Electron Push-Pull Effect of Benzotrithiophene-Based Covalent Organic Frameworks on the Photocatalytic Degradation of Pharmaceuticals and Personal Care Products
Abstract
:1. Introduction
2. Results
2.1. Structure and Morphology
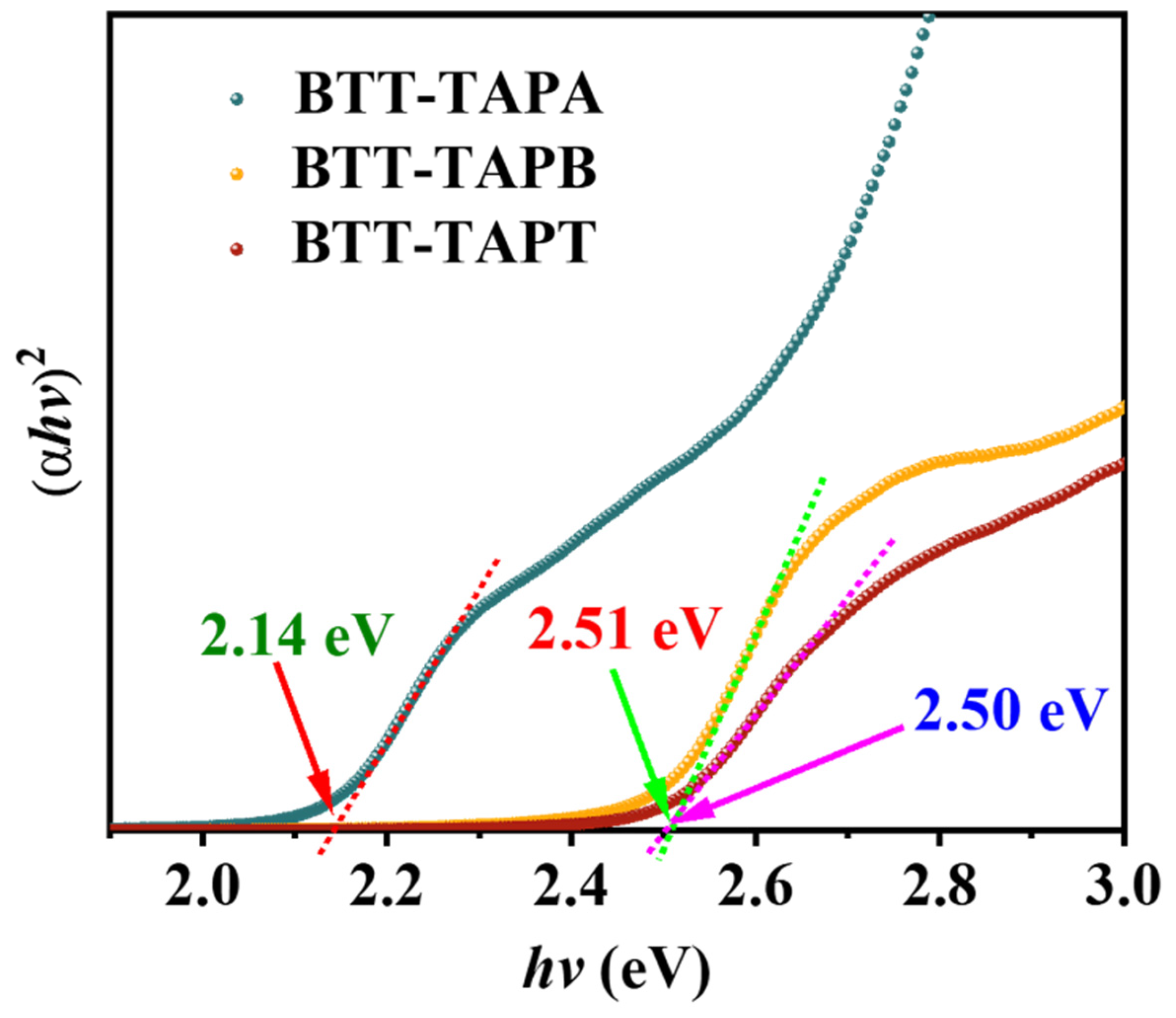
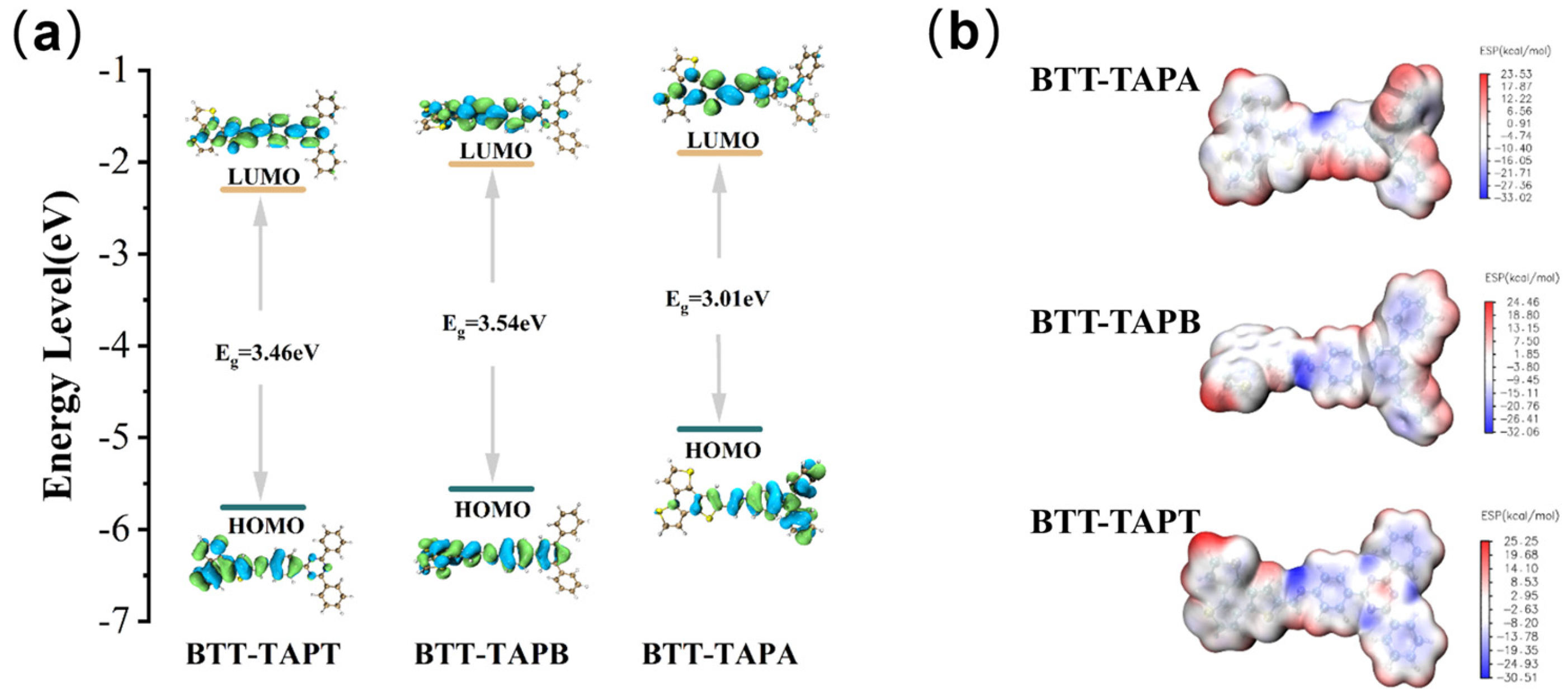
2.2. Optoelectronic Properties
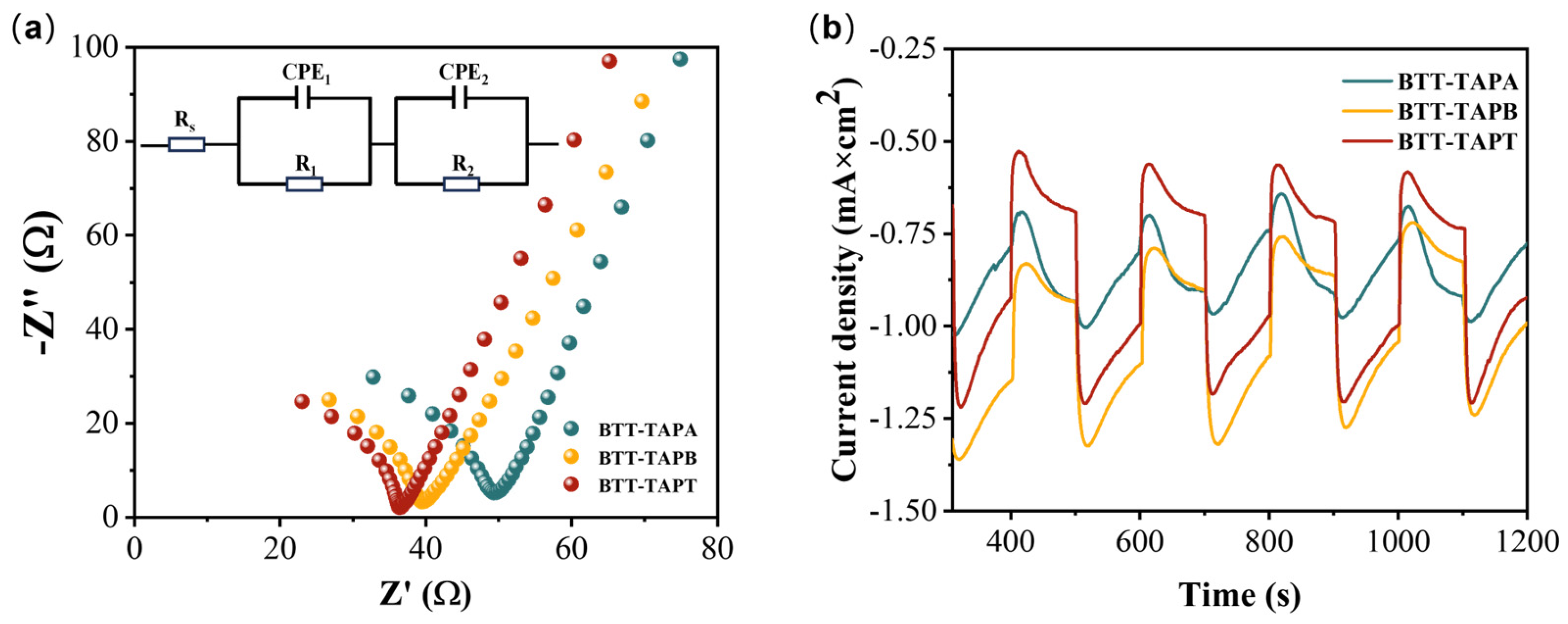
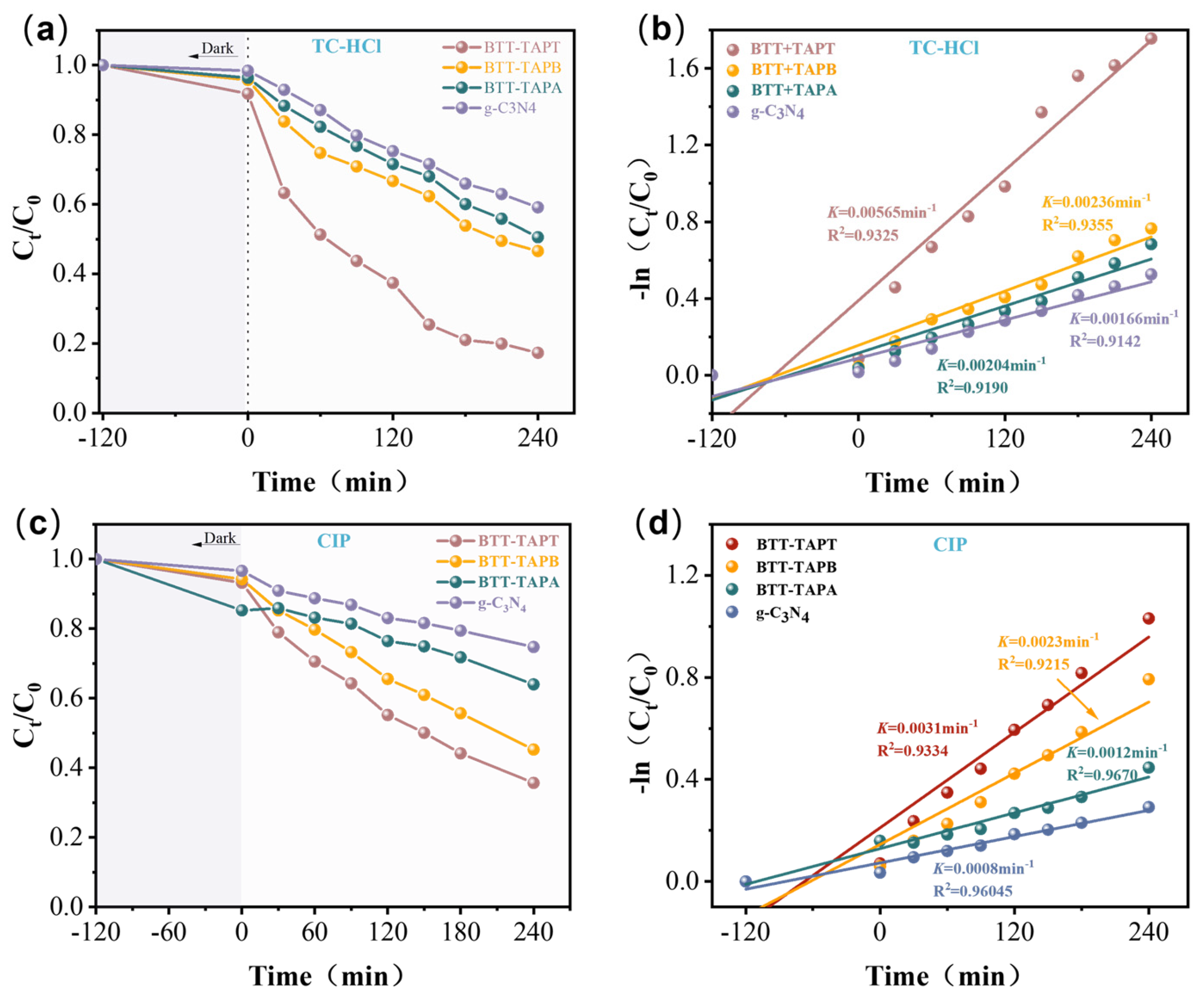
2.3. Photocatalytic Performance
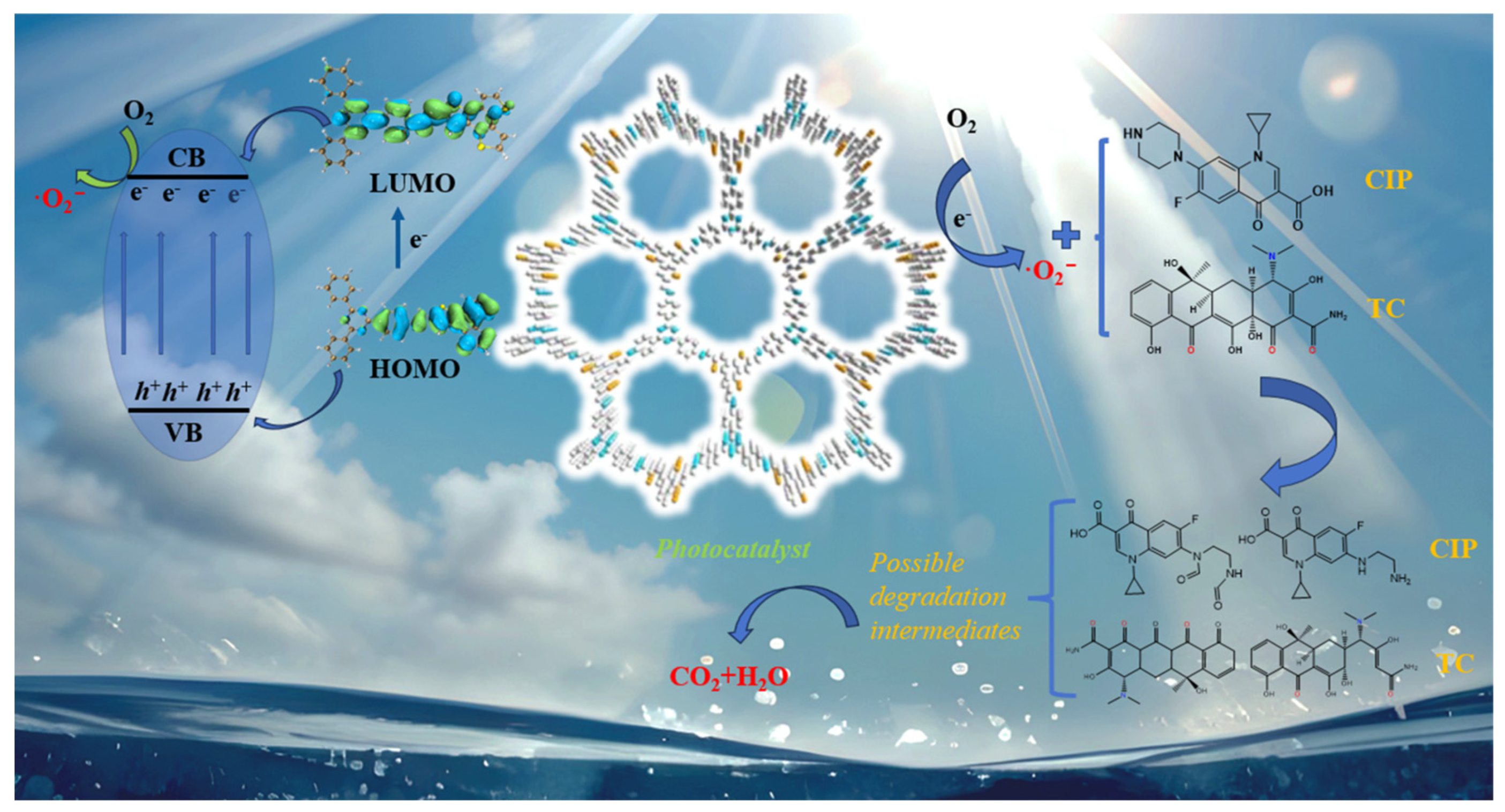
2.4. Photocatalytic Degradation Mechanism
3. Discussion
4. Materials and Methods
4.1. Synthesis of BTT-TAPT
4.2. Synthesis of BTT-TAPB
4.3. Synthesis of BTT-TAPA
4.4. Photocatalytic Degradation of TC
4.5. Photocatalytic Degradation of CIP
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tahir, M.B.; Ahmad, A.; Iqbal, T.; Ijaz, M.; Muhammad, S.; Siddeeg, S.M. Advances in photo-catalysis approach for the removal of toxic personal care product in aqueous environment. Environ. Dev. Sustain. 2020, 22, 6029–6052. [Google Scholar] [CrossRef]
- Gworek, B.; Kijenska, M.; Wrzosek, J.; Graniewska, M. Pharmaceuticals in the soil and plant environment: A Review. Water Air Soil Pollut. 2021, 232, 145. [Google Scholar] [CrossRef]
- Kim, S.; Nam, S.N.; Park, C.M.; Jang, M.; Taheri-Qazvini, N.; Yoon, Y. Effect of single and multilayered Ti3C2TX MXene as a catalyst and adsorbent on enhanced sonodegradation of diclofenac and verapamil. J. Hazard. Mater. 2022, 426, 128120. [Google Scholar] [CrossRef]
- Li, S.M.; Ao, X.W.; Li, C.; Lu, Z.D.; Cao, W.F.; Wu, F.F.; Liu, S.M.; Sun, W.J. Insight into PPCP degradation by UV/NH2Cl and comparison with UV/NaClO: Kinetics, reaction mechanism, and DBP formation. Water Res. 2020, 182, 115967. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef]
- Uzelac, M.M.; Conic, B.S.; Kladar, N.; Armakovic, S.; Armakovic, S.J. Removal of hydrochlorothiazide from drinking and environmental water: Hydrolysis, direct and indirect photolysis. Energy Environ. 2023, 34, 1243–1257. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Saheed, M.S.M.; Show, P.L.; Chang, J.S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef]
- Singh, P.; Priya, B.; Shandilya, P.; Raizada, P.; Singh, N.; Pare, B.; Jonnalagadda, S.B. Photocatalytic mineralization of antibiotics using 60%WO3/BiOCl stacked to graphene sand composite and chitosan. Arab. J. Chem. 2019, 12, 4627–4645. [Google Scholar] [CrossRef]
- Hu, A.R.; Liu, Y.L.; Zheng, J.F.; Wang, X.M.; Xia, S.J.; van der Bruggen, B. Tailoring properties and performance of thin-film composite membranes by salt additives for water treatment: A critical review. Water Res. 2023, 234, 119821. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Patial, S.; Hasija, V.; Khan, A.A.P.; Singh, P.; Gautam, S.; Kaur, M.; Nguyen, V.H. Engineering nanostructures of CuO-based photocatalysts for water treatment: Current progress and future challenges. Arab. J. Chem. 2020, 13, 8424–8457. [Google Scholar] [CrossRef]
