The Role of Ionic Liquids in Textile Processes: A Comprehensive Review
Abstract
1. Introduction
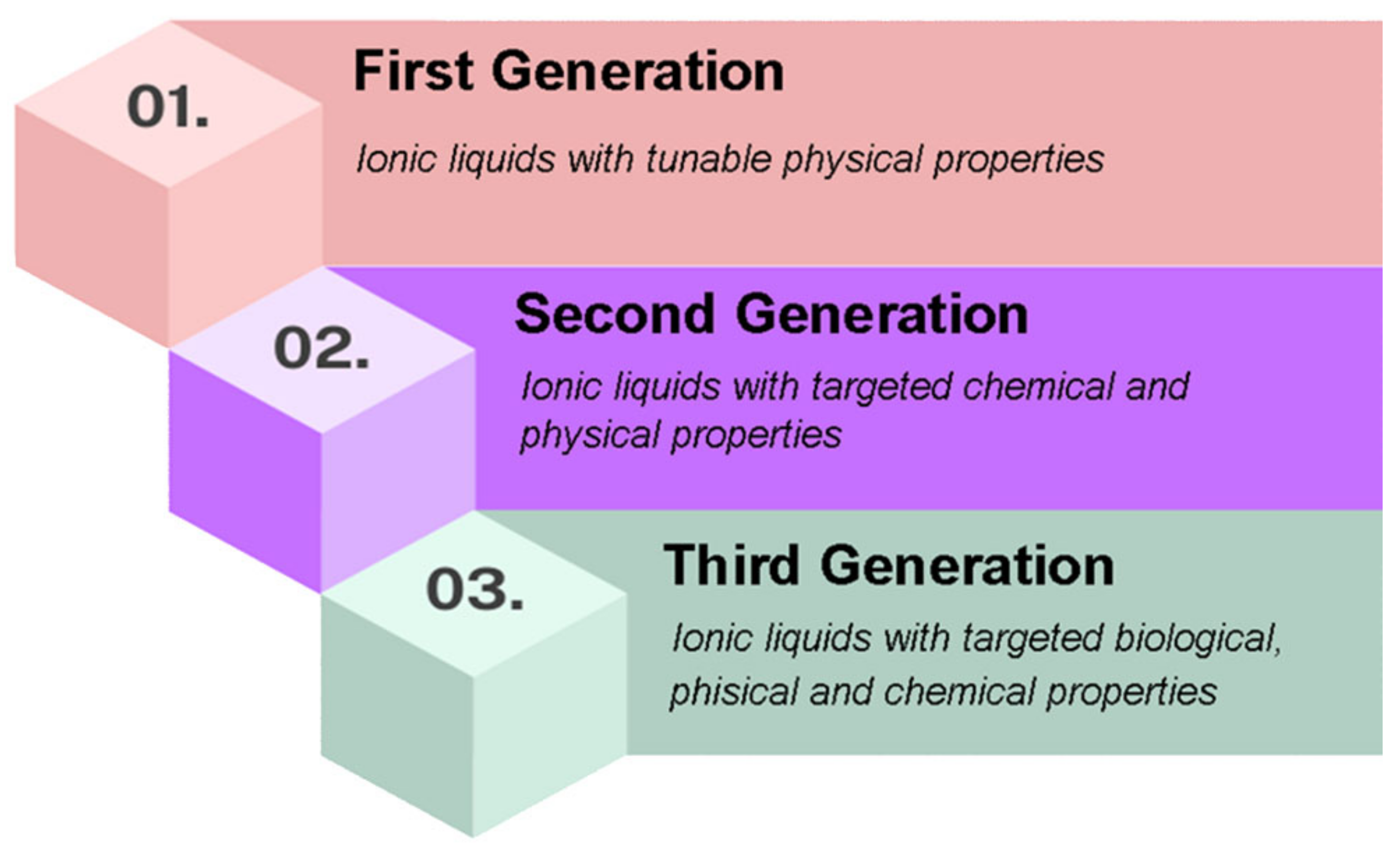
- -
- First generation
- -
- Second generation
- -
- Third generation
2. Overview of Ionic Liquids
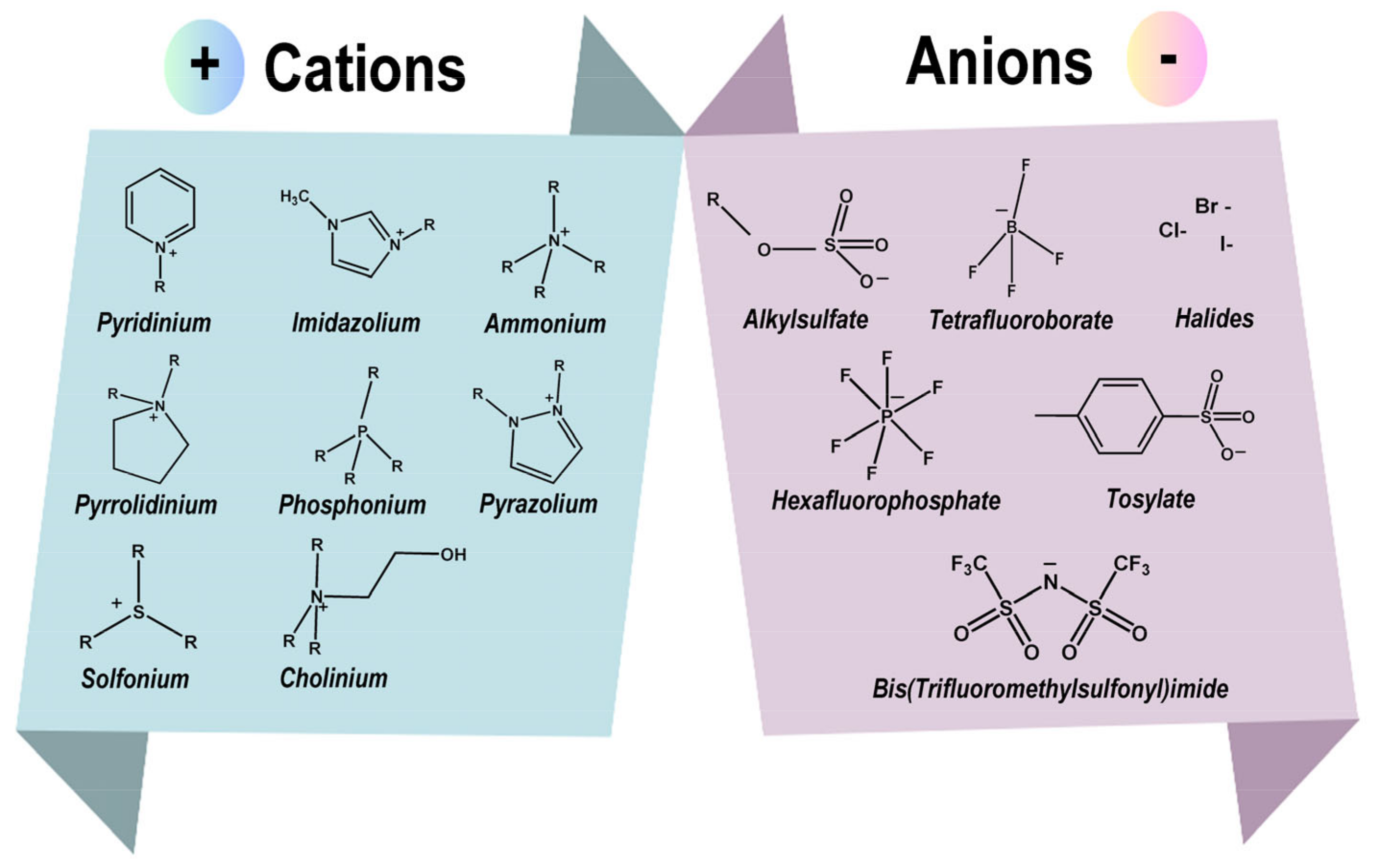
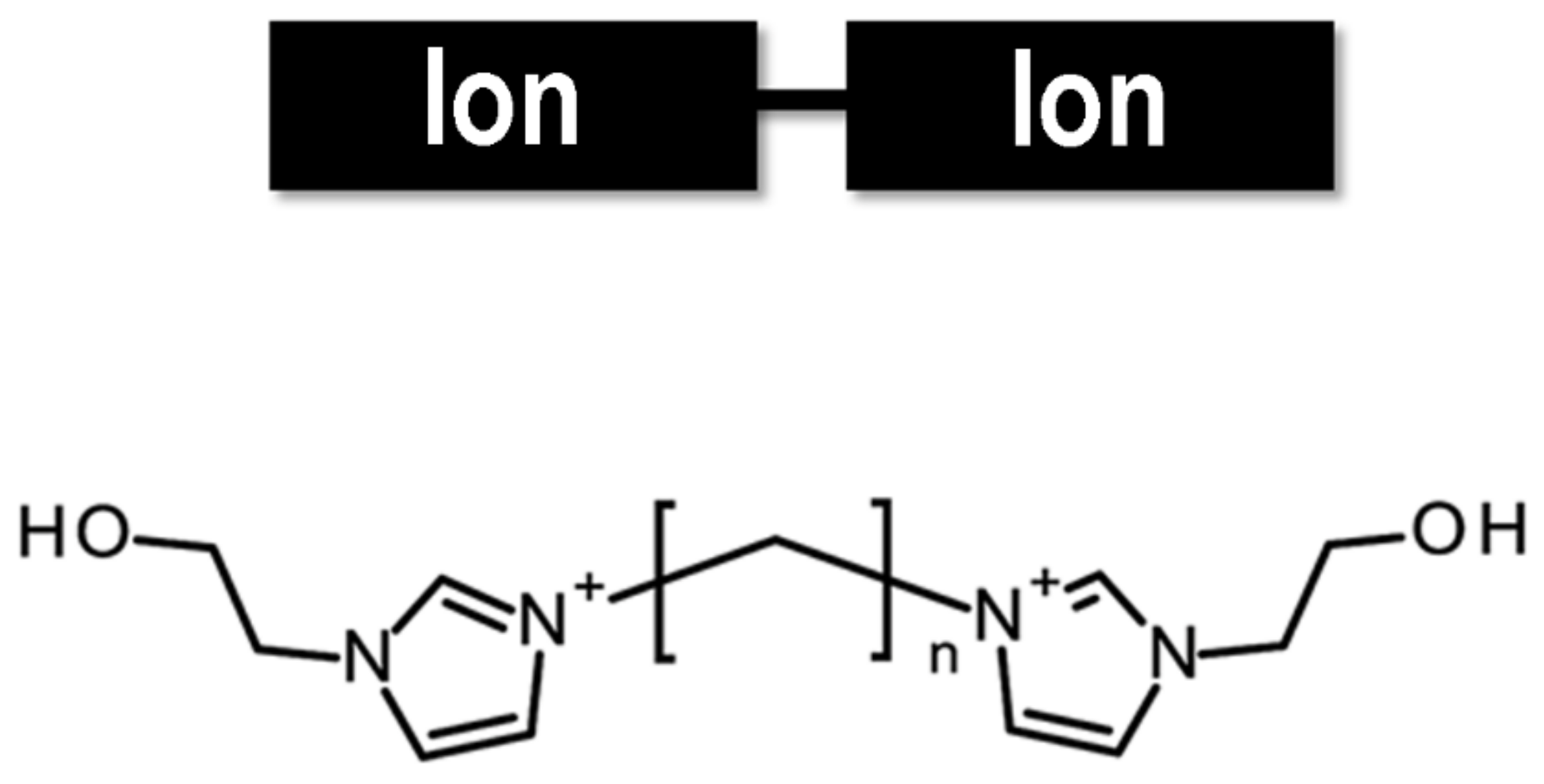
2.1. Structure of Ionic Liquids
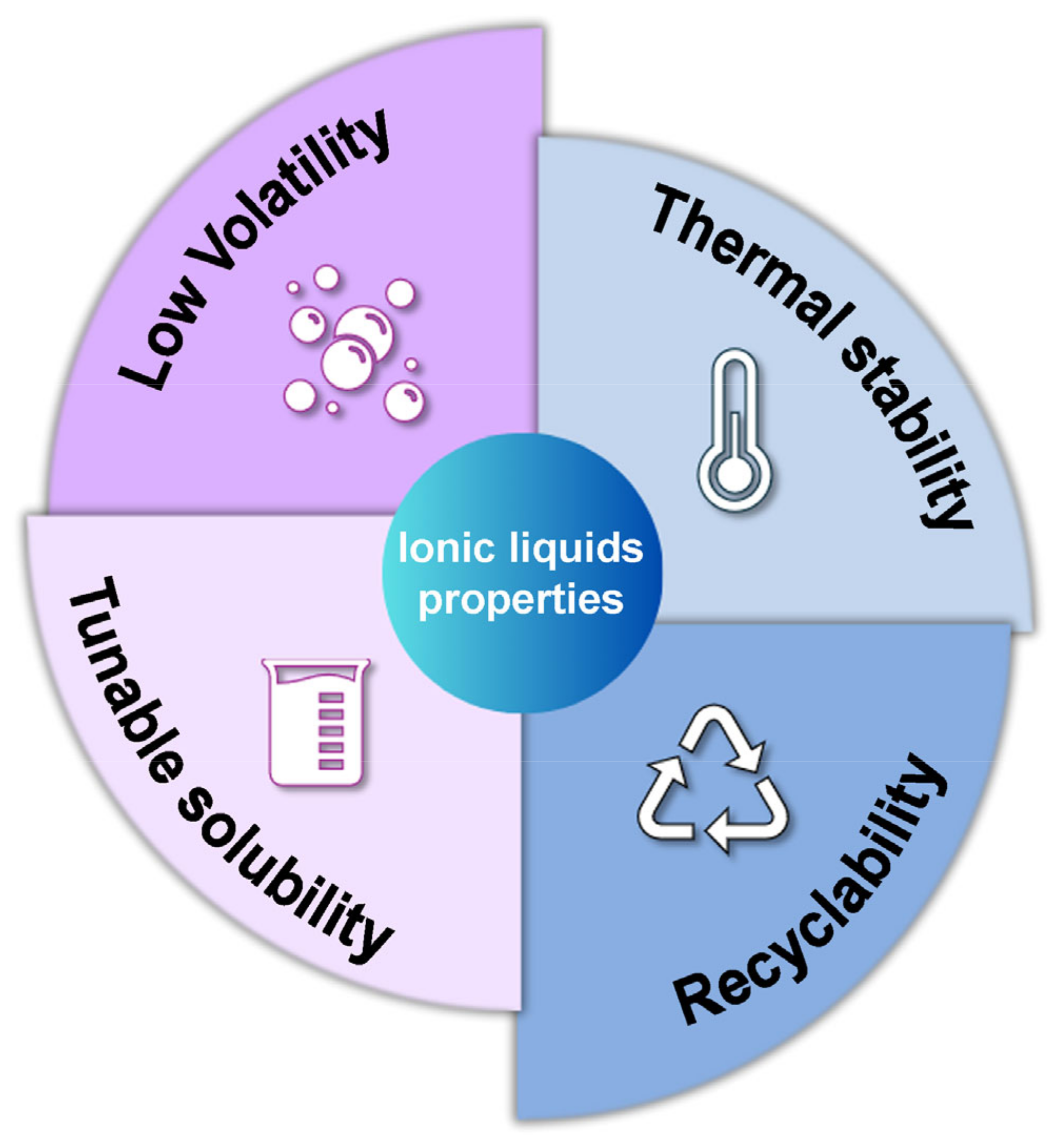
2.2. Properties of Ionic Liquids
- -
- Low Volatility
- -
- High Thermal Stability
- -
- Tunable Solubility
- -
- Recyclability
3. Applications of Ionic Liquids in Textile Processes
3.1. Dyeing Processes
- -
- Dye Solubility: Ionic liquids exhibit remarkable solvation properties attributable to their ionic composition and tunable polarity. Imidazolium-based ionic liquids, for instance, effectively dissolve a wide range of dyes, including those sparingly soluble in water, by disrupting dye aggregates and enhancing molecular dispersion [45]. This results in higher dye solubility, which is critical for achieving uniform and vibrant coloration.
