Bufalin Suppresses Colorectal Cancer Liver Metastasis by Inhibiting De Novo Fatty Acid Synthesis via the PI3K/AKT-Mediated SREBP1/FASN Pathway
Abstract
1. Introduction
2. Results
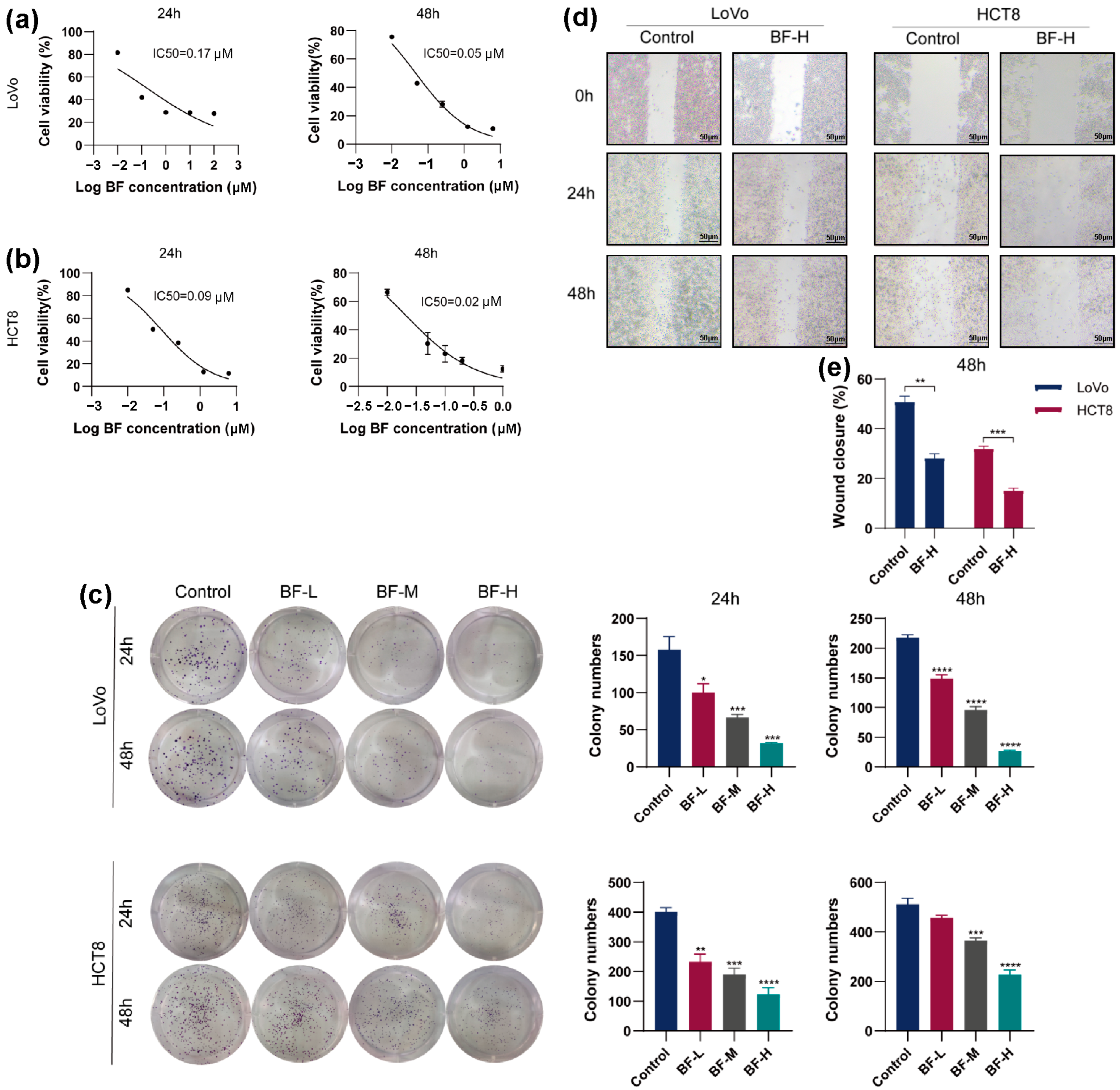
2.1. Bufalin Inhibits the Proliferation and Migration of CRC Cells
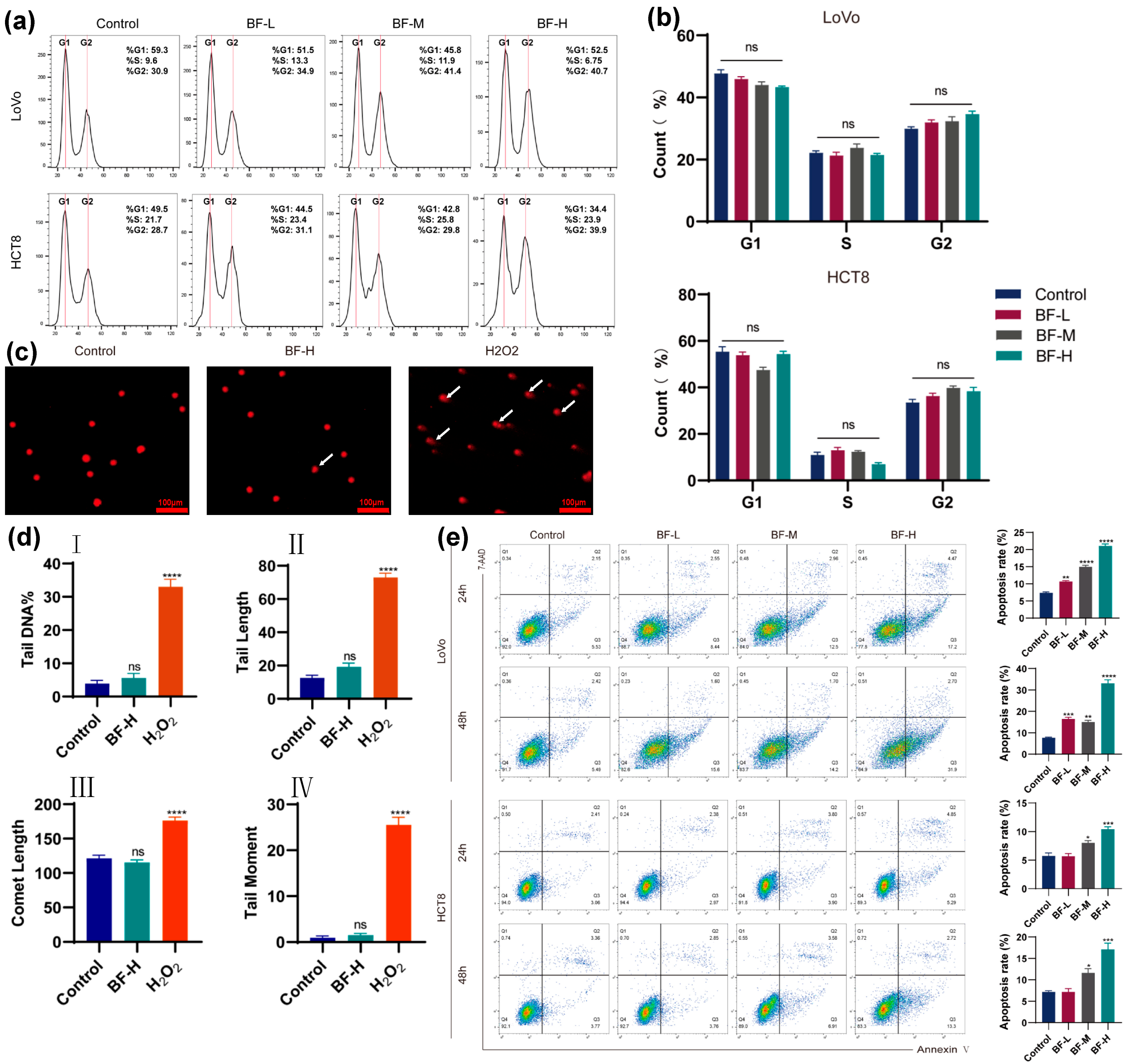
2.2. Bufalin Induces DNA Damage and Apoptosis
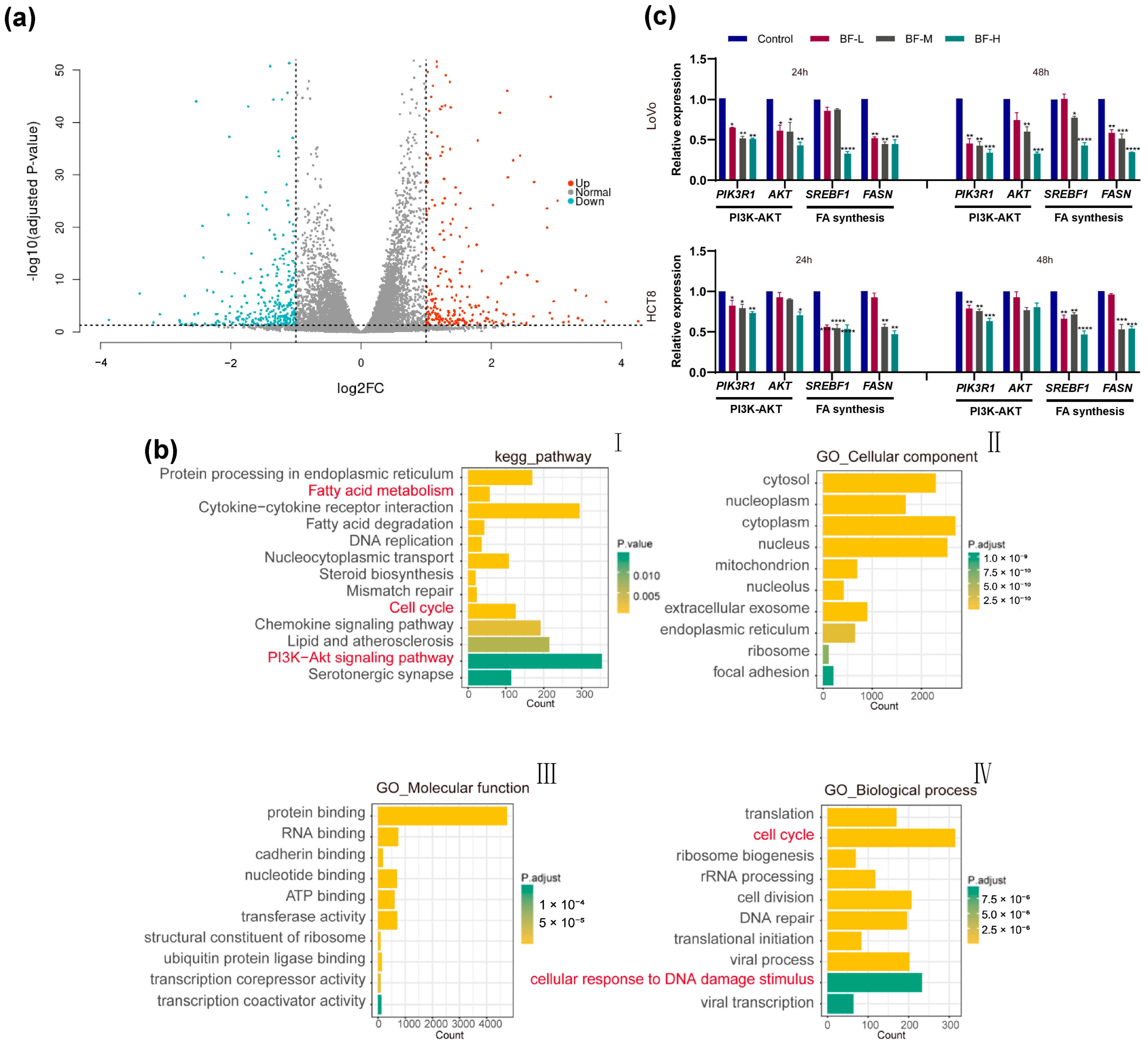
2.3. Transcriptomic Analysis Reveals the Mechanism of Bufalin Against CRC Metastasis
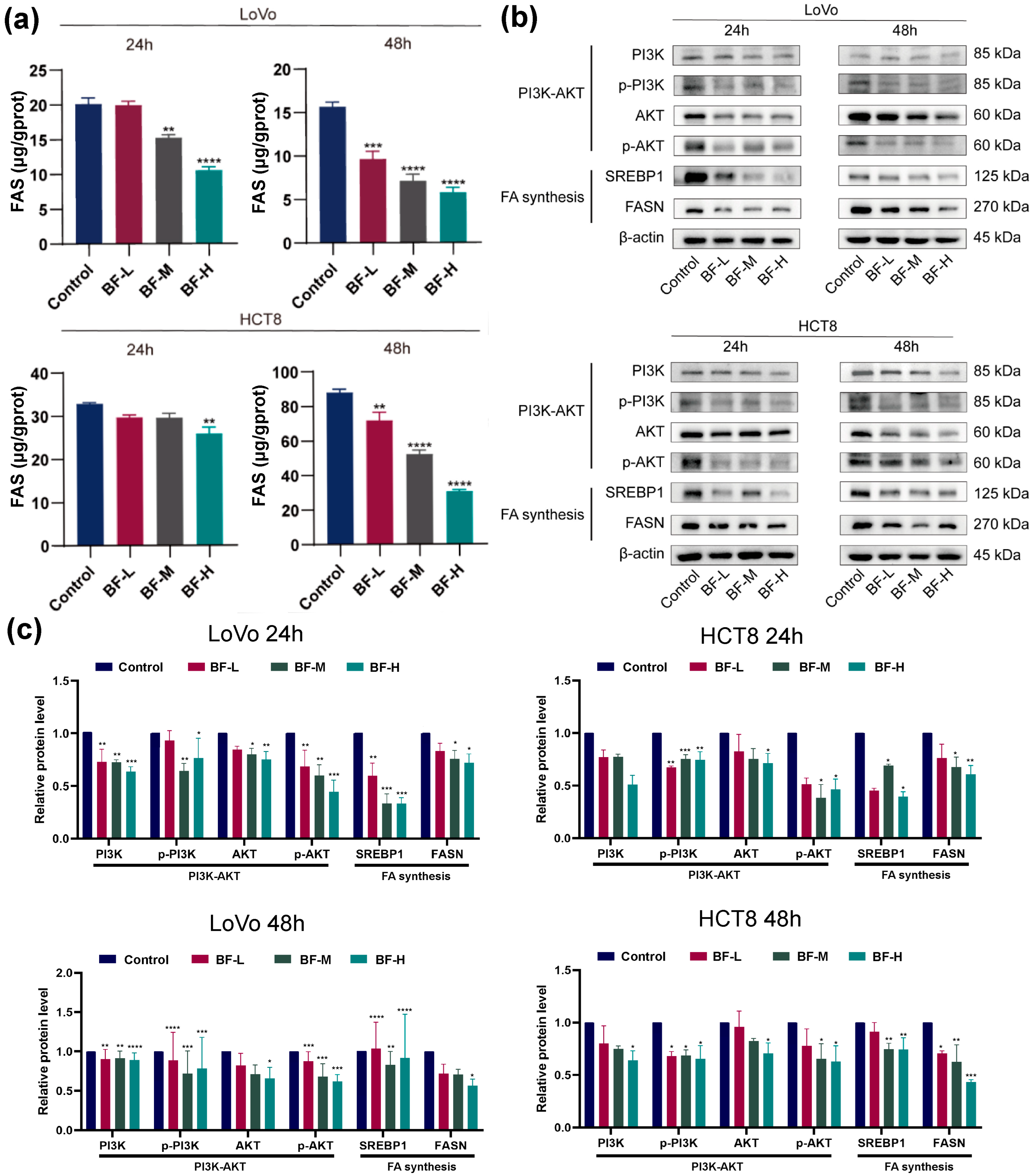
2.4. Bufalin Inhibits De Novo Fatty Acid Synthesis Through the PI3K-AKT Pathway
2.5. Bufalin Inhibits CRC Progression in Mouse Models
2.6. Bufalin Inhibits CRC Liver Metastasis in Mouse Models
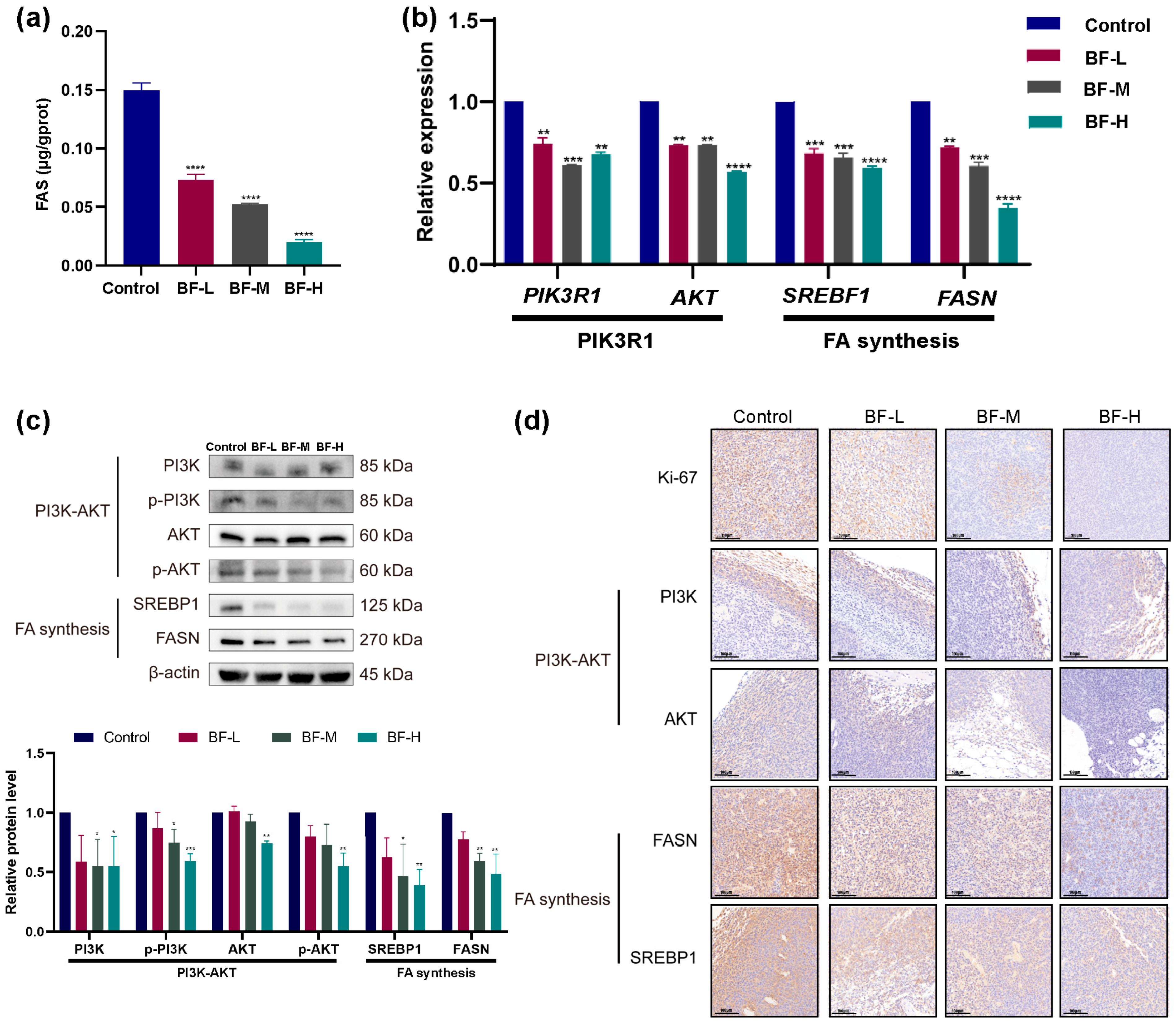
2.7. Bufalin Inhibits De Novo Fatty Acid Synthesis Through the PI3K/AKT-Mediated SREBP1/FASN Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Colony Formation Assay
4.5. Wound Healing Assay
4.6. Cell Cycle and Apoptosis Analysis
4.7. Comet Assay
4.8. RNA Sequencing
4.9. ELISA for FASN Detection
4.10. Quantitative Real-Time PCR
4.11. Western Blot
4.12. Animal Model for CRC and CRC Metastasis
4.13. Hematoxylin–Eosin (H&E)
4.14. Immunohistochemistry
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Protein Kinase B |
| BF | Bufalin |
| CCK-8 | Cell Counting Kit-8 |
| CRC | Colorectal Cancer |
| DEGs | Differentially Expressed Genes |
| DMSO | Dimethyl Sulfoxide |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FASN | Fatty Acid Synthase |
| GO | Gene Ontology |
| H&E | Hematoxylin and Eosin |
| IHC | Immunohistochemistry |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PBS | Phosphate-Buffered Saline |
