Effect of Amino Acid, Sugar, Ca2+, and Mg2+ on Maillard Reaction-Associated Products in Modified Sparkling Base Wines During Accelerated Aging
Abstract
1. Introduction
2. Results and Discussion
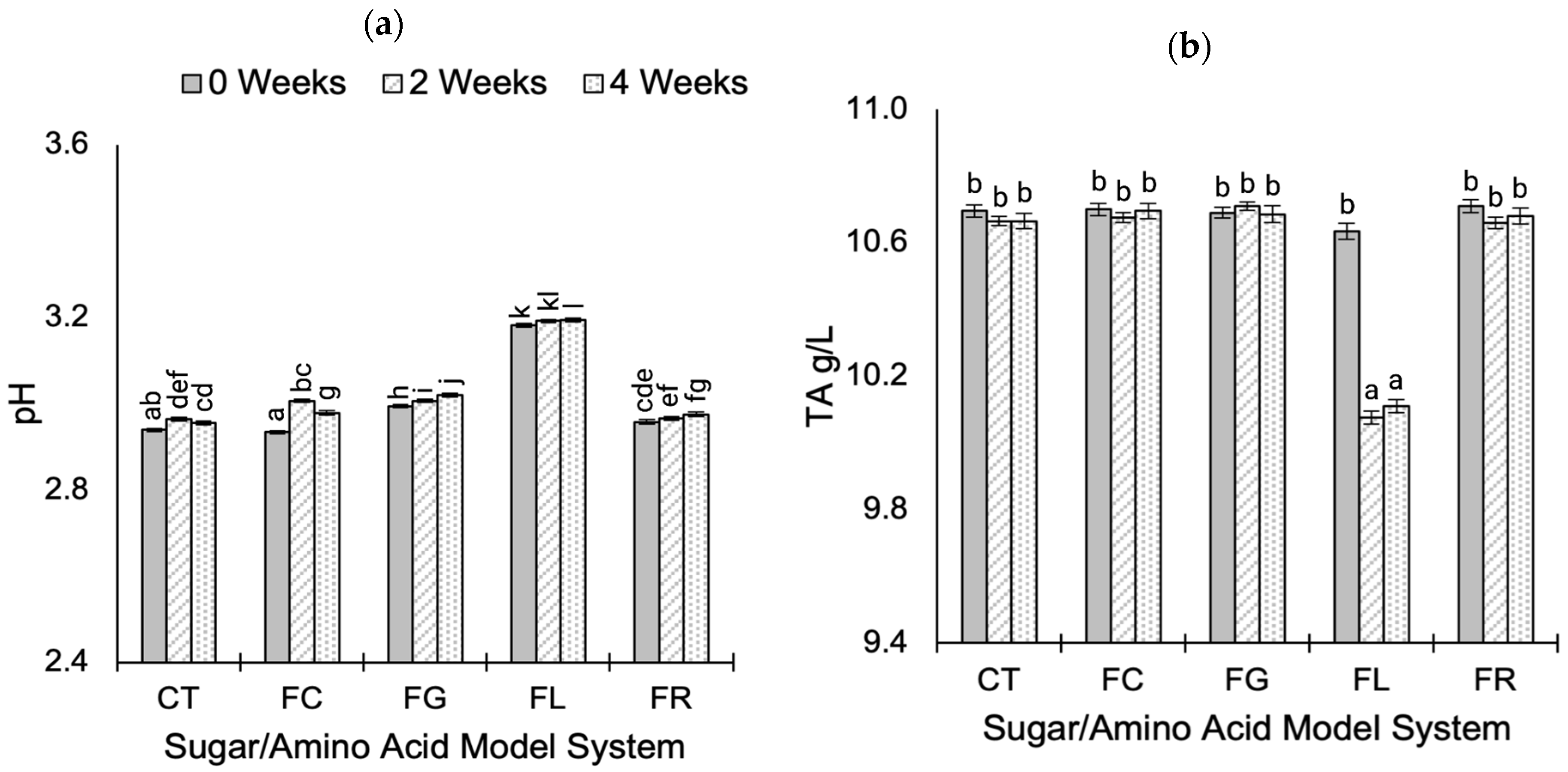
2.1. Standard Wine Chemical Parameters During Aging
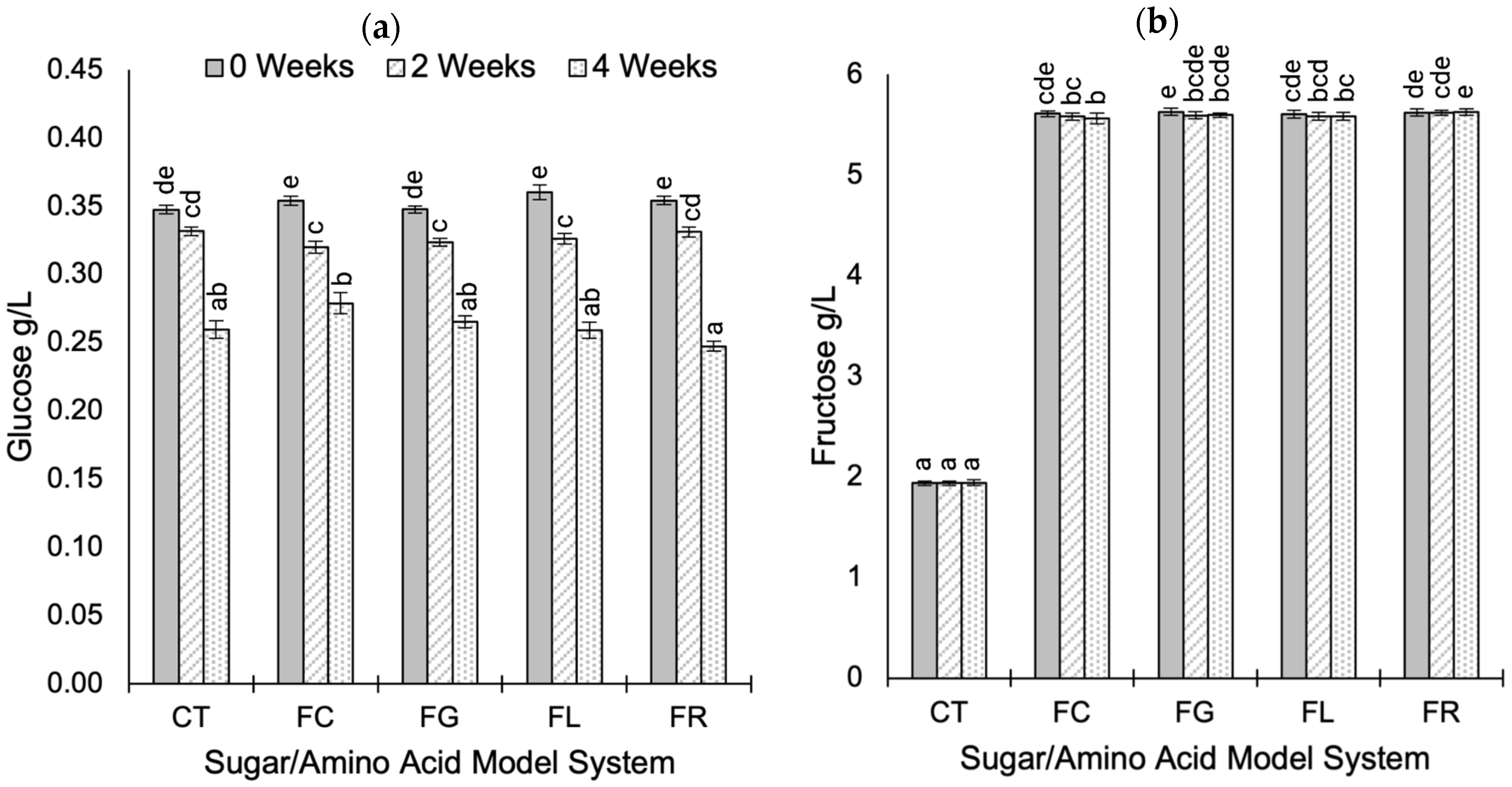
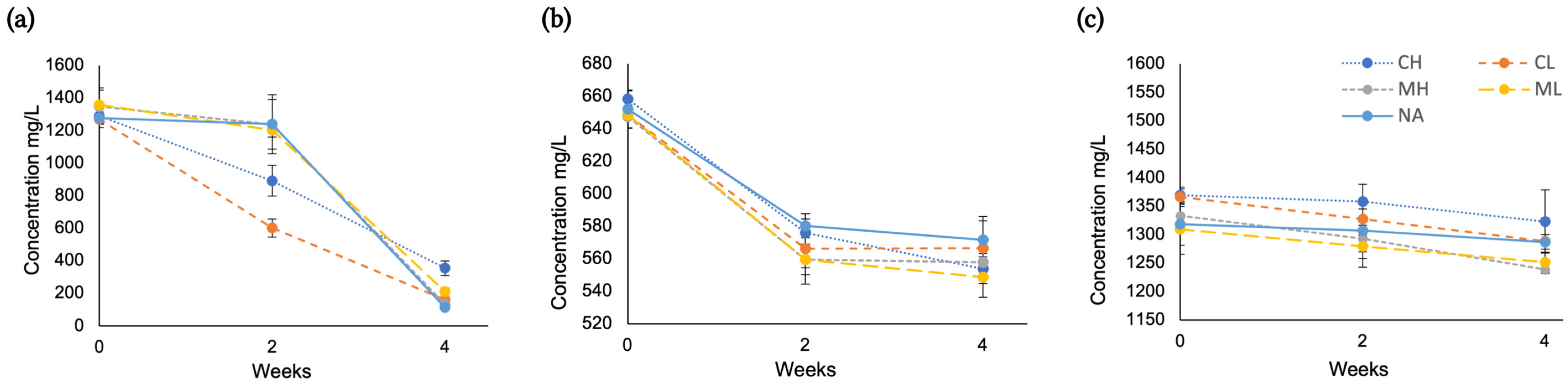
2.2. Sugar Composition During Aging
2.3. Amino Acid Composition During Aging
2.4. Ca2+ and Mg2+ During Aging
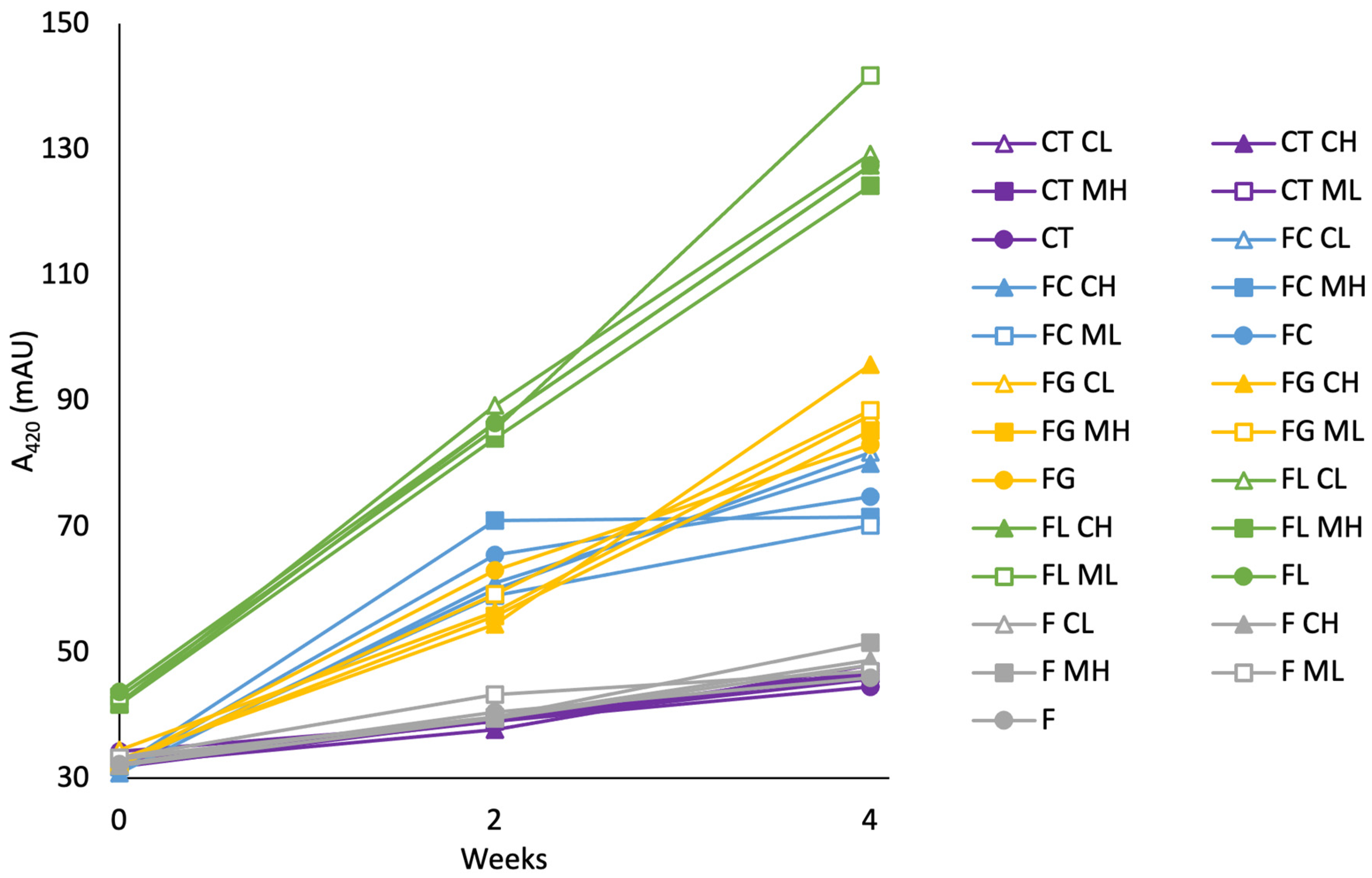
2.5. Maillard Reaction-Associated Products During Aging
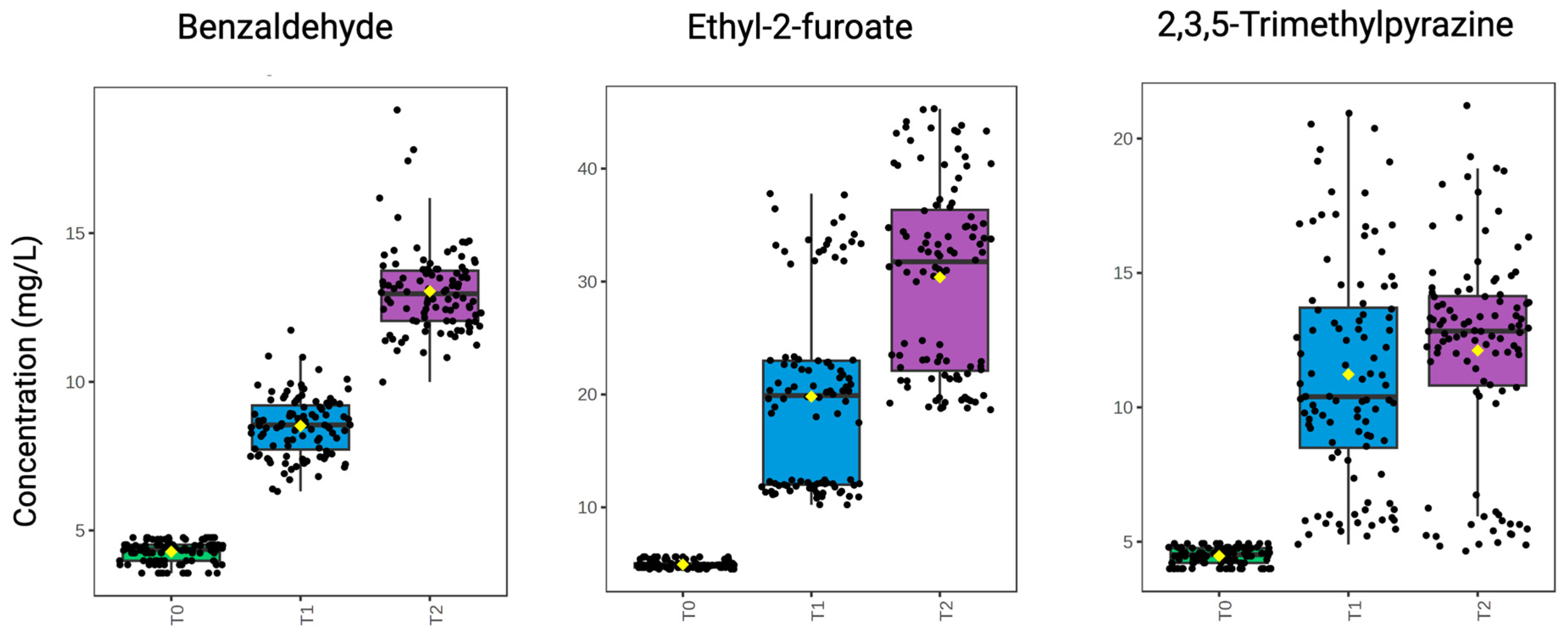
2.5.1. Influence of Time
2.5.2. Influence of Treatment
Fructose-Cysteine Treatments
Fructose-Lysine Treatments
Fructose-Glycine Treatments
2.5.3. Influence of Ca and Mg
2.5.4. Limitations of the Study and Areas of Future Research
3. Materials and Methods
3.1. Chemicals and Standards
3.2. Primary Fermentation and Winemaking
3.3. Standard Wine Chemical Analysis
3.4. Modified Base Wine Model Systems
3.5. Determination of Metals and Free Amino Acids by CE
3.5.1. Magnesium and Calcium Analysis
Instrumental Parameters
Solution and Sample Preparation
3.5.2. Free Amino Acid Analysis
Instrumental Parameters
Solution and Sample Preparation
3.5.3. Maillard Reaction-Associated Product Determination by HS-SPME-GC/MS
3.5.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ames, J.M. Control of the Maillard Reaction in Food Systems. Trends Food Sci. Technol. 1990, 1, 150–154. [Google Scholar] [CrossRef]
- Laroque, D.; Inisan, C.; Berger, C.; Vouland, É.; Dufossé, L.; Guérard, F. Kinetic Study on the Maillard Reaction. Consideration of Sugar Reactivity. Food Chem. 2008, 111, 1032–1042. [Google Scholar] [CrossRef]
- Le Menn, N.; Marchand, S.; De Revel, G.; Demarville, D.; Laborde, D.; Marchal, R. N,S,O-Heterocycles in Aged Champagne Reserve Wines and Correlation with Free Amino Acid Concentrations. J. Agric. Food Chem. 2017, 65, 2345–2356. [Google Scholar] [CrossRef]
- Keim, H.; De Revel, G.; Marchand, S.; Bertrand, A. Method for Determining Nitrogenous Heterocycle Compounds in Wine. J. Agric. Food Chem. 2002, 50, 5803–5807. [Google Scholar] [CrossRef]
- Marchand, S.; Almy, J.; de Revel, G. The Cysteine Reaction with Diacetyl Under Wine-like Conditions: Proposed Mechanisms for Mixed Origins of 2-Methylthiazole, 2-Methyl-3-Thiazoline, 2-Methylthiazolidine, and 2,4,5-Trimethyloxazole. J. Food Sci. 2011, 76, C861–C868. [Google Scholar] [CrossRef]
- Tominaga, T.; Guimbertau, G.; Dubourdieu, D. Role of Certain Volatile Thiols in the Bouquet of Aged Champagne Wines. J. Agric. Food Chem. 2003, 51, 1016–1020. [Google Scholar] [CrossRef]
- Benzing-Purdie, L.M.; Ripmeester, J.A.; Ratcliffe, C.I. Effects of Temperature on Maillard Reaction Products. J. Agric. Food Chem. 1985, 33, 31–33. [Google Scholar] [CrossRef]
- Charnock, H.M.; Pickering, G.J.; Kemp, B.S.; Kemp, B.S. The Maillard Reaction in Traditional Method Sparkling Wine. Front. Microbiol. 2022, 13, 979866. [Google Scholar] [CrossRef]
- Marchand, S.; De Revel, G.; Bertrand, A. Approaches to Wine Aroma: Release of Aroma Compounds from Reactions Between Cysteine and Carbonyl Compounds in Wine. J. Agric. Food Chem. 2000, 48, 4890–4895. [Google Scholar] [CrossRef]
