On-Resin Selenopeptide Catalysts: Synthesis and Applications of Enzyme-Mimetic Reactions and Cyclization of Unsaturated Carboxylic Acids †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of On-Resin Selenopeptide Catalysts
2.2. GPx-like Peroxidase Activity of 5
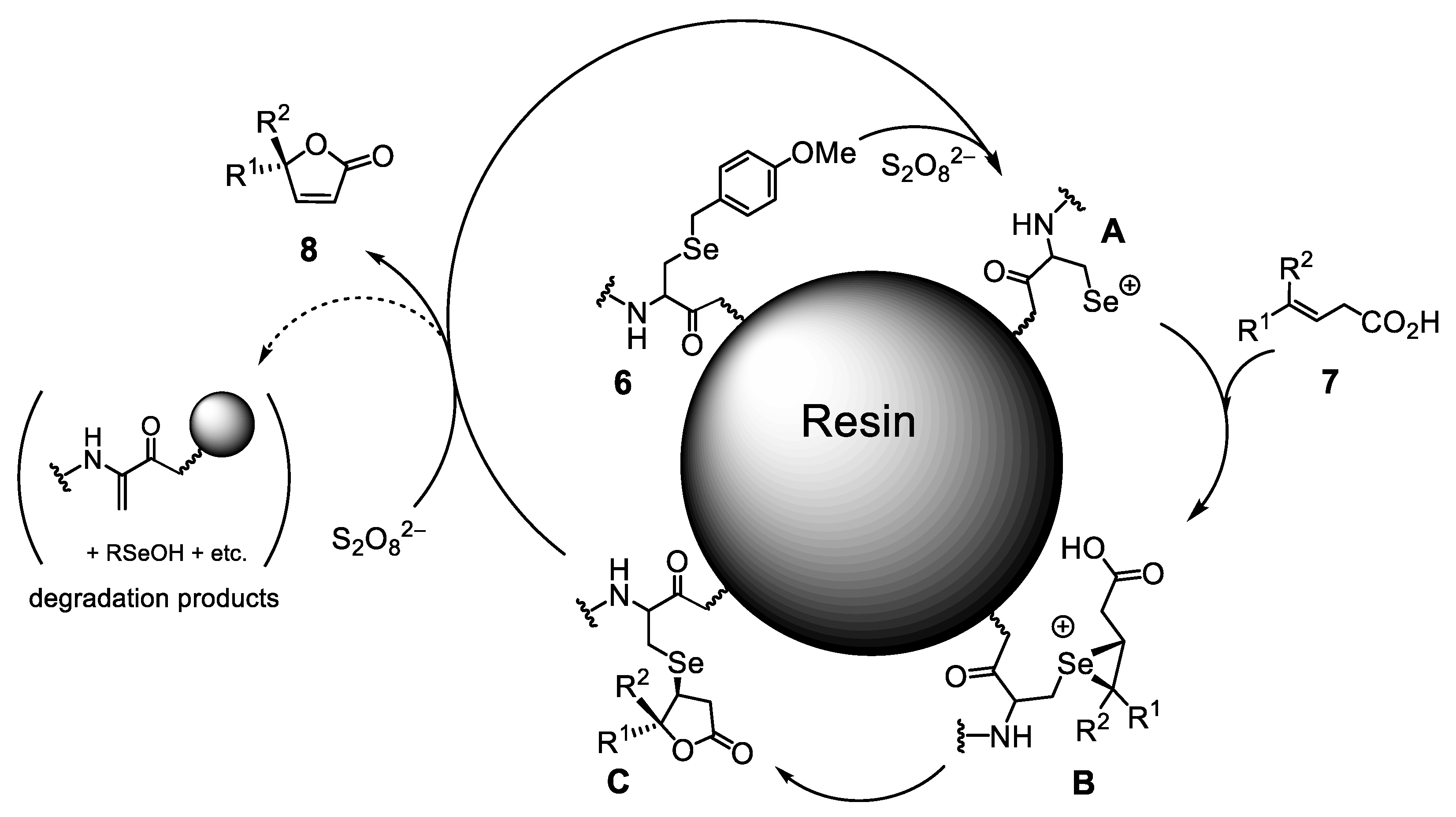
2.3. Oxidative Cyclization of β,γ-Unsaturated Acids Catalyzed by 6
2.4. Recycling of On-Resin Selenopeptide Catalysts (6)
2.5. Mechanism for the Conversion from 7 to 8 Catalyzed by On-Resin Selenopeptides (6)
3. Materials and Methods
3.1. Materials
3.2. General Procedures for the Synthesis of On-Resin Selenopeptide Catalysts
3.3. Characterization of Selenopeptides
3.4. Assessment of Peroxidase Activity
3.5. Kinetic Analysis
3.6. Statistical Analysis
3.7. Catalytic Cyclization of β,γ-Unsaturated Acids (7)
3.8. EPMA Analysis
3.9. Molecular Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, N.V.; Nogueira, C.W.; Nogara, P.A.; De Bem, A.F.; Aschner, M.; Rocha, J.B.T. Organoselenium Compounds as Mimics of Selenoproteins and Thiol Modifier Agents. Metallomics 2017, 9, 1703–1734. [Google Scholar] [CrossRef] [PubMed]
- Sands, K.N.; Tuck, T.A.; Back, T.G. Cyclic Seleninate Esters, Spirodioxyselenuranes and Related Compounds: New Classes of Biological Antioxidants That Emulate Glutathione Peroxidase. Chem. A Eur. J. 2018, 24, 9714–9728. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.A.; Park, C.W. Catalytic Antioxidants in the Kidney. Antioxidants 2021, 10, 130. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, J.; Zhao, X. Combination of Lewis Basic Selenium Catalysis and Redox Selenium Chemistry: Synthesis of Trifluoromethylthiolated Tertiary Alcohols with Alkenes. Org. Lett. 2017, 19, 4940–4943. [Google Scholar] [CrossRef]
- Singh, F.V.; Wirth, T. Selenium Reagents as Catalysts. Catal. Sci. Technol. 2019, 9, 1073–1091. [Google Scholar] [CrossRef]
- Gallo-Rodriguez, C.; Rodriguez, J.B. Organoselenium Compounds in Catalysis. Synthesis 2024, 56, 2295–2315. [Google Scholar] [CrossRef]
- Batabyal, M.; Jaiswal, S.; Jha, R.K.; Kumar, S. Directing Group Strategy for the Isolation of Organoselenium (VI) Benzoselenonates: Metal-Free Catalysts for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2024, 146, 57–61. Available online: https://pubs.acs.org/doi/10.1021/jacs.3c10572 (accessed on 26 December 2024). [CrossRef]
- Madabeni, A.; Tanini, D.; Capperucci, A.; Orian, L. Untangling the Catalytic Importance of the Se Oxidation State in Organoselenium-Mediated Oxygen-Transfer Reactions: The Conversion of Aniline to Nitrobenzene. Chem. Sci. 2024, 15, 12126–12137. [Google Scholar] [CrossRef]
