Comparative Analysis of Metabolites of Wild and Cultivated Notopterygium incisum from Different Origins and Evaluation of Their Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results
2.1. Morphological Differences Among NI from Different Areas
2.2. Chemical Composition of Volatile Oil of NI as Determined by GC–MS
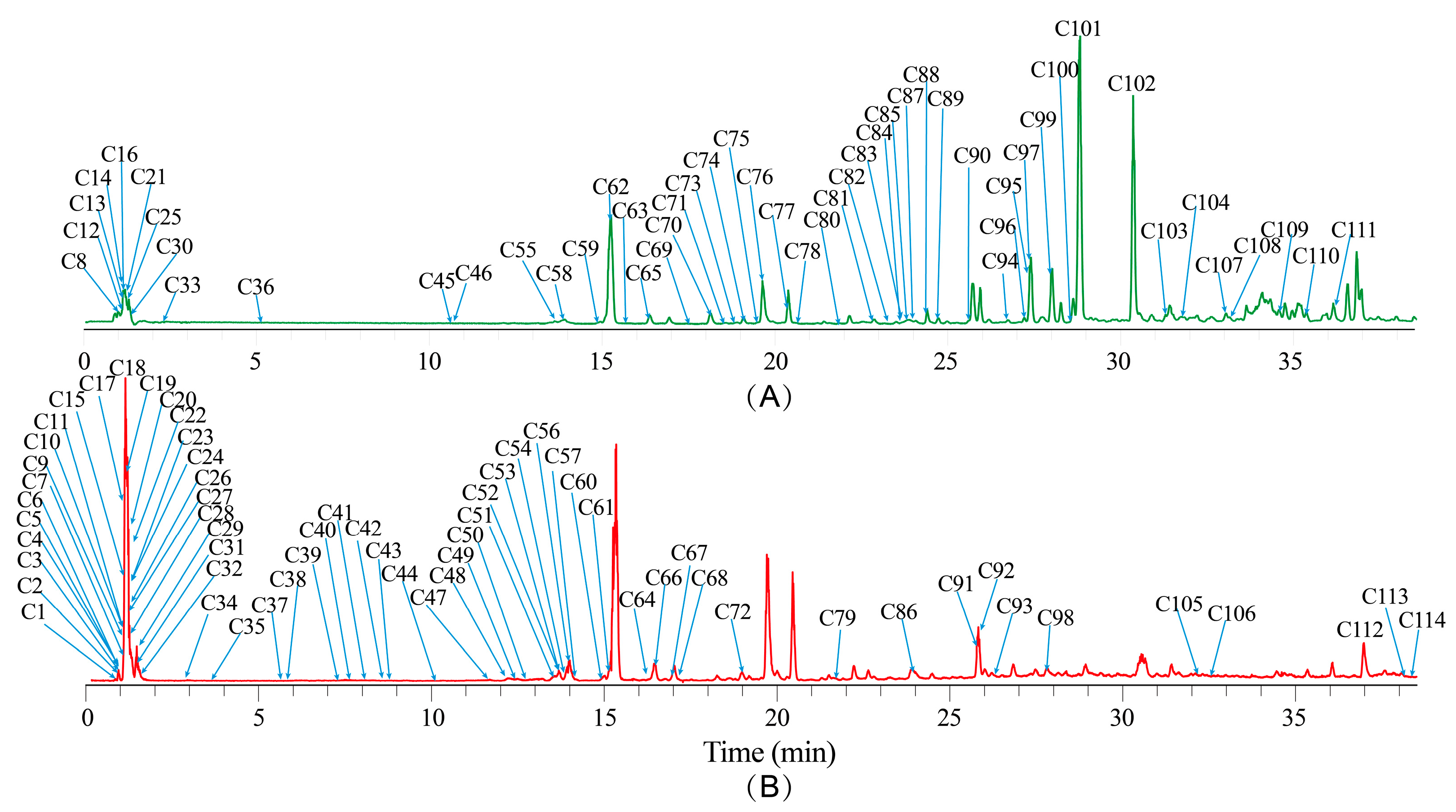
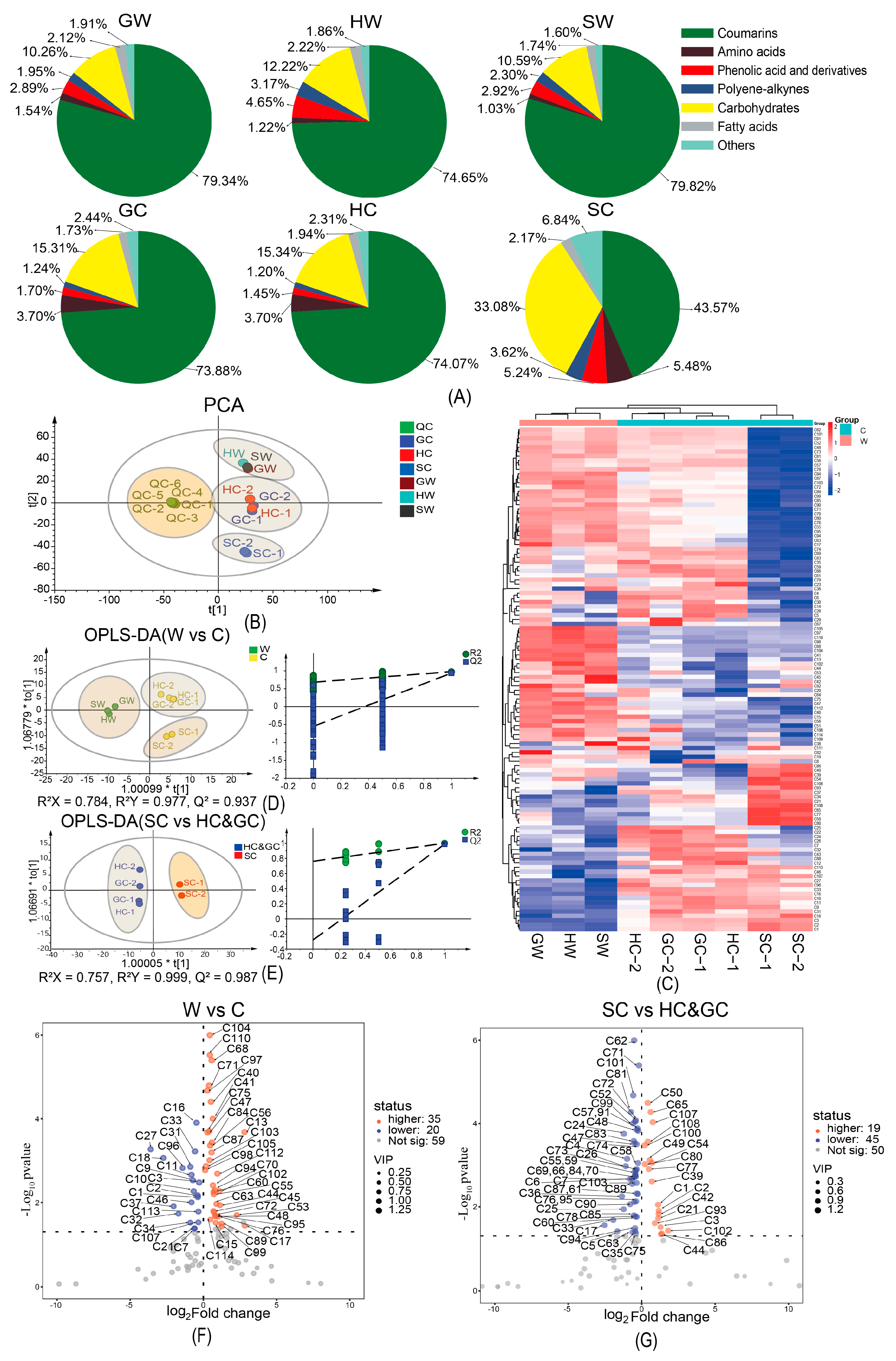
2.3. Non-Volatile Component Profiles of NI Samples as Determined by UHPLC-Orbitrap MS Analysis
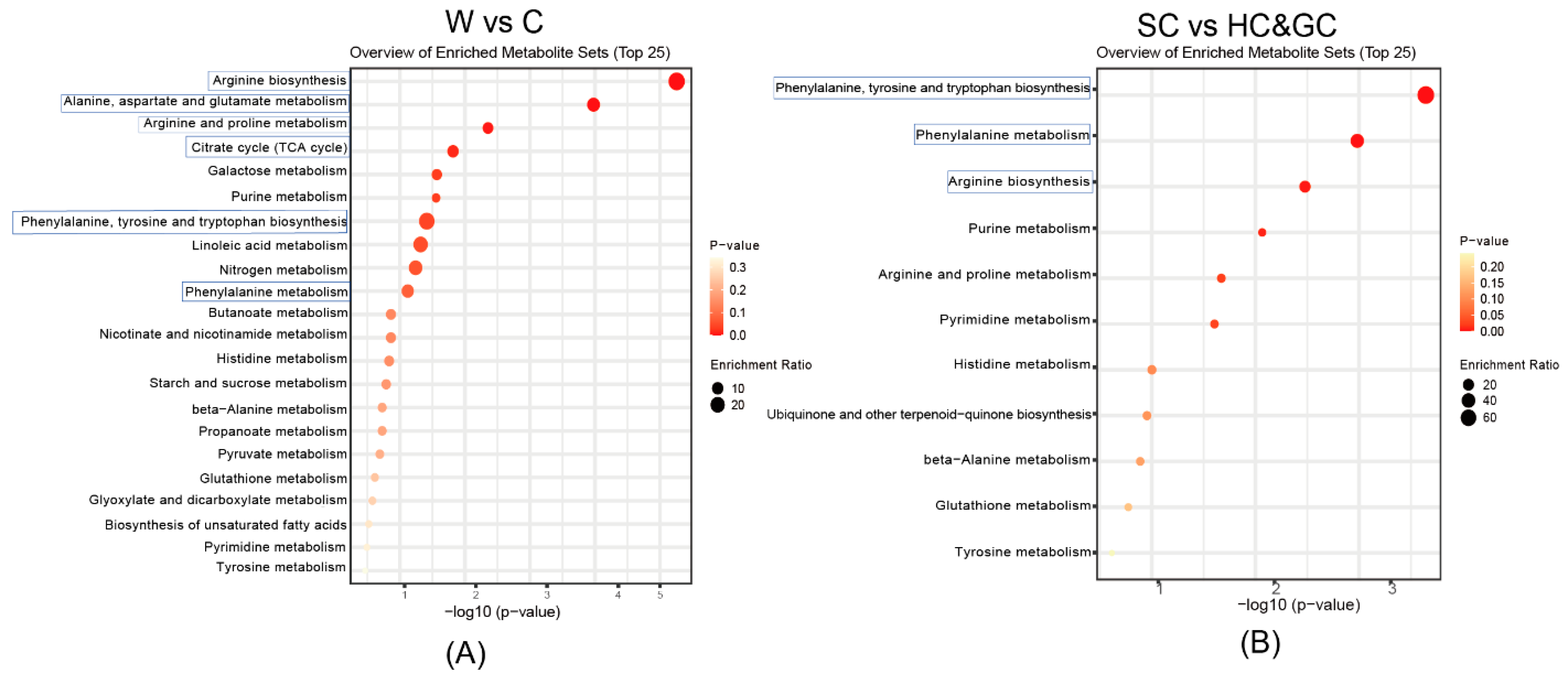
2.4. Pathway Enrichment and Metabolic Pathway Analysis of DAMs
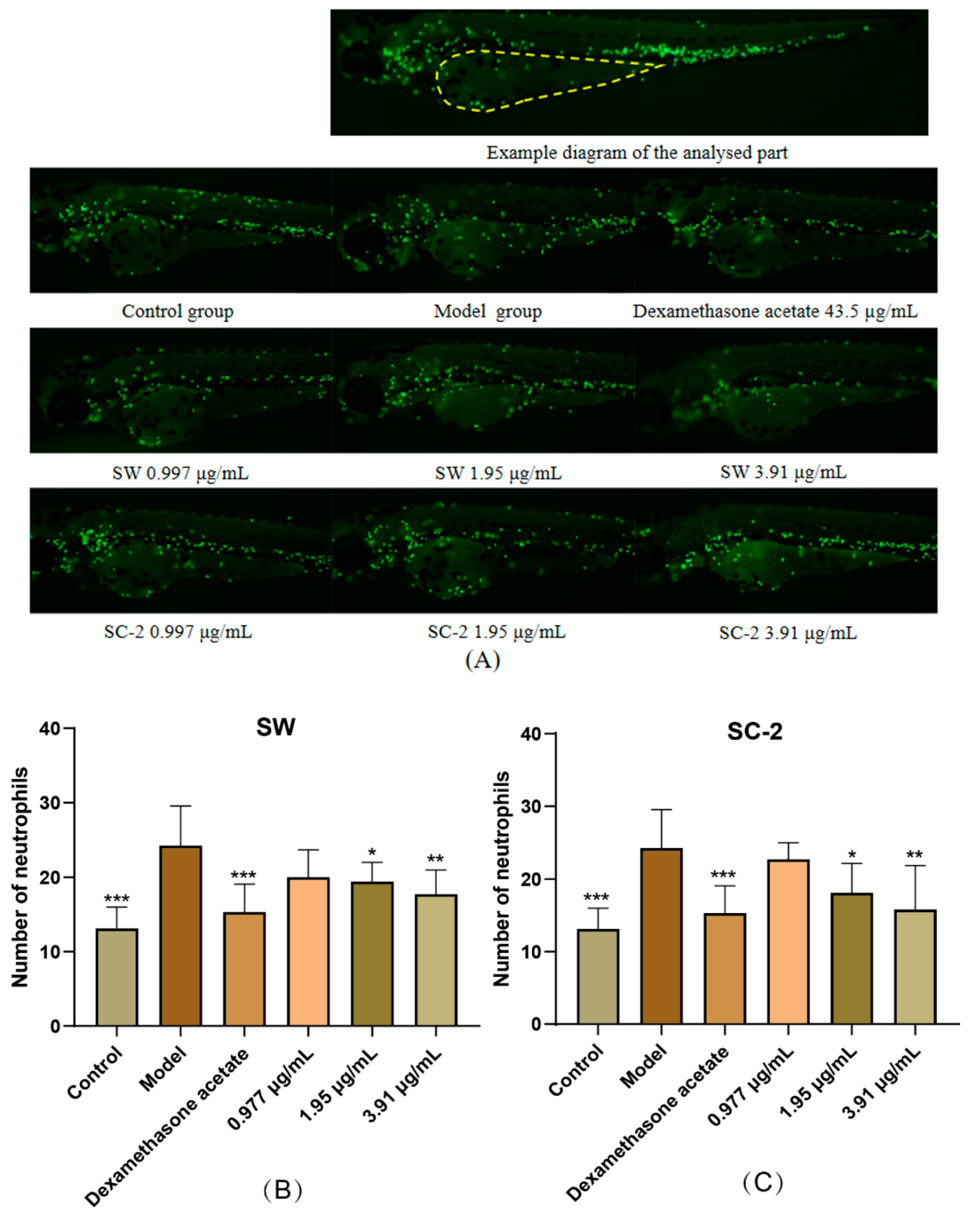
2.5. Results of Anti-Bacterial Inflammation Pharmacodynamic Study
2.5.1. Evaluation of Anti-Inflammatory Effect of NI Treatment by Neutrophil Counts
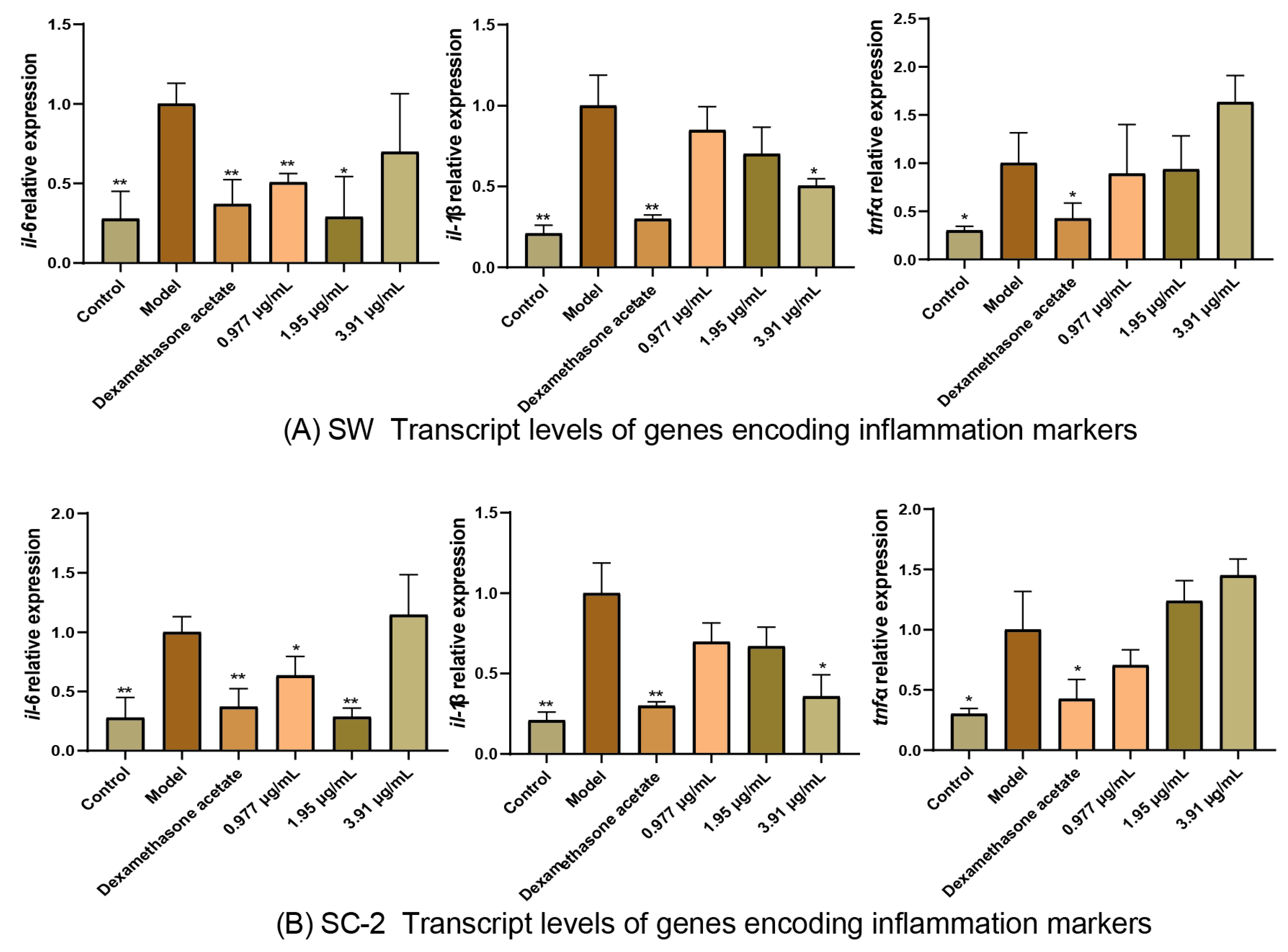
2.5.2. Effect of NI Treatments on Transcript Levels of Genes Encoding Inflammation Markers
3. Materials
4. Methods
4.1. Preparation of Samples for Chemical Components Analysis
4.2. Preparation of Samples for Analysis of Anti-Inflammatory Activity
4.3. Determination of the Chemical Components of NI Volatile Oil by GC–MS
4.4. Determination of Chemical Components of NI by UHPLC-Orbitrap MS
4.5. Anti-Inflammatory Pharmacodynamic Experiments
4.5.1. Maximum Detectable Concentration Determination
4.5.2. Effects of NI Samples on Bacterial Inflammation (Neutrophil Counts)
4.5.3. Effect of NI Samples on Gene Expression in the Zebrafish Inflammation Model
4.6. Data Analysis
4.6.1. Methods for Identification of Chemical Components
4.6.2. Phytometabolomic Research Based on Multivariate Statistical Analysis
4.6.3. Metabolic Pathway Analysis
4.6.4. Statistical Analyses of the Results of Pharmacological Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, J.; Na, Y.; Xue, J.; Gao, S.; Yang, L. A systematic review of the botany, traditional use, phytochemistry, analytical methods, pharmacological effects and pharmacokinetics of NOTOPTERYGII RHIZOMA ET RADIX. J. Ethnopharmacol. 2024, 334, 118589. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhou, Y.; Huang, Z.; Wang, M.; Jiang, J.; Yan, M.; Xiang, W.; Li, S.; Yu, Y.; Chen, L.; et al. Notopterol improves cognitive dysfunction and depression-like behavior via inhibiting STAT3/NF-kB pathway mediated inflammation in glioma-bearing mice. Int. Immunopharmacol. 2023, 118, 110041. [Google Scholar] [CrossRef]
- Huang, T.Y.; Yang, C.K.; Chen, M.Y.; Yadav, V.K.; Fong, I.H.; Yeh, C.T.; Cherng, Y.G. Furanocoumarin Notopterol: Inhibition of Hepatocellular Carcinogenesis through Suppression of Cancer Stemness Signaling and Induction of Oxidative Stress-Associated Cell Death. Nutrients 2023, 15, 2447. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, Q.; Chen, L.; Duan, K. Virulence-Inhibiting Herbal Compound Falcarindiol Significantly Reduced Mortality in Mice Infected with Pseudomonas aeruginosa. Antibiotics 2020, 9, 136. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Y.; Zeng, J.; Li, S. Nodakenetin Alleviates Inflammatory Pain Hypersensitivity by Suppressing NF-kappaB Signal Pathway. Neuroimmunomodulation 2022, 29, 486–492. [Google Scholar] [CrossRef]
