Exploring Natural Deep Eutectic Solvents (NADES) for Enhanced Essential Oil Extraction: Current Insights and Applications
Abstract
:1. Introduction
2. Essential Oils: Chemistry and Extraction

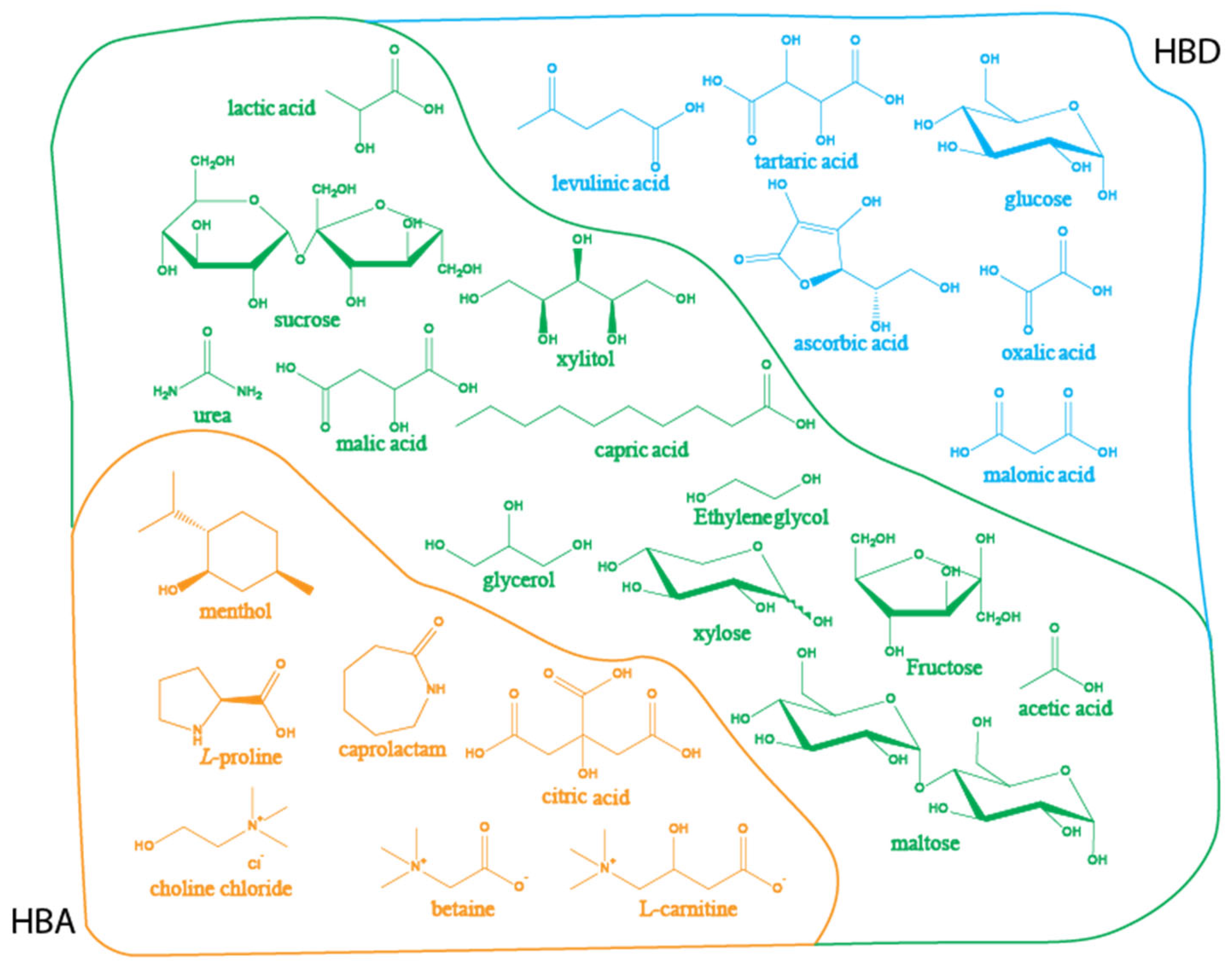
3. Application of NADES in EOs Extraction
| Plant Specie/Part Employed | Selected NADES and Extraction Technique and Conditions | Results/Main Outcomes | Main Compounds Identified | Analytical Technique | Reference | |
|---|---|---|---|---|---|---|
| Flos Chrysanthemi Indici/Flowers | Bet/Gly (1:2) at 70 °C with stirring liquid–solid ratio at 21.5 mL/g. Introduce CO2 into the liquid at a flow rate of 0.25 L/min | High-speed homogenization coupled with an SHS approach combined with CO2 and NADES extraction Rotating speed at 20,712 rpm, and extraction time of 3.23 min. | EO yield: 1.23%. High values in the extraction of TPC, TFC, and EO. Higher content of OHC | Cycloeucalenol acetate Hedycariol Cryptomeridiol | GC-MS | [101] |
| Schisandra chinensis (Turcz.) Baill/Fruits | ChCl/EG molar ratio 1:3 and solvent ratio 7:3 v/v, melted in a 80 °C oil bath. S/L: 1:30 w/v. 10.0 g of dried fruit were mixed with 300 mL, 0.75 M of DES. | Alternate UMHD at 20 min of extraction time. 400 W (UE), 250 W (MW) | EO yield: 12.2 mL/Kg. Higher free radical scavenging activity and reducing power and OHC proportion in EO composition | Ylangene α-bergamotene β-himachalene | GC-MS | [102] |
| Angelica sinensis/Roots | ChCl/CA (1:3). Water content of 40%. S/L: 1:5 w/v. | MAHD at 99 °C and the reaction time at 70 m. 600 W (MW). | EO yield: 1.39%. Higher EO yield, efficiency, and low energy consumption compared with traditional and water-based methods | Z and E-ligustilide, n-butylphthalide, 3-n-butylidenephthalide, (+)-Isovalencenol | GC-MS | [32] |
| Leptospermum scoparium/Leaves | Hydrophobic deep eutectic solvent (HDES) Me/LA (1:2). 0.75 g of Manuka powder (200 m) was mixed with 5 mL of HDES | Stirring. The mixture was stirred at 1000 rpm for 1 h at 25 °C | EO yield: n.r. Higher antioxidant and antibacterial activity as well as high TPC compared with traditional solvents | β-caryophyllene α and β-pinene, linalool, eucalyptol, α and β-selinene, α-eudesmol, α-terpineol | GC-MS | [103] |
| Cymbopogon citratus Cymbopogon flexuosus Elyonurus muticus/Leaves | ChCl/AA (1:2) | Magnetic stirring method at 60 °C, 180 min for the species C. citratus and C. flexuosus and 150 min for E. muticus species. | EO yield: n.r. Higher antioxidant, antibacterial activity, and high TPC in the NADES-based EOs compared with traditional solvents (H2O, MetOH, EtOH, and Ace) | Neral Geraniol Geranial p-menthol p and o-cymene Citronellol | GC-MS/MS | [104] |
| Coriandrum sativum L./Seed | ChCl/Ur (1:1) At 80 °C 75 g of NADES with 40% (w/w) water in a 1:5 (w/w) plant/liquid ratio. | US pretreatment followed by conventional HD. (US) at T = 25 °C, t = 30 min, and 70 W of power. Hydro-distillation for 4 h. | EO yield: 1.10% NADES positively influenced EO composition and extraction yield. The acidity of the NADES used may influence the composition of the CEO. Operational extraction conditions were optimized successfully through RSM | Linalool Limonene α-terpineol | GC-MS GC-FID (for chiral analysis) | [85] |
| Perillae folium/Leaf | ChCl/MA (2:1). 72 g of the components mixed with 48 g deionized water were placed in a beaker with an 80 °C water bath and 100 W US for 15 min L/S: 40:1 | US pretreatment followed by conventional HD. Ultrasonic extraction instrument (600 W, 5 min). In the next step, the mixture was diluted with 800 mL deionized water and put under US at 600 W for 20 min. | EO yield: 0.67% NADES components ratio influenced EO yield, TPC, antioxidant, and antibacterial activity | α-terpineol Damascenone Perillaldehyde β-caryophyllene α-bergamotene | GC-MS | [95] |
| Litsea cubeba (Lour.) Pers./Fruits | ChCl/OA (1:1) Water content (50%), liquid–solid ratio (12.5:1 mL/g) | NADES-homogenate-based MAHD. Homogenate time (2 min), and microwave power (700 W). | EO yield: 16.49% NADES led exclusively to extracting specific compounds, and the homogenate pretreatment with NADES enhanced EO yield. Antioxidant activity in NADES-based EOs was higher than those without pretreatment and water-based experiments | m-cymene trans-linalool oxide Z and E-citral Eucalyptol | GC-MS | [105] |
| Piper nigrum/Fruits | ChCl/Fr (3:2). Nades was prepared with small amount of water at 80 °C. L/S: 3:1 DES/powder ratio | Three-stage extraction: MW pretreatment, fast heating stage, and MAHD. (1) pretreatment stage: 600 W microwave power, 80 °C temperature, and 10 min duration; (2) fast heating stage: 600 W microwave power, 110 °C temperature, and 5 min duration; (3) hydro-distillation stage: 300 W microwave power, 110 C temperature, and 35 min duration. | EO yield: 1.78% The different stages and their parameters influence EO composition and yield. The optimized extraction approach allowed the identification of a higher number of compounds than HD and MAHD (solely) | Caryophyllene Eucalyptol Sabinene α-pinene | GC-MS | [106] |
| Aloysia Citriodora/Leaves | ChCl/Glu (1:1) Pretreatment: (100 g) and 80 g of NADESs were mixed with 240 mL of distilled water. HD: water ratio of 1:10 | MW pretreatment at power (600 W) and time (5 min) followed by HD. | EO yield: 0.21% MW power during the pretreatment stage influenced EO yield, and the antioxidant and antimicrobial activity varied according to NADES components | Verbenone Limonene Spathulenol | GC-MS | [107] |
| Mentha haplocalyx Briq./Leaves | ChCl/Glu stirred at 80 °C with 80% water Content. S/L: 1/12 g/mL | Enzyme-based (cellulase and pectinase 1:1, 2.0% enzyme concentration) NADES pretreatment followed by MAHD at 540 W Pretreatment temperature: 50 °C | EO yield: 2.19% The synergistic effect of the enzyme pretreatment and NADES during extraction significantly improved the components and yield from EO. Additionally, the EO extracted under those conditions showed an α-amylase and AChE inhibitory activity superior to those extracted with traditional methods and without pretreatment | Menthol Menthone Piperitone Isomenthone Germacrene D | GC-MS | [96] |
| Artemisia absinthium/Leaves | Car/MA (1:1) with 50% water S/L: 1:6 | SD Distillation time of 10 h | EO yield: 7.52% NADES inclusion in the extraction showed a higher extraction yield and a higher number of identified compounds than water-based, NaCl, and enzymatic treatments | α-terpineol l-borneol 1,8-cineole Thujone | GC-MS | [108] |
