Mononuclear Aluminum–Fluoride Ions, AlFx(+/−)—Study of Plausible Frameworks of Complexes with Biomolecules and Their In Vitro Toxicity
Abstract
:1. Introduction
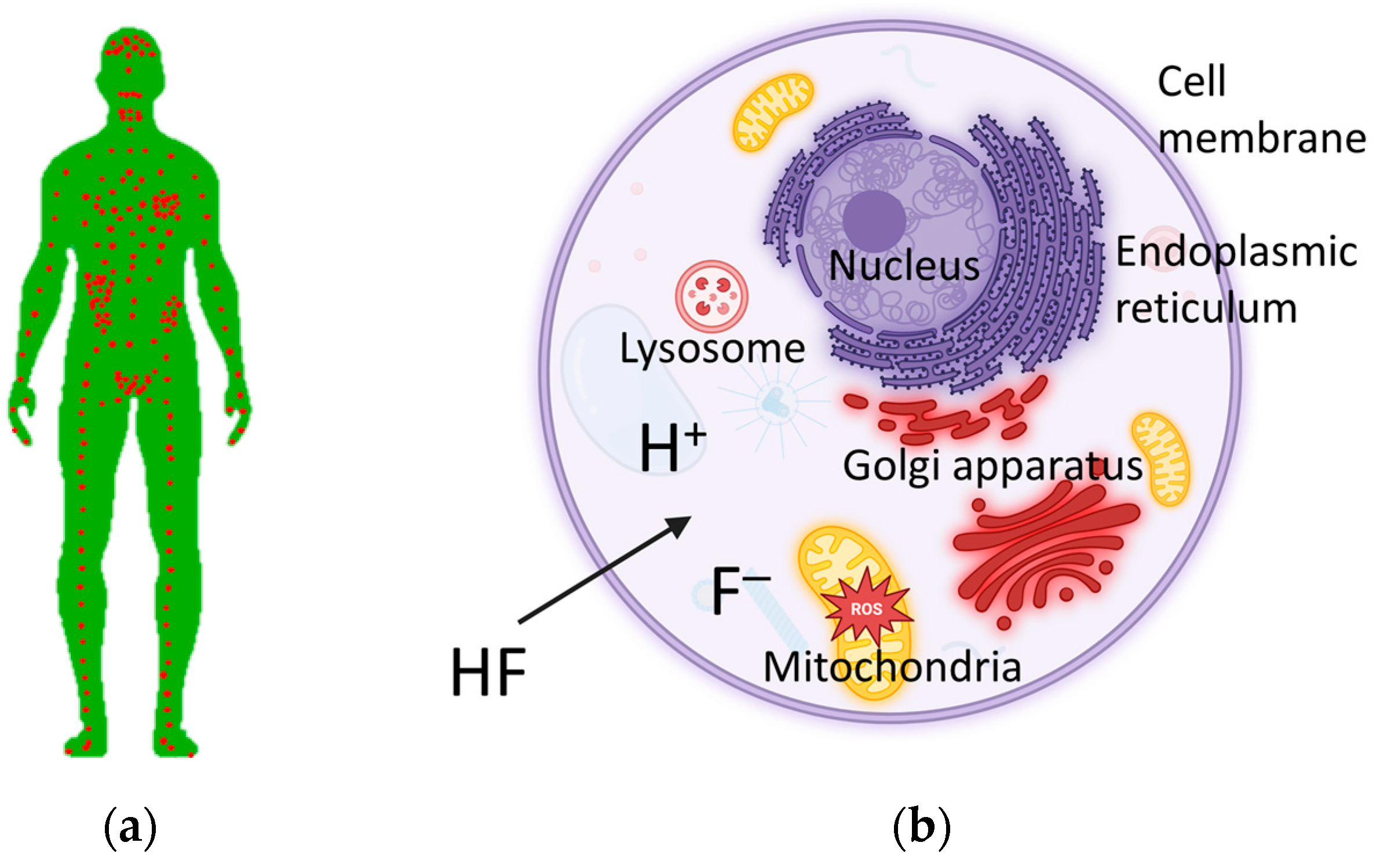
2. Human Exposure to Fluorine and Aluminum
2.1. Fluorine
2.2. Aluminum
2.3. Aqueous Chemistry of Fluoride and Aluminum
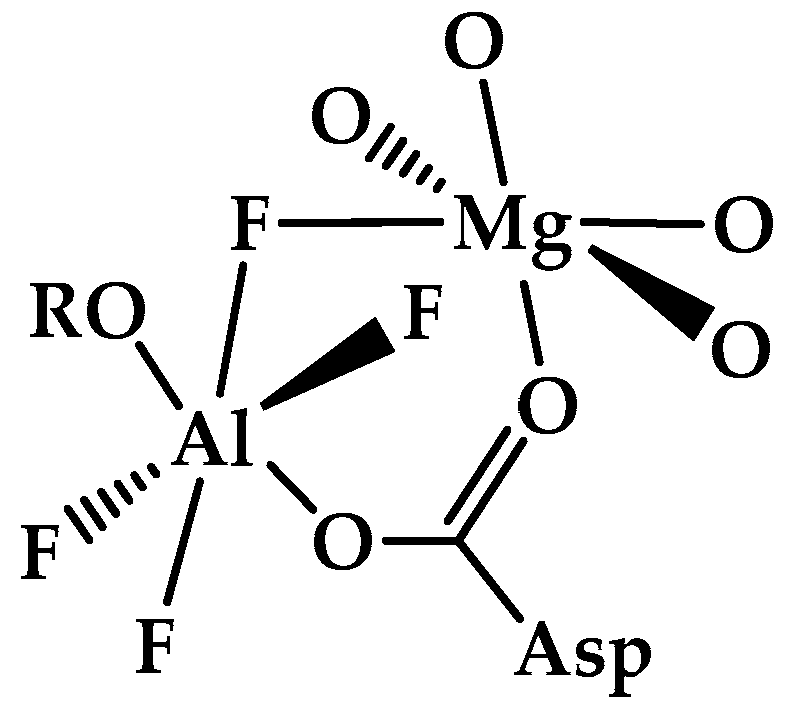
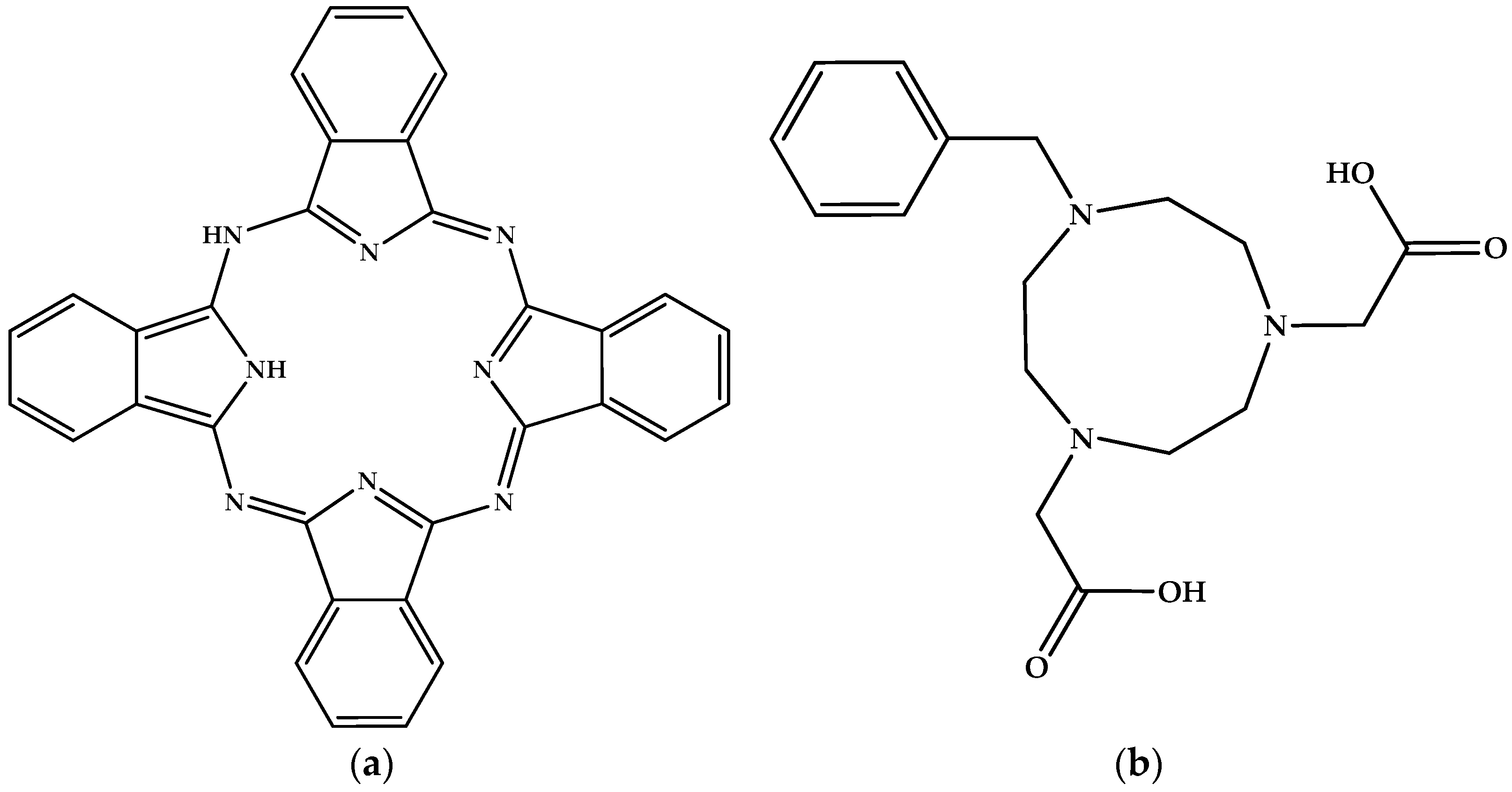
3. Well-Defined Mononuclear Aluminum–Fluoride Ions (AlFx) Plausibly Forming the Framework of Complexes with Biomolecules
3.1. The Fluoroaluminum [AlF]2+ Cation
3.2. The Difluoroaluminum [AlF2]+ Cation
3.3. The Aluminum Fluoride AlF3
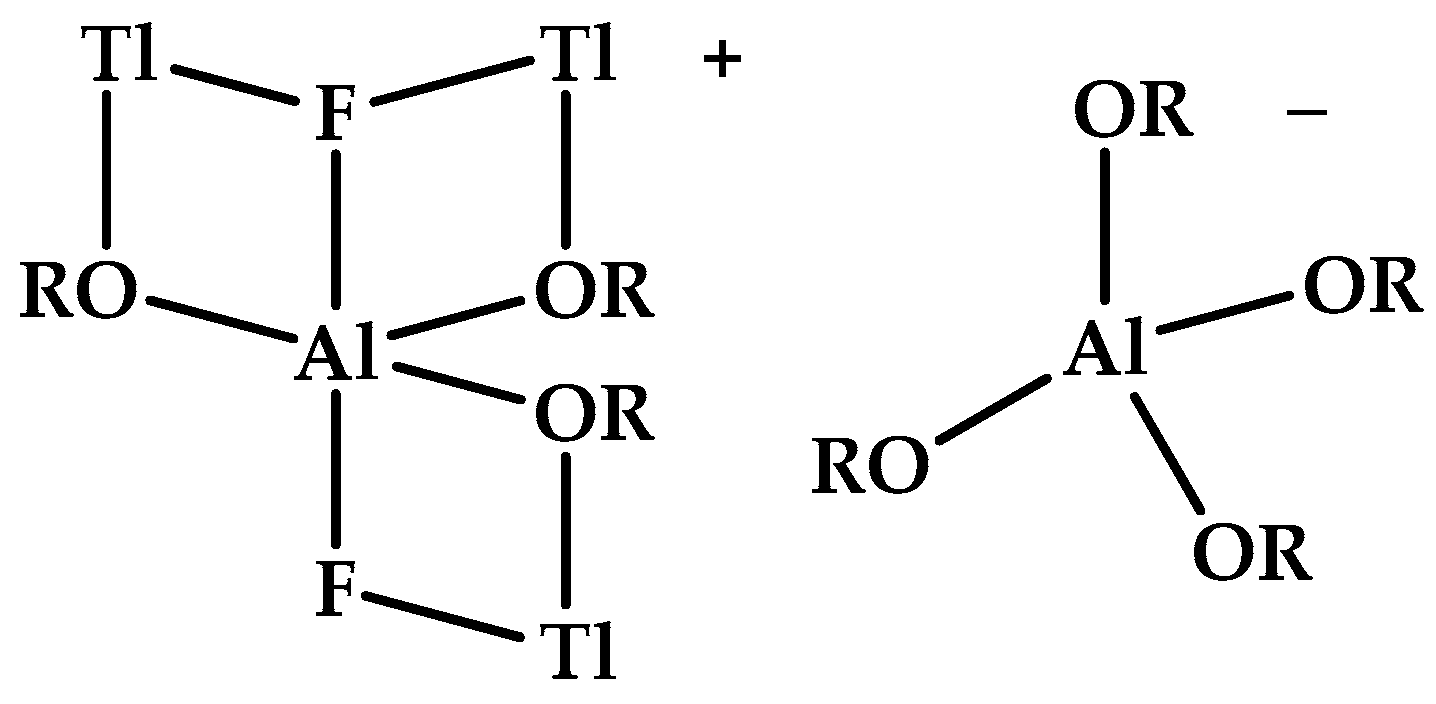
3.4. The Tetrafluoroaluminate [AlF4]− Anion
3.5. The Pentafluoroaluminate [AlF5]2− Anion
3.6. The Hexafluoroaluminate [AlF6]3− Anion
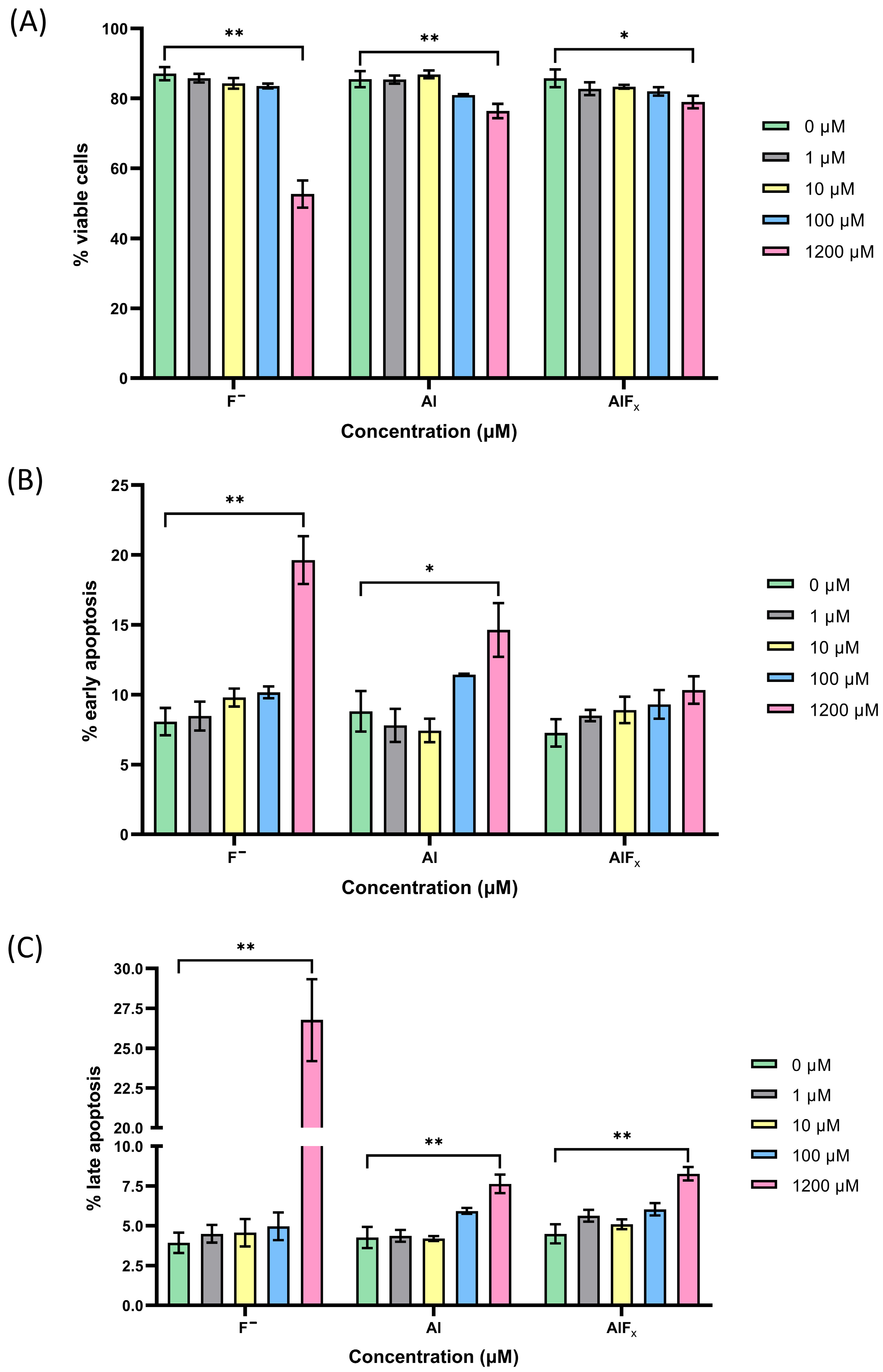
4. Results and Discussion
5. Materials and Methods
5.1. Reagents and Materials
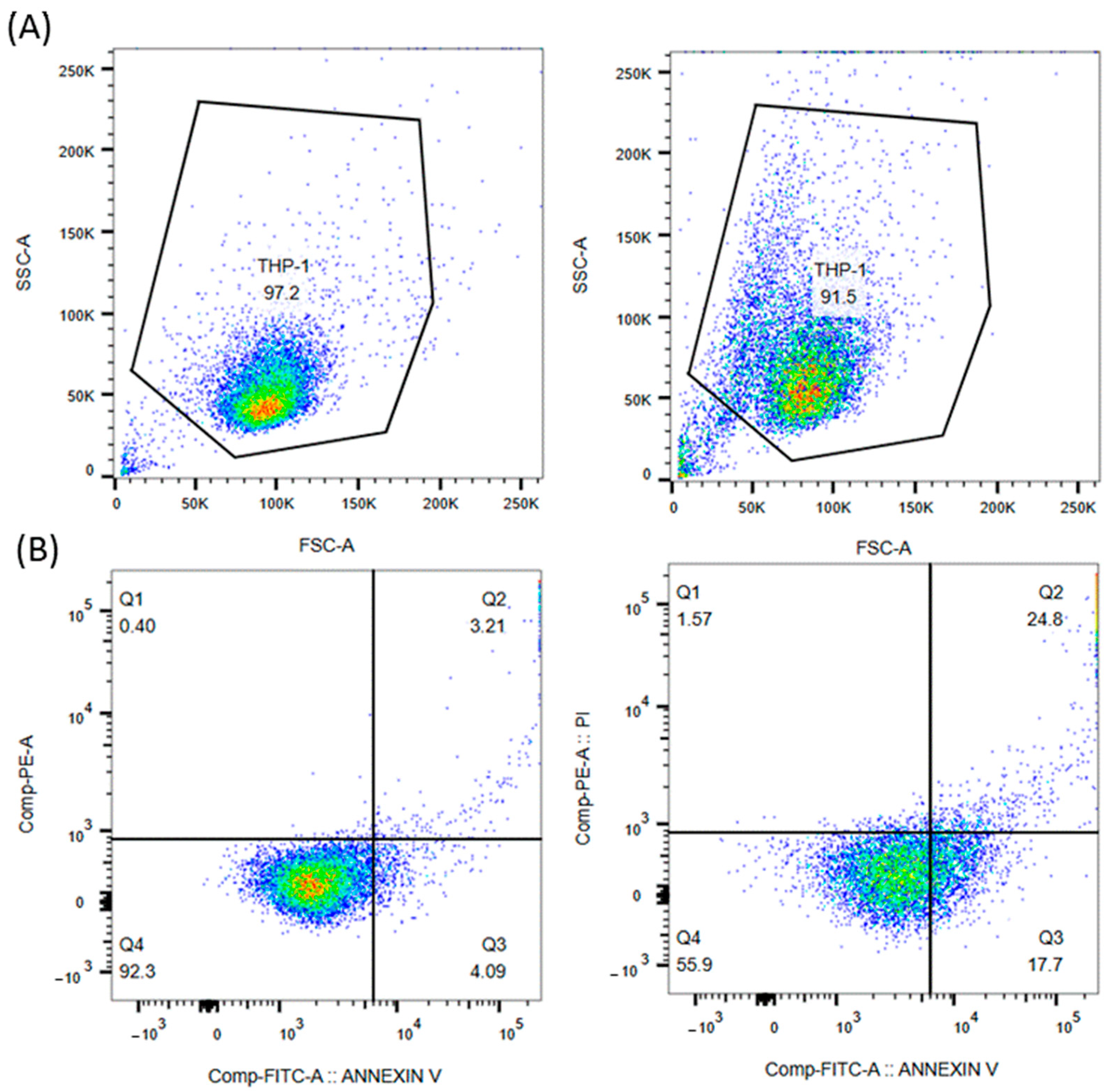
5.2. Cell Line
5.3. Apoptosis Assay
5.4. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Food Safety Authority. Scientific opinion on dietary reference values for fluoride. EFSA J. 2013, 11, 3332–3378. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academy Press: Washington, DC, USA, 1997. [Google Scholar]
- Aoba, T.; Fejerskov, O. Dental fluorosis: Chemistry and biology. Crit. Rev. Oral Biol. Med. 2002, 13, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Tadano, M.; Asanuma, S.; Tamura, K.; Matsushima, S.; Watanabe, T.; Kondo, T.; Sakurai, S.; Ji, R.; Liang, C.; et al. Health effects of indoor fluoride pollution from coal burning in China. Environ. Health Perspect. 1998, 106, 239–244. [Google Scholar] [CrossRef]
- Yi, J.; Cao, J. Tea and fluorosis. J. Fluor. Chem. 2008, 129, 76–81. [Google Scholar] [CrossRef]
- Qin, X.; Wang, S.; Yu, M.; Zhang, L.; Li, X.; Zuo, Z.; Zhang, X.; Wang, L. Child skeletal fluorosis from indoor burning of coal in southwestern China. J. Environ. Public Health 2009, 2009, 969764. [Google Scholar] [CrossRef]
- Kakumanu, N.; Rao, S.D. Images in clinical medicine. Skeletal fluorosis due to excessive tea drinking. N. Engl. J. Med. 2013, 368, 1140. [Google Scholar] [CrossRef]
- Kebede, A.; Retta, N.; Abuye, C.; Whiting, S.J.; Kassaw, M.; Zeru, T.; Tessema, M.; Kjellevold, M. Dietary fluoride intake and associated skeletal and dental fluorosis in school age children in rural Ethiopian Rift Valley. Int. J. Environ. Res. Public Health 2016, 13, 756. [Google Scholar] [CrossRef]
- Yang, N.; Tang, S.; Zhang, S.; Huang, W.; Chen, P.; Chen, Y.; Xi, Z.; Yuan, Y.; Wang, K. Fluorine in Chinese coal: A review of distribution, abundance, modes of occurrence, genetic factors and environmental effects. Minerals 2017, 7, 219. [Google Scholar] [CrossRef]
- Jin, C.; Yan, Z.; Jian-Wei, L.; Ruoden, X.; Sangbu, D.; Zhouma, Z.; Zhouma, S. Prevention and control of brick-tea type fluorosis—A 3-year observation in Dangxiong, Tibet. Ecotoxicol. Environ. Saf. 2003, 56, 222–227. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States, 1900–1999. MMWR Wkly. 1999, 48, 241–243. [Google Scholar]
- CDC Community Water Fluoridation. Available online: https://www.cdc.gov/fluoridation/php/statistics/2020-water-fluoridation-statistics.html (accessed on 25 November 2024).
- Scientific Committee on Health and Environmental Risks Scientific Committee on Health and Environmental Risks (SCHER). Critical Review of any New Evidence on the Hazard Profile, Health Effects, and Human Exposure to Fluoride and the Fluoridating Agents of Drinking Water; European Commission: Brussels, Belgium, 2010.
- Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Organic fluorine compounds: A great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.J.; Levy, S.M.; Broffitt, B.; Cavanaugh, J.E.; Kanellis, M.J.; Weber-Gasparoni, K. Considerations on optimal fluoride intake using dental fluorosis and dental caries outcomes-A longitudinal study. J. Public Health Dent. 2009, 69, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kiss, L.; Mei, H.; Remete, A.M.; Ponikvar-Svet, M.; Sedgwick, D.M.; Roman, R.; Fustero, S.; Moriwaki, H.; Soloshonok, V.A. Chemical aspects of human and environmental overload with fluorine. Chem. Rev. 2021, 121, 4678–4742. [Google Scholar] [CrossRef] [PubMed]
- Susheela, A.K. Fluoride and fluorosis mitigation: Indian contributions and its impact. In Water and Sanitation in the New Millennium; Nath, K.J., Sharma, V.P., Eds.; Springer: New Delhi, India, 2017; pp. 97–105. [Google Scholar]
- Jia, B.; Zong, L.; Lee, J.Y.; Lei, J.; Zhu, J.; Xie, H.; Clemens, J.L.; Feller, M.C.; Na, Q.; Dong, J.; et al. Maternal supplementation of low dose fluoride alleviates adverse perinatal outcomes following exposure to intrauterine inflammation. Sci. Rep. 2019, 9, 2575. [Google Scholar] [CrossRef]
- Grandjean, P. Developmental fluoride neurotoxicity: An updated review. Environ. Health Glob. Access Sci. Source 2019, 18, 110. [Google Scholar] [CrossRef]
- Barbier, O.; Arreola-Mendoza, L.; Del Razo, L.M. Molecular mechanisms of fluoride toxicity. Chem.-Biol. Interact. 2010, 188, 319–333. [Google Scholar] [CrossRef]
- Agalakova, N.I.; Gusev, G.P. Molecular mechanisms of cytotoxicity and apoptosis induced by inorganic fluoride. ISRN Cell Biol. 2012, 2012, 403835. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Cardoso, C.M.; Yujra, V.Q.; Viana, M.D.B.; Aguiar, O., Jr.; Pisani, L.P.; Oshima, C.T.F. Fluoride induces apoptosis in mammalian cells: In vitro and in vivo studies. Anticancer Res. 2017, 37, 4767–4777. [Google Scholar] [CrossRef]
- Wei, W.; Pang, S.; Sun, D. The pathogenesis of endemic fluorosis: Research progress in the last 5 years. J. Cell. Mol. Med. 2019, 23, 2333–2342. [Google Scholar] [CrossRef]
- Bigay, J.; Deterre, P.; Pfister, C.; Chabre, M. Fluoride complexes of aluminium or beryllium act on G-proteins as reversibly bound analogues of the gamma phosphate of GTP. EMBO J. 1987, 6, 2907–2913. [Google Scholar] [CrossRef]
- Chabre, M. Aluminofluoride and beryllofluoride complexes: New phosphate analogs in enzymology. Trends Biochem. Sci. 1990, 15, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Li, L. The biochemistry and physiology of metallic fluoride: Action, mechanism, and implications. Crit. Rev. Oral Biol. Med. 2002, 14, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Goličnik, M. Metallic fluoride complexes as phosphate analogues for structural and mechanistic studies of phosphoryl group transfer enzymes. Acta Chim. Slov. 2010, 57, 272–287. [Google Scholar]
- Bigay, J.; Deterre, P.; Pfister, C.; Chabre, M. Fluoroaluminates activate transducin-GDP by mimicking the γ-phosphate of GTP in its binding site. FEBS Lett. 1985, 191, 181–185. [Google Scholar] [CrossRef]
- Mullenix, P.J. A new perspective on metals and other contaminants in fluoridation chemicals. Int. J. Occup. Environ. Health 2014, 20, 157–166. [Google Scholar] [CrossRef]
- Jin, Y.; Richards, N.G.; Waltho, J.P.; Blackburn, G.M. Metal fluorides as analogues for studies on phosphoryl transfer enzymes. Angew. Chem. Int. Ed. 2017, 56, 4110–4128. [Google Scholar] [CrossRef]
- Lubkowska, A.; Zyluk, B.; Chlubek, D. Interactions between fluorine and aluminum. Fluoride 2002, 35, 73–77. [Google Scholar]
- Akinrinade, I.D.; Ogundele, O.M.; Memudu, A.E.; Adefule, A.K.; Kalejaiye, E.D. Degeneration of neuronal cells: A product of fluoride and aluminium assault to the prefrontal cortex. J. Cell Anim. Biol. 2013, 7, 63–66. [Google Scholar]
