Abstract
The aim of the study was to compare the quality of almond oils obtained using different extraction methods, including cold solvent extraction, Soxhlet extraction, and the Folch method. Oils were extracted from four commercially available almond-based products—unpeeled almonds, blanched almonds, almond flakes, and almond protein concentrate—and compared with a commercially refined almond oil. The extracted oils were analyzed for their fatty acid (FA) composition and selected quality parameters, including acid value, peroxide value, p-anisidine value, the TOTOX index, and specific extinction coefficients (K232 and K268). Based on the FA profiles, health-related indices such as atherogenic index, thrombogenic index, and hypocholesterolemic/hypercholesterolemic ratio were also calculated. Additionally, the oxidative stability of the oils was assessed using an accelerated method—pressure differential scanning calorimetry. The obtained results demonstrated that the extraction method had a stronger influence on almond oil quality than the type of raw material. Oil extracted from unpeeled almonds using Soxhlet and cold solvent techniques showed better oxidative stability and more favorable FA profiles, while oils obtained using the Folch method and commercial refined oils exhibited higher levels of primary and secondary oxidation products. These findings were further supported by statistical analyses, which revealed distinct groupings based on oxidation indices and lipid composition.
1. Introduction
The almond tree, also known as the almond (Prunus dulcis), is a fruit tree belonging to the Rosaceae family. It is native to the Middle East and Central Asia, but is now cultivated in regions with a Mediterranean climate, as well as in California, Iran, and Spain [1]. The almond tree is mainly cultivated for its edible seeds called almonds. Almonds have an elongated, ellipsoidal shape, and their surface is smooth, light beige, surrounded by brown skin. There are two varieties: sweet almonds (P. dulcis var. dulcis) and bitter almonds (P. dulcis var. amara) [2]. Both varieties, along with raspberries, apples, and pears, belong to the Rosaceae family, but sweet almonds are most often intended for consumption raw or roasted as a snack or addition to dishes. The market offers almonds with skin, blanched almonds, almond flakes, and products based on almonds, including flour, butter, almond milk, oil, and protein preparations [3]. In the food industry, they are used in confectionery and bakery products, for the production of beverages, pastes, and salads, and also as an addition to dietary snacks and high-protein products [4]. Bitter almonds, on the other hand, are not intended for direct consumption due to the presence of amygdalin, which is degraded to glucose, benzaldehyde, responsible for their bitter flavor, and toxic hydrogen cyanide [5]. The kernels are the edible part of the almonds and are considered a good source of minerals such as K, P, Ca, and Mg, which play a key role in the proper functioning of muscles, preventing them from cramps that can occur after intense physical exercise. In addition, almond seeds contain vitamin E, known for its antioxidant properties, and B vitamins, which are responsible for the proper functioning of the nervous system [6]. Almonds are also a good source of proteins (~20%), carbohydrates (~20%), dietary fiber (12.5%), and fat (~50%) [7]. Due to their high nutritional content, almonds are often used as a raw material for oil extraction, as almond oil (AO) is considered a valuable ingredient in both food and cosmetic industries due to its composition and health-promoting properties.
Literature reports indicate that the method of oil extraction from plant materials affects various properties of the oil, including chemical composition, nutritional value, purity, quality, oxidative stability, and shelf life [8]. The process of extracting oil from almonds is similar to that of extracting oils from other nuts or seeds. It can be done by different methods, depending on the desired quality of the oil and the scale of its production [9]. One of the methods used is cold-pressing, which is considered an ecological method that does not result in a qualitative change in the oil obtained [10]. In turn, conducting this process at a higher temperature can contribute to forming sulfur compounds that reduce its quality. To prevent this and improve purity, the oil should be refined. AO can also be obtained using various extraction methods, among which solvent extraction provides the highest industrial efficiency. The most commonly used solvent is hexane, one of the relatively cheap and readily available solvents that allows selective extraction of fat without affecting the presence of other components, such as fiber. However, it is necessary to remove its traces before consuming the oil so that it meets food safety standards [8].
AO has a rich and diverse chemical composition. It contains sterols, tocopherols, squalene, a significant content of monounsaturated fatty acids (MUFA), the primary representative of which is oleic acid (C18:1), a moderate content of linoleic acid (C18:2), and a relatively low level of saturated fatty acids (SFA) [11,12], which may be associated with a reduced risk of cardiovascular disease, as well as potential benefits in other diseases such as hypertension and diabetes [9,13]. Due to its mild, slightly nutty flavor, AO can be used for dressings, as an addition to salads, and in the production of almond butter or nut pastes as an agent that imparts smoothness and facilitates the right consistency, making the mass more homogeneous and easier to spread. Moreover, due to its favorable lipid profile, characterized by a high share of mono- and polyunsaturated FAs, it can act as a functional substitute for traditional fats in the technology of confectionery and bakery products, contributing to the improvement of their nutritional value.
AOs’ yield, physicochemical, and bioactive properties may vary depending on the isolation technique used. Özcan et al. [14] compared them using cold pressing and Soxhlet extraction for three almond varieties. In turn, Sayah et al. [15] determined them for oils obtained from almonds with and without skin using three extraction methods (supercritical CO2, cold press, and solvent extraction). An important factor influencing the quality of the AO obtained is also the type, degree of ripeness, and the form in which the almonds were used to get it, i.e., whether they were used in the form of raw almonds or whether they had previously been roasted or blanched. Roasting and blanching are pre-treatment methods that can significantly affect the raw material’s physicochemical properties and nutritional value, which in consequence may affect the chemical composition, quality, and functional properties of the resulting oil [16].
In this study, three solvent-based extraction techniques (Folch, Soxhlet, and cold solvent extraction) were applied to obtain oils from four different commercially available almond products, including unpeeled almonds, blanched almonds, almond flakes, and almond protein concentrate (APC). The nutritional properties, quality parameters, and oxidative stability of the extracted oils were determined and compared to those of commercially available refined AO.
2. Results and Discussion
2.1. Oil Extraction Yield
Literature data indicate that the oil extraction yield from seeds depends on several factors, including the type and composition of the raw material, the extraction method and conditions, as well as the nature and polarity of the solvent used. Moreover, the physical characteristics of the raw material, such as particle size, moisture content, and the presence of seed coat, can significantly affect oil accessibility during extraction [11,17,18].
In the present study, AO yield varied depending on both the type of raw material used (unpeeled almonds, blanched almonds, almond flakes, and APC) and the extraction method applied (Soxhlet, Folch, and cold solvent extraction), as shown in Table 1.

Table 1.
Extraction yield (w/w, %) of almond oil obtained using different extraction methods.
Soxhlet extraction proved to be the most efficient method for obtaining oil from blanched almonds, yielding nearly 86% of the oil content declared by the manufacturer. In contrast, the Soxhlet method was the least effective for APC: almost twice as much oil was obtained from APC using the Folch method (~7.51%) compared to Soxhlet extraction (~3.78%). Interestingly, the fat content extracted from APC using the Folch method (approximately 7.51%) exceeded the value declared by the manufacturer (6.6%). This discrepancy may be attributed to differences in analytical methodology, as the Folch method enables the extraction of both free and bound lipids, potentially capturing a broader range of lipid fractions than standard procedures used for nutritional labelling [19,20]. This is likely related to the higher polarity of the chloroform:methanol mixture, which facilitates the recovery of lipids bound to proteins and membranes—components that are particularly abundant in protein-rich materials such as APC.
Literature data indicate that the polarity of solvents used for extraction may play an important role in lipid recovery efficiency. The non-polar nature of hexane, used in both Soxhlet extraction and cold solvent extraction, favors the recovery of neutral lipids (triacylglycerols), which constitute the major fraction of AO [19]. Literature reports confirm that Soxhlet extraction can be more efficient than the Folch method, for example, in the case of walnut oil extraction [21]. In contrast, the more polar biphasic system of chloroform and methanol in the Folch method also enables the recovery of polar lipids (e.g., phospholipids, glycolipids). In addition, the cold maceration principle applied in the Folch method may reduce matrix degradation and facilitate the extraction of biologically active compounds, including antioxidants, due to the nature of the solvents [19,20,21]. Each of the extraction methods—Soxhlet, Folch, and cold solvent extraction—can influence oil yield and quality in positive or negative ways. While they may enhance lipid recovery and extract a wider range of compounds, they may also result in the co-extraction of undesirable substances or alter oil composition due to prolonged exposure to heat or solvents [21]. In the present study, differences in oil yield among the three extraction methods reflected not only variations in solvent polarity and extraction conditions, but also suggested the influence of other factors inherent to the raw material. This complexity underlines that solvent polarity alone cannot fully explain the observed differences in oil yield.
For unpeeled almonds, the highest oil yield was obtained by cold n-hexane extraction (~43.43%), whereas this method resulted in the lowest yield for blanched almonds (~37.02%). The opposite trend was observed for Soxhlet extraction, with the highest yield recorded for blanched almonds (~46.42%) and the lowest for unpeeled ones (~37.56%). This may be explained by the thermal degradation of heat-sensitive components present in almond skin, which could reduce oil recovery during Soxhlet extraction.
Other authors have reported different trends for oil extraction from unpeeled almonds. Miraliakbari and Shahidi [22] observed lower yields with cold n-hexane extraction (51.2%) compared to a chloroform/methanol mixture (53.5%). Similarly, Krzyczkowska et al. [20] reported higher oil yield with the Folch method (50.24%) than with Soxhlet extraction (43.75%). These discrepancies in extraction yields among studies may result from differences in raw material characteristics (such as variety or origin), solvent-to-solids ratios, and specific extraction conditions, including contact time. Both literature data and the results obtained in this study highlight the need to consider oil extraction as a process influenced by numerous interacting variables, rather than a single standardized procedure.
2.2. Fatty Acid Profile and Health Indices of Almond Oils
Plant oils are primarily composed of triacylglycerols; therefore, the FA composition and distribution within triacylglycerol molecules represent a key characteristic of the lipid fraction in plant seeds [23]. Table 2 shows the FA profiles of the tested AOs extracted by different methods and compared with the commercial, refined AO. The results revealed that oleic acid was the predominant FA in all analyzed oils, with its lowest content observed in the commercial AO (60.92%) and the highest in the oil obtained from blanched almonds via Folch extraction (69.59%). Oleic acid is the most common MUFA in commonly consumed foods. It enhances the activity of low-density lipoprotein (LDL) receptors and lowers serum cholesterol level [24]. As such, it plays a crucial role in the human diet by regulating cholesterol metabolism and contributing to a reduced risk of cardiovascular diseases. According to literature data, oils derived from olives, avocados, macadamia nuts, and hazelnuts also exhibit high oleic acid content, often exceeding 50% of their total FA composition [25,26].

