Peptide with Dual Roles in Immune and Metabolic Regulation: Liver-Expressed Antimicrobial Peptide-2 (LEAP-2)
Abstract
1. Introduction
2. LEAP-2 Evolutionary History
2.1. Mammals
2.2. Birds, Amphibians, and Reptiles
2.3. Fishes
3. The Antimicrobial and Immunoregulatory Activities of LEAP-2
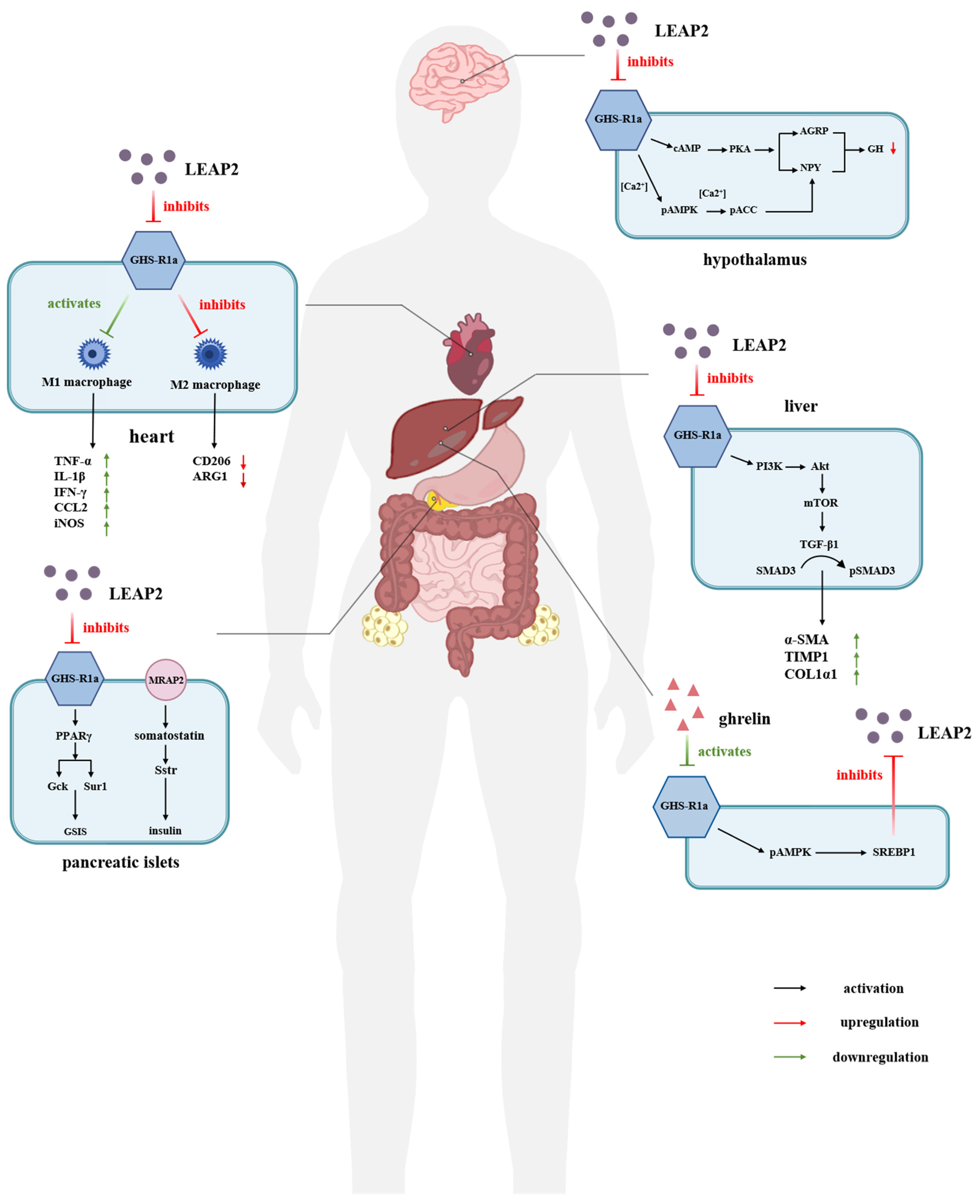
4. LEAP-2 Plays an Important Role in Energy Metabolism by Regulating the Ghrelin–GHSR1a Signaling System
4.1. LEAP-2 in Relation to Food Intake and Appetite
4.2. LEAP-2 Regulation of Blood Glucose
5. Clinical Status and Medicinal Potential of LEAP-2
5.1. LEAP-2 Clinical Application in Obesity and Type 2 Diabetes (T2D)
5.2. LEAP-2 Modulation of Cognition and Memory
5.3. LEAP-2 Regulation of Growth Hormone Secretion
5.4. LEAP-2 Regulation of Cardiovascular Function
5.5. LEAP-2 Treatment of Liver Disease
5.6. Role of LEAP2 in Addictive Disorders
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Brown, K.L.; Hancock, R.E.W. Cationic Host Defense (Antimicrobial) Peptides. Curr. Opin. Immunol. 2006, 18, 24–30. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Borregaard, N.; Cole, A.M. Antimicrobial Peptides in Innate Immune Responses. Contrib. Microbiol. 2008, 15, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.; Neitz, S.; Mägert, H.J.; Schulz, A.; Forssmann, W.G.; Schulz-Knappe, P.; Adermann, K. LEAP-1, a Novel Highly Disulfide-Bonded Human Peptide, Exhibits Antimicrobial Activity. FEBS Lett. 2000, 480, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Marguti, I. Control of Immunopathology during Plasmodium Infection by Hepcidin. Med. Hypotheses 2012, 78, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.; Sillard, R.; Kleemeier, B.; Klüver, E.; Maronde, E.; Conejo-García, J.R.; Forssmann, W.G.; Schulz-Knappe, P.; Nehls, M.C.; Wattler, F.; et al. Isolation and Biochemical Characterization of LEAP-2, a Novel Blood Peptide Expressed in the Liver. Protein Sci. 2003, 12, 143–152. [Google Scholar] [CrossRef]
- Henriques, S.T.; Tan, C.C.; Craik, D.J.; Clark, R.J. Structural and Functional Analysis of Human Liver-Expressed Antimicrobial Peptide 2. Chembiochem 2010, 11, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Hocquellet, A.; Odaert, B.; Cabanne, C.; Noubhani, A.; Dieryck, W.; Joucla, G.; Le Senechal, C.; Milenkov, M.; Chaignepain, S.; Schmitter, J.-M.; et al. Structure-Activity Relationship of Human Liver-Expressed Antimicrobial Peptide 2. Peptides 2010, 31, 58–66. [Google Scholar] [CrossRef]
- Thiébaud, P.; Garbay, B.; Auguste, P.; Sénéchal, C.L.; Maciejewska, Z.; Fédou, S.; Gauthereau, X.; Costaglioli, P.; Thézé, N. Overexpression of Leap2 Impairs Xenopus Embryonic Development and Modulates FGF and Activin Signals. Peptides 2016, 83, 21–28. [Google Scholar] [CrossRef]
- Abizaid, A.; Hougland, J.L. Ghrelin Signaling: GOAT and GHS-R1a Take a LEAP in Complexity. Trends Endocrinol. Metab. 2020, 31, 107–117. [Google Scholar] [CrossRef]
- Ge, X.; Yang, H.; Bednarek, M.A.; Galon-Tilleman, H.; Chen, P.; Chen, M.; Lichtman, J.S.; Wang, Y.; Dalmas, O.; Yin, Y.; et al. LEAP2 Is an Endogenous Antagonist of the Ghrelin Receptor. Cell Metab. 2018, 27, 461–469.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, C.-Y.; Chen, J.-Y.; Seah, R.W.X.; Zhang, L.; Ma, L.; Ding, G.-H. Host Defence Peptide LEAP2 Contributes to Antimicrobial Activity in a Mustache Toad (Leptobrachium Liui). BMC Vet. Res. 2023, 19, 47. [Google Scholar] [CrossRef]
