Isolation and Bioactivity of Natural Products from Streptomyces sp. MA37
Abstract
1. Introduction
2. Results and Discussion
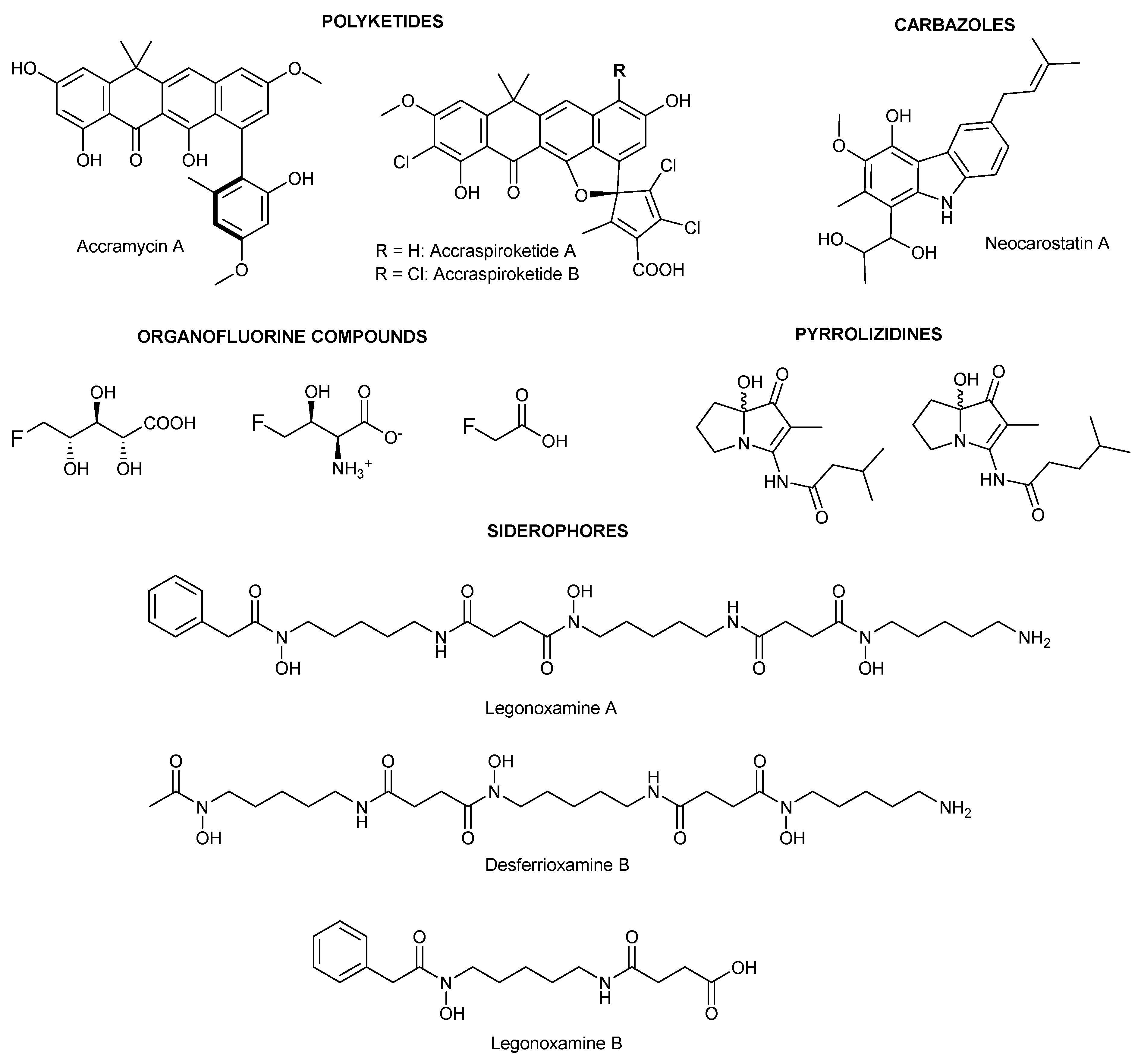
2.1. Structure Elucidation
2.2. Cell Proliferation Assay
3. Materials and Methods
3.1. Reagents and Media
3.2. Extraction and Isolation
3.3. NMR and MS Measurements
3.4. Cell Proliferation Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maglangit, F.; Wang, S.; Moser, A.; Kyeremeh, K.; Trembleau, L.; Zhou, Y.; Clark, D.J.; Tabudravu, J.; Deng, H. Accraspiroketides A-B, Phenylnaphthacenoid-Derived Polyketides with Unprecedented [6 + 6+6 + 6] + [5 + 5] Spiro-Architecture. J. Nat. Prod. 2024, 87, 831–836. [Google Scholar] [CrossRef]
- Maglangit, F.; Deng, H. Cell Factory for Phenylnaphthacenoid Polyketide Production. SynBio 2023, 1, 89–102. [Google Scholar] [CrossRef]
- Maglangit, F.; Tong, M.H.; Jaspars, M.; Kyeremeh, K.; Deng, H. Legonoxamines A-B, Two New Hydroxamate Siderophores from the Soil Bacterium, Streptomyces Sp. MA37. Tetrahedron Lett. 2019, 60, 75–79. [Google Scholar] [CrossRef]
- Maglangit, F.; Alrashdi, S.; Renault, J.; Trembleau, L.; Victoria, C.; Tong, M.H.; Wang, S.; Kyeremeh, K.; Deng, H. Characterization of the Promiscuous: N-Acyl CoA Transferase, LgoC, in Legonoxamine Biosynthesis. Org. Biomol. Chem. 2020, 18, 2219–2222. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Saha, N.; Donofrio, R.S.; Bestervelt, L.L. Microbial Siderophores: A Mini Review. J. Basic. Microbiol. 2013, 53, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Sidebottom, A.M.; Karty, J.A.; Carlson, E.E. Accurate Mass MS/MS/MS Analysis of Siderophores Ferrioxamine B and E1 by Collision-Induced Dissociation Electrospray Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2015, 26, 1899–1902. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Gu, S.; Xu, J.; Li, X.; Chen, H.; Shao, Z.; Wang, F.; Shao, J.; Yin, W.B.; Qian, L.; et al. SIDERITE: Unveiling Hidden Siderophore Diversity in the Chemical Space through Digital Exploration. iMeta 2024, 3, e192. [Google Scholar] [CrossRef] [PubMed]
- Kachadourian, R.; Dellagi, A.; Laurent, J.; Bricard, L.; Kunesch, G.; Expert, D. Desferrioxamine-Dependent Iron Transport in Erwinia Amylovora CFBP1430: Cloning of the Gene Encoding the Ferrioxamine Receptor FoxR. Biometals 1996, 9, 143–150. [Google Scholar] [CrossRef]
- Reissbrodt, R.; Rabsch, W.; Chapeaurouge, A.; Jung, G.; Winkelmann, G. Isolation and Identification of Ferrioxamine G and E in Hafnia Alvei. Biol. Metals 1990, 3, 54–60. [Google Scholar] [CrossRef]