Table 2.
Fatty acid composition (% of total fatty acids) of almond oils obtained by different extraction methods and compared with commercial refined almond oil.
The second most abundant FA in AO was linoleic acid, with the highest share found in the commercial AO (27.60%) and the lowest in the oil extracted from APC using the Folch method (19.50%). Although linoleic acid is the most abundant PUFA in the human diet, it is classified as essential, as it cannot be synthesized by mammals. Linoleic acid is vital for maintaining cell membrane integrity, regulating inflammatory processes, and supporting proper skin function and overall physiological homeostasis [27]. Similar to AO, other oils such as olive oil, avocado oil, and high-oleic sunflower oil also contain between 20% and 30% linoleic acid in their total FA profiles.
The FA composition of the analyzed AOs was compared with that reported by Maestri et al. [28]. The authors noted that AO typically contains oleic acid (50–80%), linoleic acid (10–26%), and palmitic acid (5–9%). The results obtained in this study fall within those reported ranges. The FA profile of all tested AOs was characterized by a high MUFA content (Table 2), with the highest share found in the oil extracted from blanched almonds using the cold solvent method (70.43%) and the lowest in commercial AO (61.35%). These values fall within the broad range (43.3–83%) reported by Ouzir et al. [8] for oils varying in almond origin and extraction method, as well as within the range reported by Rabadán et al. [29] for oils obtained from Spanish almond varieties (66–73.5%).
These findings are consistent with broader comparative studies evaluating MUFA levels across various plant oils. According to Tian et al. [30], AO exhibits the highest MUFA content (77.07%) among 28 tested plant oils, followed by olive oil (76.62%) and papaya seed oil (76.10%). Although MUFAs are less prone to oxidation than PUFAs, PUFAs are essential FAs that must be obtained through the diet. Both MUFAs and PUFAs play an important role in cardiovascular disease prevention.
Several dietary indices, such as the index of atherogenicity (AI), the thrombogenicity index (TI), and the hypocholesterolemic/hypercholesterolemic ratio (h/H), can be calculated based on FA profiles to assess the potential impact of dietary fats on cardiovascular health. It is recommended to consume products with a low AI (<1.0) and TI (<0.5), and a high h/H, as such profiles are associated with reduced total and LDL cholesterol levels in blood plasma. Conversely, consumption of products with a higher TI and a lower h/H ratio may increase the risk of cardiovascular diseases [23].
To provide a more comprehensive characterization of the analyzed oils, nutritional indices commonly used to assess potential health-promoting properties were calculated and are presented in Table S1 (Supplementary File). The tested AOs exhibited favorable nutritional and health-related properties, as reflected by very low AI values (0.07 and 0.08), low TI values (0.21 and 0.22, and 0.24 only for the Folch-extracted oil from APC), and a high h/H ratio (11.98–13.36). The most favorable profiles, characterized by very low AI and TI values and a high h/H ratio, were observed for the AOs obtained from APC using the Soxhlet method and the cold solvent extraction method. The obtained results suggest that, based on the applied indices, these oils may be considered the most beneficial from a cardiovascular health perspective.
Khalili Tilami and Kouřimská [31] compared AI and TI values across nine different categories of fats and oils, including nut oils. The values obtained in the present study were consistent with those reported for AO by the authors (AI = 0.07 and TI = 0.21). Notably, the AI values observed for AOs were among the lowest across all evaluated oils and comparable to those of hemp oil, chia seed oil, and hazelnut oil—products associated with low cardiovascular risk and recognized health benefits.
The TI values reported here fell within the range typical for traditional nut oils (0.16–0.35) and were similar to those for soybean oil (0.21) [31], blackcurrant seed oil, and hemp oil (0.23) [32].
The h/H index reflects the potential impact of dietary FAs on cardiovascular health by representing the balance between hypocholesterolemic FAs (cis-C18:1 and PUFA) and hypercholesterolemic FAs (C12:0, C14:0, and C16:0) [33]. The lowest h/H value was observed for the Folch-extracted oil from APC (11.98), whereas the highest h/H values were found in oil also obtained from APC but extracted using the Soxhlet method (13.36) and the cold solvent extraction method (13.32). The h/H value for refined, commercial oil was 12.64. The high h/H ratios calculated for the tested AOs indicate pronounced health-promoting potential, comparable to those of rosehip oil (11.81) [33], camelina oil (11.7–14.7) [34], dill seed oil (12.56), and blackcurrant seed oil (13.82) [32].
Given their promising nutritional profile, the overall quality of the AOs was further evaluated based on key physicochemical parameters, as discussed in the next section.
2.3. Determination of Quality Parameters
Quality parameters are among the most important factors to assess in oils, as they determine their resistance to degradation, storage stability, and functional properties. One of the main contributors to oil deterioration is the oxidation of TAGs, which occurs upon exposure to light or ambient oxygen. This process leads to the breakdown of TAG molecules and the release of free FAs [21].
The acid value (AV) serves as an indicator of oil freshness and the extent of hydrolysis, reflecting the concentration of free FAs present. According to Codex Alimentarius standards, the AV of cold-pressed vegetable oils should not exceed 4 mg KOH per gram of oil, while for refined oils, the limit is 0.6 mg KOH/g oil [35]. In the current study, almost all tested oils complied with these standards (Table 3), except for the oil extracted from APC using the Folch method, which showed a significantly elevated AV (16.77 mg KOH/g oil). When comparing the influence of extraction method on AV, the highest values were consistently observed for oils extracted using the Folch method, regardless of the raw material. In contrast, oils obtained using the cold-solvent and Soxhlet methods yielded comparable, and notably lower, AVs. In terms of raw material, the lowest AV was found in commercial refined oil (0.24 mg KOH/g oil), which is consistent with the known effect of refining processes that remove impurities and reduce rancidity-promoting compounds [36,37]. Interestingly, similar low AVs were obtained in oils extracted from unpeeled almonds and APC using both the cold-solvent and Soxhlet methods.

Table 3.
Qualitative parameters of commercial and self-extracted almond oils.
On the other hand, higher AVs were observed in oils derived from blanched almonds (0.89–1.50 mg KOH/g oil), and even higher in oils from almond flakes (1.37–2.04 mg KOH/g oil). These findings suggest that blanched and processed almond products are more susceptible to unfavorable environmental factors such as heat and humidity, which promote hydrolytic degradation. Moreover, the structural disruption occurring during blanching and flaking enhances the exposure of oils to oxygen and lipolytic enzymes, thereby accelerating hydrolysis and oxidation [38]. Additionally, the increased surface area in flaked almonds facilitates greater contact between the oil and ambient air and moisture, further contributing to AV elevation.
Following the evaluation of AV, the peroxide value (PV) provides further insight into the early stages of lipid oxidation. PV reflects the extent of oxidative changes in oil, and it is directly proportional to the concentration of primary oil oxidation products, namely peroxides. According to the Codex Alimentarius, the maximum permissible PV is 15 mEq O2/kg for cold-pressed vegetable oils and 10 mEq O2/kg for refined vegetable oils [39]. Almost all tested oils met these standards (Table 3), with the exception of oil extracted using the Folch method from unpeeled almonds (10.19 mEq O2/kg) and APC (13.07 mEq O2/kg).
Similarly to AV, PV was also consistently highest in oils extracted by the Folch method, regardless of the raw material. However, for PV, more favourable (lower) values were observed in oils extracted using the Soxhlet method (1.77–2.25 mEq O2/kg) compared to those obtained via the cold solvent method (2.05–3.87 mEq O2/kg). In contrast, an opposite trend was noted for the p-anisidine value (p-AnV), which reflects the presence of secondary oxidation products such as aldehydes. In this case, lower p-AnV was observed in oils extracted by the cold solvent method (0.30–0.75) than by Soxhlet extraction (0.51–1.54) (Table 3). These findings can be attributed to the thermal conditions of Soxhlet extraction, which is conducted at the boiling point of the solvent. Under such conditions, primary oxidation products (peroxides) may decompose into secondary products [40], leading to a reduction in PV and a corresponding increase in p-AnV. As with AV and PV, the highest p-AnVs were recorded for oils extracted using the Folch method. Interestingly, when the raw material underwent pre-treatment processes such as blanching or flaking, the oil extracted from it via the Folch method showed a PV nearly half that of the oil from unpeeled almonds, but p-AnV nearly twice as high. Additionally, the total oxidation index (TOTOX), which accounts for both primary and secondary oxidation products and thus provides a comprehensive measure of oxidative deterioration, was also highest in oils obtained by the Folch method (ranging from 13.92 to 30.11) (Table 3).
Similarly, refined oil showed relatively high values of quality parameters: PV (9.65 mEq O2/kg), p-AnV (3.24), and TOTOX (22.53). Although refining, which includes several steps (neutralization, bleaching, degumming, and deodorization), typically reduces the AVs and PVs by removing free FAs and primary oxidation products [36], the p-AnV may either decrease or, under certain conditions (e.g., excessively high temperature or prolonged deodorization), increase due to the formation or incomplete removal of secondary oxidation compounds [41,42]. Simultaneously elevated PV and p-AnV in the tested commercial oil may indicate that it had undergone both primary and secondary oxidation, reflecting advanced lipid degradation and reduced oxidative stability. This could result from factors such as improper storage, prolonged storage time, low natural antioxidant content, secondary contamination, or the presence of oxidation catalysts [43].
