Abstract
Traditional Japanese medicines, i.e., Kampo medicines, consist of crude drugs (mostly plants) that have empirical pharmacological functions (‘Yakuno’ in Japanese), such as clearing heat. Crude drugs with cold properties, such as Phellodendron bark, have the empirical function of clearing heat as they cool the body. Because we found that anti-inflammatory compounds were present in several crude drugs for clearing heat, it is speculated that the empirical function of clearing heat may be linked to anti-inflammatory activities. When 10 typical crude drugs were selected from 22 herbal crude drugs for clearing heat, we identified anti-inflammatory compounds in five crude drugs, including Phellodendron bark. In this study, the other crude drugs were extracted and partitioned with ethyl acetate (EtOAc) and n-butanol to obtain three crude fractions. All the EtOAc-soluble fractions, except that from Forsythia fruits, inhibited interleukin (IL)-1β-induced nitric oxide (NO) production in primary-cultured rat hepatocytes. Anti-inflammatory compounds were identified from these EtOAc-soluble fractions: baicalein from Scutellaria roots, (−)-nyasol from Anemarrhena rhizomes, and loniflavone from Lonicera leaves and stems. (+)-Phillygenin was purified from Forsythia fruits by removing cytotoxic oleanolic and betulinic acids. These compounds suppressed the production of NO and cytokines in hepatocytes. Anti-inflammatory compounds were not purified from the EtOAc-soluble fraction of Rehmannia roots because of their low abundance. Collectively, these findings indicate that anti-inflammatory compounds are present in all 10 crude drugs for clearing heat, confirming that these anti-inflammatory compounds in crude drugs provide the empirical functions for clearing heat. Other empirical functions of Kampo medicine can also be explained by modern pharmacological activities.
1. Introduction
Medicinal plants that grow in China, Korea, and Japan, have been used for traditional Chinese medicine (TCM) and traditional Japanese medicine, i.e., Kampo medicine, which was independently developed from traditional Chinese medicine during the 18th century [1,2]. Most ‘crude drugs’ used for Kampo medicine are specific parts of medicinal plants [3]. A Kampo formula consists of crude drugs, each of which is classified using four properties (cold, cool, warm, and heat) and five tastes (sour, bitter, sweet, spice, and salt) based on the Yin–yang and Five elements theories [1,4]. Crude drugs have empirical pharmacological functions (known as ‘Yakuno’ in Japanese), such as clearing heat to cool the body’s heat (‘Seinetsu’), replenishing blood to improve blood deficiency (‘Hoketsu’), and pus discharge (‘Haino’) [1,5]. Crude drugs are classified into 13 categories on the basis of their empirical functions in the KEGG BRITE database [6]. Although many constituents of crude drugs have been reported to date, there are few reports discussing the mechanisms of empirical functions, as well as the relationships between the constituents and empirical functions of the crude drugs used for Kampo medicine, from a modern pharmacological perspective.
Crude drugs with cold properties have the empirical function of clearing heat as they cool the body [1]. According to the KEGG BRITE database, 25 crude drugs for clearing heat are known, and 22 are derived from medicinal plants [6]. When 10 typical crude drugs were selected (Table 1), we previously purified and identified anti-inflammatory compounds from five crude drugs, i.e., Phellodendron amurense bark, Coptis chinensis rhizomes, Paeonia suffruticosa root bark, and Lonicera japonica flowers and buds [7,8,9]. In addition, the anti-inflammatory effects of the constituents of Gardenia jasminoides fruits, i.e., genipin and geniposide, were previously reported [10,11,12]. These compounds exhibited anti-inflammatory effects in primary-cultured rat hepatocytes [7,8,9,10] or the macrophage line RAW264.7 [11,12]. Accordingly, it is expected that the empirical function of clearing heat is linked to anti-inflammatory compounds in crude drugs.

Table 1.
Typical crude drugs for clearing heat 1.
The proinflammatory mediator nitric oxide (NO) is produced in hepatocytes and macrophages in response to interleukin 1β (IL-1β) and lipopolysaccharide (LPS), respectively [13]. An anti-inflammatory compound that was added to the medium suppressed NO production and downregulated proinflammatory genes in both hepatocytes and macrophages [14,15]. Therefore, the inhibition of NO production is an inflammatory response. To date, we have used primary-cultured rat hepatocytes treated with IL-1β to monitor the anti-inflammatory effects of crude drugs and their constituents and to compare their activities [7,8,9,10].
To verify the hypothesis that the crude drugs for clearing heat contain anti-inflammatory compounds, we investigated their ability to clear heat, which we did not previously examine (Table 1). We attempted to purify anti-inflammatory compounds from the five crude drugs by monitoring IL-1β-induced NO production in primary-cultured rat hepatocytes. Lastly, we discuss approaches to elucidate the empirical pharmacological function of crude drugs using modern pharmacological methods.
2. Results
2.1. Extraction and Fractionation of Crude Drugs
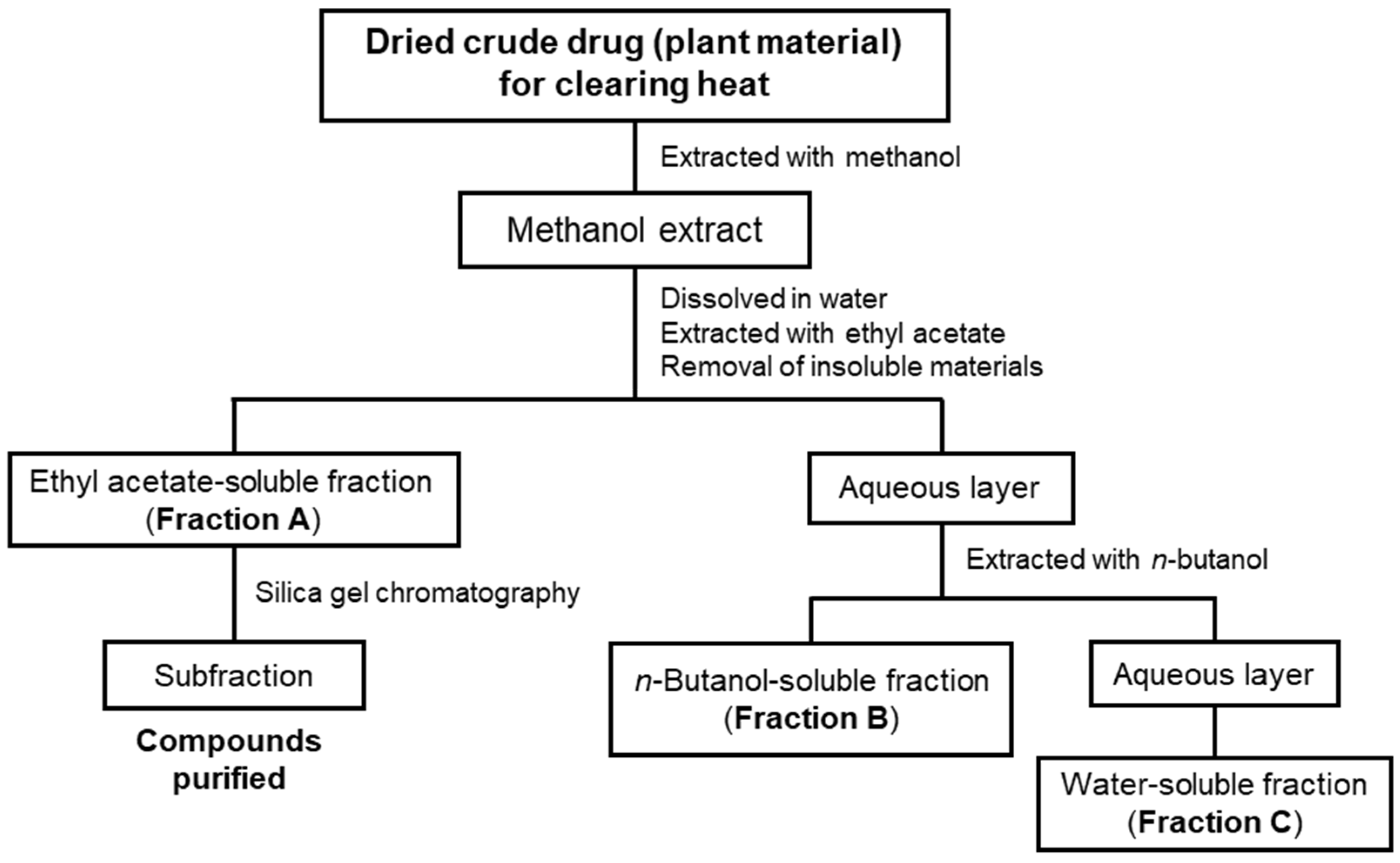
As mentioned above, we previously isolated and identified anti-inflammatory compounds from four crude drugs for clearing heat, as shown in Table 1, i.e., Phellodendron bark, Coptis rhizome, Moutan bark, and Lonicera flower are shown [7,8,9]. Therefore, we extracted other crude drugs for clearing heat with methanol and successively fractionated the extract with ethyl acetate (EtOAc) and n-butanol into three fractions (Scheme 1) in accordance with a previously published method [9].
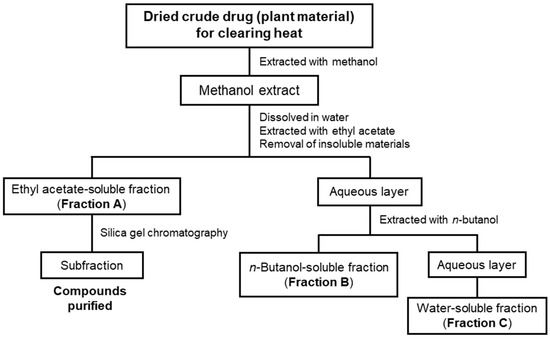
Scheme 1.
A flowchart showing the purification of compounds from crude drugs for clearing heat. Methanol extracts from crude drugs were fractionated into three fractions to purify the compounds by silica gel chromatography, preparative thin-layer chromatography (TLC), and so forth. Each fraction or compound was subjected to measurements of the nitric oxide (NO) production in interleukin (IL)-1β-treated hepatocytes.
The yields of the extraction and partitioning ratios of the fractions are shown in Table 2. Our previous data are also presented [7,8,9]. The extraction yields ranged from 8.93 to 34.6%. Because Fractions A, B, and C include hydrophobic, amphipathic, and hydrophilic compounds, respectively [9], the partitioning ratios of the fractions differ depending on the crude drug and the parts of the plants.

Table 2.
The extraction and fractionation of crude drugs for clearing heat.
2.2. Effects of the Fractions on the Suppression of NO Production in Hepatocytes
Next, the effect of each fraction on NO production was evaluated via IL-1β-treated rat hepatocytes. We determined their half-maximal inhibitory concentration (IC50) values, according to a previously established method [14]. As shown in Table 2, Fraction A of the crude drugs, excluding Forsythia fruits, presented lower IC50 values than Fractions B and C did. The results suggest that Fraction A of the crude drugs (Gardenia fruit, Scutellaria root, Anemarrhena rhizome, Lonicera leaf and stem, and Rehmannia root) contains hydrophobic compounds that suppress NO production in hepatocytes. Therefore, we attempted to purify anti-inflammatory compounds from these crude drugs (see below).
In contrast, an IC50 value could not be calculated when Fraction A of Forsythia fruits was added to the medium, because many hepatocytes detached from the dish bottom due to its cytotoxicity. The cytotoxic compounds in this fraction may mask the activity of compounds that suppress NO production. Therefore, we also tried to purify compounds from Forsythia fruit Fraction A.
2.3. Purification of Anti-Inflammatory Compounds from Crude Drugs
2.3.1. Genipin and Geniposide of Gardenia Fruits
Although there are reports that genipin and geniposide from Gardenia jasminoides fruits have anti-inflammatory effects [10,11,12], information about the fractionation of active compounds in Gardenia fruit extracts is not available. Following extraction and fractionation into three crude fractions (Table 2), only Fraction A suppressed NO production in the IL-1β-treated hepatocytes. The IC50 value of genipin (aglycone) for the suppression of NO production was 4.40 ± 0.85 μM, whereas geniposide (glycoside) did not inhibit NO production up to a final concentration of 800 μM. HPLC analysis using genipin as a standard demonstrated that genipin was present in Fraction A, indicating that genipin is at least responsible for the activity of Gardenia fruits.
2.3.2. Purification of Baicalin from Scutellaria Roots
First, we purified the constituents in Fraction A from a Scutellaria root extract, as described in the Materials and Methods. Guided by NO assays, we purified a biologically active compound from the subfraction that showed high activity against NO production in hepatocytes (Supplementary Materials Data S1.1.). This compound (5.14 mg), which was designated as Compound 1, was identified by nuclear magnetic resonance (NMR) spectroscopy analysis.
