Effect of Different Harvest Time and Microwave Aging on Aroma Characteristics of Japanese Apricot Wine
Abstract
1. Introduction
2. Results and Analysis
2.1. Color Variations in the Maceration Process of Steeped Mume Wine
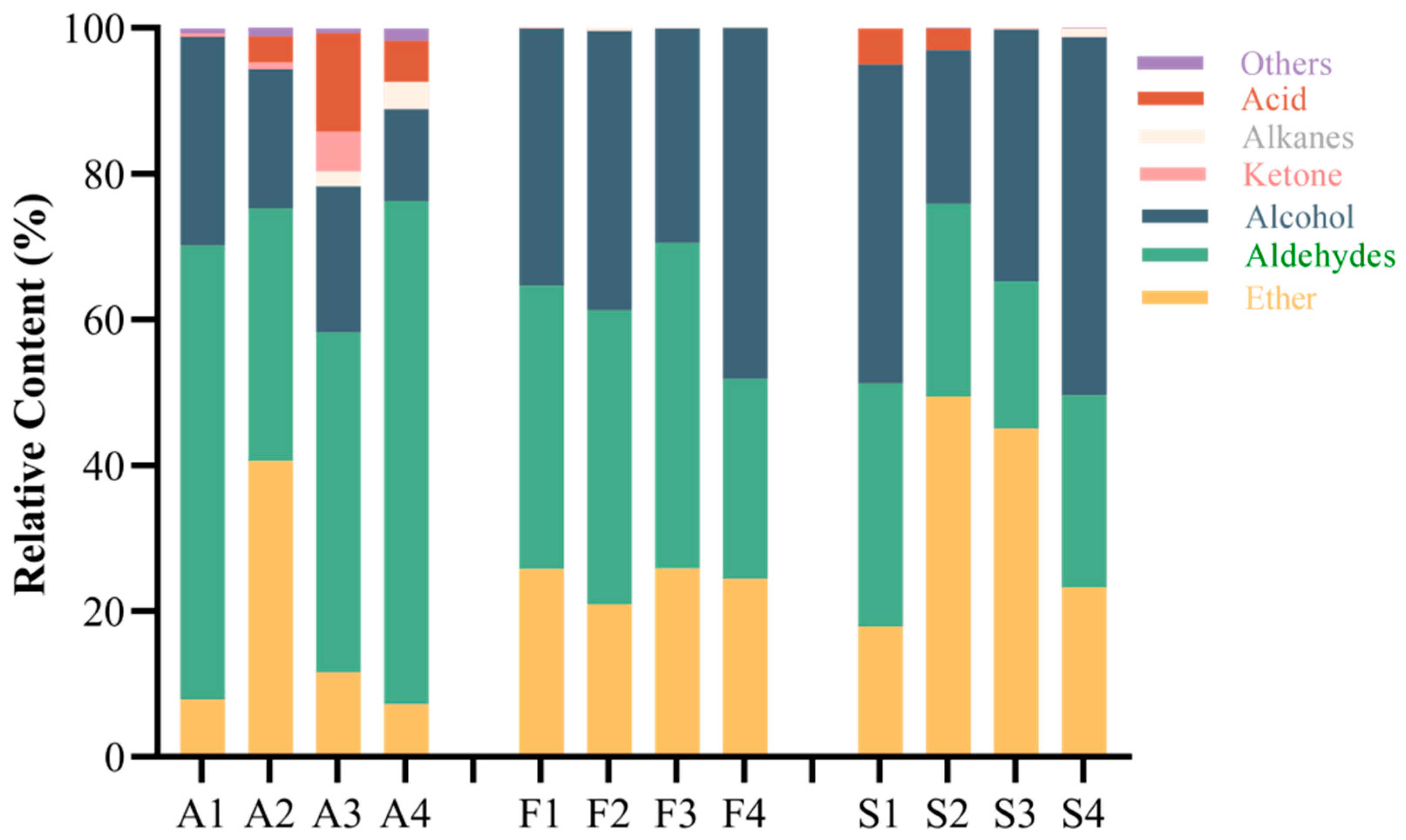
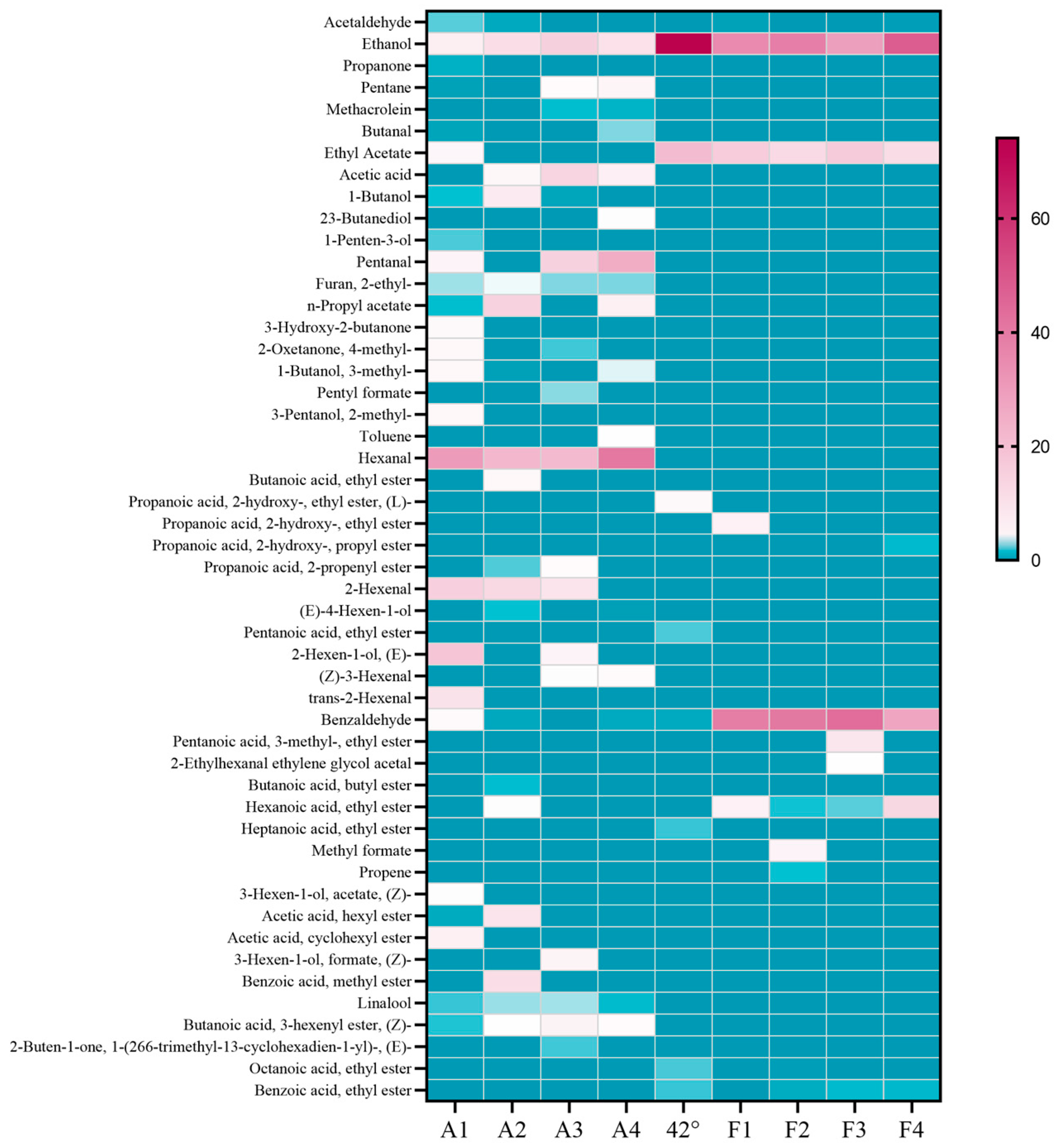
2.2. The Effect of Fruit Ripeness on the Volatile Components of Fruit and Steeped Mume Wine
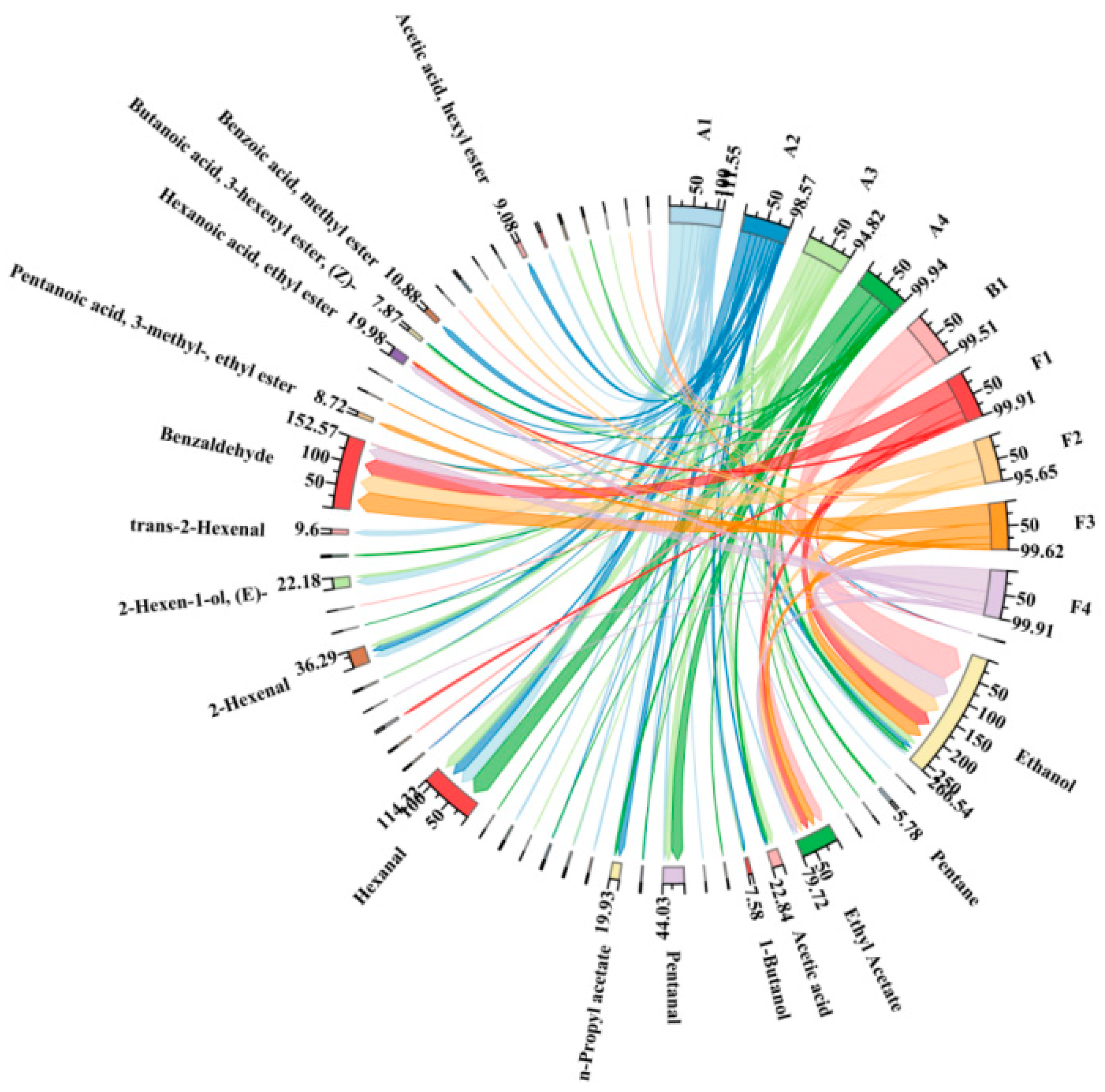
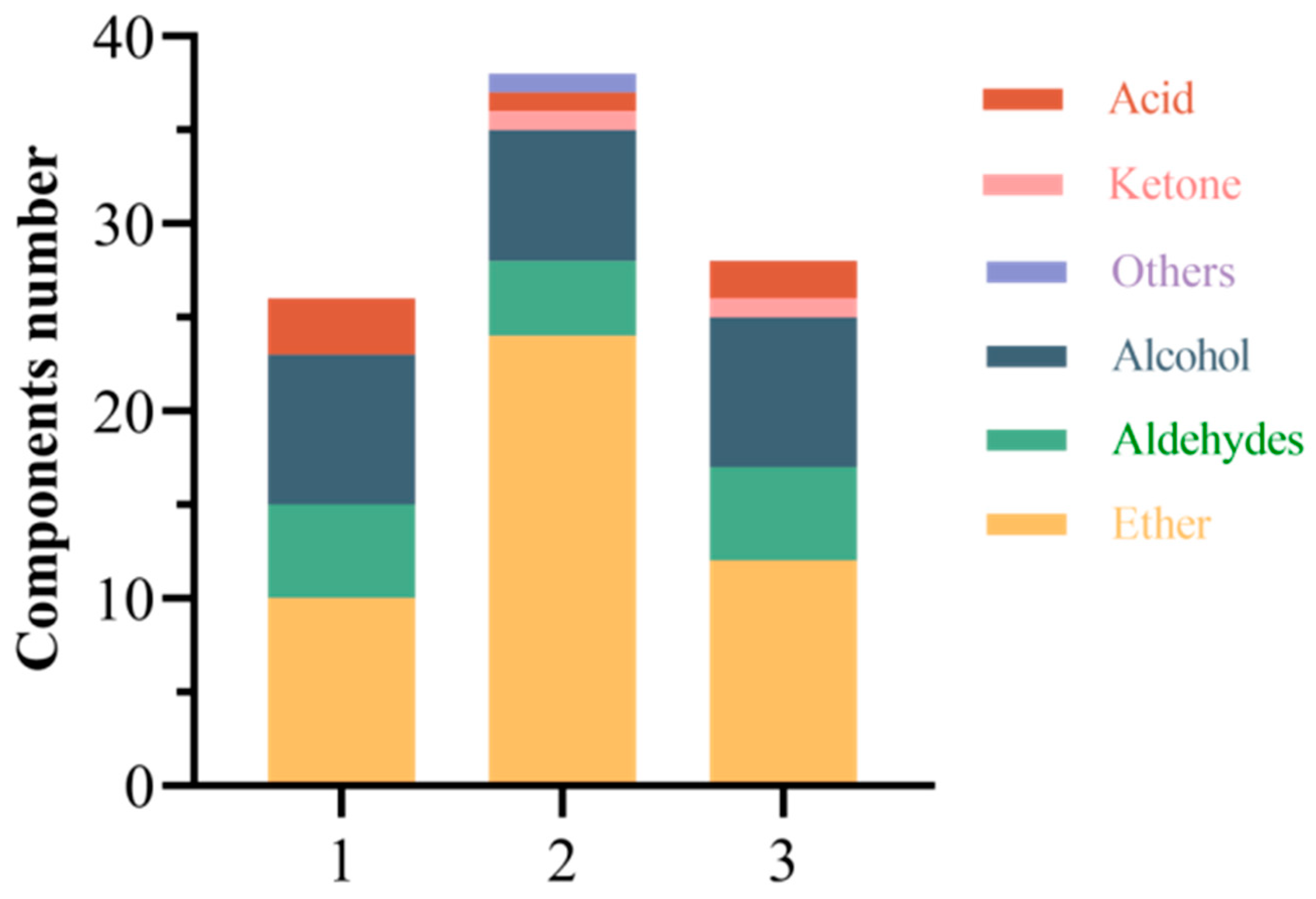
2.3. Effect of Different Fruit Development Periods on the Volatile Component of Fruit and Steeped Mume Wine
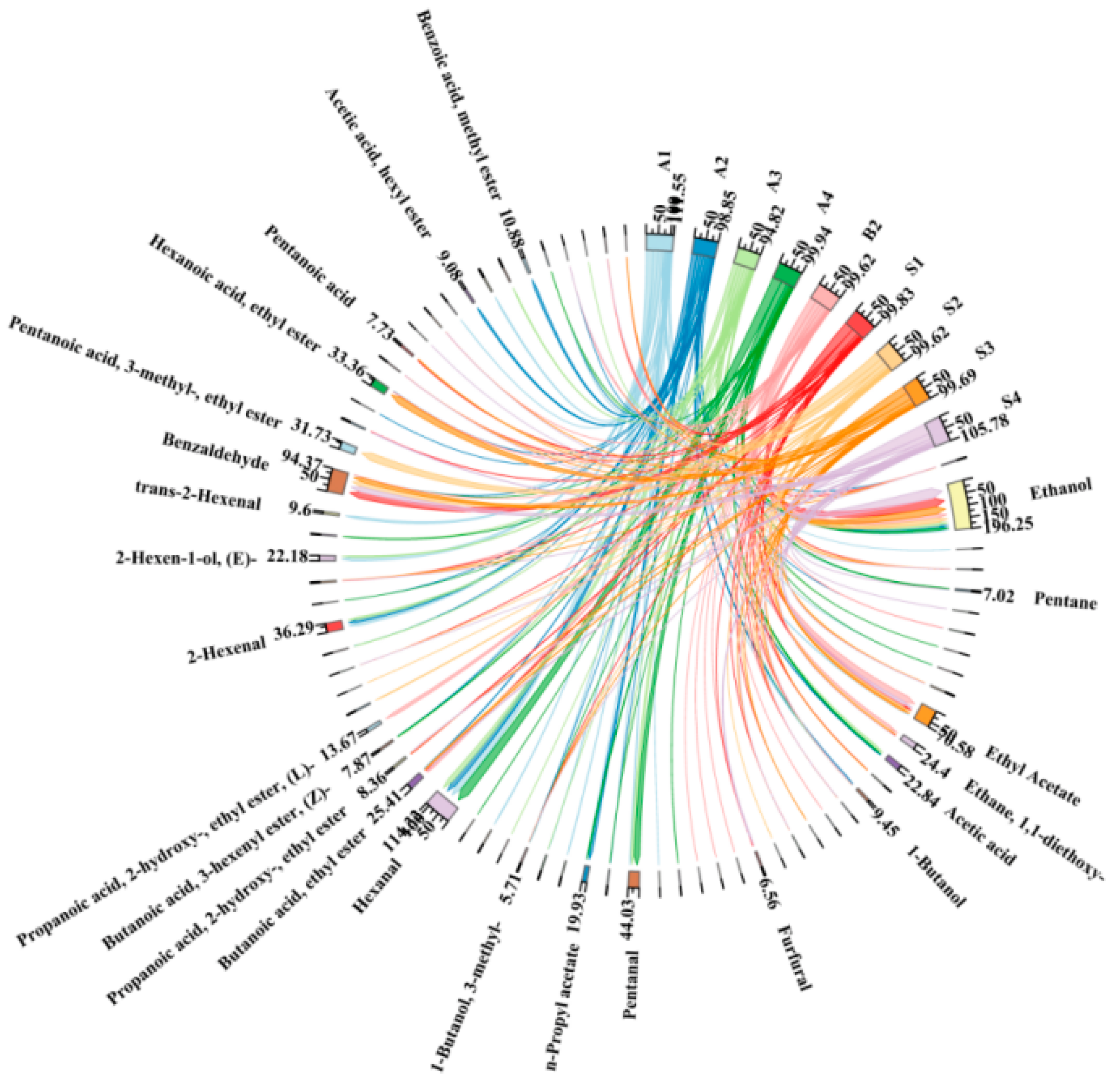
2.4. Changes in Aroma Substances During the Production of Steeped Mume Wine
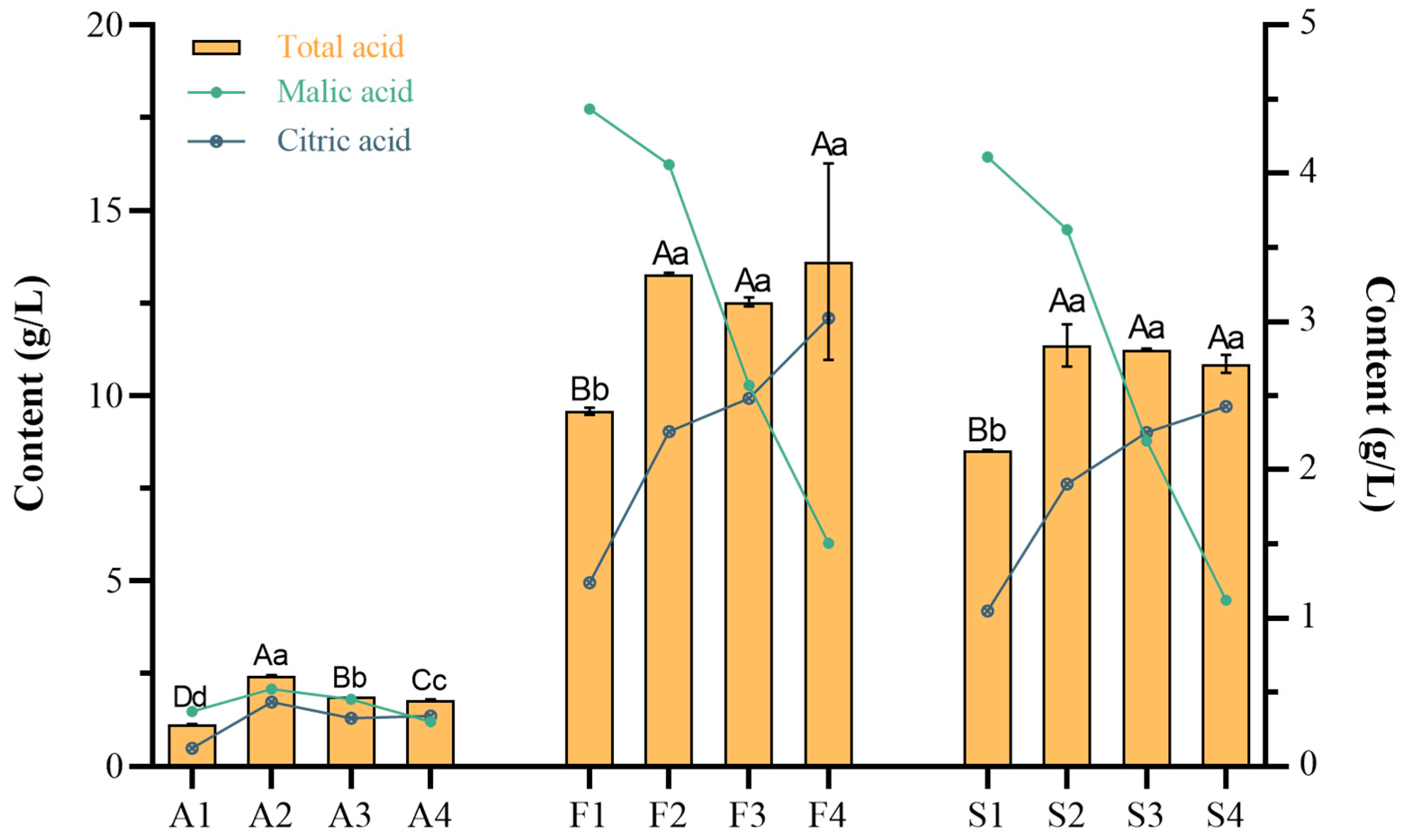
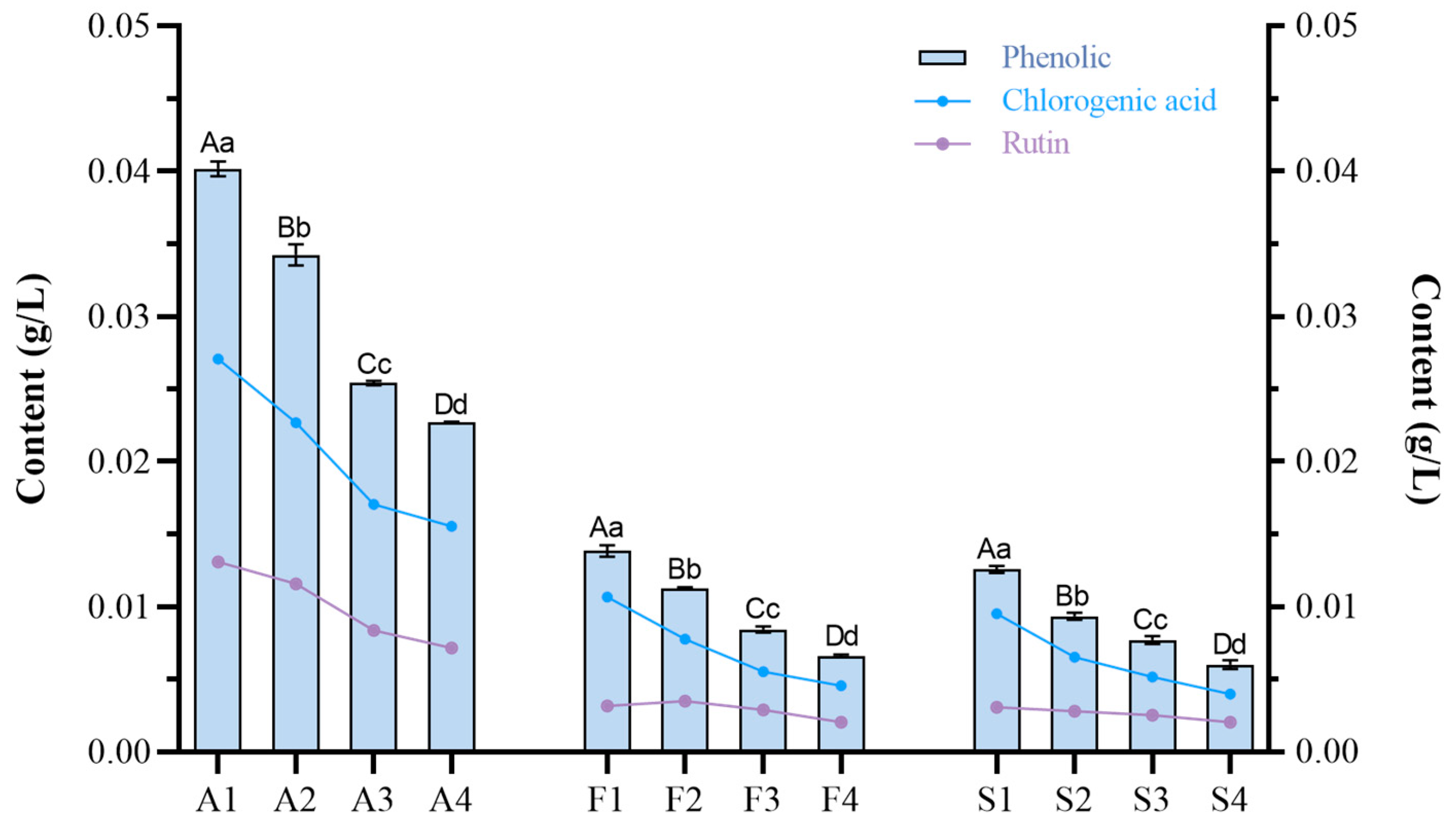
2.5. Effect of Different Fruit Development Periods on the Nutrition Content of Fruit and Steeped Mume Wine
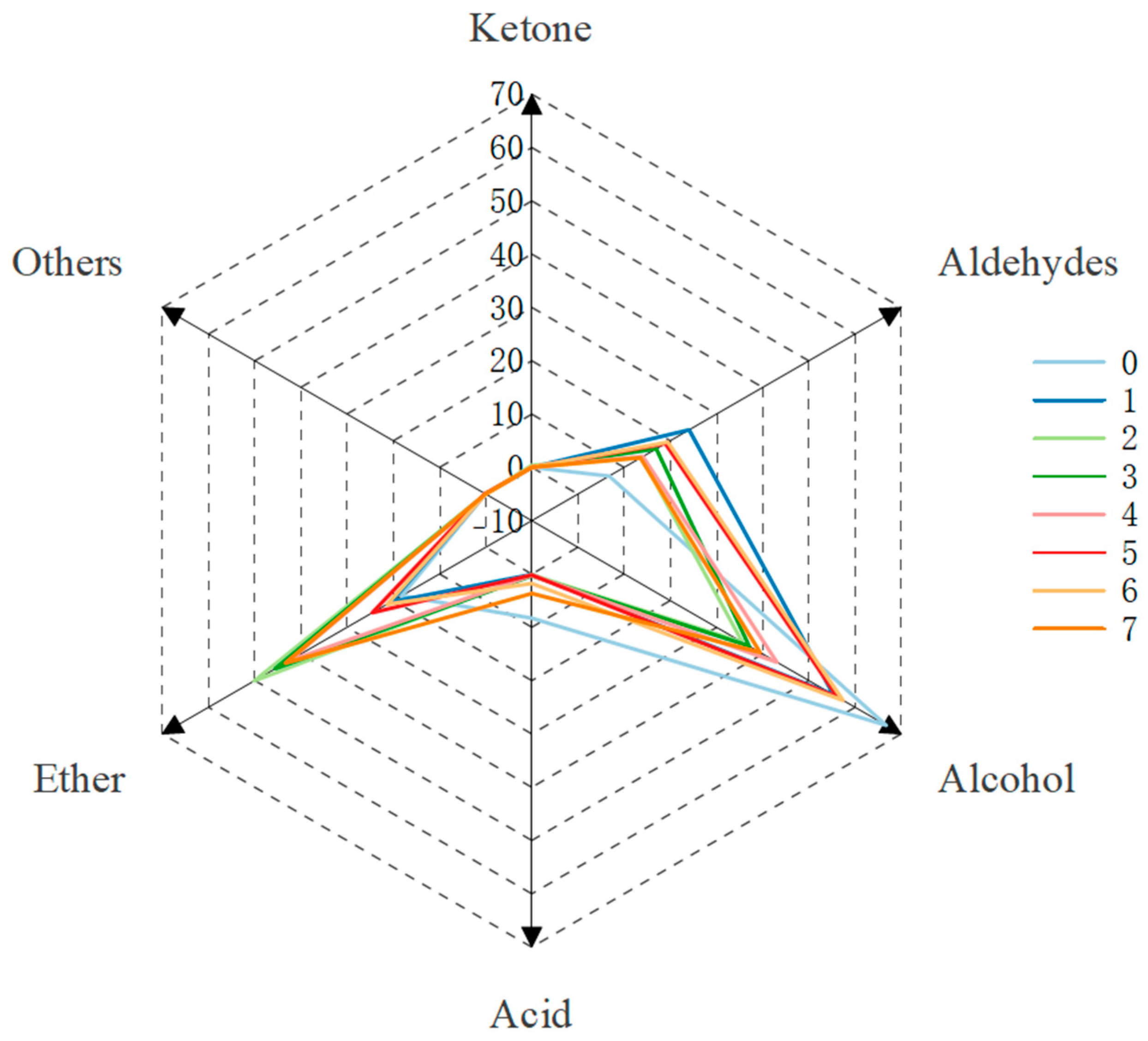
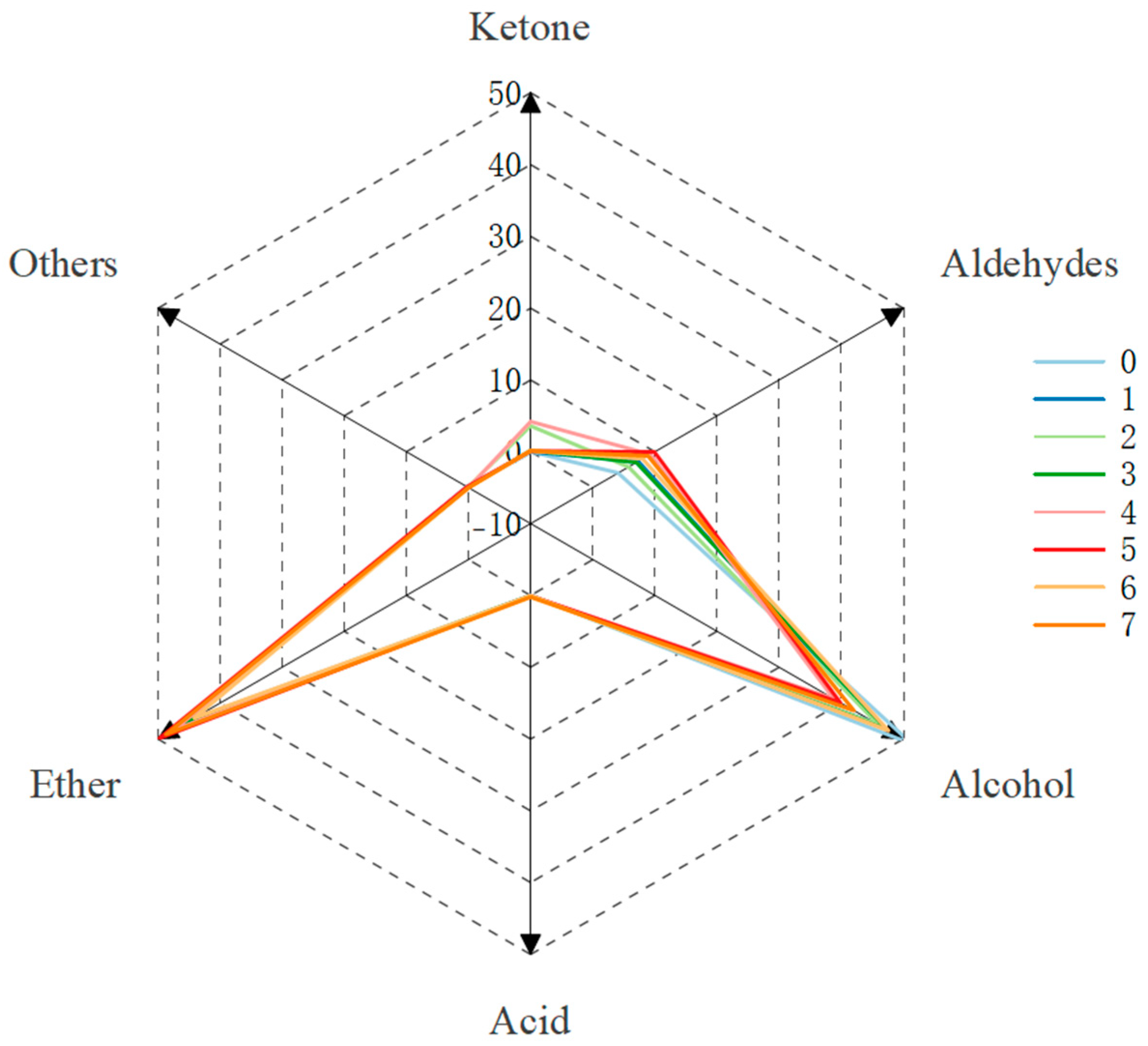
2.6. Effect of Microwave Aging on the Aroma Composition of Steeped Mume Wine
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Qualitative Analysis by HS-SPME-GC-MS