- Masuda, R.; Kimura, R.; Karasaki, T.; Sase, S.; Goto, K. Modeling the Catalytic Cycle of Glutathione Peroxidase by Nuclear Magnetic Resonance Spectroscopic Analysis of Selenocysteine Selenenic Acids. J. Am. Chem. Soc. 2021, 143, 6345–6350. [Google Scholar] [CrossRef]
- Oroz, P.; Avenoza, A.; Busto, J.H.; Corzana, F.; Zurbano, M.M.; Peregrina, J.M. Strategies for the Synthesis of Selenocysteine Derivatives. Synthesis 2022, 54, 255–270. [Google Scholar] [CrossRef]
- Pehlivan, Ö.; Waliczek, M.; Kijewska, M.; Stefanowicz, P. Selenium in Peptide Chemistry. Molecules 2023, 28, 3198. [Google Scholar] [CrossRef] [PubMed]
- Iwaoka, M.; Shimodaira, S. Synthesis and Catalytic Functions of Selenopeptides. In Organochalcogen Compounds: Synthesis, Catalysis and New Protocols with Greener Perspectives; Lenardao, E.J., Santi, C., Perin, G., Alves, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–218. ISBN 9780128194492. [Google Scholar]
- Iwaoka, M.; Oba, H.; Matsumura, K.; Yamanaka, S.; Shimodaira, S.; Kusano, S.; Asami, T. Antioxidant Activity of a Selenopeptide Modelling the Thioredoxin Reduc-Tase Active Site Is Enhanced by NH·Se Hydrogen Bond in the Mixed Se-Lenosulfide Intermediate. Curr. Chem. Biol. 2022, 16, 44–53. [Google Scholar] [CrossRef]
- Yoshida, S.; Kumakura, F.; Komatsu, I.; Arai, K.; Onuma, Y.; Hojo, H.; Singh, B.G.; Priyadarsini, K.I.; Iwaoka, M. Antioxidative Glutathione Peroxidase Activity of Selenoglutathione. Angew. Chem. Int. Ed. 2011, 50, 2125–2128. [Google Scholar] [CrossRef] [PubMed]
- Takei, T.; Urabe, Y.; Asahina, Y.; Hojo, H.; Nomura, T.; Dedachi, K.; Arai, K.; Iwaoka, M. Model Study Using Designed Selenopeptides on the Importance of the Catalytic Triad for the Antioxidative Functions of Glutathione Peroxidase. J. Phys. Chem. B 2014, 118, 492–500. [Google Scholar] [CrossRef]
- Shimodaira, S.; Takei, T.; Hojo, H.; Iwaoka, M. Synthesis of Selenocysteine-Containing Cyclic Peptides via Tandem N-to-S Acyl Migration and Intramolecular Selenocysteine-Mediated Native Chemical Ligation. Chem. Commun. 2018, 54, 11737–11740. [Google Scholar] [CrossRef]
- Shimodaira, S.; Iwaoka, M. Synthesis of Selenocysteine-Containing Dipeptides Modeling the Active Site of Thioredoxin Reductase. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 750–752. [Google Scholar] [CrossRef]