| Ipomoea cairica (L.) Sweet/Leaves | ChCl/Glu (1:1) stirred at 85 °C with 15% water S/L: 1:5 | Heating and stirring Rotating speed: 300 rpm, rotating time: 25 min | NADES solvents have shown to be efficient in the extraction and dilution of plant components and valuable for the analysis of volatile compounds | β-elemene β-caryophyllene α-humulene | Static HS-GC-MS | [109] |
| Myristica fragrans Houtt./Seeds | ChCl/CA (1:1) with 40% water (w/w) stirred at room temperature and subsequently sonicated at 25° C for 10 min. S/L: 1:15 | US pretreatment followed by HD. Pretreat: the suspension of nutmeg fruits and 40% NADES at 50 C for 30 min. Distillation time: 2 h | EO yield: 1.41% NADES inclusion improved EO yield compared with water, and the proportions of sesquiterpene hydrocarbons, monoterpene hydrocarbons, oxygenated monoterpenes and phenylpropanoids varied according to NADES composition | Elemicin Methyl eugenol Safrole Myristicin | GC-MS | [80] |
| Amomum kravanh, Amomum tsaoko Amomum villosum/Fruits | ChCl/EG (1:4) stirred at 80 °C. L/S: 7:1 | Three-stage extraction: MW pretreatment, fast heating stage, and MAHD. (1) pretreatment stage: 500 W of MW, 50 °C of temperature, and 7 min of duration; (2) fast heating stage: 600 W of MW, 110 °C of temperature, and 5 min of duration; (3) MAHD stage: 300 W of MW, 110 °C of temperature, and 30 min of duration. | EO yield: 3.64, 2.16, and 1.62%, for A. kravanh, A. tsaoko, and A. villosum The optimized method allowed a higher number of compounds to be identified than HD and MAHD (solely) and contributed to the differentiation of the three species according to their EO components | Eucalyptol Isobornyl formate Camphor | GC-MS | [110] |
| Syzygium aromaticum/Buds | ChCl/LA (1:2) with deionized water (20% w/w) and exposed MW (400 W) at 80 °C by (10–15 min. Pretreatment stage: 30 g of clove bud powder, 80 g of DES | Three-stage extraction: MW pretreatment, fast heating stage, and MAHD. Pretreatment stage: MW (600 W), temperature (80 °C) and reaction time (5 min). Fast heating stage: MW (600 W), the temperature (110 °C) and the reaction time (5 min). MAHD stage: 300 W of MW, 110 °C of temperature, and 40 min of duration. | EO yield: 4.60% The optimized method brought more compounds than others, such as HD, MAHD (solely), and water-based MAHD. Additionally, the method employed was more effective and environment friendly according to CO2 emissions and electrical consumption calculations | Eugenol β-caryophyllene Eugenyl acetate α-humulene | GC-MS | [111] |
| Curcuma longa L./Roots | ChCl/OA (1:1) exposed to microwave (400 W)at 80 °C. S/L: 1:2 | Three-stage extracti. on: MW pretreatment, fast heating stage, and MAHD. Pretreatment stage: MW (600 W), temperature (84 °C) and reaction time (5 min). Fast heating stage: MW (600 W), the temperature (110 °C) and the reaction time (5 min). MAHD stage: 300 W of MW, 110 °C of temperature, and 76 min of duration. | EO yield: 0.85% Method condition parameters optimized led to a higher extraction of EO and allowed the identification of a higher number of compounds than MAHD and HD methods | ar-turmerone α-turmerone α-himachalene | GC-MS | [112] |
| Ageratina adenophora/Flowers | ChCl/LA stirred at 85 °C. 20 g dried and powdered plant material and 72 g of Des with 850 mL of distilled water | US pretreatment followed by HD. US: at 25 °C for 25 min hydro-distillation: 70 °C, 2.5 h. | EO yield: 13.52% The incorporation of NADES not only enhanced EO yield but also influenced the chemical composition. EOs obtained with NADES led to the isolation of a new sesquiterpene in high yield and showed a potential AChE inhibitory activity | 5,11-epoxycadin-3,4-en-8-one Bornyl acetate β-bisabolene | GC-MS 1H and 13C NMR SEM SC-XRD | [99] |
| Nardostachys jatamansi (D.Don) DC/Roots | ChCl/MA (2:1) heated at 90–100 °C with stirring. Ratio plant material, NADES, and water: 1:2:4 (w/v/v). | HD; 70 °C for 3–4 h | EO yield: 1.77% v/w The NADES components highly influenced the chemical composition of EOs and, consequently, biological activities, such as insecticidal activity against A. craccivora and P. lilacinus and inhibitory activity of AChE and glutathione S-transferase | Bisabolol α-cadinol Nootkatone Valeranone Nerolidol | GC-MS GC-FID | [113] |
| Rosmarinus officinalis L./Leaves | ChCl/Gly (1:2) with 10% of water S/L 1:15 m/v | NADES soaking followed by HD. Pretreatment at 20 °C during 72 h. 2 h of distillation. | EO yield: 2.32% The pretreatment stage enhanced the EO yield, quantitative composition, and antioxidant activity compared with the non-pretreated sample | Camphor Verbenone Borneol | GC-MS GC-FID | [114] |
| Cuminum cyminum L./Seeds | ChCl/LA (1:3) with 40% (w/w) of water L/S: 6:1 | Three-stage extraction: MW pretreatment, fast heating stage, and MAHD (1) pretreatment stage: 600 W of MW, 90 °C of temperature, and 4 min of duration; (2) fast heating stage: 600 W of MW, 110 °C of temperature, and 5 min of duration; (3) MAHD stage: 300 W of MW, 110 °C of temperature, and 30 min of duration. | EO yield: 2.22% NADES inclusion resulted in more EO extraction and a greater number of compounds identified, increasing the OHC proportion in the overall composition. Additionally, MW showed to be more suitable for sample pretreatment than US | Cuminol Cuminal Moslene Terpineol | GC-MS | [115] |
| Mentha piperita L./Leaves | ChCl/Glu (5:2) diluted with water (70% w/w) S/L: 1:10 | UAE (maximum power of ~500 W, ambient temperature) | The use of NADES allows the identification of volatile compounds without the use of time-consuming methods and, organic solvents and can be applied to the differentiation of peppermint samples from different origins | Menthol Menthyl acetate Pulegone Menthone Eucalyptol | HS-SPME-GC-MS | [116] |
| Ginkgo biloba L./Leaves | ChCl/Asc diluted with deionized water (34%). S/L: 1:10 | UAE (temperature of 56 °C, 37 min) | NADES influenced the extraction of the different ginkgolides, with the highest total yield that one obtained by the selected NADES (24.60%) and allowed the lowest ginkgolic acid extraction (0.37%) Additionally, NADES solvents showed higher efficiency than traditional solvents such as MetOH and EtOH | Bilobalide Ginkgolide A, B, C, J, and K Ginkgolic acid | HPTLC-MS | [79] |
| Bet/EG (1:3) containing 40% (w/w). S/L: 1/10 | UAE at 45 °C and 100 w for 20 min | Extraction yield: 2.36% Using NADES in the extraction led to a higher yield than traditional solvents like EtOH and H2O, as well as traditional methods such as HD and solvent-reflux extraction | Triterpene lactones | HPLC-ELSD | [117] | |