4.3. Quantitative Analysis by UPLC
4.3.1. The Injection Procedures of UPLC
4.3.2. Preparation of Organic Acid Samples and Calibration Curves
4.3.3. Preparation of Amygdalin Samples and Calibration Curves
4.3.4. Preparation of Phenolics Samples and Calibration Curves
4.3.5. Preparation of Steeped Mume Wine
4.4. Aging Reaction of Steeped Mume Wine
4.5. Data Statistics and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y. On “green plum wine”. J. Beijing For. Univ. 2007, S1, 110–115. [Google Scholar]
- Pan, J.H.; Lee, K.Y.; Kim, J.H.; Shin, H.; Lee, J.H.; Kim, Y.J. Prunus mume Sieb. et Zucc. fruit ameliorates alcoholic liver injury in mice by inhibiting apoptosis and inflammation through oxidative stress. J. Funct. Foods 2016, 25, 135–148. [Google Scholar] [CrossRef]
- Chen, M. Study on the functional components and functions of main organic acids in hawthorn wine. Food Eng. 2018, 2, 50–51. [Google Scholar]
- Song, S.; Wang, X.N.; Gao, Z.H. Antibacterial activity of components of Prunus mume against foodborne pathogens. J. Chin. Inst. Food Sci. Technol. 2020, 20, 307–314. [Google Scholar]