El Bernoussi et al. [5] reported that cold-pressed sweet AO degraded significantly during storage at 60 °C for four 4 weeks, with the PV increasing from 2.4 to 24.6 mEq O2/kg. Similalry, Sidhu et al. [44] observed marked increases in PV (from 2.66, 2.55 and 2.95 to 9.02, 8.06, and 9.88 mEq O2/kg), p-AnV (from 3.67, 3.71, and 3.63 to 16.88, 16.43, 15.89), and TOTOX (from 8.99, 8.81, and 9.53 to 27.93, 26.67, and 27.99) in AOs from Australian, American, and Iranian origins, respectively, after 21 days of storage at elevated temperature. In contrast, self-extracted AOs from almond flour tested by Dias et al. [10] showed favorable quality parameters (PV = 1.8–2.0 mEq/kg oil, p-AnV = 0.1–0.4, and TOTOX = 4.0–4.3) compared to those reported in the present study. It is worth noting that the authors used environmentally friendly extraction and recovery methods. Similarly low PVs, ranging from 1.9 to 3.2 mEq/kg, were reported for different varieties of sweet AO self-obtained by Melhaoui et al. [12]. These findings suggest that the good quality parameters observed in the self-extracted oils may be attributed to their freshness and the fact that they were tested immediately after extraction. The commercial oil was analyzed during its labeled shelf life. However, the conditions of its transport and storage prior to the study were unknown and may have adversely affected its quality.
Other parameters used in the oil quality assessment are specific extinction coefficients denoted as K232 and K268. While the K232 coefficient is an indicator of the presence of conjugated dienes, which are formed as a result of the primary oxidation of unsaturated FAs, especially linoleic acid, the K268 coefficient is used to assess the content of conjugated trienes and secondary oxidation products, such as aldehydes and ketones [45]. Increased K232 values are the first signal of deteriorating oil quality, and K268 indicates the presence of more persistent and undesirable degradation compounds. The K232 values presented in Table 3 showed that for all the extracted oils, they were in the range of 1.89 ± 0.06–4.97 ± 0.19. Regardless of the type of extraction method used, the lowest K232 values were observed for oils from unpeeled almonds, amounting to 2.00 ± 0.01 (cold solvent extraction), 1.89 ± 0.06 (Soxhlet method), and 2.87 ± 0.01 (Folch method), respectively. It seems that this may be influenced by the presence of protective compounds in the brown skin of the almond [15]. In turn, oils extracted from APC were characterized by the highest K232 values, which were 3.42 ± 0.07 (cold solvent extraction), 3.18 ± 0.04 (Soxhlet method), and 4.97 ± 0.19 (Folch method), respectively. The K232 values obtained for these types of oils corresponded to a similar trend noted when discussing the PV, which may indicate the beginning of the oxidation process of the linoleic acid contained in them. Considering the method of oil extraction from the raw material used, it was observed that slightly lower K232 values were determined for oils obtained by the Soxhlet method, and higher ones by the Folch method. In contrast, no statistically significant differences were observed in the K232 values obtained for oils from unpeeled almonds and almond flakes extracted by the Soxhlet and cold solvent methods, in which the primary solvent was n-hexane. However, slight differences were observed when blanched almonds and APC were used as raw materials. The K232 values obtained for the oils extracted from almonds with n-hexane using both the Soxhlet apparatus and the cold solvent extraction method were similar to those obtained for the oils extracted from almonds with petroleum ether as extractant and not subjected to electron beam treatment [46].
About the K268 coefficient, the values obtained for the studied oils were low and close to each other. They ranged from 0.10 ± 0.01 for oil obtained from almond flakes by the cold solvent extraction method to 0.86 ± 0.02 for oil from APC obtained by the Folch method. No statistically significant differences were observed in the K268 values for the studied oils obtained using the same raw material, the Soxhlet method, and cold solvent extraction. These differences were significant when comparing oils obtained from the same material but using the Folch method. There, an increase in the K268 extinction coefficient was found. In general, the values of the two extinction coefficients K232 and K268 were higher when the oils were extracted by the Folch method, i.e., using a solvent mixture that could be too aggressive, leading to co-extraction of compounds susceptible to oxidation. What is worth emphasizing is that the highest values of K232 and K268, as well as the previously discussed PV, p-AnV, and TOTOX parameters, were observed for the commercial oil studied. This may indicate lipid degradation and reduced oxidative stability in this sample.
Overall, the results indicate that differences in quality parameters among the extraction methods were not solely attributable to solvent polarity. As mentioned earlier, both cold solvent and Soxhlet extraction employed n-hexane as the solvent, whereas the Folch method used a chloroform: methanol mixture, which differs substantially in polarity. This variation in solvent polarity likely influenced the extraction efficiency of peroxides and other oxidation products. While the extraction of highly polar oxidation products is unlikely to be the main cause of yield differences, our results indicate that such compounds may still be recovered and contribute to the higher quality parameters observed in Folch-extracted oils. In addition, factors such as extraction temperature and raw material characteristics, including prior processing (e.g., blanching or flaking), also played a significant role. These findings highlight the multifactorial nature of oil quality determination and the need to interpret oxidation indices in the context of both solvent properties and process-related variables, suggesting that both the type of raw material and the extraction method may also influence the oxidative stability of AO, which is examined in the following section.
2.4. Oxidative Stability by Pressure Differential Scanning Calorimetry
Lipid oxidation plays a crucial role in determining the final quality and nutritional value of food products, as it is the primary reaction responsible for their degradation. Oxidative stability is therefore a key quality indicator for edible oils [47]. In food chemistry, pressure differential scanning calorimetry (PDSC) is an accelerated calorimetric technique used to assess the oxidative stability of plant oils [34]. The oxidation time determined by PDSC (τmax) corresponds to the maximum oxidative changes.
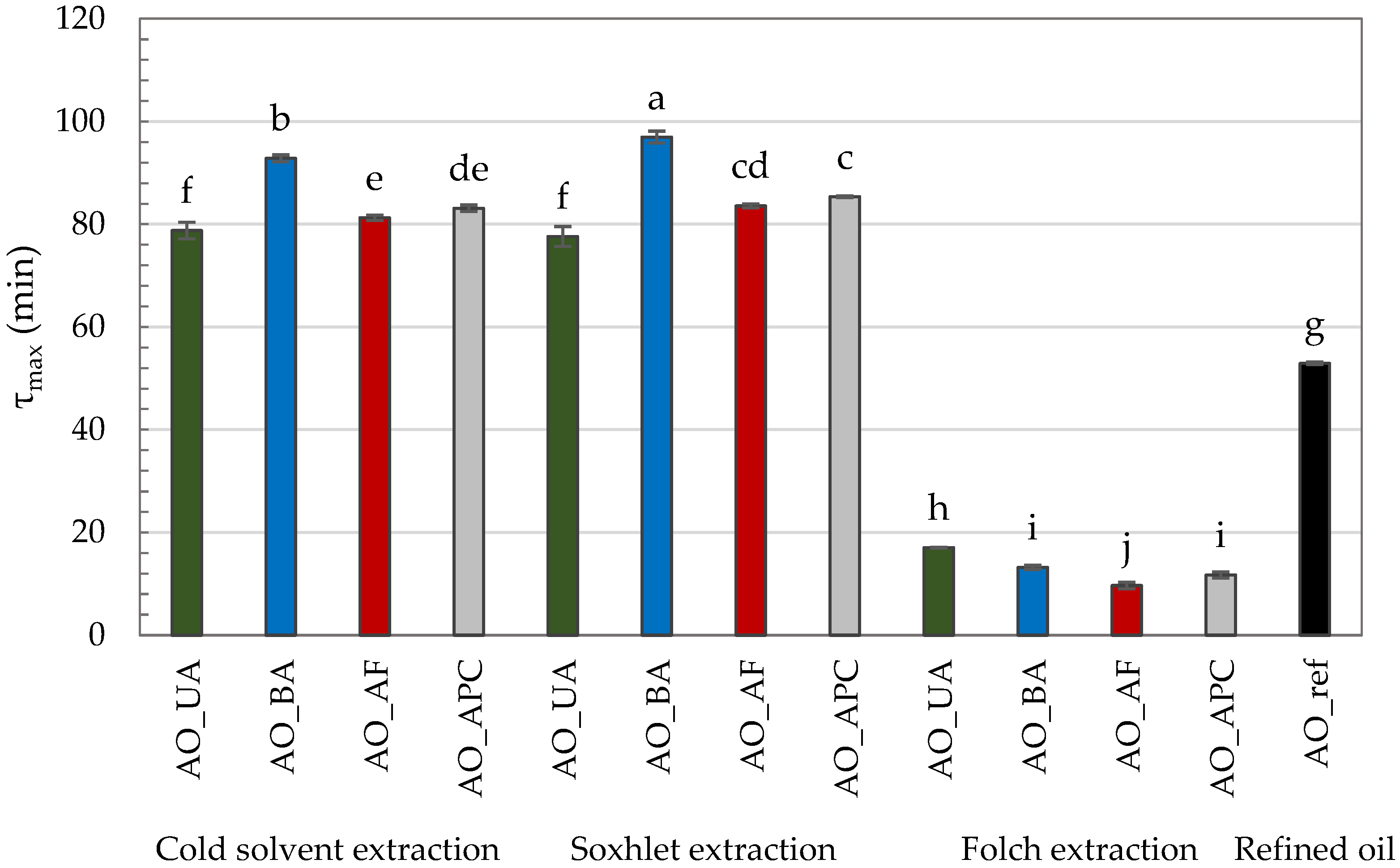
The τmax values for the tested AOs varied considerably depending on the extraction method and the raw material variant (Figure 1). The shortest τmax values (9.67–17.06 min) were recorded for oils extracted using the Folch method, indicating their markedly lower oxidation resistance. This observation is in line with their higher PV, p-AnV, and TOTOX values, which reflect both a lower initial resistance to oxidation and a more advanced oxidative deterioration, further accelerated under PDSC conditions.
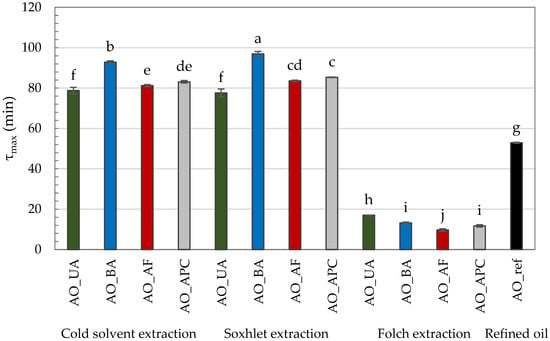
Figure 1.
