Chiral Separation of Mandelic Acid Derivatives Using Various Permethylated Cyclodextrin Selectors Containing Stationary Phases in GC
Abstract
:1. Introduction
- Numerous Chiral Centers: The asymmetric carbon atoms (e.g., 35 in β-CD) of glucose units differ from each other, each with unique configurations. Their twisted shapes create diverse functional group arrangements, enabling broad recognition spectra compared to linear molecules like amylose.
- Functional Group Modifications: Derivatized CDs possess (e.g., phosphate, sulfate, amino derivatives) enhanced interaction capabilities and selectivity.
- Structural Flexibility: CDs may alter their shape in order to adapt to the functional groups of the analyte, thus increasing chiral selectivity.
- Ionization States: Ionizable CDs exhibit variable selectivity depending on their ionization states.
- Solvent Interaction: Both analyte and solvent molecules may enter the cavities of CDs, depending on the mobile phase and buffer composition.
2. Results
- Various analytical derivatives, where mandelic acid was the starting material;
- Various alkyl chain-substituted derivatives of mandelic acid were the starting material;
- Various ring-substituted derivatives of mandelic acid were the starting material.
2.1. Results with Analytical Derivatives, Where Mandelic Acid Was the Starting Materials
2.2. Results with Analytical Derivatives, Where Alkyl Chain-Substituted Mandelic Acids Were the Starting Materials
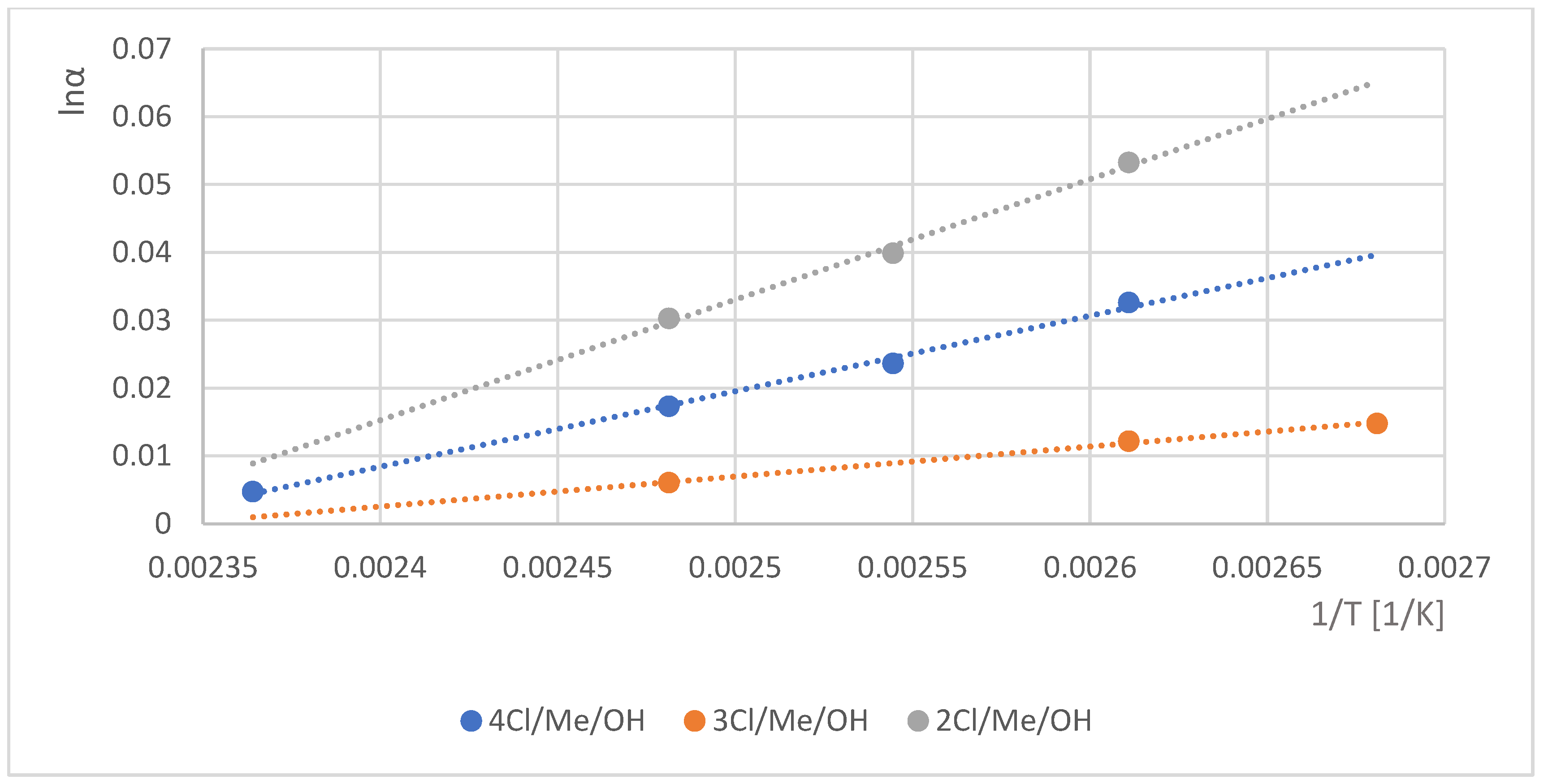
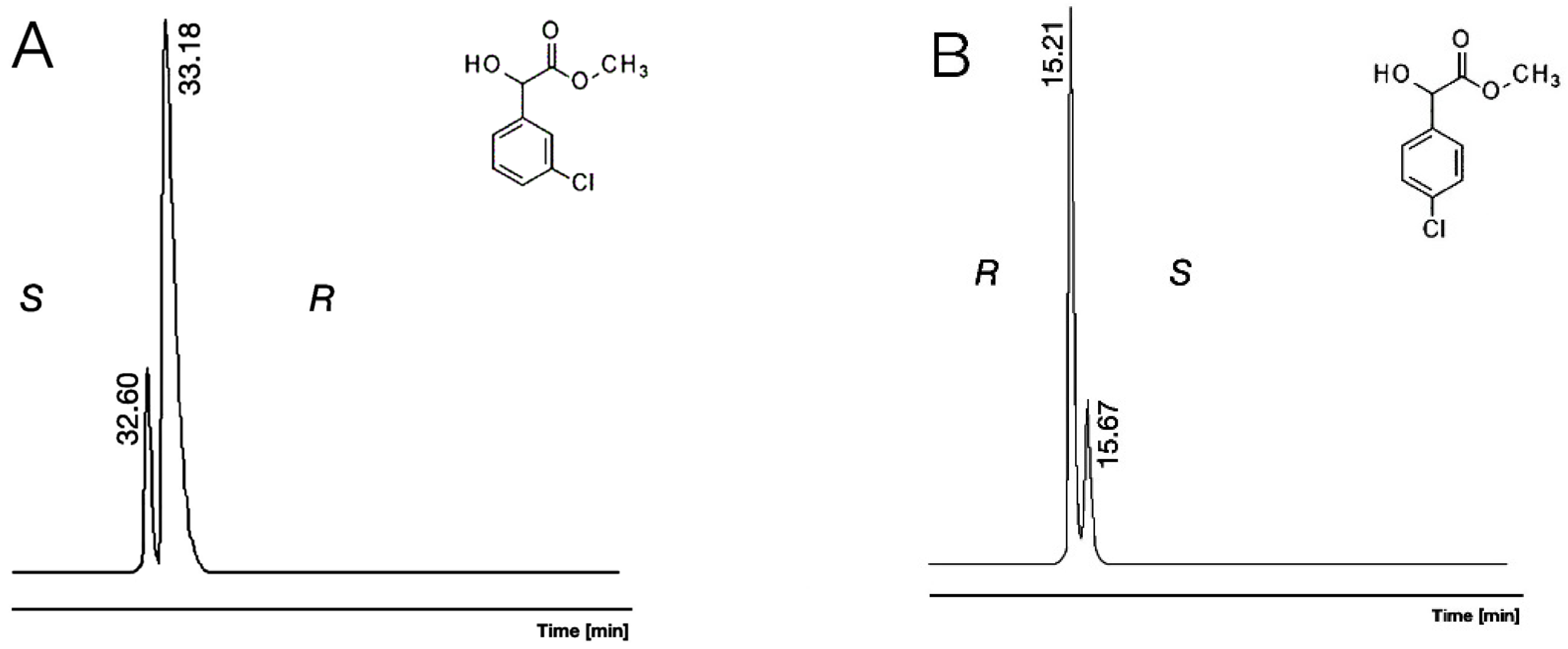
2.3. Results Where Various Ring-Substituted Derivatives of Mandelic Acid Were the Staring Materials
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Experimental Procedures
3.4. Measurements
3.5. Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palyi, G. Biological Chirality; Elsevier Science: Amszterdam, The Netherlands, 2019; ISBN 978-0-12-812212-9. [Google Scholar]
- Rainsford, K.D. (Ed.) Ibuprofen Discovery, Development and Therapeutics; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 9781118743386. [Google Scholar]
- Kim, J.H.; Scialli, A.R. Thalidomide: The tragedy of birth defects and the effective treatment of disease. Toxicol. Sci. 2011, 122, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Service of the USA. Food and Drug Administration’s Policy Statement for the Development of New Stereoisomeric Drugs, Fed. Regist. 57/2 May 1992; Volume 102. Available online: https://www.fda.gov/regulatory-information/ (accessed on 11 September 2023).
