Furan-Indole-Chromenone-Based Organic Photocatalyst for α-Arylation of Enol Acetate and Free Radical Polymerization Under LED Irradiation
Abstract
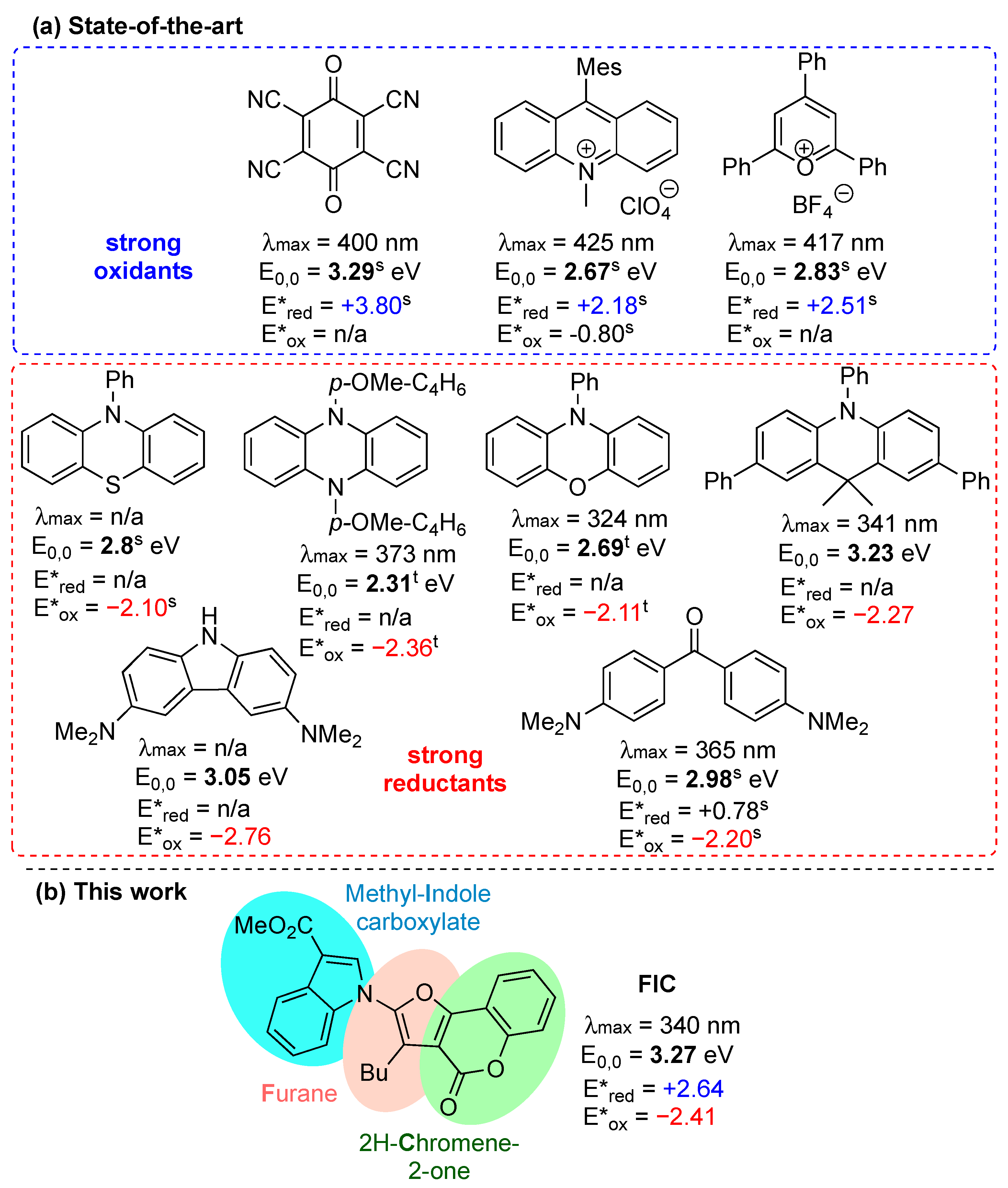
:1. Introduction
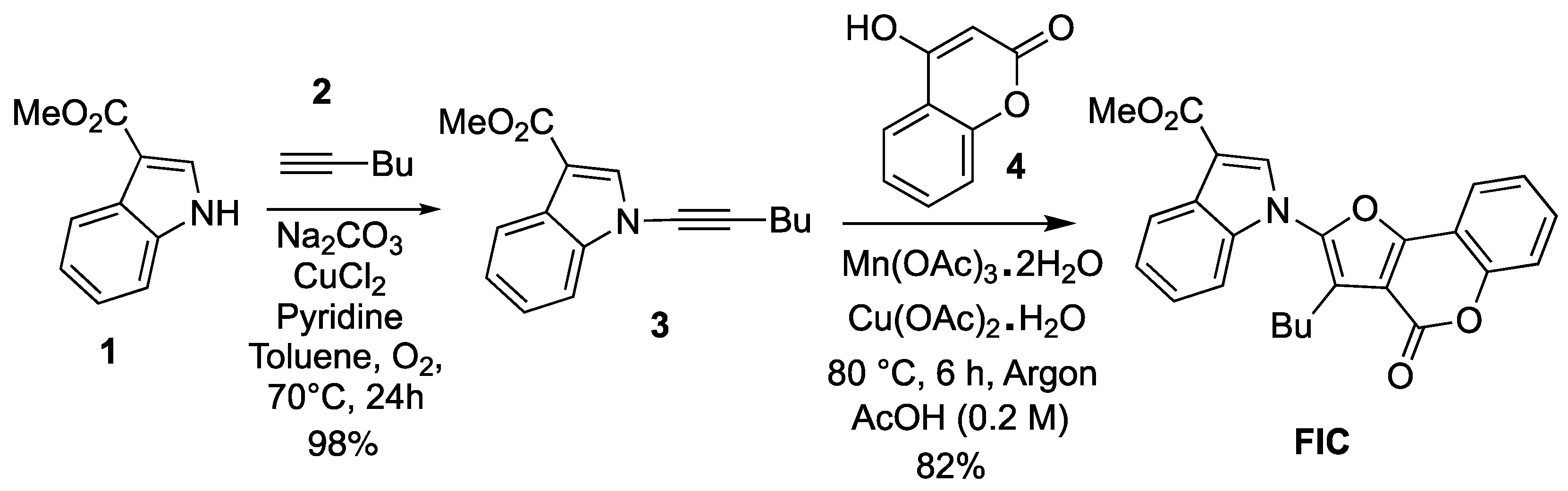
2. Results and Discussion
2.1. α-Arylation of Enol Acetate
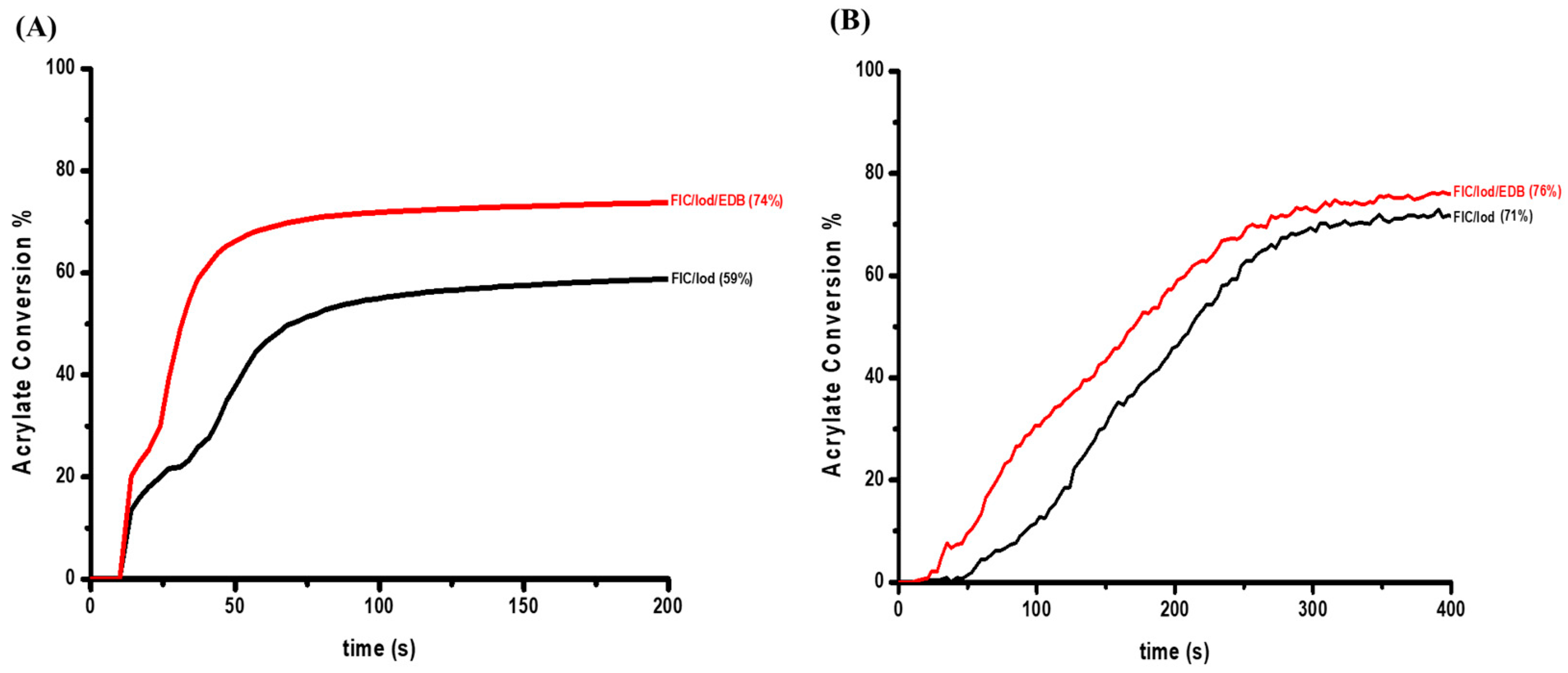
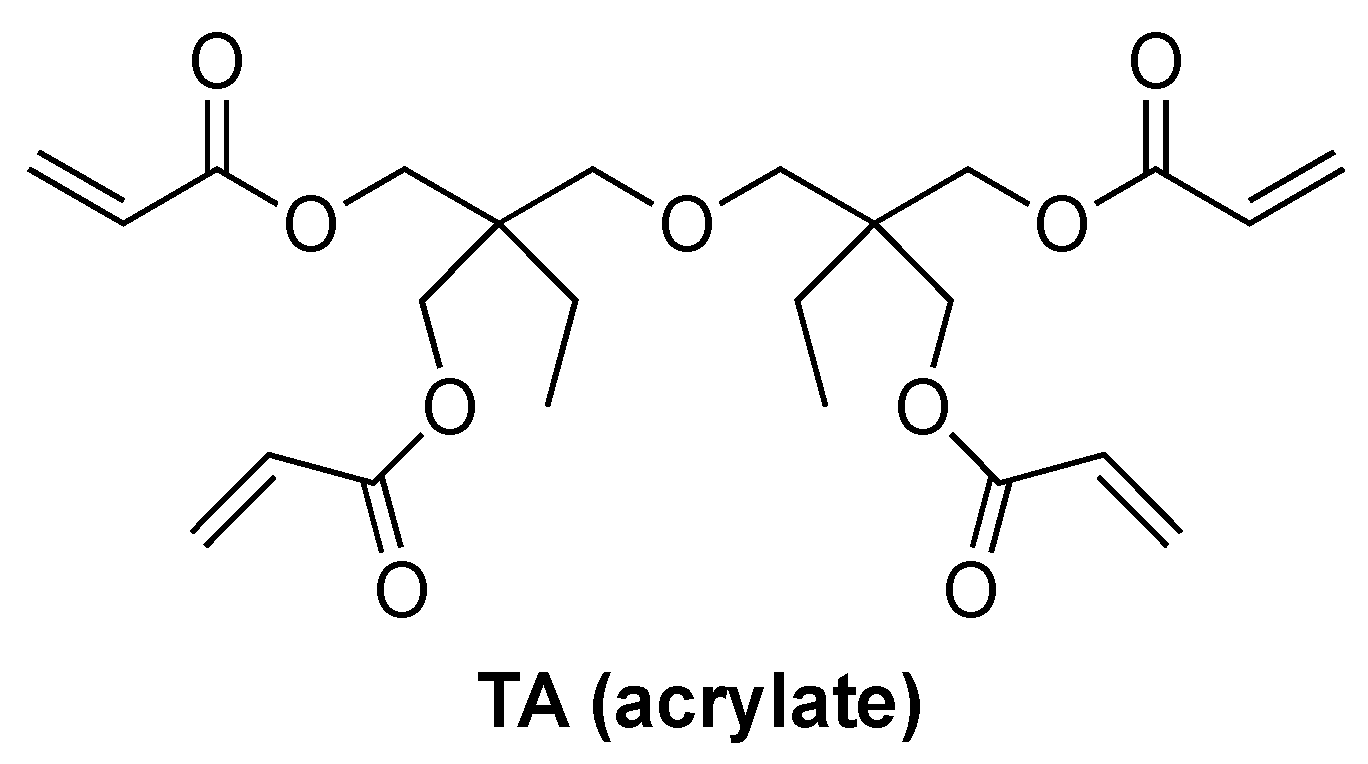
2.2. Free Radical Polymerization (FRP) of Acrylates
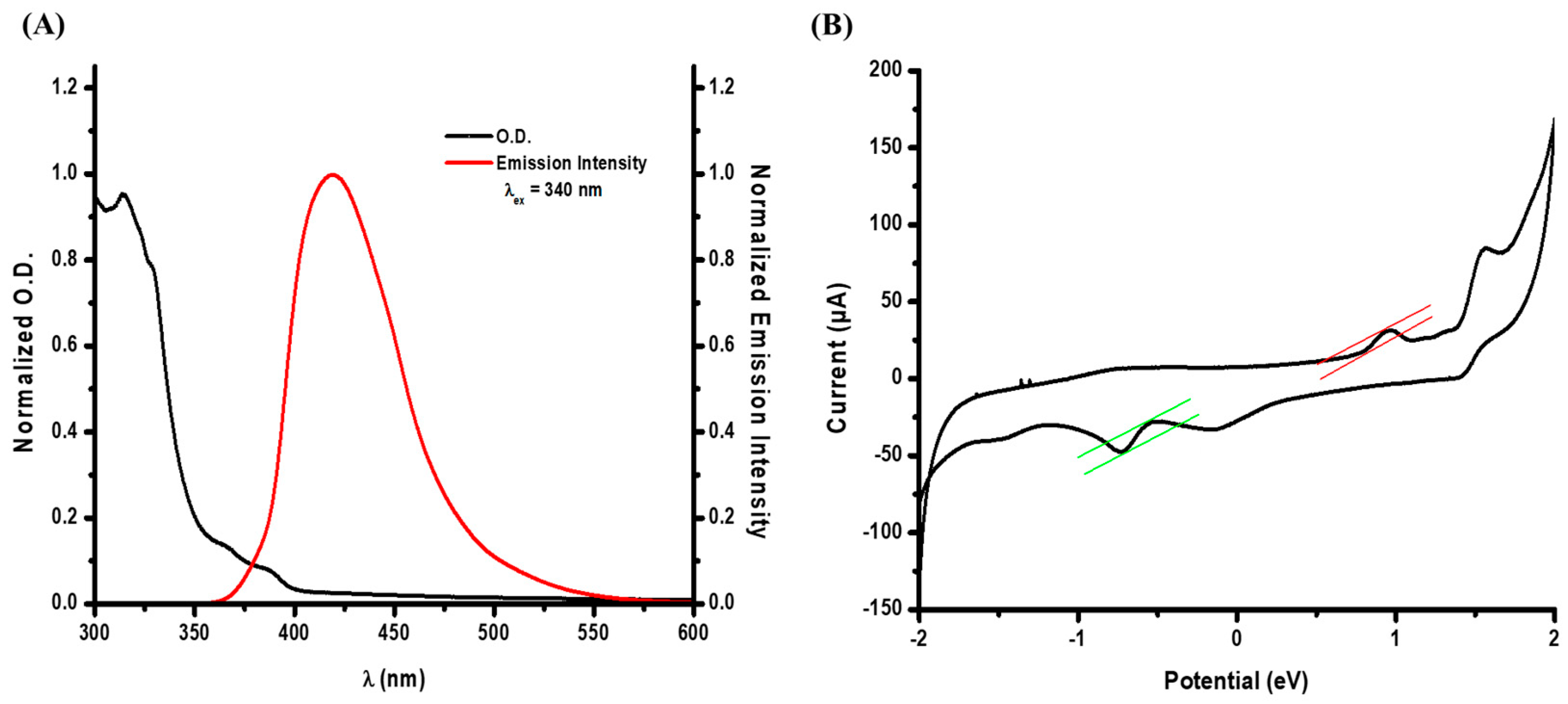
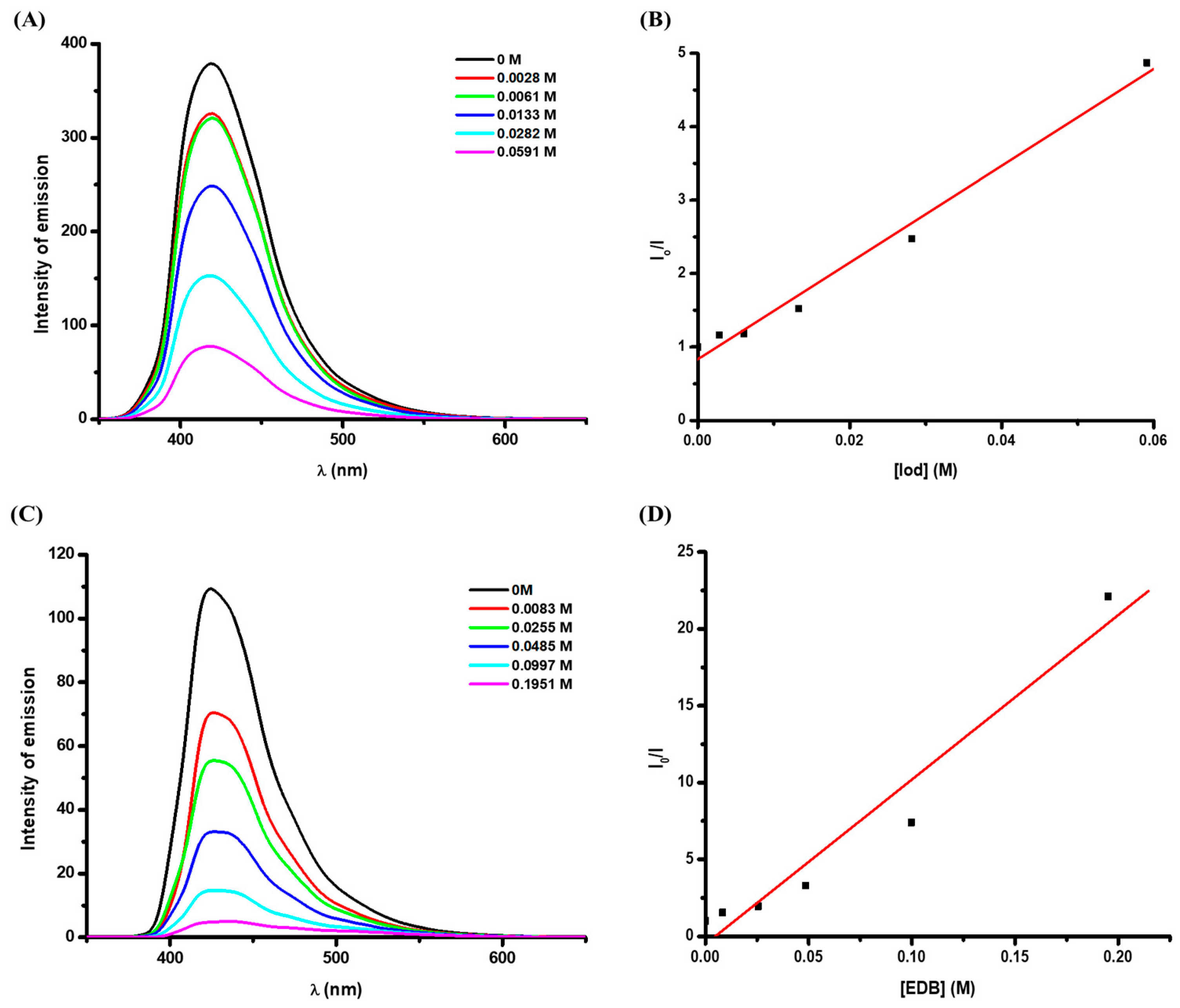
2.3. Photochemical Properties of FIC
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gadde, K.; De Vos, D.; Maes, B.U.W. Basic Concepts and Activation Modes in Visible-Light Photocatalyzed Organic Synthesis. Synthesis 2023, 55, 164–192. [Google Scholar] [CrossRef]
- De Vos, D.; Gadde, K.; Maes, B.U.W. Emerging Activation Modes and Techniques in Visible-Light-Photocatalyzed Organic Synthesis. Synthesis 2023, 55, 193–231. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kwon, M.S. Emerging Organic Photoredox Catalysts for Organic Transformations. Eur. J. Org. Chem. 2020, 2020, 6028–6043. [Google Scholar] [CrossRef]