- Fei, Y.; Wang, Q.; Lu, J.; Ouyang, L.; Hu, Q.; Chen, L. New Insights into the Antimicrobial Mechanism of LEAP2 Mutant Zebrafish under Aeromonas Hydrophila Infection Using Transcriptome Analysis. Fish. Shellfish. Immunol. 2023, 143, 109225. [Google Scholar] [CrossRef]
- Hagemann, C.A.; Jensen, M.S.; Holm, S.; Gasbjerg, L.S.; Byberg, S.; Skov-Jeppesen, K.; Hartmann, B.; Holst, J.J.; Dela, F.; Vilsbøll, T.; et al. LEAP2 Reduces Postprandial Glucose Excursions and Ad Libitum Food Intake in Healthy Men. Cell Rep. Med. 2022, 3, 100582. [Google Scholar] [CrossRef] [PubMed]
- van Hoek, M.L. Antimicrobial Peptides in Reptiles. Pharmaceuticals 2014, 7, 723–753. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, Z.; Zhuang, W.; Zhang, J.; He, J.; Xie, Y.; Chen, J. Chicken LEAP2 Level Substantially Changes with Feed Intake and May Be Regulated by CDX4 in Small Intestine. Animals 2022, 12, 3496. [Google Scholar] [CrossRef]
- Sang, Y.; Ramanathan, B.; Minton, J.E.; Ross, C.R.; Blecha, F. Porcine Liver-Expressed Antimicrobial Peptides, Hepcidin and LEAP-2: Cloning and Induction by Bacterial Infection. Dev. Comp. Immunol. 2006, 30, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.J.; Lloyd, A.T.; O’Farrelly, C. In Silico Identification of Components of the Toll-like Receptor (TLR) Signaling Pathway in Clustered Chicken Expressed Sequence Tags (ESTs). Vet. Immunol. Immunopathol. 2003, 93, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Townes, C.L.; Michailidis, G.; Nile, C.J.; Hall, J. Induction of Cationic Chicken Liver-Expressed Antimicrobial Peptide 2 in Response to Salmonella Enterica Infection. Infect. Immun. 2004, 72, 6987–6993. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Peatman, E.; Xu, P.; Li, P.; Zeng, H.; He, C.; Liu, Z. The Catfish Liver-Expressed Antimicrobial Peptide 2 (LEAP-2) Gene Is Expressed in a Wide Range of Tissues and Developmentally Regulated. Mol. Immunol. 2006, 43, 367–377. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Y.-Z.; Pan, Y.-R.; Liu, J.; Jiang, Y.-B.; Zhang, Y.-A.; Zhang, X.-J. Comparative Study on Antibacterial Characteristics of the Multiple Liver Expressed Antimicrobial Peptides (LEAPs) in Teleost Fish. Front. Immunol. 2023, 14, 1128138. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-X.; Lu, X.-J.; Li, C.-H.; Chen, J. Molecular Characterization of the Liver-Expressed Antimicrobial Peptide 2 (LEAP-2) in a Teleost Fish, Plecoglossus Altivelis: Antimicrobial Activity and Molecular Mechanism. Mol. Immunol. 2015, 65, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, Q.; Du, H.; Qi, Z.; Li, Y.; Huang, J.; Di, J.; Wei, Q. Evolution, Expression, and Characterisation of Liver-Expressed Antimicrobial Peptide Genes in Ancient Chondrostean Sturgeons. Fish. Shellfish. Immunol. 2018, 79, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Guo, H.; Li, H.; Shan, S.; Zhang, X.; Rombout, J.H.W.M.; An, L. Molecular Characterization of LEAP-2 cDNA in Common Carp (Cyprinus carpio L.) and the Differential Expression upon a Vibrio Anguillarum Stimulus; Indications for a Significant Immune Role in Skin. Fish. Shellfish. Immunol. 2014, 37, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Z.; Shou, L.-L.; Shao, X.-X.; Li, N.; Liu, Y.-L.; Xu, Z.-G.; Guo, Z.-Y. LEAP2 Has Antagonized the Ghrelin Receptor GHSR1a since Its Emergence in Ancient Fish. Amino Acids 2021, 53, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Gao, Z.; Luan, Y.; Li, Q.; Pang, Y.; Gou, M. Identification of Antibacterial Activity of Liver-Expressed Antimicrobial Peptide 2 (LEAP2) from Primitive Vertebrate Lamprey. Fish. Shellfish. Immunol. 