| PI | Propidium Iodide |
| PI3K | Phosphatidylinositol 3-Kinase |
| qRT-PCR | Quantitative Reverse Transcription Polymerase Chain Reaction |
| SREBP1 | Sterol Regulatory Element-Binding Protein 1 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
References
- Bullock, A.J.; Schlechter, B.L.; Fakih, M.G.; Tsimberidou, A.M.; Grossman, J.E.; Gordon, M.S.; Wilky, B.A.; Pimentel, A.; Mahadevan, D.; Balmanoukian, A.S.; et al. Botensilimab plus balstilimab in relapsed/refractory microsatellite stable metastatic colorectal cancer: A phase 1 trial. Nat. Med. 2024, 30, 2558–2567. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Strickler, J.H.; Cercek, A.; Siena, S.; Andre, T.; Ng, K.; Van Cutsem, E.; Wu, C.; Paulson, A.S.; Hubbard, J.M.; Coveler, A.L.; et al. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): A multicentre, open-label, phase 2 study. Lancet Oncol. 2023, 24, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Brodt, P.; Clavien, P.A.; Muschel, R.J.; D’Angelica, M.I.; Endo, I.; Parks, R.W.; Doyle, M.; de Santibanes, E.; Pawlik, T.M. Liver metastases. Nat. Rev. Dis. Primers 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Lin, Y.; Zhang, H.; Liu, C.; Cheng, Z.; Yang, X.; Zhang, J.; Xiao, Y.; Sang, N.; Qian, X.; et al. Reprogramming of lipid metabolism in cancer-associated fibroblasts potentiates migration of colorectal cancer cells. Cell Death Dis. 2020, 11, 267. [Google Scholar] [CrossRef]
- Dai, W.; Xiang, W.; Han, L.; Yuan, Z.; Wang, R.; Ma, Y.; Yang, Y.; Cai, S.; Xu, Y.; Mo, S.; et al. PTPRO represses colorectal cancer tumorigenesis and progression by reprogramming fatty acid metabolism. Cancer Commun. 2022, 42, 848–867. [Google Scholar] [CrossRef] [PubMed]