- Akinrinade, I.D.; Ogundele, O.M.; Memudu, A.E.; Obia, S. Cytoarchitecture of the cerebellum in fluoride and aluminium toxicity. J. Cell Anim. Biol. 2013, 7, 67–72. [Google Scholar] [CrossRef]
- Akinrinade, I.D.; Memudua, A.E.; Ogundelec, O.M.; Ajetunmobi, O.I. Interplay of glia activation and oxidative stress formation in fluoride and aluminium exposure. Pathophysiology 2015, 22, 39–48. [Google Scholar] [CrossRef]
- Akinrinade, I.D.; Memudu, A.E.; Ogundele, O.M. Fluoride and aluminium disturb neuronal morphology, transport functions, cholinesterase, lysosomal and cell cycle activities. Pathophysiology 2015, 22, 105–115. [Google Scholar] [CrossRef]
- Nalagoni, C.S.R.; Karnati, P.R. Protective effect of resveratrol against neuronal damage through oxidative stress in cerebral hemisphere of aluminum and fluoride treated rats. Interdiscip. Toxicol. 2016, 9, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.D.; Tan, Y.; Luo, Y.; Wang, W.J.; Zhang, H.; Xie, C. MiR-132, miR-204 and BDNF-TrkB signaling pathway may be involved in spatial learning and memory impairment of the offspring rats caused by fluorine and aluminum exposure during the embryonic stage and into adulthood. Environ. Toxicol. Pharmacol. 2018, 63, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.D.; Xie, C.; Zhang, H.; Tan, Y.; Wan, C.W.; Wang, W.J.; Jin, T.X. Differential expression of miRNAs in the hippocampi of offspring rats exposed to fluorine combined with aluminum during the embryonic stage and into adulthood. Biol. Trace Elem. Res. 2019, 189, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Kinaway, A.A. Potential toxicity of aluminum and fluoride on some biochemical aspects of male rat’s offspring. J. Basic Appl. Zool. 2019, 80, 17. [Google Scholar] [CrossRef]
- Dong, C.; Cao, J.; Cao, C.; Han, Y.; Wu, S.; Wang, S.; Wang, J. Effects of fluoride and aluminum on expressions of StAR and P450scc of related steroidogenesis in guinea pigs’ testis. Chemosphere 2016, 147, 345–351. [Google Scholar] [CrossRef]
- Singh, D.P.; Kushwah, K. Assessment of toxicity of aluminum fluoride on some selected heamatological parameters of male Wistar rats. J. Sci. Technol. Res. 2022, 3, 12–15. [Google Scholar] [CrossRef]
- Bae, Y.C.; Lee, Y.E.; Kim, H.Y.; Choi, Y.H.; Song, K.B. Fluoride intake influences the distribution of aluminum in rats. Toxicol. Environ. Chem. 2010, 92, 1149–1156. [Google Scholar] [CrossRef]
- Wasana, H.M.S.; Perera, G.D.R.K.; De Gunawardena, P.S.; Bandara, J. The impact of aluminum, fluoride, and aluminum–fluoride complexes in drinking water on chronic kidney disease. Environ. Sci. Pollut. Res. 2015, 22, 11001–11009. [Google Scholar] [CrossRef]
- Hoorang, A.; Saberzadeh, J.; Iranpak, F.; Mohammadi-Bardbori, A.; Khorsand, M.; Takhshidb, M.A. The effect of sodium fluoride on aluminum-induced oxidative stress and apoptosis of PC12 in vitro. Fluoride 2020, 53, 276–289. [Google Scholar]
- Peng, Z.; Yang, X.; Zhang, H.; Yin, M.; Luo, Y.; Xie, C. MiR-29b-3p aggravates NG108-15 cell apoptosis triggered by fluorine combined with aluminum. Ecotoxicol. Environ. Saf. 2021, 224, 112658. [Google Scholar] [CrossRef] [PubMed]
- Russ, T.C.; Killin, L.O.J.; Hannah, J.; Batty, G.D.; Deary, I.J.; Starr, J.M. Aluminium and fluoride in drinking water in relation to later dementia risk. Br. J. Psychiatry 2020, 216, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Still, C.N.; Kelley, P. Incidence of primary degenerative dementia vs. water fluoride content in South Carolina. Neurotoxicology 1980, 1, 125–131. [Google Scholar]
- Janžič, L.; Repas, J.; Pavlin, M.; Zemljić-Jokhadar, Š.; Ihan, A.; Kopitar, A.N. Macrophage polarization during Streptococcus agalactiae infection is isolate specific. Front. Microbiol. 2023, 14, 1186087. [Google Scholar] [CrossRef]