- Shi, J.; Gong, J.; Liu, J.; Wu, X.; Zhang, Y. Antioxidant capacity of extract from edible flowers of Prunus mume in China and its active components. LWT-Food Sci. Technol. 2009, 42, 477–482. [Google Scholar] [CrossRef]
- Lv, J.Z.; Deng, J.G. Research progress in pharmacological effects of amygdalin. Drugs Clin. 2012, 27, 530–535. [Google Scholar]
- Xia, Q.L.; Lu, S.M.; Wang, T.; Zheng, M.; Chen, J.; Xing, J. Studies on the Bioactivity of Amygdalin Extracted from Thinned Bayberry Kernels. J. Chin. Inst. Food Sci. Technol. 2017, 17, 58–64. [Google Scholar]
- Pang, Q.Q.; Yue, W.Y.; Liu, K.Q.; Tang, J. Content determination and chemometric analysis of 11 components in Xueli zhike syrup. China Pharm. 2023, 34, 62–66. [Google Scholar]
- Liu, Y.L.; Jin, Z.F.; Chen, H. Changes of the organic acid concentrations and the relative metabolicenzyme activities during the development of Prunus mume fruit. Acta Bot. Boreali-Occident. Sin. 2017, 37, 130–137. [Google Scholar]
- Liu, K. Identification of Aroma Characteristics of Fruits and Effect of Storage Conditions on Aroma in Japanese Apricot. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2019. [Google Scholar]