- Bell, J.D.; Murphy, J.A. Recent Advances in Visible Light-Activated Radical Coupling Reactions Triggered by (i) Ruthenium, (Ii) Iridium and (Iii) Organic Photoredox Agents. Chem. Soc. Rev. 2021, 50, 9540–9685. [Google Scholar] [CrossRef]
- Wu, Y.; Kim, D.; Teets, T.S. Photophysical Properties and Redox Potentials of Photosensitizers for Organic Photoredox Transformations. Synlett 2022, 33, 1154–1179. [Google Scholar]
- Bobo, M.V.; Kuchta, J.J.; Vannucci, A.K. Recent Advancements in the Development of Molecular Organic Photocatalysts. Org. Biomol. Chem. 2021, 19, 4816–4834. [Google Scholar] [CrossRef] [PubMed]
- Speckmeier, E.; Fischer, T.G.; Zeitler, K. A Toolbox Approach To Construct Broadly Applicable Metal-Free Catalysts for Photoredox Chemistry: Deliberate Tuning of Redox Potentials and Importance of Halogens in Donor–Acceptor Cyanoarenes. J. Am. Chem. Soc. 2018, 140, 15353–15365. [Google Scholar] [CrossRef] [PubMed]
- Zivic, N.; Kuroishi, P.K.; Dumur, F.; Gigmes, D.; Dove, A.P.; Sardon, H. Recent advances and challenges in the design of organic photoacid and photobase generators for polymerizations. Angew. Chem. Int. Ed. 2019, 58, 10410–10422. [Google Scholar] [CrossRef] [PubMed]
- Chatani, S.; Kloxin, C.J.; Bowman, C.N. The power of light in polymer science: Photochemical processes to manipulate polymer formation, structure, and properties. Polym. Chem. 2014, 5, 2187–2201. [Google Scholar] [CrossRef]