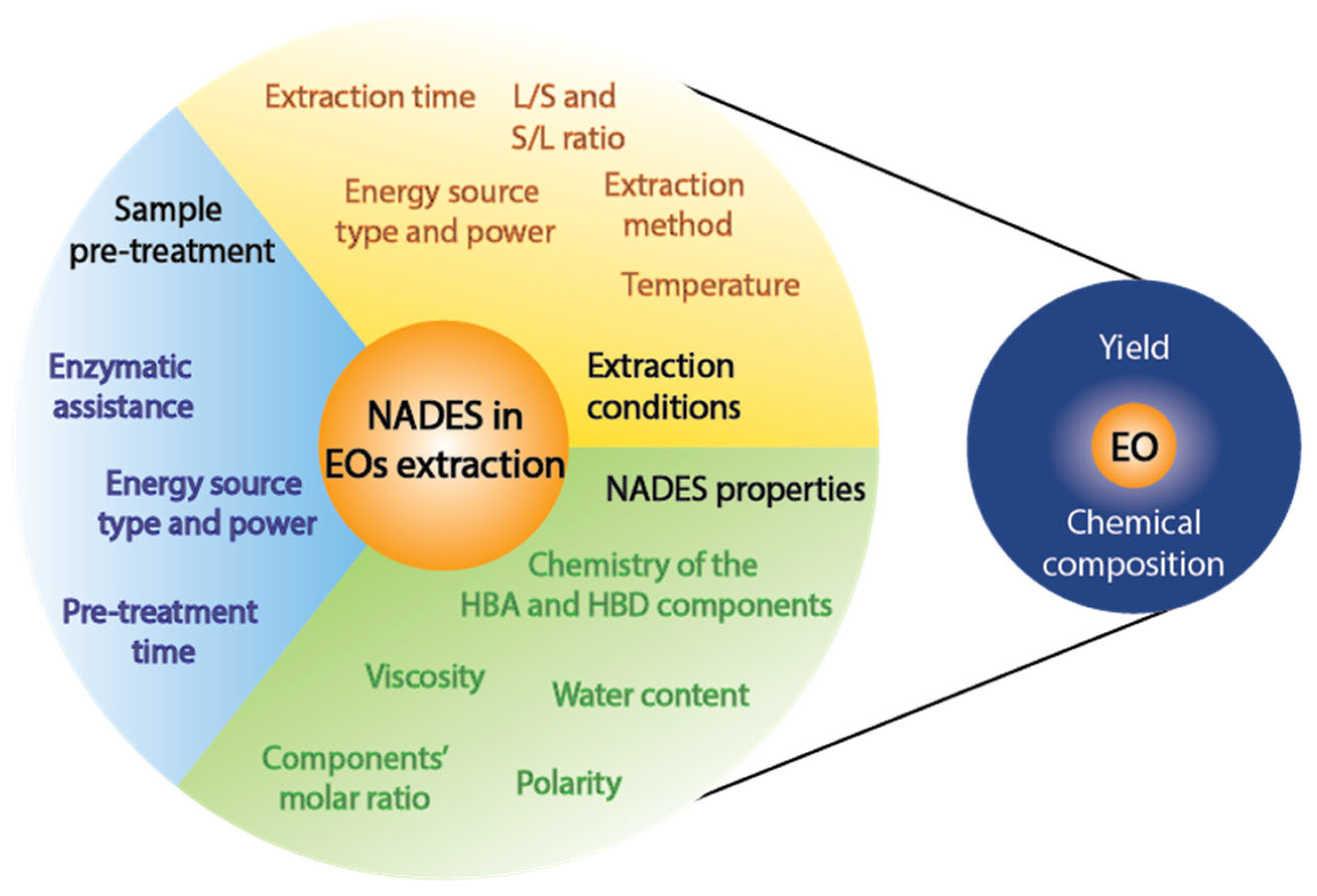
4. Factors Affecting the Extraction of Essential Oils with NADES
4.1. NADES Properties
4.2. Extraction Conditions
4.3. Sample Pretreatment
5. Analytical Methods Used for the Characterization of Essential Oils Components and Some of Their Biological Activities
6. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| ChCl | Choline chloride | Asc | Ascorbic acid |
| Bet | Betaine | Xyl | Xylose |
| Gly | Glycerol | TA | Tartaric acid |
| EG | Ethylene glycol | PG | Propylene glycol |
| CA | Citric acid | MW | Microwave radiation |
| Me | Menthol | US | Ultrasound radiation |
| LA | Lactic acid | L/S | Liquid/solid ratio |
| PW | Pure water | S/L | Solid/liquid ratio |
| Glu | Glucose | Ace | Acetone |
| AA | Acetic acid | EtOH | Ethanol |
| Ur | Urea | MetOH | Methanol |
| Mal | Malonic acid | TEAC | Tetraethyl ammonium chloride |
| MA | Malic acid | ||
| OA | Oxalic acid | ||
| Fru | Fructose | ||
| Car | L-carnitine |
References
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, Bioactivities, Mode of Action and Industrial Applications of Essential Oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.; Rodrigues, A.E. Microencapsulation of Essential Oils with Biodegradable Polymeric Carriers for Cosmetic Applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef]
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; Amin, P.D.; Gaikwad, K.K.; de Aguiar Andrade, E.H.; de Oliveira, M.S. Recent Trends in the Application of Essential Oils: The next Generation of Food Preservation and Food Packaging. Trends Food Sci. Technol. 2022, 129, 421–439. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Cavalcante, D.N.; Corrêa, R.F.; Campelo, P.H.; Sanches, E.A.; de Araújo Bezerra, J. Essential Oils from Unconventional Food Plants (Murraya Spp., Ocimum Spp., Piper Spp.) as Alternative Food Flavorings. Food Chem. Adv. 2023, 3, 100481. [Google Scholar] [CrossRef]
- Hou, T.; Sana, S.S.; Li, H.; Xing, Y.; Nanda, A.; Netala, V.R.; Zhang, Z. Essential Oils and Its Antibacterial, Antifungal and Anti-Oxidant Activity Applications: A Review. Food Biosci. 2022, 47, 101716. [Google Scholar] [CrossRef]
- Bai, X.; Chen, T.; Liu, X.; Liu, Z.; Ma, R.; Su, R.; Li, X.; Lü, X.; Xia, X.; Shi, C. Antibacterial Activity and Possible Mechanism of Litsea cubeba Essential Oil Against Shigella sonnei and Its Application in Lettuce. Foodborne Pathog. Dis. 2023, 20, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Visakh, N.U.; Pathrose, B.; Narayanankutty, A. Characterization of Secondary Metabolites from the Leaves of Curry Leaf (Murraya koenigii L.) Essential Oils with Insecticidal Activities against Stored Product Insects. Biocatal. Agric. Biotechnol. 2023, 54, 102973. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Rivas, S.; Rivas, J.; Domínguez, H. Subcritical Water Extraction of Essential Oils and Plant Oils. Sustain. Chem. Pharm. 2023, 36, 101332. [Google Scholar] [CrossRef]
- Rathore, S.; Mukhia, S.; Kumar, R.; Kumar, R. Essential Oil Composition and Antimicrobial Potential of Aromatic Plants Grown in the Mid-Hill Conditions of the Western Himalayas. Sci. Rep. 2023, 13, 4878. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The Formation and Function of Plant Volatiles: Perfumes for Pollinator Attraction and Defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Glas, J.J.; Schimmel, B.C.J.; Alba, J.M.; Escobar-Bravo, R.; Schuurink, R.C.; Kant, M.R. Plant Glandular Trichomes as Targets for Breeding or Engineering of Resistance to Herbivores. Int. J. Mol. Sci. 2012, 13, 17077–17103. [Google Scholar] [CrossRef] [PubMed]
- Tetali, S.D. Terpenes and Isoprenoids: A Wealth of Compounds for Global Use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, Y.; Chi, P.; Liu, H.; Jing, Z.; Cao, H.; Du, Y.; Zhao, Y.; Qin, X.; Zhang, W.; et al. Essential Oils: Chemical Constituents, Potential Neuropharmacological Effects and Aromatherapy—A Review. Pharmacol. Res.-Mod. Chin. Med. 2023, 6, 100210. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Hatvate, N.T.; Naren, P.; Khan, S.; Chavda, V.P.; Balar, P.C.; Gandhi, J.; Khatri, D.K. Essential Oils for Clinical Aromatherapy: A Comprehensive Review. J. Ethnopharmacol. 2024, 330, 118180. [Google Scholar] [CrossRef]
- Raghuvanshi, T.S.; Singh, P.P.; Kohar, N.; Prakash, B. Essential Oils: From Traditional to Modern-Day Applications with Special Reference to Medicinal and Aromatic Plants in India. In Plant Essential Oils: From Traditional to Modern-Day Application; Prakash, B., Dubey, N.K., Freitas Brilhante de São José, J., Eds.; Springer Nature: Singapore, 2024; pp. 1–26. ISBN 978-981-99-4370-8. [Google Scholar]
- D’Aquila, P.; Paparazzo, E.; Crudo, M.; Bonacci, S.; Procopio, A.; Passarino, G.; Bellizzi, D. Antibacterial Activity and Epigenetic Remodeling of Essential Oils from Calabrian Aromatic Plants. Nutrients 2022, 14, 391. [Google Scholar] [CrossRef] [PubMed]
- El Karkouri, J.; Bouhrim, M.; Al Kamaly, O.M.; Mechchate, H.; Kchibale, A.; Adadi, I.; Amine, S.; Ismaili, S.A.; Zair, T. Chemical Composition, Antibacterial and Antifungal Activity of the Essential Oil from Cistus ladanifer L. Plants 2021, 10, 2068. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Yang, M.; Zhao, Y.; Zang, Y.; Zhang, Z.; Chen, H. Aromatherapy with Inhalation Effectively Alleviates the Test Anxiety of College Students: A Meta-Analysis. Front. Psychol. 2023, 13, 1042553. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical and Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, A. Review on Essential Oil Extraction from Aromatic and Medicinal Plants: Techniques, Performance and Economic Analysis. Sustain. Chem. Pharm. 2022, 30, 100829. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Koidis, A. Methods for Extracting Essential Oils; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780124166417. [Google Scholar]
- Mohammed, H.H.; Laftah, W.A.; Noel Ibrahim, A.; Che Yunus, M.A. Extraction of Essential Oil from Zingiber officinale and Statistical Optimization of Process Parameters. RSC Adv. 2022, 12, 4843–4851. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.R.A.; Shahid, H.; Zahoor, A.F.; Saeed, M.; Usman, M.; Abbas, A.; Rasheed, M.U.; Hussain, T. Classical Methods for Obtaining Essential Oils. In Essential Oils; Wiley: Hoboken, NJ, USA, 2023; pp. 565–582. ISBN 9781119829614. [Google Scholar]