2024, 146, 109413. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Nijnik, A.; Philpott, D.J. Modulating Immunity as a Therapy for Bacterial Infections. Nat. Rev. Microbiol. 2012, 10, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.N.; Lawhon, S.D.; Diesel, A.B.; Bradley, C.W.; Rodrigues Hoffmann, A.; Murphy, W.J. 99 Lives Cat Genome Consortium An Ancient Haplotype Containing Antimicrobial Peptide Gene Variants Is Associated with Severe Fungal Skin Disease in Persian Cats. PLoS Genet. 2022, 18, e1010062. [Google Scholar] [CrossRef] [PubMed]
- Torrent, M.; Pulido, D.; Rivas, L.; Andreu, D. Antimicrobial Peptide Action on Parasites. Curr. Drug Targets 2012, 13, 1138–1147. [Google Scholar] [CrossRef]
- Howard, A.; Townes, C.; Milona, P.; Nile, C.J.; Michailidis, G.; Hall, J. Expression and Functional Analyses of Liver Expressed Antimicrobial Peptide-2 (LEAP-2) Variant Forms in Human Tissues. Cell Immunol. 2010, 261, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-G.; Ma, L.; Zhang, D.-N.; Ma, Y.; Wang, C.-Y.; Chen, J. Structure-Activity Relationships of the Intramolecular Disulphide Bonds in LEAP2, an Antimicrobial Peptide from Acrossocheilus Fasciatus. BMC Vet. Res. 2024, 20, 243. [Google Scholar] [CrossRef]
- Sakai, K.; Shiomi, K.; Mochizuki, H.; Islam, M.N.; Nabekura, H.; Tanida, R.; Sakoda, H.; Nakazato, M. Human Liver-Expressed Antimicrobial Peptide 2 Elevation in the Cerebrospinal Fluid in Bacterial Meningitis. Brain Behav. 2021, 11, e02111. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Yeh, I.-J.; Phan, N.N.; Yen, M.-C.; Hung, J.-H.; Chiao, C.-C.; Chen, C.-F.; Sun, Z.; Hsu, H.-P.; Wang, C.-Y.; et al. Gene Signatures and Potential Therapeutic Targets of Middle East Respiratory Syndrome Coronavirus (MERS-CoV)-Infected Human Lung Adenocarcinoma Epithelial Cells. J. Microbiol. Immunol. Infect. 2021, 54, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Trombini, J.; Scribante, A. Evaluation of Children Caries Risk Factors: A Narrative Review of Nutritional Aspects, Oral Hygiene Habits, and Bacterial Alterations. Children 2022, 9, 262. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Maiorani, C.; Molino, D.; Chiesa, A.; Preda, C.; Esposito, F.; Scribante, A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- İnce, G.; Gürsoy, H.; İpçi, Ş.D.; Cakar, G.; Emekli-Alturfan, E.; Yılmaz, S. Clinical and Biochemical Evaluation of Lozenges Containing Lactobacillus Reuteri as an Adjunct to Non-Surgical Periodontal Therapy in Chronic Periodontitis. J. Periodontol. 2015, 86, 746–754. [Google Scholar] [CrossRef]
- Scribante, A.; Butera, A.; Alovisi, M. Customized Minimally Invasive Protocols for the Clinical and Microbiological Management of the Oral Microbiota. Microorganisms 2022, 10, 675. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Q.; Lu, X.-J.; Chen, J. The Protection Effect of LEAP-2 on the Mudskipper (Boleophthalmus Pectinirostris) against Edwardsiella Tarda Infection Is Associated with Its Immunomodulatory Activity on Monocytes/Macrophages. Fish. Shellfish. Immunol. 2016, 59, 66–76. [Google Scholar] [CrossRef]