- Crivello, J.V.; Reichmanis, E. Photopolymer materials and processes for advanced technologies. Chem. Mater. 2014, 26, 533–548. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B. Photopolymerization processes. In Additive Manufacturing Technologies: Rapid Prototyping to Direct Digital Manufacturing, 1st ed.; Springer: New York, NY, USA, 2010; pp. 78–119. [Google Scholar]
- Yoshino, F.; Yoshida, A. Effects of blue-light irradiation during dental treatment. Jpn. Dent. Sci. Rev. 2018, 54, 160–168. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; Stansbury, J.W.; Zhu, X.; Wang, X.; Nie, J. Smart antibacterial surface made by photopolymerization. ACS Appl. Mater. Interfaces 2016, 8, 28047–28054. [Google Scholar] [CrossRef] [PubMed]
- Geisler, E.; Lecompère, M.; Soppera, O. 3D printing of optical materials by processes based on photopolymerization: Materials, technologies, and recent advances. Photonics Res. 2022, 10, 1344–1360. [Google Scholar] [CrossRef]
- Jandt, K.D.; Mills, R.W.; Blackwell, G.B.; Ashworth, S.H. Depth of cure and compressive strength of dental composites cured with blue light emitting diodes (LEDs). Dent. Mater. 2000, 16, 41–47. [Google Scholar] [CrossRef]
