Dry Carbonate Sorbents for CO2 Capture from Flue Gases: Role of Support in Adsorption Efficiency and Thermal Stability
Abstract
1. Introduction
2. Results
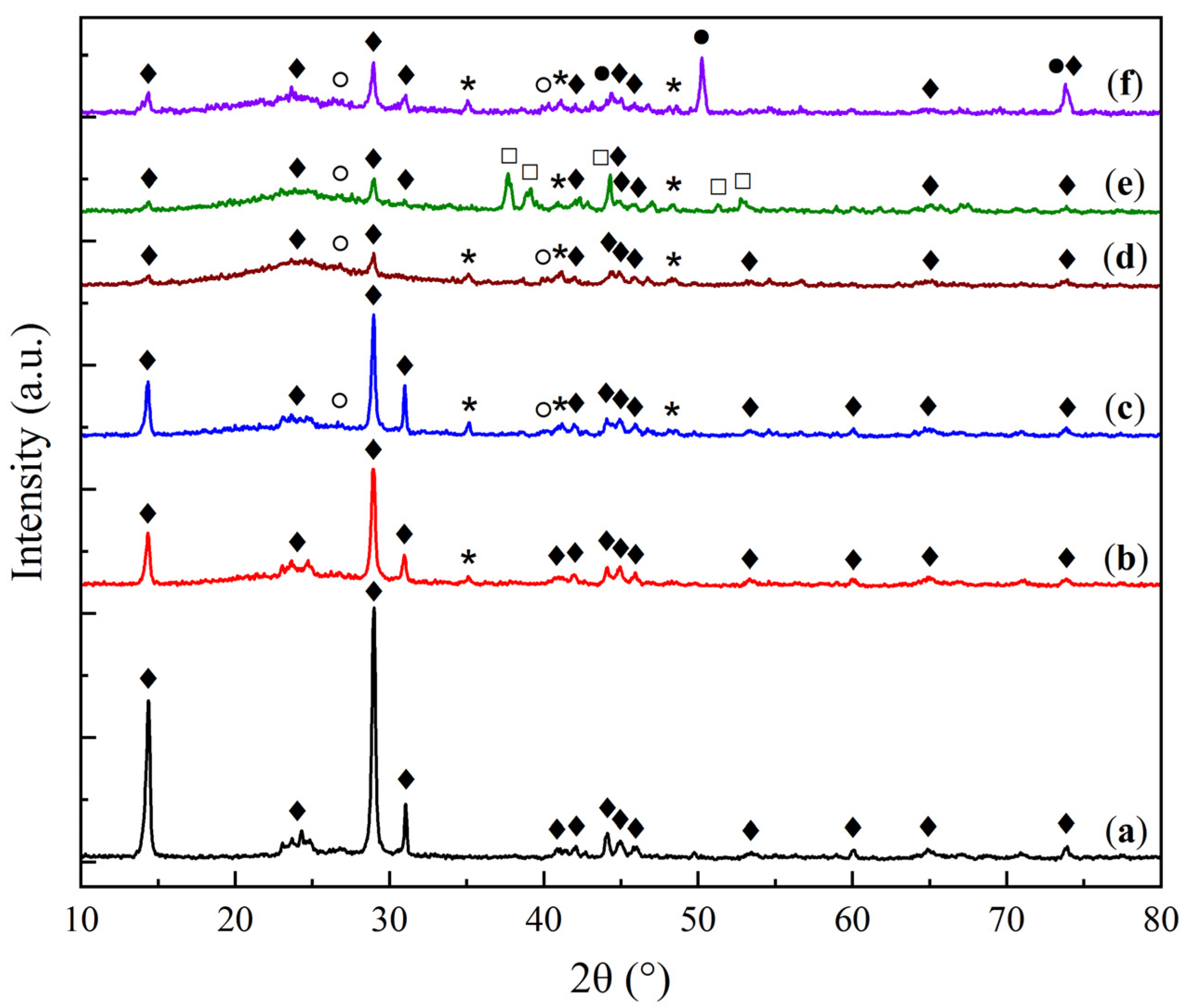
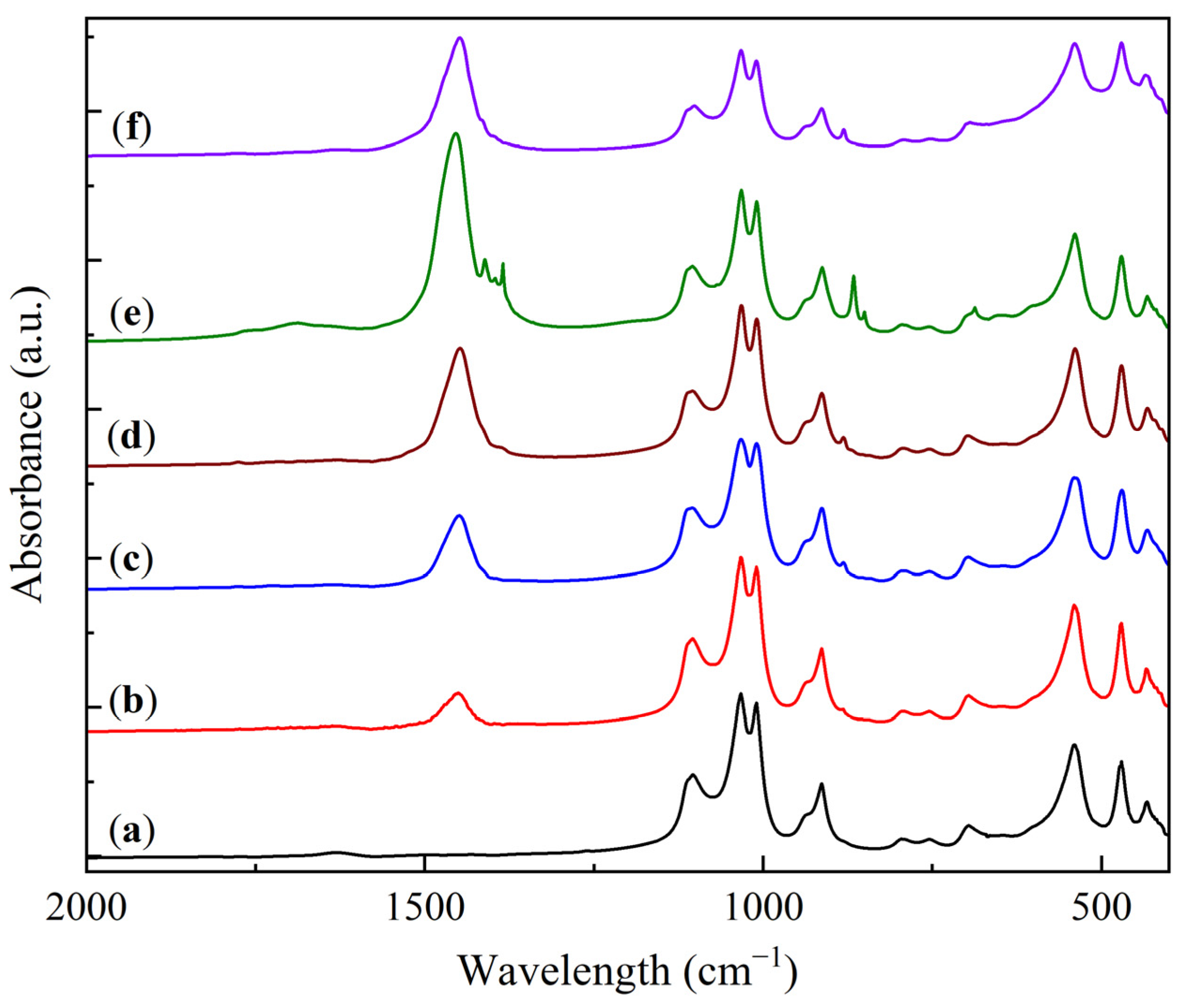
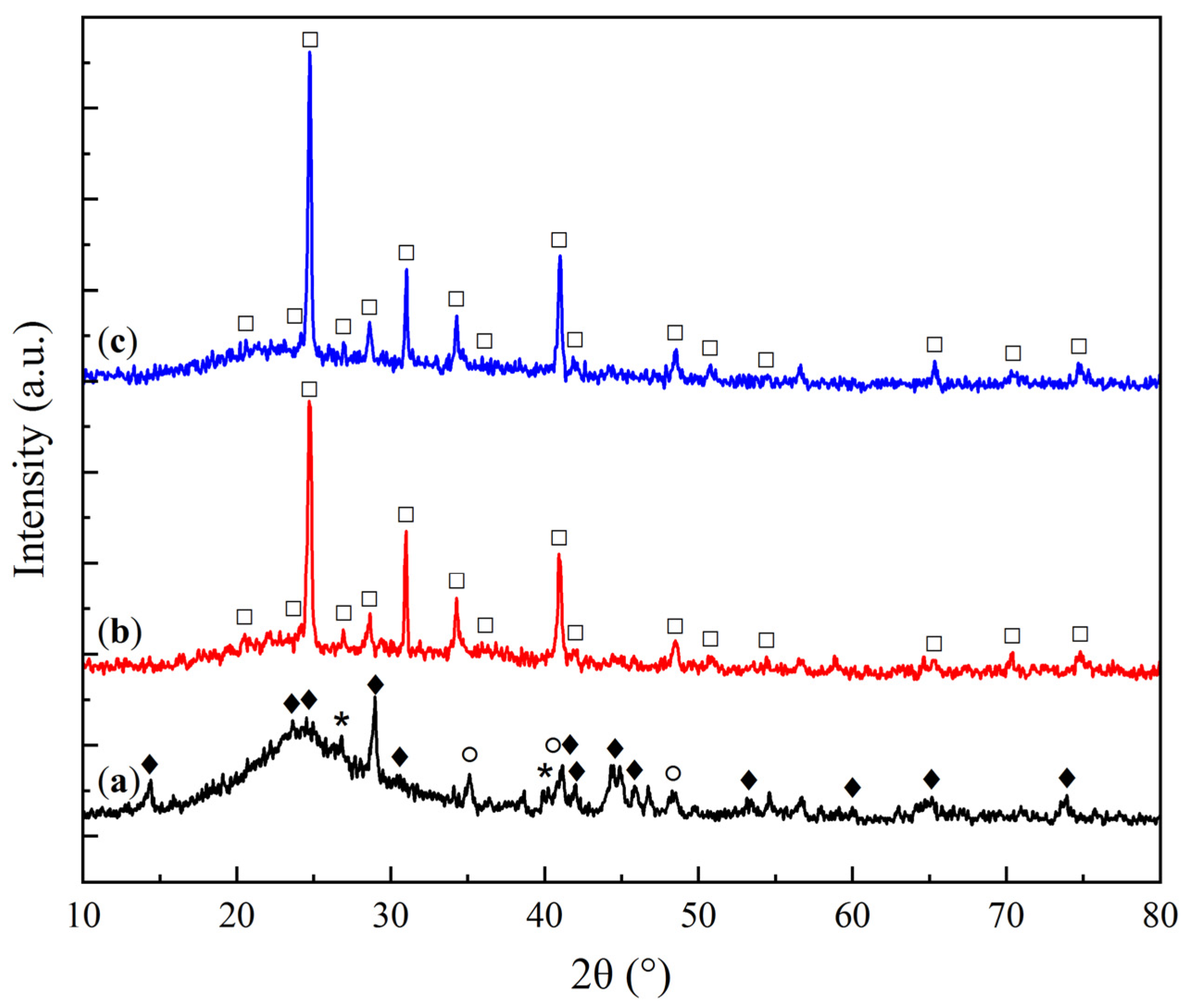
2.1. Physicochemical Characterization of Sorbent Samples
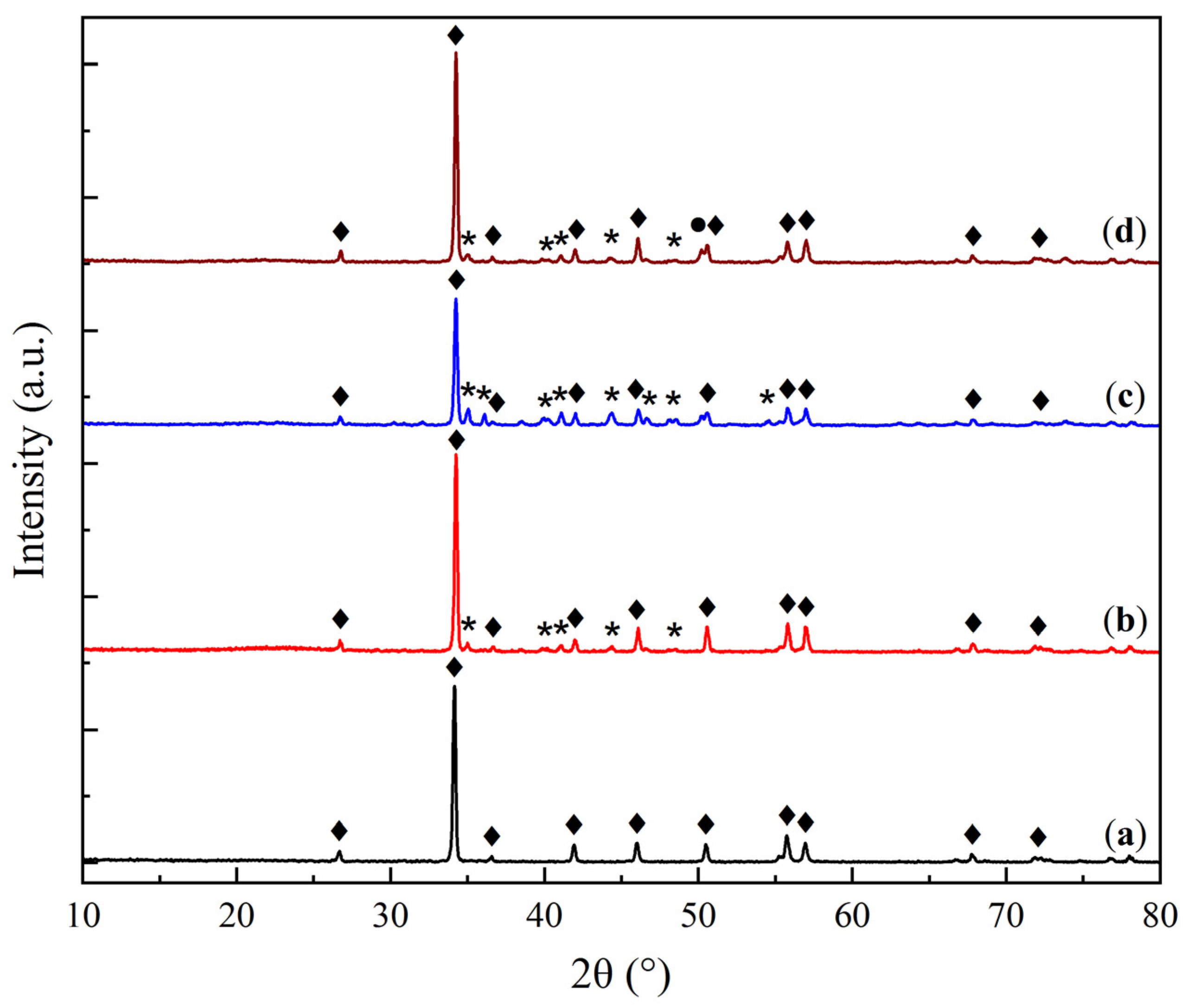
2.2. Thermal Stability and Cyclic Durability of the Supported Sorbents
2.3. Kinetic Study of CO2 Adsorption on the Na2CO3/CaCO3 Sorbent
3. Materials and Methods
3.1. Synthesis of Sorbent Samples
3.2. Characterization of Sorbent Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCUS | Carbon Capture, Utilization, and Storage |
| CCU | Carbon Capture and Utilization |
| CCS | Carbon Capture and Storage |
| DCP | Dry Carbonate Process |
| XRD | X-ray Diffraction |
| JCPDS | Joint Committee on Powder Diffraction Standards |
| SEM | Scanning Electron Microscopy |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| BET | Brunauer–Emmett–Teller Method |
| TPD | Temperature-Programmed Desorption |
| AC | Active Carbon |
| DFT | Density Functional Theory |
| TCD | Thermal Conductivity Detector |
References
- IEA. CO2 Emissions in 2023: Global Energy Review; International Energy Agency: Paris, France, 2024; Available online: https://www.iea.org/reports/co2-emissions-in-2023 (accessed on 13 May 2025).
- Hsiao, C.M. Economic growth, CO2 emissions quota and optimal allocation under uncertainty. Sustainability 2022, 14, 8706. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B. A bibliometric analysis of carbon allowances in the carbon emissions trading market. Energies 2024, 18, 57. [Google Scholar] [CrossRef]
- Zhai, X.; Yang, X.; Vukovic, D.B.; Dinets, D.A.; Liu, Q. Carbon emissions trading policy and regional energy efficiency: A quasi-natural experiment from China. Energies 2025, 18, 1161. [Google Scholar] [CrossRef]