- Iwaoka, M.; Ito, S.; Miyazaki, I.; Michibata, M. Synthesis of L-Selenocysteine and α-Methyl-l-Selenocysteine Derivatives Using Woollins’ Reagent and Their Application as Chiral Selenium Catalysts. Proc. Natl. Acad. Sci. India Sect. A—Phys. Sci. 2016, 86, 499–509. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Parrasia, S.; Vecchia, F.D. Selenium Accumulation and Metabolism in Algae. Aquat. Toxicol. 2017, 189, 1–8. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Wise, L.A. Environmental Selenium and Human Health: An Update. Curr. Environ. Heal. Rep. 2018, 5, 464–485. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Nahar, K.; Fujita, M. Selenium Toxicity in Plants and Environment: Biogeochemistry and Remediation Possibilities. Plants 2020, 9, 1711. [Google Scholar] [CrossRef] [PubMed]
- Uehlin, L.; Wirth, T. Chiral Selenium Electrophiles on Solid-Support. Chimia 2001, 55, 65–67. [Google Scholar] [CrossRef]
- Cankařová, N.; Schütznerová, E.; Krchňák, V. Traceless Solid-Phase Organic Synthesis. Chem. Rev. 2019, 119, 12089–12207. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Y.; Chen, Z.; Pan, X.; Zhang, Z.; Zhu, J.; Zhu, X. Organoselenium Chemistry-Based Polymer Synthesis. Org. Chem. Front. 2020, 7, 2815–2841. [Google Scholar] [CrossRef]
- Ruhland, T.; Andersen, K.; Pedersen, H. Selenium-Linking Strategy for Traceless Solid-Phase Synthesis: Direct Loading, Aliphatic C-H Bond Formation upon Cleavage and Reaction Monitoring by Gradient MAS NMR Spectroscopy. J. Org. Chem. 1998, 63, 9204–9211. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Pastor, J.; Barluenga, S.; Winssinger, N. Polymer-Supported Selenium Reagents for Organic Synthesis. Chem. Commun. 1998, 1947–1948. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Roecker, A.J.; Pfefferkorn, J.A.; Cao, G.-Q. A Novel Strategy for the Solid-Phase Synthesis of Substituted Indolines. J. Am. Chem. Soc. 2000, 122, 2966–2967. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Pfefferkorn, J.A.; Roecker, A.J.; Cao, G.-Q.; Barluenga, S.; Mitchell, H.J. Natural Product-like Combinatorial Libraries Based on Privileged Structures. 1. General Principles and Solid-Phase Synthesis of Benzopyrans. J. Am. Chem. Soc. 2000, 122, 9939–9953. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Watanabe, K.; Oishi, A.; Ikeda, Y.; Taguchi, Y. Preparation of Polymer-Supported Selenocyanates and Their Application to Solid-Phase Oxyselenenylation-Deselenenylation. Synlett 1999, 1999, 1760–1762. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Hashimoto, S.; Kanakubo, M.; Oishi, A.; Taguchi, Y. Solid-Phase Intramolecular Oxyselenenylation and Deselenenylation Reactions Using Polymer-Supported Selenoreagents and Their Application to Aqueous Media Organic Synthesis. Green Chem. 2003, 5, 549–553. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Hashimoto, S.; Oishi, A.; Taguchi, Y. Intramolecular Oxyselenenylation and Deselenenylation Reactions in Water, Conducted by Employing Polymer-Supported Arylselenenyl Bromide. Tetrahedron Lett. 2003, 44, 3793–3795. [Google Scholar] [CrossRef]
- Uehlin, L.; Wirth, T. Novel Polymer-Bound Chiral Selenium Electrophiles. Org. Lett. 2001, 3, 2931–2933. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.-R.; Liu, X.-L.; Wang, X.-C.; Xin, Q.; Song, C.-S. Polymer-Supported β-Bromoethyl Selenide: An Efficient Reagent for the Synthesis of Aryl Vinyl Ethers. Synthesis 2004, 2004, 2833–2836. [Google Scholar] [CrossRef]
- Xu, W.-M.; Huang, X.; Tang, E. Solid-Phase Synthesis of 1,2-Diheterocyclic-Substituted (E)-Olefins from a Supported Selenium Resin. J. Comb. Chem. 2005, 7, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wei, M.-H.; Xu, W.; Sheng, S.-R. Solid-Phase Organic Synthesis of (E)-1-Nitroalkenes Based on Polystyrene-Supported Selenonitromethane. J. Chem. Res. 2012, 36, 472–473. [Google Scholar] [CrossRef]
- Tang, E.; Huang, X.; Xu, W.-M. Polymer-Supported Selenium-Induced Electrophilic Cyclization: Solid-Phase Synthesis of Poly-Substituted Dihydrofurans and Tetrahydrofurans. Tetrahedron 2004, 60, 9963–9969. [Google Scholar] [CrossRef]
- Wang, C.-L.; Sheng, S.-R.; Cheng, X.; Cai, M.-Z. Solid-Phase Organic Synthesis of 5-Iodomethyl-Dihydrofuran-2-Ones with Recyclable Polymer-Supported Selenium Bromide. Synth. Commun. 2012, 42, 320–327. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Xu, W.-M.; Huang, X. Selenium-Based Safety-Catch Linker: Solid-Phase Synthesis of Vinyl-Substituted Oxadiazoles and Triazoles. J. Comb. Chem. 2007, 9, 513–519. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.-G. Solid-Phase Synthesis of Linked Heterocycles from a Selenopolystyrene Resin. J. Comb. Chem. 2007, 9, 121–130. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Sheng, W.-S.; Sheng, S.-R.; Li, Y.; Cai, M.-Z. Click Chemistry on Polymer Support: Synthesis of 1-Vinyl-and 1-Allyl-1,2,3-Triazoles via Selenium Linker. Synth. Commun. 2014, 44, 59–67. [Google Scholar] [CrossRef]
- Ming, P.; Liu, X.-L.; Wei, M.-H.; Sheng, S.-R. Synthesis of 4-Vinyl-1H-1,2,3-Triazoles on Solid Supports via Polystyrene-Bound But-3-Ynyl Selenide. Heteroat. Chem. 2016, 27, 184–189. [Google Scholar] [CrossRef]