Compound 1: Yellow powder. 1H NMR [500 MHz, (CD3)2SO, ppm] δ 8.06 (2H, d, J = 2.0 Hz, H-2′, H-6′), 7.59 (3H, m, H-3′, H-4′, H-5′), 6.95 (1H, s, H-3), 6.64 (1H, s, H-8); 13C NMR [125 MHz, (CD3)2SO, ppm] δ 182.0 (C-4), 162.7 (C-7), 153.6 (C-2), 149.7 (C-9), 146.8 (C-5), 131.8 (C-4′), 130.8 (C-1′), 129.2 (C-6), 129.0 (C-3′, C-5′), 126.2 (C-2′, C-6′), 104.4 (C-3), 104.1 (C-10), 93.9 (C-8). This compound was identified as baicalein on the basis of 1H and 13C NMR spectral analysis by comparison with previously published results [16], (see Figure 1).
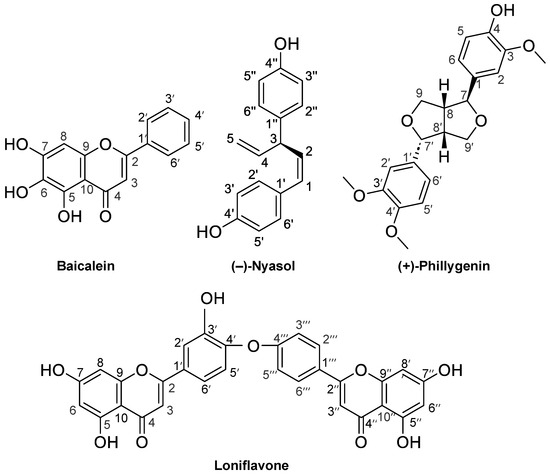
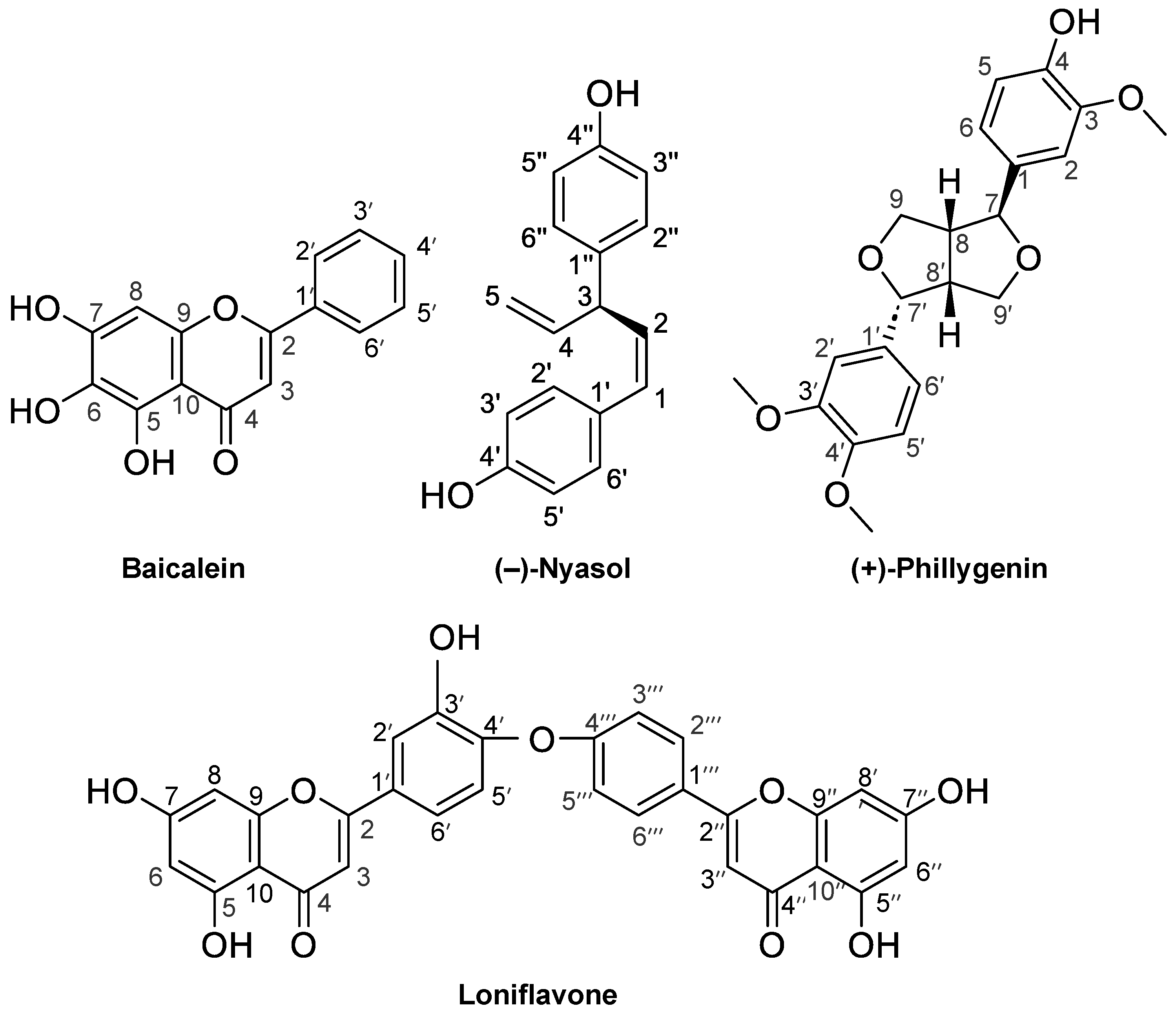
Figure 1.
The chemical structures of the compounds purified in this study. Baicalein (Compound 1) from Scutellaria roots, (−)-nyasol (2) from Anemarrhena rhizomes, loniflavone (3) from Lonicera leaves and stems, and (+)-phillygenin (4) from Forsythia fruits.
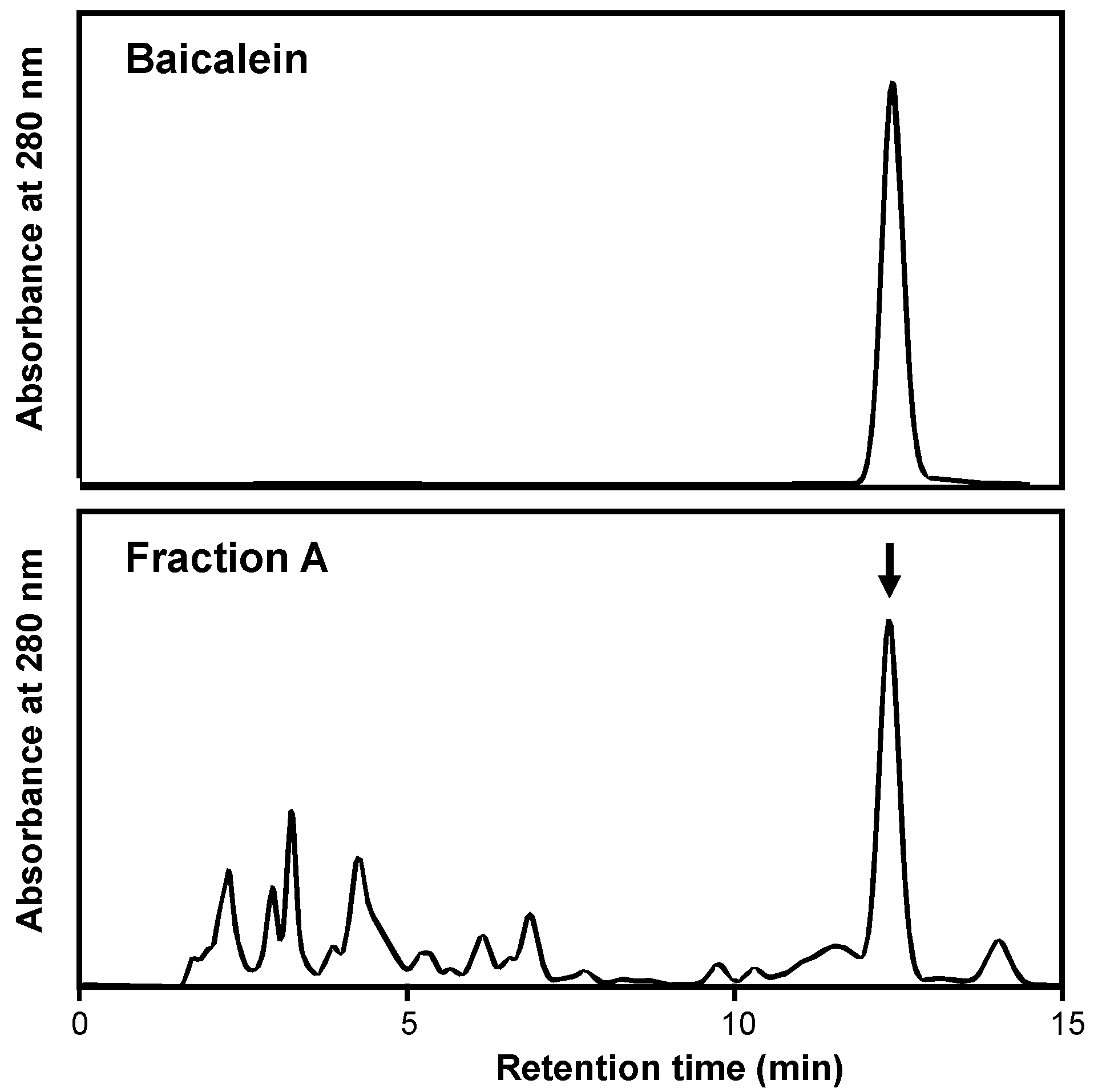
The baicalein in a Scutellaria root extract was analyzed. The HPLC chromatogram of Fraction A showed an independent peak (retention time, 12.4 min), which corresponded to that of baicalein (Figure 2). The content of baicalein was subsequently estimated by HPLC and calculated as 22.5% in Fraction A and 3.58% in the extract (Supplementary Materials Data, S2.1.). Given that the extraction yield was 34.6% (Table 1), the content of baicalein in Scutellaria roots was calculated as 1.24, i.e., 12.4 mg/g of the plant material, which was comparable to the reported values ranging from 5.747 to 15.449 mg/g [17].
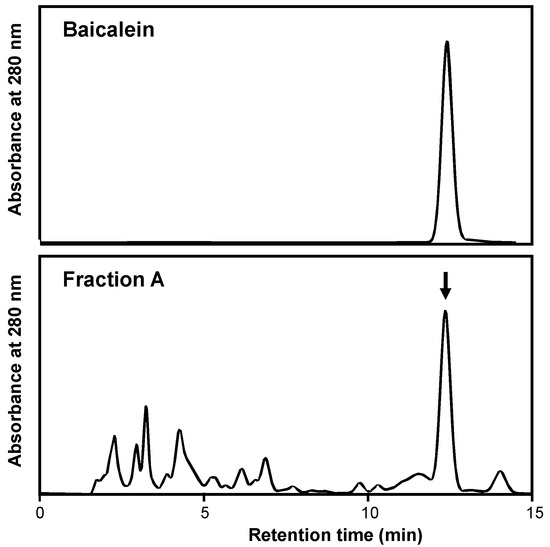
Figure 2.
HPLC chromatograms of baicalein (standard; upper) and Fraction A from the Scutellaria root extract (lower). HPLC was used for this analysis, as described in Section 4. The arrow indicates the peak of baicalein.