- Błażejowska, M.; Czarny, A.; Kowalska, I.; Michalczewski, A.; Stępień, P. The effectiveness of the EU ETS policy in changing the energy mix in selected European countries. Energies 2024, 17, 4243. [Google Scholar] [CrossRef]
- Nath, F.; Mahmood, M.N.; Yousuf, N. Recent advances in CCUS: A critical review on technologies, regulatory aspects and economics. Geoenergy Sci. Eng. 2024, 238, 212726. [Google Scholar] [CrossRef]
- Ampomah, W.; Morgan, A.; Koranteng, D.O.; Nyamekye, W.I. CCUS perspectives: Assessing historical contexts, current realities, and future prospects. Energies 2024, 17, 4248. [Google Scholar] [CrossRef]
- Saxena, A.; Gupta, J.P.; Tiwary, J.K.; Kumar, A.; Sharma, S.; Pandey, G.; Biswas, S.; Chaturvedi, K.R. Innovative pathways in carbon capture: Advancements and strategic approaches for effective carbon capture, utilization, and storage. Sustainability 2024, 16, 10132. [Google Scholar] [CrossRef]
- Raganati, F.; Ammendola, P. CO2 post-combustion capture: A critical review of current technologies and future directions. Energy Fuels 2024, 38, 13858–13905. [Google Scholar] [CrossRef]
- Chang, R.; Wu, X.; Cheung, O.; Liu, W. Synthetic solid oxide sorbents for CO2 capture: State-of-the art and future perspectives. J. Mater. Chem. A 2022, 10, 1682–1705. [Google Scholar] [CrossRef]
- Zentou, H.; Aliyu, M.; Abdalla, M.A.; Abdelaziz, O.Y.; Hoque, B.; Alloush, A.M.; Tayeb, I.M.; Patchigolla, K.; Abdelnaby, M.M. Advancements and challenges in adsorption-based carbon capture technology: From fundamentals to deployment. Chem. Rec. 2025, 25, e202400188. [Google Scholar] [CrossRef]
- Saenz Cavazos, P.A.; Hunter-Sellars, E.; Iacomi, P.; McIntyre, S.R.; Danaci, D.; Williams, D.R. Evaluating solid sorbents for CO2 capture: Linking material properties and process efficiency via adsorption performance. Front. Energy Res. 2023, 11, 1167043. [Google Scholar] [CrossRef]