PDSC oxidation time (τmax) of commercial and self-extracted almond oils, where: AO_UA—oil extracted from unpeeled almonds; AO_BA—oil extracted from blanched almonds; AO_AF—oil extracted from almond flakes; AO_APC—oil extracted from almond protein concentrate; AO_ref—commercial, refined oil. Lowercase letters (a–j) indicate a significant difference at the significance level of 0.05 (Tukey HSD).
In contrast, τmax was significantly higher and comparable for oils extracted by cold solvent and Soxhlet methods, with Soxhlet-extracted oils consistently showing the highest τmax values. The extended τmax in Soxhlet-extracted oils may be attributed to high extraction temperatures, which can inactivate prooxidant enzymes such as lipoxygenase [38,48]. Under cold extraction, such enzymes may remain active and initiate oxidative processes during oil preparation. Moreover, elevated temperatures during Soxhlet extraction may enhance the release of lipophilic antioxidants from the plant matrix [49]. Qi et al. [11] demonstrated that both phytosterols and tocopherol with tocotrienols have a significant correlation with oxidation induction time (p < 0.01).
An exception to this trend was observed for oils derived from unpeeled almonds, where no statistically significant differences in τmax were found between extraction methods. However, even among hexane-extracted oils, this variant exhibited the lowest τmax (~78 min), suggesting the least oxidative stability. Conversely, the highest τmax values were observed for oils obtained from blanched almonds—92.82 min (cold solvent extraction) and 96.96 min (Soxhlet extraction)—indicating the greatest oxidative resistance. Interestingly, this cannot be directly linked to better conventional quality indicators, as these oils did not display superior PV, p-AnV, and TOTOX values. This discrepancy highlights a key distinction: while PV, p-AnV, and TOTOX reflect the current oxidative state of the oil, τmax measures its resistance to future oxidation under accelerated conditions. Thus, an oil may already be partially oxidized yet still retain compounds that slow further oxidation. The enhanced oxidative stability of oils from blanched almonds may be explained by the blanching process itself, which may remove prooxidant components present in the peel and thermally inactivate oxidative enzymes [38,50]. Although there is a remarkable amount of antioxidants concentrated in the peels of nuts, they can interact with each other and exhibit either synergistic or inhibitory effects [51].
The value of τmax is closely related to the FA composition of the oil. It is well-established that the presence of multiple bonds significantly accelerates the rate of oil oxidation; for example, linoleic acid oxidizes 10–40 times faster than oleic acid, while α-linolenic acid oxidizes 2–4 times faster than linoleic acid [52]. In the tested AOs, α-linolenic acid was absent, and the percentage of oleic acid (60.92–69.79%) was significantly higher than that of linoleic acid (19.50–27.60%). Notably, the highest proportion of linoleic acid was found in the commercial oil (27.60%), which, along with elevated quality parameters, likely contributed to its markedly lower τmax value (52.91 min) compared to the hexane-extracted oils.
Lipid oxidation is a complex phenomenon influenced by many factors, including processing methods and parameters, exposure to light, oxygen, and elevated temperatures during storage, as well as the presence of non-glyceride components with pro- or antioxidant activity, and the overall quality of the raw material used [23]. Among these factors, FA composition and the method used to obtain the oil play a particularly important role in determining oxidative stability.
Presented results clearly indicate that τmax is shaped by a multifactorial interplay between intrinsic oil characteristics, with particular emphasis on the FA profile, and the method used to obtain it. These findings are consistent with literature data obtained under identical PDSC conditions, which allows a direct and reliable comparison of τmax values between different oils. Ratusz et al. [53] reported a τmax of 56.73 min for refined rapeseed oil and 24.55 min for sunflower oil, consistent with their respective FA profiles: rapeseed oil (~60% oleic acid, 20% linoleic acid) and sunflower oil (~30% oleic acid, ~60% linoleic acid) [54]. Similarly, Siol et al. [23] found that refined watermelon seed oil had a markedly lower τmax (23.88 min) than oil extracted with hexane at room temperature (76.55 min), highlighting the strong influence of the oil obtaining method on oxidative stability.
In the context of these findings, AO—particularly when obtained from blanched kernels—exhibits relatively high oxidative stability compared with other edible oils, positioning it favourably within the broader spectrum of vegetable oils analysed under PDSC conditions. Moreover, the application of PDSC provides an additional and valuable tool for benchmarking oxidative stability across different oils, offering predictive insights that complement conventional quality indices such as PV, p-AnV, and TOTOX.
2.5. Multivariate Analysis of Almond Oil Quality Parameters
Multivariate statistical analyses were conducted to complement the experimental assessment of AO quality. These methods aimed to uncover patterns and relationships among samples based on their composition and quality parameters.
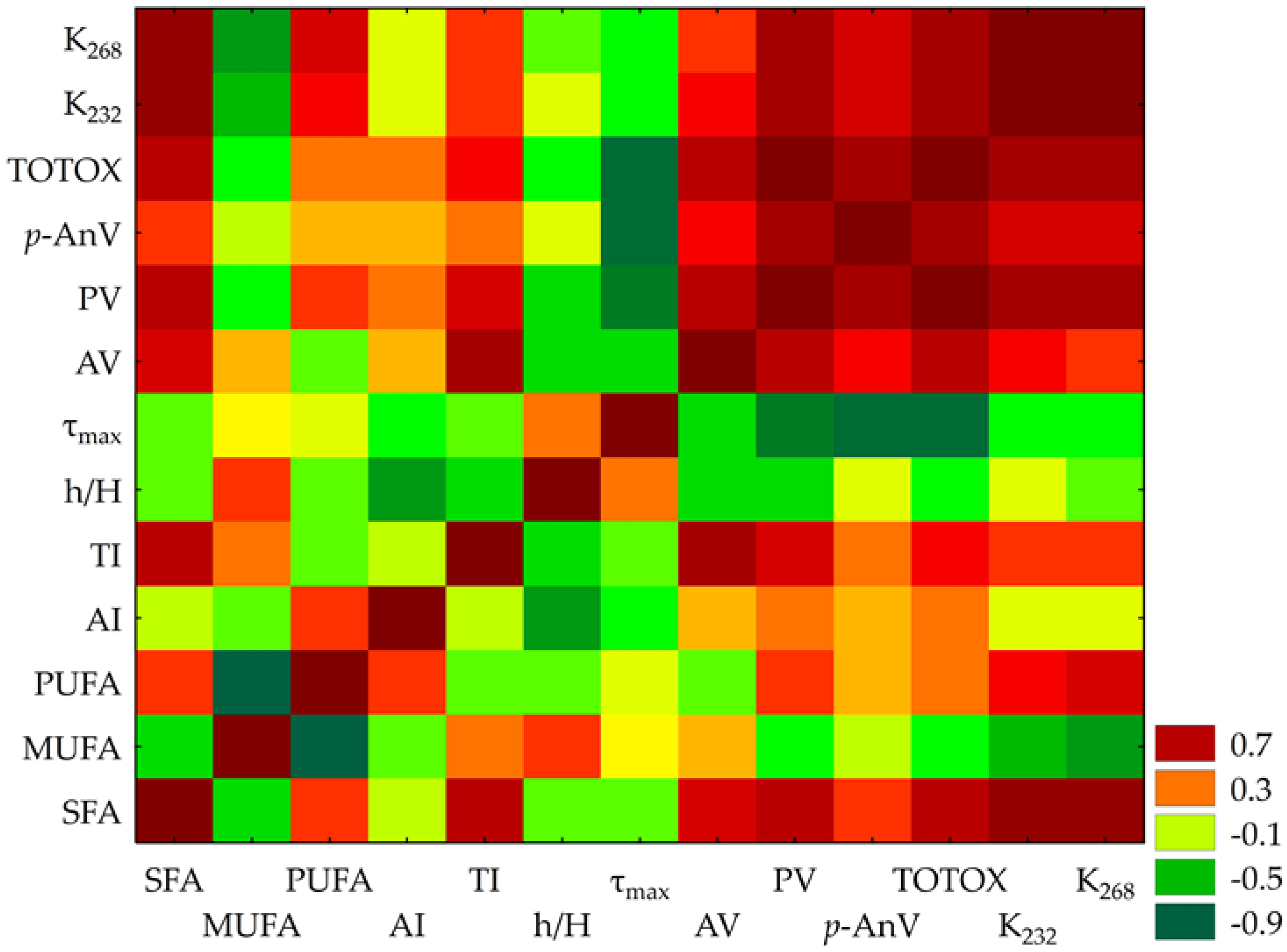
The correlation matrix (Figure 2) revealed significant relationships among 13 variables. Notably, PV and TOTOX exhibited an exceptionally strong positive correlation (r = 0.993), suggesting near-perfect alignment in their measurement of oxidative degradation. Similarly, K232 and K268, both absorbance indices linked to conjugated dienes and trienes, showed a strong positive association (r = 0.933). PV demonstrated robust correlations with K232 (r = 0.751) and K268 (r = 0.757), reinforcing their role as markers of oxidative processes.
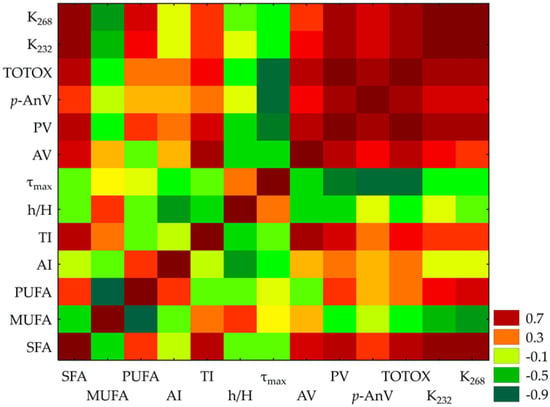
Figure 2.
Heatmap of Pearson correlation coefficients among lipid stability indices and fatty acid profiles (n = 13), where: SFA—saturated fatty acids, MUFA—monounsaturated fatty acids; PUFA—polyunsaturated fatty acids; AI—index of atherogenicity; TI—index of thrombogenicity; h/H—hypocholesterolaemic/hypercholesteraemic index; AV—acid value; PV—peroxide value; p-AnV—p-anisidine value; TOTOX—total oxidation index; K232 and K268—the specific extinction coefficients; τmax—PDSC oxidation time. Red indicates positive correlations while green denotes negative correlations, with significance at p < 0.05.