- Javaid, A.; Imran, M.; Latif, S.; Hussain, N.; Bilal, M. Functionalized magnetic nanostructured composites and hybrids for photocatalytic elimination of pharmaceuticals and personal care products. Sci. Total Environ. 2022, 849, 157683. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, H.W.; Zhang, Y.S.; Mahlknecht, J.; Wang, C.Q. A review of metallurgical slags as catalysts in advanced oxidation processes for removal of refractory organic pollutants in wastewater. J. Environ. Manag. 2024, 352, 120051. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Yoon, Y.; Oh, J. Occurrence of endocrine disrupting compounds and pharmaceuticals in 11 WWTPs in Seoul, Korea. KSCE J. Civ. Eng. 2011, 15, 57–64. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, C.; Huang, D.L.; Wang, W.J.; Zhai, Y.B.; Liang, Q.H.; Yang, Y.; Tian, S.H.; Luo, H.Z.; Qin, D.Y. Structure defined 2D Mo2C/2Dg-C3N4 Van der Waals heterojunction: Oriented charge flow in-plane and separation within the interface to collectively promote photocatalytic degradation of pharmaceutical and personal care products. Appl. Catal. B-Environ. 2022, 301, 120749. [Google Scholar] [CrossRef]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.N.; Guan, X.Y.; Jiang, H.L. Covalent organic frameworks for photocatalysis: Synthesis, structural features, fundamentals and performance. Coord. Chem. Rev. 2023, 475, 214889. [Google Scholar] [CrossRef]
- Cheng, H.; Lv, H.F.; Cheng, J.; Wang, L.; Wu, X.J.; Xu, H.X. Rational design of covalent heptazine frameworks with spatially separated redox centers for high-efficiency photocatalytic hydrogen peroxide production. Adv. Mater. 2022, 34, 2107480. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, D.S.; Pal, D.; Mishra, J.; Thakur, C. Noble metal-free doped graphitic carbon nitride (g-C3N4) for efficient photodegradation of antibiotics: Progress, limitations, and future directions. Environ. Sci. Pollut. Res. 2023, 30, 25546–25558. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yadav, P.; Yadav, M.; Gupta, R.; Rana, P.; Srivastava, A.; Zboril, R.; Varma, R.S.; Antonietti, M.; Gawande, M.B. Recent development of covalent organic frameworks (COFs): Synthesis and catalytic (organic-electro-photo) applications. Mater. Horiz. 2020, 7, 411–454. [Google Scholar] [CrossRef]
- Akyuz, L. An imine based COF as a smart carrier for targeted drug delivery: From synthesis to computational studies. Microporous Mesoporous Mat. 2020, 294, 109850. [Google Scholar] [CrossRef]
- Geng, K.Y.; He, T.; Liu, R.Y.; Dalapati, S.; Tan, K.T.; Li, Z.P.; Tao, S.S.; Gong, Y.F.; Jiang, Q.H.; Jiang, D.L. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Liu, S.S.; Qian, T.; Wang, M.F.; Ji, H.Q.; Shen, X.W.; Wang, C.; Yan, C.L. Proton-filtering covalent organic frameworks with superior nitrogen penetration flux promote ambient ammonia synthesis. Nat. Catal. 2021, 4, 322–331. [Google Scholar] [CrossRef]