- Nelson, T.O.; Coleman, L.J.; Green, D.A.; Gupta, R.P. The dry carbonate process: Carbon dioxide recovery from power plant flue gas. Energy Procedia 2009, 1, 1305–1311. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, X.; Anthony, E.J.; Jiang, X.; Duan, L.; Wu, Y.; Dong, W.; Zhao, C. Capturing CO2 in flue gas from fossil fuel-fired power plants using dry regenerable alkali metal-based sorbent. Prog. Energy Combust. Sci. 2013, 39, 515–534. [Google Scholar] [CrossRef]
- Medina-Carrasco, S.; Valverde, J.M. Crystallographic and morphological transformation of natrite and nahcolite in the dry carbonate process for CO2 capture. Cryst. Growth Des. 2018, 18, 4578–4592. [Google Scholar] [CrossRef]
- Ma, J.; Zhong, J.; Bao, X.; Chen, X.; Wu, Y.; Cai, T.; Liu, D.; Liang, C. Continuous CO2 capture performance of K2CO3/Al2O3 sorbents in a novel two-stage integrated bubbling-transport fluidized reactor. Chem. Eng. J. 2021, 404, 126465. [Google Scholar] [CrossRef]
- Lee, S.C.; Choi, B.Y.; Lee, T.J.; Ryu, C.K.; Ahn, Y.S.; Kim, J.C. CO2 absorption and regeneration of alkali metal-based solid sorbents. Catal. Today 2006, 111, 385–390. [Google Scholar] [CrossRef]
- Qin, C.; Yin, J.; Ran, J.; Zhang, L.; Feng, B. Effect of support material on the performance of K2CO3-based pellets for cyclic CO2 capture. Appl. Energy 2014, 136, 280–288. [Google Scholar] [CrossRef]
- Masoud, N.; Bordanaba-Florit, G.; Van Haasterecht, T.; Bitter, J.H. Effect of support surface properties on CO2 capture from air by carbon-supported potassium carbonate. Ind. Eng. Chem. Res. 2021, 60, 13749–13755. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, J.; Chen, X.; Wang, C.; Tao, Z.; Liu, D.; Liang, C. CO2 capture performance of Na2CO3/γ-Al2O3 sorbent modified with organic acid. Energy Fuels 2024, 38, 7148–7157. [Google Scholar] [CrossRef]
- Baizhumanova, T.; Tungatarova, S.; Kaumenova, G.; Zhumabek, M.; Kassymkan, K. Catalytic oxidation of C3–C4 mixture into industrial important chemical products. Orient. J. Chem. 2019, 35, 404–408. [Google Scholar] [CrossRef]