A striking inverse relationship emerged between p-AnV and τmax (r = −0.893), suggesting that shorter τmax corresponds with higher secondary oxidation products.
SFAs demonstrated moderate-to-strong positive correlations with K232 (r = 0.859) and K268 (r = 0.866). Conversely, MUFA showed a weak negative correlation with the atherogenic index (r = −0.283). Meanwhile, AI and h/H exhibited a moderate negative correlation (r = −0.600), indicating a significant relationship between FA profiles and cardiovascular risk indices.
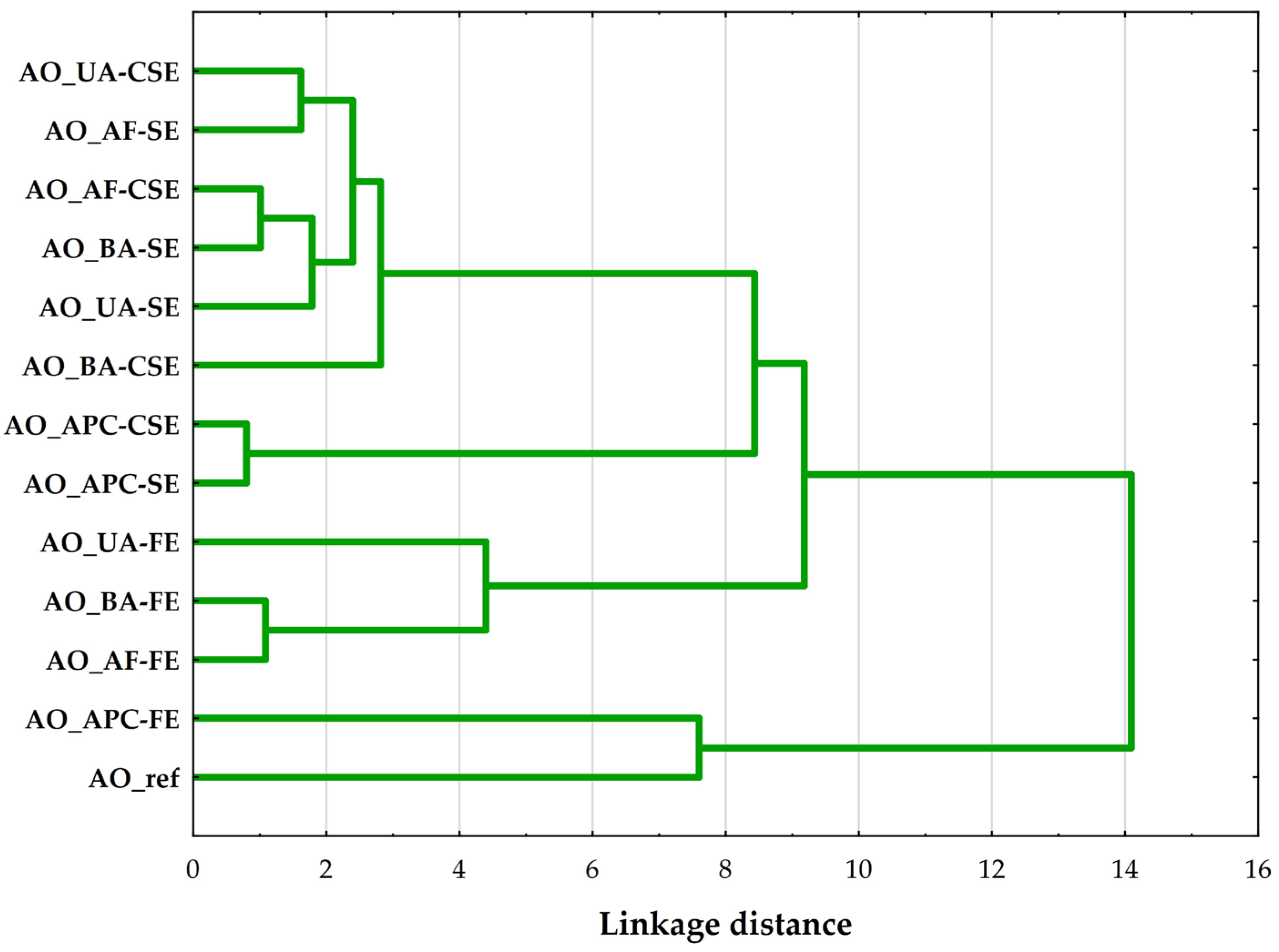
A hierarchical cluster analysis (HCA) was performed on the AO samples using Ward’s method with Euclidean distance as the similarity metric. The resulting dendrogram (Figure 3) shows the presence of four main clusters, suggesting significant compositional and quality differences among the AO samples.
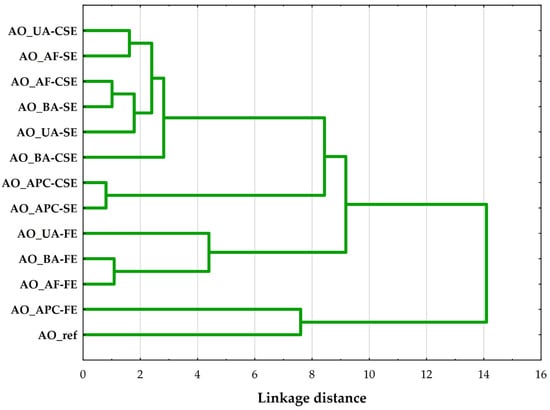
Figure 3.
Hierarchical cluster analysis (Ward’s method, Euclidean distance) dendrogram of compared almond oils, where: AO_UA—oil extracted from unpeeled almonds; AO_BA—oil extracted from blanched almonds; AO_AF—oil extracted from almond flakes; AO_APC—oil extracted from almond protein concentrate; AO_ref—commercial, refined oil; CSE—cold solvent extraction; SE—Soxhlet extraction; FE—Folch extraction.
The first cluster groups oils extracted using cold solvent and Soxhlet methods from whole almond forms (unpeeled, flaked, and blanched). These oils likely share similar FA profiles and low oxidative degradation, suggesting that such extraction conditions preserve the intrinsic properties of the source material. The similarity across different almond types indicates that the extraction technique has a greater influence on the oil’s profile than the almond’s processing form in this group [15].
The second cluster includes oils derived from APC, extracted using both cold solvent and Soxhlet methods. The close grouping reflects a unique compositional profile distinct from that of whole almond forms, likely due to prior protein isolation, which alters the matrix and residual lipid content. These oils exhibited a lower PUFA content compared to oils from whole almonds.
The oils in the third cluster were all obtained through Folch extraction, regardless of the form of the almond. The clustering indicates that the Folch method, which utilizes a chloroform–methanol solvent system, results in a distinct oil quality, possibly due to its higher extraction efficiency for polar lipids [22]. These differences suggest that the extraction method plays a significant role in shaping the oil characteristics in this case, overshadowing variations attributed to almond form.
The final and most distinct cluster includes Folch-extracted oil from APC and a commercial refined AO. Despite their different origins and extraction processes, these oils clustered together due to their significantly altered composition. Notably, this group was characterized by low overall oil quality, as indicated by elevated PV, p-AnV, and TOTOX index, along with K232 and K268 indices showing higher levels of primary and secondary oxidation products.
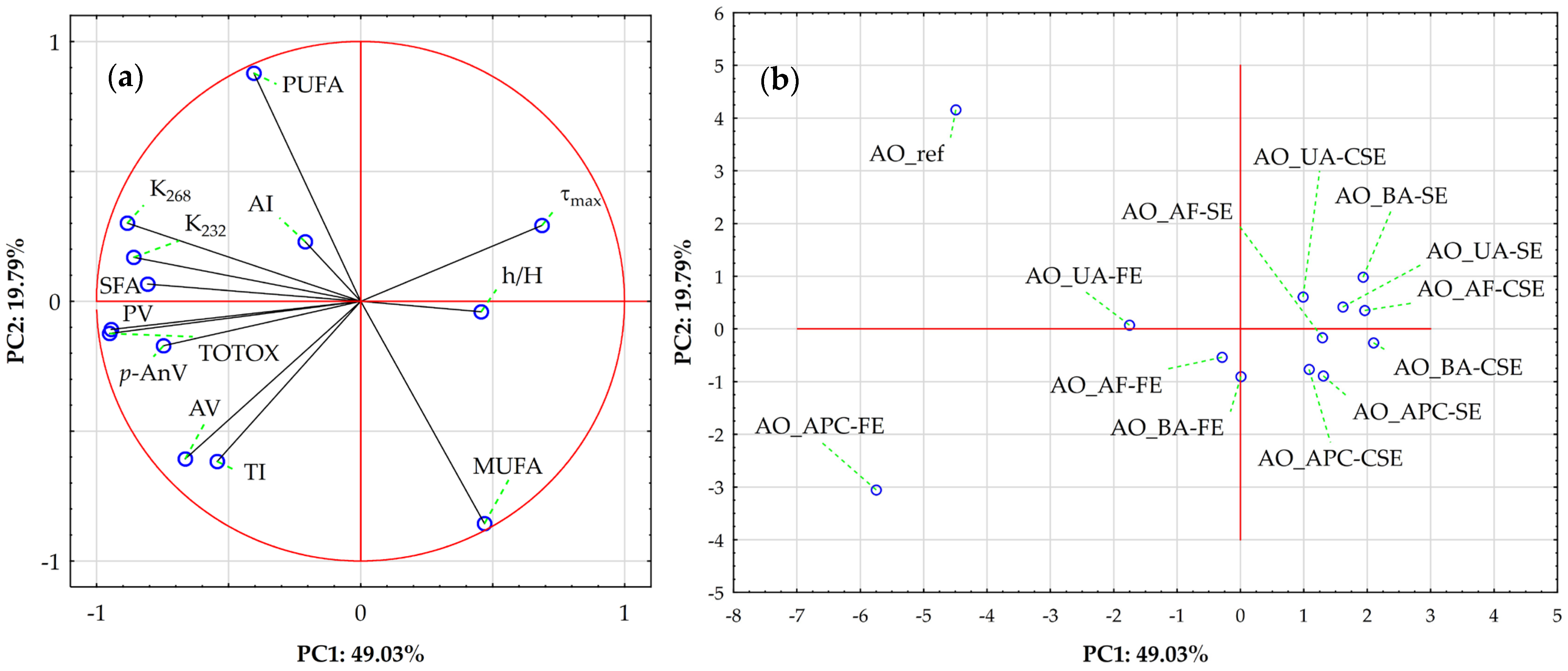
Finally, Figure 4 illustrates the results of a Principal Component Analysis (PCA) to explore sample variation driven by lipid stability indices and FA profiles. Together, the first two principal components explain 68.82% of the total variance (PC1: 49.03%; PC2: 19.79%). The correlation circle plot (Figure 4a) shows PC1 is mainly influenced by strong negative loadings from TOTOX (−0.951), PV (−0.945), K268 (−0.884), K232 (−0.860), SFA (−0.807), p-AnV (−0.747), and AV (−0.664), and strong positive loadings from τmax (0.687), MUFA (0.469), and h/H (0.457). This plot reveals distinct groupings of variables, with the length of vectors indicating each variable’s relative influence. A tight cluster of oxidation markers (TOTOX, PV, p-AnV, K232, K268, and SFA) mainly influences PC1 in the negative direction, emphasizing their strong positive correlation and role as key factors in variation linked to oxidative degradation. These markers are primary indicators of oil quality deterioration. In contrast, MUFA, τmax, and h/H are projected in the positive PC1 direction, directly opposing the oxidation markers, suggesting an inverse relationship with oxidation severity. PC2 is driven primarily by a strong positive loading from PUFA (0.878), with smaller positive contributions from τmax (0.292) and K268 (0.301), and strong negative contributions from MUFA (−0.856), TI (−0.616), and AV (−0.607), indicating that PUFA variation occurs largely independently of the oxidation markers.