- Jiang, C.C.; Ye, X.F.; Lin, Y.J.; Fang, Z.Z.; Zhou, D.R. Analysis and evaluation of fruit nutritional quality and aroma components of four major japanese apricot (Prunus mume sieb. et zucc.) cultivars infujian province. Food Sci. 2021, 42, 276–283. [Google Scholar]
- Muñoz-García, R.; Díaz-Maroto, M.C.; Villena, M.A.; Pérez-Coello, M.S.; Alañón, M.E. Ultrasound and microwave techniques as physical methods to accelerate oak wood aged aroma in red wines. LWT 2023, 179, 114597. [Google Scholar] [CrossRef]
- Liu, S.Y. The study of the Color and Flavor in Blueberry Wine Promoted by High Power Pulsed Microwave. Ph.D. Thesis, Shanghai Ocean University, Shanghai, China, 2022. [Google Scholar]
- Nie, L.C.; Sun, J.S.; Chen, H.J.; Zou, X.W. Study on fruit aroma of different apple cultivars. Sci. Agric. Sin. 2006, 03, 641–646. [Google Scholar]
- Drawert, F.; Heimann, W.; Emberger, R.; Tressl, R. Biogenesis of aroma compounds in plant and fruit. II. Enzymic formation of 2-hexen-1-al, hexanal, and their precursors. Justus Liebigs Ann. Der Chem. 1966, 694, 200–208. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Wu, H.C.; Liu, Q.B.; Wang, L.; Dou, X.; Yang, L.; Yang, J. Study on the Aromatic Components of Green Plum Wine by HS-SPME-GC-MS. J. Anhui Agric. Sci. 2015, 43, 349–353. [Google Scholar]