- Dreyer, C.; Mildner, F. Application of LEDs for UV-curing. In III-Nitride Ultraviolet Emitters: Technology and Applications, 1st ed.; Springer: Cham, Switzerland, 2016; pp. 415–434. [Google Scholar]
- Galibert-Guijarro, A.; Mouysset, D.; Mimoun, L.; Bertrand, M.P.; Feray, L. Ynamides in Radical Reactions: A Route to Original Persubstituted 2-Aminofurans. J. Org. Chem. 2023, 88, 2464–2473. [Google Scholar] [CrossRef] [PubMed]
- Galli, C. Radical Reactions of Arenediazonium Ions: An Easy Entry into the Chemistry of the Aryl Radical. Chem. Rev. 1988, 88, 765–792. [Google Scholar] [CrossRef]
- De Souza, E.L.S.; Oliveira, C.C. Selective Radical Transformations with Aryldiazonium Salts. Eur. J. Org. Chem. 2023, 26, e202300073. [Google Scholar] [CrossRef]
- Schroll, P.; Hari, D.P.; König, B. Photocatalytic Arylation of Alkenes, Alkynes and Enones with Diazonium Salts. ChemistryOpen 2012, 1, 130–133. [Google Scholar] [CrossRef]
- Hering, T.; Hari, D.P.; König, B. Visible-Light-Mediated α-Arylation of Enol Acetates Using Aryl Diazonium Salts. J. Org. Chem. 2012, 77, 10347–10352. [Google Scholar] [CrossRef]