- Tong, K.; Xin, C.; Chen, W. Isoimperatorin induces apoptosis of the SGC-7901 human gastric cancer cell line via the mitochondria-mediated pathway. Oncol. Lett. 2017, 13, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Q.; Li, Y.; Sun, P.; Kuek, V.; Yuan, J.; Yang, J.; Wen, L.; Wang, H.; Xu, J.; et al. Notopterol Attenuates Estrogen Deficiency-Induced Osteoporosis via Repressing RANKL Signaling and Reactive Oxygen Species. Front. Pharmacol. 2021, 12, 664836. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.W.; Liu, W.W.; Wu, Y.T.; Wu, Q.; Lu, H.Y.; Xu, Z.H.; Gao, H.Y.; Zhao, Q.C. Notopterygium incisum extract (NRE) rescues cognitive deficits in APP/PS1 Alzhneimer’s disease mice by attenuating amyloid-beta, tau, and neuroinflammation pathology. J. Ethnopharmacol. 2020, 249, 112433. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.Y.; Wang, T.; Ma, H.X.; Chen, S.; Chang, Z.Y.; Li, F. Synergistic effect of osthole and notopterol combination against Alzheimer’s disease and osteoporosis by applying zebrafish AD/OP comorbidity model. Eur. J. Pharmacol. 2024, 979, 176829. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jang, S.Y.; Cheng, S.L.; Zhou, Y.; Xie, C.X.; Ma, X.J.; Chen, T.Z. Studies on habitats suitability of endangered medicinal plant Notopterygium incisum. China J. Chin. Mater. Med. 2009, 34, 535–538. [Google Scholar]
- Bi, J.P.; Li, P.; Xu, X.X.; Wang, T.; Li, F. Anti-rheumatoid arthritic effect of volatile components in notopterygium incisum in rats via anti-inflammatory and anti-angiogenic activities. Chin. J. Nat. Med. 2018, 16, 926–935. [Google Scholar] [CrossRef]
- Li, S.P.; Sa, L.; Zhao, Y.W.; Xu, Z.L.; Huang, W.Z.; Wang, Z.Z.; Xiao, W. Advance in research on chemical constituents from Notopterygii Rhizoma et Radix. China J. Chin. Mater. Med. 2015, 40, 2952–2963. [Google Scholar]
- Wu, X.W.; Wei, W.; Yang, X.W.; Zhang, Y.B.; Xu, W.; Yang, Y.F.; Zhong, G.Y.; Liu, H.N.; Yang, S.L. Anti-Inflammatory Phenolic Acid Esters from the Roots and Rhizomes of Notopterygium incisium and Their Permeability in the Human Caco-2 Monolayer Cell Model. Molecules 2017, 22, 935. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Yang, L.; Zhao, Y.; Chew, Z.; Xiao, J.; Liu, C.; Zheng, X.; Zheng, Y.; Shi, Q.; et al. The Natural Compound Notopterol Binds and Targets JAK2/3 to Ameliorate Inflammation and Arthritis. Cell Rep. 2020, 32, 108158. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Kwon, H.S.; Kang, M.; Leem, H.H.; Lee, K.H.; Kim, D.Y. The Antitumor Natural Compound Falcarindiol Disrupts Neural Stem Cell Homeostasis by Suppressing Notch Pathway. Int. J. Mol. Sci. 2018, 19, 3432. [Google Scholar] [CrossRef]