- Huang, X.; Tang, E.; Xu, W.-M.; Cao, J. Lewis Acid Catalyzed Solid-Phase Synthesis of Flavonoids Using Selenium-Bound Resin. J. Comb. Chem. 2005, 7, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Mogemark, M.; Gustafsson, L.; Bengtsson, C.; Elofsson, M.; Kihlberg, J. A Fluorinated Selenide Linker for Solid-Phase Synthesis of n-Pentenyl Glycosides. Org. Lett. 2004, 6, 4885–4888. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.; Chen, B.; Zhang, L.; Li, W.; Lin, J. ZnCl2-Catalyzed Intramolecular Cyclization Reaction of 2-Aminochalcones Using Polymer-Supported Selenium Reagent: Synthesis of 2-Phenyl-4-Quinolones and 2-Phenyl-2,3-Dihydroquinolin-4(1 H)-One. Synlett 2011, 2011, 707–711. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, Y.G. An Efficient Solid-Phase Synthesis of Substituted Benzofuran Using Selenium-Bound Resin. Appl. Organomet. Chem. 2012, 26, 212–216. [Google Scholar] [CrossRef]
- Barrero, A.F.; Del Moral, J.F.Q.; Herrador, M.M.; Cortés, M.; Arteaga, P.; Catalán, J.V.; Sánchez, E.M.; Arteaga, J.F. Solid-Phase Selenium-Catalyzed Selective Allylic Chlorination of Polyprenoids: Facile Syntheses of Biologically Active Terpenoids. J. Org. Chem. 2006, 71, 5811–5814. [Google Scholar] [CrossRef]
- Tan, K.H.; Xu, W.; Stefka, S.; Demco, D.E.; Kharandiuk, T.; Ivasiv, V.; Nebesnyi, R.; Petrovskii, V.S.; Potemkin, I.I.; Pich, A. Selenium-Modified Microgels as Bio-Inspired Oxidation Catalysts. Angew. Chem. Int. Ed. 2019, 58, 9791–9796. [Google Scholar] [CrossRef]
- Mitchell, A.R.; Erickson, B.W.; Ryabtsev, M.N.; Hodges, R.S.; Merrifield, R.B. Tert-Butoxycarbonylaminoacyl-4-(Oxymethyl)Phenylacetamidomethyl-Resin, a More Acid-Resistant Support for Solid-Phase Peptide Synthesis. J. Am. Chem. Soc. 1976, 98, 7357–7362. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Kent, S.B.H.; Engelhard, M.; Merrifield, R.B. A New Synthetic Route to Tert-Butyloxycarbonylaminoacyl-4-(Oxymethyl)Phenylacetamidomethyl-Resin, an Improved Support for Solid-Phase Peptide Synthesis1. J. Org. Chem. 1978, 43, 2845–2852. [Google Scholar] [CrossRef]
- Kumakura, F.; Mishra, B.; Priyadarsini, K.I.; Iwaoka, M. A Water-Soluble Cyclic Selenide with Enhanced Glutathione Peroxidase-like Catalytic Activities. Eur. J. Org. Chem. 2010, 2010, 440–445. [Google Scholar] [CrossRef]