- Dalgliesh, C.E. The optical resolution of aromatic amino-acids on paper. J. Chem. Soc. 1952, 137, 3940–3942. [Google Scholar] [CrossRef]
- Betzenbichler, G.; Huber, L.; Kräh, S.; Morkos, M.L.K.; Siegle, A.F.; Trapp, O. Chiral stationary phases and applications in gas chromatography. Chirality 2022, 34, 732–759. [Google Scholar] [CrossRef] [PubMed]
- Scriba, G.K.E. (Ed.) Chiral Separations, Methods and Protocols, 3rd ed.; Humana Press: Totowa, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Şarkaya, K.; Göktürk, İ.; Yılmaz, F.; Denizli, A. Chiral Separations by Capillary Electrophoresis and Related Techniques with Different Chiral Selectors: A Review. Hacet. J. Biol. Chem. 2021, 49, 253–303. [Google Scholar] [CrossRef]
- Qiu, H.; Zhu, B.; Zhu, J.; Chen, S.; Ma, C.; Xiang, H.; Tong, S. Recent advances in application of cyclodextrin-based chiral materials for enantioseparation. TrAC Trends Anal. Chem. 2024, 175, 117708. [Google Scholar] [CrossRef]
- Tang, W.; Ng, S.-N.; Sun, D. Modified Cyclodextrins for Chiral Separations; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-37647-4. [Google Scholar]
- Szejtli, J.; Osa, T. (Eds.) Comprehensive Supramolecular Chemistry, Volume 3: Cyclodextrins; Pergamon: London, UK, 1999; ISBN 9780080427157. [Google Scholar]