- Cao, L.; Huang, X.; Zhu, J.; Xiao, J.; Xie, L. Falcarindiol improves functional recovery and alleviates neuroinflammation after spinal cord injury by inhibiting STAT/MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2024, 736, 150860. [Google Scholar] [CrossRef]
- Khoshnazar, S.M.; Kazemi, M.; Amirheidari, B. Neuroprotective Effects of γ-Terpinene in Rats with Acute Cerebral Ischemia: Modulation of Inflammation, Apoptosis, and Oxidation. Neurochem Res. 2024, 49, 1863–1878. [Google Scholar] [CrossRef]
- Pina, L.T.S.; Rabelo, T.K.; Trindade, G.G.G.; Almeida, I.K.S.; Oliveira, M.A.; Dos Santos, P.L.; Souza, D.S.; de Menezes-Filho, J.E.R.; de Vasconcelos, C.M.L.; Santos, S.L.; et al. gamma-Terpinene complexed with beta-cyclodextrin attenuates spinal neuroactivity in animals with cancer pain by Ca2+ channel block. J. Pharm. Pharmacol. 2022, 74, 1629–1639. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of alpha- and beta-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Bagińska, S.; Golonko, A.; Świsłocka, R.; Lewandowski, W. Monoterpenes as Medicinal Agents: Exploring the Pharmaceutical Potential of p-Cymene, p-Cymenene, and γ-Terpinene. Acta Pol. Pharm.-Drug Res. 2024, 80, 879–892. [Google Scholar] [CrossRef]
- Nait Irahal, I.; azzahra Lahlou, F.; Hmimid, F.; Errami, A.; Guenaou, I.; Diawara, I.; Kettani-Halabi, M.; Fahde, S.; Ouafik, L.H.; Bourhim, N. Identification of the chemical composition of six essential oils with mass spectroscopy and evaluation of their antibacterial and antioxidant potential. Flavour Fragr. J. 2021, 36, 465–476. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Benelli, G. δ-Cadinene,Calarene and δ-4-Carene from Kadsura heteroclita Essential Oil as Novel Larvicides Against Malaria, Dengue and Filariasis Mosquitoes. Comb. Chem. High Throughput Screen. 2016, 19, 565–571. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Cui, H.-F.; Cao, C.-Y.; Zhang, Y.-B. 4-Terpineol exhibits potent in vitro and in vivo anticancer effects in Hep-G2 hepatocellular carcinoma cells by suppressing cell migration and inducing apoptosis and sub-G1 cell cycle arrest. J. BUON 2016, 21, 1195–1202. [Google Scholar]
- Zhao, Z.J.; Sun, Y.L.; Ruan, X.F. Bornyl acetate: A promising agent in phytomedicine for inflammation and immune modulation. Phytomedicine 2023, 114, 154781. [Google Scholar] [CrossRef]