- Li, K.; Chen, W.H.; Bruner, S.D. Microbial Siderophore-Based Iron Assimilation and Therapeutic Applications. BioMetals 2016, 29, 377–388. [Google Scholar] [CrossRef]
- Fujita, M.J.; Nakano, K.; Sakai, R. Bisucaberin B, a Linear Hydroxamate Class Siderophore from the Marine Bacterium Tenacibaculum Mesophilum. Molecules 2013, 18, 3917–3926. [Google Scholar] [CrossRef]
- Elyashberg, M.; Williams, A. ACD/Structure Elucidator: 20 Years in the History of Development. Molecules 2021, 26, 6623. [Google Scholar] [CrossRef]
- Sasaki, R. Neural Network Algorithms vs. HOSE Code Algorithms. ACD Labs. Available online: https://www.acdlabs.com/blog/neural-network/ (accessed on 29 October 2023).
- Elyashberg, M.E.; Williams, A.; Blinov, K. Contemporary Computer-Assisted Approaches to Molecular Structure Elucidation; The Royal Society of Chemistry: London, UK, 2011; ISBN 978-1-84973-432-5. [Google Scholar]
- Elyashberg, M.; Williams, A.J.; Blinov, K. Structural Revisions of Natural Products by Computer-Assisted Structure Elucidation (CASE) Systems. Nat. Prod. Rep. 2010, 27, 1296–1328. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Moon, S.S.; Hwang, B.K. Isolation, Antifungal Activity, and Structure Elucidation of the Glutarimide Antibiotic, Streptimidone, Produced by Micromonospora Coerulea. J. Agric. Food Chem. 1999, 47, 3372–3380. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Oritani, T.; Kiyota, H. Synthesis and Antifungal Activity of the Four Stereoisomers of Streptimidone, a Glutarimide Antibiotic from Streptomyces Rimosus Forma Paromomycinus. European J. Org. Chem. 2000, 2000, 3459–3462. [Google Scholar] [CrossRef]
- Becker, A.M.; Rickards, R.W. The Absolute Configuration of the Glutarimide Antibiotics Streptimidone and 9-Methylstreptimidone. Helv. Chim. Acta 1976, 59, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Son, S.; Lee, J.K.; Jang, M.; Heo, K.T.; Ko, S.K.; Park, D.J.; Park, C.S.; Kim, C.J.; Ahn, J.S.; et al. Isolation of New Streptimidone Derivatives, Glutarimide Antibiotics from Streptomyces sp. W3002 Using LC-MS-Guided Screening. J. Antibiot. 2020, 73, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Maglangit, F.; Kyeremeh, K.; Deng, H. Deletion of the Accramycin Pathway-Specific Regulatory Gene AccJ Activates the Production of Unrelated Polyketide Metabolites. Nat. Prod. Res. 2022, 37, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Maglangit, F.; Wu, L.; Ebel, R.; Kyeremeh, K.; Andersen, J.H.; Annang, F.; Pérez-Moreno, G.; Reyes, F.; Deng, H. Signalling and Bioactive Metabolites from Streptomyces Sp. RK44. Molecules 2020, 25, 460. [Google Scholar] [CrossRef] [PubMed]