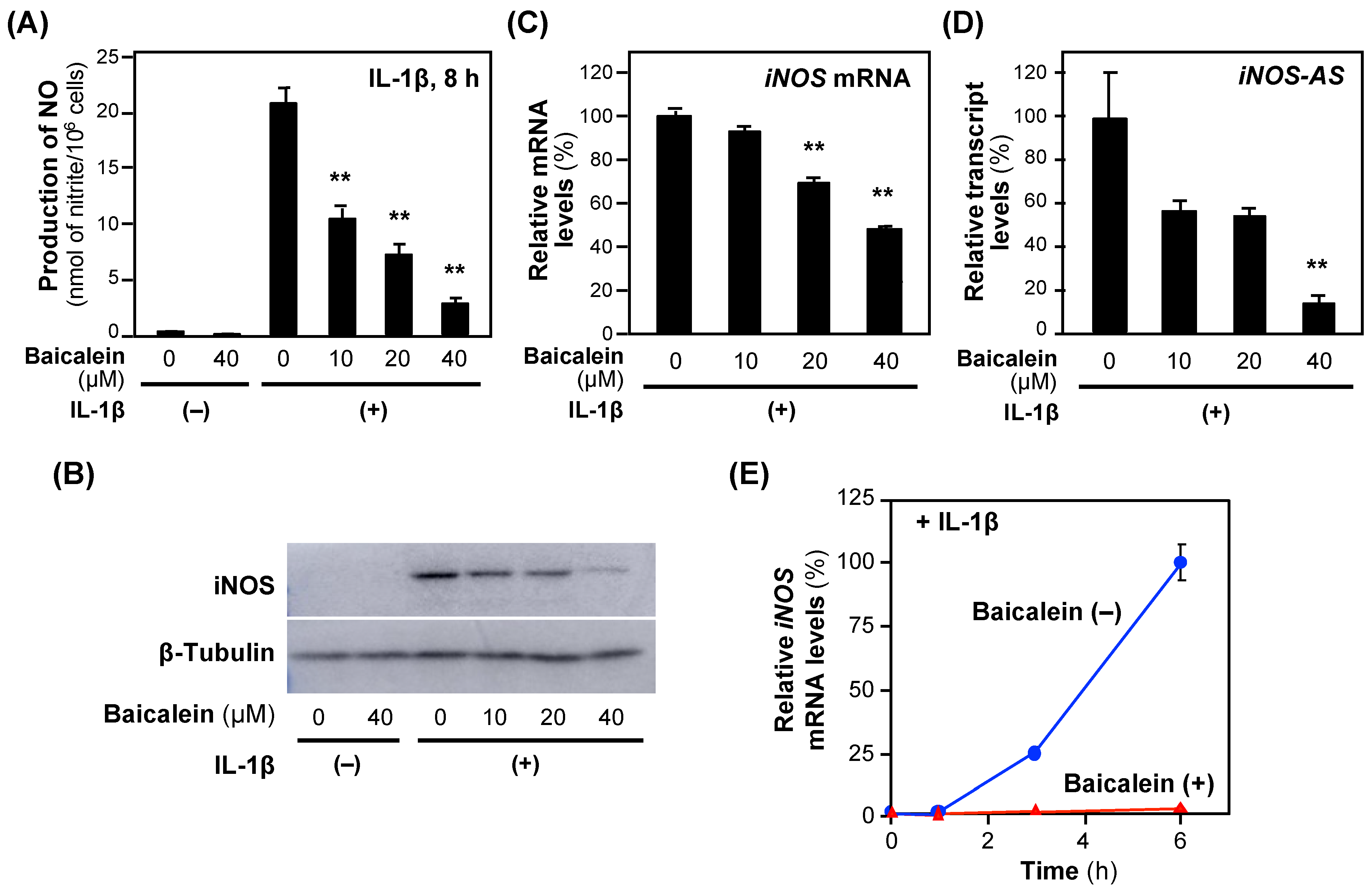
Next, the effects of baicalein on NO production in hepatocytes were examined. When baicalein was added to the medium of the rat hepatocytes together with IL-1β, the NO concentration decreased in a concentration-dependent fashion without showing cytotoxicity, i.e., <5% of the activity of the whole-cell extract (Figure 3). The calculated IC50 value of baicalein for suppressing NO production was 16.8 ± 7.93 μM.
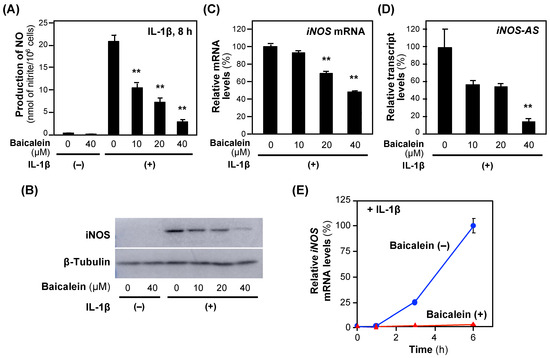
Figure 3.
The effects of baicalein on the expression of the iNOS gene in hepatocytes. (A) The effects of baicalein on NO production. Baicalein and IL-1β were added to the medium of primary-cultured rat hepatocytes and incubated for 8 h. The nitrate concentrations in the medium were measured as NO. Cytotoxicity was not observed at the concentrations applied. (B) An immunoblot analysis of the iNOS protein. Hepatocyte extracts were prepared from hepatocytes in (A) and analyzed by immunoblotting to detect the iNOS (130 kDa) and the internal control β-tubulin (55 kDa). (C) The levels of iNOS mRNA and (D) iNOS antisense transcript (iNOS-AS). Total RNA was extracted 3 h after the addition of baicalein and subjected to quantitative reverse transcription–polymerase chain reaction (RT–qPCR). The level of each mRNA was measured and normalized to the elongation factor 1α (Ef1a) mRNA level. Relative mRNA levels (%) are presented as the means ± SDs (n = 3) when the measured mRNA level was set as 100% in the presence of IL-1β alone. ** p < 0.01 versus IL-1β alone. (E) The time course of the iNOS mRNA levels. After the addition of 40 μM baicalein (t = 0 h), total RNA was extracted at the indicated times and subjected to RT–qPCR. Relative mRNA levels (%) were normalized to Ef1a mRNA levels and are presented as the means ± SDs (n = 3), and the mRNA level measured 6 h after the addition of IL-1β was set as 100%. ** p < 0.01 versus IL-1β alone.
Next, we investigated the iNOS gene expression. Baicalein reduced both iNOS protein and iNOS mRNA levels (Figure 3B,C), suggesting that the iNOS gene expression is regulated at a transcriptional step. Because a natural antisense transcript (iNOS-AS) transcribed from the iNOS gene interacts with and stabilizes iNOS mRNA [18], we analyzed the effects of baicalein on iNOS-AS expression. Baicalein inhibited iNOS-AS in a concentration-dependent manner (Figure 3D). The iNOS mRNA level increased in the presence of IL-1β, whereas it was inhibited by baicalein for 6 h after the addition of IL-1β (Figure 3E). Similarly to chlorogenic acid [9], baicalein inhibits the induction of the iNOS gene expression at both the transcriptional and post-transcriptional levels.
2.3.3. Purification of (−)-Nyasol from Anemarrhena Rhizomes
To purify and identify the constituents of the Anemarrhena rhizome extract, Fraction A was fractionated into subfractions via silica gel column chromatography and preparative thin-layer chromatography (TLC). We purified an active compound from the subfraction that showed high activity against NO production. Lastly, Compound 2 (18.5 mg) was isolated (Supplementary Materials Data, S1.2.) and identified by NMR spectroscopy and mass spectrometry (MS) analysis.
Compound 2: Pale yellow oil. [α −72.8° (c 0.257, CHCl3); EI-MS m/z (%): 252 (M+, 100), 237 (20), 158 (32), 145 (29), 107 (28) HR-EI-MS m/z 252.1156 (M+) (calculated for C17H16O2: 252.1150); 1H NMR (400 MHz, CD3OD, ppm) δ 7.16 (2H, d, J = 8.8 Hz, H-2′,6′), 7.09 (2H, d, J = 8.0 Hz, H-2″,6″), 6.78 (2H, d, J = 8.8 Hz, H-3′,5′), 6.77 (2H, d, J = 8.0 Hz, H-3″,5″), 6.51 (1H, d, J = 11.6 Hz, H-1), 6.00 (1H, ddd, J = 17.2, 10.8, 6.4 Hz, H-4), 5.66 (1H, dd, J = 11.6, 10.0 Hz, H-2), 5.16(2H, ddd, J = 1.2, 4.8, 11.7 Hz, H-5), 4.48 (1H, dd, J = 10.0, 6.4 Hz, H-3). 13C NMR (100 MHz, CD3OD, ppm) δ 154.61 (C-4′), 154.14 (C-4″), 140.77 (C-4), 135.66 (C-1″), 131.78 (C-2), 130.11 (C-2′ and 6′), 129.91 (C-1′), 128.97 (C-2″ and 6″), 128.68 (C-1), 115.47 (C-3″ and 5″), 115.20 (C-3′,5′ and 5), 46.69 (C-3). These data were almost the same as the previously published results for (−)-nyasol (=cis-hinokiresinol) isolated from Anemarrhena asphodeloides rhizomes, on the basis of 1H and 13C NMR spectral analysis and optical rotation [α −67° (c 0.1, C3H6O) [19,20]. Therefore, this compound was identified as (−)-nyasol.
To determine the (−)-nyasol content in Anemarrhena rhizome extract, HPLC analysis was performed. The HPLC results revealed several independent peaks in the chromatogram of Fraction A, and the (−)-nyasol content was subsequently estimated; it was 5.56% in Fraction A and 0.773% in the extract (Supplementary Materials Data, S2.2.).
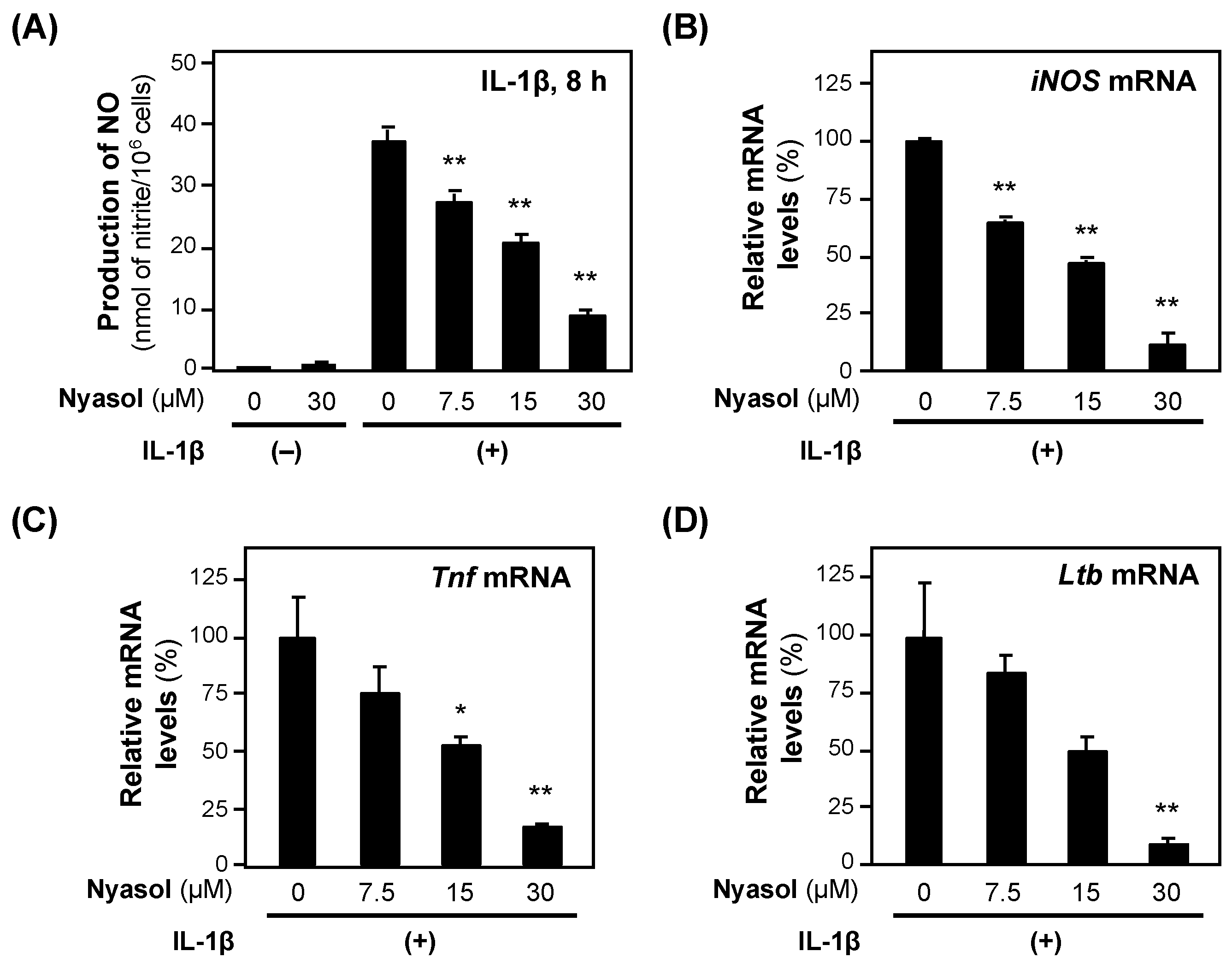
When (−)-nyasol was added to the medium of the rat hepatocytes together with IL-1β, the NO concentration decreased in a concentration-dependent manner without showing cytotoxicity, i.e., <5% of the activity of the whole-cell extract (Figure 4). The calculated IC50 value of (−)-nyasol for the suppression of NO production was 18.3 ± 2.99 μM. (−)-Nyasol significantly decreased the levels of iNOS mRNA. Furthermore, (−)-nyasol significantly reduced the levels of mRNAs encoded by proinflammatory genes, such as tumor necrosis factor α (Tnf) and lymphotoxin β (Ltb) mRNAs (Figure 4).