- Nursten, H. The Maillard Reaction: Chemistry, Biochemistry and Implications; The Royal Society of Chemistry: Cambridge, UK, 2005. [Google Scholar]
- Yaylayan, V.A. Classification of the Maillard Reaction: A Conceptual Approach. Trends Food Sci. Technol. 1997, 8, 13–18. [Google Scholar] [CrossRef]
- Ivit, N.N.; Kemp, B. The Impact of Non-Saccharomyces Yeast on Traditional Method Sparkling Wine. Fermentation 2018, 4, 73. [Google Scholar] [CrossRef]
- Medeiros, J.; Xu, S.; Pickering, G.J.; Kemp, B.S. Influence of Caffeic and Caftaric Acid, Fructose, and Storage Temperature on Furan Derivatives in Base Wine. Molecules 2022, 27, 7891. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, T.; Russell, I.; Stewart, G.G. Sugar Utilization by Yeast During Fermentation. J. Ind. Microbiol. 1989, 4, 315–323. [Google Scholar] [CrossRef]
- Omari, I.O.; Charnock, H.M.; Fugina, A.L.; Thomson, E.L.; McIndoe, J.S. Magnesium-Accelerated Maillard Reactions Drive Differences in Adjunct and All-Malt Brewing. J. Am. Soc. Brew. Chem. 2021, 79, 145–155. [Google Scholar] [CrossRef]
- Rizzi, G.P. Effects of Cationic Species on Visual Color Formation in Model Maillard Reactions of Pentose Sugars and Amino Acids. J. Agric. Food Chem. 2008, 56, 7160–7164. [Google Scholar] [CrossRef]
- Hayase, F.; Shibuya, T.; Sato, L.; Yamamoto, M. Effects of Oxygen and Transition Metals on the Advanced Maillard Reaction of Proteins with Glucose. Biosci. Biotechnol. Biochem. 1996, 60, 1820–1825. [Google Scholar] [CrossRef]
- Kwak, E.J.; Lim, S.I. The Effect of Sugar, Amino Acid, Metal Ion, and NaCl on Model Maillard Reaction Under PH Control. Amino Acids 2004, 27, 85–90. [Google Scholar] [CrossRef]
- Tariba, B. Metals in Wine—Impact on Wine Quality and Health Outcomes. Biol. Trace Elem. Res. 2011, 144, 143–156. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV). International Code of Oenological Practices: Annex—Maximum Acceptable Limits; International Organisation of Vine and Wine (OIV): Dijon, France, 2015. [Google Scholar]
- Jos, A.; Moreno, I.; González, A.G.; Repetto, G.; Cameán, A.M. Differentiation of Sparkling Wines (Cava and Champagne) According to Their Mineral Content. Talanta 2004, 63, 377–382. [Google Scholar] [CrossRef]
- Jos, A.; Moreno, I.; González, A.G.; López-Artíguez, M.; Cameán, A.M. Study of the Mineral Profile of Catalonian “Brut” Cava Using Atomic Spectrometric Methods. Eur. Food Res. Technol. 2004, 218, 448–451. [Google Scholar] [CrossRef]
- Yamashita, G.H.; Anzanello, M.J.; Soares, F.; Rocha, M.K.; Fogliatto, F.S.; Rodrigues, N.P.; Rodrigues, E.; Celso, P.G.; Manfroi, V.; Hertz, P.F. Hierarchical Classification of Sparkling Wine Samples According to the Country of Origin Based on the Most Informative Chemical Elements. Food Control 2019, 106, 106737. [Google Scholar] [CrossRef]
- Rodrigues, N.P.; Rodrigues, E.; Celso, P.G.; Kahmann, A.; Yamashita, G.H.; Anzanello, M.J.; Manfroi, V.; Hertz, P.F. Discrimination of Sparkling Wines Samples According to the Country of Origin by ICP-OES Coupled with Multivariate Analysis. LWT 2020, 131, 109760. [Google Scholar] [CrossRef]