- Piccinelli, A.C.; Santos, J.A.; Konkiewitz, E.C.; Oesterreich, S.A.; Formagio, A.S.; Croda, J.; Ziff, E.B.; Kassuya, C.A. Antihyperalgesic and antidepressive actions of (R)-(+)-limonene, alpha-phellandrene, and essential oil from Schinus terebinthifolius fruits in a neuropathic pain model. Nutr. Neurosci. 2015, 18, 217–224. [Google Scholar] [CrossRef]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activities of the sesquiterpene fraction from Annona reticulata L. bark. Nat. Prod. Res. 2012, 26, 1515–1518. [Google Scholar] [CrossRef]
- de Christo Scherer, M.M.; Marques, F.M.; Figueira, M.M.; Peisino, M.C.O.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Wound healing activity of terpinolene and alpha-phellandrene by attenuating inflammation and oxidative stress in vitro. J. Tissue Viability 2019, 28, 94–99. [Google Scholar] [CrossRef]
- Ames-Sibin, A.P.; Barizao, C.L.; Castro-Ghizoni, C.V.; Silva, F.M.S.; Sa-Nakanishi, A.B.; Bracht, L.; Bersani-Amado, C.A.; Marcal-Natali, M.R.; Bracht, A.; Comar, J.F. beta-Caryophyllene, the major constituent of copaiba oil, reduces systemic inflammation and oxidative stress in arthritic rats. J. Cell. Biochem. 2018, 119, 10262–10277. [Google Scholar] [CrossRef]
- Tao, N.; Jia, L.; Zhou, H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef]
- Xing, S.; Gao, Y.; Li, X.; Ren, H.; Gao, Y.; Yang, H.; Liu, Y.; He, S.; Huang, Q. Antifungal activity of volatile components from Ceratocystis fimbriata and its potential biocontrol mechanism on Alternaria alternata in postharvest cherry tomato fruit. Microbiol. Spectr. 2023, 11, 02713–02722. [Google Scholar] [CrossRef]
- Hajaji, S.; Sifaoui, I.; Lopez-Arencibia, A.; Reyes-Batlle, M.; Valladares, B.; Pinero, J.E.; Lorenzo-Morales, J.; Akkari, H. Amoebicidal activity of alpha-bisabolol, the main sesquiterpene in chamomile (Matricaria recutita L.) essential oil against the trophozoite stage of Acanthamoeba castellani Neff. Acta Parasitol. 2017, 62, 290–295. [Google Scholar] [CrossRef]
- Garcia, M.C.F.; Soares, D.C.; Santana, R.C.; Saraiva, E.M.; Siani, A.C.; Ramos, M.F.S.; Danelli, M.; Souto-Padron, T.C.; Pinto-da-Silva, L.H. The in vitro antileishmanial activity of essential oil from Aloysia gratissima and guaiol, its major sesquiterpene against Leishmania amazonensis. Parasitology 2018, 145, 1219–1227. [Google Scholar] [CrossRef]
- Munoz-Perez, V.M.; Ortiz, M.I.; Ponce-Monter, H.A.; Monter-Perez, V.; Barragan-Ramirez, G. Anti-inflammatory and utero-relaxant effect of alpha-bisabolol on the pregnant human uterus. Korean J. Physiol. Pharmacol. 2018, 22, 391–398. [Google Scholar] [CrossRef]
- Mendes, F.B.; Bergamin, L.S.; Dos Santos Stuepp, C.; Braganhol, E.; Terroso, T.; Pohlmann, A.R.; Guterres, S.S.; Battastini, A.M. Alpha-bisabolol Promotes Glioma Cell Death by Modulating the Adenosinergic System. Anticancer Res. 2017, 37, 1819–1823. [Google Scholar]
- He, L.; Chen, S.L. Research progresses on synthesis of chlorogenic acid in plants. Pharm. Biotechnol. 2013, 20, 463–466. [Google Scholar]