- -
- Fiber Modification: Ionic liquids can chemically modify fiber surfaces to enhance dye absorption. For example, when used on polyamide fibers, ionic liquids can generate positive charges on the fiber surface, strengthening electrostatic interactions with anionic dyes [38]. Similarly, for natural fibers like wool and silk, ionic liquids partially disrupt the keratin or cellulose network, allowing deeper penetration of dye molecules [39].
- -
- Temperature and Energy Efficiency: Ionic liquids facilitate dyeing at reduced temperatures, minimizing thermal degradation of both dye and fiber. This property not only conserves energy but also supports the preservation of fabric integrity, particularly for heat-sensitive textiles [35].
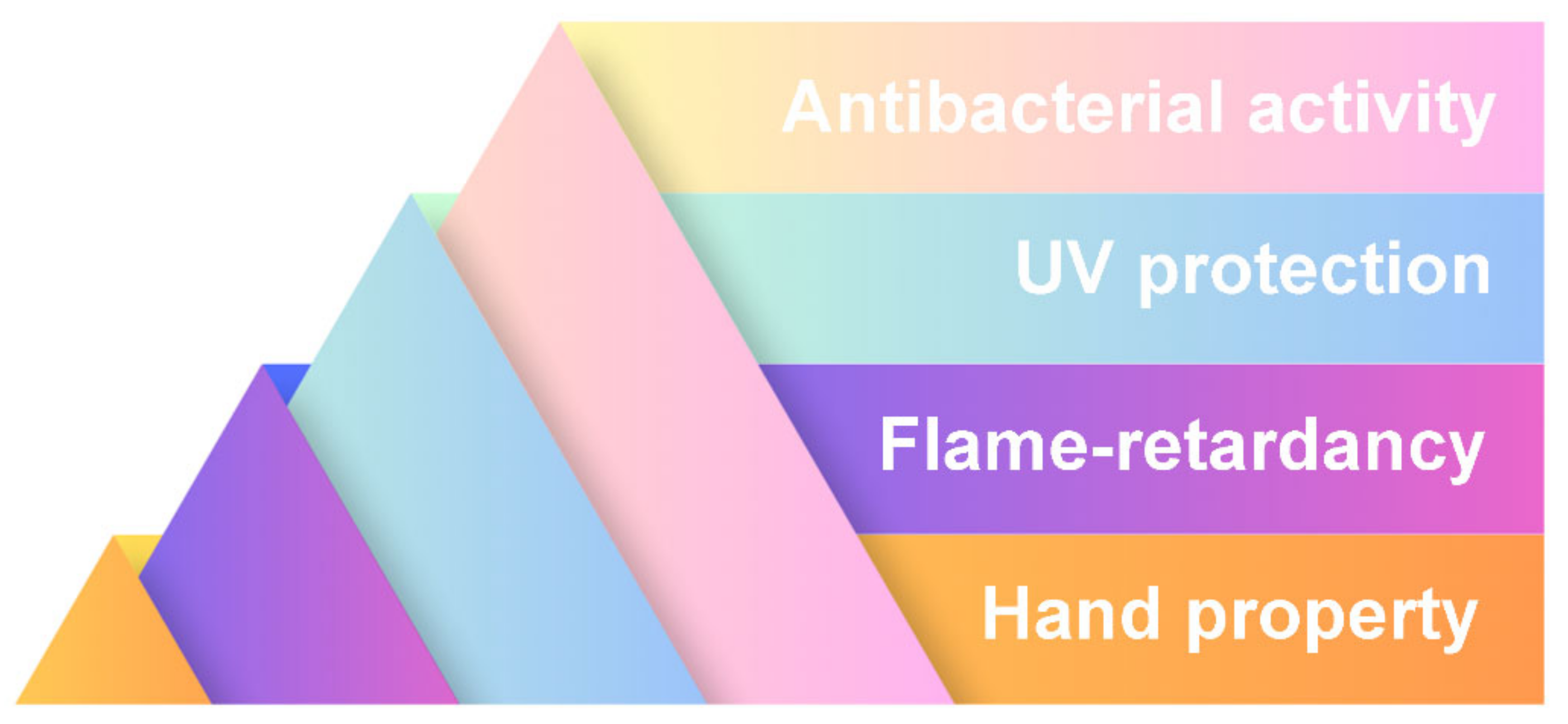
3.2. Fiber Preparation and Textile Finishing
- -
- Antibacterial properties: Imidazolium-based ionic liquids chemically bond with keratin in wool, forming stable complexes that exhibit durable antibacterial effects against both Gram-positive and Gram-negative bacteria, even after repeated washing [55].
- -
- -
- Surface modification: Ionic liquids enable surface-level transformations of synthetic fibers, such as imparting a cotton-like texture to polyester through deposition of cellulosic material [58]. These interactions are facilitated by the unique solvating and catalytic properties of ionic liquids, making them versatile tools for advanced textile finishing.