- Mejri, J.; Aydi, A.; Abderrabba, M.; Mejri, M. Emerging Extraction Processes of Essential Oils: A Review. Asian J. Green Chem. 2018, 2, 246–267. [Google Scholar] [CrossRef]
- Pandey, V.K.; Tripathi, A.; Srivastava, S.; Dar, A.H.; Singh, R.; Farooqui, A.; Pandey, S. Exploiting the Bioactive Properties of Essential Oils and Their Potential Applications in Food Industry. Food Sci. Biotechnol. 2023, 32, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, Q. An Efficient Extraction Method for Essential Oil from Angelica Sinensis Radix by Natural Deep Eutectic Solvents-Assisted Microwave Hydrodistillation. Sustain. Chem. Pharm. 2022, 29, 100792. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Dai, Y.; Varypataki, E.M.; Golovina, E.A.; Jiskoot, W.; Witkamp, G.-J.; Choi, Y.H.; Verpoorte, R. Chapter Seven—Natural Deep Eutectic Solvents in Plants and Plant Cells: In Vitro Evidence for Their Possible Functions. In Eutectic Solvents and Stress in Plants; Verpoorte, R., Witkamp, G.-J., Choi, Y.H., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 97, pp. 159–184. ISBN 0065-2296. [Google Scholar]
- Gonzalez, C.G.; Choi, Y.H.; Verpoorte, R. Preanalytical Treatments: Extraction with Deep Eutectic Solvents; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128169117. [Google Scholar]
- Zhang, M.; Zhang, X.; Liu, Y.; Wu, K.; Zhu, Y.; Lu, H.; Liang, B. Insights into the Relationships between Physicochemical Properties, Solvent Performance, and Applications of Deep Eutectic Solvents. Environ. Sci. Pollut. Res. 2021, 28, 35537–35563. [Google Scholar] [CrossRef] [PubMed]
- Anmol; Sharma, M.; Suresh, P.S.; Gupta, S.S.; Sharma, U. NADES-Based Selective Extraction of Bioactive Molecules: A Case Study with Commercially Important Himalayan Medicinal Plant Aconitum Heterophyllum. Sustain. Chem. Pharm. 2023, 36, 101305. [Google Scholar] [CrossRef]
- Schuh, L.; Reginato, M.; Florêncio, I.; Falcao, L.; Boron, L.; Gris, E.F.; Mello, V.; Báo, S.N. From Nature to Innovation: The Uncharted Potential of Natural Deep Eutectic Solvents. Molecules 2023, 28, 7653. [Google Scholar] [CrossRef]
- Wils, L.; Hilali, S.; Boudesocque-Delaye, L. Biomass Valorization Using Natural Deep Eutectic Solvents: What’s New in France? Molecules 2021, 26, 6556. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Sarraguça, M. A Comprehensive Review on Deep Eutectic Solvents and Its Use to Extract Bioactive Compounds of Pharmaceutical Interest. Pharmaceuticals 2024, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- de los Ángeles Fernández, M.; Boiteux, J.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Natural Deep Eutectic Solvents-Mediated Extractions: The Way Forward for Sustainable Analytical Developments. Anal. Chim. Acta 2018, 1038, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cannavacciuolo, C.; Pagliari, S.; Celano, R.; Campone, L.; Rastrelli, L. Critical Analysis of Green Extraction Techniques Used for Botanicals: Trends, Priorities, and Optimization Strategies—A Review. TrAC-Trends Anal. Chem. 2024, 173, 117627. [Google Scholar] [CrossRef]
- Mansour, F.R.; Bedair, A.; Hamed, M.; Magdy, G.; Ali, I.; Locatelli, M. Applications of (Natural) Deep Eutectic Solvents in Liquid Phase Microextraction: A Review. Microchem. J. 2024, 198, 110178. [Google Scholar] [CrossRef]
- Koh, Q.Q.; Kua, Y.L.; Gan, S.; Tan, K.W.; Lee, T.Z.E.; Cheng, W.K.; Lau, H.L.N. Sugar-Based Natural Deep Eutectic Solvent (NADES): Physicochemical Properties, Antimicrobial Activity, Toxicity, Biodegradability and Potential Use as Green Extraction Media for Phytonutrients. Sustain. Chem. Pharm. 2023, 35, 101218. [Google Scholar] [CrossRef]
- Liu, J.Z.; Lyu, H.C.; Fu, Y.J.; Jiang, J.C.; Cui, Q. Simultaneous Extraction of Natural Organic Acid and Flavonoid Antioxidants from Hibiscus manihot L. Flower by Tailor-Made Deep Eutectic Solvent. Lwt 2022, 163, 113533. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, A.; Panesar, P.S.; Thakur, A. Potential of Deep Eutectic Solvents in the Extraction of Value-added Compounds from Agro-industrial By-products. Appl. Food Res. 2022, 2, 100211. [Google Scholar] [CrossRef]
- Moghaddam, M.; Mehdizadeh, L. Chapter 13—Chemistry of Essential Oils and Factors Influencing Their Constituents. In Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 379–419. ISBN 978-0-12-811412-4. [Google Scholar]
- Boaro, C.S.F.; Vieira, M.A.R.; Campos, F.G.; Ferreira, G.; De-la-Cruz-Chacón, I.; Marques, M.O.M. Factors Influencing the Production and Chemical Composition of Essential Oils in Aromatic Plants from Brazil. In Essential Oil Research; Malik, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 19–47. ISBN 978-3-030-16546-8. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental Chemistry of Essential Oils and Volatile Organic Compounds, Methods of Analysis and Authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Jeliazkova, E.A.; Astatkie, T. Allelopathic Effects of Essential Oils on Seed Germination of Barley and Wheat. Plants 2021, 10, 2728. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Da Silveira E Sá, R.D.C.; Andrade, L.N.; De Oliveira, R.D.R.B.; De Sousa, D.P. A Review on Anti-Inflammatory Activity of Phenylpropanoids Found in Essential Oils. Molecules 2014, 19, 1459–1480. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, T.; Khan, M.U.; Sharma, V.; Gupta, K. Terpenoids in Essential Oils: Chemistry, Classification, and Potential Impact on Human Health and Industry. Phytomedicine Plus 2024, 4, 100549. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Das, A.; Pandita, D.; Jain, G.K.; Agarwal, P.; Grewal, A.S.; Khar, R.K.; Lather, V. Role of Phytoconstituents in the Management of COVID-19. Chem. Biol. Interact. 2021, 341, 109449. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef] [PubMed]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid.-Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef] [PubMed]
- Elhawary, E.A.; Nilofar, N.; Zengin, G.; Eldahshan, O.A. Variation of the Essential Oil Components of Citrus aurantium Leaves upon Using Different Distillation Techniques and Evaluation of Their Antioxidant, Antidiabetic, and Neuroprotective Effect against Alzheimer’s Disease. BMC Complement. Med. Ther. 2024, 24, 73. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Khalil, A.A.; Rahman, U.U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential Oil Eugenol: Sources, Extraction Techniques and Nutraceutical Perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef]