- Debastiani, R.; Dos Santos, C.E.I.; Dias, J.F. Elemental Characterization of Sparkling Wine and Cork Stoppers. Curr. Res. Food Sci. 2021, 4, 670–678. [Google Scholar] [CrossRef]
- Charnock, H.M.; Cairns, G.; Pickering, G.J.; Kemp, B.S. Production Method and Wine Style Influence Metal Profiles in Sparkling Wines. Am. J. Enol. Vitic. 2022, 73, 170–182. [Google Scholar] [CrossRef]
- Pohl, P. What Do Metals Tell Us About Wine? TrAC Trends Anal. Chem. 2007, 26, 941–949. [Google Scholar] [CrossRef]
- Tarasov, A.; Garzelli, F.; Schuessler, C.; Fritsch, S.; Loisel, C.; Pons, A.; Patz, C.D.; Rauhut, D.; Jung, R. Wine Storage at Cellar vs. Room Conditions: Changes in the Aroma Composition of Riesling Wine. Molecules 2021, 26, 6256. [Google Scholar] [CrossRef]
- Carlin, S.; Lotti, C.; Correggi, L.; Mattivi, F.; Arapitsas, P.; Vrhovšek, U. Measurement of the Effect of Accelerated Aging on the Aromatic Compounds of Gewürztraminer and Teroldego Wines, Using a SPE-GC-MS/MS Protocol. Metabolites 2022, 12, 180. [Google Scholar] [CrossRef]
- Oliveira, I.; Ferreira, V. Modulating Fermentative, Varietal and Aging Aromas of Wine Using Non-Saccharomyces Yeasts in a Sequential Inoculation Approach. Microorganisms 2019, 7, 164. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Hermosín-Gutiérrez, I.; Pérez-Coello, M.S. Accelerated Aging Against Conventional Storage: Effects on the Volatile Composition of Chardonnay White Wines. J. Food Sci. 2013, 78, C507–C513. [Google Scholar] [CrossRef]
- Cutzach, I.; Chatonnet, P.; Dubourdieu, D. Study of the Formation Mechanisms of Some Volatile Compounds During the Aging of Sweet Fortified Wines. J. Agric. Food Chem. 1999, 47, 2837–2846. [Google Scholar] [CrossRef]
- de Lerma, N.L.; Peinado, J.; Moreno, J.; Peinado, R.A. Antioxidant Activity, Browning and Volatile Maillard Compounds in Pedro Ximénez Sweet Wines Under Accelerated Oxidative Aging. LWT Food Sci. Technol. 2010, 43, 1557–1563. [Google Scholar] [CrossRef]
- Clark, A.C.; Scollary, G.R. Copper(II)-Mediated Oxidation of (+)-Catechin in a Model White Wine System. Aust. J. Grape Wine Res. 2002, 8, 186–195. [Google Scholar] [CrossRef]
- Clark, A.C.; Scollary, G.R. Influence of Light Exposure, Ethanol and Copper(II) on the Formation of a Precursor for Xanthylium Cations from Tartaric Acid. Aust. J. Grape Wine Res. 2003, 9, 64–71. [Google Scholar] [CrossRef]
- Pickering, G.J.; Heatherbell, D.A.; Barnes, M.F. The production of reduced-alcohol wine using glucose oxidase-treated juice. Part II. Stability and SO2-binding. Am. J. Enol. Vitic. 1999, 50, 299–306. [Google Scholar] [CrossRef]
- Ames, J.M.; Guy, R.C.E.; Kipping, G.J. Effect of PH and Temperature on the Formation of Volatile Compounds in Cysteine/Reducing Sugar/Starch Mixtures During Extrusion Cooking. J. Agric. Food Chem. 2001, 49, 1885–1894. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Van Boekel, M.A.J.S. Kinetics of the Glucose/Glycine Maillard Reaction Pathways: Influences of PH and Reactant Initial Concentrations. Food Chem. 2005, 92, 437–448. [Google Scholar] [CrossRef]
- Rydberg, P.; Eriksson, S.; Tareke, E.; Karlsson, P.; Ehrenberg, L.; Törnqvist, M. Factors That Influence the Acrylamide Content of Heated Foods. Adv. Exp. Med. Biol. 2005, 561, 317–328. [Google Scholar] [CrossRef]