- Tomin, V.I. Studying reactions that proceed from highest excited states of molecules using fluorescence quenching. Opt. Spectrosc. 2008, 105, 496–510. [Google Scholar] [CrossRef]
- Noon, D.; Hammoud, F.; Graff, B.; Hamieh, T.; Toufaily, J.; Morlet-Savary, F.; Schmitt, M.; Bui, T.-T.; Rico, A.; Goubard, F.; et al. Photoinitiation Mechanisms of Novel Phenothiazine-Based Oxime and Oxime Esters Acting as Visible Light Sensitive Type I and Multicomponent Photoinitiators. Adv. Mater. Technol. 2023, 8, 2300205. [Google Scholar] [CrossRef]
- Fehér, P.P.; Madarász, Á.; Stirling, A. Prediction of Redox Power for Photocatalysts: Synergistic Combination of DFT and Machine Learning. J. Chem. Theory Comput. 2023, 19, 4125–4135. [Google Scholar] [CrossRef]
- Mateos, J.; Rigodanza, F.; Vega-Peñaloza, A.; Sartorel, A.; Natali, M.; Bortolato, T.; Pelosi, G.; Companyó, X.; Bonchio, M.; Dell’Amico, L. Naphthochromenones: Organic Bimodal Photocatalysts Engaging in Both Oxidative and Reductive Quenching Processes. Angew. Chem. Int. Ed. 2020, 59, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Roe, A. Preparation of aromatic fluorine compounds from diazonium fluoborates. The Schiemann reaction. Org. React. 1949, 5, 193. [Google Scholar]