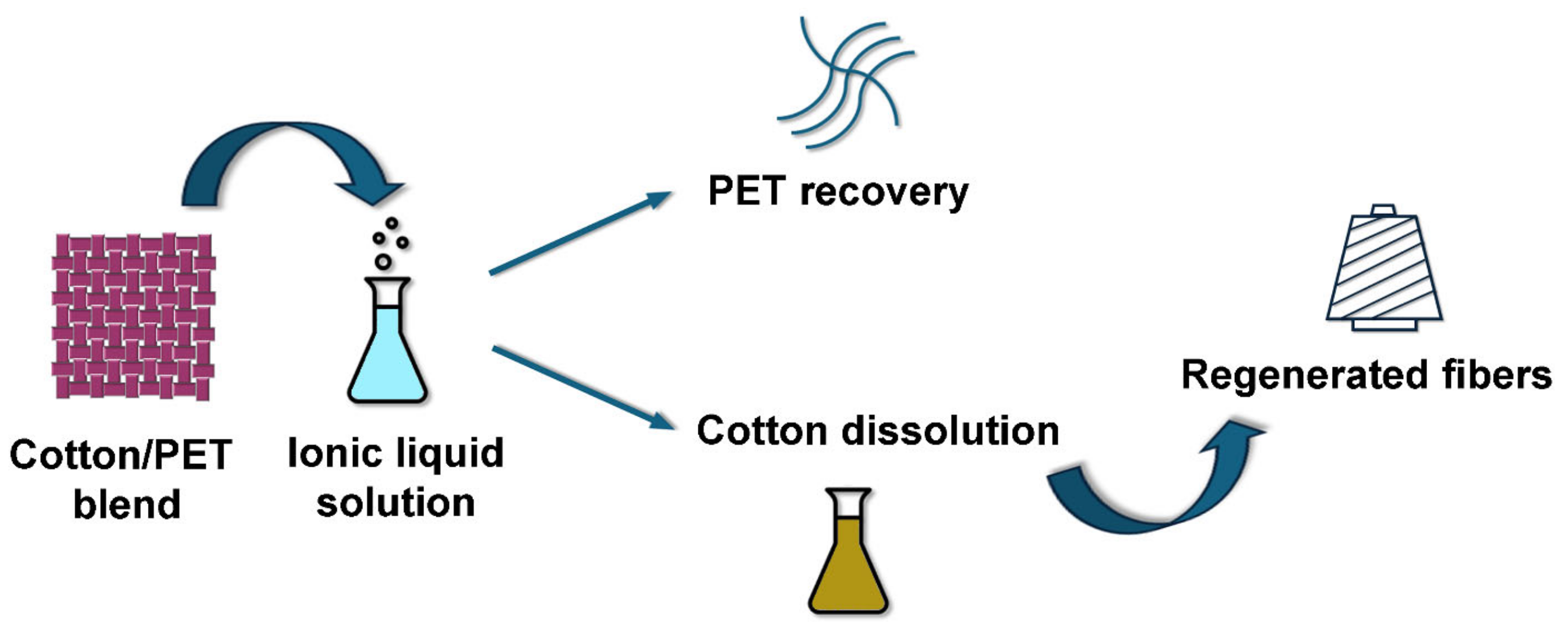
3.3. Recycling of Textile Waste
3.4. Dyed Textiles and Wastewater Treatment
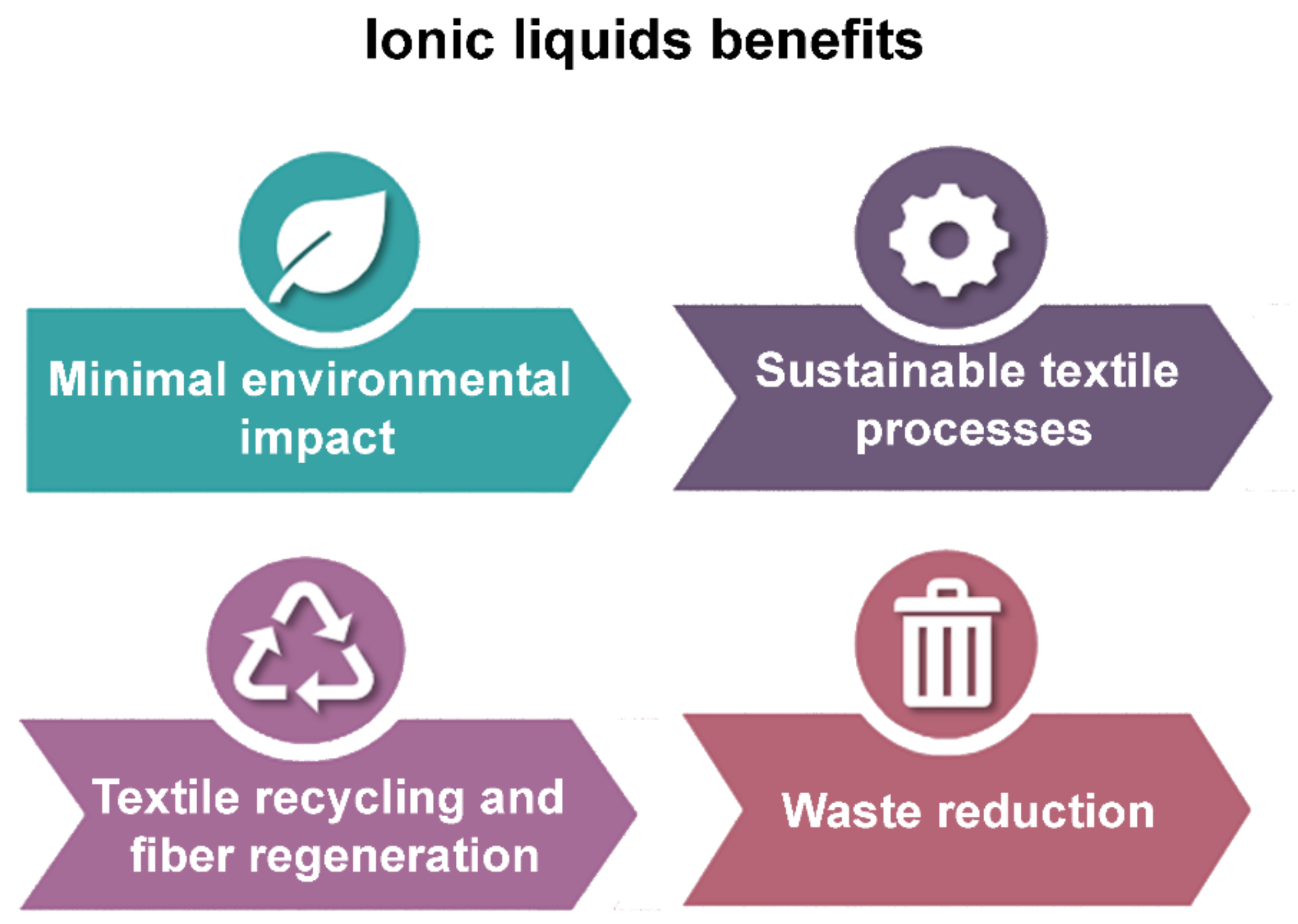
4. Benefits of Ionic Liquids in Textile Applications
5. Ecological Concerns of Ionic Liquids
6. Future Research Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kalb, R.S. Toward Industrialization of Ionic Liquids; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9783030352448. [Google Scholar]
- Pernak, J.; Rzemieniecki, T.; Materna, K. Ionic liquids “in a nutshell” (history, properties and development). ChemiK 2016, 70, 476–480. [Google Scholar]
- Freemantle, M.; Welton, T.; Rogers, R. An Introduction to Ionic Liquids; RSC Publishing: Cambridge, UK, 2019; ISBN 978-1-84755-161-0. [Google Scholar]
- Nambu, N.; Hiraoka, N.; Shigemura, K.; Hamanaka, S.; Ogawa, M. A Study of the 1,3,5-Trialkylbenzenes with Aluminum Chloride-Hydrogen Chloride Systems. Bull. Chem. Soc. Jpn. 1976, 49, 3637–3640. [Google Scholar] [CrossRef]
- Ramsay, W. XXXIV. On picoline and its derivatives. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1876, 2, 269–281. [Google Scholar] [CrossRef]
- Laus, G.; Bentivoglio, G.; Schottenberger, H.; Kahlenberg, V.; Kopacka, H.; Roeder, T.; Sixta, H. Ionic Liquids: Current Dev Elopments, Potential and Drawbacks for Industr Ial Applications. Lenzing. Ber. 2005, 84, 71–85. [Google Scholar]
- Barrer, R.M. The viscosity of pure liquids. II. Polymerised ionic melts. Trans. Faraday Soc. 1943, 39, 59–67. [Google Scholar] [CrossRef]
- Silva, W.; Zanatta, M.; Ferreira, A.; Corvo, M.; Cabrita, E. Revisiting Ionic Liquid Structure-Property Relationship: A Critical Analysis. Int. J. Mol. Sci. 2020, 21, 7745. [Google Scholar] [CrossRef]
- Walden, P.T. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914, 1800. [Google Scholar]
- Gabriel, S.; Weiner, J. Ueber einige abkömmlinge des propylamins. Berichte Der Dtsch. Chem. Ges. 1888, 21, 2669–2679. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J. Structures and Interactions of Ionic Liquids; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783642386183. [Google Scholar]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef]
- Hough, W.L.; Smiglak, M.; Rodríguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The third evolution of ionic liquids: Active pharmaceutical ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Turguła, A.; Stęsik, K.; Materna, K.; Klejdysz, T.; Praczyk, T.; Pernak, J. Third-generation ionic liquids with N-alkylated 1{,}4-diazabicyclo[2.2.2]octane cations and pelargonate anions. RSC Adv. 2020, 10, 8653–8663. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, W.J.; Hong, S.H.; Kim, J.E.; Suh, K.S. Ionic-Liquid-Assisted Formation of Silver Nanowires. Angew. Chem. Int. Ed. 2009, 48, 3806–3809. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M. New developments in catalysis using ionic liquids. Appl. Catal. A Gen. 2001, 222, 101–117. [Google Scholar] [CrossRef]
- Fischer, T.; Sethi, A.; Welton, T.; Woolf, J. Diels-Alder reactions in room-temperature ionic liquids. Tetrahedron Lett. 1999, 40, 793–796. [Google Scholar] [CrossRef]
- Tzschucke, C.C.; Markert, C.; Bannwarth, W.; Roller, S.; Hebel, A.; Haag, R. Modern Separation Techniques for the Efficient Workup in Organic Synthesis. Angew. Chem. Int. Ed. 2002, 41, 3964–4000. [Google Scholar] [CrossRef]
- Dupont, J.; de Souza, R.F.; Suarez, P.A.Z. Ionic Liquid (Molten Salt) Phase Organometallic Catalysis. Chem. Rev. 2002, 102, 3667–3692. [Google Scholar] [CrossRef]
- Borra, E.F.; Seddiki, O.; Angel, R.; Eisenstein, D.; Hickson, P.; Seddon, K.R.; Worden, S.P. Deposition of metal films on an ionic liquid as a basis for a lunar telescope. Nature 2007, 447, 979–981. [Google Scholar] [CrossRef]
- Doroodian, A.; Dengler, J.E.; Genest, A.; Rösch, N.; Rieger, B. Methylguanidinium borohydride: An ionic-liquid-based hydrogen-storage material. Angew. Chem. Int. Ed. 2010, 49, 1871–1873. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Lau, R.M.; Sorgedrager, M.J.; van Rantwijk, F.; Seddon, K.R. Biocatalysis in ionic liquids. Green Chem. 2002, 4, 147–151. [Google Scholar] [CrossRef]
- Meksi, N.; Moussa, A. A review of progress in the ecological application of ionic liquids in textile processes. J. Clean. Prod. 2017, 161, 105–126. [Google Scholar] [CrossRef]
- Salas, R.; Villa, R.; Velasco, F.; Cirujano, F.G.; Nieto, S.; Martin, N.; Garcia-Verdugo, E.; Dupont, J.; Lozano, P. Ionic liquids in polymer technology. Green Chem. 2024. [Google Scholar] [CrossRef]