- Ling, C.; Wang, Z.; Gui, C.; Tang, Z. High temperature CO2 capture performance and kinetic analysis of Na4SiO4 ceramics. Ceram. Int. 2022, 48, 33048–33057. [Google Scholar] [CrossRef]
- Shang, H.; Ouyang, T.; Yang, F.; Kou, Y. A biomass-supported Na2CO3 sorbent for flue gas desulfurization. Environ. Sci. Technol. 2003, 37, 2596–2599. [Google Scholar] [CrossRef]
- Meshkani, F.; Rezaei, M. Facile synthesis of nanocrystalline magnesium oxide with high surface area. Powder Technol. 2009, 196, 85–88. [Google Scholar] [CrossRef]
- Lekatou, A.G.; Tsouli, S. Cyclic polarization of corrugated austenitic stainless steel rebars in acid rain: Effect of fly ash, pH and steel type. Corros. Mater. Degrad. 2022, 3, 75–100. [Google Scholar] [CrossRef]
- Kristóf, J.; Frost, R.L.; Felinger, A.; Mink, J. FTIR spectroscopic study of intercalated kaolinite. J. Mol. Struct. 1997, 410, 119–122. [Google Scholar] [CrossRef]
- Zich, D.; Zacher, T.; Darmo, J.; Szöcs, V.; Lorenc, D.; Janek, M. Far-infrared investigation of kaolinite and halloysite intercalates using terahertz time-domain spectroscopy. Vib. Spectrosc. 2013, 69, 1–7. [Google Scholar] [CrossRef]
- Joshi, S.; Kalyanasundaram, S.; Balasubramanian, V. Quantitative analysis of sodium carbonate and sodium bicarbonate in solid mixtures using Fourier transform infrared spectroscopy (FT–IR). Appl. Spectrosc. 2013, 67, 841–845. [Google Scholar] [CrossRef]
- Đorđević, N.; Vlahović, M.; Mihajlović, S.; Martinović, S.; Vušović, N.M.; Šajić, J.L. Fourier-transform infrared spectroscopy analysis of mechanochemical transformation kinetics of sodium carbonate to bicarbonate. Sci. Sinter. 2022, 54, 481–494. [Google Scholar] [CrossRef]
- Hofmeister, A.M.; Keppel, E.; Speck, A.K. Absorption and reflection infrared spectra of MgO and other diatomic compounds. Mon. Not. R. Astron. Soc. 2003, 345, 16–38. [Google Scholar] [CrossRef]