- Leishman, S.; Aljadeed, N.M.; Qian, L.; Cockcroft, S.; Behmoaras, J.; Anand, P.K. Fatty acid synthesis promotes inflammasome activation through NLRP3 palmitoylation. Cell Rep. 2024, 43, 114516. [Google Scholar] [CrossRef]
- Bai, R.; Cui, J. Regulation of fatty acid synthase on tumor and progress in the development of related therapies. Chin. Med. J. 2024, 137, 1894–1902. [Google Scholar] [CrossRef]
- Wei, W.; Qin, B.; Wen, W.; Zhang, B.; Luo, H.; Wang, Y.; Xu, H.; Xie, X.; Liu, S.; Jiang, X.; et al. FBXW7beta loss-of-function enhances FASN-mediated lipogenesis and promotes colorectal cancer growth. Signal Transduct. Target. Ther. 2023, 8, 187. [Google Scholar] [CrossRef]
- Zaytseva, Y.Y.; Harris, J.W.; Mitov, M.I.; Kim, J.T.; Butterfield, D.A.; Lee, E.Y.; Weiss, H.L.; Gao, T.; Evers, B.M. Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. Oncotarget 2015, 6, 18891–18904. [Google Scholar] [CrossRef]
- Zhan, X.; Wu, H.; Wu, H.; Wang, R.; Luo, C.; Gao, B.; Chen, Z.; Li, Q. Metabolites from Bufo gargarizans (Cantor, 1842): A review of traditional uses, pharmacological activity, toxicity and quality control. J. Ethnopharmacol. 2020, 246, 112178. [Google Scholar] [CrossRef] [PubMed]
- Soumoy, L.; Ghanem, G.E.; Saussez, S.; Journe, F. Bufalin for an innovative therapeutic approach against cancer. Pharmacol. Res. 2022, 184, 106442. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.; Li, S.; Yang, J.; Tang, D.; Wu, W.; Yu, K.; Cao, Y.; Xu, K.; Yin, P.; et al. Bufalin reverses cancer-associated fibroblast-mediated colorectal cancer metastasis by inhibiting the STAT3 signaling pathway. Apoptosis 2023, 28, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, C.; Sun, Y.; Li, Y.; Wu, H.; Ma, S.; Shang, J.; Zhan, Y.; Yin, P.; Gao, F. Bufalin exacerbates Photodynamic therapy of colorectal cancer by targeting SRC-3/HIF-1alpha pathway. Int. J. Pharm. 