- Zheng, L.-B.; Mao, Y.; Wang, J.; Chen, R.-N.; Su, Y.-Q.; Hong, Y.-Q.; Hong, Y.-J.; Hong, Y.-C. Excavating Differentially Expressed Antimicrobial Peptides from Transcriptome of Larimichthys Crocea Liver in Response to Cryptocaryon Irritans. Fish. Shellfish. Immunol. 2018, 75, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, J.; Cheng, H.; Dai, Y.; Wang, Y.; Yang, H.; Xiong, F.; Xu, W.; Wei, L. Anti-Infective Effects of a Fish-Derived Antimicrobial Peptide Against Drug-Resistant Bacteria and Its Synergistic Effects With Antibiotic. Front. Microbiol. 2020, 11, 602412. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Wang, Q.; Lu, J.; Ouyang, L.; Li, W.; Hu, R.; Chen, L. Identification of Antibacterial Activity of LEAP2 from Antarctic Icefish Chionodraco Hamatus. J. Fish. Dis. 2023, 46, 905–916. [Google Scholar] [CrossRef]
- Howard, A.D.; Feighner, S.D.; Cully, D.F.; Arena, J.P.; Liberator, P.A.; Rosenblum, C.I.; Hamelin, M.; Hreniuk, D.L.; Palyha, O.C.; Anderson, J.; et al. A Receptor in Pituitary and Hypothalamus That Functions in Growth Hormone Release. Science 1996, 273, 974–977. [Google Scholar] [CrossRef]
- Wang, J.-H.; Li, H.-Z.; Shao, X.-X.; Nie, W.-H.; Liu, Y.-L.; Xu, Z.-G.; Guo, Z.-Y. Identifying the Binding Mechanism of LEAP2 to Receptor GHSR1a. FEBS J. 2019, 286, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- M’Kadmi, C.; Cabral, A.; Barrile, F.; Giribaldi, J.; Cantel, S.; Damian, M.; Mary, S.; Denoyelle, S.; Dutertre, S.; Péraldi-Roux, S.; et al. N-Terminal Liver-Expressed Antimicrobial Peptide 2 (LEAP2) Region Exhibits Inverse Agonist Activity toward the Ghrelin Receptor. J. Med. Chem. 2019, 62, 965–973. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin Is a Growth-Hormone-Releasing Acylated Peptide from Stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Metzger, N.P.; Singh, O.; Mani, B.K.; Osborne-Lawrence, S.; Varshney, S.; Gupta, D.; Ogden, S.B.; Takemi, S.; Richard, C.P.; et al. LEAP2 Deletion in Mice Enhances Ghrelin’s Actions as an Orexigen and Growth Hormone Secretagogue. Mol. Metab. 2021, 53, 101327. [Google Scholar] [CrossRef] [PubMed]
- Casado, S.; Varela-Miguéns, M.; de Oliveira Diz, T.; Quintela-Vilariño, C.; Nogueiras, R.; Diéguez, C.; Tovar, S. The Effects of Ghrelin and LEAP-2 in Energy Homeostasis Are Modulated by Thermoneutrality, High-Fat Diet and Aging. J. Endocrinol. Invest. 2024, 47, 2061–2074. [Google Scholar] [CrossRef] [PubMed]
- Andrews, Z.B. The next Big LEAP2 Understanding Ghrelin Function. J. Clin. Invest. 2019, 129, 3542–3544. [Google Scholar] [CrossRef]
- Fernandez, G.; Cabral, A.; De Francesco, P.N.; Uriarte, M.; Reynaldo, M.; Castrogiovanni, D.; Zubiría, G.; Giovambattista, A.; Cantel, S.; Denoyelle, S.; et al. GHSR Controls Food Deprivation-Induced Activation of CRF Neurons of the Hypothalamic Paraventricular Nucleus in a LEAP2-Dependent Manner. Cell Mol. Life Sci. 2022, 79, 277. [Google Scholar] [CrossRef] [PubMed]
- Wald, H.S.; Ghidewon, M.Y.; Hayes, M.R.; Grill, H.J. Hindbrain Ghrelin and Liver-Expressed Antimicrobial Peptide 2, Ligands for Growth Hormone Secretagogue Receptor, Bidirectionally Control Food Intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 324, R547–R555. [Google Scholar] [CrossRef] [PubMed]
- Mani, B.K.; Puzziferri, N.; He, Z.; Rodriguez, J.A.; Osborne-Lawrence, S.; Metzger, N.P.; Chhina, N.; Gaylinn, B.; Thorner, M.O.; Thomas, E.L.; et al. LEAP2 Changes with Body Mass and Food Intake in Humans and Mice. J. Clin. Invest. 2019, 129, 3909–3923. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Asakawa, A.; Fujimiya, M.; Lee, S.-D.; Inui, A. Ghrelin Gene Products and the Regulation of Food Intake and Gut Motility. Pharmacol. Rev. 2009, 61, 430–481. [Google Scholar] [CrossRef] [PubMed]