- Mauricio, J.C.; Valero, E.; Millán, C.; Ortega, J.M. Changes in nitrogen compounds in must and wine during fermentation and biological aging by flor yeasts. J. Agric. Food Chem. 2001, 49, 3310–3315. [Google Scholar] [CrossRef]
- Guo, Z.J. The Application Prospect of Artificial Ageing of the Wine in the Chinese Northwest Region. Jiuquan Steel Technol. 2015, 2, 56–59. [Google Scholar]
- Paulus, R.M.; Erdmenger, T.; Becer, C.R.; Hoogenboom, R.; Schubert, U.S. Scale-up of microwave-assisted polymerizations in continuous-flow mode: Cationic ring-opening polymerization of 2-ethyl-2-oxazoline. Macromol. Rapid Commun. 2007, 28, 484–491. [Google Scholar] [CrossRef]
- Chen, L.S. Countermeasures on the Phenomenon of Turning Crudity in accelerative white spirit. J. Guiyang Univ. (Nat. Sci.) 2005, 03, 106–108. [Google Scholar]
- Li, Y.R.; Zhao, D.Q.; Gao, J.; Guang, Y.Y.; Wang, S.N.; Wen, Y.; Fang, Y.L.; Sun, X.Y. Research progress of physical aging in fruit wine and analysis of industry development status. Food Ferment. Ind. 2024, 50, 349–360. [Google Scholar]