2022, 624, 122018. [Google Scholar] [CrossRef]
- Yang, J.; Shay, C.; Saba, N.F.; Teng, Y. Cancer metabolism and carcinogenesis. Exp. Hematol. Oncol. 2024, 13, 10. [Google Scholar] [CrossRef]
- Jiang, T.; Qi, J.; Xue, Z.; Liu, B.; Liu, J.; Hu, Q.; Li, Y.; Ren, J.; Song, H.; Xu, Y.; et al. The m(6)A modification mediated-lncRNA POU6F2-AS1 reprograms fatty acid metabolism and facilitates the growth of colorectal cancer via upregulation of FASN. Mol. Cancer 2024, 23, 55. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, Y.; Xiong, H.; Dong, G. The implications of FASN in immune cell biology and related diseases. Cell Death Dis. 2024, 15, 88. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Y.; Li, Y.; Zhang, J.; Li, M.; Ji, L.; Tang, Y.; Zheng, Y.; Sheng, J.; Han, Q.; et al. Bufalin stimulates antitumor immune response by driving tumor-infiltrating macrophage toward M1 phenotype in hepatocellular carcinoma. J. Immunother. Cancer 2022, 10, e004297. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.; Shi, J.; Li, Y.; Li, Y.; Tao, R.; Huang, L.; Tang, Y.; Zhu, X.; Li, M.; et al. Bufalin suppresses hepatocellular carcinogenesis by targeting M2 macrophage-governed Wnt1/beta-catenin signaling. Phytomedicine 2024, 126, 155395. [Google Scholar] [CrossRef]
- Dunkenberger, L.; Reiss, K.; Del Valle, L. Comet Assay for the Detection of Single and Double-Strand DNA Breaks. In Immunohistochemistry and Immunocytochemistry: Methods in Molecular Biology; Humana: New York, NY, USA, 2022; Volume 2422, pp. 263–269. [Google Scholar] [CrossRef]