- Jeandet, P.; Heinzmann, S.S.; Roullier-Gall, C.; Cilindre, C.; Aron, A.; Deville, M.A.; Moritz, F.; Karbowiak, T.; Demarville, D.; Brun, C.; et al. Chemical Messages in 170-Year-Old Champagne Bottles from the Baltic Sea: Revealing Tastes from the Past. Proc. Natl. Acad. Sci. USA 2015, 112, 5893–5898. [Google Scholar] [CrossRef]
- Serra-Cayuela, A.; Castellari, M.; Bosch-Fusté, J.; Riu-Aumatell, M.; Buxaderas, S.; López-Tamames, E. Identification of 5-Hydroxymethyl-2-Furfural (5-HMF) in Cava Sparkling Wines by LC-DAD-MS/MS and NMR Spectrometry. Food Chem. 2013, 141, 3373–3380. [Google Scholar] [CrossRef]
- Sawyer, S.; Longo, R.; Solomon, M.; Nicolotti, L.; Westmore, H.; Merry, A.; Gnoinski, G.; Ylia, A.; Dambergs, R.; Kerslake, F. Autolysis and the Duration of Ageing on Lees Independently Influence the Aroma Composition of Traditional Method Sparkling Wine. Aust. J. Grape Wine Res. 2022, 28, 146–159. [Google Scholar] [CrossRef]
- Ramonaityte, D.T.; Keršiene, M.; Adams, A.; Tehrani, K.A.; Kimpe, N. De The Interaction of Metal Ions with Maillard Reaction Products in a Lactose-Glycine Model System. Food Res. Int. 2009, 42, 331–336. [Google Scholar] [CrossRef]
- Hemmler, D.; Roullier-Gall, C.; Marshall, J.W.; Rychlik, M.; Taylor, A.J.; Schmitt-Kopplin, P. Insights into the Chemistry of Non-Enzymatic Browning Reactions in Different Ribose-Amino Acid Model Systems. Sci. Rep. 2018, 8, 16879. [Google Scholar] [CrossRef] [PubMed]
- Pripis-Nicolau, L.; De Revel, G.; Bertrand, A.; Maujean, A. Formation of Flavor Components by the Reaction of Amino Acid and Carbonyl Compounds in Mild Conditions. J. Agric. Food Chem. 2000, 48, 3761–3766. [Google Scholar] [CrossRef]
- Ajandouz, E.H.; Tchiakpe, L.S.; Dalle Ore, F.; Benajiba, A.; Puigserver, A. Effects of PH on Caramelization and Maillard Reaction Kinetics in Fructose-Lysine Model Systems. J. Food Sci. 2001, 66, 926–931. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; Van Boekel, M.A.J.S. A Review of Maillard Reaction in Food and Implications to Kinetic Modelling. Trends Food Sci. Technol. 2001, 11, 364–373. [Google Scholar] [CrossRef]
- Ashoor, S.H.; Zent, J.B. Maillard Browning of Common Amino Acids and Sugars. J. Food Sci. 1984, 49, 1206–1207. [Google Scholar] [CrossRef]
- Leiva, G.E.; Naranjo, G.B.; Malec, L.S. A Study of Different Indicators of Maillard Reaction with Whey Proteins and Different Carbohydrates Under Adverse Storage Conditions. Food Chem. 2017, 215, 410–416. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Karangwa, E.; Xia, S. Correlating Enzymatic Browning Inhibition and Antioxidant Ability of Maillard Reaction Products Derived from Different Amino Acids. J. Sci. Food Agric. 2017, 97, 4210–4218. [Google Scholar] [CrossRef]
- Pereira, V.; Santos, M.; Cacho, J.; Marques, J.C. Assessment of the Development of Browning, Antioxidant Activity and Volatile Organic Compounds in Thermally Processed Sugar Model Wines. LWT Food Sci. Technol. 2017, 75, 719–726. [Google Scholar] [CrossRef]
- De Revel, G.; Pripis-Nicolau, L.; Barbe, J.C.; Bertrand, A. The Detection of α-Dicarbonyl Compounds in Wine by Formation of Quinoxaline Derivatives. J. Sci. Food Agric. 2000, 80, 102–108. [Google Scholar] [CrossRef]