- Zhang, X.; Li, C.; Hao, Z.; Liu, Y. Transcriptome analysis provides insights into coumarin biosynthesis in the medicinal plant Angelica dahurica cv. Yubaizhi. Gene 2023, 888, 147757. [Google Scholar] [CrossRef]
- Huang, X.C.; Tang, H.; Wei, X.; He, Y.; Hu, S.; Wu, J.Y.; Xu, D.; Qiao, F.; Xue, J.Y.; Zhao, Y. The gradual establishment of complex coumarin biosynthetic pathway in Apiaceae. Nat. Commun. 2024, 15, 6864. [Google Scholar] [CrossRef]
- Zhang, T.J.; Ma, Z.; Ma, H.J.; Tian, X.S.; Guo, W.L.; Zhang, C. Metabolic pathways modulated by coumarin to inhibit seed germination and early seedling growth in Eleusine indica. Plant Physiol. Biochem. 2023, 203, 108035. [Google Scholar] [CrossRef]
- Moller, B.; Villiger, P.M. Inhibition of IL-1, IL-6, and TNF-alpha in immune-mediated inflammatory diseases. Springer Semin. Immunopathol. 2006, 27, 391–408. [Google Scholar] [CrossRef]
- Wu, M.R.; Tang, L.H.; Chen, Y.Y.; Shu, L.X.; Xu, Y.Y.; Yao, Y.Q.; Li, Y.B. Systematic characterization of the chemical constituents in vitro and in vivo of Qianghuo by UPLC-Q-TOF-MS/MS. Fitoterapia 2024, 172, 105758. [Google Scholar] [CrossRef]
- Liu, Y.M.; Wang, S.; Dickenson, A.; Mao, J.; Bai, X.; Liao, X. An on-line SPE-LC-MS/MS method for quantification of nucleobases and nucleosides present in biological fluids. Anal. Methods 2024, 16, 2505–2512. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, Q.; Chen, D.; Zhang, Z.; Pan, Q.; Yu, J.; Cao, H.; Li, L.; Li, L. Serum metabonomics reveal the effectiveness of human placental mesenchymal stem cell therapy for primary sclerosing cholangitis. Stem Cell Res. Ther. 2024, 15, 346. [Google Scholar] [CrossRef] [PubMed]
- Ndolo, V.U.; Fulcher, R.G.; Beta, T. Application of LC–MS–MS to identify niacin in aleurone layers of yellow corn, barley and wheat kernels. J. Cereal Sci. 2015, 65, 88–95. [Google Scholar] [CrossRef]
- Shevchuk, A.; Megias-Perez, R.; Zemedie, Y.; Kuhnert, N. Evaluation of carbohydrates and quality parameters in six types of commercial teas by targeted statistical analysis. Food Res. Int. 2020, 133, 109122. [Google Scholar] [CrossRef]
- Choi, J.N.; Kim, J.; Ponnusamy, K.; Lim, C.; Kim, J.G.; Muthaiya, M.J.; Lee, C. Metabolic changes of Phomopsis longicolla fermentation and its effect on antimicrobial activity against Xanthomonas oryzae. J. Microbiol. Biotechnol. 2013, 23, 177–183. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.W. Studies on chemical constituents in roots and rhizomes of Notopterygium incisum. China J. Chin. Mater. Med. 2008, 33, 2918–2921. [Google Scholar]
- Ma, X.; Wu, Y.; Li, Y.; Huang, Y.; Liu, Y.; Luo, P.; Zhang, Z. Rapid discrimination of Notopterygium incisum and Notopterygium franchetii based on characteristic compound profiles detected by UHPLC-QTOF-MS/MS coupled with multivariate analysis. Phytochem. Anal. 2020, 31, 355–365. [Google Scholar] [CrossRef]