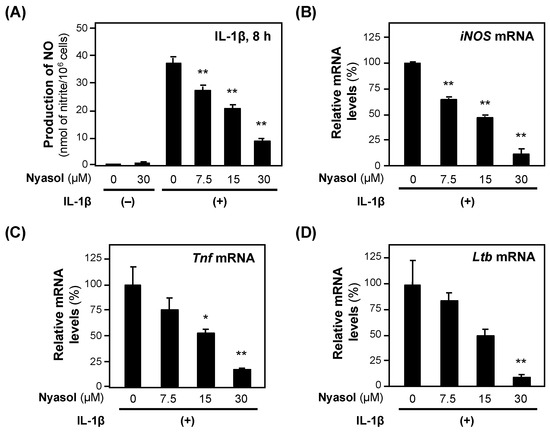
Figure 4.
The effects of (−)-nyasol on the expression of proinflammatory genes. (A) NO levels. (−)-Nyasol and IL-1β were added to the hepatocyte medium and incubated. Cytotoxicity was not observed at the concentrations applied. After 4 h, the total RNA was extracted and subjected to RT–qPCR. Each mRNA level was measured in triplicate and normalized to the Ef1a mRNA level: (B) iNOS mRNA; (C) Tnf mRNA; and (D) lymphotoxin β (Ltb) mRNA. The relative mRNA levels (%) are presented as the means ± SDs (n = 3) when the mRNA level was set at 100% in the presence of IL-1β alone. * p < 0.05 and ** p < 0.01 versus IL-1β alone.
2.3.4. Purification of Loniflavone from Lonicera Leaves and Stems
To purify the anti-inflammatory compounds, Fraction A of the extract from Lonicera leaves and stems was purified, as described in Materials and Methods. Subfractions that suppressed NO production in hepatocytes were further purified to obtain Compound 3 (5.73 mg) (Supplementary Materials Data, S1.3.).
Compound 3: Pale yellow powder. 1H NMR [400 MHz, (CD3)2CO, ppm] δ 12.92 (1H, s, H-5), 12.91 (1H, s, H-5″), 8.03 (2H, d, J = 9.1 Hz, H-2‴/H-6‴), 7.89 (1H, dd, J = 8.5, 2.1 Hz, H-6′), 7.87 (1H, d, J = 2.1 Hz, H-2′), 7.24 (1H, d, J = 8.5 Hz, H-5′), 7.10 (2H, d, J = 8.8 Hz, H-3‴/H-5‴), 6.70 (1H, s, H-3″), 6.69 (1H, s, H-3), 6.52 (1H, d, J = 2.1 Hz, H-8), 6.51 (1H, d, J = 2.1 Hz, H-8″), 6.23 (1H, d, J = 1.8 Hz, H-6), 6.22 (1H, d, J = 2.1 Hz, H-6″). 13C NMR [100 MHz, (CD3)2CO, ppm] δ 182.2 (C-4), 182.2 (C-4′), 164.2 (C-7), 164.2 (C-7′), 163.5 (C-2), 163.1 (C-2′), 162.5 (C-5), 162.5 (C-5′), 161.1 (C-4‴), 158.0 (C-9″), 157.9 (C-9), 153.1 (C-3′), 142.4 (C-4′), 128.4 (C-2‴/C-6‴), 125.4 (C-1‴), 125.1 (C-6′), 123.7 (C-1′), 120.9 (C-2′), 118.2 (C-5′), 116.6 (C-3‴/C-5‴), 104.6 (C-10″), 104.5 (C-10), 104.3 (C-3″), 104.0 (C-3), 99.0 (C-6), 99.0 (C-6″), 94.0 (C-8), 94.0 (C-8″). This compound was identified as loniflavone (Figure 1) on the basis of 1H and 13C NMR spectral analysis in comparison with a previous report on loniflavone isolated from Caesalpinia pyramidalis leaves [21].
We first measured the NMR spectrum of Compound 3 in (CD3)2SO. 1H NMR [400 MHz, (CD3)2SO, ppm] δ 12.88 (1H, s, H-5), 12.85 (1H, s, H-5″), 8.01 (2H, d, J = 9.8 Hz, H-2‴/H-6‴), 7.88 (1H, dd, J = 9.1, 2.4 Hz, H-6′), 7.88 (1H, d, J = 2.1 Hz, H-2′), 7.12 (1H, d, J = 9.1 Hz, H-5′), 6.99 (2H, d, J = 9.1 Hz, H-3‴/H-5‴), 6.85 (1H, s, H-3″), 6.85 (1H, s, H-3), 6.46 (1H, d, J = 2.1 Hz, H-8), 6.45 (1H, d, J = 2.1 Hz, H-8″), 6.16 (1H, d, J = 2.1 Hz, H-6), 6.15 (1H, d, J = 2.1 Hz, H-6″). 13C NMR [100 MHz, (CD3)2SO, ppm] δ 182.3 (C-4), 182.3 (C-4′), 164.8 (C-7), 164.7 (C-7′), 163.6 (C-2), 163.2 (C-2′), 162.0 (C-5), 161.9 (C-5′), 161.3 (C-4‴), 157.9 (C-9″), 157.8 (C-9), 153.9 (C-3′), 142.1 (C-4′), 129.0 (C-2‴/ C-6‴), 125.9 (C-6′), 124.9 (C-1‴), 122.8 (C-1′), 121.8 (C-2′), 118.4 (C-5′), 116.6 (C-3‴/C-5‴), 104.5 (C-10″), 104.3 (C-10), 104.3 (C-3″), 104.1 (C-3), 99.4 (C-6), 99.4 (C-6″), 94.6 (C-8), 94.6 (C-8″).
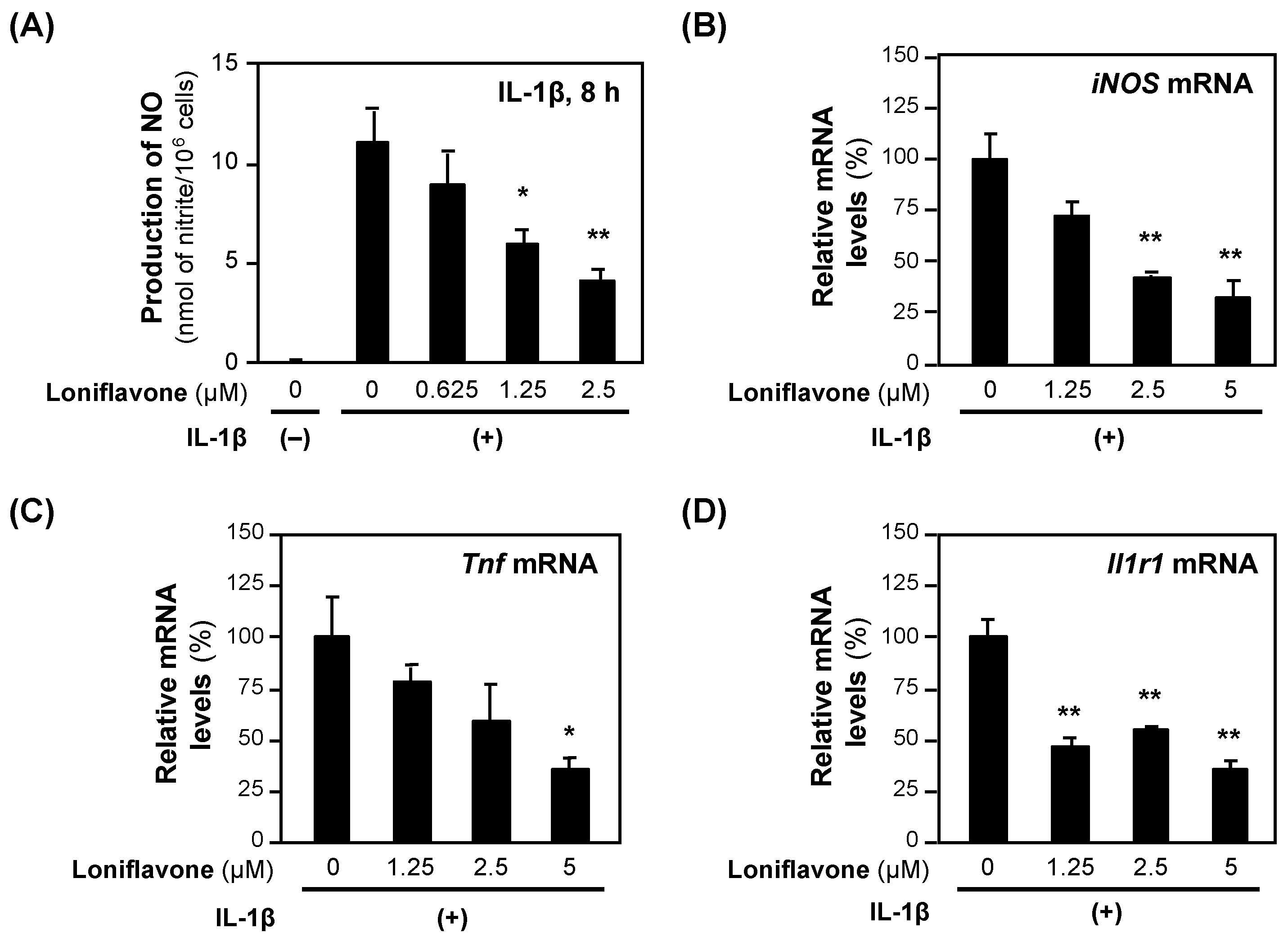
Next, we examined the effects of loniflavone on the expression of the mRNAs encoding proinflammatory genes. Loniflavone decreased the levels of iNOS and Tnf mRNAs in a concentration-dependent manner (Figure 5). IL-1β induces the expression of type 1 interleukin 1 receptor (IL1R1), which regulates the expression of proinflammatory genes, including the iNOS gene [22]. When we measured the Il1r1 mRNA levels, loniflavone significantly decreased the Il1r1 mRNA levels (Figure 5).
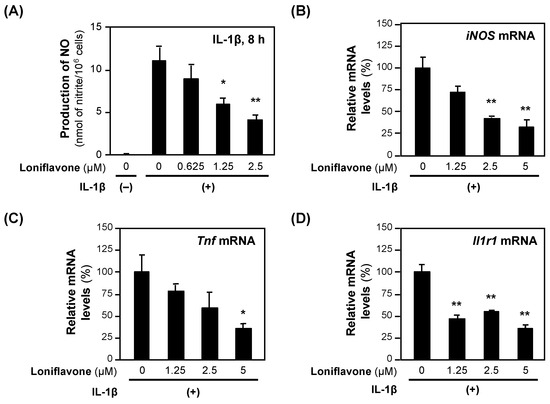
Figure 5.
The effects of loniflavone on the expression of proinflammatory genes. (A) Decreased NO production by baicalein in hepatocytes. Loniflavone and IL-1β were added to the medium of primary-cultured rat hepatocytes and incubated for 8 h until the NO concentration was measured. Cytotoxicity was not observed at the concentrations applied. (B–D) mRNA levels in hepatocytes. After incubation with loniflavone and IL-1β, the total RNA was extracted and subjected to RT–qPCR. The levels of each mRNA were measured in triplicate and normalized to the Ef1a mRNA level. The relative mRNA levels (%) of iNOS (B), Tnf mRNA (C), and Il1r1 (D) are presented as the means ± SDs (n = 3), when the measured mRNA level was set at 100% in the presence of IL-1β alone. * p < 0.05 and ** p < 0.01 versus IL-1β alone.
2.4. Unsuccessful Purification of Anti-Inflammatory Compounds
Because Rehmannia root Fraction A strongly suppressed NO production in hepatocytes (Table 2), we attempted to purify the anti-inflammatory compounds. When Fraction A from the Rehmannia root extract was separated by silica gel column chromatography, the compounds were eluted stepwise using an n-hexane–EtOAc mixture (100:0 ---> 0:100) to provide subfractions A1 to A14. When each subfraction was added to the medium at a final concentration of 50 μg/mL, six subfractions (A2 to A7) reduced NO production to less than 50% of that in the presence of IL-1β. Furthermore, many spots were visualized by spraying p-anisaldehyde–sulfuric acid solution, indicating that several anti-inflammatory compounds were present in Fraction A.