- Martínez-Lapuente, L.; Apolinar-Valiente, R.; Guadalupe, Z.; Ayestarán, B.; Pérez-Magariño, S.; Williams, P.; Doco, T. Polysaccharides, Oligosaccharides and Nitrogenous Compounds Change During the Ageing of Tempranillo and Verdejo Sparkling Wines. J. Sci. Food Agric. 2018, 98, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Ortega-Heras, M.; Pérez-Magariño, S. Changes in Polysaccharide Composition during Sparkling Wine Making and Aging. J. Agric. Food Chem. 2013, 61, 12362–12373. [Google Scholar] [CrossRef] [PubMed]
- OIV. Method for the Determination of Dicarbonyl Compounds of Wine by HPLC; OIV: Dijon, France, 2010. [Google Scholar]
- Bartowsky, E.J.; Henschke, P.A. The ‘Buttery’ Attribute of Wine—Diacetyl—Desirability, Spoilage and Beyond. Int. J. Food Microbiol. 2004, 96, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Culbert, J.A.; McRae, J.M.; Condé, B.C.; Schmidtke, L.M.; Nicholson, E.L.; Smith, P.A.; Howell, K.S.; Boss, P.K.; Wilkinson, K.L. Influence of Production Method on the Chemical Composition, Foaming Properties, and Quality of Australian Carbonated and Sparkling White Wines. J. Agric. Food Chem. 2017, 65, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Sartor, S.; Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Profiling of Free Amino Acids in Sparkling Wines During Over-Lees Aging and Evaluation of Sensory Properties. LWT Food Sci. Technol. 2021, 140, 110847. [Google Scholar] [CrossRef]
- Zhang, T.; Ye, M.; Hu, Z.; Pan, G.; Liang, L.; Wu, S. Determination of Cations in Wines and Beverages Based on Capillary Ion Exchange Chromatography. Sepu 2012, 30, 400–403. [Google Scholar]
- Nunez, M.; Pena, R.M.; Herrero, C.; Garcia-Martin, S. Analysis of Some Metals in Wine by Means of Capillary Electrophoresis. Application to the Differentiation of Ribeira Sacra Spanish Red Wines. Analusis 2000, 28, 432–437. [Google Scholar] [CrossRef]
- Rovio, S.; Sirén, K.; Sirén, H. Application of Capillary Electrophoresis to Determine Metal Cations, Anions, Organic Acids, and Carbohydrates in Some Pinot Noir Red Wines. Food Chem. 2011, 124, 1194–1200. [Google Scholar] [CrossRef]
- Qin, W.; Wei, H.; Li, S.F.Y. 1,3-Dialkylimidazolium-Based Room-Temperature Ionic Liquids as Background Electrolyte and Coating Material in Aqueous Capillary Electrophoresis. J. Chromatogr. A 2003, 985, 447–454. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chen, C.M.; Chang, G.L.; Lin, C.H.; Fu, L.M. Fabrication and Characterization of Semicircular Detection Electrodes for Contactless Conductivity Detector—CE Microchips. Electrophoresis 2006, 27, 5043–5050. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Monasterio, R.P.; Vargas, V.C.S.; Silva, M.F. Analytical Characterization of Wine and Its Precursors by Capillary Electrophoresis. Electrophoresis 2012, 33, 2240–2252. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.M.; Lönnberg, H. Complexing of Sugars and Sugar Alcohols with Metal Ions: A Comparative Study by Ion-Exchange Chromatography. Carbohydr. Res. 1991, 215, 357–360. [Google Scholar] [CrossRef]
