Green Synthesis of Antibacterial CuO Nanoparticles Based on the Synergy Between Cornu aspersum Snail Mucus and Ascorbic Acid
Abstract
:1. Introduction
2. Results
2.1. Green Synthesis of CuONPs-Muc AsA
2.1.1. Isolation and Characterization of a Mucus Extract from the Garden Snail C. aspersum
2.1.2. Green Synthesis of CuONPs in Mucus Matrix with MW > 20 kDa and L-Ascorbic Acid
2.2. Characterization of the Obtained CuONPs-MucAsA
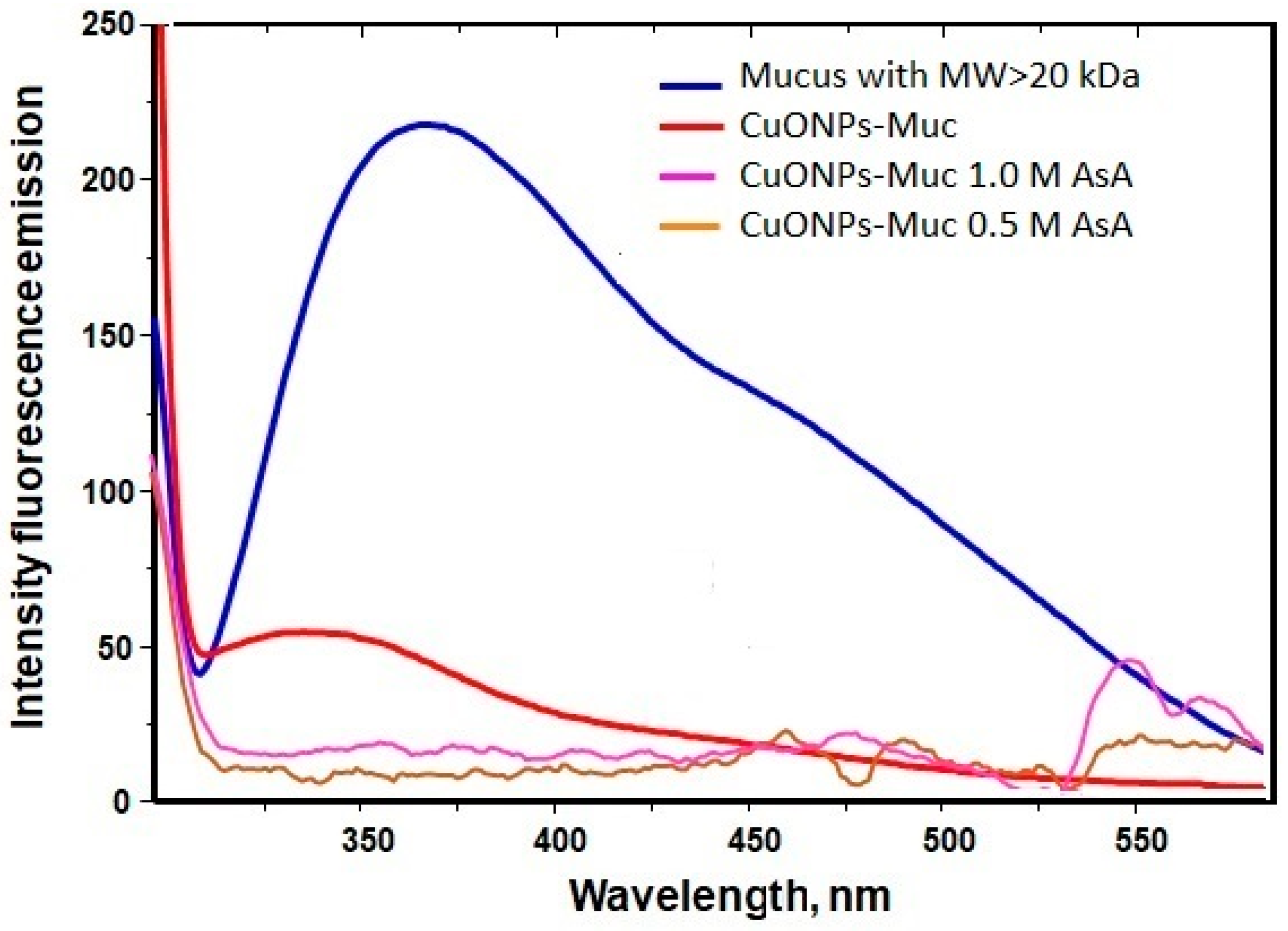
2.2.1. UV–Vis and Fluorescence Analyses of CuONPs
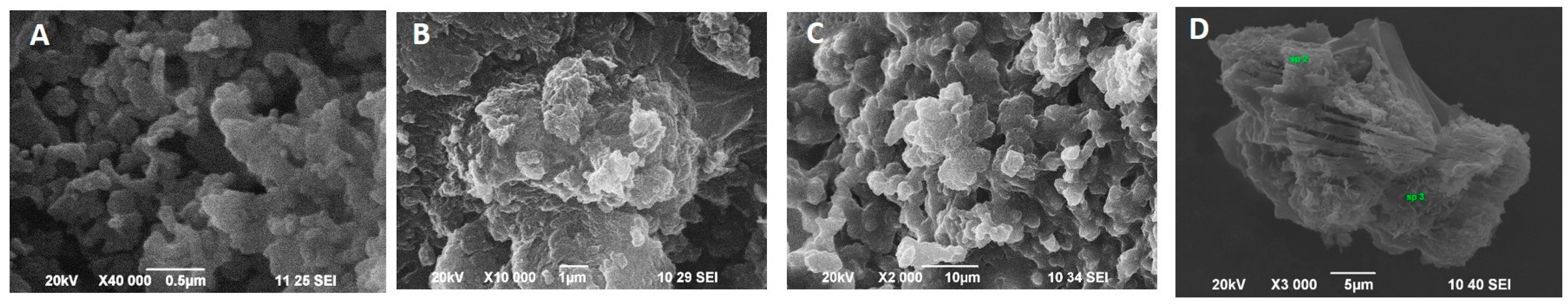
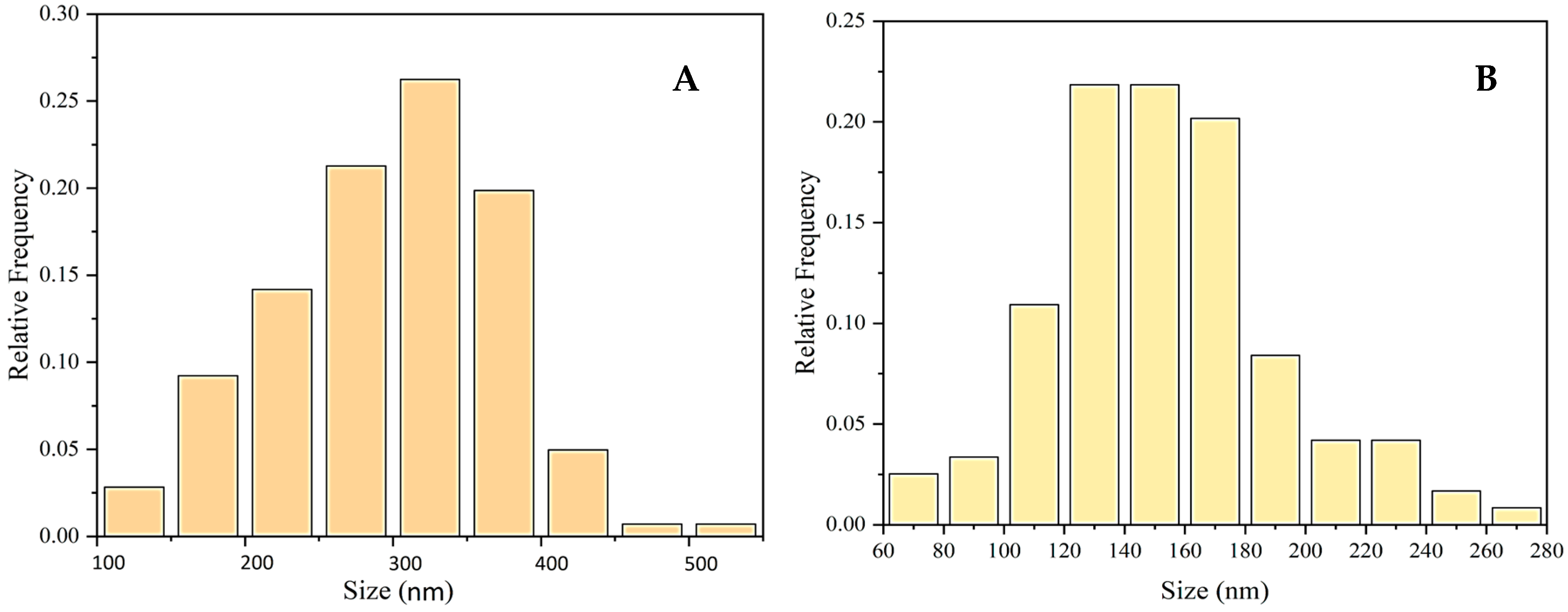
2.2.2. Characterization of the CuONPs-Muc AsA by Scanning Electron Microscopy Combined with Energy-Dispersive Spectroscopy (SEM/EDS)
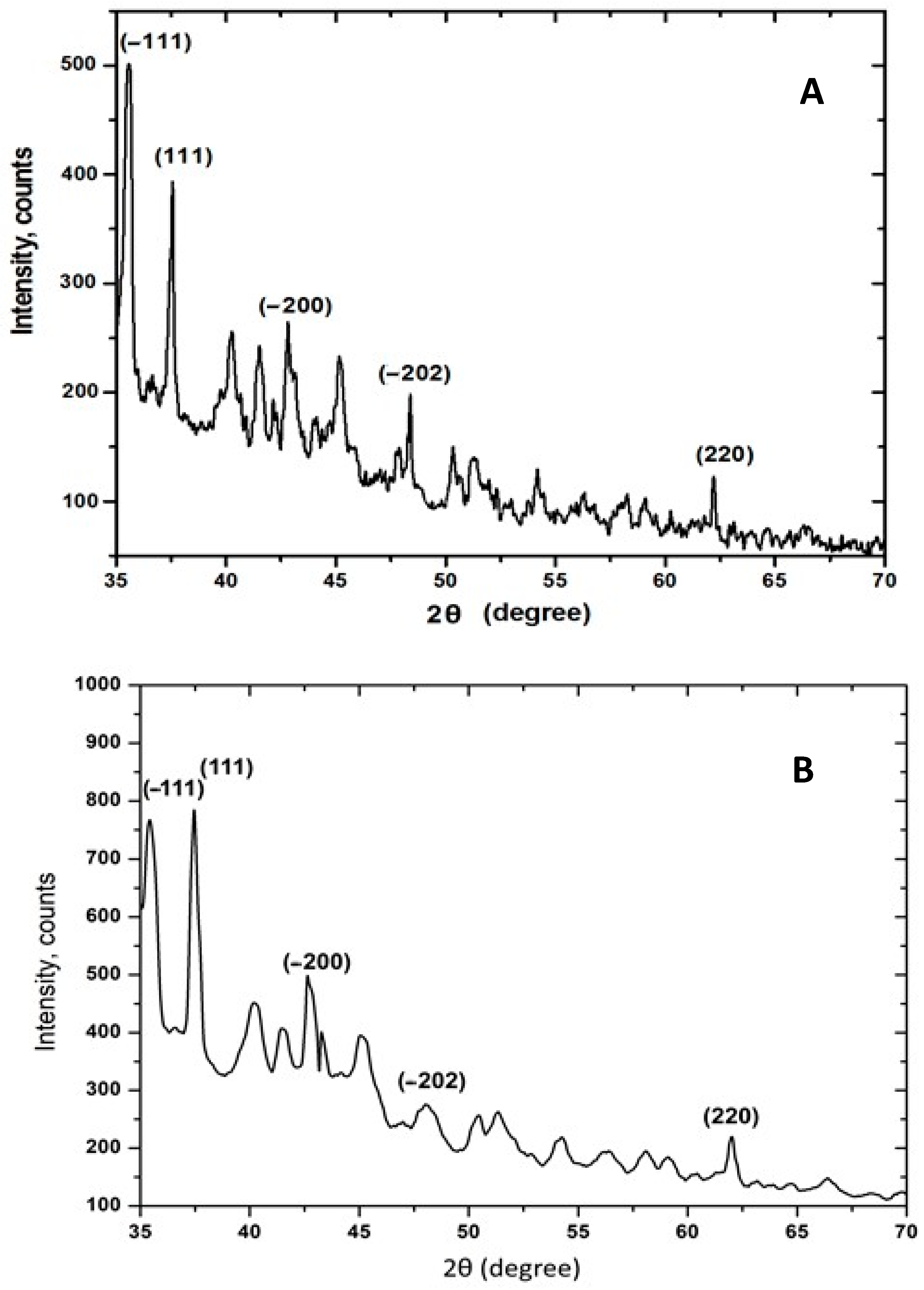
2.2.3. Characterization of CuONPs-Muc AsA by X-Ray Diffraction (XRD) Technique
2.2.4. Characterization of CuONPs Using Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.5. Stability of CuONPs-Muc Analyzed by Thermogravimetric Analyses (TG/DSC-MS)
2.3. Antibacterial Activity of CuONPs-MucAsA
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Green Synthesis of CuONPs from Snail Mucus
4.3. Characterization of Copper Nanoparticles
4.3.1. Characterization of CuONPs by UV-Vis Spectroscopy
4.3.2. Fluorescence Spectroscopy Analyses of CuONPs
4.3.3. Characterization of CuONPs by Means of SEM
4.3.4. X-Ray Diffraction Analysis (XRD)
4.3.5. Characterization of CuONPs-Muc by FT-IR
4.3.6. Characterization of CuONPs by DTG
4.4. Antimicrobial Activity of CuONPs-Muc
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussein, A.A.; Abd El-latif, M.; Saad El-Din, M.I.; El-Shenawy, N.S.; Hammam, O.; Ibrahim, A.M. The Molluscicidal Activity of Green Synthesized Copper Oxide–Based Annona squamosa Seed Extract Nanoparticles on the Feeding Behavior, Biochemical, Molecular, and Immunohistochemical Alterations of Biomphalaria alexandrina Snails. Biol. Trace Elem. Res. 2024, 202, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, M.; Obodo, R.M.S.; Mukhtar, S.; Ilyas, S.Z.; Aziz, F.; Thovhogi, N. Green synthesis of copper oxide nanoparticles using Cedrus deodara aqueous extract for antibacterial activity. Mater. Today Proc. 2021, 36, 576–581. [Google Scholar] [CrossRef]
- Dolashka, P.; Marinova, K.; Petrov, P.; Petrova, V.; Ranguelov, B.; Atanasova-Vladimirova, S.; Kaynarov, D.; Stoycheva, I.; Pisareva, E.; Tomova, A.; et al. Development of CuO Nanoparticles from the Mucus of Garden Snail Cornu aspersum as New Antimicrobial Agents. Pharmaceuticals 2024, 17, 506. [Google Scholar] [CrossRef]