- Gupta, U.; Singh, V.K.; Kumar, V.; Khajuria, Y. Experimental and theoretical spectroscopic studies of calcium carbonate (CaCO3). Mater. Focus 2015, 4, 164–169. [Google Scholar] [CrossRef]
- Zbik, M.; Smart, R.S.C. Nanomorphology of kaolinites: Comparative SEM and AFM studies. Clays Clay Miner. 1998, 46, 153–160. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Leonard, B. Use of multifunctional nanoporous TiO(OH)2 for catalytic NaHCO3 decomposition-eventually for Na2CO3/NaHCO3 based CO2 separation technology. Sep. Purif. Technol. 2011, 80, 364–374. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Mohamedali, M.; Sedghkerdar, M.H.; Mahinpey, N. Stability of CaO-based sorbents under realistic calcination conditions: Effect of metal oxide supports. ACS Sustain. Chem. Eng. 2022, 10, 9760–9769. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, C.; Harrison, A.; Alsouleman, K.; Gao, M.; Huang, Z.; Chen, Q.; Nie, B. Enhancement strategies of calcium looping technology and CaO-based sorbents for carbon capture. Small 2025, 21, 2412463. [Google Scholar] [CrossRef]
- Scardi, P.; Sartori, N.; Giachello, A.; Demaestri, P.P.; Branda, F. Influence of calcium oxide and sodium oxide on the microstructure of cordierite catalyst supports. Ceram. Int. 1993, 19, 105–111. [Google Scholar] [CrossRef]
- Gavryushkin, P.N.; Thomas, V.G.; Bolotina, N.B.; Bakakin, V.V.; Golovin, A.V.; Seryotkin, Y.V.; Fursenko, D.A.; Litasov, K.D. Hydrothermal synthesis and structure solution of Na2Ca(CO3)2: “Synthetic analogue” of mineral nyerereite. Cryst. Growth Des. 2016, 16, 1893–1902. [Google Scholar] [CrossRef]
- Temuujin, J.; Ruescher, C.; Minjigmaa, A.; Darkhijav, B.; Davaabal, B.; Battsetseg, B.E. Characterization of efflorescences of ambient and elevated temperature cured fly ash based geopolymer type concretes. Adv. Mater. Res. 2016, 1139, 25–29. [Google Scholar] [CrossRef]
- Cooper, A.F.; Gittins, J.; Tuttle, O.F. The system Na2CO3–K2CO3–CaCO3 at 1 kilobar and its significance in carbonatite petrogenesis. Am. J. Sci. 1975, 275, 534–560. [Google Scholar] [CrossRef]
