Factor Rev. 2014, 25, 285–300. [Google Scholar] [CrossRef]
- Liang, Z.; Huang, Y.; Wang, S.; Deng, X. Synthesis and Biological Evaluation of Some Pyrazole Derivatives, Containing (Thio) Semicarbazide, as Dual Anti-Inflammatory Antimicrobial Agents. Lett. Drug Des. Discov. 2019, 16, 1020–1030. [Google Scholar] [CrossRef]
- Du, X.; Yin, D.; Ge, Z.; Wang, X.; Li, R. Asymmetric Michael addition reactions of pyrrolones with chalcones catalyzed by vicinal primary-diamine salts. RSC Adv. 2017, 7, 24547–24550. [Google Scholar] [CrossRef]
- Ramzan, F.; Nabi, S.A.; Lone, M.S.; Bonardi, A.; Hamid, A.; Bano, S.; Sharma, K.; Shafi, S.; Samim, M.; Javed, K.; et al. Synthesis, biological evaluation and theoretical studies of (E)-1-(4-sulfamoyl-phenylethyl)-3-arylidene-5-aryl-1H-pyrrol-2(3H)-ones as human carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2023, 38, 2189126. [Google Scholar] [CrossRef]
- Abdelbaset, M.S.; Abuo-Rahma, G.E.D.A.; Abdelrahman, M.H.; Ramadan, M.; Youssif, B.G.M.; Bukhari, S.N.A.; Mohamed, M.F.A.; Abdel-Aziz, M. Novel pyrrol-2(3H)-ones and pyridazin-3(2H)-ones carrying quinoline scaffold as anti-proliferative tubulin polymerization inhibitors. Bioorg. Chem. 2018, 80, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.H.; Abuo-Rahma, G.E.D.A.A.; Abdel-Aziz, M.; Aly, O.M.; Beshr, E.A.; Gamal-Eldeen, A.M. Synthesis, cytotoxic activity, and tubulin polymerization inhibitory activity of new pyrrol-2(3H)-ones and pyridazin-3(2H)-ones. Bioorg. Chem. 2016, 66, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elmagd, W.S.I.; EL-Ziaty, A.K.; Elzahar, M.I.; Ramadan, S.K.; Hashem, A.I. Synthesis and antitumor activity evaluation of some N-heterocycles derived from pyrazolyl-substituted 2(3H)-furanone. Synth. Commun. 2016, 46, 1197–1208. [Google Scholar] [CrossRef]
- Murugesan, D.; Mital, A.; Kaiser, M.; Shackleford, D.M.; Morizzi, J.; Katneni, K.; Campbell, M.; Hudson, A.; Charman, S.A.; Yeates, C.; et al. Discovery and structure-activity relationships of pyrrolone antimalarials. J. Med. Chem. 2013, 56, 2975–2990. [Google Scholar] [CrossRef]
- Alam, M.M.; Husain, A.; Hasan, S.M.; Suruchi; Anwer, T. Synthesis and pharmacological evaluation of 2(3H)-furanones and 2(3H)-pyrrolones, combining analgesic and anti-inflammatory properties with reduced gastrointestinal toxicity and lipid peroxidation. Eur. J. Med. Chem. 2009, 44, 2636–2642. [Google Scholar] [CrossRef]
- Youssef, Y.M.; Azab, M.E.; Elsayed, G.A.; El-Sayed, A.A.; Hassaballah, A.I.; El-Helw, E.A.E. Synthesis and antioxidant activity of some pyrazole-based heterocycles using a 2(3H)-furanone building block. Synth. Commun. 2023, 53, 402–413. [Google Scholar] [CrossRef]
- Pelkey, E.T.; Pelkey, S.J.; Greger, J.G. De Novo Synthesis of 3-Pyrrolin-2-Ones. In Advances in Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, C.A., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 115, pp. 151–285. ISBN 9780128021293. [Google Scholar]
- Ji Ram, V.; Sethi, A.; Nath, M.; Pratap, R. Five-Membered Heterocycles. In The Chemistry of Heterocycles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–478. ISBN 9780081010334. [Google Scholar]
- Ríos, M.C.; Portilla, J. Recent Advances in Synthesis and Properties of Pyrazoles. Chemistry 2022, 4, 940–968. [Google Scholar] [CrossRef]
- Brown, A.W. Recent Developments in the Chemistry of Pyrazoles, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 126. [Google Scholar]