- Tang, Z.Y.; Zhang, Y.; Wang, T.; Wang, W. Rhodium (I)-catalyzed synthesis of aryltriethoxysilanes from arenediazonium tosylate salts with triethoxysilane. Synlett 2010, 5, 804–808. [Google Scholar]
- Xu, Y.; Alcock, N.W.; Clarkson, G.J.; Docherty, G.; Woodward, G.; Wills, M. Asymmetric hydrogenation of ketones using a ruthenium (II) catalyst containing binol-derived monodonor phosphorus-donor ligands. Org. Lett. 2004, 6, 4105–4107. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cai, P.; Guo, Q.; Xue, S.J. Et2Zn-mediated rearrangement of bromohydrins. Org. Chem. 2008, 73, 3516–3522. [Google Scholar] [CrossRef]
- Molinaro, C.; Mowat, J.; Gosselin, F.; O’Shea, P.D.; Marcoux, J.-F.; Angelaud, R.; Davies, I.W.J. A practical synthesis of α-aryl methyl ketones via a transition-metal-free meerwein arylation. Org. Chem. 2007, 72, 1856–1858. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.Z.; Zhao, G.; Hamze, A.; Alami, M.; Provot, O. Chlorotrimethylsilane and Sodium Iodide: A Useful Combination for the Regioselective Deoxygenation of Arylalkyl-α-Diketones. Adv. Synth. Catal. 2017, 359, 2682–2691. [Google Scholar] [CrossRef]