- Christis, M.; Vercalsteren, A.; Arnold, M.; Nicolau, M.; Lafond, E.; Mortensen, L.; Coscieme, L.; Manshoven, S. Textiles and the Environment in a Circular Economy; ETC/WMGE: Copenhagen, Denmark, 2019. [Google Scholar]
- Keskin, S.; Kayrak-Talay, D.; Akman, U.; Hortaçsu, Ö. A review of ionic liquids towards supercritical fluid applications. J. Supercrit. Fluids 2007, 43, 150–180. [Google Scholar] [CrossRef]
- Cruz, C.; Ciach, A. Phase Transitions and Electrochemical Properties of Ionic Liquids and Ionic Liquid—Solvent Mixtures. Molecules 2021, 26, 3668. [Google Scholar] [CrossRef]
- Al Hassan, M.K.; Alfarsi, A.; Nasser, M.S.; Hussein, I.A.; Khan, I. Ionic liquids and NADES for removal of organic pollutants and heavy metals in wastewater: A comprehensive review. J. Mol. Liq. 2023, 391, 123163. [Google Scholar] [CrossRef]
- Atashnezhad, A.; Akhtarmanesh, S.; Al Dushaishi, M.F. Rheology characterization of ionic liquids under high pressure and high temperature. J. Ion. Liq. 2024, 4, 100105. [Google Scholar] [CrossRef]
- Yu, L.; Li, Z.; Huang, W.; Ali, A.; Chen, Y.; Zhao, G.; Yao, S. Recovery and post-treatment processes for ionic liquids and deep eutectic solvents. J. Mol. Liq. 2024, 402, 124767. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar]
- Johnson, K. What’s an Ionic Liquid? Electrochem. Soc. Interface 2007, 16, 38. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Exploring the potential of ionic liquid-based electrochemical biosensors for real-time biomolecule monitoring in pharmaceutical applications: From lab to life. Results Eng. 2023, 20, 101533. [Google Scholar] [CrossRef]
- Bianchini, R.; Cevasco, G.; Chiappe, C.; Pomelli, C.S.; Rodríguez Douton, M.J. Ionic Liquids Can Significantly Improve Textile Dyeing: An Innovative Application Assuring Economic and Environmental Benefits. ACS Sustain. Chem. Eng. 2015, 3, 2303–2308. [Google Scholar] [CrossRef]
- Barros, S.M.; Andrade, R.S.; Torres, D.; Chiari-Andrèo, B.G.; Veloso, G.B.R.; Gonzalez, C.; Iglesias, M. Eco-Friendly Technology for Reactive Dyeing of Cationized Fabrics: Protic Ionic Liquids as Innovative Media. Cellul. Chem. Technol. 2022, 56, 403–425. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Q.; Fan, X.; Wang, P. Enhancing Dye Adsorption of Wool Fibers with 1-butyl-3-methylimidazolium Chloride Ionic Liquid Processing. Text. Res. J. 2010, 80, 1898–1904. [Google Scholar] [CrossRef]
- Dong, Z.Q. Influence of ionic liquids on the sorption of acid dyes by nylon fibers. Adv. Mater. Res. 2011, 175, 602–607. [Google Scholar] [CrossRef]
- Kantouch, A.; Khalil, E.M.; Mowafi, S.; Allam, O.G.; El-Sayed, H. Utilization of ionic liquids in improving dyeability of proteinic fabrics with acid and reactive dyes. Egypt. J. Chem. 2011, 54, 189–203. [Google Scholar]
- Ribeiro, F.R.G.; Cabral, V.F.; Silva, C.; Andreaus, J.; Cardozo-Filho, L.; Croscato, G.S.; Silva, A.B.; Moraes, M.R. Alternative sustainable dyeing of textiles with ionic liquid. Integrating Cleaner Production into Sustainability Strategies. In Proceedings of the 4th International Workshop Advances in Cleaner Production, São Paulo, Brazil, 22–24 May 2013; p. 27. [Google Scholar]
- Opwis, K.; Celik, B.; Benken, R.; Knittel, D.; Gutmann, J.S. Dyeing of m-Aramid Fibers in Ionic Liquids. Polymers 2020, 12, 1824. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Dyeing Process in Ionic Liquid Solvents. WO Patent 2009024766A2, 26 February 2009. [Google Scholar]
- Dong, Z.; Tang, R.; Chen, G. The retarding action of imidazole ionic liquid in acrylic dyeing. China Dye. Finish. 2011, 37, 1–5. [Google Scholar]
- Farbeverfahren, D.T.N.-W.E. V Für Synthese-Und Naturfasermaterial Mit Hilfe Ionischer Flüssigkeiten. DE Patent 102006040075A1, 15 August 2008. [Google Scholar]
- Buettner, C.S.; Cognigni, A.; Schröder, C.; Bica-Schröder, K. Surface-active ionic liquids: A review. J. Mol. Liq. 2022, 347, 118160. [Google Scholar] [CrossRef]
- Kawai, R.; Niki, M.; Yada, S.; Yoshimura, T. Physicochemical and solution properties of quaternary-ammonium-salt-type amphiphilic gemini ionic liquids with spacers containing oxygen or nitrogen. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125218. [Google Scholar] [CrossRef]
- Zhuang, L.; Zheng, C.; Sun, J.; Yuan, A.; Wang, G. Performances of ramie fiber pretreated with dicationic imidazolium ionic liquid. Fibers Polym. 2014, 15, 226–233. [Google Scholar] [CrossRef]
- Cheng, D. Application of ionic liquid in silk dyeing. Adv. Mater. Res. 2011, 331, 253–256. [Google Scholar] [CrossRef]
- Dyeing Method of Reactive Dye Containing Ionic Liquid. Patent CN102493222A, 8 November 2011.