- Burin, V.M.; Marchand, S.; De Revel, G.; Bordignon-Luiz, M.T. Development and Validation of Method for Heterocyclic Compounds in Wine: Optimization of HS-SPME Conditions Applying a Response Surface Methodology. Talanta 2013, 117, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Charnock, H.M.; Pickering, G.J.; Kemp, B.S. The Impact of Dosage Sugar-Type and Aging on Maillard Reaction-Associated Products in Traditional Method Sparkling Wines. OENO One 2023, 57, 303–322. [Google Scholar] [CrossRef]
- de Souza Nascimento, A.; de Souza, J.; dos Santos Lima, M.; Pereira, G. Volatile Profiles of Sparkling Wines Produced by the Traditional Method from a Semi-Arid Region. Beverages 2018, 4, 103. [Google Scholar] [CrossRef]
- Delfini, C.; Di Stefano, R.; Istituto Sperimentale per l’ Enologia Italy. Benzaldehyde, an Aromatic Substance Responsible for the Smell of Bitter Almond in Wines. Riv. Vitic. Enol. 1984, 11, 9–10. [Google Scholar]
- Delfini, C. Experimental Observations on the Origin and Disappearance of Benzylic Alcohol and Benzoic Aldehyde in Musts and Wine. Bull. Int. Off. Vine Wine 1987, 675–676, 463–473. [Google Scholar]
- Bemis-Young, G.L.; Huang, J.; Bernhard, R.A. Effect of PH on Pyrazine Formation in Glucose-Glycine Model Systems. Food Chem. 1993, 46, 383–387. [Google Scholar] [CrossRef]
- Martín-Garcia, A.; Abarca-Rivas, C.; Riu-Aumatell, M.; López-Tamames, E. Comparison of Volatile Compounds During Biological Ageing and Commercial Storage of Cava (Spanish Sparkling Wine): The Role of Lees. Heliyon 2023, 9, e19306. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Z.; Han, Y.; Duan, Y.; Shi, B.; Ma, W. A Review on Wine Flavour Profiles Altered by Bottle Aging. Molecules 2023, 28, 6522. [Google Scholar] [CrossRef]
- De Revel, G.; Marchand, S.; Bertrand, A. Identification of Maillard-Type Aroma Compounds in Winelike Model Systems of Cysteine—Carbonyls: Occurrence in Wine. Proc. ACS Symp. Ser. 2003, 871, 353–364. [Google Scholar]
- Hodge, J.E. Dehydrated Foods, Chemistry of Browning Reactions in Model Systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Torrens, J.; Rlu-Aumatell, M.; Vichi, S.; López-Tamames, E.; Buxaderas, S. Assessment of Volatlle and Sensory Profiles Between Base and Sparkling Wines. J. Agric. Food Chem. 2010, 58, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Spillman, P.J.; Pollnitz, A.P.; Liacopoulos, D.; Pardon, K.H.; Sefton, M.A. Formation and Degradation of Furfuryl Alcohol, 5-Methylfurfuryl Alcohol, Vanillyl Alcohol, and Their Ethyl Ethers in Barrel-Aged Wines. J. Agric. Food Chem. 1998, 46, 657–663. [Google Scholar] [CrossRef]
- Kotseridis, Y.; Razungles, A.; Bertrand, A.; Baumes, R. Differentiation of the Aromas of Merlot and Cabernet Sauvignon Wines Using Sensory and Instrumental Analysis. J. Agric. Food Chem. 2000, 48, 5383–5388. [Google Scholar] [CrossRef]
- Escudero, A.; Charpentier, M.; Etievant, P. Characterization of Aged Champagne Wine Aroma by GC-O and Descriptive Profile Analyses. Sci. Aliment. 2000, 20, 331–346. [Google Scholar] [CrossRef]
- Van Lancker, F.; Adams, A.; Owczarek-Fendor, A.; De Meulenaer, B.; De Kimpe, N. Mechanistic Insights into Furan Formation in Maillard Model Systems. J. Agric. Food Chem. 2011, 59, 229–235. [Google Scholar] [CrossRef]