- Juvancz, Z.; Bodáné-Kendrovics, R.; Szente, L.; Maklári, D. Cyclodextrins are the Dominant Chiral Selective Agents in the Capillary Separation Techniques. Period. Polytech. Chem. Eng. 2021, 65, 580–594. [Google Scholar] [CrossRef]
- Repassy, L.; Juvancz, Z.; Bodane-Kendrovics, R.; Kaleta, Z.; Hunyadi, C.; Riszter, G. Structure–Chiral Selectivity Relationships of Various Mandelic Acid Derivatives on Octakis 2,3-di-O-acetyl-6-O-tert-butyldimethylsilyl-gamma-cyclodextrin Containing Gas Chromatographic Stationary. Int. J. Mol. Sci. 2023, 24, 15051. [Google Scholar] [CrossRef] [PubMed]
- Juvancz, Z.; Bodane-Kendrovics, R.; Agoston, C.; Czegledi, B.; Kaleta, Z.; Jicsinszky, L.; Riszter, G. Chiral Selectivities of Permethylated α-, β-, and γ-Cyclodextrins Containing Gas Chromatographic Stationary Phases towards Ibuprofen and Its Derivatives. Int. J. Mol. Sci. 2024, 25, 7802. [Google Scholar] [CrossRef] [PubMed]
- Sharon, M.; Durve, A.; Pandey, A.; Pathak, M. Mandelic Acid; Partridge Publishing: Bloomington, India, 2018; ISBN 9781482816723/1482816725. [Google Scholar]