- Kabir, A.; Cacciagrano, F.; Tartaglia, A.; Lipsi, M.; Ulusoy, H.I.; Locatelli, M. Analysis of Monoterpenes and Monoterpenoids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 274–286. [Google Scholar] [CrossRef]
- Silva, R.C.E.; Da Costa, J.S.; De Figueiredo, R.O.; Setzer, W.N.; Da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Monoterpenes and Sesquiterpenes of Essential Oils from Psidium Species and Their Biological Properties. Molecules 2021, 26, 965. [Google Scholar] [CrossRef] [PubMed]
- Nowrouzi, B.; Li, R.A.; Walls, L.E.; d’Espaux, L.; Malcı, K.; Liang, L.; Jonguitud-Borrego, N.; Lerma-Escalera, A.I.; Morones-Ramirez, J.R.; Keasling, J.D.; et al. Enhanced Production of Taxadiene in Saccharomyces cerevisiae. Microb. Cell Fact. 2020, 19, 200. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical Fluid Extraction of Essential Oils. TrAC-Trends Anal. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Moradi, S.; Fazlali, A.; Hamedi, H. Microwave-Assisted Hydro-Distillation of Essential Oil from Rosemary: Comparison with Traditional Distillation. Avicenna J. Med. Biotechnol. 2018, 10, 22–28. [Google Scholar]
- Chen, F.; Guo, Y.; Kang, J.; Yang, X.; Zhao, Z.; Liu, S.; Ma, Y.; Gao, W.; Luo, D. Insight into the Essential Oil Isolation from Foeniculum vulgare Mill. Fruits Using Double-Condensed Microwave-Assisted Hydrodistillation and Evaluation of Its Antioxidant, Antifungal and Cytotoxic Activity. Ind. Crop. Prod. 2020, 144, 112052. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Ghazanfari, N.; Mortazavi, S.A.; Yazdi, F.T.; Mohammadi, M. Microwave-Assisted Hydrodistillation Extraction of Essential Oil from Coriander Seeds and Evaluation of Their Composition, Antioxidant and Antimicrobial Activity. Heliyon 2020, 6, e04893. [Google Scholar] [CrossRef] [PubMed]
- Drinić, Z.; Pljevljakušić, D.; Živković, J.; Bigović, D.; Šavikin, K. Microwave-Assisted Extraction of O. vulgare L. Spp. Hirtum Essential Oil: Comparison with Conventional Hydro-Distillation. Food Bioprod. Process. 2020, 120, 158–165. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Guzmán-Lorite, M.; Marina, M.L.; García, M.C. Successive Extraction Using Natural Deep Eutectic Solvents and Pressurized Liquids for a Greener and Holistic Recovery of Proteins from Pomegranate Seeds. Food Res. Int. 2022, 161, 111862. [Google Scholar] [CrossRef]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of Deep Eutectic Solvents in Analytical Chemistry. A Review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2023, 12, 56. [Google Scholar] [CrossRef]
- Vladić, J.; Kovačević, S.; Aladić, K.; Rebocho, S.; Jokić, S.; Podunavac-Kuzmanović, S.; Duarte, A.R.C.; Jerković, I. Novel Insights Into the Recovery and Stabilization of Rosmarinus officinalis Volatile Aroma Compounds Using Green Solvents. Food Bioprocess Technol. 2024, 17, 1215–1230. [Google Scholar] [CrossRef]
- Ozturk, B.; Gonzalez-Miquel, M. Alkanediol-Based Deep Eutectic Solvents for Isolation of Terpenoids from Citrus Essential Oil: Experimental Evaluation and COSMO-RS Studies. Sep. Purif. Technol. 2019, 227, 115707. [Google Scholar] [CrossRef]
- Abouheif, S.A.; Sallam, S.M.; El Sohafy, S.M.; Kassem, F.F.; Shawky, E. Optimization of Terpene Lactones and Ginkgolic Acids Extraction from Ginkgo biloba L. Leaves by Natural Deep Eutectic Solvents Using Experimental Design and HPTLC-MS Analysis. Microchem. J. 2022, 176, 107246. [Google Scholar] [CrossRef]
- Lanari, D.; Zadra, C.; Negro, F.; Njem, R.; Marcotullio, M.C. Influence of Choline Chloride-Based NADES on the Composition of Myristica fragrans Houtt. Essential Oil. Heliyon 2022, 8, e09531. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.Q.; Jin, X.Z.; Zhang, Z.H.; Yi, Y.X.; Zhang, J.Y.; Li, Z.G. Extraction of Eugenol from Essential Oils by In Situ Formation of Deep Eutectic Solvents: A Green Recyclable Process. J. Anal. Test. 2024, 8, 63–73. [Google Scholar] [CrossRef]
- Liu, Y.; Garzon, J.; Friesen, J.B.; Zhang, Y.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Countercurrent Assisted Quantitative Recovery of Metabolites from Plant-Associated Natural Deep Eutectic Solvents. Fitoterapia 2016, 112, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and Thermal Behavior of Natural Deep Eutectic Solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Alsaud, N.; Shahbaz, K.; Farid, M. Evaluation of Deep Eutectic Solvents in the Extraction of β-Caryophyllene from New Zealand Manuka Leaves (Leptospermum scoparium). Chem. Eng. Res. Des. 2021, 166, 97–108. [Google Scholar] [CrossRef]
- Scandar, S.; Zadra, C.; Lanari, D.; Marcotullio, M.C. Boosting the Essential Oil Yield of Coriandrum sativum L. Using Choline Chloride-Based NADES: An Optimized and Systematic Study Employing Response Surface Methodology (RSM). Ind. Crop. Prod. 2024, 208, 117920. [Google Scholar] [CrossRef]
- Tang, B.; Bi, W.; Zhang, H.; Row, K.H. Deep Eutectic Solvent-Based HS-SME Coupled with GC for the Analysis of Bioactive Terpenoids in Chamaecyparis obtusa Leaves. Chromatographia 2014, 77, 373–377. [Google Scholar] [CrossRef]
- Bragagnolo, F.S.; Strieder, M.M.; Pizani, R.S.; de Souza Mesquita, L.M.; González-Miquel, M.; Rostagno, M.A. Revisiting Natural Deep Eutectic Solvents (NADES) as Extraction Media and Ready-to-Use Purposes. TrAC-Trends Anal. Chem. 2024, 175, 117726. [Google Scholar] [CrossRef]
- Ayres, L.B.; Gomez, F.J.V.; Silva, M.F.; Linton, J.R.; Garcia, C.D. Predicting the Formation of NADES Using a Transformer—Based Model. Sci. Rep. 2024, 14, 2175. [Google Scholar] [CrossRef] [PubMed]
- Van Osch, D.J.G.P.; Dietz, C.H.J.T.; Van Spronsen, J.; Kroon, M.C.; Gallucci, F.; Van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Li, S.; Wang, G.; Zhao, J.; Ou, P.; Yao, Q.; Wang, W. Ultrasound-Assisted Extraction of Phenolic Compounds from Celtuce (Lactuca sativa Var. Augustana) Leaves Using Natural Deep Eutectic Solvents (NADES): Process Optimization and Extraction Mechanism Research. Molecules 2024, 29, 2385. [Google Scholar] [CrossRef]
- González-Laredo, R.F.; Sayago-Monreal, V.I.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Landeros-Macías, L.F.; Rosales-Castro, M. Natural Deep Eutectic Solvents (NaDES) as an Emerging Technology for the Valorisation of Natural Products and Agro-Food Residues: A Review. Int. J. Food Sci. Technol. 2023, 58, 6660–6673. [Google Scholar] [CrossRef]