- Wang, X.Y.; Sun, B.H.; Ye, Z.Q.; Zhang, W.J.; Xu, W.; Gao, S.R.; Zhou, N.L.; Wu, F.; Shen, J. Enzyme-responsive COF-based thiol-targeting nanoinhibitor for curing bacterial infections. ACS Appl. Mater. Interfaces 2022, 14, 38483–38496. [Google Scholar] [CrossRef]
- Zhi, Y.F.; Wang, Z.R.; Zhang, H.L.; Zhang, Q.C. Recent progress in metal-free covalent organic frameworks as heterogeneous catalysts. Small 2020, 16, 2001070. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Bi, J.H.; Zhang, Z.T.; Tian, J.J.; Huang, G.C. Interface engineering in a nitrogen-rich COF/BiOBr S-scheme heterojunction triggering efficient photocatalytic degradation of tetracycline antibiotics. J. Colloid Interface Sci. 2024, 661, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.X.; Chen, Z.; Zhang, Y.; Wang, X.N.; Lu, T.T.; Wang, Q.; Zhan, Z.L.; Zhang, P. COF TzDa/Ag/AgBr Z-scheme heterojunction photocatalyst for efficient visible light driven elimination of antibiotics tetracycline and heavy metal ion Cr(VI). Sep. Purif. Technol. 2022, 288, 120717. [Google Scholar] [CrossRef]
- Subudhi, S.; Tripathy, S.P.; Parida, K. Highlights of the characterization techniques on inorganic, organic (COF) and hybrid (MOF) photocatalytic semiconductors. Catal. Sci. Technol. 2021, 11, 392–415. [Google Scholar] [CrossRef]
- Hu, S.Y.; Sun, Y.N.; Feng, Z.W.; Wang, F.O.; Lv, Y.K. Design and construction strategies to improve covalent organic frameworks photocatalyst’s performance for degradation of organic pollutants. Chemosphere 2022, 286, 131646. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cai, A.Q.; Li, T.T. Covalent organic framework-semiconductor-based heterostructures for photocatalytic applications. ChemSusChem 2023, 16, e202300021. [Google Scholar] [CrossRef]
- Yang, Y.L.; Niu, H.Y.; Xu, L.; Zhang, H.; Cai, Y.Q. Triazine functionalized fully conjugated covalent organic framework for efficient photocatalysis. Appl. Catal. B-Environ. 2020, 269, 118799. [Google Scholar] [CrossRef]
- Guo, L.P.; Jin, S.B. Stable covalent organic frameworks for photochemical applications. ChemPhotoChem 2019, 3, 973–983. [Google Scholar] [CrossRef]
- Dong, P.F.; Lv, H.F.; Luo, R.A.; Li, Z.; Wu, X.J.; Lei, J.P. Reticular polarization engineering of covalent organic frameworks for accelerated generation of superoxide anion radicals. Chem. Eng. J. 2023, 461, 141817. [Google Scholar] [CrossRef]
- Zhang, K.K.; Huang, F.W.; Dong, X.Y.; Xiong, K.H.; Lang, X.J. Benzotrithiophene-based sp2 carbon-conjugated microporous polymers for green light-triggered oxidation of amines to imines. Mater. Today Chem. 2024, 35, 101879. [Google Scholar] [CrossRef]