- Luo, Q.; Cui, H.; Deng, H.; Kuang, P.; Liu, H.; Lu, Y.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; et al. Histopathological findings of renal tissue induced by oxidative stress due to different concentrations of fluoride. Oncotarget 2017, 8, 50430–50446. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; p. 14. [Google Scholar]
- Pavlovič, A.; Tavčar, G.; Ponikvar-Svet, M. Fluoride and aluminium in tea (Camellia sinensis L.)—Tea quality indicators and risk factors for consumers. Molecules 2023, 28, 6396. [Google Scholar] [CrossRef]
- Štepec, D.; Ponikvar-Svet, M. Fluoride in human health and nutrition. Acta Chim. Slov. 2019, 66, 255–275. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Ponikvar, M. Exposure of humans to fluorine and its assessment. In Fluorine and Health: Molecular Imaging, Biomedical Materials and Pharmaceuticals, 1st ed.; Tressaud, A., Haufe, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 488–549. [Google Scholar]
- Abuhelwa, A.Y.; Foster, D.J.R.; Upton, R.N. A quantitative review and meta-models of the variability and factors affecting oral drug absorption—Part I: Gastrointestinal pH. AAPS J. 2016, 18, 1309–1321. [Google Scholar] [CrossRef]
- Whitford, G.M.; Pashley, D.H. Fluoride absorption: The influence of gastric acidity. Calcif. Tissue Int. 1984, 36, 302–307. [Google Scholar] [CrossRef]
- Nopakun, J.; Messer, H.H.; Voller, V. Fluoride absorption from the gastrointestinal tract of rats. J. Nutr. 1989, 119, 1411–1417. [Google Scholar] [CrossRef]
- Nopakun, J.; Messer, H.H. Mechanism of fluoride absorption from the rat small intestine. Nutr. Res. 1990, 10, 771–779. [Google Scholar] [CrossRef]
- Dharmaratne, R.W. Exploring the role of excess fluoride in chronic kidney disease: A review. Hum. Exp. Toxicol. 2019, 38, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Flaten, T.P. Aluminium in tea—Concentrations, speciation and bioavailability. Coord. Chem. Rev. 2002, 228, 385–395. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). European Food Safety Authority. Safety of aluminium from dietary intake—Scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC). EFSA J. 2008, 6, 754. [Google Scholar] [CrossRef]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium toxicosis: A review of toxic actions and effects. Interdiscip. Toxicol. 2019, 12, 45–70. [Google Scholar] [CrossRef]
- Wills, M.R.; Savory, J. Aluminum and chronic renal failure: Sources, absorption, transport, and toxicity. Crit. Rev. Clin. Lab. Sci. 1989, 27, 59–107. [Google Scholar] [CrossRef]
- Becaria, A.; Campbell, A.; Bondy, S. Aluminum as a toxicant. Toxicol. Ind. Health 2002, 18, 309–320. [Google Scholar] [CrossRef]
- Kandimalla, R.; Vallamkondu, J.; Corgiat, E.B.; Gill, K.D. Understanding aspects of aluminum exposure in Alzheimer’s disease development. Brain Pathol. 2016, 26, 139–154. [Google Scholar] [CrossRef]
- Martin, B. Ternary complexes of Al3+ and F− with a third ligand. Coord. Chem. Rev. 1996, 149, 23–32. [Google Scholar] [CrossRef]
- Marion, S.P.; Thomas, A.W. Effect of diverse anions on the pH of maximum precipitation of “aluminum hydroxide”. J. Colloid Sci. 1946, 1, 221–234. [Google Scholar] [CrossRef]
- Sternweis, P.C.; Gilman, A.G. Aluminum: A requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc. Natl. Acad. Sci. USA 1982, 79, 4888–4891. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.J.; Girardet, J.L.; Morat, C. Multinuclear NMR study of fluoroaluminate complexes in aqueous solution. Inorg. Chem. 1996, 35, 706–710. [Google Scholar] [CrossRef]
- Schlichting, I.; Reinstein, J. pH influences fluoride coordination number of the AlFx phosphoryl transfer transition state analog. Nat. Struct. Mol. Biol. 1999, 6, 721–723. [Google Scholar] [CrossRef]