- Brahmkshatriya, P.P.; Brahmkshatriya, P.S. Terpenes: Chemistry, Biological Role, and Therapeutic Applications. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2665–2691. ISBN 978-3-642-22144-6. [Google Scholar]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Kempinski, C.; Chappell, J. Extraction and Analysis of Terpenes/Terpenoids. Curr. Protoc. Plant Biol. 2016, 1, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, K.; Zhu, W.; Wang, Y.; Su, C.; Yi, F. Chemical Compositions and Bioactivities of Essential Oil from Perilla Leaf (Perillae Folium) Obtained by Ultrasonic-Assisted Hydro-Distillation with Natural Deep Eutectic Solvents. Food Chem. 2022, 375, 131834. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Pan, X.D.; Guo, Y.; Gao, W.B.; Wang, J.; Dong, B.J.; Duan, M.Y.; Yin, H.Y.; Zhang, Q.; et al. Enzyme-Deep Eutectic Solvent Pre-Treatment for Extraction of Essential Oil from Mentha haplocalyx Briq. Leaves: Kinetic, Chemical Composition and Inhibitory Enzyme Activity. Ind. Crop. Prod. 2022, 177, 114429. [Google Scholar] [CrossRef]
- Mori, N.; Usuki, T. Extraction of Essential Oils from Tea Tree (Melaleuca alternifolia) and Lemon Grass (Cymbopogon citratus) Using Betaine-Based Deep Eutectic Solvent (DES). Phytochem. Anal. 2022, 33, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Wang, J.W.; Wu, M.Y.; Cheng, H.Y.; Chen, L.F.; Qi, Z.W. Deep Deterpenation of Citrus Essential Oils Intensified by In Situ Formation of a Deep Eutectic Solvent in Associative Extraction. Ind. Eng. Chem. Res. 2020, 59, 9223–9232. [Google Scholar] [CrossRef]
- Aggarwal, G.; Singh, P.P.; Gupta, M.K.; Sharma, U. NADES-Based Essential Oil Extraction and Isolation of New Epoxysesquiterpene from Ageratina adenophora Flowers. J. Mol. Struct. 2023, 1292, 136077. [Google Scholar] [CrossRef]
- Yue, Y.; Huang, Q.; Fu, Y.; Chang, J. A Quick Selection of Natural Deep Eutectic Solvents for the Extraction of Chlorogenic Acid from Herba Artemisiae Scopariae. RSC Adv. 2020, 10, 23403–23409. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, S.; Zhou, J.; Lu, T.; Ruan, K.; Xia, Y.; Wang, T. A Novel Deep Eutectic Solvent/Switchable-Hydrophilicity Solvent/H2O System with Enhanced CO2 Switchability for Integrated Extraction of Phenolics, Flavonoids and Essential Oil from Flos Chrysanthemi Indici Flower. Sep. Purif. Technol. 2024, 336, 126315. [Google Scholar] [CrossRef]
- Li, J.H.; Li, W.; Luo, S.; Ma, C.H.; Liu, S.X. Alternate Ultrasound/Microwave Digestion for Deep Eutectic Hydro-Distillation Extraction of Essential Oil and Polysaccharide from Schisandra chinensis (Turcz.) Baill. Molecules 2019, 24, 1288. [Google Scholar] [CrossRef]
- Alsaud, N.; Shahbaz, K.; Farid, M. Antioxidant and Antibacterial Evaluation of Manuka Leaves (Leptospermum scoparium) Extracted by Hydrophobic Deep Eutectic Solvent. Chem. Eng. Res. Des. 2021, 174, 96–106. [Google Scholar] [CrossRef]
- Toazza, C.E.B.; Leal, F.C.; Marques, C.; Oliveira, G.; Farias, F.O.; Belan, A.L.D.; Leite, N.F.; Mafra, M.R.; Igarashi-Mafra, L.; Masson, M.L. Bioactive Compounds Extraction from Different Lemongrass Species: Strategies and Deep Eutectic Solvents Evaluation. J. Food Process Eng. 2022, 45, e14033. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Li, Z.; Jiang, L.; Cao, X.; Gao, W.; Wang, J.; Luo, D.; Chen, F. Deep Eutectic Solvent-Homogenate Based Microwave-Assisted Hydrodistillation of Essential Oil from Litsea cubeba (Lour.) Pers. Fruits and Its Chemical Composition and Biological Activity. J. Chromatogr. A 2021, 1646, 462089. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.W.; Cheng, Q.; Nie, J.; Wang, P.; Wang, X.J.; Li, Z.G.; Lee, M.R. DES-Based Microwave Hydrodistillation Coupled with GC-MS for Analysis of Essential Oil from Black Pepper (Piper nigrum) and White Pepper. Anal. Methods 2017, 9, 6777–6784. [Google Scholar] [CrossRef]
- Recio-Cázares, S.L.; Jiménez-González, O.; López-Malo, A.; Palou, E.; Ramírez-Corona, N. Enhancing the Extraction of Essential Oil from Mexican Lippia (Aloysia citriodora) Leaves Obtained by Hydro-Distillation Aided by Natural Deep Eutectic Solvents (NADES). Chem. Eng. Process.-Process Intensif. 2024, 195, 109623. [Google Scholar] [CrossRef]
- Hu, M.; Feng, G.; Xie, L.; Shi, X.; Lu, B.; Li, Y.; Shi, S.; Zhang, J. Green and Efficient Extraction of Wormwood Essential Oil Using Natural Deep Eutectic Solvent: Process Optimization and Compositional Analysis. J. Mol. Liq. 2023, 382, 121977. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, X.R. Headspace Gas Chromatography-Mass Spectrometry for Volatile Components Analysis in Ipomoea cairica (L.) Sweet Leaves: Natural Deep Eutectic Solvents as Green Extraction and Dilution Matrix. Foods 2019, 8, 205. [Google Scholar] [CrossRef]
- Yu, G.W.; Cheng, Q.; Nie, J.; Wang, X.J.; Wang, P.; Li, Z.G.; Lee, M.R. Microwave Hydrodistillation Based on Deep Eutectic Solvent for Extraction and Analysis of Essential Oil from Three Amomum Species Using Gas Chromatography-Mass Spectrometry. Chromatographia 2018, 81, 657–667. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, F.; Pang, M.; Jin, X.; Lv, H.; Li, Z.; Lee, M. Microwave-Assisted Hydrodistillation Extraction Based on Microwave-Assisted Preparation of Deep Eutectic Solvents Coupled with GC-MS for Analysis of Essential Oils from Clove Buds. Sustain. Chem. Pharm. 2022, 27, 100695. [Google Scholar] [CrossRef]
- Xu, F.X.; Zhang, J.Y.; Jin, J.; Li, Z.G.; She, Y.B.; Lee, M.R. Microwave-Assisted Natural Deep Eutectic Solvents Pretreatment Followed by Hydrodistillation Coupled with GC-MS for Analysis of Essential Oil from Turmeric (Curcuma longa L.). J. Oleo Sci. 2021, 70, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Arokiyaraj, C.; Anmol; Rana, S.; Sharma, U.; Reddy, S.G.E. Natural Deep Eutectic Solvents (NADESs) Assisted Extraction of Essential Oil from Nardostachys jatamansi (D.Don) DC with Insecticidal Activities. Ind. Crop. Prod. 2023, 202, 117040. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Todorovic, Z.B.; Stanojevic, K.S.; Stanojevic, J.S.; Troter, D.Z.; Nikolic, L.B.; Dordevic, B. The Influence of Natural Deep Eutectic Solvent Glyceline on the Yield, Chemical Composition and Antioxidative Activity of Essential Oil from Rosemary (Rosmarinus officinalis L.) Leaves. J. Essent. Oil Res. 2021, 33, 247–255. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Zheng, W.; Yu, G.; Li, Z.; She, Y.; Lee, M. Three-Stage Microwave Extraction of Cumin (Cuminum cyminum L.) Seed Essential Oil with Natural Deep Eutectic Solvents. Ind. Crop. Prod. 2019, 140, 111660. [Google Scholar] [CrossRef]