- Hisano, N.; Kamei, Y.; Kansaku, Y.; Yamanaka, M.; Mori, K. Construction of 1, 3-Dithio-substituted Tetralins by [1, 5]-alkylthio group transfer mediated skeletal rearrangement. Org. Lett. 2018, 20, 4223–4226. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, J.; Yang, S.; Liu, W.; Chen, Q.; He, M. C–H arylation reactions through aniline activation catalysed by a PANI-gC3N4-TiO2 composite under visible light in aqueous medium. Green Chem. 2018, 20, 1290–1296. [Google Scholar] [CrossRef]

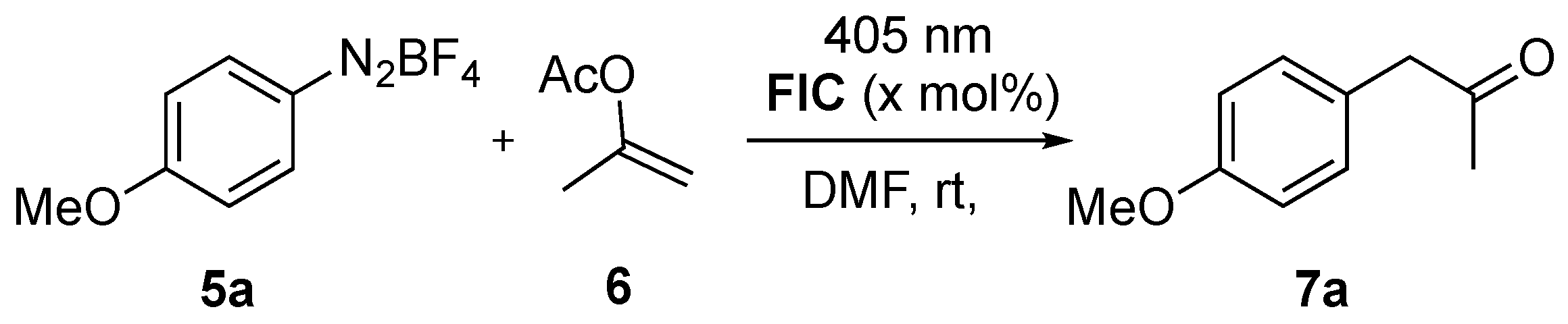
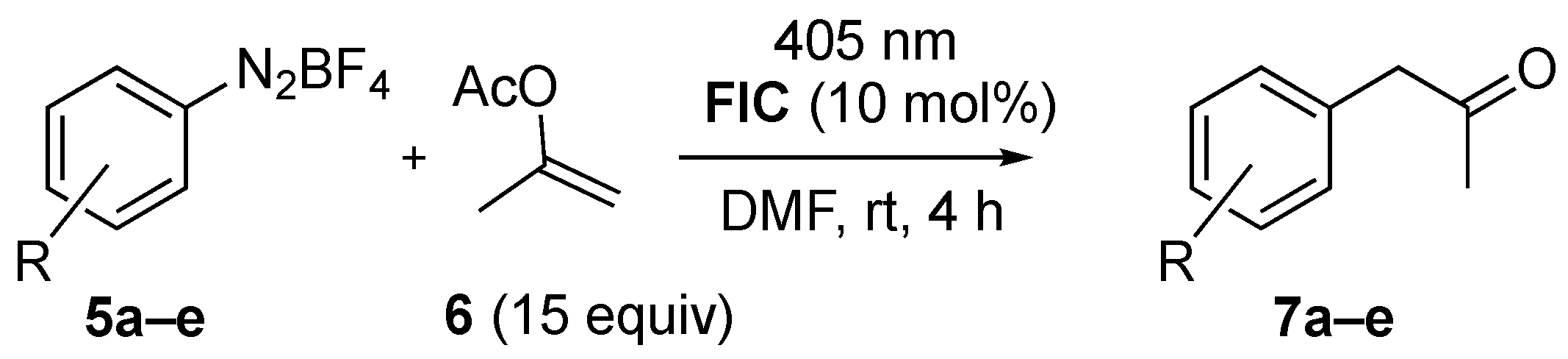


| Entry | 6 (Equiv) | t (h) | FIC (mol%) | 7a:5a 1 | 7a (%) 2 |
|---|---|---|---|---|---|
| 1 | 10 | 2 | - | 20:80 | nd 3 |
| 2 | 10 | 2 | 10 | 70:30 4 | nd 3 |
| 3 | 15 | 4 | - | - | 30 |
| 4 | 15 | 4 | 10 | - | 80 5 |
| Entry | 5 | R | 7 (%) 1 |
|---|---|---|---|
| 1 | 5a | 4-OMe | 7a (80) |
| 2 | 5b | 4-NO2 | 7b (77) |
| 3 | 5c | H | 7c (88) |
| 4 | 5d | 2-Br | 7d (61) |
| 5 | 5e | 3-CN | 7e (75) |
| ES1 (eV) | Eox (V) | ΔGS1(FIC/Iod) (eV) a | Ered (V) | ΔGS1(FIC/EDB) (eV) b | Ksv (Iod) (M−1) c | Φet (FIC/Iod) d | Ksv (EDB) (M−1) c | Φet (FIC/EDB) e | |
|---|---|---|---|---|---|---|---|---|---|
| FIC | 3.27 | 0.86 | −1.71 | −0.63 | −1.64 | 66 | 0.8 | 107 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galibert-Guijarro, A.; Noon, A.; Toufaily, J.; Hamieh, T.; Besson, E.; Gastaldi, S.; Lalevée, J.; Feray, L. Furan-Indole-Chromenone-Based