- Khatri, A.; Peerzada, M.; Mohsin, M.; White, M. A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J. Clean. Prod. 2015, 87, 50–57. [Google Scholar] [CrossRef]
- Lindström, F. Chemical and Physical Changes in PET Fibres Due to Exhaust Dyeing. Master’s Thesis, University of Borås, Borås, Sweden, 2018. [Google Scholar]
- El-Sayed, H. Chapter 6—Wool Dyeing. In The Wool Handbook; The Textile Institute Book Series; Jose, S., Thomas, S., Basu, G., Eds.; Woodhead Publishing: Sawston, UK, 2024; pp. 139–158. ISBN 978-0-323-99598-6. [Google Scholar]
- Schwarz, W.M. Ink Compositions Containing Ionic Liquid Solvents. US Patent 6,048,388, 11 April 2000. [Google Scholar]
- Hibbert, R. 19–What Textile Fibres are Applicable for the Layering System for the Active Ageing? In Woodhead Publishing Series in Textiles, Proceedings of the MRS Spring Meeting & Exhibition San Francisco, CA, USA, 1–5 April 2013; Woodhead Publishing: Sawston, UK, 2015; pp. 329–359. ISBN 978-0-85709-538-1. [Google Scholar]
- Kantouch, A.; Khalil, E.M.; El-Sayed, H.; Mowafi, S. A novel application of ionic liquid in improvement of the felting resistance of wool. Egypt. J. Chem. 2011, 54, 481–493. [Google Scholar]
- Opwis, K.; Textor, T.; Gutmann, J. Use of Ionic Liquids in Textile Finishing; ResearchGate: Berlin, Germany, 2013. [Google Scholar]
- Asaadi, S.; Hummel, M.; Hellsten, S.; Härkäsalmi, T.; Ma, Y.; Michud, A.; Sixta, H. Renewable High-Performance Fibers from the Chemical Recycling of Cotton Waste Utilizing an Ionic Liquid. ChemSusChem 2016, 9, 3250–3258. [Google Scholar] [CrossRef]
- Musale, R.M.; Shukla, S.R. Weight reduction of polyester fabric using sodium hydroxide solutions with additives cetyltrimethylammonium bromide and [BMIM]Cl. J. Text. Inst. 2017, 108, 467–471. [Google Scholar] [CrossRef]
- Kantouch, A.; Khalil, E.M.; Mowafi, S.; El-Sayed, H. Antimicrobial finishing of wool fabric using ionic liquids. J. Text. Inst. 2013, 104, 363–369. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kantouch, A.; El-Sayed, H. An approach to impart bactericidal effect to viscose fabric using ionic liquids. Egypt. J. Chem. 2011, 54, 495–507. [Google Scholar]
- Bentis, A.; Boukhriss, A.; Grancaric, A.M.; El Bouchti, M.; El Achaby, M.; Gmouh, S. Flammability and combustion behavior of cotton fabrics treated by the sol gel method using ionic liquids combined with different anions. Cellulose 2019, 26, 2139–2153. [Google Scholar] [CrossRef]
- Boukhriss, A.; Gmouh, S.; Hannach, H.; Roblin, J.-P.; Cherkaoui, O.; Boyer, D. Treatment of cotton fabrics by ionic liquid with PF6− anion for enhancing their flame retardancy and water repellency. Cellulose 2016, 23, 3355–3364. [Google Scholar] [CrossRef]
- Arputharaj, A.; Prasad, V.; Saxena, S.; Nadanathangam, V.; Shukla, S.R. Ionic liquid mediated application of nano zinc oxide on cotton fabric for multi-functional properties. J. Text. Inst. 2017, 108, 1189–1197. [Google Scholar] [CrossRef]
- Jiugang, Y.; Qiang, W.; Ping, W.; Li, C.; Xuerong, F. Promotional effect of 1-butyl-3-methylimidazolium chloride ionic liquid on the enzymatic finishing of wool. Eng. Life Sci. 2012, 12, 209–215. [Google Scholar] [CrossRef]
- Vyas, S.K.; Shukla, S.R. Degumming of eri silk using ionic liquids and optimization through response surface methodology. J. Text. Inst. 2016, 107, 1096–1111. [Google Scholar] [CrossRef]
- De Barros, A.G.; Tomber, D.D. Quantitative analysis of passenger and baggage security screening at airports. J. Adv. Transp. 2007, 41, 171–193. [Google Scholar] [CrossRef]
- Moazzem, S.; Wang, L.; Daver, F.; Crossin, E. Environmental impact of discarded apparel landfilling and recycling. Resour. Conserv. Recycl. 2021, 166, 105338. [Google Scholar] [CrossRef]
- Hoti, G.; Caldera, F.; Trotta, F.; Zoccola, M.; Patrucco, A.; Anceschi, A. A Novel Approach for Nanosponge: Wool Waste as a Building Block for the Synthesis of Keratin-Based Nanosponge and Perspective Application in Wastewater Treatment. ACS Omega 2024, 9, 43319–43330. [Google Scholar] [CrossRef] [PubMed]
- Anceschi, A.; Trotta, F.; Zoccola, M.; Caldera, F.; Magnacca, G.; Patrucco, A. From Waste to Worth: Innovative Pyrolysis of Textile Waste into Microporous Carbons for Enhanced Environmental Sustainability. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4960149 (accessed on 9 January 2025).