- Cadiau, A.; Le Bail, A.; Hemon-Ribaud, A.; Leblanc, M.; Body, M.; Fayon, F.; Durand, E.; Boulou, J.C.; Maisonneuve, V. Evolution of guanazolium fluoroaluminates within the composition-space diagram and with the temperature. Cryst. Growth Des. 2010, 10, 5159–5168. [Google Scholar] [CrossRef]
- Latimer, W.M.; Jolly, W.L. Heats and entropies of successive steps in the formation of AlF6---. J. Am. Chem. Soc. 1953, 75, 1548–1550. [Google Scholar] [CrossRef]
- Bodor, A.; Toth, I.; Banyai, I.; Szabo, Z.; Hefter, G.T. 19F NMR study of the equilibria and dynamics of the Al3+/F− system. Inorg. Chem. 2000, 39, 2530–2537. [Google Scholar] [CrossRef]
- Yu, P.; Phillips, B.L.; Casey, W.H. Water exchange in fluoroaluminate complexes in aqueous solution: A variable temperature multinuclear NMR study. Inorg. Chem. 2001, 40, 4750–4754. [Google Scholar] [CrossRef]
- Burguera, S.; Vidal, L.; Bauzá, A. Aluminum fluorides as noncovalent Lewis acids in proteins: The case of phosphoryl transfer enzymes. ChemPlusChem 2024, 90, e202400578. [Google Scholar] [CrossRef]
- Heinemann, C.; Schröder, D.; Schwarz, H. Generation of the thermodynamically stable dications AlF2+ and SiF2+ via charge-stripping mass spectrometry. J. Phys. Chem. 1995, 99, 16195–16198. [Google Scholar] [CrossRef]
- Vasiliev, A.D.; Melnikova, S.V.; Isaenko, L.I. Orthorhombic aluminum oxyfluoride, AlOF. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2009, 65, i20–i22. [Google Scholar] [CrossRef]
- Wen, M.; Medel, R.; Deng, G.; Tsegaw, Y.A.; Lu, Y.; Riedel, S. Infrared spectroscopic and theoretical investigations of group 13 oxyfluorides OMF2 and OMF (M = B, Al, Ga, In). Chem. Eur. J. 2023, 29, e202301676. [Google Scholar] [CrossRef] [PubMed]
- Bachet, B.; Cesbron, F.; Chevalier, R. Crystal structure of khademite Al(SO4)F.5H2O. Bull. Mineral. 1981, 104, 19–22. [Google Scholar]
- Shetty, D.; Choi, S.Y.; Jeong, J.M.; Lee, J.Y.; Hoigebazar, L.; Lee, Y.S.; Lee, D.S.; Chung, J.K.; Lee, M.C.; Chung, Y.K. Stable aluminum fluoride chelates with triazacyclononane derivatives proved by X-ray crystallography and 18F-labeling study. Chem. Commun. 2011, 47, 9732–9734. [Google Scholar] [CrossRef]
- Brant, P.; Weber, D.C.; Haupt, S.G.; Nohr, R.S.; Wynne, K.J. Partially oxidized group 3A fluorometallophthalocyanines. J. Chem. Soc. Dalton Trans. 1985, 2, 269–274. [Google Scholar] [CrossRef]
- Kitao, S.; Seto, M.; Maeda, Y.; Hiromitsu, I. Mossbauer study of iodine-doped fluoro-aluminum phthalocyanine. J. Phys. Soc. Jpn. 1997, 66, 850–852. [Google Scholar] [CrossRef]
- Grant, T.M.; McIntyre, V.; Vestfrid, J.; Raboui, H.; White, R.T.; Lu, Z.H.; Lessard, B.H.; Bender, T.P. Straightforward and relatively safe process for the fluoride exchange of trivalent and tetravalent group 13 and 14 phthalocyanines. ACS Omega 2019, 4, 5317–5326. [Google Scholar] [CrossRef]
- Yang, Y. Effects induced by axial ligands binding to tetrapyrrole-based aromatic metallomacrocycles. J. Phys. Chem. A 2011, 115, 9043–9054. [Google Scholar] [CrossRef]
- Hiromitsu, I.; Ohkubo, S.; Takeuchi, J.; Ito, T. Crystal structure of the antiferromagnetic phase in AlPcF-Ix. Solid State Commun. 1994, 89, 605–610. [Google Scholar] [CrossRef]
- Hiromitsu, I.; Yamamoto, H.; Ito, T. Three-dimensional antiferromagnetic transition of iodine-doped fluoro-aluminum phthalocyanine with a disordered polymer chain. Phys. Rev. B 1995, 52, 7252–7259. [Google Scholar] [CrossRef]
- Schmidt, A.; Schlaf, R.; Louder, D.; Chau, L.K.; Chen, S.Y.; Fritz, T.; Lawrence, M.F.; Parkinson, B.A.; Armstrong, N.R. Epitaxial growth of the ionic polymer fluoroaluminum phthalocyanine on the basal plane of single crystal tin disulfide. Chem. Mater. 1995, 7, 2127–2135. [Google Scholar] [CrossRef]