- Davidek, T.; Clety, N.; Devaud, S.; Robert, F.; Blank, I. Simultaneous Quantitative Analysis of Maillard Reaction Precursors and Products by High-Performance Anion Exchange Chromatography. J. Agric. Food Chem. 2003, 51, 7259–7265. [Google Scholar] [CrossRef]
- Hou, L.; Xie, J.; Zhao, J.; Zhao, M.; Fan, M.; Xiao, Q.; Liang, J.; Chen, F. Roles of Different Initial Maillard Intermediates and Pathways in Meat Flavor Formation for Cysteine-Xylose-Glycine Model Reaction Systems. Food Chem. 2017, 232, 135–144. [Google Scholar] [CrossRef]
- Véstia, J.; Barroso, J.M.; Ferreira, H.; Gaspar, L.; Rato, A.E. Predicting Calcium in Grape Must and Base Wine by FT-NIR Spectroscopy. Food Chem. 2019, 276, 71–76. [Google Scholar] [CrossRef]
- McKinnon, A.J.; Scollary, G.R.; Solomon, D.H.; Williams, P.J. The Influence of Wine Components on the Spontaneous Precipitation of Calcium L(+)-Tartrate in a Model Wine Solution. Am. J. Enol. Vitic. 1995, 46, 509–517. [Google Scholar] [CrossRef]
- Ryan, D.; Prenzler, P.D.; Saliba, A.J.; Scollary, G.R. The Significance of Low Impact Odorants in Global Odour Perception. Trends Food Sci. Technol. 2008, 19, 383–389. [Google Scholar] [CrossRef]
- Nurgel, C.; Pickering, G.J.; Inglis, D.L. Sensory and Chemical Characteristics of Canadian Ice Wines. J. Sci. Food Agric. 2004, 84, 1675–1684. [Google Scholar] [CrossRef]
- Iland, P.; Bruer, N.; Edwards, G.; Caloghiris, S.; Wilkes, E. Chemical Analysis of Grapes and Wine: Techniques and Concepts, 2nd ed.; Cargill, M., Ed.; Patrick Iland Wine Promotions Pty Ltd.: Campbelltown, Australia, 2015; ISBN 9780958160582. [Google Scholar]
- Viviers, M.Z.; Smith, M.E.; Wilkes, E.; Smith, P. Effects of Five Metals on the Evolution of Hydrogen Sulfide, Methanethiol, and Dimethyl Sulfide During Anaerobic Storage of Chardonnay and Shiraz Wines. J. Agric. Food Chem. 2013, 61, 12385–12396. [Google Scholar] [CrossRef] [PubMed]
- Ageeva, N.M.; Guguchkina, T.I.; Guguchkin, A.A. Analysis of Metal Cations in Kuban Wines by Capillary Electrophoresis. Grapes Wine Russ. 2001, 4, 47. (In Russian) [Google Scholar]
- Komarova, N.V.; Kamentsev, J.S.; Solomonova, A.P.; Anufrieva, R.M. Determination of Amino Acids in Fodders and Raw Materials Using Capillary Zone Electrophoresis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 800, 135–143. [Google Scholar] [CrossRef]
- Prudhviraju, C.; Swaminathan, J.; Sri Nataraj, K.; Rajasekhar, B. Pre and Post Column Derivatization of Amino Acid—A Systematic Review of HPLC. Acta Sci. Pharm. Sci. 2021, 5, 104–115. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and Reporting Effect Sizes to Facilitate Cumulative Science: A Practical Primer for t-Tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
| Model System | Reaction Time (Weeks) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 0 | 2 | 4 | 0 | 2 | 4 | 0 | 2 | 4 | |
| Benzaldehyde | 2-Acetylfuran | Furfural | 5-Methylfurfural | |||||||||
| CT | 4.2 ± 0.2 | 7.1 ± 0.9 | 11.0 ± 0.8 | n.q. | 1.9 ± 0.2 | 4.6 ± 0.5 | n.d. | 48.0 ± 1.9 | 98.0 ± 3.8 | n.q. | 4.1 ± 0.2 | 6.8 ± 0.4 |
| CT ML | 7.0 ± 0.6 | 11.3 ± 0.4 | 2.0 ± 0.2 | 4.5 ± 0.6 | 45.8 ± 2.1 | 101.1 ± 8.3 | 4.1 ± 0.1 | 7.2 ± 0.4 | ||||