- Xie, P.; Peng, Z.; Chen, Y.; Li, H.; Du, M.; Tan, Y.; Zhang, X.; Lu, Z.; Cui, C.P.; Liu, C.H.; et al. Neddylation of PTEN regulates its nuclear import and promotes tumor development. Cell Res. 2021, 31, 291–311. [Google Scholar] [CrossRef]
- Stewart, C.L.; Warner, S.; Ito, K.; Raoof, M.; Wu, G.X.; Kessler, J.; Kim, J.Y.; Fong, Y. Cytoreduction for colorectal metastases: Liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr. Probl. Surg. 2018, 55, 330–379. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Han, Y.; Ouyang, Y.; Li, H.; Li, L.; Wu, X.; Yang, L.; Gao, J.; Zhang, L.; Zhou, J.; et al. Kaempferol inhibits colorectal cancer metastasis through circ_0000345 mediated JMJD2C/beta-catenin signaling pathway. Phytomedicine 2024, 128, 155261. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, W.Y.; Lin, X.T.; Fang, L.; Xie, C.M. Bufalin induces apoptosis via mitochondrial ROS-mediated caspase-3 activation in HCT-116 and SW620 human colon cancer cells. Drug Chem. Toxicol. 2019, 42, 444–450. [Google Scholar] [CrossRef]
- Zhang, N.; Xie, Y.; Tai, Y.; Gao, Y.; Guo, W.; Yu, W.; Li, J.; Feng, X.; Hao, J.; Gao, Y.; et al. Bufalin Inhibits hTERT Expression and Colorectal Cancer Cell Growth by Targeting CPSF4. Cell Physiol. Biochem. 2016, 40, 1559–1569. [Google Scholar] [CrossRef]
- Xie, C.M.; Chan, W.Y.; Yu, S.; Zhao, J.; Cheng, C.H. Bufalin induces autophagy-mediated cell death in human colon cancer cells through reactive oxygen species generation and JNK activation. Free Radic. Biol. Med. 2011, 51, 1365–1375. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Huang, C.; Zhang, J.; Wu, J. Bufalin inhibits epithelial-mesenchymal transition and increases radiosensitivity of non-small cell lung cancer via inhibition of the Src signaling. J. Thorac. Dis. 2023, 15, 123–134. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Jia, L.; He, J.; Li, Y.; Liu, H.; Wu, R.; Qiu, Y.; Zhan, Y.; Yuan, Z.; et al. Bufalin targets the SRC-3/MIF pathway in chemoresistant cells to regulate M2 macrophage polarization in colorectal cancer. Cancer Lett. 2021, 513, 63–74. [Google Scholar] [CrossRef]
- Fang, K.; Zhan, Y.; Zhu, R.; Wang, Y.; Wu, C.; Sun, M.; Qiu, Y.; Yuan, Z.; Liang, X.; Yin, P.; et al. Bufalin suppresses tumour microenvironment-mediated angiogenesis by inhibiting the STAT3 signaling pathway. J. Transl. Med. 2021, 19, 383. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, G.Y.; Chang, Y.C.; Moon, S.K.; Kim, W.J.; Choi, Y.H. Bufalin prevents the migration and invasion of T24 bladder carcinoma cells through the inactivation of matrix metalloproteinases and modulation of tight junctions. Int. J. Oncol. 2013, 42, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, S.; Che, X.; Hou, K.; Ma, Y.; Li, C.; Wen, T.; Fan, Y.; Hu, X.; Liu, Y.; et al. Bufalin inhibits TGF-beta-induced epithelial-to-mesenchymal transition and migration in human lung cancer A549 cells by downregulating TGF-beta receptors. Int. J. Mol. Med. 2015, 36, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Feng, L.X.; Liu, M.; Jin, W.H.; Luo, J.; Nie, A.Y.; Zhou, Y.; Li, Y.; Wu, W.Y.; Jiang, B.H.; et al. Possible target-related proteins and signal network of bufalin in A549 cells suggested by both iTRAQ-based and label-free proteomic analysis. Proteomics 2016, 16, 935–945. [Google Scholar] [CrossRef]