- Arai, K.; Ueno, H.; Asano, Y.; Chakrabarty, G.; Shimodaira, S.; Mugesh, G.; Iwaoka, M. Protein Folding in the Presence of Water-Soluble Cyclic Diselenides with Novel Oxidoreductase and Isomerase Activities. ChemBioChem 2018, 19, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Iwaoka, M.; Tomoda, S. A Model Study on the Effect of an Amino Group on the Antioxidant Activity of Glutathione Peroxidase. J. Am. Chem. Soc. 1994, 116, 2557–2561. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Mugesh, G. Synthesis and Structure—Activity Correlation Studies of Secondary- and Tertiary-Amine-Based Glutathione Peroxidase Mimics. Chem. A Eur. J. 2009, 15, 9846–9854. [Google Scholar] [CrossRef] [PubMed]
- Hodage, A.S.; Phadnis, P.P.; Wadawale, A.; Priyadarsini, K.I.; Jain, V.K. Synthesis, Characterization, and X-Ray Structures of 2-(3,5—Dimethylpyrazol-1-Yl)Phenyl-Based Organoselenium Compounds and Their Glutathione Peroxidase (Gpx) like Activity. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 1419–1433. [Google Scholar] [CrossRef]
- Arai, K.; Kumakura, F.; Takahira, M.; Sekiyama, N.; Kuroda, N.; Suzuki, T.; Iwaoka, M. Effects of Ring Size and Polar Functional Groups on the Glutathione Peroxidase-like Antioxidant Activity of Water-Soluble Cyclic Selenides. J. Org. Chem. 2015, 80, 5633–5642. [Google Scholar] [CrossRef]
- Ibrahim, M.; Muhammad, N.; Naeem, M.; Deobald, A.M.; Kamdem, J.P.; Rocha, J.B.T. In Vitro Evaluation of Glutathione Peroxidase (GPx)-like Activity and Antioxidant Properties of an Organoselenium Compound. Toxicol. Vitr. 2015, 29, 947–952. [Google Scholar] [CrossRef]
- Arai, K.; Matsunaga, T.; Ueno, H.; Akahoshi, N.; Sato, Y.; Chakrabarty, G.; Mugesh, G.; Iwaoka, M. Modeling Thioredoxin Reductase-Like Activity with Cyclic Selenenyl Sulfides: Participation of an NH⋅Se Hydrogen Bond through Stabilization of the Mixed Se−S Intermediate. Chem. A Eur. J. 2019, 25, 12751–12760. [Google Scholar] [CrossRef]
- Iwaoka, M.; Yoshida, K.; Shimosato, T. Application of a Distance-Dependent Sigmoidal Dielectric Constant to the REMC/SAAP3D Simulations of Chignolin, Trp-Cage, and the G10q Mutant. Protein J. 2020, 39, 402–410. [Google Scholar] [CrossRef]
- Bhowmick, D.; Mugesh, G. Introduction of a Catalytic Triad Increases the Glutathione Peroxidase-like Activity of Diaryl Diselenides. Org. Biomol. Chem. 2015, 13, 9072–9082. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Tingoli, M.; Bagnoli, L.; Santi, C. Selenium Catalysed Conversion of β,γ-Unsaturated Acids into Butenolides. Synlett 1993, 1993, 798–800. [Google Scholar] [CrossRef]