- Fontal, M.; van Drooge, B.L.; Lopez, J.F.; Fernandez, P.; Grimalt, J.O. Broad spectrum analysis of polar and apolar organic compounds in submicron atmospheric particles. J. Chromatogr. A 2015, 1404, 28–38. [Google Scholar] [CrossRef]
- Duan, H.; Wang, W.; Li, Y.; Jilany Khan, G.; Chen, Y.; Shen, T.; Bao, N.; Hua, J.; Xue, Z.; Zhai, K.; et al. Identification of phytochemicals and antioxidant activity of Premna microphylla Turcz. stem through UPLC-LTQ-Orbitrap-MS. Food Chem. 2022, 373, 131482. [Google Scholar] [CrossRef]
- Tian, Y.J.; Li, J.N.; Feng, J.L.; Wang, Q.; Zhang, C.Y.; Zhong, H.G.; Cheng, W.Q.; Yang, C. Chemical constituents from Notopterygium Root. Liaoning J. Tradit. Chin. Med. 2013, 15, 40–42. [Google Scholar] [CrossRef]
- Li, L.M.; Liang, B.D.; Yu, S.W.; Sun, H.D. Chemical constituents of Notopterygium incisum. Chin. J. Nat. Med. 2007, 5, 351–354. [Google Scholar]
- Dadabay, C.Y.; Spaulding, P.B.; Valenzuela, E.; Turner, M.; Eckert, K.E.; Julkunen-Tiitto, R.; Noblit, N.; Mansfield, D.H. Polyphenols from the sagebrush Artemisia tridentata ssp. tridentata affect the redox state of cultured hepatocytes by direct and indirect mechanisms. Curr. Top. Phytochem. 2019, 15, 15–25. [Google Scholar]
- Cao, L.; Zhang, S.; Cao, J.; Chang, R.; Qu, C.; Li, C.; Yan, J.; Quan, X.; Xu, Z.; Liu, G. Nitrogen modifies wood composition in poplar seedlings by regulating carbon and nitrogen metabolism. Ind. Crops Prod. 2024, 219, 119118. [Google Scholar] [CrossRef]
- Yang, J.; Fu, C.W.; Qin, H.L.; Qin, D.J.; Qin, Z.L. Geographical origin traceability of Desmodium caudatum (Thunb.) DC. By UPLC MS/MS coupled with BP neural network. Chin. J. Pharm. Anal. 2024, 44, 1176–1185. [Google Scholar]
- Su, X.; Wu, Y.; Li, Y.; Huang, Y.; Liu, Y.; Luo, P.; Zhang, Z. Effect of Different Post-Harvest Processing Methods on the Chemical Constituents of Notopterygium franchetii by an UHPLC-QTOF-MS-MS Metabolomics Approach. Molecules 2019, 24, 3188. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Xiong, J.; Zhao, Y.; Cao, L.; Wu, S.B.; Xia, G.; Hu, J.F. Glycosides from the methanol extract of Notopterygium incisum. Planta Med. 2011, 77, 1939–1943. [Google Scholar] [CrossRef]
- Wu, X.W.; Zhang, Y.B.; Zhang, L.; Yang, X.W. Simultaneous quantification of 33 active components in Notopterygii Rhizoma et Radix using ultra high performance liquid chromatography with tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1092, 244–251. [Google Scholar] [CrossRef]
- Xu, K.; Jiang, S.; Zhou, Y.; Zhang, Y.; Xia, B.; Xu, X.; Zhou, Y.; Li, Y.; Wang, M.; Ding, L. Discrimination of the seeds of Notopterygium incisum and Notopterygium franchetii by validated HPLC-DAD-ESI-MS method and principal component analysis. J. Pharm. Biomed. Anal. 2011, 56, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- González-Trujano, M.; Ventura-Martínez, R.; Chávez, M.; Díaz-Reval, I.; Pellicer, F. Spasmolytic and Antinociceptive Activities of Ursolic Acid and Acacetin Identified in Agastache mexicana. Planta Med. 2012, 78, 793–796. [Google Scholar] [CrossRef]