- Jin, S.B.; Ding, X.S.; Feng, X.; Supur, M.; Furukawa, K.; Takahashi, S.; Addicoat, M.; El-Khouly, M.E.; Nakamura, T.; Irle, S.; et al. Charge dynamics in a donor-acceptor covalent organic framework with periodically ordered bicontinuous heterojunctions. Angew. Chem.-Int. Ed. 2013, 52, 2017–2021. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.W.; Wang, Y.X.; Dong, X.Y.; Lang, X.J. Imine-linked 2D covalent organic frameworks based on benzotrithiophene for visible-light-driven selective aerobic sulfoxidation. J. Mater. Chem. A 2024, 12, 7036–7046. [Google Scholar] [CrossRef]
- Qin, C.C.; Wu, X.D.; Tang, L.; Chen, X.H.; Li, M.; Mou, Y.; Su, B.; Wang, S.B.; Feng, C.Y.; Liu, J.W.; et al. Dual donor-acceptor covalent organic frameworks for hydrogen peroxide photosynthesis. Nat. Commun. 2023, 14, 5238. [Google Scholar] [CrossRef]
- Zhao, X.; Shang, S.; Liu, H.; Peng, C.; Hu, J. Dipole moment regulation for enhancing internal electric field in covalent organic frameworks photocatalysts. Chemosphere 2024, 356, 141947. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.C.; Yang, Y.; Wu, X.D.; Chen, L.; Liu, Z.L.; Tang, L.; Lyu, L.; Huang, D.L.; Wang, D.B.; Zhang, C.; et al. Twistedly hydrophobic basis with suitable aromatic metrics in covalent organic networks govern micropollutant decontamination. Nat. Commun. 2023, 14, 6470. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Liu, Y.Y.; Kaczmarek, M.K.; Liu, H.S.; Artizzu, F.; Carlos, L.D.; Van Der Voort, P. Developing luminescent ratiometric thermometers based on a covalent organic framework (COF). Angew. Chem.-Int. Ed. 2020, 59, 1932–1940. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.Y.; Kuang, L.J.; Li, Y.H.; Chen, L.L.; Lin, C.H.; Wang, L.; Song, Y.H. Benzotrithiophene-based covalent organic frameworks for real-time visual onsite assays of enrofloxacin. Biosens. Bioelectron. 2022, 214, 114527. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.H.; Huang, A.X.; Yan, X.C.; Liu, Y.H.; Wang, Y.; Jin, X.; Zhang, F.M. Constructing tightly integrated conductive metal-organic framework/covalent triazine framework heterostructure by coordination bonds for photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2023, 633, 233–242. [Google Scholar] [CrossRef]
- Pei, L.S.; Su, J.P.; Yang, H.L.; Wu, Y.; Du, Y.; Zhu, Y.M. A novel covalent-organic framework for highly sensitive detection of Cd2+, Pb2+, Cu2+ and Hg2+. Microporous Mesoporous Mat. 2022, 333, 111742. [Google Scholar] [CrossRef]
- Wang, Z.; Ang, J.M.; Liu, J.; Ma, X.Y.D.; Kong, J.H.; Zhang, Y.F.; Yan, T.; Lu, X.H. FeNi alloys encapsulated in N-doped CNTs-tangled porous carbon fibers as highly efficient and durable bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Appl. Catal. B Environ. 2020, 263, 118344. [Google Scholar] [CrossRef]