- Markovic, S.; Dondur, V.; Dimitrijevic, R. FTIR spectroscopy of framework aluminosilicate structures: Carnegieite and pure sodium nepheline. J. Mol. Struct. 2003, 654, 223–234. [Google Scholar] [CrossRef]
- Böttcher, M.E.; Reutel, C. The Raman spectrum of α-Na2Ca(CO3)2. J. Raman Spectrosc. 1996, 27, 859–861. [Google Scholar] [CrossRef]
- Frost, R.L.; Dickfos, M. Hydrated double carbonates—A Raman and infrared spectroscopic study. Polyhedron 2007, 26, 4503–4508. [Google Scholar] [CrossRef]
- Kumazawa, H.; Choi, B.S.; Park, S.W.; Sung, D.H.; Lee, J.W. Carbonation kinetics of potassium carbonate by carbon dioxide. J. Ind. Eng. Chem. 2006, 12, 522–530. [Google Scholar]
- Guo, Y.; Zhao, C.; Li, C.; Wu, Y. CO2 sorption and reaction kinetic performance of K2CO3/AC in low temperature and CO2 concentration. Chem. Eng. J. 2015, 260, 596–604. [Google Scholar] [CrossRef]
- Guo, B.; Wang, Y.; Shen, X.; Qiao, X.; Jia, L.; Xiang, J.; Jin, Y. Study on CO2 capture characteristics and kinetics of modified potassium-based adsorbents. Materials 2020, 13, 877. [Google Scholar] [CrossRef]
- Cai, T.; Johnson, J.K.; Wu, Y.; Chen, X. Toward understanding the kinetics of CO2 capture on sodium carbonate. ACS Appl. Mater. Interfaces 2019, 11, 9033–9041. [Google Scholar] [CrossRef]
- Cai, T.; Chen, X.; Johnson, J.K.; Wu, Y.; Ma, J.; Liu, D.; Liang, C. Understanding and improving the kinetics of bulk carbonation on sodium carbonate. J. Phys. Chem. C 2020, 124, 23106–23115. [Google Scholar] [CrossRef]
- Frank-Kamenetskii, D.A. Diffusion and Heat Transfer in Chemical Kinetics, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 1969; 384p, ISBN 978-0-306-30349-4. [Google Scholar]
- Froment, G.F.; Bischoff, K.B.; De Wilde, J. Chemical Reactor Analysis and Design, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; 900p, ISBN 978-0-470-48167-6. [Google Scholar]