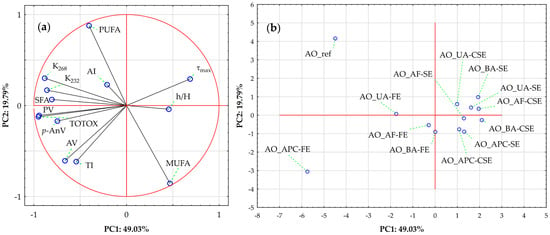
Figure 4.
Principal component analysis (PCA) of almond oils based on lipid stability indices and fatty acid profiles. (a) Loading plot for PC1 vs. PC2; where symbol designations are as in Figure 2. (b) Score plot showing sample distribution on PC1 vs. PC2; where symbol designations are as in Figure 3.
Then, the Score Plot (Figure 4b) visualizes sample distribution in the PC1-PC2 space. The extraction method (SE—Soxhlet extraction, FE—Folch extraction, CSE—cold solvent extraction) emerges as a key differentiator, with clear clustering visible despite some overlap—a finding strongly aligned with the hierarchical results (Figure 3). Refined and Folch extracted-protein concentrate oils occupy the extreme negative end of PC1, confirming their association with advanced oxidation. In contrast, samples near the center exhibit intermediate profiles.
3. Materials and Methods
3.1. Materials and Chemicals
Commercial, refined sweet AO (Prunus dulcis) of pharmaceutical quality (Greenaction, Kielce, Poland) was purchased within its shelf life and tested prior to expiration. The product originated from the USA.
Certified organic almonds, almond flakes, and blanched almonds were purchased from Bio Planet (Leszno, Poland). The unpeeled Italian almonds contained 22% protein, 53% fat (including 5.1% SFA), 3.9% carbohydrates (including 3.5% sugars), 13% fiber, and 0.03% salt. The blanched almonds (also from Italy) had a composition of 22% protein, 54% fat (including 3.9% SFA), and 5.4% carbohydrates (including 4.8% sugars). Spanish almond flakes contained 22% protein, 53% fat (5.1% SFA), 3.9% carbohydrates (3.5% sugars), and 13% fiber.
A commercially available APC (Smart Organic, Sofia, Bulgaria), with a minimum protein content of 50% (dry basis), was used in this study. According to the manufacturer’s data, APC contained 6.6% fat (0.6% SFA), 9.6% carbohydrates (9.0% sugars), 14% fiber, and 0.03% salt, and was obtained through a gentle, low-temperature, solvent-free extraction process.
All solvents and reagents used were of chromatographic or analytical grade, sourced from Avantor (Gliwice, Poland), except for standard compounds, which were supplied by Sigma–Aldrich (Saint Louis, MO, USA). All analyses were carried out after oil extraction and prior to the expiration date of commercial oil.
3.2. Methods
3.2.1. Oil Extraction
Extractions were performed immediately after opening commercial products. Raw materials (unpeeled almonds, blanched almonds, and almond flakes) were ground separately using a laboratory mill to facilitate solvent penetration. Each extraction was carried out using 30 g of ground raw material or 60 g of protein powder.
Cold Solvent Method
Samples were extracted with 300 mL of n-hexane (200 mL for protein powder) by mechanical shaking at room temperature for 120 min. After extraction, the mixtures were centrifuged at 5000 rpm for 20 min. The supernatants were dried with anhydrous magnesium sulfate, which was then filtered off. Solvent was removed under reduced pressure at 40 °C using a rotary vacuum evaporator (Rotavapor® R-300, BUCHI, Uster, Switzerland), followed by nitrogen purging to eliminate residual solvent.
Soxhlet Method
Samples were wrapped in filter paper, placed into extraction thimbles, and extracted with n-hexane (as in Section Cold Solvent Method) using a Soxhlet apparatus for 4 h at the solvent’s boiling point. After extraction, the solvent was removed by rotary evaporation under reduced pressure, and residual hexane was eliminated by purging with nitrogen.
Folch Method
Oil extraction was performed using the Folch method [55], modified according to Boselli and Caboni [56]. Samples were mixed with 100 mL of a chloroform:methanol (1:1, v/v) solution, shaken for several minutes, and incubated in a laboratory dryer at 60 °C for 20 min. After cooling, 100 mL of chloroform was added to re-extract remaining lipids, followed by mechanical shaking. The mixtures were filtered using a Büchner funnel to recover solid residues. Subsequently, 70 mL of aqueous potassium chloride was added, and the mixtures were left overnight at 4 °C. The following day, the biphasic systems were allowed to reach room temperature and were separated using a separatory funnel. The lower (chloroform) phase was collected and dried over anhydrous sodium sulfate for 2 h, then filtered to remove the drying agent. The solvent was evaporated under reduced pressure, and residual solvent was removed by purging the oil samples under a stream of nitrogen.
3.2.2. Oil Yield Determination and Fatty Acid Composition Analysis
Oil yield from the raw materials was determined gravimetrically and expressed as a percentage, calculated as the ratio of the extracted oil mass to the initial sample mass, according to Equation (1):
where mo is the mass of the extracted oil and ms is the mass of the raw material.
The FA composition of each oil sample was determined by gas chromatography using a YL6100 GC Clarity system (Young Lin Bldg., Anyang, Hogye-dong, Republic of Korea) equipped with a flame ionization detector and a BPX-70 capillary column (SGE Analytical Science, Milton Keynes, UK). FA methyl esters were prepared according to EN ISO 5509:2001 [57], and nitrogen was used as the carrier gas.
The chromatographic conditions were as follows: initial oven temperature of 70 °C (held for 30 s), increased to 160 °C at 15 °C/min, then to 200 °C at 1.1 °C/min, and finally to 225 °C at 30 °C/min (held for 1 min). The injector and detector temperatures were set at 225 °C and 250 °C, respectively.
FAs were identified by comparing their retention times with those of a standard FA methyl esters mixture (Supelco 37 Component FAME Mix, Sigma-Aldrich, Bellefonte, PA, USA). The relative content of each FA was expressed as a percentage of the total identified FAs.
3.2.3. Health Indices of Oils
The FA composition was used to calculate the health-related lipid indices of the tested oils. The atherogenic index and thrombogenic index were calculated according to the equations proposed by Ulbricht and Southgate (Equations (2) and (3)) [58], while the hypocholesterolaemic/hypercholesterolaemic ratio was determined from Equation (4) [59]:
where: FA—fatty acids; UFA—unsaturated fatty acids; SFA—saturated fatty acids; MUFA—monounsaturated fatty acids; PUFA—polyunsaturated fatty acids. The calculations were based on the percentage area of each FA relative to the total FA content.
3.2.4. Quality Parameters Determination
The physicochemical quality of the tested oils was evaluated based on standard lipid quality parameters. The AV, reflecting the extent of hydrolytic degradation, was determined using the AOCS Official Method Te 1a-64 [60]. The PV, representing the content of primary oxidation products, was assessed according to AOCS Cd 8b-90 [61]. Both AV and PV were measured using an automatic titrator (TitraLab AT1000 Series, Hach Lange, Wroclaw, Poland).
The degree of secondary oxidation was determined based on the p-anisidine value, following AOCS Cd 18–90 [62]. The TOTOX index was calculated using the following formula: TOTOX index = (2 × PV) + p-AnV.
The determination of the specific extinction coefficients, K232 and K268, was carried out by preparing a 0.3% and a 1% (m/v) sample oil solution in isooctane [63]. Absorbance at 232 nm and 268 nm was measured using a UV/VIS double-beam scanning spectrophotometer (Shimadzu, Kyoto, Japan). K232 and K268 were calculated using Equation (5):
where Kλ is the specific extinction coefficient at wavelength λ, Eλ is the measured absorbance at wavelength λ, c is the concentration of the oil solution (g/100 mL), and s is the cuvette thickness (cm).
3.2.5. Oxidative Stability Determination
Oxidative stability was assessed using pressure differential scanning calorimetry with a DSC Q20P thermal analyser (TA Instruments, New Castle, DE, USA). Oil sample (3.0–4.0 mg) was placed in an open aluminium pan in a cell with an empty reference pan and analysed at a constant temperature of 120 °C under a pressure of approximately 1400 kPa. The PDSC oxidation time, corresponding to the maximum rate of heat flow, was recorded and used as a measure of oxidative stability.