- Anbalagan, G.; Subramanian, B.; Suresh, V.; Sivaperumal, P. Green Synthesis of Copper and Copper Oxide Nanoparticles from Brown Algae Turbinaria Species‘ Aqueous Extract and Its Antibacterial Properties. Cureus 2024, 16, e57366. [Google Scholar] [CrossRef]
- Rathnakumar, S.S.; Noluthando, K.; Kulandaiswamy, A.J.; Rayappan, J.B.B.; Kasinathan, K.; Kennedy, J.; Maaza, M. Stalling behaviour of chloride ions: A non-enzymatic electrochemical detection of a-Endosulfan using CuO interface. Sens. Actuat. B 2019, 293, 100–106. [Google Scholar] [CrossRef]
- Deng, C.; Hu, H.; Zhu, W.; Han, C.; Shao, G. Green and facile synthesis of hierarchical cocoon shaped CuO hollow architectures. Mater. Lett. 2011, 65, 575–578. [Google Scholar] [CrossRef]
- Tilaki, R.M.; Iraji Zad, A.; Mahdavi, S.M. Size, composition and optical properties of copper nanoparticles prepared by laser ablation in liquids. Appl. Phys. A 2007, 88, 415–419. [Google Scholar] [CrossRef]
- Ayi, A.A.; Khare, V.; Strauch, P.; Girard, J.; Fromm, K.M.; Taubert, A. On the chemical synthesis of titanium nanoparticles from ionic liquids. Monatshefte Für Chem.-Chem. Mon. 2010, 141, 1273–1278. [Google Scholar] [CrossRef]
- Charinpanitkul, T.; Soottitantawat, A.; Tonanon, N.; Tanthapanichakoon, W. Single-step synthesis of nanocomposite of copper and arbon nanoparticles using arc discharge in liquid nitrogen. Mater. Chem. Phys. 2009, 116, 125–128. [Google Scholar] [CrossRef]
- Solanki, J.N.; Sengupta, R.; Murthy, Z. Synthesis of copper sulphide and copper nanoparticles with microemulsion method. Solid State Sci. 2010, 12, 1560–1566. [Google Scholar] [CrossRef]
- Liu, Q.M.; Yasunami, T.; Kurida, K.; Okido, M. Preparation of Cu nanoparticles with ascorbic acid by aqueous solution reduction method. Trans. Nonferrous Met. Soc. China 2012, 22, 2198–2203. [Google Scholar] [CrossRef]
- Nwanya, A.C.; Ndipingwi, M.M.; Ikpo, C.O.; Obodo, R.M.; Nwanya, S.C.; Botha, S.; Ezema, F.I.; Iwuoha, E.I.; Maaza, M. Zea mays lea silk extract mediated synthesis of nickel oxide nanoparticles as positive electrode material for asymmetric supercabattery. J. Alloys Compd. 2020, 822, 153581. [Google Scholar] [CrossRef]
- Nwanya, A.C.; Ndipingwi, M.M.; Mayedwa, N.; Razanamahandry, L.C.; Ikpo, C.O.; Waryo, T.; Ntwampe, S.K.O.; Malenga, E.; Fosso-Kankeu, E.; Ezema, F.I.; et al. Maize (Zea mays L.) fresh husk mediated biosynthesis of copper oxides: Potentials for pseudo capacitive energy storage. Electrochim. Acta 2019, 301, 436–448. [Google Scholar] [CrossRef]
- Noothuan, N.; Apitanyasai, K.; Panha, S.; Tassanakajon, A. Snail Mucus From the Mantle and Foot of Two Land Snails, Lissachatina Fulica and Hemiplecta Distincta, Exhibits Different Protein Profile and Biological Activity. BMC Res. Notes 2021, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Mane, C.; Kadam, D.; Khadse, N. Green adeptness in synthesis of non-toxic copper and cobalt oxide nanocomposites with multifaceted bioactivities. Cancer Nano 2023, 14, 79. [Google Scholar] [CrossRef]