Organic Photocatalyst for α-Arylation of Enol Acetate and Free Radical Polymerization Under LED Irradiation. Molecules 2025, 30, 265. https://doi.org/10.3390/molecules30020265
Galibert-Guijarro A, Noon A, Toufaily J, Hamieh T, Besson E, Gastaldi S, Lalevée J, Feray L. Furan-Indole-Chromenone-Based Organic Photocatalyst for α-Arylation of Enol Acetate and Free Radical Polymerization Under LED Irradiation. Molecules. 2025; 30(2):265. https://doi.org/10.3390/molecules30020265
Chicago/Turabian StyleGalibert-Guijarro, Aurélien, Adel Noon, Joumana Toufaily, Tayssir Hamieh, Eric Besson, Stéphane Gastaldi, Jacques Lalevée, and Laurence Feray. 2025. "Furan-Indole-Chromenone-Based Organic Photocatalyst for α-Arylation of Enol Acetate and Free Radical Polymerization Under LED Irradiation" Molecules 30, no. 2: 265. https://doi.org/10.3390/molecules30020265
APA StyleGalibert-Guijarro, A., Noon, A., Toufaily, J., Hamieh, T., Besson, E., Gastaldi, S., Lalevée, J., & Feray, L. (2025). Furan-Indole-Chromenone-Based Organic Photocatalyst for α-Arylation of Enol Acetate and Free Radical Polymerization Under LED Irradiation. Molecules, 30(2), 265. https://doi.org/10.3390/molecules30020265