- Fuller, C.A.; Quintero-Castro, D.L.; Bosak, A.; Dyadkin, V.; Chernyshov, D. Correlated disorder and crystal structure of β-VOSO4, Correlated disorder and crystal structure of β-VOSO4. Acta Cryst. B 2022, 78, 842–847. [Google Scholar] [CrossRef]
- Pozin, M.E.; Kopylev, B.A.; Zinyuk, R.Y. Formation of aluminum fluorocomplexes during the acid treatment of phosphates. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 1963, 6, 98–105. [Google Scholar]
- Nikolaev, N.S. Aluminum fluorosulfate and its use in preparing fluorides. Zhur. Khim. Prom. 1937, 14, 1087–1097. [Google Scholar]
- Lynton, H.; Passmore, J. Crystal and molecular structure of difluoro chlorine(III) hexafluoroarsenate(V), ClF2AsF6. Canad. J. Chem. 1971, 49, 2539–2543. [Google Scholar] [CrossRef]
- Edwards, A.J.; Jones, G.R. Fluoride crystal structures. VI. Difluorobromine(III) hexafluoroantimonate(V). J. Chem. Soc. A 1969, 1467–1470. [Google Scholar] [CrossRef]
- Christe, K.O.; Guertin, J.P. Structure of the tetrafluorochlorate(III) anion, ClF4−. Inorg. Chem. 1966, 5, 473–476. [Google Scholar] [CrossRef]
- Edwards, A.J.; Jones, G.R. Fluoride crystal structures. VIII. Neutron diffraction studies of potassium tetrafluorobromate(III) and potassium tetrafluoroaurate(III). J. Chem. Soc. A 1996, 1936–1938. [Google Scholar] [CrossRef]
- Gonsior, M.; Krossing, I.; Mitzel, N. A thallium coated dianion: Trigonal bipyramidal [F2Al(OR)3]2− coordinated to three Tl+ cations in the ion pair [Tl3F2Al(OR)3]+[Al(OR)4]− [R = CH(CF3)2]. Z. Anorg. Allgem. Chem. 2002, 628, 1821–1830. [Google Scholar] [CrossRef]
- Dyke, J.M.; Kirby, C.; Morris, A.; Gravenor, B.W.J.; Klein, R.; Rosmus, P. A study of aluminium monofluoride and aluminium trifluoride by high-temperature photoelectron spectroscopy. Chem. Phys. 1984, 88, 289–298. [Google Scholar] [CrossRef]
- Nakamoto, M.; Yamasaki, T.; Sekiguchi, A. Stable mononuclear radical anions of heavier group 13 elements: [(tBu2MeSi)3E−]·[K−(2.2.2-Cryptand)] (E = Al, Ga). J. Am. Chem. Soc. 2005, 127, 6954–6955. [Google Scholar] [CrossRef]
- Kemnitz, E.; Gross, U.; Rüdiger, S.; Scholz, G.; Heidemann, D.; Troyanov, S.I.; Morosov, I.V.; Lemée-Cailleau, M.H. Comparative structural investigation of aluminium fluoride solvates. Solid Sate Sci. 2006, 8, 143–152. [Google Scholar] [CrossRef]
- Herron, N.; Harlow, R.L.; Thorn, D.L. Novel coordination geometries in fluoroaluminate salts. Inorg. Chem. 1993, 32, 2985–2986. [Google Scholar] [CrossRef]
- Herron, N.; Thorn, D.L.; Harlow, R.L.; Davidson, F. Organic cation salts of the tetrafluoroaluminate anion. Yes, it does exist, and yes, it is tetrahedral. J. Am. Chem. Soc. 1993, 115, 3028–3029. [Google Scholar] [CrossRef]
- Cadiau, A.; Adil, K.; Hemon-Ribaud, A.; Leblanc, M.; Jouanneaux, A.; Slawin, A.M.Z.; Lightfoot, P.; Maisonneuve, V. Fluoroaluminates of purine and DNA bases, adenine, guanine. [Hpur]2.(AlF5), [Hade]3.(AlF6).6.5H2O, [Hguan]3.(Al3F12). Solid State Sci. 2011, 13, 151–157. [Google Scholar] [CrossRef]
- Gross, U.; Mueller, D.; Kemnitz, E. Coordination geometry of the discrete pentafluoroaluminate dianion [AlF5]2−. Angew. Chem. Int. Ed. 2003, 42, 2626–2629. [Google Scholar] [CrossRef] [PubMed]
- Veryasov, G.; Matsumoto, K.; Hagiwara, R. The discrete AlF52− fluoroaluminate anion in the structure of [tetraethylammonium]2[AlF5](H2O)2. Eur. J. Inorg. Chem. 2015, 32, 5306–5310. [Google Scholar] [CrossRef]
- de Kozak, A.; Gredin, P.; Pierrard, A.; Renaudin, J. The crystal structure of a new form of the dipotassium pentafluoroaluminate hydrate, K2AlF5·H2O, and of its dehydrate, K2AlF5. J. Fluor. Chem. 1996, 77, 39–44. [Google Scholar] [CrossRef]
- Griffiths, J.E.; Carter, R.P., Jr.; Holmes, R.R. Molecular structures of PCl4F, PCl3F2, PCl2F3, and PF5: Infrared and low-temperature Raman vibrational spectra. J. Chem. Phys. 1964, 41, 863–876. [Google Scholar] [CrossRef]