Many constituents in the Rehmannia roots have been reported [23], and most of them are iridoid glycosides (e.g., glutinoside and catalpol) and sugars (e.g., stachyose), which seem to be partitioned to Fractions B and C, respectively. Conversely, the ratio of Fraction A was 1.71%, which was the lowest among the 10 crude drugs (Table 2). Because hydrophobic compounds are expected to be present at low abundances in Fraction A, we stopped purifying anti-inflammatory compounds from Fraction A of Rehmannia roots.
2.5. Identification of an Anti-Inflammatory Compound by Removing Masking Compounds
As shown in Table 2, Fraction A of the Forsythia fruit extract was cytotoxic, suggesting that the cytotoxic compounds in this fraction may mask the activity of anti-inflammatory compounds that suppress IL-1β-induced NO production. Fraction A from Forsythia fruits was subjected to silica gel column chromatography, and the compounds were eluted stepwise using an n-hexane–EtOAc mixture to provide subfractions A1 to A13. When each subfraction was added to the medium, only subfractions A3 and A4 exhibited high LDH activity.
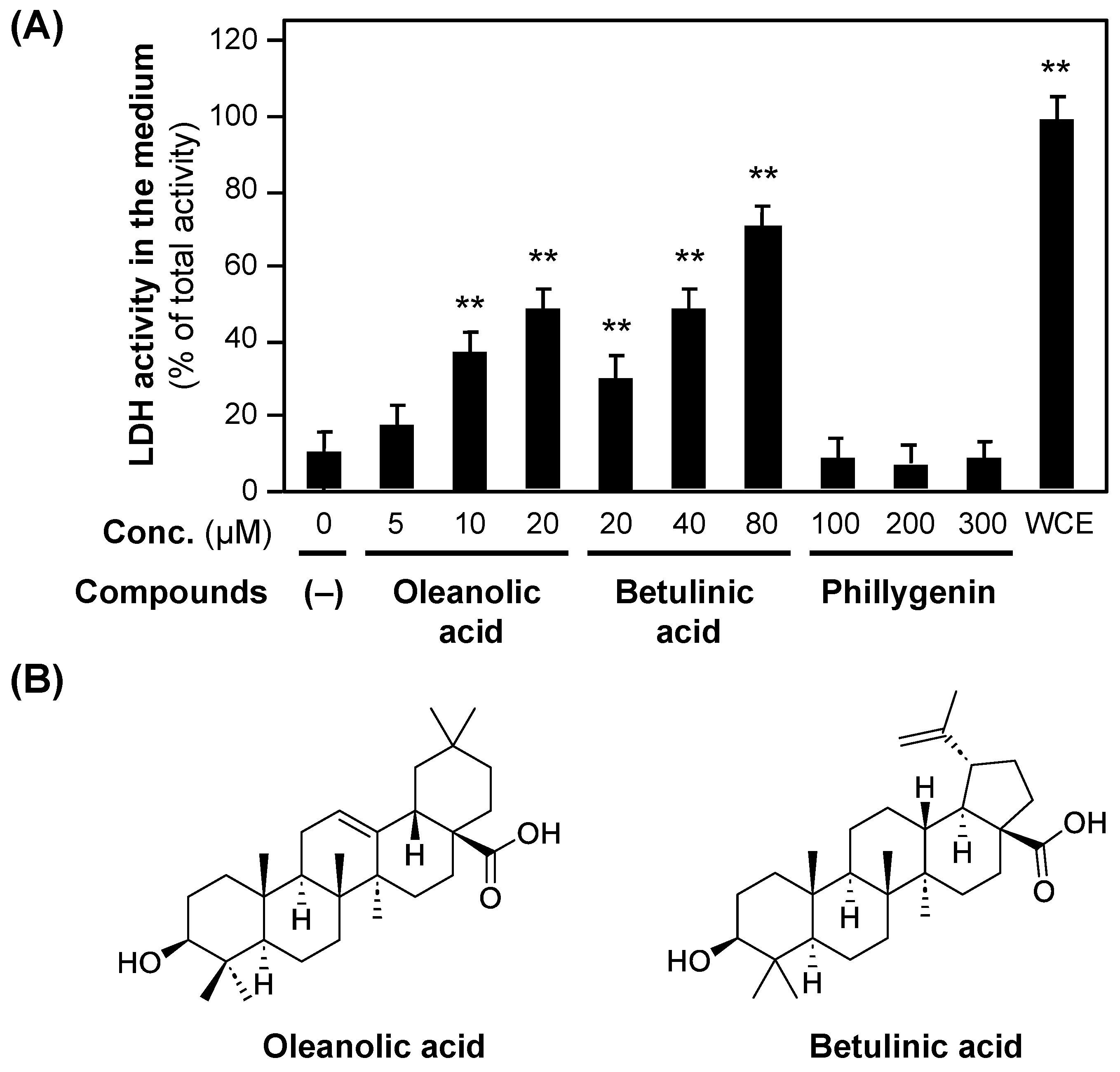
Triterpenoids, e.g., oleanolic acid, are present in Forsythia suspensa fruits [24] and Zizyphus jujuba fruits [25], and the cytotoxicity of betulinic acid has been reported [25,26]. The subfractions were developed by TLC using the standards of oleanolic and betulinic acids. Spots with the same Rf as those of oleanolic (Rf = 0.13) and betulinic acid (Rf = 0.20) were visualized by using the p-anisaldehyde–sulfuric acid coloring reaction. The spots observed via TLC indicated that oleanolic and betulinic acids were present in subfractions A3 and A4.
To investigate whether these triterpenoids are cytotoxic, the LDH activity of the medium was measured in the presence of oleanolic or betulinic acid. As shown in Figure 6, both oleanolic and betulinic acids increased LDH activity in a concentration-dependent manner. In contrast, (+)-phillygenin resulted in background LDH activity at concentrations up to 300 μM, indicating no cytotoxicity.
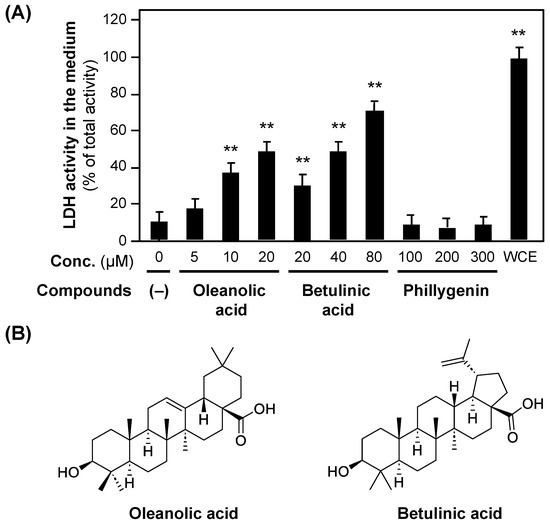
Figure 6.
The cytotoxicity of oleanolic acid and betulinic acid. (A) The cytotoxicity of oleanolic acid and betulinic acid, measured by LDH activity in the hepatocyte medium. Oleanolic acid, betulinic acid, or (+)-phillygenin was added to the medium at the indicated concentrations. After incubation for 8 h, the LDH activity of the medium was measured. When the LDH activity of the whole-cell extract (WCE) of hepatocytes on a dish was set at 100%, each LDH activity was recorded. ** p < 0.01 versus LDH activity at 0 μM. (B) The chemical structures of oleanolic acid and betulinic acid.
The other subfractions, excluding A3 and A4, which did not include oleanolic or betulinic acid, were eluted by silica gel column chromatography. These subfractions were examined to determine whether they inhibited NO production in hepatocytes. One subfraction that markedly inhibited NO production was further purified to obtain a compound (174 mg) (Supplementary Materials Data S1.4.), which was designated as Compound 4 and analyzed by NMR spectroscopy and MS analyses.
Compound 4: White powder. [α +95.4° (c 0.763, CH3OH); EI–MS spectrum m/z 372.1562 (M+) (calculated for C21H24O6; 372.1573). 1H NMR (400 MHz, CDCl3, ppm) δ 2.92 (1H, m, H-8), 3.33 (1H, m, H-9′ ax), 3.35 (1H, m, H-8′), 3.85 (2H, m, H-9 eq, H-9′ eq), 3.87(3H, s, -OCH3), 3.89 (3H, s, -OCH3), 3.90 (3H, s, -OCH3), 4.13 (1H, d, J = 9.6 Hz, H-9), 4.42 (1H, d, J = 7.2 Hz, H-7), 4.87 (1H, d, J = 5.9 Hz, H-7′), 5.60 (1H, s, -OH), 6.82–6.92 (6H, m, H-2, 5, 6, 2′, 5′, 6′). 13C NMR (400 MHz, CDCl3, ppm) δ 50.2 (C-8′), 54.5 (C-8), 55. 9 (OCH3), 56.0 × 2 (-OCH3 × 2), 69.7 (C-9′), 71.0 (C-9), 82.1 (C-7′), 87.8 (C-7), 108.6 (C-2′), 109.0 (C-2), 111.0 (C-5′), 114.3 (C-5), 117.8 (C-6′), 119.3 (C-6), 131.0 (C-1′), 133.1 (C-1), 145.4 (C-4), 146.8 (C-4′), 148.0 (C-3), 148.9 (C-3′). The 1H and 13C NMR spectra of this compound were identical to previously published spectral data for (+)-phillygenin [27]. The EI–MS spectrum data indicated that this compound was calculated for C21H24O6 (372.1573), which is identical to the molecular formula of phillygenin. The optical rotation measured was close to the reported value [α + 90.0° (c 0.2, CH3OH) of (+)-phillygenin [28]. Overall, we concluded that Compound 4 is (+)-phillygenin.
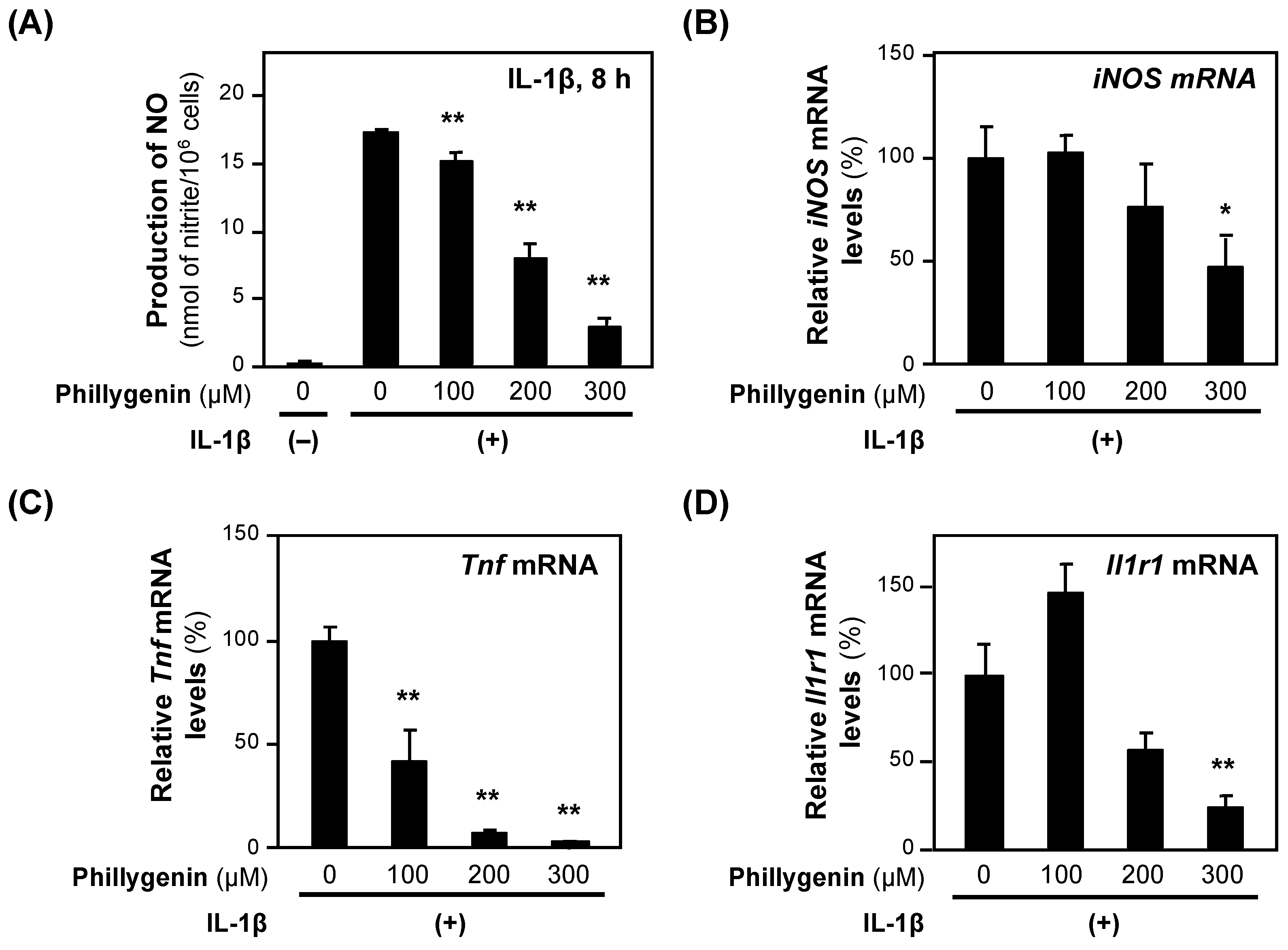
When (+)-phillygenin was added to the medium of the rat hepatocytes together with IL-1β, the NO concentration decreased in a concentration-dependent manner without showing cytotoxicity (Figure 7A). The calculated IC50 value of (+)-phillygenin for the suppression of NO production was 193.0 ± 47.6 μM. Like other anti-inflammatory compounds, (+)-phillygenin decreased the levels of iNOS mRNA and mRNAs transcribed from proinflammatory genes, such as Tnf and Il1r1 mRNAs (Figure 7B–D). These results indicate that (+)-phillygenin is an anti-inflammatory compound.