- Wu, X.Y.; Tian, F.; Su, M.H.; Wu, M.; Huang, Y.; Hu, L.H.; Jin, L.; Zhu, X.J. BF211, a derivative of bufalin, enhances the cytocidal effects in multiple myeloma cells by inhibiting the IL-6/JAK2/STAT3 pathway. Int. Immunopharmacol. 2018, 64, 24–32. [Google Scholar] [CrossRef]
- Menendez, J.A.; Cuyas, E.; Encinar, J.A.; Vander Steen, T.; Verdura, S.; Llop-Hernandez, A.; Lopez, J.; Serrano-Hervas, E.; Osuna, S.; Martin-Castillo, B.; et al. Fatty acid synthase (FASN) signalome: A molecular guide for precision oncology. Mol. Oncol. 2024, 18, 479–516. [Google Scholar] [CrossRef]
- Drury, J.; Geisen, M.E.; Tessmann, J.W.; Rychahou, P.G.; Kelson, C.O.; He, D.; Wang, C.; Evers, B.M.; Zaytseva, Y.Y. Overexpression of Fatty Acid Synthase Upregulates Glutamine-Fructose-6-Phosphate Transaminase 1 and O-Linked N-Acetylglucosamine Transferase to Increase O-GlcNAc Protein Glycosylation and Promote Colorectal Cancer Growth. Int. J. Mol. Sci. 2024, 25, 4883. [Google Scholar] [CrossRef]
- Tang, D.; Wang, H.; Deng, W.; Wang, J.; Shen, D.; Wang, L.; Lu, J.; Feng, Y.; Cao, S.; Li, W.; et al. Mechanism of bufalin inhibition of colon cancer liver metastasis by regulating M2-type polarization of Kupffer cells induced by highly metastatic colon cancer cells. Apoptosis 2024, 29, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Liu, S.; He, F.; Li, X.; Saira, B.; Zheng, T.; Chen, J.; Dong, K.; Pei, X.F. Anticancer activities of Zanthoxylum bungeanum seed oil on malignant melanoma. J. Ethnopharmacol. 