- Mane, P.C.; Sayyed, S.A.R.; Kadam, D.D.; Shinde, M. Terrestrial snail-mucus mediated green synthesis of silver nanoparticles and in vitro investigations on their antimicrobial and anticancer activities. Sci. Rep. 2021, 11, 13068–13083. [Google Scholar] [CrossRef] [PubMed]
- Dolashki, A.; Velkova, L.; Daskalova, E.; Zheleva, N.; Topalova, Y.; Atanasov, V.; Voelter, W.; Dolashka, P. Antimicrobial activities of different fractions from mucus of the garden snail Cornu aspersum. Biomedicines 2020, 8, 315. [Google Scholar] [CrossRef]
- Belouhova, M.; Daskalova, E.; Yotinov, I.; Topalova, Y.; Velkova, L.; Dolashki, A.; Dolashka, P. Microbial diversity of garden snail mucus. Microbiology 2020, 11, e1263. [Google Scholar] [CrossRef] [PubMed]
- Velkova, L.; Dolashki, A.; Petrova, V.; Pisareva, E.; Kaynarov, D.; Kermedchiev, M.; Todorova, M.; Dolashka, P. Antibacterial properties of peptide and protein fractions from Cornu aspersum mucus. Molecules 2024, 29, 2886. [Google Scholar] [CrossRef]
- Topalova, Y.; Belouhova, M.; Velkova, L.; Dolashki, A.; Zheleva, N.; Daskalova, E.; Kaynarov, D.; Dolashka, P. Effect and mechanisms of antibacterial peptide fraction from mucus of Cornu aspersum against Escherichia coli NBIMCC 8785. Biomedicines 2022, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Cerullo, A.R.; McDermott, M.B.; Pepi, L.E.; Liu, Z.L.; Barry, D.; Zhang, S.; Yang, X.; Chen, X.; Azadi, P.; Holford, M.; et al. Comparative mucomic analysis of three functionally distinct Cornu aspersum secretions. Nat. Commun. 2023, 14, 5361. [Google Scholar] [CrossRef] [PubMed]
- Conte, R. Recent advances on nano delivery of Helix mucus pharmacologically active components. Int. J. Nano Dimens. 2016, 7, 181–185. [Google Scholar] [CrossRef]
- Wargala, E.; Zalewska, A.; Sławska, M.; Kot, I. Snail mucus as an innovative ingredient used in the cosmetology and medical industry. Aesth. Cosmetol. Med. 2023, 12, 45–49. [Google Scholar] [CrossRef]
- Aouji, M.; Rkhaila, A.; Bouhaddioui, B.; Khalid, G.; Lrhorfi, L.A.; Bengueddour, R. Antioxidant activity, biochemical composition and physicochemical properties of Helix aspersa Muller snail slime. Int. J. Chem. Biochem. Sci. 2023, 23, 53–62. [Google Scholar]
- Aouji, M.; Rkhaila, A.; Bouhaddioui, B.; Ziraric, M.; Harifid, H.; Taboza, Y.; Lrhorfia, A.L.; Bengueddour, R. Chemical composition, mineral profile, anti-bacterial and wound healing properties of snail slime of Helix aspersa Müller. BioMedicine 2023, 13, 10–19. [Google Scholar] [CrossRef]