- Jeong, K.M.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Kim, E.M.; Lee, J. One-Step Sample Preparation for Convenient Examination of Volatile Monoterpenes and Phenolic Compounds in Peppermint Leaves Using Deep Eutectic Solvents. Food Chem. 2018, 251, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Su, E.; Yang, M.; Cao, J.; Lu, C.; Wang, J.; Cao, F. Deep Eutectic Solvents as Green Media for Efficient Extraction of Terpene Trilactones from Ginkgo biloba Leaves. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 385–391. [Google Scholar] [CrossRef]
- Su, G.; Yu, Z.; Wang, H.; Zhao, M.; Zhao, T.; Zhang, J. Impact of Ternary NADES Prepared from Proline, Glucose and Water on the Maillard Reaction: Reaction Activity, Amadori Compound Yield, and Taste-Enhancing Ability. Food Chem. X 2023, 20, 100905. [Google Scholar] [CrossRef] [PubMed]
- Bajkacz, S.; Rusin, K.; Wolny, A.; Adamek, J.; Erfurt, K.; Chrobok, A. Highly Efficient Extraction Procedures Based on Natural Deep Eutectic Solvents or Ionic Liquids for Determination of 20-Hydroxyecdysone in Spinach. Molecules 2020, 25, 4736. [Google Scholar] [CrossRef] [PubMed]
- Sazali, A.L.; AlMasoud, N.; Amran, S.K.; Alomar, T.S.; Pa’ee, K.F.; El-Bahy, Z.M.; Yong, T.L.K.; Dailin, D.J.; Chuah, L.F. Physicochemical and Thermal Characteristics of Choline Chloride-Based Deep Eutectic Solvents. Chemosphere 2023, 338, 139485. [Google Scholar] [CrossRef]
- Costa, F.S.; Moreira, L.S.; Silva, A.M.; Silva, R.J.; dos Santos, M.P.; da Silva, E.G.P.; Grassi, M.T.; Gonzalez, M.H.; Amaral, C.D.B. Natural Deep Eutectic Solvent-Based Microwave-Assisted Extraction in the Medicinal Herb Sample Preparation and Elemental Determination by ICP OES. J. Food Compos. Anal. 2022, 109, 104510. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Li, Y.; Pan, Z.; Chen, Z.; Lu, J.; Yuan, J.; Zhu, Z.; Zhang, J. Ultrasonication-Assisted Synthesis of Alcohol-Based Deep Eutectic Solvents for Extraction of Active Compounds from Ginger. Ultrason. Sonochem. 2020, 63, 104915. [Google Scholar] [CrossRef] [PubMed]
- García-Roldán, A.; Piriou, L.; Jauregi, P. Natural Deep Eutectic Solvents as a Green Extraction of Polyphenols from Spent Coffee Ground with Enhanced Bioactivities. Front. Plant Sci. 2023, 13, 1072592. [Google Scholar] [CrossRef] [PubMed]
- Pires, I.V.; Sakurai, Y.C.N.; Ferreira, N.R.; Moreira, S.G.C.; da Cruz Rodrigues, A.M.; da Silva, L.H.M. Elaboration and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from Pitaya. Molecules 2022, 27, 8310. [Google Scholar] [CrossRef]
- Suresh, P.S.; Singh, P.P.; Sharma, M.; Sharma, U. Multicomponent Natural Deep Eutectic Solvents: Super Solvents for the Efficient Extraction of Steviol Glycosides (Rebaudioside A) from Stevia rebaudiana. J. Clean. Prod. 2023, 385, 135639. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Granados, M.; Sentellas, S.; Saurina, J. Microwave-Assisted Extraction with Natural Deep Eutectic Solvents for Polyphenol Recovery from Agrifood Waste: Mature for Scaling-Up? Sci. Total Environ. 2024, 912, 168716. [Google Scholar] [CrossRef] [PubMed]
- Sahraoui, N.; Boutekedjiret, C. Innovative Process of Essential Oil Extraction: Steam Distillation Assisted by Microwave. In Progress in Clean Energy, Volume 1: Analysis and Modeling; Dincer, I., Colpan, C.O., Kizilkan, O., Ezan, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 831–841. ISBN 978-3-319-16709-1. [Google Scholar]
- Biswas, R.; Sarkar, A.; Alam, M.; Roy, M.; Mahdi Hasan, M.M. Microwave and Ultrasound-Assisted Extraction of Bioactive Compounds from Papaya: A Sustainable Green Process. Ultrason. Sonochem. 2023, 101, 106677. [Google Scholar] [CrossRef] [PubMed]
- Golmakani, M.T.; Rezaei, K. Comparison of Microwave-Assisted Hydrodistillation Withthe Traditional Hydrodistillation Method in the Extractionof Essential Oils from Thymus vulgaris L. Food Chem. 2008, 109, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Taklimi, S.M.; Divsalar, A.; Ghalandari, B.; Ding, X.; Di Gioia, M.L.; Omar, K.A.; Saboury, A.A. Effects of Deep Eutectic Solvents on the Activity and Stability of Enzymes. J. Mol. Liq. 2023, 377, 121562. [Google Scholar] [CrossRef]
- Ferreira, N.; Viana, T.; Henriques, B.; Tavares, D.S.; Jacinto, J.; Colónia, J.; Pinto, J.; Pereira, E. Application of Response Surface Methodology and Box–Behnken Design for the Optimization of Mercury Removal by Ulva Sp. J. Hazard. Mater. 2023, 445, 130405. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, Y.; Zhao, J.; Wang, Y.; Yao, Q.; Li, S. Development and Optimization of Green Extraction of Polyphenols in Michelia alba Using Natural Deep Eutectic Solvents (NADES) and Evaluation of Bioactivity. Sustain. Chem. Pharm. 2024, 37, 101425. [Google Scholar] [CrossRef]
- Katekar, V.P.; Rao, A.B.; Sardeshpande, V.R. A Hydrodistillation-Based Essential Oils Extraction: A Quest for the Most Effective and Cleaner Technology. Sustain. Chem. Pharm. 2023, 36, 101270. [Google Scholar] [CrossRef]
- Vo, T.P.; Ho, M.T.; Nguyen Nguyen, P.U.; Pham, N.D.; Truong, K.V.; Yen Nguyen, T.H.; Nguyen, D.Q.; Huong Vo, T.T. Extracting Phenolics, Flavonoids, and Terpenoids from Codonopsis pilosula Using Green Solvents. Sustain. Chem. Pharm. 2024, 37, 101395. [Google Scholar] [CrossRef]
- Zhang, Y.; Bian, S.; Hu, J.; Liu, G.; Peng, S.; Chen, H.; Jian, Z.; Wang, T.; Ye, Q.Y.; Zhu, H. Natural Deep Eutectic Solvent-Based Microwave-Assisted Extraction of Total Flavonoid Compounds from Spent Sweet Potato (Ipomoea batatas L.) Leaves: Optimization and Antioxidant and Bacteriostatic Activity. Molecules 2022, 27, 5985. [Google Scholar] [CrossRef]
- Vo, T.P.; Pham, T.V.; Tran, T.N.H.; Vo, L.T.V.; Vu, T.T.; Pham, N.D.; Nguyen, D.Q. Ultrasonic-Assisted and Microwave-Assisted Extraction of Phenolics and Terpenoids from Abelmoschus sagittifolius (Kurz) Merr Roots Using Natural Deep Eutectic Solvents. ACS Omega 2023, 8, 29704–29716. [Google Scholar] [CrossRef] [PubMed]
- Riyamol; Jeevitha, G.C. Microwave and Ultrasound-Assisted Natural Deep Eutectic Solvents-Based Extraction of Pectin from Onion Peel Wastes. CYTA-J. Food 2024, 22, 2311215. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, L.; Xie, L.; Zheng, Y.; Liu, K.; Tang, H.; Liao, Y.; Li, X. Z-Ligustilide: A Review of Its Pharmacokinetics and Pharmacology. Phyther. Res. 2020, 34, 1966–1991. [Google Scholar] [CrossRef]
- Yasutomi, R.; Anzawa, R.; Urakawa, M.; Usuki, T. Effective Extraction of Limonene and Hibaene from Hinoki (Chamaecyparis obtusa) Using Ionic Liquid and Deep Eutectic Solvent. Molecules 2021, 26, 4271. [Google Scholar] [CrossRef]