- Bentivoglio, G.; Röder, T.; Fasching, M.; Buchberger, M.; Schottenberger, H.; Sixta, H. Cellulose processing with chloride-based ionic liquids. Lenzing. Ber. 2006, 86, 154–161. [Google Scholar]
- Ma, Y.; Zeng, B.; Wang, X.; Byrne, N. Circular Textiles: Closed Loop Fiber to Fiber Wet Spun Process for Recycling Cotton from Denim. ACS Sustain. Chem. Eng. 2019, 7, 11937–11943. [Google Scholar] [CrossRef]
- Haslinger, S.; Hummel, M.; Anghelescu-Hakala, A.; Määttänen, M.; Sixta, H. Upcycling of cotton polyester blended textile waste to new man-made cellulose fibers. Waste Manag. 2019, 97, 88–96. [Google Scholar] [CrossRef]
- Villalta, E.; Riba-Moliner, M.; Lis, M. Recovery of cellulose from polyester/cotton fabrics making use of ionic liquids. Polym. Sci. Peer Rev. J. 2022, 4, 000590. [Google Scholar]
- Wu, H.; Wang, B.; Li, T.; Wu, Y.; Yang, R.; Gao, H.; Nie, Y. Efficient recycle of waste poly-cotton and preparation of cellulose and polyester fibers using the system of ionic liquid and dimethyl sulfoxide. J. Mol. Liq. 2023, 388, 122757. [Google Scholar] [CrossRef]
- Idris, A.; Vijayaraghavan, R.; Rana, U.A.; Patti, A.F.; MacFarlane, D.R. Dissolution and regeneration of wool keratin in ionic liquids. Green Chem. 2014, 16, 2857–2864. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Sun, F.; Tian, M.; Qu, L.; Perry, P.; Owens, H.; Liu, X. Textile Waste Fiber Regeneration via a Green Chemistry Approach: A Molecular Strategy for Sustainable Fashion. Adv. Mater. 2021, 33, 2105174. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, M.; Schlapp-Hackl, I.; Sawada, D.; Raiskio, S.; Ojha, K.; Smith, E.; Sixta, H. Chemical recycling of hemp waste textiles via the ionic liquid based dry-jet-wet spinning technology. Text. Res. J. 2022, 93, 2545–2557. [Google Scholar] [CrossRef]
- Swatloski, R.; Spear, S.; Hobrey, J.D.; Rogers, R. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4947–4975. [Google Scholar] [CrossRef]
- Acharya, S.; Liyanage, S.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L.; Abidi, N. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. [Google Scholar] [CrossRef]
- Arshi, S.; Zhao, Y.; Seyen, T.; Vreckem, E.; Buelens, L. A comparative study between ionic liquid and acidic pretreatment of waste cotton textiles with simultaneous saccharification and fermentation. Bangladesh J. Sci. Ind. Res. 2022, 57, 41–48. [Google Scholar] [CrossRef]
- Hong, F.; Guo, X.; Zhang, S.; Han, S.; Yang, G.; Jönsson, L.J. Bacterial cellulose production from cotton-based waste textiles: Enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour. Technol. 2012, 104, 503–508. [Google Scholar] [CrossRef]
- Azanaw, A.; Birlie, B.; Teshome, B.; Jemberie, M. Textile effluent treatment methods and eco-friendly resolution of textile wastewater. Case Stud. Chem. Environ. Eng. 2022, 6, 100230. [Google Scholar] [CrossRef]
- Singh, B.J.; Chakraborty, A.; Sehgal, R. A systematic review of industrial wastewater management: Evaluating challenges and enablers. J. Environ. Manag. 2023, 348, 119230. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; Vedaraman, N.; Surianarayanan, M.; MacFarlane, D.R. Extraction and recovery of azo dyes into an ionic liquid. Talanta 2006, 69, 1059–1062. [Google Scholar] [CrossRef]
- Li, C.; Xin, B. Extraction and Mechanisms of Acid Dyes into a Room Temperature Ionic Liquid. In Proceedings of the 2008 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008; pp. 3519–3522. [Google Scholar]
- Li, C.; Xin, B.; Xu, W.; Zhang, Q. Study on the extraction of dyes into a room-temperature ionic liquid and their mechanisms. J. Chem. Technol. Biotechnol. 2007, 82, 196–204. [Google Scholar] [CrossRef]
- Pei, Y.C.; Wang, J.J.; Xuan, X.P.; Fan, J.; Fan, M. Factors Affecting Ionic Liquids Based Removal of Anionic Dyes from Water. Environ. Sci. Technol. 2007, 41, 5090–5095. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Fan, Y.; Zhang, S.; Wang, J. Extraction of Azo Dyes from Aqueous Solutions with Room Temperature Ionic Liquids. Sep. Sci. Technol. 2011, 46, 1172–1177. [Google Scholar] [CrossRef]
- Fat’hi, M.R.; Parham, H.; Pourreza, N.; Golmohammadi, H. Removal of indigo carmine from water samples using ionic liquid immobilized on alumina as a new adsorbent. Fresenius Environ. Bull. 2012, 21, 1873–1878. [Google Scholar]
- Zarezadeh-Mehrizi, M.; Badiei, A.; Mehrabadi, A.R. Ionic liquid functionalized nanoporous silica for removal of anionic dye. J. Mol. Liq. 2013, 180, 95–100. [Google Scholar] [CrossRef]
- Tkachenko, O.; Panteleimonov, A.; Padalko, I.; Korobov, A.; Gushikem, Y.; Kholin, Y. Silica functionalized with 1-propyl-3-methylimidazolium chloride as an efficient adsorbent for the removal of Eosin Yellow and Reactive Blue 4. Chem. Eng. J. 2014, 254, 324–332. [Google Scholar] [CrossRef]
- Lawal, I.A.; Moodley, B. Synthesis, characterisation and application of imidazolium based ionic liquid modified montmorillonite sorbents for the removal of amaranth dye. RSC Adv. 2015, 5, 61913–61924. [Google Scholar] [CrossRef]
- Cheng, J.; Shi, L.; Lu, J. Amino ionic liquids-modified magnetic core/shell nanocomposite as an efficient adsorbent for dye removal. J. Ind. Eng. Chem. 2016, 36, 206–214. [Google Scholar] [CrossRef]
- Gao, H.; Kan, T.; Zhao, S.; Qian, Y.; Cheng, X.; Wu, W.; Wang, X.; Zheng, L. Removal of anionic azo dyes from aqueous solution by functional ionic liquid cross-linked polymer. J. Hazard. Mater. 2013, 261, 83–90. [Google Scholar] [CrossRef]
- Kamran, S.; Tavallali, H.; Azad, A. Fast Removal of Reactive Red 141 and Reactive Yellow 81 From Aqueous Solution by Fe3O4 Magnetic Nanoparticles Modified With Ionic Liquid 1-Octyl-3-methylimidazolium Bromide. Iran. J. Anal. Chem. 2014, 1, 78–86. [Google Scholar]
- Chen, X.; Li, F.; Asumana, C.; Yu, G. Extraction of soluble dyes from aqueous solutions with quaternary ammonium-based ionic liquids. Sep. Purif. Technol. 2013, 106, 105–109. [Google Scholar] [CrossRef]
- Mu, B.; Yang, Y. Complete separation of colorants from polymeric materials for cost-effective recycling of waste textiles. Chem. Eng. J. 2022, 427, 131570. [Google Scholar] [CrossRef]
- Talbi, Z.; Haddou, B.; Ghouas, H.; Kameche, M.; Derriche, Z.; Gourdon, C. Cationic Dye Removal From Aqueous Solutions Using Ionic Liquids AND nonionic Surfactant-Ionic Liquids Systems: A Comparative Study Based Upon Experimental Design. Chem. Eng. Commun. 2014, 201, 41–52. [Google Scholar] [CrossRef]
- Inman, G.; Nlebedim, I.C.; Prodius, D. Application of Ionic Liquids for the Recycling and Recovery of Technologically Critical and Valuable Metals. Energies 2022, 15, 628. [Google Scholar] [CrossRef]
- Khoo, Y.S.; Tjong, T.C.; Chew, J.W.; Hu, X. Techniques for recovery and recycling of ionic liquids: A review. Sci. Total Environ. 2024, 922, 171238. [Google Scholar] [CrossRef]