2019, 229, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, X.; Wang, Q.; Chen, Z.; Li, L.; Chen, H.; Qi, H. Bufalin induces ferroptosis by modulating the 2,4-dienoyl-CoA reductase (DECR1)-SLC7A11 axis in breast cancer. Phytomedicine 2024, 135, 156130. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, T.; Liu, J.; Wang, Y.; Zhang, C.; Guo, L.; Shi, D.; Zhang, T.; Wang, X.; Li, J. FGF19-Induced Inflammatory CAF Promoted Neutrophil Extracellular Trap Formation in the Liver Metastasis of Colorectal Cancer. Adv. Sci. 2023, 10, e2302613. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, W.; Li, X.; Yan, S.; Zhang, J.; Wu, P.; Yu, H.; Zhang, B.; Zhang, C. Bufalin Suppresses Colorectal Cancer Liver Metastasis by Inhibiting De Novo Fatty Acid Synthesis via the PI3K/AKT-Mediated SREBP1/FASN Pathway. Molecules 2025, 30, 3634. https://doi.org/10.3390/molecules30173634
Pang W, Li X, Yan S, Zhang J, Wu P, Yu H, Zhang B, Zhang C. Bufalin Suppresses Colorectal Cancer Liver Metastasis by Inhibiting De Novo Fatty Acid Synthesis via the PI3K/AKT-Mediated SREBP1/FASN Pathway. Molecules. 2025; 30(17):3634. https://doi.org/10.3390/molecules30173634
Chicago/Turabian StylePang, Wenwen, Xiang Li, Suying Yan, Junshi Zhang, Ping Wu, Haiyang Yu, Bowei Zhang, and Chunze Zhang. 2025. "Bufalin Suppresses Colorectal Cancer Liver Metastasis by Inhibiting De Novo Fatty Acid Synthesis via the PI3K/AKT-Mediated SREBP1/FASN Pathway" Molecules 30, no. 17: 3634. https://doi.org/10.3390/molecules30173634
APA StylePang, W., Li, X., Yan, S., Zhang, J., Wu, P., Yu, H., Zhang, B., & Zhang, C. (2025). Bufalin Suppresses Colorectal Cancer Liver Metastasis by Inhibiting De Novo Fatty Acid Synthesis via the PI3K/AKT-Mediated SREBP1/FASN Pathway. Molecules, 30(17), 3634. https://doi.org/10.3390/molecules30173634