- Deng, T.; Gao, D.; Song, X.; Zhou, Z.; Zhou, L.; Tao, M.; Jiang, Z.; Yang, L.; Luo, L.; Zhou, A.; et al. A natural biological adhesive from snail mucus for wound repair. Nat. Commun. 2023, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Trapella, C.; Rizzo, R.; Gallo, S.; Alogna, A.; Bortolotti, D.; Casciano, F.; Zauli, G.; Secchiero, P.; Voltan, R. HelixComplex snail mucus exhibits pro-survival, proliferative and pro-migration effects on mammalian fibroblasts. Sci. Rep. 2018, 8, 17665. [Google Scholar] [CrossRef]
- Gentili, V.; Bortolotti, D.; Benedusi, M.; Alogna, A.; Fantinati, A.; Guiotto, A.; Turrin, G.; Cervellati, C.; Trapella, C.; Rizzo, R.; et al. HelixComplex snail mucus as a potential technology against O3 induced skin damage. PLoS ONE 2020, 15, e0229613. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.F.; Di Matteo, V.; Dolci, L.S.; Albertini, B.; Ballarin, B.; Cassani, M.C.; Passerini, N.; Gentilomi, G.A.; Bonvicini, F.; Panzavolta, S. Effectiveness of Snail Slime in the Green Synthesis of Silver Nanoparticles. Nanomaterials 2022, 12, 3447. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.; Burmistrov, D.; Fomina, P.; Validov, S.; Kozlov, V. Antibacterial Properties of Copper Oxide Nanoparticles (Review). Int. J. Mol. Sci. 2024, 25, 11563. [Google Scholar] [CrossRef] [PubMed]
- Pitt, S.; Hawthorne, J.; Garcia-Maya, M.; Alexandrovich, A.; Symonds, R.; Gunn, A. Identification and characterisation of anti-Pseudomonas aeruginosa proteins in mucus of the brown garden snail, Cornu aspersum. Br. J. Biomed. Sci. 2019, 76, 129–136. [Google Scholar] [CrossRef]
- Pitt, S.J.; Graham, M.A.; Dedi, C.G.; Taylor-Harris, P.M.; Gunn, A. Antimicrobial properties of mucus from the brown garden snail Helix aspersa. Br. J. Biomed. Sci. 2015, 72, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Alhalili, Z. Green synthesis of copper oxide nanoparticles CuO NPs from Eucalyptus Globoulus leaf extract Adsorption and design of experiments. Arab. J. Chem. 2022, 15, 103739. [Google Scholar] [CrossRef]
- Dolashka, P.; Atanasov, D. Device for Collecting Extracts from Garden Snail. BG Utility Model Application Number 2656, 08.11.2013. Patent Number 2097, 31 August 2015. Available online: https://portal.bpo.bg/bpo_online/-/bpo/utility-model-detail (accessed on 8 January 2025).
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.; Dhawale, D.S.; Salunkhe, R.; Jamdade, V.; Lokhande, C.D. Fabrication of copper oxide multilayer nanosheets for supercapacitor application. J. Alloys Compd. 2010, 492, 26–30. [Google Scholar] [CrossRef]