- He, S.J.; Yin, B.; Niu, H.Y.; Cai, Y.Q. Targeted synthesis of visible-light-driven covalent organic framework photocatalyst via molecular design and precise construction. Appl. Catal. B Environ. 2018, 239, 147–153. [Google Scholar] [CrossRef]
- Liu, X.D.; Zhao, F.; Ming, S.L.; Zhang, Y.; Zhao, J.S. Construction of two-dimensional porous polymers with crystalline or amorphous forms for near-infrared electrochromism. Eur. Polym. J. 2024, 208, 112853. [Google Scholar] [CrossRef]
- Lu, W.; Wang, C.; Bai, Y.X.; Xie, C.D.; Zhang, Z.X.; Song, W.H.; Wang, J.J. A novel covalent organic framework for efficient photocatalytic reduction of Cr(vi) and synergistic removal of organic pollutants under visible light irradiation. Environ. Sci. Nano 2024, 11, 229–240. [Google Scholar] [CrossRef]
- Qian, Y.; Li, J.; Ji, M.; Li, J.; Liu, A.; Ma, D.; Zhu, Y. Fluorescence sensing of picomolar ammonia by covalent organic framework. ChemRxiv 2022. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, C.; Song, S.; Zeng, T.; Li, L.X.Y.; Cai, Z.W. Defect-abundant covalent triazine frameworks as sunlight-driven self-cleaning adsorbents for volatile aromatic pollutants in Water. Environ. Sci. Technol. 2019, 53, 9091–9101. [Google Scholar] [CrossRef]
- Pan, X.W.; Qin, X.H.; Zhang, Q.H.; Ge, Y.S.; Ke, H.Z.; Cheng, G.E. N-and S-rich covalent organic framework for highly efficient removal of indigo carmine and reversible iodine capture. Microporous Mesoporous Mater. 2020, 296, 109990. [Google Scholar] [CrossRef]
- Guo, S.Y.; Luo, H.H.; Li, Y.; Chen, J.Z.; Mou, B.; Shi, X.Q.; Sun, G.X. Structure-controlled three-dimensional BiOI/MoS2 microspheres for boosting visible-light photocatalytic degradation of tetracycline. J. Alloys Compd. 2021, 852, 157026. [Google Scholar] [CrossRef]
- Wang, K.W.; Wu, Z.X.; Ji, N.; Wang, T.X.; Gu, Y.X.; Zhao, Z.X.; Guo, Y.; Wang, X.Y.; Jia, Z.F.; Tan, B. Robust thiazole-linked covalent organic frameworks for water sensing with high selectivity and sensitivity. Molecules 2024, 29, 1677. [Google Scholar] [CrossRef]
- Xiao, J.D.; Hang, Q.Z.; Cao, H.B.; Rabeah, J.; Yang, J.; Guo, Z.; Zhou, L.B.; Xie, Y.B.; Brückner, A. Number of reactive charge carriers-a hidden linker between band structure and catalytic performance in photocatalysts. ACS Catal. 2019, 9, 8852–8861. [Google Scholar] [CrossRef]
- Heris, S.Z.; Etemadi, M.; Mousavi, S.B.; Mohammadpourfard, M.; Ramavandi, B. Preparation and characterizations of TiO2/ZnO nanohybrid and its application in photocatalytic degradation of tetracycline in wastewater. J. Photochem. Photobiol. A Chem. 2023, 443, 114893. [Google Scholar]
- Barrio, J.; Lin, L.H.; Wang, X.C.; Shalom, M. Design of a unique energy-band structure and morphology in a carbon nitride photocatalyst for improved charge separation and hydrogen production. ACS Sustain. Chem. Eng. 2018, 6, 519–530. [Google Scholar] [CrossRef]