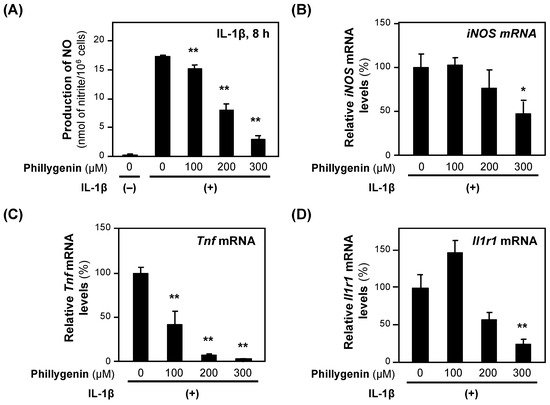
Figure 7.
The effects of (+)-phillygenin on the expression of proinflammatory genes. (A) A decrease in NO production by (+)-phillygenin in hepatocytes. (+)-Phillygenin and IL-1β were added to the medium of primary-cultured rat hepatocytes and incubated for 8 h until the NO concentration was measured. Cytotoxicity was not observed at the concentrations applied. (B–D) mRNA levels in hepatocytes. After incubation with (+)-phillygenin and IL-1β, the total RNA was extracted. The level of each mRNA was measured by RT–qPCR and normalized to the Ef1a mRNA level. The relative mRNA levels (%) of iNOS mRNA (B), Tnf mRNA (C), and Il1r1 mRNA are presented as the means ± SDs (n = 3) when the mRNA level measured was set at 100% in the presence of IL-1β alone. * p < 0.05 and ** p < 0.01 versus IL-1β alone.
3. Discussion
Empirical pharmacological function is an important concept in Kampo medicine. In this study, we investigated 10 typical crude drugs for clearing heat and demonstrated that the empirical function of clearing heat involves many anti-inflammatory compounds. Inflammation is characterized by four cardinal signs, i.e., calor (heat), dolor (pain), rubor (redness), and tumor (swelling) [29], and the anti-inflammatory compounds attenuate inflammatory responses, resulting in the cooling of the body.
During the purification of the compounds from Fraction A, we selected the most active subfraction, i.e., one that prominently suppressed NO production. Consequently, we identified constituents that are responsible for anti-inflammatory effects, such as baicalein in Scutellaria roots [30,31] and (+)-phillygenin in Forsythia fruits [32]. The isolation of loniflavone from Lonicera japonica (stems and leaves collected in China) is second, followed by the first isolation from Lonicera japonica (leaves collected in India) [33]. We first reported the anti-inflammatory effects of loniflavone in this study. Anti-inflammatory compounds may be isolated from Fraction A of Rehmannia roots, when a large amount of plant material is used for the purification. At least several anti-inflammatory compounds are expected to be present in Fraction A. The contribution of each compound to the suppression of NO production depends on its IC50 value and content in the extract.
Bioactive compounds are not always major constituents of a crude drug. Baicalin (a baicalein glucuronide), which is present in Fraction B of the Scutellaria root extract, reduced NO production, similar to the baicalein in Fraction A. Baicalin had an IC50 value of 55.0 μM, which was 3.3-fold greater than that of baicalein in IL-1β-treated hepatocytes (A.F., K.I., M.N. unpublished data). In contrast, geniposide (an iridoid glucoside), which is a major constituent primarily present in Fraction B of Gardenia fruit extract, did not decrease NO production in IL-1β-treated hepatocytes. In LPS-treated RAW264.7 cells, geniposide reduced NO production with an IC50 value of 136 µM [12], which may be caused by differences in the cell types [14]. Because bacterial β-glucosidase hydrolyzes geniposide to genipin in the intestine [34,35], geniposide functions as genipin in vivo. The bioavailability of glycosides in crude drugs may be improved by the bacterial conversion. Although the amount of each crude drug in a Kampo formula is determined by the Japanese Pharmacopoeia [3], effective doses of the compounds in the crude drugs may be investigated in future.
The IC50 value of Fraction A from Forsythia fruit extract could not be determined because hepatocytes partly detached from the dish bottom, suggesting the cytotoxicity of betulinic and oleanolic acids to hepatocytes (Figure 6). Oleanolic acid is abundant in Forsythia suspensa fruits [24], and betulinic acid is widely distributed in plants, such as Betula platyphylla and Ziziphus jujuba Miller var. spinosa Hu ex H. F. Chou. In accordance with our findings, betulinic acid is cytotoxic to tumor cell lines [25,26], and these triterpenoids induced apoptosis in hepatocellular carcinoma cell lines [36,37]. Therefore, small amounts of these triterpenoids co-existing in an extract or Fraction A may mask the anti-inflammatory effects of other compounds, including (+)-phillygenin. Because cytotoxic compounds are sometimes present in crude drugs, separation by silica gel chromatography may help identify the compounds of interest.
When the IC50 values of the identified anti-inflammatory compounds for the suppression of NO production in hepatocytes were compared, they presented variable values ranging from 2.6 μM (obakunone) to 652 μM (chlorogenic acid) (Table 3). It is difficult to identify common structures among them. Our previous studies revealed other anti-inflammatory compounds; for example, atractylodin (polyacetylene) from Atractylodes chinensis rhizomes inhibited NO production with an IC50 value of 8.25 μM in hepatocytes [38]. In contrast, β-eudesmol and (+)-hinesol, which are major constituents of A. chinensis rhizome Fraction A, exhibited low activity, and their IC50 values could not be calculated.

Table 3.
The IC50 values of anti-inflammatory compounds in crude drugs for clearing heat.
The compounds identified in this study decreased the expression of the iNOS genes, at least at a transcriptional step. Because baicalein reduced the NO production, iNOS protein, and iNOS mRNA in hepatocytes (Figure 3), it inhibits the iNOS gene expression at a transcriptional step through transcription factors. The other compounds identified in this study and 1,2,3,4,6-pentagalloyl-β-D-glucose [8] reduced the NO production by decreasing both iNOS protein and mRNA (Figure 4, Figure 5 and Figure 7), suggesting they inhibit the iNOS gene expression at a transcriptional step. Furthermore, anti-inflammatory compounds, including obakunone [7], chlorogenic acid [9], genipin [10], and baicalein inhibited the iNOS gene expression at transcriptional and post-transcriptional steps by reducing iNOS-AS [18]. Collectively, the compounds purified from the crude drugs for clearing heat downregulate the iNOS gene expression, at least at a transcriptional step.
How do the anti-inflammatory compounds in the crude drugs exert anti-inflammatory effects? Anti-inflammatory compounds or drugs, such as baicalein, attenuate the expression of the Il1r1 gene (Figure 3). IL-1β induces the expression of the Il1r1 gene [22], and nonsteroidal anti-inflammatory drugs (NSAIDs), such as aspirin and loxoprofen, suppress Il1r1 gene expression in rat hepatocytes [14]. NSAIDs modulate the nuclear factor κB (NF-κB) signaling pathway to inhibit the iNOS gene expression by reducing iNOS mRNA and antisense transcript (iNOS-AS) levels [14,18]. Nyasol and phillygenin inhibited inflammatory responses via the NF-κB pathway in the LPS-treated RAW264.7 macrophage line [39,40]. Phillygenin inhibited LPS-induced inflammatory responses through the Toll-like receptor 4 (TLR4)–NF-κB signaling pathway in the human hepatic stellate cell line LX-2 [41]. Furthermore, when the nonalkaloid fraction of Phellodendron bark, which includes the strong anti-inflammatory compound obakunone, was administered to healthy mice, prominent decreases in iNOS and Il1r1 mRNA levels were observed in their livers [7]. Baicalein showed anti-inflammatory effects by inhibiting the translocation of the phosphorylated p65 subunit of NF-κB to the nucleus [42,43]. Baicalein attenuated rat liver injuries caused by ischemia–reperfusion and partial hepatectomy by suppressing inflammatory responses through NF-κB and Akt signaling [44]. Sakuranetin [(S)-(–)-4′,5-dihydroxy-7-methoxyflavanone], which is an anti-inflammatory compound of cherry bark (a crude drug of Kampo medicines), exhibited similar effects. Sakuranetin decreases both Il1r1 gene expression and the phosphorylation of the activator isoforms of the transcription factor CCAAT/enhancer-binding protein β (C/EBPβ), which synergistically activates the transcription of the iNOS gene with NF-κB [22]. Because IL1R1 is a key protein that regulates proinflammatory genes, repressing the expression of the Il1r1 gene by anti-inflammatory compounds results in decreased proinflammatory responses.
Our method using hepatocytes is one of the in vitro approaches to examine anti-inflammatory effects of the crude drugs for clearing heat, although it is difficult to elucidate their multiple interacting mechanisms or synergies [45]. Animals may be used to investigate various anti-inflammatory mechanisms. The administration of non-alkaloid fractions of Phellodendron bark and Coptis rhizome downregulated a variety of genes that are involved in inflammation [7], such as activating transcription factor 2 (Atf2) [46], Krüppel-like factor 10 (Klf10) [47], and suppressor of cytokine signaling 3 (Socs 3) [48]. They are key molecules in the signaling pathways, other than the NF-κB pathway, and may modulate inflammatory responses and interacting mechanisms. In addition, isocoumarines inhibited the enzymes in the leukotriene and prostaglandin pathways [49,50]. Therefore, compounds of the crude drugs for clearing heat might affect various biological activities, including anti-oxidant, antimicrobial, neuroprotective, and anticancer activities.
A. chinensis rhizomes are classified as crude drugs that circulate fluid retention (‘Risui’) [1,5,6], and this empirical function is closely related to the treatment of kidney disease. The administration of A. chinensis rhizome Fraction A to high immunoglobulin A (HIGA) mice [51], a model of human immunoglobulin A (IgA) nephropathy, prominently reduced IgA deposition in renal glomeruli [38]. Atractylodin, β-eudesmol, and (+)-hinesol are present in this Fraction A and may improve the glomerular IgA deposition [38]. Therefore, the empirical function of circulating fluid retention cannot be simply explained by anti-inflammatory effects alone.
An empirical function classified as ‘Hoketsu’ involves replenishing blood to improve blood deficiency [1,5,6]. Polygonum multiflorum roots are classified as a crude drug that replenishes blood. We found that this crude drug promoted the renal production of erythropoietin, which stimulates red blood cell production in healthy mice [52], which may explain its empirical function in replenishing blood. There are many other empirical functions in the concept of Kampo medicine, such as Hoki (reinforcing qi to improve qi deficiency). Here, we elucidate aspects of the empirical function Seinetsu. Using other systems or model animals, the empirical functions of Kampo medicine may be explained by modern pharmacological functions in the future.