- Ardakani, L.S.; Mosslemin, M.H.; Hassanabadi, A.; Hashemian, S. Reaction between Benzoic Acid N′-(2-Oxo-2-Phenyl-Ethyl)Hydrazide and Acetylenic Esters in the Presence of Alkyl Isocyanides: One-Pot Synthesis of Highly Functionalized 2,3-Dihydro-1H-Pyrazoles. Polycycl. Aromat. Compd. 2022, 42, 6861–6867. [Google Scholar] [CrossRef]
- Rawat, P.; Bharati, P.; Gautam, A.; Kumar, M.; Singh, R.; Prakash; Ram, A.; Gautam, S.; Darwari, A.; Mishra, A.; et al. Design and synthesis of pyrazole, pyrazolone and 1,3,4-oxadiazole derivatives having pyrrole motif as a source of new antimicrobial and anticancer agents. J. Mol. Struct. 2023, 1272, 134087. [Google Scholar] [CrossRef]
- Deivasigamani, P.; Rubavathy, S.M.E.; Jayasankar, N.; Saravanan, V.; Thilagavathi, R.; Prakash, M.; Selvam, C.; Rajagopal, R.; Alfarhan, A.; Kathiravan, M.K.; et al. Dual Anti-Inflammatory and Anticancer Activity of Novel 1,5-Diaryl Pyrazole Derivatives: Molecular Modeling, Synthesis, In Vitro Activity, and Dynamics Study. Biomedicines 2024, 12, 788. [Google Scholar] [CrossRef]
- Lato, A.M.; Burke, S.J.; Ducote, M.P.; Kennedy, B.J.; Collier, J.J.; Campagna, S.R. Stereoisomers of an Aryl Pyrazole Glucocorticoid Receptor Agonist Scaffold Elicit Differing Anti-inflammatory Responses. ACS Med. Chem. Lett. 2022, 13, 1493–1499. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.; Faouzi, M.E.A. Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line. Bioorg. Chem. 2020, 97, 103470. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.B.; Tang, W.J.; Qi, X.B.; Li, R.; Liu, X.H. Novel pyrazole-5-carboxamide and pyrazole–pyrimidine derivatives: Synthesis and anticancer activity. Eur. J. Med. Chem. 2015, 90, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.F.; Mahmoud, W.R.; Abdelgawad, N.M.; Fouad, M.A.; Said, M.F. Exploring novel anticancer pyrazole benzenesulfonamides featuring tail approach strategy as carbonic anhydrase inhibitors. Eur. J. Med. Chem. 2023, 261, 115805. [Google Scholar] [CrossRef]
- Stecoza, C.E.; Nitulescu, G.M.; Draghici, C.; Caproiu, M.T.; Olaru, O.T.; Bostan, M.; Mihaila, M. Synthesis and Anticancer Evaluation of New 1,3,4-Oxadiazole Derivatives. Pharmaceuticals 2021, 14, 438. [Google Scholar] [CrossRef]
- Paidi, K.R.; Kolli, M.K.; Reddy, E.K.; Pedakotla, V.R. Sodium hypochlorite-mediated synthesis of 2,5-disubstituted 1,3,4-oxadiazoles from hydrazides and aldehydes. Chem. Heterocycl. Compd. 2020, 56, 371–376. [Google Scholar] [CrossRef]
- Paruch, K.; Popiołek, Ł.; Biernasiuk, A.; Hordyjewska, A.; Malm, A.; Wujec, M. Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines: Synthesis and Biological Activity. Molecules 2020, 25, 5844. [Google Scholar] [CrossRef]
- Chauhan, J.; Ravva, M.K.; Sen, S. Harnessing Autoxidation of Aldehydes: In Situ Iodoarene Catalyzed Synthesis of Substituted 1,3,4-Oxadiazole, in the Presence of Molecular Oxygen. Org. Lett. 2019, 21, 6562–6565. [Google Scholar] [CrossRef]
- Izgi, S.; Sengul, I.F.; Şahin, E.; Koca, M.S.; Cebeci, F.; Kandemir, H. Synthesis of 7-azaindole based carbohydrazides and 1,3,4-oxadiazoles; Antioxidant activity, α-glucosidase inhibition properties and docking study. J. Mol. Struct. 2022, 1247, 131343. [Google Scholar] [CrossRef]
- Babalola, B.A.; Sharma, L.; Olowokere, O.; Malik, M.; Folajimi, O. Advancing drug discovery: Thiadiazole derivatives as multifaceted agents in medicinal chemistry and pharmacology. Bioorg. Med. Chem. 2024, 112, 117876. [Google Scholar] [CrossRef]