- Ma, S.; Caprioli, R.M.; Hill, K.E.; Burk, R.F. Loss of Selenium from Selenoproteins: Conversion of Selenocysteine to Dehydroalanine in Vitro. J. Am. Soc. Mass Spectrom. 2003, 14, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, Y.; Hashimoto, T.; Maruoka, K. A Chiral Electrophilic Selenium Catalyst for Highly Enantioselective Oxidative Cyclization. J. Am. Chem. Soc. 2016, 138, 5206–5209. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, S.; Iwaoka, M. Improved Synthetic Routes to the Selenocysteine Derivatives Useful for Boc-Based Peptide Synthesis with Benzylic Protection on the Selenium Atom. Arkivoc 2016, 2017, 260–271. [Google Scholar] [CrossRef]
- Brown, D.M.; Niyomura, O.; Wirth, T. Catalytic Addition-Elimination Reactions towards Butenolides. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 1026–1035. [Google Scholar] [CrossRef]
- Hillas, P.J.; Soto Del Alba, F.; Oyarzabal, J.; Wilks, A.; Ortiz De Montellano, P.R. The AhpC and AhpD Antioxidant Defense System of Mycobacterium Tuberculosis. J. Biol. Chem. 2000, 275, 18801–18809. [Google Scholar] [CrossRef]
- Tan, C.K.; Er, J.C.; Yeung, Y.-Y. Synthesis of Chiral Butenolides Using Amino-Thiocarbamate-Catalyzed Asymmetric Bromolactonization. Tetrahedron Lett. 2014, 55, 1243–1246. [Google Scholar] [CrossRef]
- Devalankar, D.A.; Chouthaiwale, P.V.; Sudalai, A. Organocatalytic Sequential α-Aminoxylation and Cis-Wittig Olefination of Aldehydes: Synthesis of Enantiopure γ-Butenolides. Tetrahedron Asymmetry 2012, 23, 240–244. [Google Scholar] [CrossRef]
- Iwaoka, M.; Kimura, N.; Yosida, D.; Minezaki, T. The SAAP Force Field: Development of the Single Amino Acid Potentials for 20 Proteinogenic Amino Acids and Monte Carlo Molecular Simulation for Short Peptides. J. Comput. Chem. 2009, 30, 2039–2055. [Google Scholar] [CrossRef]
- Iwaoka, M.; Suzuki, T.; Shoji, Y.; Dedachi, K.; Shimosato, T.; Minezaki, T.; Hojo, H.; Onuki, H.; Hirota, H. Development of SAAP3D Force Field and the Application to Replica-Exchange Monte Carlo Simulation for Chignolin and C-Peptide. J. Comput. Aided. Mol. Des. 2017, 31, 1039–1052. [Google Scholar] [CrossRef]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium Nanoparticles for Biomedical Applications: From Development and Characterization to Therapeutics. Adv. Healthc. Mater. 2021, 10, 2100598. [Google Scholar] [CrossRef]
- Kiyokawa, K.; Takemoto, K.; Yahata, S.; Kojima, T.; Minakata, S. Oxidative Cyclization of β,γ-Unsaturated Carboxylic Acids Using Hypervalent Iodine Reagents: An Efficient Synthesis of 4-Substituted Furan-2-Ones. Synthesis 2017, 49, 2907–2912. [Google Scholar] [CrossRef]