- Leng, H.; Cong, N.; Ying, M.; Sheng, L.; Wen, T. Influence of ultra high pressure on quality of Cabernet Sauvignon wine. Sino-Overseas Grapevine Wine. 2014, 2, 12–17. [Google Scholar]
- He, Q.; Lin, Y.H.; Long, B.; Liu, X.Y.; Chen, J.R.; Chen, J.; Chen, A.J. Response surface optimization for microwave aging fig wine and the quality evaluation for its products. J. Hunan Agric. Univ. (Nat. Sci.) 2023, 49, 241–250. [Google Scholar]
- Jiang, Q.; Liu, J.X.; Li, P.Y.; Bo, Y.L.; Li, X.; Xu, B.C.; Luo, D.L.; Guo, J.Y. Effect of Microwave Radiation Treatment on Volatile Substances in Luzhou-Flavor Liquor. J. Nucl. Agric. Sci. 2022, 36, 341–349. [Google Scholar]





| Component Type | BP Area Relative Content (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Ether | 18.15 | 20.47 | 50.16 | 45.58 | 42.57 | 24.53 | 21.33 | 43.36 |
| Aldehydes | 6.76 | 24.08 | 13.72 | 16.98 | 14.09 | 18.93 | 19.47 | 13.54 |
| Alcohol | 66.78 | 55.30 | 35.61 | 37.11 | 43.10 | 56.25 | 57.39 | 39.44 |
| Ketone | / | / | 0.38 | / | / | / | / | / |
| Acid | 8.30 | 0.09 | 0.13 | 0.34 | 0.24 | 0.17 | 1.81 | 3.65 |