- Ethiraj, A.S.; Kang, D.J. Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res. Lett. 2012, 7, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Alhumaimess, M.S.; Essawy, A.A.; Kamel, M.M.; Alsohaimi, I.H.; Hassan, H.M.A. Biogenic-mediated synthesis of mesoporous Cu2O/CuO nano-architectures of superior catalytic reductive towards nitroaromatics. Nanomaterials 2020, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Debut, A.; Cumbal, L. Andean sacha inchi (Plukenetia volubilis L.) leaf-mediated synthesis of Cu2O Nanoparticles: A low-cost approach. Bioengineering 2020, 7, 54. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim Biophys Acta. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Laurenzana, A.; Fibbi, G.; Veiga-Villauriz, C.; Fanelli, F.; FrAsAssi, F.; Onzo, A.; Bianco, G.; et al. Biomolecules from snail mucus (Helix Aspersa) conjugated gold nanoparticles, exhibiting potential wound healing and anti-inflammatory activity. Soft Matter. 2020, 16, 10876–10888. [Google Scholar] [CrossRef] [PubMed]
- Onzo, A.; Pascale, R.; Acquavia, M.A.; Cosma, P.; Gubitosa, J.; Gaeta, C.; Iannece, P.; Tsybin, Y.; Rizzi, V.; Guerrieri, A.; et al. Untargeted analysis of pure snail slime and snail slime-induced Au nanoparticles metabolome with MALDI FT-ICR MS. J. Mass Spectrom. 2021, 56, e4722. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Nuzzo, S.; Agostiano, A.; Cosma, P. Snail Slime-Based Gold Nanoparticles: An Interesting Potential Ingredient in Cosmetics as an Antioxidant, Sunscreen, and Tyrosinase Inhibitor. Photochem. Photobiol. B 2021, 224, 112309. [Google Scholar] [CrossRef] [PubMed]
- Umer, A.; Naveed, S.; Ramzan, N.; Rafique, M.S.; Imran, M. A green method for the synthesis of Copper Nanoparticles using L-ascorbic acid. Matéria 2014, 19, 197–203. [Google Scholar] [CrossRef]
- Antonio-Pérez, A.; Durán-Armenta, L.F.; Pérez-Loredo, M.G.; Torres-Huerta, A.L. Biosynthesis of Copper Nanoparticles with Medicinal Plants Extracts: From Extraction Methods to Applications. Micromachines 2023, 14, 1882. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Mandal, T.K. Tryptophan-based peptides to synthesize gold and silver nanoparticles: A mechanistic and kinetic study. Chemistry 2007, 13, 3160–3168. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Siddiquee, M.A.; ud din Parray, M.; Kamli, M.R.; Malik, M.A.; Mehdi, S.H.; Imtiyaz, K.; Rizvi, M.M.A.; Rajor, H.K.; Patel, R. Biogenic synthesis, in-vitro cytotoxicity, esterase activity and interaction studies of copper oxide nanoparticles with lysozyme. J. Mater Res. Technol. 2021, 13, 2066–2077. [Google Scholar] [CrossRef]
- Malassis, L.; Dreyfus, R.; Murphy, R.J.; Hough, L.A.; Donnio, B.; Murray, C.B. One-Step Green Synthesis of Gold and Silver Nanoparticles with Ascorbic Acid and Their Versatile Surface Post-Functionalization. RSC Adv. 2016, 6, 33092–33100. [Google Scholar] [CrossRef]