- Wu, S.B.; Zhao, Y.; Fan, H.; Hu, Y.H.; Hamann, M.T.; Peng, J.N.; Starks, C.M.; O'Neil-Johnson, M.; Hu, J.F. New guaiane sesquiterpenes and furanocoumarins from Notopterygium incisum. Planta Med. 2008, 74, 1812–1817. [Google Scholar] [CrossRef]
- Ding, X.-F.; Feng, X.; Dong, Y.-F.; Zhao, X.-Z.; Chen, Y.; Wang, M. Studies on chemical constituents of the roots of Angelica pubescens. China J. Chin. Mater. Med. 2008, 31, 516–518. [Google Scholar]
- Matano, Y.; Okuyama, T.; Shibata, S.; Hoson, M.; Kawada, T.; Osada, H.; Noguchi, T. Studies on Coumarins of a Chinese Drug “Qian-Hu”; VII1. Structures of New Coumarin-Glycosides of Zi-Huan Quian-Hu and Effect of Coumarin-Glycosides on Human Platelet Aggregation. Planta Med. 1986, 52, 135–138. [Google Scholar] [CrossRef]
- Xiao, L.; Zhou, Y.M.; Zhang, X.F.; Du, F.Y. Notopterygium incisum extract and associated secondary metabolites inhibit apple fruit fungal pathogens. Pestic. Biochem. Physiol. 2018, 150, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Xu, W.; Liu-Chen, L.Y.; Lee, D.Y. Novel coumarin glycoside and phenethyl vanillate from Notopterygium forbesii and their binding affinities for opioid and dopamine receptors. Bioorg. Med. Chem. 2008, 16, 3218–3223. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liu, X.; Lu, Y.; Ma, X.; Wu, X.; Wang, X.; Jia, M.; Su, P.; Tong, Y.; Guan, H.; et al. A specific UDP-glucosyltransferase catalyzes the formation of triptophenolide glucoside from Tripterygium wilfordii Hook. f. Phytochemistry 2019, 166, 112062. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.-G.; Yang, X.-W.; Zhang, Y.-B.; Xu, W.; Wei, W.; Chen, T.-L. [Chemical constituents from lipophilic parts in roots of Angelica dahurica var. formosana cv. Chuanbaizhi]. China J. Chin. Mater. Med. 2015, 40, 2148–2156. [Google Scholar]
- Hu, Z.; Chen, J.-T.; Jiang, S.-C.; Liu, Z.; Ge, S.-B.; Zhang, Z. Chemical components and functions of Taxus chinensis extract. JKSU-Science 2020, 32, 1562–1568. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, P.; Zhuang, Y.; Liu, T. Two O-Methyltransferases Mediate Multiple Methylation Steps in the Biosynthesis of Coumarins in Cnidium monnieri. J. Nat. Prod. 2022, 85, 2116–2121. [Google Scholar] [CrossRef]
- Ayoub, N.; Al-Azizi, M.; König, W.; Kubeczka, K.H. Essential oils and a novel polyacetylene from Eryngium yuccifolium Michaux. (Apiaceae). Flavour Frag. J. 2006, 21, 864–868. [Google Scholar] [CrossRef]