- Kacprzak, K.; Maier, N.; Lindner, W. Unexpected enantioseparation of mandelic acids and their derivatives on 1,2,3-triazolo-linked quinine tert-butyl carbamate anion exchange-type chiral stationary phase. J. Sep. Sci. 2010, 33, 2590–82010. [Google Scholar] [CrossRef] [PubMed]
- Juvancz, Z.; Bodáné-Kendrovics, R.; Laczkó, Z.; Iványi, R.; Varga, E. Chiral Separations of Pyrethroic Acids Using Cyclodextrin Selectors. Molecules 2022, 27, 8718. [Google Scholar] [CrossRef] [PubMed]
- Felinger, A. Data Analysis and Signal Processing in Chromatography; Elsevier: Amsterdam, The Netherland, 1998; ISBN 9780080525563. [Google Scholar]
- Kovats, E. Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphati-scher Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Stein, S.E.; Brown, R.L. Estimation of Normal Boiling Points from Group Contribution. J. Chem. Inf. Comput. Sci. 1994, 34, 581–587. [Google Scholar] [CrossRef]
- König, W.A. Gas Chromatographic Enantiomer Separation with Modified Cyclodextrins; Hüthig: Heidelberg, Germany, 1992; ISBN 13:978-3778520260. [Google Scholar]
- Schurig, V. Use of derivatized cyclodextrins as chiral selectors for the separation of enantiomers by gas chromatography. Pharm. Françaises 2010, 68, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Blau, K.; Halket, J.M. (Eds.) Handbook of Derivatives for Chromatography, 2nd ed.; Wiley: New York, NY, USA, 1993; ISBN 978-0-471-92699-3. [Google Scholar]