- Uthayakumar, V.; Ramasubramanian, V.; Senthilkumar, D.; Priyadarisini, V.B.; Harikrishanan, R. Biochemical characterization, antimicrobial and hemolytic studies on skin mucus of freshwater spiny eel Mastacembelus armatus. Asian Pac. J. Trop. Biomed. 2012, 2, 863–869. [Google Scholar] [CrossRef]
- Gabriel, U.I.; Mirela, S.; Ionel, J. Quantification of mucoproteins (glycoproteins) from snails mucus, Helix aspersa and Helix Pomatia. J. Agroaliment. Process. Technol. 2011, 17, 410–413. [Google Scholar]
- Drozd, M.; Duszczyk, A.; Ivanova, P.; Pietrzak, M. Interactions of proteins with metal-based nanoparticles from a point of view of analytical chemistry—Challenges and opportunities. Adv. Colloid Interface Sci. 2022, 304, 102656. [Google Scholar] [CrossRef]
- Rashad, M.; Sampò, S.; Cataldi, A.; Zara, S. Biological activities of gastropods secretions: Snail and slug slime. Nat. Prod. Bioprospect. 2023, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, S.; Karishma, S.; Vo, D.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A Review on Biosynthesis of Metal Nanoparticles and Its Environmental Applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef] [PubMed]
- Márquez, A.; Berger, T.; Feinle, A.; Hüsing, N.; Himly, M.; Duschl, A.; Diwald, O. Bovine Serum Albumin Adsorption on TiO2 Colloids: The Effect of Particle Agglomeration and Surface Composition. Langmuir 2017, 33, 2251–2258. [Google Scholar] [CrossRef]
- Umar, A.; Alshahrani, A.; Algarni, H.; Kumar, R. CuO Nanosheets as Potential Scaffolds for Gas Sensing Applications. Sens. Actuators B Chem. 2017, 250, 24–31. [Google Scholar] [CrossRef]
- Baldisserri, C.; Costa, A.L. Electrochemical detection of copper ions leached from CuO nanoparticles in saline buffers and biological media using a gold wire working electrode. Nanopart. Res. 2016, 18, 96. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Derylo-Marczewska, A. Mesoporous silica/protein biocomposites: Surface, topography, thermal properties. Int. J. Biol. Macromol. 2019, 139, 531–542. [Google Scholar] [CrossRef]
- Andrade, J.D.; Hlady, V.; Wei, A.P. Adsorption of complex proteins at interfaces. Pure Appl. Chem. 1992, 64, 1777–1781. [Google Scholar] [CrossRef]
- Dee, K.C.; Puleo, D.A.; Bizios, R. Chapter 3—Protein-surface interactions. An Introduction to Tissue-Biomaterial Interactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 37–52. [Google Scholar] [CrossRef]
- Marin-Silva, D.A.; Romano, N.; Damonte, L.; Giannuzzi, L.; Pinotti, A. Hybrid materials based on chitosan functionalized with green synthesized copper nanoparticles: Physico-chemical and antimicrobial analysis. Int. J. Biol. Macromol. 2023, 242, 124898. [Google Scholar] [CrossRef]