4. Materials and Methods
4.1. General Experimental Procedures and Reagents
The NMR spectra were recorded using a JNM-ECS400 NMR spectrometer (JEOL Ltd., Akishima, Tokyo, Japan), which was operated at 400 MHz (1H) and 100 MHz (13C). Deuterated chloroform (CDCl3), methanol (CD3OD), dimethyl sulfoxide [(CD3)2SO], and tetramethylsilane (internal standard) were purchased from Eurisotop (Saint-Aubin, France). The EI–MS spectra were obtained with a JMS-700 MStation mass spectrometer (JEOL Ltd.). The optical rotations of the compounds were measured using a DIP-1000 polarimeter (JASCO Corporation, Hachioji, Tokyo, Japan). The standards baicalein, genipin, geniposide, and betulinic acid were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), and the oleanolic acid 4-methoxybenzaldehyde (=p-anisaldehyde) was purchased from Fujifilm Wako Pure Chemical Corporation (Osaka, Japan).
4.2. Plant Materials
The crude drugs, authenticated by Dr. Yutaka Yamamoto (Tochimoto Tenkaido Co., Ltd., Osaka, Japan) were obtained from Tochimoto Tenkaido Co., Ltd., and the voucher samples were deposited in the Ritsumeikan Herbarium of Pharmacognosy, Ritsumeikan University, under the following code numbers. Gardenia jaminoides fruits were collected in Zhejiang Province, China (No. RIN-GJ-46), Scutellaria baicalensis roots were collected in the Inner Mongolia Autonomous Region, China (No. RIN-SB-52), Anemarrhena asphodeloides rhizomes were collected in Hebei Province, China (No. RIN-AS-53), Forsythia suspensa fruits were collected in Shaanxi Province, China (No. RIN-FS-54), the leaves and stems of Lonicera japonica were collected in Shandong Province, China (No. RIN-LJ-55), and Rehmannia glutinosa roots were collected in Shaanxi Province, China (No. RIN-RG-56).
4.3. Extraction and Crude Fractionation
The dried crude drugs were extracted with methanol and the resulting extracts were successively extracted with EtOAc and n-butanol to obtain EtOAc-soluble (Fraction A), n-butanol-soluble (Fraction B), and water-soluble fractions (Fraction C), as previously described [9] (Scheme 1). The yield of the extracts and the partitioning ratios of the crude fractions are shown in Table 1.
4.4. Purification of Anti-Inflammatory Compounds
The compounds were purified from Fraction A using several methods guided by NO assays in hepatocytes. Silica gel column chromatography was performed with silica gel 60 (Nacalai Tesque Inc., Kyoto, Japan) or Wakogel C-300 HG (Fujifilm Wako Pure Chemical Corporation). PLC silica gel 60 F254, 0.5 mm glass plates (Merck KGaA, Darmstadt, Germany) were used to perform preparative TLC. The subfraction that suppressed the NO production was further purified to obtain compounds. The details are described in Supplementary Materials Data, S1.
4.5. Measuring the Content of Compounds
The content of each compound was estimated using HPLC according to a previously published method [22], HPLC analysis was performed to estimate the content of compounds. An HPLC system with an LC-20AT pump equipped with an SPD-20A UV/VIS detector (Shimadzu Corporation, Kyoto, Japan) and a Cosmosil 5C18 MS-II column (4.6 mm internal diameter × 150 mm; Nacalai Tesque Inc.) was used. The details are described in Supplementary Materials Data, S2.
4.6. TLC and p-Anisaldehyde–Sulfuric Acid Color Reactions
The constituents were developed on a silica gel 60 F254 plate (Fujifilm Wako Pure Chemical Corporation). The plate was sprayed with a solution prepared from 2.6 mL p-anisaldehyde in 1.6 mL glacial acetic acid, 3.4 mL 97% sulfuric acid, and 92 mL ethanol and heated to 105 °C for 10 min to visualize spots of phenols, sugars, steroids, and terpenoids.
4.7. Animals and Primary-Cultured Rat Hepatocytes
Specific pathogen-free male Wistar rats (5–6 weeks old, Charles River Laboratories Japan, Inc., Yokohama, Japan) were purchased and maintained at 21–23 °C under a 12 h light–dark cycle. A γ-ray-irradiated CRF-1 diet (Charles River Laboratories Japan, Inc.) was provided with water available ad libitum. The acclimatization time was at least one week. All procedures involving animal care and experiments were performed in accordance with the laws and guidelines of the Japanese government and were approved by the Animal Care Committee of Ritsumeikan University, Biwako-Kusatsu Campus under Nos. BKC2020-045 and BKC2023-052.
After acclimatization, the Wistar rats were euthanized by the intraperitoneal administration of an anesthetic combination of medetomidine hydrochloride, midazolam, and butorphanol tartrate. Rat livers were used to prepare hepatocytes as previously described [53,54]. In brief, the liver was perfused with collagenase. The cells were resuspended in Williams’ E (WE) medium supplemented with 10% newborn calf serum, 10 nM dexamethasone, and 10 nM human insulin and seeded at 1.2 ×106 cells per 35 mm diameter dish. After the medium was replaced with serum-free WE medium, the cells were incubated overnight. The next day, the medium was replaced with WE medium containing 1 nM recombinant rat IL-1β [54] and/or a fraction or compound. The hepatocytes were further incubated at 37 °C for 4 h before RNA extraction or for 8 h before measuring the NO levels and performing a Western blot analysis of the cell lysates.
4.8. NO Assay and LDH Activity
After 8 h of incubation with 1 nM IL-1β and/or a fraction or compound, nitrite (a stable metabolite of NO) was measured in triplicate using the Griess method [14,55]. The NO concentration in the medium alone was set at 0%, whereas the concentration in the medium including IL-1β alone was set at 100%. Unless a fraction or compound exhibited cytotoxicity, the IC50 values of nitrite were calculated for three different concentrations [14]. Cytotoxicity was monitored by estimating the LDH activity in the medium using LDH Cytotoxicity Detection Kits (Takara Bio Inc., Kusatsu, Japan). The Griess reagent [14,55] was added to the medium and incubated at 20–23 °C for 5 min, and the absorbance at 540 nm was measured to determine the decrease in nitrite. The LDH activity of the whole-cell extract was assumed to be 100%, whereas the LDH activity of the medium alone was assumed to have no cytotoxicity (i.e., less than 5% of the LDH activity of the whole-cell extract).
4.9. Immunoblot Analysis
Hepatocyte lysates were prepared, resolved on a 10% sodium dodecyl sulfate–polyacrylamide gel, and transferred onto a membrane, as previously described [22]. After the samples were blocked with 5% skim milk, immunostaining was performed with antibodies against iNOS (BD Biosciences, San Jose, CA, USA) and β-tubulin, and with a horseradish peroxidase-conjugated anti-immunoglobulin Fc antibody (Cell Signaling Technology Inc., Danvers, MA, USA). The protein of interest was visualized by ECL Western blotting Detection Reagents (Cytiva, Tokyo, Japan) and detected using an Amersham Imager 600 (Cytiva).
4.10. RT–qPCR
Hepatocytes were lysed in Sepasol I Super G solution (Nacalai Tesque, Inc.) to extract total RNA, which was purified using an RNAqueous Kit and a TURBO DNA-free Kit (Applied Biosystems, Austin, TX, USA). The resulting RNA was converted to cDNA, which was amplified by PCR in triplicate with primers [54], SYBR Green I, and the Thermal Cycler Dice Real-Time System (Takara Bio Inc.) [18]. Ct values were calculated by the ΔΔCt method and then normalized to elongation factor 1α (Ef1a) mRNA [18,54]. The mRNA levels normalized to the total RNA from the livers of the mice fed a diet alone were set at 100%.
4.11. Statistical Analysis
The data are representative of at least three independent experiments that provided similar findings. The values are presented as the means ± SDs. The differences were analyzed using Student’s t test followed by the Bonferroni correction. The statistical significance was set at 0.05 or 0.01.
5. Conclusions
Based on the concepts of Kampo medicine, 10 typical crude drugs for clearing heat were examined for their anti-inflammatory effects. The EtOAc-soluble fraction from the crude drugs, except Forsythia fruits, exhibited anti-inflammatory effects, and anti-inflammatory compounds were identified from these extracts, with the exception of that from Rehmannia roots. Through the removal of cytotoxic compounds, the anti-inflammatory compound (+)-phillygenin was purified from Fraction A of the Forsythia fruit extract. Our approach may support the idea that anti-inflammatory compounds in crude drugs provide the empirical function of clearing heat to cool the body. Other empirical functions of Kampo medicine may also be explained by modern pharmacological activities in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30020416/s1, Purification of anti-inflammatory compounds from crude drugs. Measuring the content of compounds.
Author Contributions
Conceptualization, A.F., Y.I. and M.N.; methodology, Y.N., Y.I. and M.N.; validation, A.F. and S.S.; formal analysis, A.F. and M.N.; investigation, A.F., S.O., N.W., M.K., K.I. and K.M.; resources, M.N.; data curation, A.F., Y.I. and M.N.; writing—original draft preparation, A.F. and M.N.; writing—review and editing, Y.N., Y.I. and M.N.; visualization, A.F. and S.S.; supervision, Y.I. and M.N.; project administration, M.N.; funding acquisition, M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by the Asia-Japan Research Institute, Ritsumeikan Asia-Japan Research Organization, Ritsumeikan University (Ibaraki, Osaka, Japan).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care Committee of Ritsumeikan University, Biwako-Kusatsu Campus (Nos. BKC2020-045 and BKC2023-052).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Yuji Hasegawa (Daiichi University of Pharmacy) for MS analyses, Tadayoshi Okumura and Tetsuya Okuyama (Kansai Medical University, Hirakata, Osaka, Japan) for their invaluable advice, Ashley Polak (Illinois College, IL, USA), many undergraduate students in the Medical Chemistry Division, Department of Biomedical Sciences, Faculty of Life Sciences, Ritsumeikan University for their technical assistance, and Noriko Kanazawa for her secretarial assistance. We also thank the Asia-Japan Research Institute (AJI), Asia-Japan Research Organization, Ritsumeikan University (Ibaraki, Osaka, Japan) for supporting this study.
Conflicts of Interest
Saki Onishi, Nodoka Watanabe, Mizuki Kajimura, and Keita Minamisaka performed the experiments as graduate students of the Graduate School of Life Sciences, Ritsumeikan University. Kentaro Ito performed the experiments as an undergraduate student of the Faculty of Life Sciences, Ritsumeikan University. The authors declare no conflicts of interest.
References
- Ikeya, Y.; Epp, D.A.; Nishizawa, M. Anti-inflammatory effect of foods and crude drugs in relation to bitter and spicy tastes. Bioact. Compd. Health Dis. 2019, 2, 77–93. [Google Scholar] [CrossRef]
- Borchardt, J.K. Traditional Chinese drug therapy. Drug News Perspect. 2003, 16, 698–702. [Google Scholar] [CrossRef] [PubMed]
- The Committee on the Japanese Pharmacopoeia. The Japanese Pharmacopoeia, 18th ed.; The Minister of Health, Labour and Welfare: Tokyo, Japan, 2021; Available online: https://www.mhlw.go.jp/content/11120000/000912390.pdf (accessed on 7 January 2025).
- Cheng, J.T. Review: Drug therapy in Chinese traditional medicine. J. Clin. Pharmacol. 2000, 40, 445–450. [Google Scholar] [CrossRef]
- Nemoto, Y. (Ed.) Explanation of Crude Drugs in 294 Kampo Formulae, 2nd ed.; Jiho, Inc.: Tokyo, Japan, 2021; ISBN 978-4840754002. (In Japanese) [Google Scholar]
- KEGG BRITE, Traditional Chinese Medicine in Japan. Available online: https://www.kegg.jp/kegg-bin/show_brite?br08304.keg (accessed on 7 January 2025).