- Li, M.; Tao, Y.; Tang, J.; Wang, Y.; Zhang, X.; Tao, Y.; Wang, X. Synergetic Organocatalysis for Eliminating Epimerization in Ring-Opening Polymerizations Enables Synthesis of Stereoregular Isotactic Polyester. J. Am. Chem. Soc. 2019, 141, 281–289. [Google Scholar] [CrossRef] [PubMed]


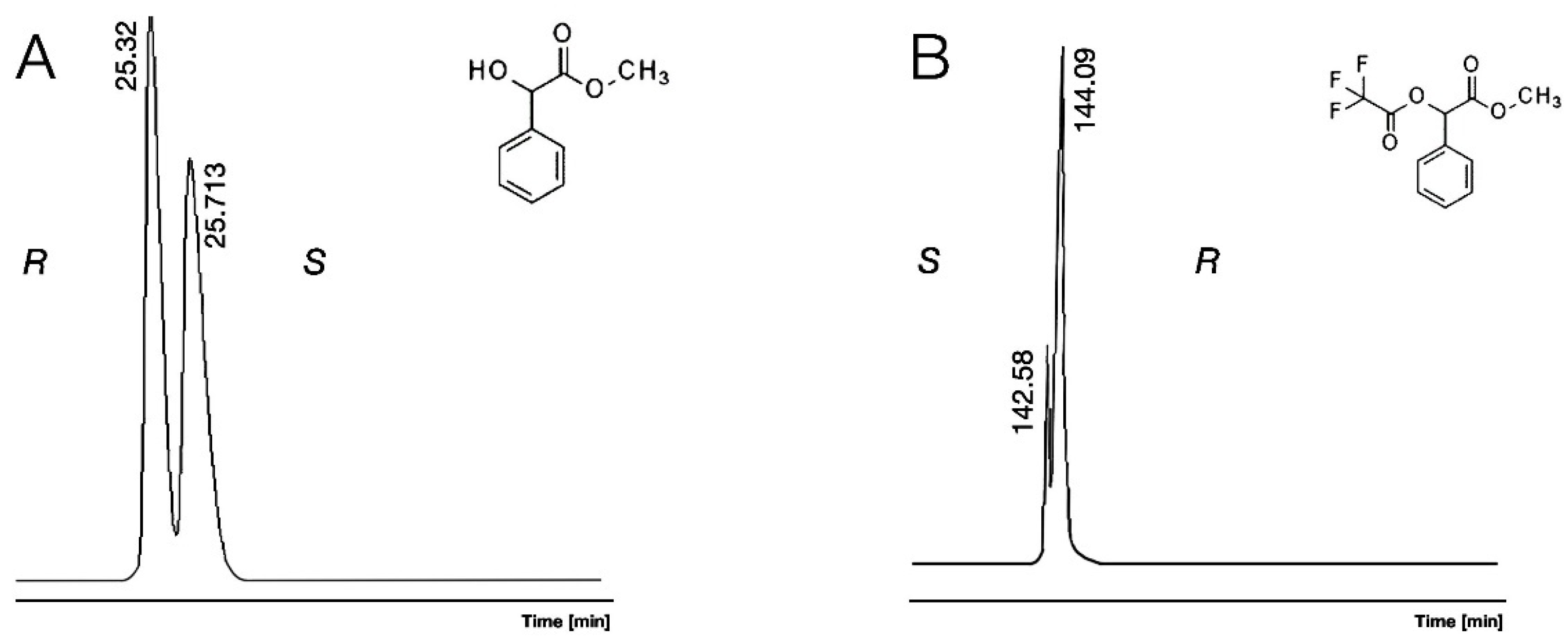
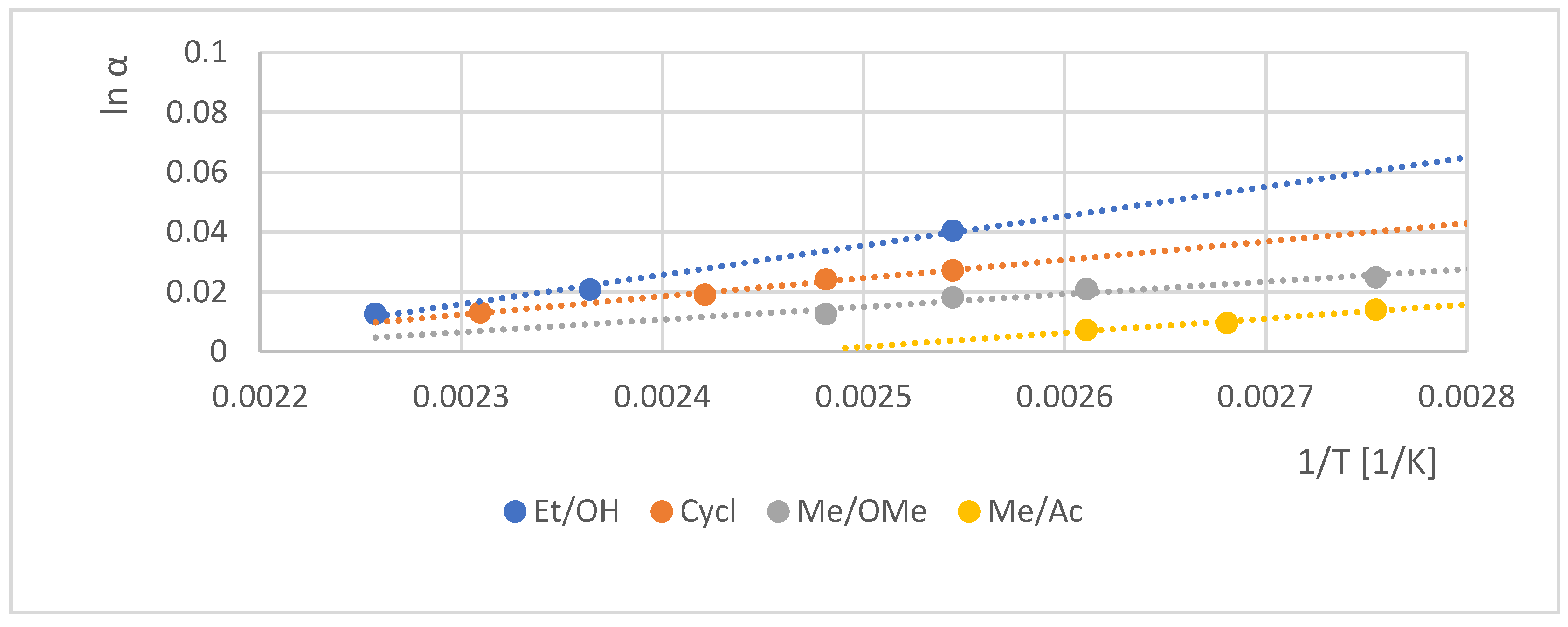
| ID | Abr. | Acid a | Alcohol b | α-CD e | β-CD f | γ-CD g | bp d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selectivity c | KI | Elution Order | Selectivity c | KI | Elution Order | Selectivity c | KI | Elution Order | |||||
| 1 | H/OMe | H | CH3 | <1.01 | 1767 | 1.085 | 2114 | R | 1.053 | 3310 | R | 289.51 | |
| 2 | Me/OH | CH3 | / | 1.035 | 1589 | S | 1.045 | 1554 | R | 1.029 | 1650 | R | 266.08 |
| 3 | Me/OMe | CH3 | CH3 | 1.011 | 1578 | S | 1.021 | 1547 | R | <1.01 | 1348 | R | 242.20 |
| 4 | Me/Ac | CH3 | OCH2CH3 | 1.021 | 1709 | S | 1.010 | 1630 | S | 1.015 | 1814 | S | 255.74 |
| 5 | Me/TFA | CH3 | OCH2CF3 | 1.016 | 1577 | S | 1.007 | 1409 | S | 1.01 | 1514 | S | 243.38 |
| 6 | Et/OH | CH2CH3 | / | 1.036 | 1579 | S | 1.054 | 1552 | R | 1.025 | 1649 | R | 282.27 |