- Zhou, J.; Sui, H.; Jia, Z.; Yang, Z.; He, L.; Li, X. Recovery and purification of ionic liquids from solutions: A review. RSC Adv. 2018, 8, 32832–32864. [Google Scholar] [CrossRef]
- Bento, R.M.F.; Almeida, M.R.; Bharmoria, P.; Freire, M.G.; Tavares, A.P.M. Improvements in the enzymatic degradation of textile dyes using ionic-liquid-based surfactants. Sep. Purif. Technol. 2020, 235, 116191. [Google Scholar] [CrossRef]
- Latifi, S.; Saoiabi, S.; Boukhriss, A.; Gmouh, S.; Saoiabi, A. Ionic Liquids as an Ecological Flame-retardant Agent for Textile Fabrics. NanoWorld J. 2023, 9, S157–S162. [Google Scholar]
- Gonçalves, A.R.P.; Paredes, X.; Cristino, A.F.; Santos, F.J.V.; Queirós, C.S.G.P. Ionic liquids—A review of their toxicity to living organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Zhang, D.; Zhang, Y.; Xu, H.; Sun, Y.; Gu, X.; Luo, J.; Gao, B. Environmental applications and toxicity of ionic liquids. J. Environ. Chem. Eng. 2024, 12, 114638. [Google Scholar] [CrossRef]
- Docherty, K.M.; Aiello, S.W.; Buehler, B.K.; Jones, S.E.; Szymczyna, B.R.; Walker, K.A. Ionic liquid biodegradability depends on specific wastewater microbial consortia. Chemosphere 2015, 136, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Pan, X.; Chen, C.-Y.; Li, H.-Y.; Yan, X.; Li, C.; Chu, Y.-H.; Yan, B. Emerging impacts of ionic liquids on eco-environmental safety and human health. Chem. Soc. Rev. 2021, 50, 13609–13627. [Google Scholar] [CrossRef] [PubMed]
- Paparella, A.N.; Perrone, S.; Salomone, A.; Messa, F.; Cicco, L.; Capriati, V.; Perna, F.M.; Vitale, P. Use of Deep Eutectic Solvents in Plastic Depolymerization. Catalysts 2023, 13, 1035. [Google Scholar] [CrossRef]
- Ali, S.S.; Abdelkarim, E.A.; Elsamahy, T.; Al-Tohamy, R.; Li, F.; Kornaros, M.; Zuorro, A.; Zhu, D.; Sun, J. Bioplastic production in terms of life cycle assessment: A state-of-the-art review. Environ. Sci. Ecotechnol. 2023, 15, 100254. [Google Scholar] [CrossRef]
- Baaqel, H.; Tulus, V.; Chachuat, B.; Guillén-Gosálbez, G.; Hallett, J. Uncovering the True Cost of Ionic Liquids using Monetization. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2020; Volume 48, pp. 1825–1830. ISBN 9780128233771. [Google Scholar]

| Dye Type | Type of Material | Ionic Liquids | Dyeing Process | Process Condition | Role of Ionic Liquid | Reference |
|---|---|---|---|---|---|---|
| Acid dyes | Wool | Methylimidazolium-based ionic liquids | Exhaustion | 60 °C, 60 min | Pretreatment | [37] |
| Diethylethanolamine-based ionic liquids | ||||||
| Polyamide 6.6 | Methylimidazolium-based ionic liquids | Exhaustion | 80 °C, 45 min | Dyeing medium | [38] | |
| Diethylethanolamine-based ionic liquids | ||||||
| Silk | Methylimidazolium-based ionic liquids | Exhaustion | 60 °C, 30 min | Pretreatment | [39] | |
| Cotton | Diethylethanolamine-based ionic liquids | / | / | Dyeing medium | [40] | |
| Polyester | Diethylethanolamine-based ionic liquids | / | / | Dyeing medium | [40] | |
| Polyacrylonitrile | Diethylethanolamine-based ionic liquids | / | / | Dyeing medium | [40] | |
| Imidazole-based ionic liquids | ||||||
| m-aramid | Methylimidazolium-based ionic liquids | Exhaustion | 180 °C, 60 min | Dyeing medium | [41] | |
| Reactive dyes | Wool | Methylimidazolium-based ionic liquids | Exhaustion | 80 °C, 60 min | Pretreatment | [39] |
| Silk | Methylimidazolium-based ionic liquids | Exhaustion | 80 °C, 60 min | Pretreatment | [39] | |
| Cotton | Quaternary ammonium salt ionic liquids | Pad-dry-cure | 60 °C, 10–20 min | Auxiliary | [36] | |
| Flax | Methylimidazolium-based ionic liquids | / | 40–80 °C, 150 min | Auxiliary | [42] | |
| Basic dyes | Polyacrylonitrile | Methylimidazolium-based ionic liquids | Exhaustion | 85 °C, 50 min | Retardant | [43] |
| Disperse dyes | Polyester | Methylimidazolium-based ionic liquids | Open-vessel | 95 °C | Additives | [35,44] |
| Wool | Methylimidazolium-based ionic liquids | Open-vessel | 95 °C | Additives | [35] | |
| Cotton | Methylimidazolium-based ionic liquids | Open-vessel | 95 °C | Additives | [35] | |
| Direct dyes | Cotton | Methylimidazolium-based ionic liquids | Impregnation | 50 °C, 30 min | Dyeing medium | [44] |
| Application | Material | Ionic Liquids | Process | Outcome | Reference |
|---|---|---|---|---|---|
| Antimicrobial properties | Wool | Imidazolium-based ionic liquids | Pretreatment with ionic liquids to bind keratin. | Longlasting effect against E. coli. | [59] |
| Viscose | Imidazolium-based ionic liquids | Treated with anionic agents followed by ionic liquids. | Antibacterial resistance against Gram-positive and Gram-negative bacteria. | [60] | |
| Flame retardancy | Cotton | Methylimidazolium and pyridinium-based ionic liquids | Coating applied via sol-gel method. | Improved LOI (limiting oxygen index) and flame retardancy. | [61] |
| Cotton | Various (e.g., PF6−) | Ionic liquid applied in finishing bath. | Enhanced flame retardant and water repellency properties. | [62] | |
| UV protection | Cotton | 1-Butyl-3-methylimidazolium chloride + nano-ZnO | Nano-zinc oxide applied to cotton fabric using ionic liquids. | Excellent UV protection and bacteriostatic activity. | [63] |
| Surface Tactility | Polyester | Cetyltrimethylammonium bromide and 1-butyl-3-methylimidazolium chloride | Deposition of cellulosic materials on polyester fibers. | Imparts a cotton-like feel to synthetic fibers. | [58] |
| Material Recycled | Type of Ionic Liquids | Reference |
|---|---|---|
| Cotton | 1-allyl-3-methylimidazolium chloride | [70] |
| 1,5-diazabicyclo[4.3.0]non-5-enium acetate | [57] | |
| 1-butyl-3-methylimidazolium acetate and DMSO as a cosolvent. | [71] | |
| Cotton/PET | Superbase-based ionic liquid | [72] |
| 1-allyl-3-Methylimidazolium chloride | [73] | |
| 1-ethyl-3-methylimidazolium diethyl phosphate DMSO system | [74] | |
| 1-allyl-3-methylimidazole chloride in conjunction with DMSO | [74] | |
| 1-allyl-3-methylimidazolium dicyanamide | [75] | |
| Wool | 1-allyl-3-methylimidazolium dicyanamide | [75] |
| Wool/Polyester | 1,3-dimethylimidazolium dimethyl phosphate | [76] |
| Hemp | 1,5-diazabicyclo[4.3.0]non-5-enium acetate | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anceschi, A.; Riccardi, C.; Patrucco, A. The Role of Ionic Liquids in Textile Processes: A Comprehensive Review. Molecules 2025, 30, 353. https://doi.org/10.3390/molecules30020353
Anceschi A, Riccardi C, Patrucco A. The Role of Ionic Liquids in Textile Processes: A Comprehensive Review. Molecules. 2025; 30(2):353. https://doi.org/10.3390/molecules30020353
Chicago/Turabian StyleAnceschi, Anastasia, Claudia Riccardi, and Alessia Patrucco. 2025. "The Role of Ionic Liquids in Textile Processes: A Comprehensive Review" Molecules 30, no. 2: 353. https://doi.org/10.3390/molecules30020353
APA StyleAnceschi, A., Riccardi, C., & Patrucco, A. (2025). The Role of Ionic Liquids in Textile Processes: A Comprehensive Review. Molecules, 30(2), 353. https://doi.org/10.3390/molecules30020353