3.3. Statistical Analysis
All oil samples were analyzed in triplicate, and the results are expressed as mean ± standard deviation (SD). Statistical analysis was performed using Statistica software, version 13.3 (StatSoft, Krakow, Poland). Differences between means were evaluated using two-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. Statistical significance was considered at p ≤ 0.05. Furthermore, multivariate analyses were employed to examine relationships between variables and to characterize the AO samples. These included PCA, HCA performed on standardized data using Ward’s method with Euclidean distance, and correlation analysis, presented as a heatmap of Pearson correlation coefficients.
4. Conclusions
This study demonstrated that the type of almond raw material used and the extraction method influenced the yield and quality of the obtained oils. The Folch and cold solvent extraction methods applied to blanched almonds yielded oils with the highest oleic acid and MUFA content, while commercially refined oil showed the lowest. All extracted oils had a favorable FA profile, as reflected by low indices of atherogenicity and thrombogenicity and a high h/H ratio, confirming their health-promoting potential. However, the extraction method significantly influenced the quality parameters of the obtained AOs. Those extracted by the Folch method consistently showed the highest AV, PV, p-AnV, TOTOX, K232, and K268 values, as well as the shortest τmax, indicating both their reduced oxidative stability and a more advanced state of lipid degradation. In contrast, oils obtained by the Soxhlet and cold solvent extraction methods were characterized by lower oxidation rates, with Soxhlet-extracted oils generally having the most favorable PV and K232 values. These studies highlight the crucial impact of the extraction method on oil stability and the need for careful selection of extraction conditions to maintain its quality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30173519/s1, Table S1. Health indices of almond oils obtained by different extraction methods and compared 1 with commercial refined almond oil.
Author Contributions
Conceptualization, M.K. and D.M.-J.; methodology, M.K., D.M.-J., and B.Z.; software, M.K., D.M.-J., and B.Z.; validation, M.K., D.M.-J., and B.Z.; formal analysis, M.K., D.M.-J., and B.Z.; investigation, M.K., D.M.-J., and B.Z.; resources, M.K., D.M.-J., and B.Z.; data curation, M.K., D.M.-J., and B.Z.; writing—original draft preparation, M.K., D.M.-J., and B.Z.; writing—review and editing, M.K., D.M.-J., B.Z., and M.R.; visualization, D.M.-J. and B.Z.; supervision, M.R.; project administration, M.K. and D.M.-J.; funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The research for this publication was carried out with the use of equipment purchased as part of the “Food and Nutrition Centre—modernisation of the WULS campus to create a Food and Nutrition Research and Development Centre (CŻiŻ)” co-financed by the European Union from the European Regional Development Fund under the Regional Operational Programme of the Mazowieckie Voivodeship for 2014–2020 (Project No. RPMA.01.01.00-14-8276/17).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Index of atherogenicity |
| AO | Almond oil |
| AO_AF | Oil extracted from almond flakes |
| AO_APC | Oil extracted from almond protein concentrate |
| AO_BA | Oil extracted from blanched almonds |
| AO_ref | Commercial, refined almond oil |
| AO_UA | Oil extracted from unpeeled almonds |
| AOCS | American Oil Chemists’ Society |
| APC | Almond protein concentrate |
| AV | Acid value |
| CSE | Cold solvent extraction |
| FA | Fatty acid |
| FE | Folch extraction |
| h/H | Hypocholesterolaemic/hypercholesteraemic index |
| HCA | Hierarchical Cluster Analysis |
| K232 | Specific extinction coefficient at 232 nm |
| K268 | Specific extinction coefficient at 268 nm |
| LDL | Low-density lipoprotein |
| MUFA | Monounsaturated fatty acids |
| ND | Not detected |
| p-AnV | p-Anisidine value |
| PC1 | First principal component |
| PC2 | Second principal component |
| PCA | Principal Component Analysis |
| PDSC | Pressure Differential Scanning Calorimetry |
| PUFA | Polyunsaturated fatty acids |
| PV | Peroxide value |
| SD | Standard deviation |
| SE | Soxhlet extraction |
| SFA | Saturated fatty acids |
| TI | Index of thrombogenicity |
| TOTOX | Total oxidation index |
| τmax | PDSC oxidation time |
References
- Gharehyakheh, S. Optimization of Bitter Almond Oil (BAO) Extraction Conditions Using Natural Enzymes and Ultrasound Waves. Iran. J. Chem. Eng. 2022, 41, 2000–2012. [Google Scholar] [CrossRef]
- Berkkan, A.; Türk, B.N.D.; Pekacar, S.; Ulutaş, O.K.; Orhan, D.D. Evaluation of Marketed Almond Oils [Prunus dulcis (Mill.) D.A. Webb] in Terms of European Pharmacopoeia Criteria. Turk. J. Pharm. Sci. 2022, 19, 322–329. [Google Scholar] [CrossRef]
- Tian, L.; You, X.; Zhang, S.; Zhu, Z.; Yi, J.; Jin, G. Enhancing Functional Properties and Protein Structure of Almond Protein Isolate Using High-Power Ultrasound Treatment. Molecules 2024, 29, 3590. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.N.; Akinci-Yildirim, F.; San, B.; Sesli, Y. Total Oil Content and Fatty Acid Profile of Some Almond (Amygdalus communis L.) Cultivars. Pol. J. Food Nutr. Sci. 2016, 66, 173–178. [Google Scholar] [CrossRef]
- El Bernoussi, S.; Boujemaa, I.; Harhar, H.; Belmaghraoui, W.; Matthäus, B.; Tabyaoui, M. Evaluation of Oxidative Stability of Sweet and Bitter Almond Oils under Accelerated Storage Conditions. J. Stored Prod. Res. 2020, 88, 101662. [Google Scholar] [CrossRef]
- Siddiqua, A.; Hussain, S.; Syed, S.K. Phytochemistry, Nutritional and Medicinal Importance of Almond. Postep. Biol. Komorki 2021, 48, 167–180. [Google Scholar]
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Aires, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Phenolic and Fatty Acid Profiles, α-Tocopherol and Sucrose Contents, and Antioxidant Capacities of Understudied Portuguese Almond Cultivars. J. Food Biochem. 2019, 43, e12887. [Google Scholar] [CrossRef]
- Ouzir, M.; Bernoussi, S.E.; Tabyaoui, M.; Taghzouti, K. Almond Oil: A Comprehensive Review of Chemical Composition, Extraction Methods, Preservation Conditions, Potential Health Benefits, and Safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3344–3387. [Google Scholar] [CrossRef]
- Martín-Tornero, E.; Simón-García, D.; Álvarez-Ortí, M.; Pardo, J.E.; Durán-Merás, I.; Martín-Vertedor, D. Non-Destructive Fluorescence Spectroscopy for Quality Evaluation of Almond Oils Extracted from Roasted Kernel. Talanta Open 2024, 9, 100334. [Google Scholar] [CrossRef]
- Dias, F.F.G.; Teixeira, B.F.; Taha, A.Y.; Bell, J.M.L.N.d.M. Integrated Impact of Environmentally Friendly Extraction and Recovery Methods on Almond Oil Quality: Insights from a Lipidomic Perspective. J. Am. Oil Chem. Soc. 2025, 102, 995–1004. [Google Scholar] [CrossRef]
- Qi, Z.; Xiao, J.; Ye, L.; Chuyun, W.; Chang, Z.; Shugang, L.; Fenghong, H. The Effect of the Subcritical Fluid Extraction on the Quality of Almond Oils: Compared to Conventional Mechanical Pressing Method. Food Sci. Nutr. 2019, 27, 2231–2241. [Google Scholar] [CrossRef]
- Melhaoui, R.; Kodad, S.; Houmy, N.; Belhaj, K.; Mansouri, F.; Abid, M.; Addi, M.; Mihamou, A.; Sindic, M.; Serghini-Caid, H.; et al. Characterization of Sweet Almond Oil Content of Four European Cultivars (Ferragnes, Ferraduel, Fournat, and Marcona) Recently Introduced in Morocco. Scientifica 2021, 9141695. [Google Scholar] [CrossRef]
- Ruchi, V.; Nayanjeet, C.; Kalra, P.; Nair, N.S.; Prabhakar, B. Effects of Almond Consumption Compared with the Consumption of Traditional Isocaloric Cereal/Pulse-Based Snacks on Glycaemic Control and Gut Health in Adults with Pre-Diabetes in Rural India: Protocol for a 16-Week, Parallel-Arm, Cluster Randomised Controlled Trial. BMJ Open 2024, 14, e076934. [Google Scholar] [CrossRef]
- Özcan, M.M.; Al Juhaimi, F.; Ghafoor, K.; Babiker, E.E.; Özcan, M.M. Characterization of Physico-Chemical and Bioactive Properties of Oils of some Important Almond Cultivars by Cold Press and Soxhlet Extraction. J. Food Sci. Technol. 2020, 57, 955–961. [Google Scholar] [CrossRef]
- Sayah, O.; Taibi, S.; Bouakline, H.; El Yousfi, R.; Tayebi, A.; Ziani, I.; Tahani, A.; El Bachiri, A. Comparison of Sweet and Bitter Almond Oil Quality: Impact of Kernel Skin and Green Supercritical CO2 Extraction Method. Food Chem. 2025, 486, 144540. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Sequeira, A.; Vilela, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Effects of Different Processing Treatments on Almond (Prunus dulcis) Bioactive Compounds, Antioxidant Activities, Fatty Acids, and Sensorial Characteristics. Plants 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Nde, D.B.; Foncha, A.C. Optimization Methods for the Extraction of Vegetable Oils: A Review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Danlami, J.M.; Arsad, A.; Zaini, M.A.A.; Sulaiman, H. A Comparative Study of Various Oil Extraction Techniques from Plants. Rev. Chem. Eng. 2014, 30, 605–626. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczyńska, E.; Ścibisz, I.; Rudzińska, M. Fatty Acids and Sterols Composition, and Antioxidant Activity of Oils Extracted from Plant Seeds. Food Chem. 2016, 213, 450–456. [Google Scholar] [CrossRef]