| 7 | cyclic | 1,3-dioxolane-2,4 dione | 1.107 | 1709 | S | 1.041 | 1625 | S | <1.01 | 1769 | 269.28 | ||
| ID: | Abrev. | α-CD d | β-CD e | γ-CD f | bp c | |||
|---|---|---|---|---|---|---|---|---|
| Selectivity a | KI b | Selectivity a | KI b | Selectivity a | KI b | |||
| 2 | Me/OH | 1.035 | 1589 | 1.045 | 1554 | 1.029 | 1650 | 266.08 |
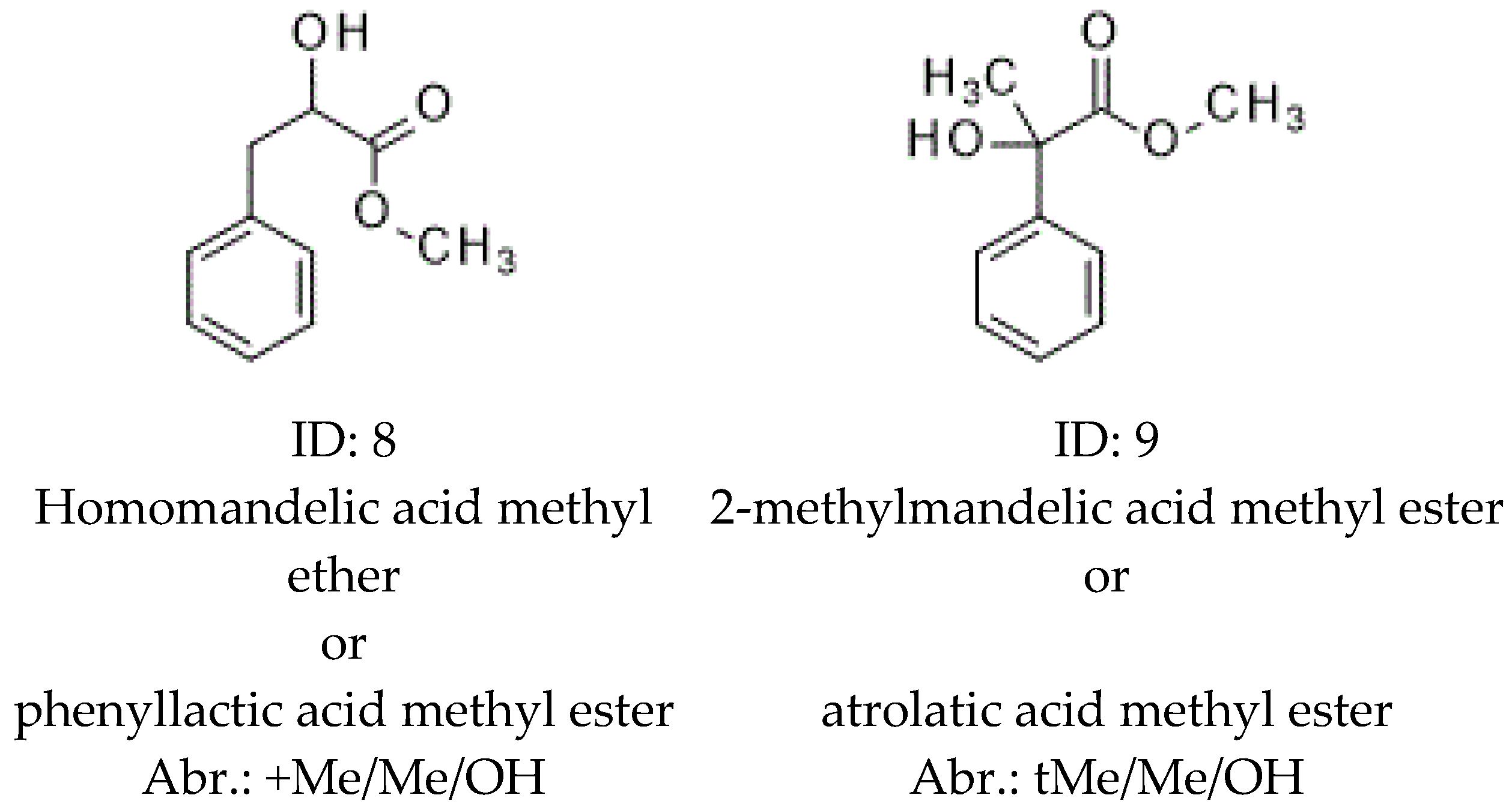
| 8 | +Me/Me/OH | <1.01 | 1717 | 1.015 | 1623 | <1.01 | 1754 | 282.27 |
| 9 | tMe/Me/OH | 1.048 | 1557 | 1.047 | 1522 | 1.012 | 1604 | 268.34 |
| ID | Abr. | α-CD d | β-CD e | γ-CD f | bp c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Selectivity a | KI b | Elution Order | Selectivity a | KI b | Elution Order | Selectivity a | KI b | Elution Order | |||
| 2 | Me/OH | 1.035 | 1589 | S | 1.045 | 1554 | R | 1.029 | 1650 | R | 266.08 |
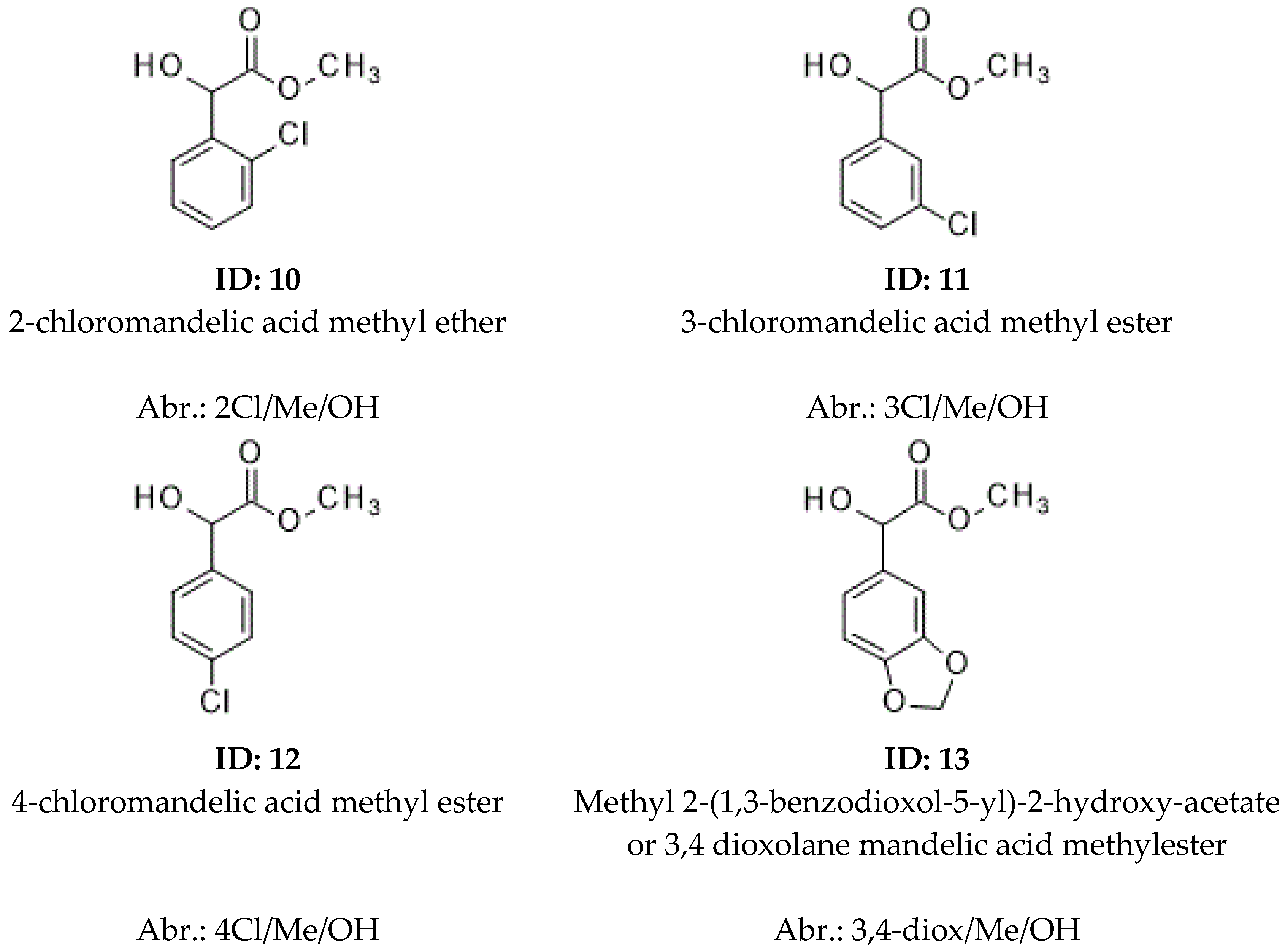
| 10 | 2Cl/Me/OH | <1.01 | 1740 | <1.01 | 1727 | 1.028 | 1803 | R | 291.72 | ||
| 11 | 3Cl/Me/OH | 1.027 | 1829 | S | 1.141 | 1745 | S | 1.015 | 1741 | R | 291.72 |
| 12 | 4Cl/Me/OH | 1.024 | 1807 | S | 1.170 | 1725 | R | 1.067 | 1866 | R | 291.72 |