- Lugilde, J.; Casado, S.; Beiroa, D.; Cuñarro, J.; Garcia-Lavandeira, M.; Álvarez, C.V.; Nogueiras, R.; Diéguez, C.; Tovar, S. LEAP-2 Counteracts Ghrelin-Induced Food Intake in a Nutrient, Growth Hormone and Age Independent Manner. Cells 2022, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Luur, S.; Rodriguez Flores, M.; Emini, M.; Prechtl, C.G.; Goldstone, A.P. Postprandial Increases in Liver-Gut Hormone LEAP2 Correlate with Attenuated Eating Behavior in Adults Without Obesity. J. Endocr. Soc. 2023, 7, bvad061. [Google Scholar] [CrossRef]
- Gradel, A.K.J.; Holm, S.K.; Byberg, S.; Merkestein, M.; Hogendorf, W.F.J.; Lund, M.L.; Buijink, J.A.; Damgaard, J.; Lykkesfeldt, J.; Holst, B. The Dietary Regulation of LEAP2 Depends on Meal Composition in Mice. FASEB J. 2023, 37, e22923. [Google Scholar] [CrossRef]
- Holá, L.; Tureckiuová, T.; Kuneš, J.; Železná, B.; Maletínská, L. High-Fat Diet Induces Resistance to Ghrelin and LEAP2 Peptide Analogs in Mice. Physiol. Res. 2023, 72, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Tezenas du Montcel, C.; Duriez, P.; Cao, J.; Lebrun, N.; Ramoz, N.; Viltart, O.; Gorwood, P.; Tolle, V. The Role of Dysregulated Ghrelin/LEAP-2 Balance in Anorexia Nervosa. iScience 2023, 26, 107996. [Google Scholar] [CrossRef]
- Ragland, T.J.; Malin, S.K. Plasma LEAP-2 Following a Low-Calorie Diet with or without Interval Exercise in Women with Obesity. Nutrients 2023, 15, 655. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.G.B.; Lauritzen, E.S.; Svart, M.V.; Støy, J.; Søndergaard, E.; Thomsen, H.H.; Kampmann, U.; Bjerre, M.; Jessen, N.; Møller, N.; et al. Nutrient Sensing: LEAP2 Concentration in Response to Fasting, Glucose, Lactate, and β-Hydroxybutyrate in Healthy Young Males. Am. J. Clin. Nutr. 2023, 118, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Shcherbina, L.; Prasad, R.B.; Miskelly, M.G.; Abels, M.; Martínez-Lopéz, J.A.; Fred, R.G.; Nergård, B.J.; Hedenbro, J.; Groop, L.; et al. Ghrelin Suppresses Insulin Secretion in Human Islets and Type 2 Diabetes Patients Have Diminished Islet Ghrelin Cell Number and Lower Plasma Ghrelin Levels. Mol. Cell. Endocrinol. 2020, 511, 110835. [Google Scholar] [CrossRef]
- Bayle, M.; Péraldi-Roux, S.; Gautheron, G.; Cros, G.; Oiry, C.; Neasta, J. Liver-Expressed Antimicrobial Peptide 2 Antagonizes the Insulinostatic Effect of Ghrelin in Rat Isolated Pancreatic Islets. Fundam. Clin. Pharmacol. 2022, 36, 375–377. [Google Scholar] [CrossRef]
- Chen, R.-B.; Wang, Q.-Y.; Wang, Y.-Y.; Wang, Y.-D.; Liu, J.-H.; Liao, Z.-Z.; Xiao, X.-H. Feeding-Induced Hepatokines and Crosstalk with Multi-Organ: A Novel Therapeutic Target for Type 2 Diabetes. Front. Endocrinol. 2023, 14, 1094458. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Ogden, S.B.; Shankar, K.; Varshney, S.; Zigman, J.M. A LEAP 2 Conclusions? Targeting the Ghrelin System to Treat Obesity and Diabetes. Mol. Metab. 2021, 46, 101128. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and Diabetes: A Two-Way Relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Aslanipour, B.; Alan, M.; Demir, I. Decreased Levels of Liver-Expressed Antimicrobial Peptide-2 and Ghrelin Are Related to Insulin Resistance in Women with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2020, 36, 222–225. [Google Scholar] [CrossRef]