- Hansen, K.W.; Bartell, L.S. Electron diffraction study of the structure of PF5. Inorg. Chem. 1965, 4, 1775–1776. [Google Scholar] [CrossRef]
- Romanov, G.V.; Spiridonov, V.P. Electron diffraction study of phosphorus pentafluoride and pentachloride. Zhur. Strukt. Khim. 1967, 8, 159–160. [Google Scholar]
- Clark, D.; Powell, H.M.; Wells, A.F. Crystal structure of phosphorus pentachloride. J. Chem. Soc. 1942, 145, 642–645. [Google Scholar] [CrossRef]
- Ripmeester, J.A.; Wong, P.T.T.; Davidson, D.W.; Whalley, E. The ionization of hexachlorophosphate in the solid state under pressure. III. Phosphorus-31 NMR spectra of three phases of phosphorus pentachloride. J. Chem. Phys. 1979, 70, 5545–5549. [Google Scholar] [CrossRef]
- Finch, A.; Gates, P.N.; Jenkins, H.D.B.; Thakur, K.P. Ionic isomerism in phosphorus(V) chloride. J. Chem. Soc. Chem. Commun. 1980, 12, 579–580. [Google Scholar] [CrossRef]
- Garton, G.; Wanklyn, B.M. Polymorphism in Li3AlF6. J. Inorg. Nucl. Chem. 1965, 27, 2466–2469. [Google Scholar] [CrossRef]
- Zhou, Q.; Kennedy, B.J. High-temperature powder synchrotron diffraction studies of synthetic cryolite Na3AlF6. J. Solid State Chem. 2004, 177, 654–659. [Google Scholar] [CrossRef]
- King, G.; Abakumov, A.M.; Woodward, P.M.; Llobet, A.; Tsirlin, A.A.; Batuk, D.; Antipov, E.V. The high-temperature polymorphs of K3AlF6. Inorg. Chem. 2011, 50, 7792–7801. [Google Scholar] [CrossRef]
- Holm, J. Phase transitions and structure of the high-temperature phases of some compounds of the cryolite family. Acta Chem. Scand. 1965, 19, 261–263. [Google Scholar] [CrossRef]
- Schwarzmann, S. The polymorphism of ammonium aluminum-, ammonium gallium-, and ammonium indium hexafluorides. Fortsch. Mineral. 1966, 42, 231. [Google Scholar]
- Hefter, G.; Bodor, A.; Töth, I. Does [AlF6]3− exist in aqueous solution? Austr. J. Chem. 2000, 53, 625–626. [Google Scholar] [CrossRef]
- Liebman, J.F.; Romm, M.J.; Meot-Ner, M.; Cybulski, S.M.; Scheiner, S. Isotropy in ionic interactions. 2. How spherical is the ammonium ion? Comparison of the gas-phase clustering energies and condensed-phase thermochemistry of K+ and NH4+. J. Phys. Chem. 1991, 95, 1112–1119. [Google Scholar] [CrossRef]
- Hourri, A.; St-Arnaud, J.M.; Bose, T.K. Dielectric and pressure virial coefficients of imperfect gases: CO2–SF6 mixtures. J. Chem. Phys. 1997, 106, 1780–1785. [Google Scholar] [CrossRef]
- Nicholson, L.B. The immune system. Essays Biochem. 2016, 60, 275. [Google Scholar] [CrossRef] [PubMed]
- Austermann, J.; Roth, J.; Barczyk-Kahlert, K. The good and the bad: Monocytes’ and macrophages’ diverse functions in inflammation. Cells 2022, 11, 1979. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Eubank, T.D.; Doseff, A.I. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J. Innate Immun. 2010, 2, 204. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Wlodkowic, D.; Telford, W.; Skommer, J.; Darzynkiewicz, Z. Apoptosis and beyond: Cytometry in studies of programmed cell death. Methods Cell Biol. 2011, 103, 55. [Google Scholar] [CrossRef]
- McGuigan, M.A. Chronic poisoning: Trace metals and others. In Goldman’s Cecil Medicine, 24th ed.; Forsmark, C.E., Goldman, L., Eds.; Elsevier: Philadelphia, PA, USA, 2012; Volume 1, pp. 88–95. [Google Scholar]
- He, H.; Wang, H.; Jiao, Y.; Ma, C.; Zhang, H.; Zhou, Z. Effect of sodium fluoride on the proliferation and gene differential expression in human RPMI8226 cells. Biol. Trace Elem. Res. 2015, 167, 11–17. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, A.; He, W.; He, P.; Xu, B.; Xia, T.; Chen, X.; Yang, K. Effects of fluoride on the expression of NCAM, oxidative stress, and apoptosis in primary cultured hippocampal neurons. Toxicology 2007, 236, 208–216. [Google Scholar] [CrossRef]