- Alsaud, N.; Shahbaz, K.; Farid, M. Application of Deep Eutectic Solvents in the Extraction of Polyphenolic Antioxidants from New Zealand Manuka Leaves (Leptospermum scoparium): Optimization and Antioxidant Activity. J. Mol. Liq. 2021, 337, 116385. [Google Scholar] [CrossRef]
- Bradić, B.; Novak, U.; Likozar, B. Crustacean Shell Bio-Refining to Chitin by Natural Deep Eutectic Solvents. Green Process. Synth. 2020, 9, 13–25. [Google Scholar] [CrossRef]
- Akhbari, M.; Masoum, S.; Aghababaei, F.; Hamedi, S. Optimization of Microwave Assisted Extraction of Essential Oils from Iranian Rosmarinus officinalis L. Using RSM. J. Food Sci. Technol. 2018, 55, 2197–2207. [Google Scholar] [CrossRef]
- Cori, O.; Chayet, L.; Perez, L.M.; Bunton, C.A.; Hachey, D. Rearrangement of Linalool, Geraniol, and Nerol and Their Derivatives. J. Org. Chem. 1986, 51, 1310–1316. [Google Scholar] [CrossRef]
- Roberts, M.; Steam, U.; Wainer, J.; Thomas, A.; Chimhau, T.; Harding, K.G. Extraction of Essential Oils from Lavandula × Intermedia ‘Margaret Roberts’ Using Steam Distillation, Hydrodistillation, and Cellulase-Assisted Hydrodistillation: Experimentation and Cost Analysis. Plants 2022, 11, 3479. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, L.; Wang, Z.; Tao, Y.; Chen, L.; Zhang, W. Effects of Natural Deep Eutectic Solvent on the Extraction Efficiency and Antioxidant Activities of Toona sinensis Seed Polyphenols: Composition and Mechanism. Food Biosci. 2023, 56, 103151. [Google Scholar] [CrossRef]
- Cajnko, M.M.; Vicente, F.A.; Novak, U.; Likozar, B. Natural Deep Eutectic Solvents (NaDES): Translating Cell Biology to Processing. Green Chem. 2023, 25, 9045–9062. [Google Scholar] [CrossRef]
- Grillo, G.; Gaudino, E.C.; Rosa, R.; Leonelli, C.; Timonina, A.; Grygiškis, S.; Tabasso, S.; Cravotto, G. Green Deep Eutectic Solvents for Microwave-Assisted Biomass Delignification and Valorisation. Molecules 2021, 26, 798. [Google Scholar] [CrossRef] [PubMed]
- Vovk, H.; Karnpakdee, K.; Ludwig, R.; Nosenko, T. Enzymatic Pretreatment of Plant Cells for Oil Extraction. Food Technol. Biotechnol. 2023, 61, 160–178. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Yusupov, M.; Cornet, I.; Billen, P.; Neyts, E.C. Effect of Natural Deep Eutectic Solvents of Non-Eutectic Compositions on Enzyme Stability. J. Mol. Liq. 2022, 366, 120180. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Li, J.; Wang, A.; Li, G.; Ren, X.; Yin, W. Ultrasound-Assisted Deep Eutectic Solvent Extraction of Echinacoside and Oleuropein from Syringa Pubescens Turcz. Ind. Crop. Prod. 2020, 151, 112442. [Google Scholar] [CrossRef]
- Cagliero, C.; Bicchi, C.; Marengo, A.; Rubiolo, P.; Sgorbini, B. Gas Chromatography of Essential Oil: State-of-the-Art, Recent Advances, and Perspectives. J. Sep. Sci. 2022, 45, 94–112. [Google Scholar] [CrossRef]
- Nie, J.; Yu, G.W.; Song, Z.Y.; Wang, X.J.; Li, Z.G.; She, Y.B.; Lee, M. Microwave-Assisted Deep Eutectic Solvent Extraction Coupled with Headspace Solid-Phase Microextraction Followed by GC-MS for the Analysis of Volatile Compounds from Tobacco. Anal. Methods 2017, 9, 856–863. [Google Scholar] [CrossRef]
- Liang, S.Q.; Hu, W.; Cheng, W.S.; Zhang, S.; Zou, R.S. Zanthoxylum Bungeanum Essential Oil: Extraction and Component Analysis for α-Glucosidase Inhibitory Activity and the Underlying Mechanism Based on Molecular Docking. Appl. Sci. 2023, 13, 2627. [Google Scholar] [CrossRef]
- Rants’o, T.A.; Koekemoer, L.L.; Panayides, J.L.; van Zyl, R.L. Potential of Essential Oil-Based Anticholinesterase Insecticides against Anopheles Vectors: A Review. Molecules 2022, 27, 7026. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, G.; Amador-Luna, V.M.; Benešová, K.; Pernica, M.; Parada-Alfonso, F.; Ibáñez, E. Biorefinery Approach with Green Solvents for the Valorization of Citrus reticulata Leaves to Obtain Antioxidant and Anticholinergic Extracts. Food Chem. 2024, 456, 140034. [Google Scholar] [CrossRef] [PubMed]
- Jauregi, P.; Esnal-Yeregi, L.; Labidi, J. Natural Deep Eutectic Solvents (NADES) for the Extraction of Bioactives: Emerging Opportunities in Biorefinery Applications. PeerJ Anal. Chem. 2024, 6, e32. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (Nades): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- García, C.B.; Concha, J.; Culleré, L.; Lomba, L.; Sangüesa, E.; Ribate, M.P. Has the Toxicity of Therapeutic Deep Eutectic Systems Been Assessed? Appl. Sci. 2023, 13, 5980. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Tripathi, M.; Lukk, T.; Karpichev, Y.; Gathergood, N.; Singh, B.N.; Thakur, V.K.; Tabatabaei, M.; Gupta, V.K. Biobased Natural Deep Eutectic System as Versatile Solvents: Structure, Interaction and Advanced Applications. Sci. Total Environ. 2023, 881, 163002. [Google Scholar] [CrossRef] [PubMed]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Cokdinleyen, M.; Domínguez-Rodríguez, G.; Kara, H.; Ibáñez, E.; Cifuentes, A. New Green Biorefinery Strategies to Valorize Bioactive Fractions from Palmaria palmata. Mar. Drugs 2024, 22, 467. [Google Scholar] [CrossRef] [PubMed]
- Della Posta, S.; Gallo, V.; Gentili, A.; Fanali, C. Strategies for the Recovery of Bioactive Molecules from Deep Eutectic Solvents Extracts. TrAC-Trends Anal. Chem. 2022, 157, 116798. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Vega, L.; Cifuentes, A.; Ibáñez, E.; Galeano Garcia, P. Exploring Natural Deep Eutectic Solvents (NADES) for Enhanced Essential Oil Extraction: Current Insights and Applications. Molecules 2025, 30, 284. https://doi.org/10.3390/molecules30020284
Acosta-Vega L, Cifuentes A, Ibáñez E, Galeano Garcia P. Exploring Natural Deep Eutectic Solvents (NADES) for Enhanced Essential Oil Extraction: Current Insights and Applications. Molecules. 2025; 30(2):284. https://doi.org/10.3390/molecules30020284
Chicago/Turabian StyleAcosta-Vega, Luis, Alejandro Cifuentes, Elena Ibáñez, and Paula Galeano Garcia. 2025. "Exploring Natural Deep Eutectic Solvents (NADES) for Enhanced Essential Oil Extraction: Current Insights and Applications" Molecules 30, no. 2: 284. https://doi.org/10.3390/molecules30020284
APA StyleAcosta-Vega, L., Cifuentes, A., Ibáñez, E., & Galeano Garcia, P. (2025). Exploring Natural Deep Eutectic Solvents (NADES) for Enhanced Essential Oil Extraction: Current Insights and Applications. Molecules, 30(2), 284. https://doi.org/10.3390/molecules30020284