- Feng, S.; Zhu, J.; Wang, R.; Qu, Z.; Song, L.; Wang, H. The influence of CaO and MgO on the mechanical properties of alkali-activated blast furnace slag powder. Materials 2022, 15, 6128. [Google Scholar] [CrossRef]
- Wang, T.; Li, H.; Wei, F.; Xue, Z.; Wang, M.; Guo, Y.; Sun, J.; Lu, P. Impregnated layer solution combustion synthesized CaO-based composite pellets with enhanced CO2 sorption property. Carbon Neutrality 2024, 3, 38. [Google Scholar] [CrossRef]
- De Jong, A.M.; Niemantsverdriet, J.W. Thermal desorption analysis: Comparative test of ten commonly applied procedures. Surf. Sci. 1990, 233, 355–365. [Google Scholar] [CrossRef]





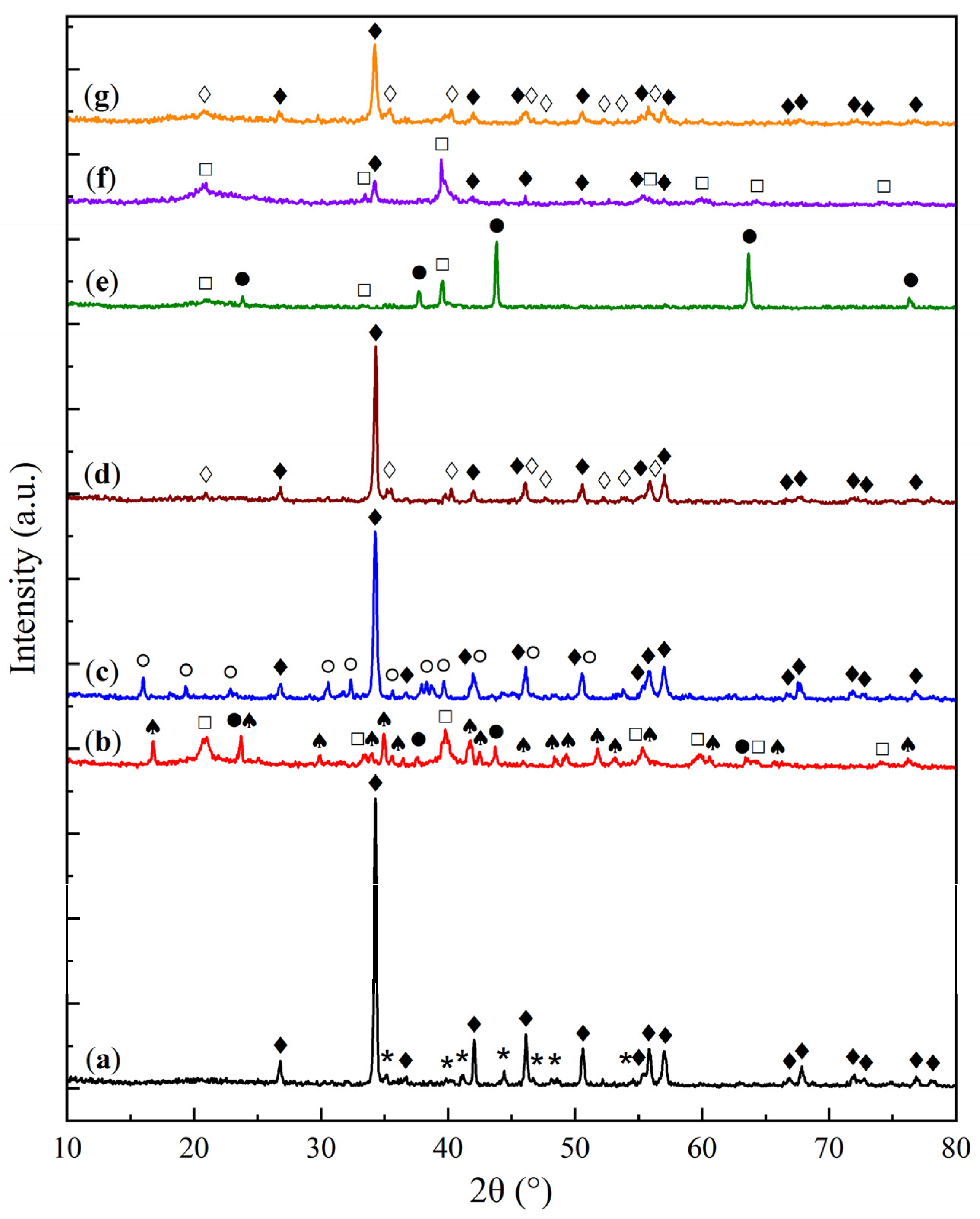

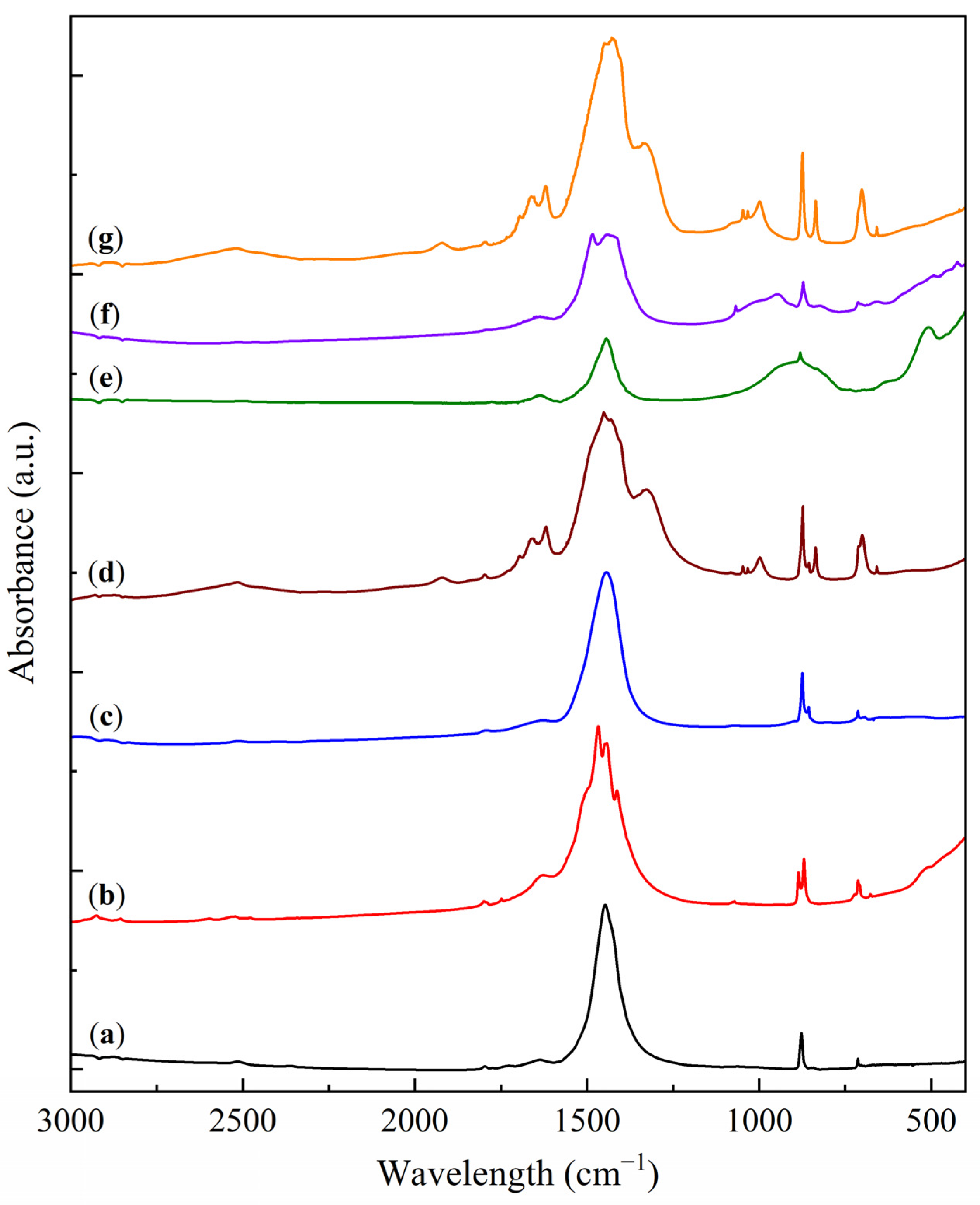
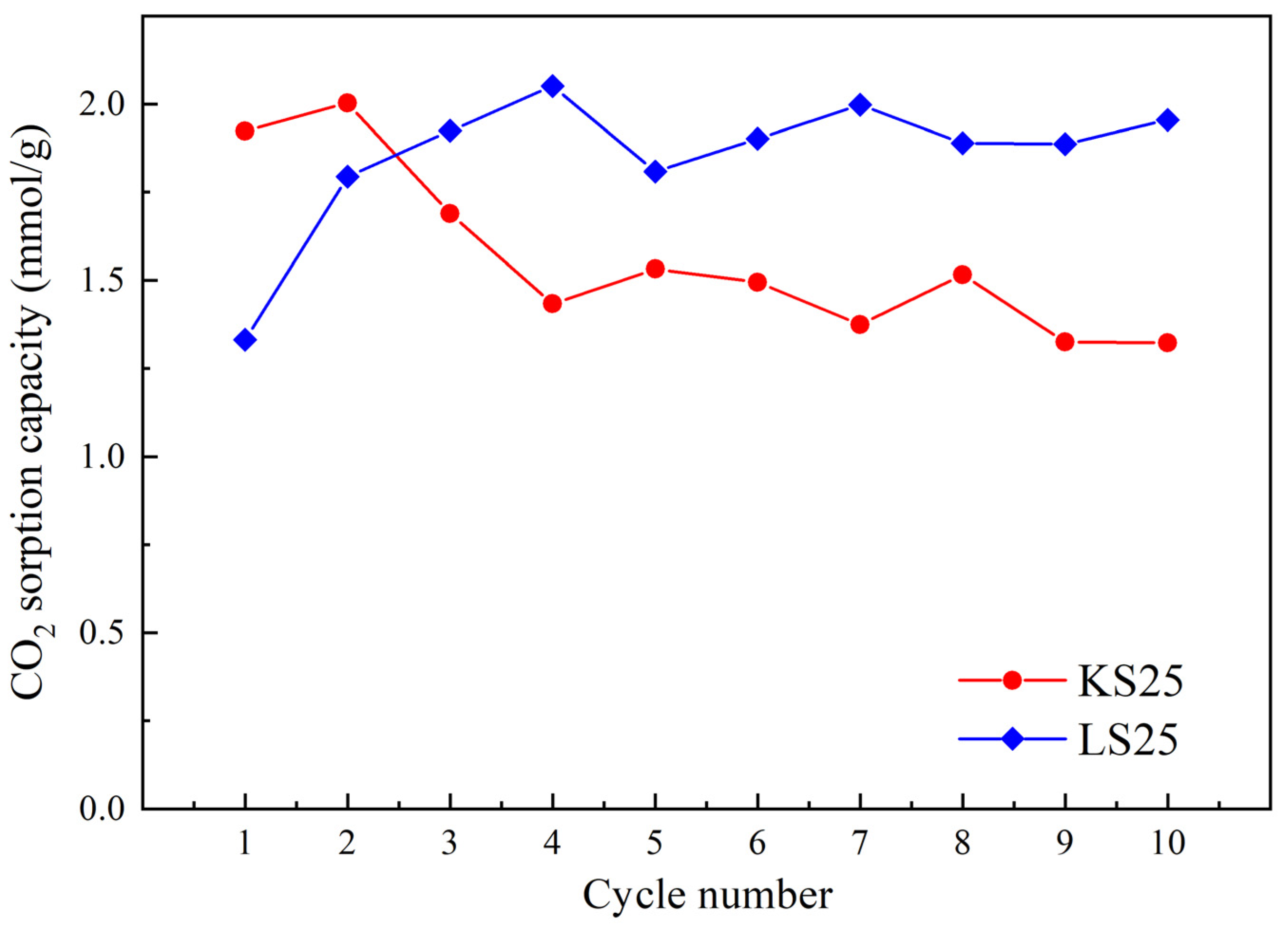

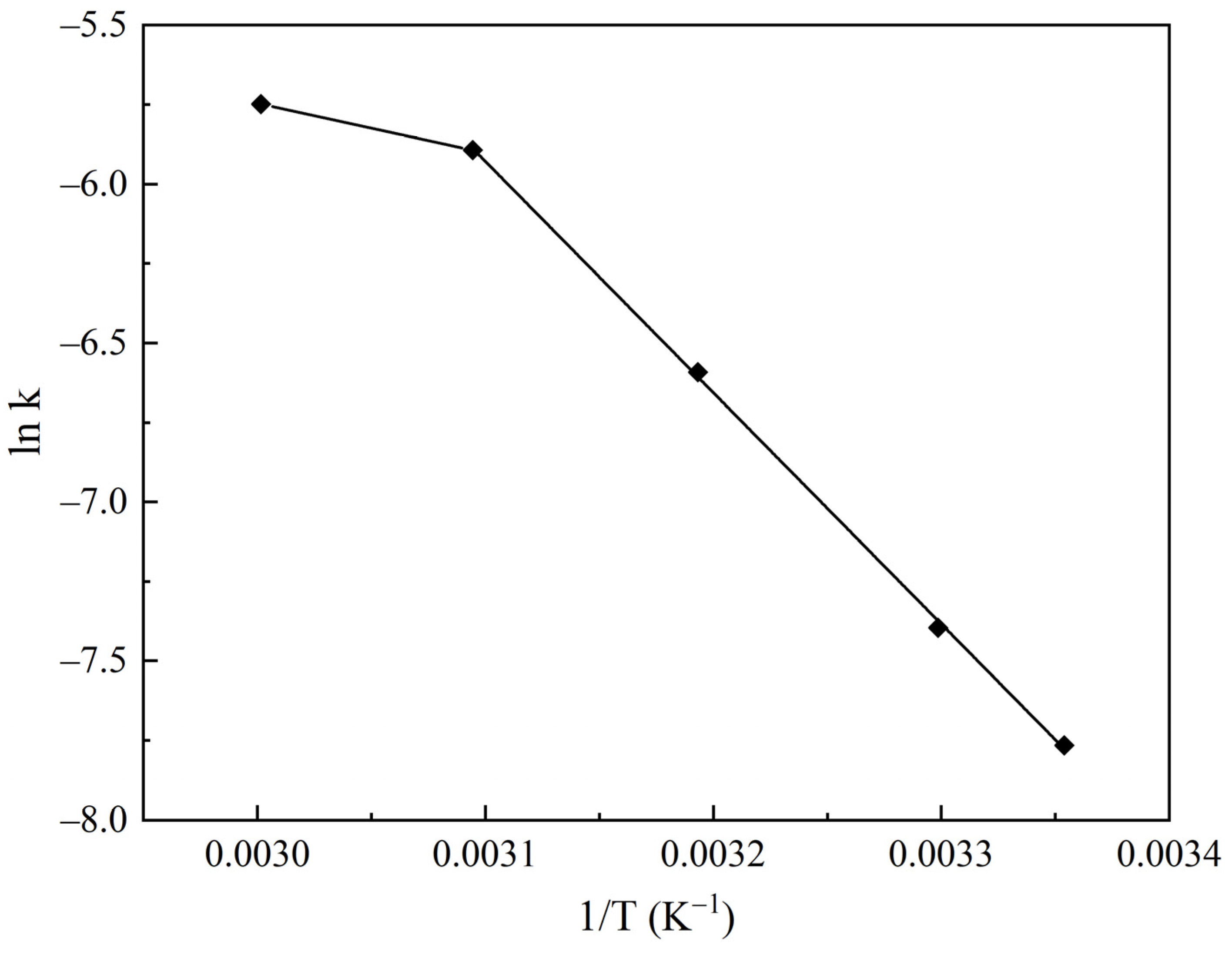
| Sample | O | Al | Si | Na | Mg | Ca |
|---|---|---|---|---|---|---|
| KS5 | 49.93 | 8.15 | 9.23 | 32.69 | — | — |
| KS10 | 48.85 | 8.23 | 7.90 | 35.02 | — | — |
| KS15 | 49.78 | 9.62 | 9.58 | 31.02 | — | — |
| KS20 | 49.24 | 7.80 | 7.80 | 35.16 | — | — |
| KS25 | 53.06 | 5.62 | 4.92 | 36.40 | — | — |
| KS50 | 51.01 | 7.02 | 6.57 | 35.40 | — | — |
| KS25M | 46.51 | 5.51 | 5.69 | 33.45 | 8.84 | — |
| LS15 | 52.95 | 0.83 | 0.25 | 6.95 | 0.16 | 38.86 |
| LS25 | 55.78 | 0.27 | 1.42 | 8.48 | 0.34 | 33.71 |
| LS50 | 54.62 | 0.41 | 0.55 | 25.38 | 0.69 | 18.35 |
| LS25M | 52.99 | 0.33 | 0.43 | 10.03 | 9.01 | 27.21 |
| Sample | Bulk Density, g/cm3 | Surface Area, m2/g | Pore Volume, cm3/g | Adsorption Capacity, mmol/g |
|---|---|---|---|---|
| KS5 | 0.85 | 13.3 | 0.084 | 0.482 |
| KS10 | 0.76 | 13.7 | 0.086 | 0.662 |
| KS15 | 0.72 | 16.2 | 0.102 | 1.156 |
| KS20 | 0.70 | 17.9 | 0.112 | 1.664 |
| KS25 | 0.68 | 20.7 | 0.130 | 1.923 |
| KS50 | 0.63 | 25.1 | 0.149 | 2.529 |
| KS25M | 0.74 | 18.1 | 0.108 | 1.282 |
| LS15 | 0.94 | 8.4 | 0.038 | 0.792 |
| LS25 | 0.90 | 11.7 | 0.065 | 1.331 |
| LS50 | 0.87 | 13.5 | 0.076 | 1.941 |