- Fujii, A.; Okuyama, T.; Wakame, K.; Okumura, T.; Ikeya, Y.; Nishizawa, M. Identification of anti-inflammatory constituents in Phellodendri Cortex and Coptidis Rhizoma by monitoring the suppression of nitric oxide production. J. Nat. Med. 2017, 71, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Okuyama, T.; Ikeya, Y.; Nishizawa, M. Anti-inflammatory effects of hydrophobic constituents in the extract of the root cortex of Paeonia suffruticosa. Bioact. Compd. Health Dis. 2022, 5, 160–173. [Google Scholar] [CrossRef]
- Ohno, N.; Yoshigai, E.; Okuyama, T.; Yamamoto, Y.; Okumura, T.; Sato, K.; Ikeya, Y.; Nishizawa, M. Chlorogenic acid from the Japanese herbal medicine Kinginka (Flos Lonicerae japonicae) suppresses the expression of inducible nitric oxide synthase in rat hepatocytes. HOAJ Biol. 2012, 1, 2. [Google Scholar] [CrossRef]
- Nakatake, R.; Tsuda, T.; Matsuura, T.; Miki, H.; Hishikawa, H.; Matsushima, H.; Ishizaki, M.; Matsui, K.; Kaibori, K.; Nishizawa, M.; et al. Genipin Inhibits the Induction of Inducible Nitric Oxide Synthase Through the Inhibition of NF-κB Activation in Rat Hepatocytes. Drug Metab. Lett. 2017, 10, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Song, Y.S.; Kim, H.J.; Lee, Y.H.; Hong, S.M.; Kim, S.J.; Kim, B.C.; Jin, C.; Lim, C.J.; Park, E.H. Antiinflammatory effects of genipin, an active principle of gardenia. Eur. J. Pharmacol. 2004, 495, 201–208. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, W. Alleviative Effect of Geniposide on Lipopolysaccharide-Stimulated Macrophages via Calcium Pathway. Int. J. Mol. Sci. 2024, 25, 1728. [Google Scholar] [CrossRef] [PubMed]
- Kitade, H.; Sakitani, K.; Indue, K.; Masu, Y.; Kawada, N.; Hiramatsu, Y.; Kamiyama, Y.; Okumura, T.; Ito, S. Interleukin 1β Markedly Stimulates Nitric Oxide Formation in the Absence of Other Cytokines or Lipopolysaccharide in Primary Cultured Rat Hepatocytes but Not in Kupffer Cells. Hepatology 1996, 23, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Yoshigai, E.; Okuyama, T.; Murakoshi, M.; Sugiyama, K.; Nishino, H.; Nishizawa, M. Antipyretic Analgesic Drugs Have Different Mechanisms for Regulation of the Expression of Inducible Nitric Oxide Synthase in Hepatocytes and Macrophages. Nitric Oxide 2015, 44, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Facchin, B.M.; Dos Reis, G.O.; Vieira, G.N.; Mohr, E.T.B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and meta-analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Y.; Li, X.C.; Liu, W.Y.; Xu, Y.H.; Feng, F.; Qu, W. An efficient, scalable approach to hydrolyze flavonoid glucuronides via activation of glycoside bond. Tetrahedron 2017, 73, 1895–1903. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, S.; Zhang, H.; Lu, Z.; Deng, R.; Feng, Y.; Liu, P. Comparative Study of the Flavonoid Content in Radix Scutellaria from Different Cultivation Areas in China. Int. J. Anal. Chem. 2023, 2023, 3754549. [Google Scholar] [CrossRef]
- Matsui, K.; Nishizawa, M.; Ozaki, T.; Kimura, T.; Hashimoto, I.; Yamada, M.; Kaibori, M.; Kamiyama, Y.; Ito, S.; Okumura, T. Natural Antisense Transcript Stabilizes Inducible Nitric Oxide Synthase Messenger RNA in Rat Hepatocytes. Hepatology 2008, 30, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.; Yu, J.R.; Lee, J.; Chang, J.; Seo, E.K. Identification of nyasol and structurally related compounds as the active principles from Anemarrhena asphodeloides against respiratory syncytial virus (RSV). Chem. Biodivers. 2007, 4, 2231–2235. [Google Scholar] [CrossRef]
- Minami, E.; Taki, M.; Takaishi, S.; Iijima, Y.; Tsutumi, S.; Akiyama, T. Stereochemistry of cis- and trans-Hinokiresinol and Their Estrogen-like Activity. Chem. Pharm. Bull. 2000, 48, 389–392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bahia, M.V.; David, J.P.; David, J.M. Occurrence of biflavones in leaves of Caesalpinia pyramidalis specimens. Quím. Nova 2010, 33, 1297–1300. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Okuyama, T.; Ishii, T.; Okumura, T.; Ikeya, Y.; Nishizawa, M. Sakuranetin Downregulates Inducible Nitric Oxide Synthase Expression by Affecting Interleukin-1 Receptor and CCAAT/Enhancer-Binding Protein β. J. Nat. Med. 2019, 73, 353–368. [Google Scholar] [CrossRef]
- KEGG, DRUG: Rehmannia Root. Available online: https://www.kegg.jp/entry/D06736 (accessed on 7 January 2025).
- Ren, T.; Xu, Z. Study of isomeric pentacyclic triterpene acids in traditional Chinese medicine of Forsythiae Fructus and their binding constants with β-cyclodextrin by capillary electrophoresis. Electrophoresis 2018, 39, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Min, B.S.; Lee, C.G.; Kim, K.S.; Kho, Y.H. Cytotoxic triterpenoids from the fruits of Zizyphus jujuba. Planta Med. 2003, 69, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhang, H.; Liu, Q.; Zhao, Y.; Cui, X.; Guo, S.; Zhang, L.; Ho, C.T.; Bai, N. Chemical characterization of the main bioactive constituents from fruits of Ziziphus jujuba. Food Funct. 2016, 7, 2870–2877. [Google Scholar] [CrossRef]
- Rahman, M.M.A.; Dewick, P.M.; Jackson, D.E.; Lucas, J.A. Lignans of Forsythia intermedia. Phytochemistry 1990, 29, 1971–1980. [Google Scholar] [CrossRef]
- Chang, M.J.; Hung, T.M.; Min, B.S.; Kim, J.C.; Woo, M.H.; Choi, J.S.; Lee, H.K.; Bae, K.H. Lignans from the Fruits of Forsythia suspensa (Thunb.) Vahl Protect High-Density Lipoprotein during Oxidative Stress. Biosci. Biotechnol. Biochem. 2008, 72, 2750–2755. [Google Scholar] [CrossRef] [PubMed]
- Tracy, R.P. The five cardinal signs of inflammation: Calor, Dolor, Rubor, Tumor … and Penuria (Apologies to Aulus Cornelius Celsus, De medicina, c. A.D. 25). J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1051–1052. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Sabry, K.; Jamshidi, Z.; Emami, S.A.; Sahebka, A. Potential therapeutic effects of baicalin and baicalein. Avicenna J. Phytomed. 2024, 14, 23–49. [Google Scholar] [CrossRef]
- Wang, C.; Wu, R.; Zhang, S.; Gong, L.; Fu, K.; Yao, C.; Peng, C.; Li, Y. A comprehensive review on pharmacological, toxicity, and pharmacokinetic properties of phillygenin: Current landscape and future perspectives. Biomed. Pharmacother. 2023, 166, 115410. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, B.; Bhandari, P.; Gupta, A.P.; Uniyal, S.K.; Kaul, V.K. Biflavonoids from Lonicera japonica. Phytochemistry 2005, 66, 2740–2744. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Kobashi, K.; Aburada, M. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol. Pharm. Bull. 1994, 17, 1573–1576. [Google Scholar] [CrossRef]
- Yim, J.S.; Kim, Y.S.; Moon, S.K.; Cho, K.H.; Bae, H.S.; Kim, J.J.; Park, E.K.; Kim, D.H. Metabolic activities of ginsenoside Rb1, baicalin, glycyrrhizin and geniposide to their bioactive compounds by human intestinal microflora. Biol. Pharm. Bull. 2004, 27, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Liu, M.; Zhang, Y.; Yang, T.; Li, D.; Huang, Y.; Li, Q.; Bai, G.; Shi, L. Betulinic acid induces apoptosis and suppresses metastasis in hepatocellular carcinoma cell line in vitro and in vivo. J. Cell. Mol. Med. 2019, 23, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, X.; Dong, S.; Zhou, W. Betulinic acid in the treatment of tumour diseases: Application and research progress. Biomed. Pharmacother. 2021, 142, 111990. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Okuyama, T.; Noguchi, N.; Nishidono, Y.; Okumura, T.; Kaibori, M.; Tanaka, K.; Terabayashi, S.; Ikeya, Y.; Nishizawa, M. Antiinflammatory constituents of Atractylodes chinensis rhizome improve glomerular lesions in immunoglobulin A nephropathy model mice. J. Nat. Med. 2020, 74, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Chung, H.J.; Pyee, Y.; Hong, J.Y.; Youn, U.J.; Seo, E.K.; Lee, S.K. Suppression of Inducible Nitric Oxide Synthase Expression by Nyasol and Broussonin A, Two Phenolic Compounds from Anemarrhena asphodeloides, through NF-kB Transcriptional Regulation in vitro and in vivo. Chem. Biodivers. 2014, 11, 749–759. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, Y.; Liao, L.; Liu, M.; Deng, Y.; Zhao, X.; Li, Y. Phillygenin inhibited LPS-induced RAW 264.7 cell inflammation by NF-κB pathway. Eur. J. Pharmacol. 2021, 899, 174043. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Wang, C.; Dai, X.; Zhou, M.; Gong, L.; Yu, L.; Peng, C.; Li, Y. Phillygenin inhibits LPS-induced activation and inflammation of LX2 cells by TLR4/MyD88/NF-κB signaling pathway. J. Ethnopharmacol. 2020, 248, 112361. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Song, X.; Bai, H.; Ma, T.; Zhang, Z.; Li, X.; Jiang, R.; Wang, G.; Fan, X.; et al. Effects of baicalein on IL-1β-induced inflammation and apoptosis in rat articular chondrocytes. Oncotarget 2017, 8, 90781–90795. [Google Scholar] [CrossRef]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, T.; Nakatake, R.; Ito, K.; Ishizaki, M.; Yanagida, H.; Kitade, H.; Yoshizawa, K.; Ikeya, Y.; Nishizawa, M.; Sekimoto, M. Hepatoprotective effects of baicalein against liver ischemia-reperfusion injury and partial hepatectomy in a rat model. Mol. Biol. Rep. 2024, 51, 643. [Google Scholar] [CrossRef]
- Leonti, M.; Casu, L. Traditional medicines and globalization: Current and future perspectives in ethnopharmacology. Front. Pharmacol. 2013, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, Y.; Yang, Y.; Qiu, Y.; Wang, Z.; Li, X.; Zhang, W. Emerging roles of activating transcription factor (ATF) family members in tumourigenesis and immunity: Implications in cancer immunotherapy. Genes Dis. 2021, 9, 981–999. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M.; Hawse, J.R.; Rajamannan, N.M.; Ingle, J.N.; Spelsberg, T.C. Functional role of KLF10 in multiple disease processes. Biofactors 2010, 36, 8–18. [Google Scholar] [CrossRef]
- Galic, S.; Sachithanandan, N.; Kay, T.W.; Steinberg, G.R. Suppressor of cytokine signalling (SOCS) proteins as guardians of inflammatory responses critical for regulating insulin sensitivity. Biochem. J. 2014, 461, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Aidhen, I.S.; Doble, M. Inhibition of the enzymes in the leukotriene and prostaglandin pathways in inflammation by 3-aryl isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar] [CrossRef]
- Sudarshan, K.; Boda, A.K.; Dogra, S.; Bose, I.; Yadav, P.N.; Aidhen, I.S. Discovery of an isocoumarin analogue that modulates neuronal functions via neurotrophin receptor TrkB. Bioorg. Med. Chem. Lett. 2019, 29, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Muso, E.; Yoshida, H.; Takeuchi, E.; Yashiro, M.; Matsushima, H.; Oyama, A.; Suyama, K.; Kawamura, T.; Kamata, T.; Miyawaki, S.; et al. Enhanced production of glomerular extracellular matrix in a new mouse strain of high serum IgA ddY mice. Kidney Int. 1996, 50, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Shirako, S.; Ulfa, S.M.; Nishidono, Y.; Dwijayanti, D.R.; Okuyama, T.; Nakatake, R.; Tanaka, K.; Ikeya, Y.; Nishizawa, M. Hydrophobic constituents of Polygonum multiflorum roots promote renal erythropoietin expression in healthy mice. J. Nat. Med. 2023, 77, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Kanemaki, T.; Kitade, H.; Hiramatsu, Y.; Kamiyama, Y.; Okumura, T. Stimulation of Glycogen Degradation by Prostaglandin E2 in Primary Cultured Rat Hepatocytes. Prostaglandins 1993, 45, 459–474. [Google Scholar] [CrossRef]
- Ozaki, H.; Nishidono, Y.; Fujii, A.; Okuyama, T.; Nakamura, K.; Maesako, T.; Shirako, S.; Nakatake, R.; Tanaka, K.; Ikeya, Y.; et al. Identification of Anti-Inflammatory Compounds from Peucedanum praeruptorum Roots by Using Nitric Oxide-Producing Rat Hepatocytes Stimulated by Interleukin 1β. Molecules 2023, 28, 5076. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N]Nitrate in Biological Fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).