| Others | 0.00 | 0.05 | / | / | 0.00 | 0.11 | / | 0.02 |
| Component Type | BP Area Relative Content (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Ether | 44.34 | 46.37 | 44.45 | 45.33 | 47.56 | 49.78 | 44.17 | 48.72 |
| Aldehydes | 4.11 | 7.32 | 5.76 | 7.03 | 9.28 | 9.94 | 8.03 | 8.88 |
| Alcohol | 51.39 | 46.00 | 46.01 | 47.39 | 38.82 | 39.89 | 47.62 | 42.04 |
| Ketone | 0.01 | 0.02 | 3.61 | 0.19 | 4.22 | 0.11 | 0.13 | 0.14 |
| Acid | 0.02 | 0.04 | 0.03 | 0.04 | 0.12 | 0.07 | 0.05 | 0.22 |
| Others | 0.12 | 0.24 | 0.13 | 0.01 | / | 0.22 | / | / |
| Sample | Material | Sample | Material | Sample | Material |
|---|---|---|---|---|---|
| A1 | 74 DAF | F1 | 2 L 42%vol BW + 1 kg A1 | S1 | 2 L 65%vol BW + 1 kg A1 |
| A2 | 81 DAF | F2 | 2 L 42%vol BW + 1 kg A2 | S2 | 2 L 65%vol BW + 1 kg A2 |
| A3 | 88 DAF | F3 | 2 L 42%vol BW + 1 kg A3 | S3 | 2 L 65%vol BW + 1 kg A3 |
| A4 | 95 DAF | F4 | 2 L 42%vol BW + 1 kg A4 | S4 | 2 L 65%vol BW + 1 kg A4 |
| Group Number | Temperature | Power (Watt) | Time |
|---|---|---|---|
| Control Group (0) | / | / | / |
| 1 | 40 °C | 500 W | 80 min |
| 2 | 45 °C | 500 W | 80 min |
| 3 | 50 °C | 500 W | 80 min |