- Yang, J.; Chen, C. Hormonal Changes in PCOS. J. Endocrinol. 2024, 261, e230342. [Google Scholar] [CrossRef] [PubMed]
- Schalla, M.A.; Stengel, A. LEAP2: A Novel Regulator of Food Intake and Body Weight? Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 711–712. [Google Scholar] [CrossRef]
- Cornejo, M.P.; Castrogiovanni, D.; Schiöth, H.B.; Reynaldo, M.; Marie, J.; Fehrentz, J.-A.; Perello, M. Growth Hormone Secretagogue Receptor Signalling Affects High-Fat Intake Independently of Plasma Levels of Ghrelin and LEAP2, in a 4-Day Binge Eating Model. J. Neuroendocrinol. 2019, 31, e12785. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Mita, Y.; Maruyama, K.; Tanida, R.; Zhang, W.; Sakoda, H.; Nakazato, M. Liver-Expressed Antimicrobial Peptide 2 Antagonizes the Effect of Ghrelin in Rodents. J. Endocrinol. 2020, 244, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, S. LEAP2: Next Game-Changer of Pharmacotherapy for Overweight and Obesity? Cell Rep. Med. 2022, 3, 100612. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.N.; Liu, C.M.; Cortella, A.M.; Noble, E.E.; Kanoski, S.E. Ghrelin and Orexin Interact to Increase Meal Size Through a Descending Hippocampus to Hindbrain Signaling Pathway. Biol. Psychiatry 2020, 87, 1001–1011. [Google Scholar] [CrossRef]
- Tian, J.; Guo, L.; Wang, T.; Jia, K.; Swerdlow, R.H.; Zigman, J.M.; Du, H. Liver-Expressed Antimicrobial Peptide 2 Elevation Contributes to Age-Associated Cognitive Decline. JCI Insight 2023, 8, e166175. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Arosio, M.; Kreitschmann-Andermahr, I.; Persani, L. Editorial: New Insights and Controversies in Diagnosis and Treatment of Adult Growth Hormone Deficiency. Front. Endocrinol. 2021, 12, 819527. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Clemmons, D.R.; Malozowski, S.; Merriam, G.R.; Vance, M.L. Endocrine Society Evaluation and Treatment of Adult Growth Hormone Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1587–1609. [Google Scholar] [CrossRef]
- Vergani, E.; Bruno, C.; Gavotti, C.; Aversa, L.S.; Martire, M.; Mancini, A.; Currò, D. LEAP-2/Ghrelin Interplay in Adult Growth Hormone Deficiency: Cause or Consequence? A Pilot Study. IUBMB Life 2021, 73, 978–984. [Google Scholar] [CrossRef]
- Ohta, H.; Kimura, I.; Konishi, M.; Itoh, N. Neudesin as a Unique Secreted Protein with Multi-Functional Roles in Neural Functions, Energy Metabolism, and Tumorigenesis. Front. Mol. Biosci. 2015, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Vergani, E.; Bruno, C.; Gavotti, C.; Oliva, A.; Currò, D.; Mancini, A. Increased Levels of Plasma Neudesin in Adult Growth Hormone Deficiency and Their Relationship with Plasma Liver-Expressed Antimicrobial Peptide-2 Levels: A Cross-Sectional Study. J. Endocrinol. Invest. 2023, 46, 1187–1195. [Google Scholar] [CrossRef]
- Thakker, G.D.; Frangogiannis, N.G.; Bujak, M.; Zymek, P.; Gaubatz, J.W.; Reddy, A.K.; Taffet, G.; Michael, L.H.; Entman, M.L.; Ballantyne, C.M. Effects of Diet-Induced Obesity on Inflammation and Remodeling after Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2504-2514. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.-Y.; Xu, X.-Y.; Liu, Y.-L.; Ye, C.-F.; Hu, N.; Yao, Q.; Cheng, W.-S.; Cheng, Z.-G.; Liu, Y. Ghrelin Relieves Obesity-Induced Myocardial Injury by Regulating the Epigenetic Suppression of miR-196b Mediated by lncRNA HOTAIR. Obes. Facts 2022, 15, 540–549. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.-Y.; Shen, Y.; Ye, C.-F.; Hu, N.; Yao, Q.; Lv, X.-Z.; Long, S.-L.; Ren, C.; Lang, Y.-Y.; et al. Ghrelin Protects against Obesity-Induced Myocardial Injury by Regulating the lncRNA H19/miR-29a/IGF-1 Signalling Axis. Exp. Mol. Pathol. 2020, 114, 104405. [Google Scholar] [CrossRef]