| No. | 13C ppm * | 1H ppm, mult. | COSY | HMBC |
|---|---|---|---|---|
| 1-N | - | - | - | - |
| 2 | 39.0, CH2 | 2.92, tr | 3, 4, 5, 6 | 3, 4 |
| 3 | 28.3, CH2 | 1.54, m | 2, 4, 5, 6 | 2, 4 |
| 4 | 22.8, CH2 | 1.39, m | 2, 3, 5, 6 | 3, 5, 6 |
| 5 | 25.9, CH2 | 1.68, m | 2, 3, 4, 6 | 2, 3, 4, 6 |
| 6 | 50.5, CH2 | 3.21, m | 2, 3, 4, 5 | 4, 8, 9 |
| 7-NOH | - | - | - | |
| 8 | 173.4, C | - | - | |
| 9 | 29.8, CH2 | 2.46, m | 10 | 8, 9, 11 |
| 10 | 28.3, CH2 | 2.59, m | 9 | 8, 10, 11 |
| 11 | 173.4, C | - | - | - |
| 12-NH | - | - | - | - |
| 13 | 39.4, CH2 | 3.19, m | 14, 15, 16, 17 | 14, 15 |
| 14 | 28.3, CH2 | 1.54, m | 13, 15, 16, 17 | 13, 15 |
| 15 | 22.8, CH2 | 1.39, m | 13, 14, 16, 17 | 14, 16, 17 |
| 16 | 25.9, CH2 | 1.68, m | 13, 14, 15, 17 | 13, 14, 15, 17 |
| 17 | 50.5, CH2 | 3.21, m | 13, 14, 15, 16 | 15, 19, 20 |
| 18-NOH | - | - | - | - |
| 19 | 173.6, C | - | - | - |
| 20 | 29.8, CH2 | 2.46, m | 21 | 19, 21, 22 |
| 21 | 28.3, CH2 | 2.59, m | 20 | 19, 20, 22 |
| 22 | 174.2, C | - | - | - |
| 23-NH | - | - | - | - |
| 24 | 39.4, CH2 | 3.19, m | 25, 26, 27, 28 | 25, 26 |
| 25 | 28.3, CH2 | 1.54, m | 24, 26, 27, 28 | 24, 26 |
| 26 | 22.8, CH2 | 1.39, m | 24, 25, 27, 28 | 24, 27, 28 |
| 27 | 28.3, CH2 | 1.54, m | 24, 25, 26, 28 | 24, 25, 26, 28 |
| 28 | 46.8, CH2 | 3.63, t | 24, 25, 26, 27 | 26, 27 |
| 29-NO | - | - | - |
| Compound Name | IC50 | ||
|---|---|---|---|
| A2058 ATCC CRL-11147 (Skin Cancer) | MCF-7 ATCC HTB-22 (Breast Cancer) | ATCC CCL-171 (Lung Normal Cell) | |
| Legonoxamine A | - | 2.2 µM | - |
| Desferrioxamine B | - | 61.1 µM | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maglangit, F.; Fang, Q.; Tabudravu, J.N.; Kyeremeh, K.; Jaspars, M.; Deng, H. Isolation and Bioactivity of Natural Products from Streptomyces sp. MA37. Molecules 2025, 30, 306. https://doi.org/10.3390/molecules30020306
Maglangit F, Fang Q, Tabudravu JN, Kyeremeh K, Jaspars M, Deng H. Isolation and Bioactivity of Natural Products from Streptomyces sp. MA37. Molecules. 2025; 30(2):306. https://doi.org/10.3390/molecules30020306
Chicago/Turabian StyleMaglangit, Fleurdeliz, Qing Fang, Jioji N. Tabudravu, Kwaku Kyeremeh, Marcel Jaspars, and Hai Deng. 2025. "Isolation and Bioactivity of Natural Products from Streptomyces sp. MA37" Molecules 30, no. 2: 306. https://doi.org/10.3390/molecules30020306
APA StyleMaglangit, F., Fang, Q., Tabudravu, J. N., Kyeremeh, K., Jaspars, M., & Deng, H. (2025). Isolation and Bioactivity of Natural Products from Streptomyces sp. MA37. Molecules, 30(2), 306. https://doi.org/10.3390/molecules30020306