| LS25M | 0.72 | 17.9 | 0.100 | 1.324 |
| Treatment Conditions | CO2 Sorption Capacity, mmol/g | |
|---|---|---|
| KS25 | LS25 | |
| Initial | 1.923 | 1.331 |
| After thermal treatment at 750 °C | 0.113 | 0.170 |
| After regeneration | 0.119 | 1.244 |
| After thermal treatment at 1000 °C | — | 0.147 |
| After regeneration | — | 0.916 |
| Temperature, °C | 25 | 30 | 40 | 50 | 60 |
| k · 103, cm/min | 0.424 | 0.614 | 1.370 | 2.757 | 3.186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khussain, B.; Sass, A.; Brodskiy, A.; Zhurinov, M.; Torlopov, I.; Rakhmetova, K.; Zhumadullaev, D.; Boleubayev, Y.; Khussain, A.; Kenessary, A.; et al. Dry Carbonate Sorbents for CO2 Capture from Flue Gases: Role of Support in Adsorption Efficiency and Thermal Stability. Molecules 2025, 30, 2859. https://doi.org/10.3390/molecules30132859
Khussain B, Sass A, Brodskiy A, Zhurinov M, Torlopov I, Rakhmetova K, Zhumadullaev D, Boleubayev Y, Khussain A, Kenessary A, et al. Dry Carbonate Sorbents for CO2 Capture from Flue Gases: Role of Support in Adsorption Efficiency and Thermal Stability. Molecules. 2025; 30(13):2859. https://doi.org/10.3390/molecules30132859
Chicago/Turabian StyleKhussain, Bolatbek, Alexandr Sass, Alexandr Brodskiy, Murat Zhurinov, Ivan Torlopov, Kenzhegul Rakhmetova, Daulet Zhumadullaev, Yerzhan Boleubayev, Atabek Khussain, Abzal Kenessary, and et al. 2025. "Dry Carbonate Sorbents for CO2 Capture from Flue Gases: Role of Support in Adsorption Efficiency and Thermal Stability" Molecules 30, no. 13: 2859. https://doi.org/10.3390/molecules30132859
APA StyleKhussain, B., Sass, A., Brodskiy, A., Zhurinov, M., Torlopov, I., Rakhmetova, K., Zhumadullaev, D., Boleubayev, Y., Khussain, A., Kenessary, A., Sarsenova, A., & Darzhokov, T. (2025). Dry Carbonate Sorbents for CO2 Capture from Flue Gases: Role of Support in Adsorption Efficiency and Thermal Stability. Molecules, 30(13), 2859. https://doi.org/10.3390/molecules30132859