- Lang, Y.; Liu, Y.; Ye, C.; Tang, X.; Cheng, Z.; Xie, L.; Feng, L.; Liu, Y. Loss of LEAP-2 Alleviates Obesity-Induced Myocardial Injury by Regulating Macrophage Polarization. Exp. Cell Res. 2023, 430, 113702. [Google Scholar] [CrossRef] [PubMed]
- Ezquerro, S.; Tuero, C.; Becerril, S.; Valentí, V.; Moncada, R.; Landecho, M.F.; Catalán, V.; Gómez-Ambrosi, J.; Mocha, F.; Silva, C.; et al. Antagonic Effect of Ghrelin and LEAP-2 on Hepatic Stellate Cell Activation and Liver Fibrosis in Obesity-Associated Nonalcoholic Fatty Liver Disease. Eur. J. Endocrinol. 2023, 188, 564–577. [Google Scholar] [CrossRef]
- Ma, X.; Xue, X.; Zhang, J.; Liang, S.; Xu, C.; Wang, Y.; Zhu, J. Liver Expressed Antimicrobial Peptide 2 Is Associated with Steatosis in Mice and Humans. Exp. Clin. Endocrinol. Diabetes 2021, 129, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, R.; Qu, Y.; Zhao, J.; Tong, L.; Ye, S.; Qin, Y. Ghrelin Ameliorates Transformation of Hepatic Ischemia-Reperfusion Injury to Liver Fibrosis by Blocking Smad and ERK Signalling Pathways, and Promoting Anti-Inflammation and Anti-Oxidation Effects. Transpl. Immunol. 2022, 73, 101597. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Q.; Mu, H.; Zeng, Y.; An, Z.; He, H. Preliminary Study on the Diagnostic Value of LEAP-2 and CK18 in Biopsy-Proven MAFLD. BMC Gastroenterol. 2024, 24, 182. [Google Scholar] [CrossRef]
- Voigt, K.; Giddens, E.; Stark, R.; Frisch, E.; Moskovsky, N.; Kakoschke, N.; Stout, J.C.; Bellgrove, M.A.; Andrews, Z.B.; Verdejo-Garcia, A. The Hunger Games: Homeostatic State-Dependent Fluctuations in Disinhibition Measured with a Novel Gamified Test Battery. Nutrients 2021, 13, 2001. [Google Scholar] [CrossRef] [PubMed]
- Etxandi, M.; Baenas, I.; Mora-Maltas, B.; Granero, R.; Fernández-Aranda, F.; Tovar, S.; Solé-Morata, N.; Lucas, I.; Casado, S.; Gómez-Peña, M.; et al. Are Signals Regulating Energy Homeostasis Related to Neuropsychological and Clinical Features of Gambling Disorder? A Case-Control Study. Nutrients 2022, 14, 5084. [Google Scholar] [CrossRef]
- Engel, J.A.; Jerlhag, E. Role of Appetite-Regulating Peptides in the Pathophysiology of Addiction: Implications for Pharmacotherapy. CNS Drugs 2014, 28, 875–886. [Google Scholar] [CrossRef]
- Sztainert, T.; Hay, R.; Wohl, M.J.A.; Abizaid, A. Hungry to Gamble? Ghrelin as a Predictor of Persistent Gambling in the Face of Loss. Biol. Psychol. 2018, 139, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Baenas, I.; Mora-Maltas, B.; Etxandi, M.; Lucas, I.; Granero, R.; Fernández-Aranda, F.; Tovar, S.; Solé-Morata, N.; Gómez-Peña, M.; Moragas, L.; et al. Cluster Analysis in Gambling Disorder Based on Sociodemographic, Neuropsychological, and Neuroendocrine Features Regulating Energy Homeostasis. Compr. Psychiatry 2024, 128, 152435. [Google Scholar] [CrossRef]
- Mora-Maltas, B.; Baenas, I.; Etxandi, M.; Lucas, I.; Granero, R.; Fernández-Aranda, F.; Tovar, S.; Solé-Morata, N.; Gómez-Peña, M.; Moragas, L.; et al. Association between Endocrine and Neuropsychological Endophenotypes and Gambling Disorder Severity. Addict. Behav. 2024, 153, 107968. [Google Scholar] [CrossRef]