| 13 | 3,4-diox/Me/OH | 1023 | 1813 | 1.015 | 1949 | 1.007 | 1878 | 269.28 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juvancz, Z.; Bodáne-Kendrovics, R.; Ágoston, C.; Maklári, D.; Voller, C.C.; Kaleta, Z. Chiral Separation of Mandelic Acid Derivatives Using Various Permethylated Cyclodextrin Selectors Containing Stationary Phases in GC. Molecules 2025, 30, 451. https://doi.org/10.3390/molecules30030451
Juvancz Z, Bodáne-Kendrovics R, Ágoston C, Maklári D, Voller CC, Kaleta Z. Chiral Separation of Mandelic Acid Derivatives Using Various Permethylated Cyclodextrin Selectors Containing Stationary Phases in GC. Molecules. 2025; 30(3):451. https://doi.org/10.3390/molecules30030451
Chicago/Turabian StyleJuvancz, Zoltan, Rita Bodáne-Kendrovics, Csaba Ágoston, Dóra Maklári, Csanad Csaba Voller, and Zoltan Kaleta. 2025. "Chiral Separation of Mandelic Acid Derivatives Using Various Permethylated Cyclodextrin Selectors Containing Stationary Phases in GC" Molecules 30, no. 3: 451. https://doi.org/10.3390/molecules30030451
APA StyleJuvancz, Z., Bodáne-Kendrovics, R., Ágoston, C., Maklári, D., Voller, C. C., & Kaleta, Z. (2025). Chiral Separation of Mandelic Acid Derivatives Using Various Permethylated Cyclodextrin Selectors Containing Stationary Phases in GC. Molecules, 30(3), 451. https://doi.org/10.3390/molecules30030451