- Ahmad, S.; Alam, M.Z.; Salma, U.; Mohasin, M.; Rahaman, P.F.; Parveen, H.; Khan, S.A. A review on recent progress in synthesis and biological activities of thiadiazole and its derivatives. J. Mol. Struct. 2024, 1312, 138438. [Google Scholar] [CrossRef]
- Kandemir, L.; Karakus, S.; Özbas, S.; Rollas, S.; Akbuga, J. Synthesis, structure elucidation and cytotoxic activities of 2,5-disubstituted-1,3,4-thiadiazole and l,2,4-triazole-3-thione derivatives. J. Res. Pharm. 2022, 26, 941–953. [Google Scholar] [CrossRef]
- Kashyap, A.; Silakari, O. Triazoles. In Key Heterocycle Cores for Designing Multitargeting Molecules; Elsevier: Amsterdam, The Netherlands, 2018; pp. 323–342. ISBN 9780081020838. [Google Scholar]
- Hassani, I.A.E.; Rouzi, K.; Hassani, A.A.E.; Karrouchi, K.; Ansar, M. Recent Developments Towards the Synthesis of Triazole Derivatives: A Review. Organics 2024, 5, 450–471. [Google Scholar] [CrossRef]
- Ren, M.T.; Li, M.; Wang, A.J.; Gao, J.; Zhang, X.X.; Shu, W.M. Iodine-Mediated Condensation–Cyclization of α-Azido Ketones with p-Toluenesulfonyl Hydrazide for Synthesis of 4-Aryl-NH-1,2,3-Triazoles. Eur. J. Org. Chem. 2020, 2020, 2233–2236. [Google Scholar] [CrossRef]
- Clark, P.R.; Williams, G.D.; Hayes, J.F.; Tomkinson, N.C.O. A Scalable Metal-, Azide-, and Halogen-Free Method for the Preparation of Triazoles. Angew. Chem. Int. Ed. 2020, 59, 6740–6744. [Google Scholar] [CrossRef]
- Patterson, S.J.M.; Clark, P.R.; Williams, G.D.; Tomkinson, N.C.O. An azide and acetylene free synthesis of 1-substituted 1,2,3-triazoles. Tetrahedron Lett. 2020, 61, 152483. [Google Scholar] [CrossRef]
- Han, M.İ.; Bekçi, H.; Uba, A.I.; Yıldırım, Y.; Karasulu, E.; Cumaoğlu, A.; Karasulu, H.Y.; Yelekçi, K.; Yılmaz, Ö.; Küçükgüzel, Ş.G. Synthesis, molecular modeling, in vivo study, and anticancer activity of 1,2,4-triazole containing hydrazide–hydrazones derived from (S)-naproxen. Arch. Pharm. 2019, 352, 1800365. [Google Scholar] [CrossRef]
- Zhu, J.; He, L.; Luo, J.; Xiong, J.; Wang, T. Design, synthesis, and herbicidal activity of novel pyrimidine derivatives containing 1,2,4-triazole. Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 948–953. [Google Scholar] [CrossRef]
- Hussein, B.R.M.; Khodairy, A. Utility of [4-(3-methoxyphenyl)pyrimidin-2-yl]cyanamide in synthesis of some heterocyclic compounds. J. Heterocycl. Chem. 2021, 58, 1983–1991. [Google Scholar] [CrossRef]
- Umapathi, A.; PN, N.; Madhyastha, H.; Singh, M.; Madhyastha, R.; Maruyama, M.; Daima, H.K. Curcumin and isonicotinic acid hydrazide functionalized gold nanoparticles for selective anticancer action. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125484. [Google Scholar] [CrossRef]
- Guo, F.; Xia, T.; Xiao, P.; Wang, Q.; Deng, Z.; Zhang, W.; Diao, G. A supramolecular complex of hydrazide-pillar[5]arene and bisdemethoxycurcumin with potential anti-cancer activity. Bioorg. Chem. 2021, 110, 104764. [Google Scholar] [CrossRef]
- Qurrat-ul-Ain; Abid, A.; Lateef, M.; Rafiq, N.; Eijaz, S.; Tauseef, S. Multi-activity tetracoordinated pallado-oxadiazole thiones as anti-inflammatory, anti-Alzheimer, and anti-microbial agents: Structure, stability and bioactivity comparison with pallado-hydrazides. Biomed. Pharmacother. 2022, 146, 112561. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).