- Bo, J.; Yang, Y.; Zheng, R.; Fang, C.; Jiang, Y.; Liu, J.; Chen, M.; Hong, F.; Bailey, C.; Segner, H.; et al. Antimicrobial Activity and Mechanisms of Multiple Antimicrobial Peptides Isolated from Rockfish Sebastiscus Marmoratus. Fish. Shellfish. Immunol. 2019, 93, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Niyonsaba, F.; Ushio, H.; Okuda, D.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Synergistic Effect of Antibacterial Agents Human Beta-Defensins, Cathelicidin LL-37 and Lysozyme against Staphylococcus Aureus and Escherichia Coli. J. Dermatol. Sci. 2005, 40, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Peteláková, M.; Neprašová, B.; Šmotková, Z.; Myšková, A.; Holá, L.; Petelák, A.; Áčová, A.; Cantel, S.; Fehrentz, J.-A.; Sýkora, D.; et al. Simultaneous Treatment with Palm-LEAP2(1-14) and Feeding High-Fat Diet Attenuates Liver Lipid Metabolism but Not Obesity: Sign of Selective Resistance to Palm-LEAP2(1-14). Mol. Cell Endocrinol. 2024, 597, 112442. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Vandvik, P.O.; Lytvyn, L.; Guyatt, G.H.; Palmer, S.C.; Rodriguez-Gutierrez, R.; Foroutan, F.; Agoritsas, T.; Siemieniuk, R.A.C.; Walsh, M.; et al. SGLT-2 Inhibitors or GLP-1 Receptor Agonists for Adults with Type 2 Diabetes: A Clinical Practice Guideline. BMJ 2021, 373, n1091. [Google Scholar] [CrossRef]
- Li, C.-H.; Chen, J.; Nie, L.; Chen, J. MOSPD2 Is a Receptor Mediating the LEAP-2 Effect on Monocytes/Macrophages in a Teleost, Boleophthalmus Pectinirostris. Zool. Res. 2020, 41, 644–655. [Google Scholar] [CrossRef] [PubMed]
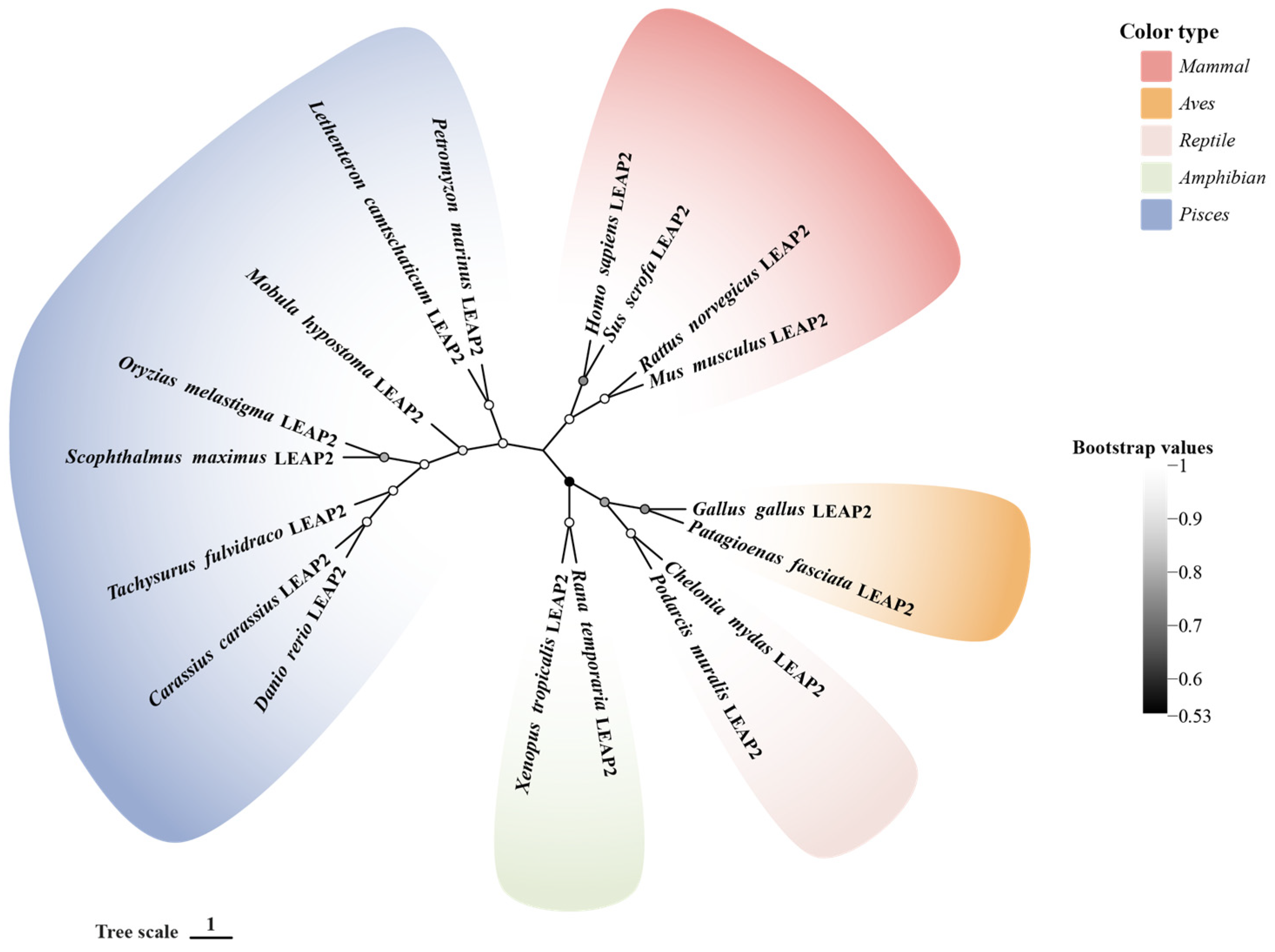
| Species | Protein | GenBank Accession No. |
|---|---|---|
| Homo sapiens | LEAP-2 | NP_443203.1 |
| Sus scrofa | LEAP-2 | NP_998953.1 |
| Rattus norvegicus | LEAP-2 | NP_001380270.1 |
| Mus musculus | LEAP-2 | NP_694709.1 |
| Gallus gallus | LEAP-2 | NP_001001606.1 |
| Patagioenas fasciata | LEAP-2 | XP_065705459.1 |
| Chelonia mydas | LEAP-2 | XP_007063679.3 |
| Podarcis muralis | LEAP-2 | XP_028573793.1 |
| Rana temporaria | LEAP-2 | XP_040200558.1 |
| Xenopus tropicalis | LEAP-2 | NP_001106385.1 |
| Danio rerio | LEAP-2 | NP_001122249.1 |
| Oryzias melastigma | LEAP-2 | XP_024144419.1 |
| Scophthalmus maximus | LEAP-2 | XP_035465006.1 |
| Carassius carassius | LEAP-2 | XP_059379122.1 |
| Tachysurus fulvidraco | LEAP-2 | XP_026990153.1 |
| Mobula hypostoma | LEAP-2 | XP_062917438.1 |
| Petromyzon marinus | LEAP-2 | XP_032813599.1 |
| Lethenteron camtschaticum | LEAP-2 | OR882686 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, Y.; Gou, M. Peptide with Dual Roles in Immune and Metabolic Regulation: Liver-Expressed Antimicrobial Peptide-2 (LEAP-2). Molecules 2025, 30, 429. https://doi.org/10.3390/molecules30020429
Li Y, Liu Y, Gou M. Peptide with Dual Roles in Immune and Metabolic Regulation: Liver-Expressed Antimicrobial Peptide-2 (LEAP-2). Molecules. 2025; 30(2):429. https://doi.org/10.3390/molecules30020429
Chicago/Turabian StyleLi, Yitong, Ying Liu, and Meng Gou. 2025. "Peptide with Dual Roles in Immune and Metabolic Regulation: Liver-Expressed Antimicrobial Peptide-2 (LEAP-2)" Molecules 30, no. 2: 429. https://doi.org/10.3390/molecules30020429
APA StyleLi, Y., Liu, Y., & Gou, M. (2025). Peptide with Dual Roles in Immune and Metabolic Regulation: Liver-Expressed Antimicrobial Peptide-2 (LEAP-2). Molecules, 30(2), 429. https://doi.org/10.3390/molecules30020429