- Raja, F.N.S.; Worthington, T.; Martin, R.A. The antimicrobial efficacy of copper, cobalt, zinc and silver nanoparticles: Alone and in combination. Biomed. Mater. 2023, 18, 045003. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Gabrielyan, L.; Trchounian, A. Antibacterial activities of transient metals nanoparticles and membranous mechanisms of action. World J. Microbiol. Biotechnol. 2019, 35, 162. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.; Katas, H.; Samsudin, S.N.; Zin, N.M. Regioselective Sequential Modification of Chitosan via Azide-Alkyne Click Reaction: Synthesis, Characterization, and Antimicrobial Activity of Chitosan Derivatives and Nanoparticles. PLoS ONE 2015, 10, e0123084. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.G.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L. Antimicrobial metal-based nanoparticles: A review on their synthesis, types and antimicrobial action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed]




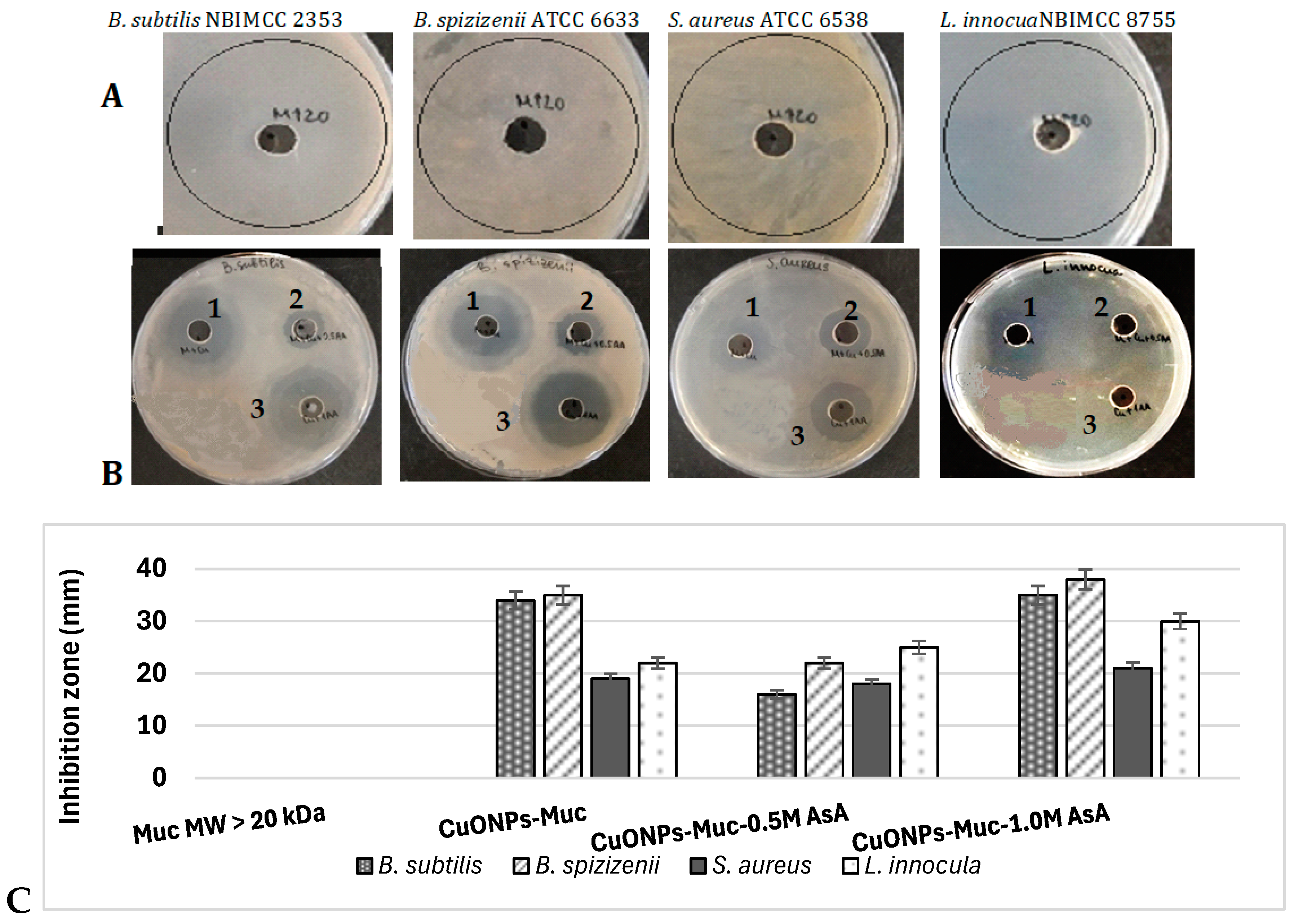

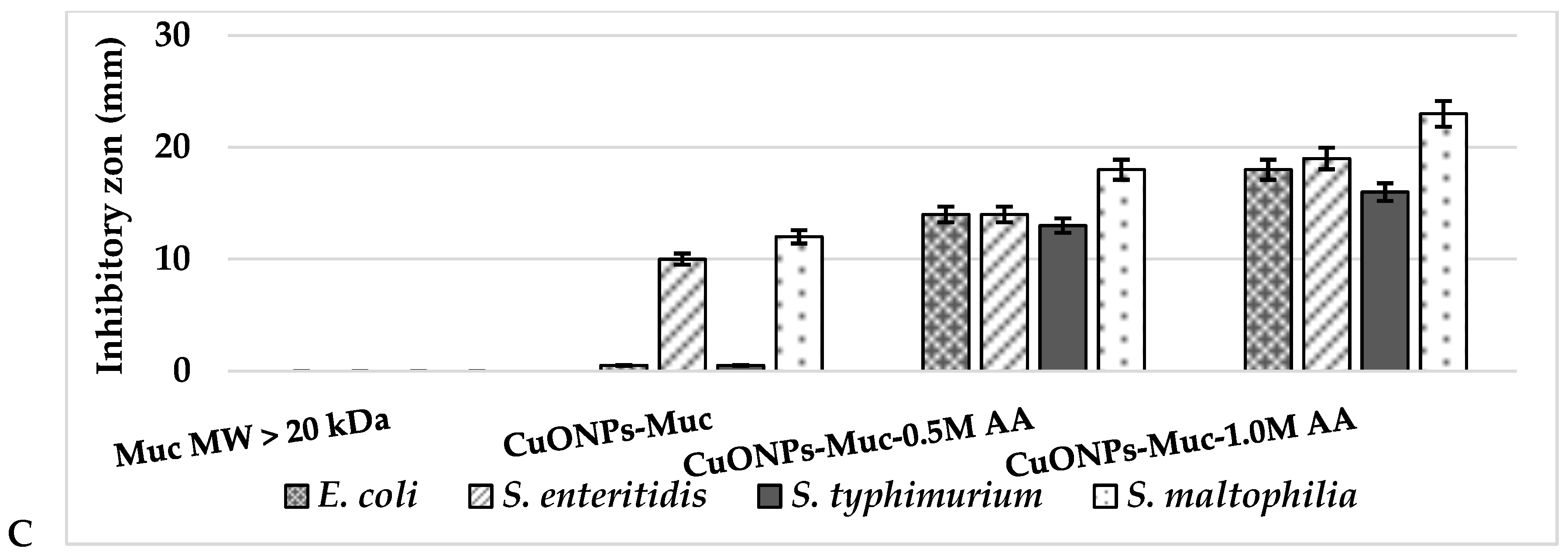
| Mucus Fraction with MW > 20 kDa | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Element | C | O | S | Cu | Cl | Ca | Mg | Na | Total |
| wt% | 46.08 | 43.24 | 2.33 | 2 | 0.71 | 1.06 | 1.44 | 3.15 | 100 |
| at % | 56.55 | 38.5 | 1.03 | 0.47 | 0.28 | 0.38 | 0.84 | 1.95 | 100 |
| CuONPs-Muc | |||||||||
| wt % | 49.92 | 43.29 | 2.51 | 4.27 | - | - | - | - | 100 |
| at % | 59.31 | 38.61 | 1.12 | 0.96 | - | - | - | - | 100 |
| CuONPs-Muc 0.5 M AsA | |||||||||
| wt % | 42.47 | 50.32 | 2.67 | 4.53 | - | - | - | - | 100 |
| at % | 51.73 | 46.01 | 1.22 | 1.04 | - | - | - | - | 100 |
| CuONPs-Muc 1.0 M AsA | |||||||||
| wt % | 42.81 | 46.32 | 3.55 | 7.32 | - | - | - | - | 100 |
| at % | 53.31 | 43.31 | 1.66 | 1.72 | - | - | - | - | 100 |
| Wavenumber [cm−1] | Interaction |
|---|---|
| 467 | with Cu–O stretching vibration |
| 597 | the Cu(II)–O bond in CuO [36] |
| 611 | stretching of Cu(I)–O in Cu2O particles [37] |
| 1022 | C–C stretching vibration and O–H bending vibration |
| 1067 | Cu(II)–O bond in CuO [36] |
| 1099 | C–N and C–H stretching |
| 1318 | C–H stretching |
| 1621 | O–H bending and NH bond |
| 1780 | C=O bond in CHO group amide I and amide II |
| 2978 | C–H stretching [38] |
| 3293 | O–H stretching [38] |
| Sample | TG | DTG | |||||
|---|---|---|---|---|---|---|---|
| Mloss IDS [%] 30–120 [°C] | Mloss1 [%] 120–200 [°C] | Mloss2 [%] 200–300 [°C] | MlossTOTAL [%] | Tmax,IDS [°C] | Tmax1 [°C] | Tmax2 [°C] | |
| Mucus with MW > 20 kDa | 16.94 | 75.75 | 0.03 | 92.72 | 73.3 | 123.0 | 183.0 |