- Agalakova, N.I.; Gusev, G.P. Fluoride-induced death of rat erythrocytes in vitro. Toxicol. In Vitro 2011, 25, 1609–1618. [Google Scholar] [CrossRef]
- Francisco, L.F.V.; Baldivia, D.d.S.; Crispim, B.d.A.; Baranoski, A.; Klafke, S.M.F.F.; dos Santos, E.L.; Oliveira, R.J.; Barufatti, A. In vitro evaluation of the cytotoxic and genotoxic effects of Al and Mn in ambient concentrations detected in groundwater intended for human consumption. Ecotoxicol. Environ. Saf. 2023, 264, 1154154. [Google Scholar] [CrossRef]
- Francisco, L.F.V.; Baldivia, D.d.S.; Crispim, B.D.A.; Klafke, S.M.F.F.; de Castilho, P.F.; Viana, L.F.; Dos Santos, E.L.; de Oliveira, K.M.P.; Barufatti, A. Acute toxic and genotoxic effects of aluminum and manganese using in vitro models. Toxics 2021, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Tenan, M.R.; Nicolle, A.; Moralli, D.; Verbouwe, E.; Jankowska, J.D.; Durin, M.A.; Green, C.M.; Mandriota, S.J.; Sappino, A.P. Aluminum enters mammalian cells and destabilizes chromosome structure and number. Int. J. Mol. Sci. 2021, 22, 9515. [Google Scholar] [CrossRef] [PubMed]
- Shuhua, X.; Ziyou, L.; Ling, Y.; Fei, W.; Sun, G. A Role of fluoride on free radical generation and oxidative stress in BV-2 microglia cells. Mediat. Inflamm. 2012, 1, 102954. [Google Scholar] [CrossRef] [PubMed]
- Badran, G.; Grare, C.; Masson, J.D.; David, M.O.; Achour, D.; Guidice, J.M.L.; Garçon, G.; Crépeaux, G. Difference in the cellular response following THP-1 derived phagocytic monocyte cells exposure to commercial aluminum-based adjuvants and aluminum-containing vaccines. J. Trace Elem. Med. Biol. 2024, 83, 127394. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cui, H.; Deng, H.; Kuang, P.; Liu, H.; Lu, Y.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; et al. Sodium fluoride induces renal inflammatory responses by activating NF-ΚB signaling pathway and reducing anti-inflammatory cytokine expression in mice. Oncotarget 2017, 8, 80192. [Google Scholar] [CrossRef]
- Ligi, D.; Santi, M.; Croce, L.; Mannello, F. Aluminum induces inflammatory and proteolytic alterations in human monocytic cell line. J. Inorg. Biochem. 2015, 152, 190–198. [Google Scholar] [CrossRef]
- Van Engeland, M.; Ramaekers, F.C.S.; Schutte, B.; Reutelingsperger, P.M. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 1996, 24, 131–139. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlovič, A.; Janžič, L.; Sršen, L.; Kopitar, A.N.; Edwards, K.F.; Liebman, J.F.; Ponikvar-Svet, M. Mononuclear Aluminum–Fluoride Ions, AlFx(+/−)—Study of Plausible Frameworks of Complexes with Biomolecules and Their In Vitro Toxicity. Molecules 2025, 30, 389. https://doi.org/10.3390/molecules30020389
Pavlovič A, Janžič L, Sršen L, Kopitar AN, Edwards KF, Liebman JF, Ponikvar-Svet M. Mononuclear Aluminum–Fluoride Ions, AlFx(+/−)—Study of Plausible Frameworks of Complexes with Biomolecules and Their In Vitro Toxicity. Molecules. 2025; 30(2):389. https://doi.org/10.3390/molecules30020389
Chicago/Turabian StylePavlovič, Anja, Larisa Janžič, Lucija Sršen, Andreja Nataša Kopitar, Kathleen F. Edwards, Joel F. Liebman, and Maja Ponikvar-Svet. 2025. "Mononuclear Aluminum–Fluoride Ions, AlFx(+/−)—Study of Plausible Frameworks of Complexes with Biomolecules and Their In Vitro Toxicity" Molecules 30, no. 2: 389. https://doi.org/10.3390/molecules30020389
APA StylePavlovič, A., Janžič, L., Sršen, L., Kopitar, A. N., Edwards, K. F., Liebman, J. F., & Ponikvar-Svet, M. (2025). Mononuclear Aluminum–Fluoride Ions, AlFx(+/−)—Study of Plausible Frameworks of Complexes with Biomolecules and Their In Vitro Toxicity. Molecules, 30(2), 389. https://doi.org/10.3390/molecules30020389