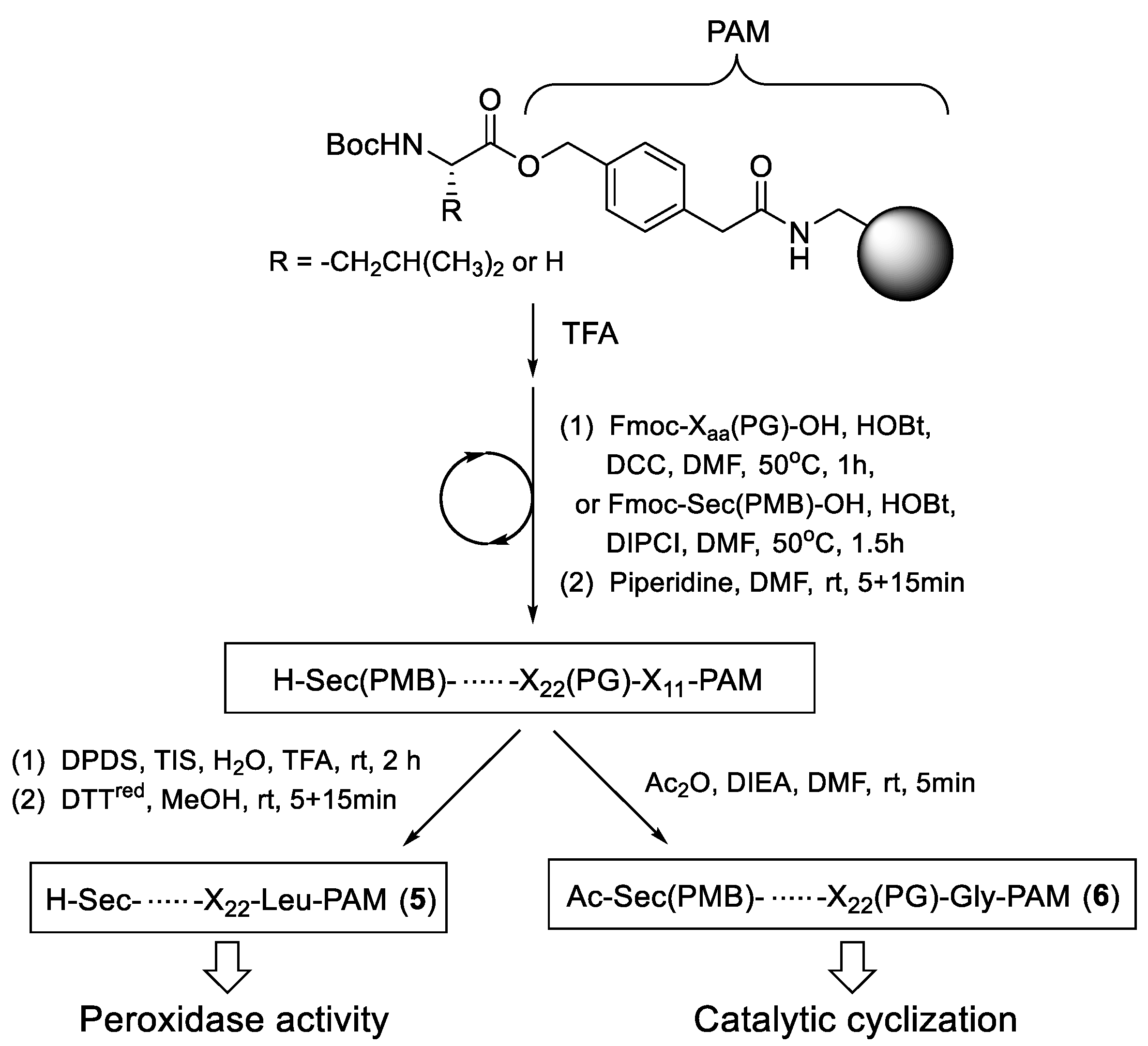

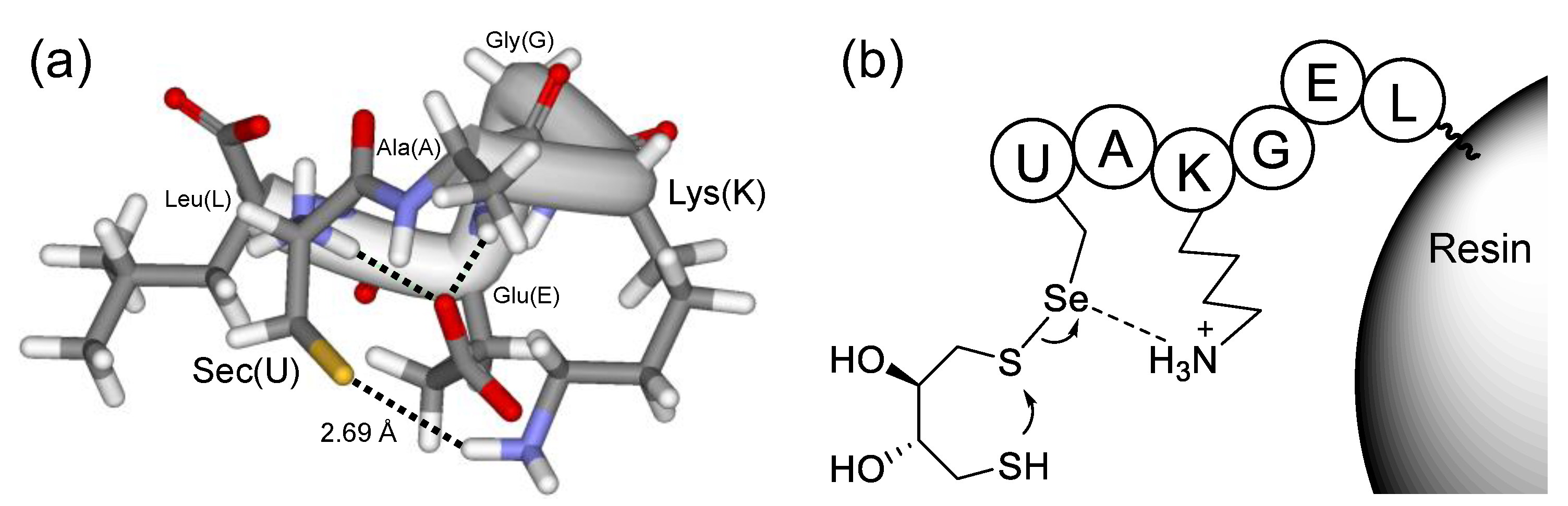

| Resins | Amino Acid Sequence 1 | Resins | Amino Acid Sequence 1 |
|---|---|---|---|
| 5a | H-UHGEL-PAM | 6a | Ac-U*AAAG-PAM |
| 5b | H-UAHGEL-PAM | 6b | Ac-U*AAAGGG-PAM |
| 5c | H-UAAAHGEL-PAM | 6c | Fmoc-U*AAAG-PAM |
| 5d | H-UAAAAHGEL-PAM | 6d | Ac-AU*AAG-PAM |
| 5e | H-UAKGEL-PAM | ||
| 5f | H-UAPGEL-PAM | ||
| 5g | H-AAHGEL-PAM |
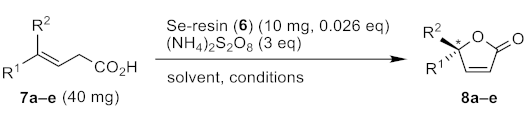 | ||||||
|---|---|---|---|---|---|---|
| Entry | Substrate | Se-Resin | Solvent | Conditions | Round | Yields of 8 |
| 1 | 7a (R1 = Ph, R2 = Me) | 6a | MeCN (10 mL) | 60 °C, 18 h | 1 | 51% |
| 2 | 7a | 6b | MeCN (10 mL) | 60 °C, 18 h | 1 | 25% |
| 3 | 7a | 6a | MeCN (1 mL) | 60 °C, 18 h | 1 2 3 4 | 79% 73% 27% 2 0% |
| 4 | 7a | 6b | MeCN (1 mL) | 60 °C, 18 h | 1 2 3 4 | 33% 51% 14% 2 0% |
| 5 | 7a | 6b | 1,4-dioxane (1 mL) | 60 °C, 48 h | 1 2 3 | 33% 18% 0% |
| 6 | 7a | 6c | MeCN (1 mL) | 60 °C, 18 h | 1 | 47% |
| 7 | 7a | 6d | MeCN (1 mL) | 60 °C, 18 h | 1 2 3 | 90% 83% trace |
| 8 | 7b (R1 = Ph, R2 = H) | 6a | MeCN (1 mL) | 60 °C, 18 h | 1 | 53% |
| 9 | 7c (R1 = 4-(MeO)Ph, R2 = H) | 6a | MeCN (1 mL) | 60 °C, 48 h | 1 | 0% |
| 10 | 7d (R1 = CH2Ph, R2 = H) | 6a | MeCN (1 mL) | 60 °C, 18 h | 1 | 45% |
| 11 | 7e (R1 = Et, R2 = H) | 6a | MeCN (1 mL) | 60 °C, 18 h | 1 | 68% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwaoka, M.; Maese, Y.; Abe, K. On-Resin Selenopeptide Catalysts: Synthesis and Applications of Enzyme-Mimetic Reactions and Cyclization of Unsaturated Carboxylic Acids. Molecules 2025, 30, 480. https://doi.org/10.3390/molecules30030480
Iwaoka M, Maese Y, Abe K. On-Resin Selenopeptide Catalysts: Synthesis and Applications of Enzyme-Mimetic Reactions and Cyclization of Unsaturated Carboxylic Acids. Molecules. 2025; 30(3):480. https://doi.org/10.3390/molecules30030480
Chicago/Turabian StyleIwaoka, Michio, Yua Maese, and Kasumi Abe. 2025. "On-Resin Selenopeptide Catalysts: Synthesis and Applications of Enzyme-Mimetic Reactions and Cyclization of Unsaturated Carboxylic Acids" Molecules 30, no. 3: 480. https://doi.org/10.3390/molecules30030480
APA StyleIwaoka, M., Maese, Y., & Abe, K. (2025). On-Resin Selenopeptide Catalysts: Synthesis and Applications of Enzyme-Mimetic Reactions and Cyclization of Unsaturated Carboxylic Acids. Molecules, 30(3), 480. https://doi.org/10.3390/molecules30030480