| 4 | 45 °C | 500 W | 40 min |
| 5 | 45 °C | 500 W | 60 min |
| 6 | 45 °C | 400 W | 80 min |
| 7 | 45 °C | 600 W | 80 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.; Song, J.; Wang, Y.; Huang, X.; Segbo, S.; Gao, Z. Effect of Different Harvest Time and Microwave Aging on Aroma Characteristics of Japanese Apricot Wine. Molecules 2025, 30, 392. https://doi.org/10.3390/molecules30020392
Li A, Song J, Wang Y, Huang X, Segbo S, Gao Z. Effect of Different Harvest Time and Microwave Aging on Aroma Characteristics of Japanese Apricot Wine. Molecules. 2025; 30(2):392. https://doi.org/10.3390/molecules30020392
Chicago/Turabian StyleLi, Aoting, Jiangfeng Song, Yike Wang, Xiao Huang, Silas Segbo, and Zhihong Gao. 2025. "Effect of Different Harvest Time and Microwave Aging on Aroma Characteristics of Japanese Apricot Wine" Molecules 30, no. 2: 392. https://doi.org/10.3390/molecules30020392
APA StyleLi, A., Song, J., Wang, Y., Huang, X., Segbo, S., & Gao, Z. (2025). Effect of Different Harvest Time and Microwave Aging on Aroma Characteristics of Japanese Apricot Wine. Molecules, 30(2), 392. https://doi.org/10.3390/molecules30020392