- Gou, N.; Yang, W.Y.; Gao, S.; Li, Q. Incorporation of ultrathin porous metal-free graphite carbon nitride nanosheets in polyvinyl chloride for efficient photodegradation. J. Hazard. Mater. 2023, 447, 130795. [Google Scholar] [CrossRef] [PubMed]
- Alamgir; Ullah, R.; Talha, K.; Yang, H.Y.; Mushtaq, N.; Ahmad, A.; Fan, L.K.; Li, L.; Zhao, G.X.; Wang, X.S.; et al. MOF-derived In2O3/TiO2 S-scheme heterojunction for efficient photocatalytic degradation of tetracycline. J. Alloys Compd. 2024, 1002, 175398. [Google Scholar]
- Li, Z.P.; Deng, T.Q.; Ma, S.; Zhang, Z.W.; Wu, G.; Wang, J.A.; Li, Q.Z.; Xia, H.; Yang, S.W.; Liu, X.M. Three-component donor-π-acceptor covalent-organic frameworks for boosting photocatalytic hydrogen evolution. J. Am. Chem. Soc. 2023, 145, 8364–8374. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Geerlings, P.; Langenaeker, W.; De Proft, F.; Baeten, A. Molecular electrostatic potentials vs. DFT descriptors of reactivity. In Theoretical and Computational Chemistry; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Suresh, C.H.; Remya, G.S.; Anjalikrishna, P.K. Molecular electrostatic potential analysis: A powerful tool to interpret and predict chemical reactivity. WIREs Comput. Mol. Sci. 2022, 12, e1601. [Google Scholar] [CrossRef]
- Zhuo, L.G.; Liao, W.; Yu, Z.X. A Frontier molecular orbital theory approach to understanding the mayr equation and to quantifying nucleophilicity and electrophilicity by using HOMO and LUMO energies. Asian J. Org. Chem. 2012, 1, 336–345. [Google Scholar] [CrossRef]
- Wang, N.A.; Ye, S.Q.; Li, M.J.; Pan, H.; Wang, G.W. Benzotrithiophene-based covalent organic frameworks: Synthesis and application to perovskite solar cells. Sol. RRL 2024, 8, 2300790. [Google Scholar] [CrossRef]
- Kruszyk, M.; Jessing, M.; Kristensen, J.L.; Jorgensen, M. Computational methods to predict the regioselectivity of electrophilic aromatic substitution reactions of heteroaromatic systems. J. Org. Chem. 2016, 81, 5128–5134. [Google Scholar] [CrossRef]
- Roy, R.K. Stockholders charge partitioning technique. A reliable electron population analysis scheme to predict intramolecular reactivity sequence. J. Phys. Chem. A 2003, 107, 10428–10434. [Google Scholar] [CrossRef]
- Gu, Q.; Gao, Z.W.; Zhao, H.G.; Lou, Z.Z.; Liao, Y.S.; Xue, C. Temperature-controlled morphology evolution of graphitic carbon nitride nanostructures and their photocatalytic activities under visible light. RSC Adv. 2015, 5, 49317–49325. [Google Scholar] [CrossRef]
- Luo, L.B.; Dong, S.Y.; Chen, H.; Jin, H.J.; Huang, T.L. Construction of MgIn2S4/ZnIn2S4 micro-flowers: Efficient degradation of tetracycline hydrochloride over a wide pH range. Appl. Surf. Sci. 2022, 581, 152417. [Google Scholar] [CrossRef]