- Krzyczkowska, J.; Kozłowska, M. Effect of Oils Extracted from Plant Seeds on the Growth and Lipolytic Activity of Yarrowia lipolytica Yeast. J. Am. Oil Chem. Soc. 2017, 94, 661–671. [Google Scholar] [CrossRef]
- Gambert, A.; Niţu, S.; Tămaș, A.; Fanani, M.; Dupré, J.; Delepine, C.; Chaveriat, L.; Martin, P.; Rusnac, L. Influence of the Extraction Process on the Characteristics of Romanian Mountain Walnut Oil. Am. J. Plant Sci. 2024, 15, 940–967. [Google Scholar] [CrossRef]
- Miraliakbari, H.; Shahidi, F. Lipid Class Compositions, Tocopherols and Sterols of Tree Nut Oils Extracted with Different Solvents. J. Food Lipids 2008, 15, 81–96. [Google Scholar] [CrossRef]
- Siol, M.; Witkowska, B.; Mańko-Jurkowska, D.; Makouie, S.; Bryś, J. Comprehensive Evaluation of the Nutritional Quality of Stored Watermelon Seed Oils. Appl. Sci. 2025, 15, 830. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Stolp, L.J.; Kodali, D.R. Chapter 2—Naturally occurring high-oleic oils: Avocado, macadamia, and olive oils. In High Oleic Oils; Flider, F.J., Ed.; AOCS Press: Champaign, IL, USA, 2022; pp. 7–52. [Google Scholar] [CrossRef]
- Gülsoy, E.; Kaya, E.D.; Türkhan, A.; Bulut, M.; Koyuncu, M.; Güler, E.; Sayın, F.; Muradoğlu, F. The Effect of Altitude on Phenolic, Antioxidant and Fatty Acid Compositions of Some Turkish Hazelnut (Coryllus avellana L.) Cultivars. Molecules 2023, 28, 5067. [Google Scholar] [CrossRef]
- Poli, A.; Agostoni, C.; Visioli, F. Dietary Fatty Acids and Inflammation: Focus on the n-6 Series. Int. J. Mol. Sci. 2023, 24, 4567. [Google Scholar] [CrossRef]
- Maestri, D.; Cittadini, M.C.; Bodoira, R.; Martínez, M. Tree Nut Oils: Chemical Profiles, Extraction, Stability, and Quality Concerns. Eur. J. Lipid Sci. Technol. 2020, 122, 1900450. [Google Scholar] [CrossRef]
- Rabadán, A.; Pardo, J.E.; Gómez, R.; Álvarez-Ortí, M. Effect of Almond Roasting, Light Exposure and Addition of Different Garlic Cultivars on Almond Oil Stability. Eur. Food Res. Technol. 2018, 244, 219–224. [Google Scholar] [CrossRef]
- Tian, M.; Bai, Y.; Tian, H.; Zhao, X. The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review. Molecules 2023, 28, 6393. [Google Scholar] [CrossRef]
- Tilami, S.K.; Kouřimská, L. Assessment of the Nutritional Quality of Plant Lipids Using Atherogenicity and Thrombogenicity Indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef]
- Ying, Q.; Wojciechowska, P.; Siger, A.; Kaczmarek, A.; Rudzińska, M. Phytochemical Content, Oxidative Stability, and Nutritional Properties of Unconventional Cold-pressed Edible Oils. J. Food Nutr. Res. 2018, 6, 476–485. [Google Scholar] [CrossRef]
- Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Górska, A.; Brzezińska, R.; Piasecka, I. Assessment of the Nutritional Potential and Resistance to Oxidation of Sea Buckthorn and Rosehip Oils. Appl. Sci. 2024, 14, 1867. [Google Scholar] [CrossRef]
- Ratusz, K.; Symoniuk, E.; Wroniak, M.; Rudzińska, M. Bioactive Compounds, Nutritional Quality and Oxidative Stability of Cold-Pressed Camelina (Camelina sativa L.) Oils. Appl. Sci. 2018, 8, 2606. [Google Scholar] [CrossRef]
- Codex-ALINORM 09/32/17; Codex Alimentarius 2009. Codex Standard for Named Vegetable Oils. Codex Alimentarius Commission: Rome, Italy, 2009.
- Gharby, S.; Hajib, A.; Ibourki, M.; Sakar, E.H.; Nounah, I.; Moudden, H.E.; Elibrahimi, M.; Harhar, H. Induced Changes in Olive Oil Subjected to Various Chemical Refining Steps: A Comparative Study of Quality Indices, Fatty Acids, Bioactive Minor Components, and Oxidation Stability Kinetic Parameters. Chem. Data Collect. 2021, 33, 100702. [Google Scholar] [CrossRef]
- Gharby, S. Refining Vegetable Oils: Chemical and Physical Refining. Sci. World J. 2022, 6627013. [Google Scholar] [CrossRef]
- Atamyradova, N.; Özkılıç, S.Y.; Arslan, D. Blanching of Olive Fruits Before Storage at Different Conditions: Effects on Oil Yield, Lipase Activity and Oxidation. J. Agric. Food Res. 2024, 18, 101509. [Google Scholar] [CrossRef]
- PN-EN ISO 3960:2017-03; Vegetable and Animal Oils and Fats. Determination of Peroxide Number (Reference Method). Polish Committee for Standardization: Warsaw, Poland, 2017.
- Rezvankhah, A.; Emam-Djomeh, Z.; Safari, M.; Askari, G.; Salami, M. Microwave-Assisted Extraction of Hempseed Oil: Studying and Comparing of Fatty Acid Composition, Antioxidant Activity, Physiochemical and Thermal Properties with Soxhlet Extraction. J. Food Sci. Technol. 2019, 56, 4198–4210. [Google Scholar] [CrossRef]
- Mortensen, G.; Sørensen, J.; Stapelfeldt, H. Comparison of Peroxide Value Methods used for Semihard Cheeses. J. Agric. Food Chem. 2002, 50, 5007–5011. [Google Scholar] [CrossRef]
- Esfahani, S.T.; Zamindar, N.; Esmaeili, Y.; Sharifian, S. Effect of Initial Quality of Oil and Thermal Processing on Oxidation Indexes in Canned Tuna. Appl. Food Res. 2024, 4, 100553. [Google Scholar] [CrossRef]
- Čolić, S.; Zec, G.; Natić, M.; Fotirić-Akšić, M. Almond (Prunus dulcis) oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Sidhu, A.R.; Naz, S.; Mahesar, S.A.; Kandhro, A.A.; Khaskheli, A.R.; Ali, Z.; Memon, H.D.; Shoaib, H.; Mahesar, H.R. Effect of Storage at Elevated Temperature on the Quality and Stability of Different Almond Oils: A Comprehensive Study. Food Mater. Res. 2023, 3, 30. [Google Scholar] [CrossRef]
- Petkova, Z.; Antova, G. A Comparative Study on Quality Parameters of Pumpkin, Melon and Sunflower Oils During Thermal Treatment. OCL 2019, 26, 32. [Google Scholar] [CrossRef]
- Sánchez-Bel, P.; Martínez-Madrid, M.C.; Egea, I.; Romojaro, F. Quality and Sensory Evaluation of Almond (Prunus amygdalus) Stored after Electron Beam Processing. J. Agric. Food Chem. 2005, 53, 2567–2573. [Google Scholar] [CrossRef]
- Tan, C.P.; Man, Y.B.C.; Selamat, J.; Yusoff, M.S.A. Comparative Studies of Oxidative Stability of Edible Oils by Differential Scanning Calorimetry and Oxidative Stability Index Methods. Food Chem. 2002, 76, 385–389. [Google Scholar] [CrossRef]
- Buranasompob, A.; Tang, J.; Powers, J.R.; Reyes, J.; Clark, S.; Swanson, B.G. Lipoxygenase Activity in Walnuts and Almonds. LWT Food Sci. Technol. 2007, 40, 893–899. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel Oil Extraction Technologies: Process Conditions, Quality Parameters, and Optimization. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Magangana, T.P.; Makunga, N.P.; la Grange, C.; Stander, M.A.; Fawole, O.A.; Opara, U.L. Blanching Pre-Treatment Promotes High Yields, Bioactive Compounds, Antioxidants, Enzyme Inactivation and Antibacterial Activity of ‘Wonderful’ Pomegranate Peel Extracts at Three Different Harvest Maturities. Antioxidants 2021, 10, 1119. [Google Scholar] [CrossRef]
- Salcedo, C.L.; de Mishima, B.A.L.; Nazareno, M.A. Walnuts and Almonds as Model Systems of Foods Constituted by Oxidisable, Pro-Oxidant and Antioxidant Factors. Food Res. Int. 2010, 43, 1187–1197. [Google Scholar] [CrossRef]
- Symoniuk, E.; Wroniak, M.; Napiórkowska, K.; Brzezińska, R.; Ratusz, K. Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures. Foods 2022, 11, 1597. [Google Scholar] [CrossRef]
- Ratusz, K.; Kowalski, B.; Bekas, W.; Wirkowska, M. Monitorowanie Autooksydacji Oleju Rzepakowego i Słonecznikowego. Rośliny Oleiste Oilseed Crops 2005, 26, 211–220. [Google Scholar]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Boselli, E.; Velazco, V.; Caboni, M.F.; Lercker, G. Pressurized Liquid Extraction of Lipids for the Determination of Oxysterols in Egg-Containing Food. J. Chromatogr. A 2001, 917, 239–244. [Google Scholar] [CrossRef]
- PN-EN ISO 5509:2001; Vegetable and Animal Oils and Fats. Preparation of Fatty Acid Methyl Esters. Polish Committee for Standardization: Warsaw, Poland, 2001.
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of Genotype, Feeding System and Slaughter Weight on the Quality of Light Lambs: II. Fatty Acid Composition of Meat. Livest. Prod. Sci. 2022, 77, 187–194. [Google Scholar] [CrossRef]
- AOCS Official Method Te 1a-64; Acid Value Official Methods and Recommended Practices of the AOCS. AOCS Press: Champaign, IL, USA, 2009.
- AOCS Official Method Cd 8b-90; Peroxide Value Acetic Acid-Isooctane Method Official Methods and Recommended Practices of the AOCS. AOCS Press: Champaign, IL, USA, 2009.
- AOCS Official Method Cd 18-90. p-Anisidine Value. In Official Methods and Recommended Practices of the AOCS; American Oil Chemists Society Press: Champaign, IL, USA, 2011. [Google Scholar]
- ISO 3656:2011; Animal and Vegetable Fats and Oils-Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction. International Organization for Standardization (ISO): Geneva, Switzerland, 2011.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).