- Bindila, L.; Lutz, B. Extraction and Simultaneous Quantification of Endocannabinoids and Endocannabinoid-Like Lipids in Biological Tissues. Methods Mol. Biol. 2016, 1412, 9–18. [Google Scholar] [PubMed]
- Tsochatzis, E.; Lopes, J.; Gika, H.; Theodoridis, G. Polystyrene Biodegradation by Tenebrio molitor Larvae: Identification of Generated Substances Using a GC-MS Untargeted Screening Method. Polymers 2020, 13, 17. [Google Scholar] [CrossRef]
- Zhang, S.; You, J.; Zhou, G.; Li, C.; Suo, Y. Analysis of free fatty acids in Notopterygium forbesii Boiss by a novel HPLC method with fluorescence detection. Talanta 2012, 98, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Yen, M.H.; Teng, C.M.; Tsai, I.L.; Chen, I.S. Cerebrosides from the Rhizomes of Gynura Japonica. J. Chin. Chem. Soc.-Tai. 2004, 51, 1429–1434. [Google Scholar] [CrossRef]





| Serial Number | Sample Name | Code Name | Harvest Site | Harvest Time |
|---|---|---|---|---|
| 1 | Sichuan wild product | SW | Aba, Sichuan | 2023.11 |
| 2 | Qinghai wild product | HW | Tanggula Mountains | 2023.11 |
| 3 | Gansu wild product | GW | Min County, Gansu | 2023.12 |
| 4 | Sichuan cultivated product (4-year-old) | SC-1 | Aba, Sichuan | 2023.11 |
| 5 | Sichuan cultivated product (4-year-old) | SC-2 | Aba, Sichuan | 2023.11 |
| 6 | Qinghai cultivated product (4-year-old) | HC-1 | Haixi Mongol and Tibet Autonomous Prefecture | 2023.11 |
| 7 | Qinghai cultivated product (4-year-old) | HC-2 | Haixi Mongol and Tibet Autonomous Prefecture | 2023.11 |
| 8 | Gansu cultivated product (4-year-old) | GC-1 | Longnan, Gansu | 2023.12 |
| 9 | Gansu cultivated product (4-year-old) | GC-2 | Longnan, Gansu | 2023.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, F.; Kou, Y.; Zhang, X.; Tian, Y.; Yang, B.; Wang, W. Comparative Analysis of Metabolites of Wild and Cultivated Notopterygium incisum from Different Origins and Evaluation of Their Anti-Inflammatory Activity. Molecules 2025, 30, 468. https://doi.org/10.3390/molecules30030468
Kong F, Kou Y, Zhang X, Tian Y, Yang B, Wang W. Comparative Analysis of Metabolites of Wild and Cultivated Notopterygium incisum from Different Origins and Evaluation of Their Anti-Inflammatory Activity. Molecules. 2025; 30(3):468. https://doi.org/10.3390/molecules30030468
Chicago/Turabian StyleKong, Fukang, Yannan Kou, Xu Zhang, Yue Tian, Bin Yang, and Weihao Wang. 2025. "Comparative Analysis of Metabolites of Wild and Cultivated Notopterygium incisum from Different Origins and Evaluation of Their Anti-Inflammatory Activity" Molecules 30, no. 3: 468. https://doi.org/10.3390/molecules30030468
APA StyleKong, F., Kou, Y., Zhang, X., Tian, Y., Yang, B., & Wang, W. (2025). Comparative Analysis of Metabolites of Wild and Cultivated Notopterygium incisum from Different Origins and Evaluation of Their Anti-Inflammatory Activity. Molecules, 30(3), 468. https://doi.org/10.3390/molecules30030468