- Kang, N.J.; Xu, D.B.; Shi, W.D. Synthesis plasmonic Bi/BiVO4 photocatalysts with enhanced photocatalytic activity for degradation of tetracycline (TC). Chin. J. Chem. Eng. 2019, 27, 3053–3059. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, F.; Zhu, Z.Y.; Zhan, S.; He, Q.C. Enhanced photocatalytic activities of Ag3PO4/GO in tetracycline degradation. Chem. Phys. Lett. 2019, 724, 90–95. [Google Scholar] [CrossRef]
- Nagamine, M.; Osial, M.; Jackowska, K.; Krysinski, P.; Widera-Kalinowska, J. Tetracycline photocatalytic degradation under CdS treatment. J. Mar. Sci. Eng. 2020, 8, 483. [Google Scholar] [CrossRef]
- Hu, L.X.; Ren, X.H.; Yang, M.; Guo, W.L. Facet-controlled activation of persulfate by magnetite nanoparticles for the degradation of tetracycline. Sep. Purif. Technol. 2021, 258, 118014. [Google Scholar] [CrossRef]
- Li, D.; Liu, H.; Niu, C.Q.; Yuan, J.R.; Xu, F.G. Mpg-C3N4-ZIF-8 composites for the degradation of tetracycline hydrochloride using visible light. Water Sci. Technol. 2019, 80, 2206–2217. [Google Scholar] [CrossRef] [PubMed]
- Min, F.; Wei, Z.Q.; Yu, Z.; Xiao, Y.T.; Guo, S.E.; Song, R.J.; Li, J.H. Construction of a hierarchical ZnIn2S4/C3N4 heterojunction for the enhanced photocatalytic degradation of tetracycline. Dalton Trans. 2022, 51, 2323–2330. [Google Scholar] [CrossRef]
- Song, J.H.; Zhao, K.; Yin, X.B.; Liu, Y.; Khan, I.; Liu, S.Y. Photocatalytic degradation of tetracycline hydrochloride with g-C3N4/Ag/AgBr composites. Front. Chem. 2022, 10, 1069816. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, C.H.; Hu, J. Ultrasonic degradation of tetracycline combining peroxymonosulfate and BiVO4 microspheres. J. Water Process. Eng. 2023, 56, 104428. [Google Scholar] [CrossRef]
- Li, K.Y.; Li, J.; Luo, F.Y.; Yu, Y.H.; Yang, Y.P.; Li, Y.Z. Heterogeneous photocatalytic degradation of selected pharmaceuticals and personal care products (PPCPs) using tungsten doped TiO2: Effect of the tungsten precursors and solvents. Molecules 2024, 29, 4164. [Google Scholar] [CrossRef]
- Jv, A.; Wu, L.N.; Lu, J.L.; Feng, B.; Che, G.B.; Guan, R.Q.; Zhang, Y.F.; Liu, C.B.; Yu, J.K.; Sun, T.T. BiOBr/Bi2O2CO3 heterojunction photocatalyst for the degradation of tetracycline. ACS Appl. Nano Mater. 2024, 7, 12722–12733. [Google Scholar] [CrossRef]
- Liu, M.J.; He, P.P.; Gong, H.T.; Zhao, Z.H.; Li, Y.M.; Zhou, K.; Lin, Y.M.; Li, J.; Bao, Z.B.; Yang, Q.W.; et al. Benzotrithiophene-based covalent organic frameworks as efficient catalysts for artificial photosynthesis of H2O2 in pure water. Chem. Eng. J. 2024, 482, 148922. [Google Scholar] [CrossRef]
- Wang, L.L.; Fu, Y.; Li, Q.C.; Wang, Z.H. EPR evidence for mechanistic diversity of Cu(II)/peroxygen oxidation systems by tracing the origin of DMPO spin adducts. Environ. Sci. Technol. 2022, 56, 8796–8806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Zhu, M.Y.; Zhang, S.; Cai, Y.W.; Lv, Z.M.; Fang, M.; Tan, X.L.; Wang, X.K. Highly efficient removal of U(VI) by the photoreduction of SnO2/CdCO3/CdS nanocomposite under visible light irradiation. Appl. Catal. B-Environ. 2020, 279, 119390. [Google Scholar] [CrossRef]