| CuONPs-Mucus | 23.47 | 69.44 | 0.04 | 92.95 | 62.2 | 134.9 | 160.9 |
| CuONPs-Muc 0.5 M AsA | 20.91 | 70.21 | 0.20 | 91.31 | 63.2 | 151.5 | 170.4 |
| CuONPs-Muc 1.0 M AsA | 16.37 | 72.84 | 1.27 | 90.48 | 88.6 | 120.3 | 177.2 |
| Sample | DSC | |||||||
|---|---|---|---|---|---|---|---|---|
| Tonset [°C] | Tpeak [°C] | Tend [°C] | ∆H J/g | Tonset [°C] | Tpeak [°C] | Tend [°C] | ∆H J/g | |
| Mucus with MW > 20 kDa | 58.2 | 80.8 | 91.1 | −289.2 | 127.9 | 181.9 | 189.0 | −1216 |
| CuONPs-Mucus | 41.1 | 65.2 | 85.4 | −183.8 | 125.3 | 147.3 | 170.7 | −1058 |
| CuONPs-Muc 0.5 M AsA | 42.3 | 57.2 | 74.7 | −139.3 | 130.0 | 159.3 | 185.6 | −1076 |
| CuONPs-Muc 1.0 M AsA | 58.7 | 83.6 | 99.6 | −119.6 | 106.4 | 163.2 | 175.4 | −1119 |
| Taxonomic Affiliation | Culture Collection | Number |
|---|---|---|
| Gram-positive | ||
| Bacillus subtilis | NBIMCC | 2353 |
| Bacillus spizizenii | ATCC | 6633 |
| Staphylococcus aureus | ATCC | 6538 |
| Listeria innocua | NBIMCC | 8755 |
| Gram-negative | ||
| Escherichia coli | ATCC | 8739 |
| Salmonella enteritidis | NBIMCC | 8691 |
| Salmonella typhimurium | ATCC | 14,028 |
| Stenotrophomonas maltophilia | ATCC | 17,666 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, M.; Kosateva, A.; Petrova, V.; Ranguelov, B.; Atanasova-Vladimirova, S.; Avdeev, G.; Stoycheva, I.; Pisareva, E.; Tomova, A.; Velkova, L.; et al. Green Synthesis of Antibacterial CuO Nanoparticles Based on the Synergy Between Cornu aspersum Snail Mucus and Ascorbic Acid. Molecules 2025, 30, 291. https://doi.org/10.3390/molecules30020291
Todorova M, Kosateva A, Petrova V, Ranguelov B, Atanasova-Vladimirova S, Avdeev G, Stoycheva I, Pisareva E, Tomova A, Velkova L, et al. Green Synthesis of Antibacterial CuO Nanoparticles Based on the Synergy Between Cornu aspersum Snail Mucus and Ascorbic Acid. Molecules. 2025; 30(2):291. https://doi.org/10.3390/molecules30020291
Chicago/Turabian StyleTodorova, Maria, Angelina Kosateva, Ventsislava Petrova, Bogdan Ranguelov, Stela Atanasova-Vladimirova, Georgi Avdeev, Ivanka Stoycheva, Emiliya Pisareva, Anna Tomova, Lyudmila Velkova, and et al. 2025. "Green Synthesis of Antibacterial CuO Nanoparticles Based on the Synergy Between Cornu aspersum Snail Mucus and Ascorbic Acid" Molecules 30, no. 2: 291. https://doi.org/10.3390/molecules30020291
APA StyleTodorova, M., Kosateva, A., Petrova, V., Ranguelov, B., Atanasova-Vladimirova, S., Avdeev, G., Stoycheva, I., Pisareva, E., Tomova, A., Velkova, L., Dolashki, A., & Dolashka, P. (2025). Green Synthesis of Antibacterial CuO Nanoparticles Based on the Synergy Between Cornu aspersum Snail Mucus and Ascorbic Acid. Molecules, 30(2), 291. https://doi.org/10.3390/molecules30020291