- Zhang, B.; Li, T.T.; Mao, Z.C.; Jiang, M.; Zhang, Z.H.; Zhao, K.; Qu, W.Y.; Xiao, W.J.; Chen, J.R. Enantioselective cyanofunctionalization of aromatic alkenes via radical anions. J. Am. Chem. Soc. 2024, 146, 1410–1422. [Google Scholar] [CrossRef]
- Ding, L.C.; Yang, G.H.; Luo, L.; Ma, Y.C.; Shi, J.F.; Liang, D.Q.; Li, Y.N. DABCO-mediated photoelectrochemical three-component sulfonocyclization of 3-Aza-1,5-dienes. Chin. J. Chem. 2024. [Google Scholar] [CrossRef]
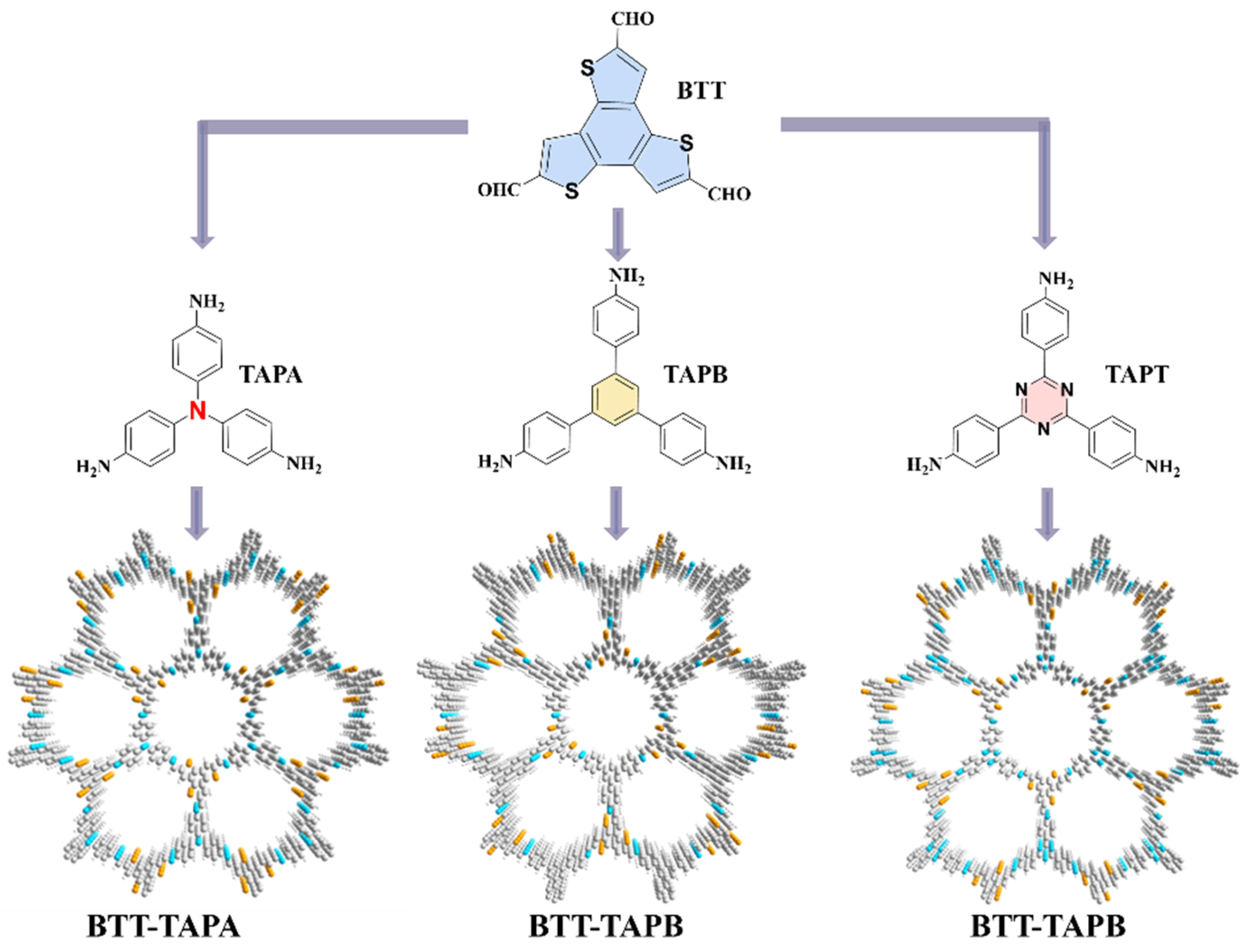
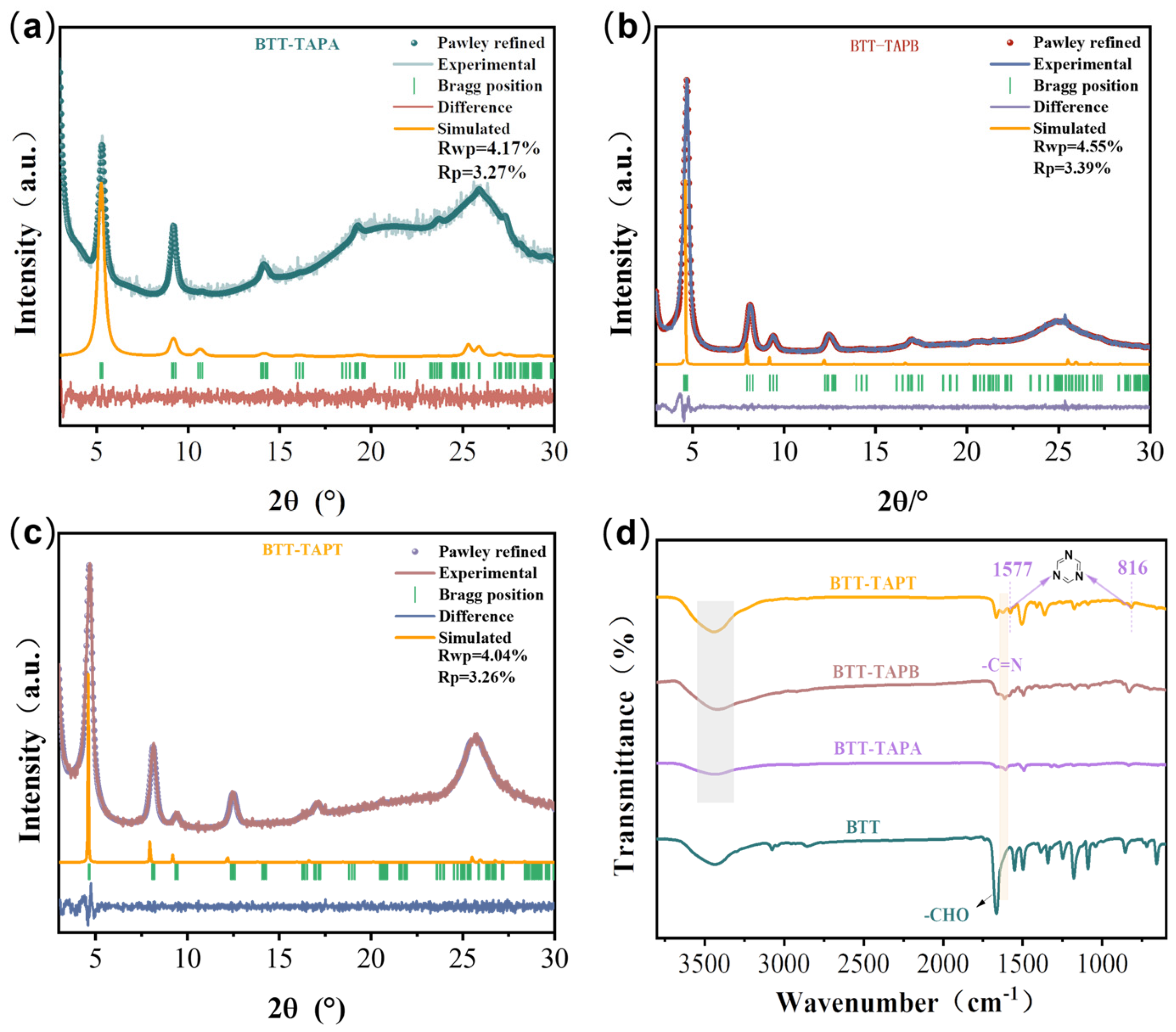



| Model | a (Å) | b (Å) | c (Å) | α | β | γ | Rwp | RP |
|---|---|---|---|---|---|---|---|---|
| BTT-TAPA | 19.18 | 19.18 | 3.52 | 90° | 90° | 120° | 4.17% | 3.27% |
| BTT-TAPB | 21.35 | 21.35 | 3.50 | 90° | 90° | 120° | 4.55% | 3.39% |
| BTT-TAPT | 22.20 | 22.20 | 3.49 | 90° | 90° | 120° | 4.04% | 3.26% |
| Sample | Dosage (g/L) | Concentration (mg/L) | Time (min) | Removal Rate (%) | Year | Ref. |
|---|---|---|---|---|---|---|
| Bi/BiVO4 | 0.5 | 10 | 60 | 74.7% | 2019 | [68] |
| Ag3PO4/5GO | 0.25 | 10 | 30 | 69.8% | 2019 | [69] |
| CdS | 0.4 | 100 | 60 | 80.0% | 2020 | [70] |
| PS/MNPs | 0.05 | 50 | 240 | 74.4% | 2020 | [71] |
| mpg-C3N4–ZIF-8 | 0.5 | 40 | 180 | 74.8% | 2020 | [72] |
| ZIS-TCN/3 | 0.1 | 20 | 60 | 86.1% | 2021 | [73] |
| g-C3N4/Ag/AgBr-8% | 0.32 | 20 | 30 | 57.5% | 2022 | [74] |
| US/BiVO4/PMS | >1 | 20 | 60 | 78.6% | 2023 | [75] |
| W1-TiO2-Et | 0.4 | 50 | 360 | 77.2% | 2024 | [76] |
| BiOBr/Bi2O2CO3 | 0.2 | 40 | 40 | 63.0% | 2024 | [77] |
| BTT-TAPT | 0.1 | 50 | 240 | 82.7% | 2024 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; He, J.; Guo, Y.; Chang, Y.; Ju, H.; Li, Y. Electron Push-Pull Effect of Benzotrithiophene-Based Covalent Organic Frameworks on the Photocatalytic Degradation of Pharmaceuticals and Personal Care Products. Molecules 2025, 30, 336. https://doi.org/10.3390/molecules30020336
Guo H, He J, Guo Y, Chang Y, Ju H, Li Y. Electron Push-Pull Effect of Benzotrithiophene-Based Covalent Organic Frameworks on the Photocatalytic Degradation of Pharmaceuticals and Personal Care Products. Molecules. 2025; 30(2):336. https://doi.org/10.3390/molecules30020336
Chicago/Turabian StyleGuo, Hongguang, Jiaqin He, Yixi Guo, Yunxi Chang, Haidong Ju, and Yizhou Li. 2025. "Electron Push-Pull Effect of Benzotrithiophene-Based Covalent Organic Frameworks on the Photocatalytic Degradation of Pharmaceuticals and Personal Care Products" Molecules 30, no. 2: 336. https://doi.org/10.3390/molecules30020336
APA StyleGuo, H., He, J., Guo, Y., Chang, Y., Ju, H., & Li, Y. (2025). Electron Push-Pull Effect of Benzotrithiophene-Based Covalent Organic Frameworks on the Photocatalytic Degradation of Pharmaceuticals and Personal Care Products. Molecules, 30(2), 336. https://doi.org/10.3390/molecules30020336