| CT MH | 7.7 ± 0.7 | 11.7 ± 0.5 | 2.3 ± 0.1 | 4.4 ± 0.4 | 46.0 ± 2.2 | 102.5 ± 8.4 | 4.1 ± 0.1 | 7.5 ± 0.4 | ||||
| CT CL | 8.3 ± 0.8 | 12.1 ± 0.5 | 2.2 ± 0.1 | 4.4 ± 0.4 | 42.3 ± 1.6 | 102.6 ± 8.8 | 4.0 ± 0.2 | 6.9 ± 0.4 | ||||
| CT CH | 8.5 ± 0.8 | 12.0 ± 0.5 | 2.0 ± 0.2 | 4.2 ± 0.3 | 37.2 ± 1.4 | 98.4 ± 10.7 | 3.8 ± 0.2 | 7.0 ± 0.4 | ||||
| F | 4.4 ± 0.2 | 7.6 ± 0.6 | 12.1 ± 0.6 | n.q. | 2.2 ± 0.1 | 4.5 ± 0.3 | n.d. | 47.6 ± 1.9 | 99.4 ± 12.1 | n.q. | 4.1 ± 0.1 | 7.1 ± 0.4 |
| F ML | 8.4 ± 0.9 | 12.5 ± 0.5 | 2.3 ± 0.2 | 4.7 ± 0.5 | 46.1 ± 1.6 | 99.5 ± 10.7 | 4.0 ± 0.2 | 7.1 ± 0.4 | ||||
| F MH | 8.2 ± 0.6 | 12.4 ± 0.5 | 2.3 ± 0.1 | 4.4 ± 0.4 | 45.6 ± 1.5 | 100.8 ± 10.4 | 4.1 ± 0.2 | 7.0 ± 0.2 | ||||
| F CL | 8.6 ± 0.6 | 11.9 ± 0.5 | 2.2 ± 0.1 | 4.7 ± 0.4 | 42.3 ± 1.3 | 99.4 ± 8.3 | 4.0 ± 0.1 | 7.1 ± 0.5 | ||||
| F CH | 8.6 ± 0.8 | 12.3 ± 0.4 | 2.0 ± 0.2 | 5.0 ± 0.5 | 36.3 ± 2.3 | 101.2 ± 8.4 | 3.8 ± 0.2 | 7.1 ± 0.6 | ||||
| FG | 4.6 ± 0.2 | 8.4 ± 0.2 | 13.7 ± 0.4 | n.q. | 6.8 ± 0.5 | 17.9 ± 2.3 | n.d. | 207.0 ± 2.0 | 192.7 ± 19.4 | n.q. | 10.2 ± 0.7 | 25.3 ± 2.0 |
| FG ML | 8.3 ± 0.6 | 13.6 ± 1.1 | 7.6 ± 0.1 | 16.8 ± 1.6 | 223.3 ± 6.4 | 214.6 ± 31.0 | 10.8 ± 0.4 | 25.6 ± 1.8 | ||||
| FG MH | 8.6 ± 0.9 | 12.8 ± 0.3 | 7.5 ± 0.5 | 18.5 ± 1.8 | 228.3 ± 6.6 | 201.2 ± 20.8 | 11.7 ± 0.8 | 26.7 ± 1.8 | ||||
| FG CL | 8.7 ± 1.1 | 12.9 ± 0.6 | 7.6 ± 0.2 | 16.8 ± 1.4 | 222.7 ± 1.8 | 199.5 ± 17.6 | 11.2 ± 0.6 | 27.0 ± 0.9 | ||||
| FG CH | 8.8 ± 0.9 | 13.4 ± 0.3 | 7.5 ± 0.5 | 16.8 ± 1.5 | 225.0 ± 3.4 | 205.6 ± 28.4 | 11.2 ± 0.5 | 26.0 ± 2.0 | ||||
| FL | 4.6 ± 0.1 | 9.8 ± 0.8 | 13.8 ± 0.5 | n.q. | 6.8 ± 0.5 | 16.6 ± 1.6 | n.d. | 279.8 ± 5.2 | 251.2 ± 17.9 | n.q. | 12.2 ± 0.8 | 31.1 ± 2.0 |
| FL ML | 9.4 ± 0.5 | 13.4 ± 0.8 | 6.4 ± 0.4 | 17.9 ± 1.7 | 273.1 ± 4.1 | 235.8 ± 18.3 | 11.8 ± 0.6 | 31.4 ± 2.3 | ||||
| FL MH | 9.1 ± 0.7 | 12.8 ± 0.6 | 6.6 ± 0.3 | 16.5 ± 1.6 | 280.1 ± 6.7 | 244.8 ± 27.3 | 11.5 ± 0.6 | 30.8 ± 1.9 | ||||
| FL CL | 9.2 ± 0.3 | 13.9 ± 0.4 | 6.7 ± 0.3 | 15.8 ± 1.7 | 282.4 ± 7.8 | 251.1 ± 21.7 | 12.0 ± 0.7 | 29.2 ± 0.7 | ||||
| FL CH | 9.1 ± 0.7 | 14.0 ± 0.7 | 6.5 ± 0.4 | 15.2 ± 1.2 | 270.0 ± 1.7 | 258.8 ± 32.2 | 11.1 ± 0.5 | 30.4 ± 1.6 | ||||
| FC | 3.7 ± 0.2 | 7.7 ± 0.6 | 13.9 ± 0.5 | n.q. | 5.6 ± 0.3 | 12.0 ± 0.8 | n.d. | n.d. | 4.4 ± 0.5 | n.q. | 5.7 ± 0.3 | 8.5 ± 0.4 |
| FC ML | 8.2 ± 0.7 | 14.3 ± 1.2 | 5.6 ± 0.4 | 11.4 ± 0.8 | n.d. | 4.6 ± 0.4 | 5.3 ± 0.2 | 8.6 ± 0.4 | ||||
| FC MH | 8.7 ± 0.6 | 13.5 ± 0.9 | 5.3 ± 0.4 | 11.2 ± 0.9 | n.d. | 5.7 ± 0.4 | 5.3 ± 0.2 | 8.5 ± 0.8 | ||||
| FC CL | 8.3 ± 1.0 | 13.5 ± 1.1 | 5.6 ± 0.6 | 11.5 ± 0.7 | n.d. | n.d. | 5.3 ± 0.2 | 8.6 ± 0.3 | ||||
| FC CH | 10.7 ± 0.9 | 17.5 ± 1.5 | 6.0 ± 0.4 | 11.7 ± 1.2 | n.d. | n.d. | 5.3 ± 0.2 | 8.5 ± 0.3 | ||||
| Time | A | B | C | A | B | C | A | B | C | A | B | C |
| Homofuraneol | Furfuryl ethyl ether | Ethyl-2-furoate * | 2,3-Dihydrobenzofuran * | |||||||||
| CT | n.q. | 2.5 ± 0.0 | 3.6 ± 0.1 | n.q. | 1.9 ± 0.1 | 2.0 ± 0.1 | 4.6 ± 0.1 | 11.6 ± 0.4 | 23.2 ± 1.3 | n.q. | 4.1 ± 0.2 | 6.8 ± 0.4 |
| CT ML | 2.4 ± 0.1 | 3.6 ± 0.0 | 1.9 ± 0.1 | 2.1 ± 0.2 | 11.7 ± 0.4 | 23.8 ± 0.9 | 4.1 ± 0.1 | 7.2 ± 0.4 | ||||
| CT MH | 2.3 ± 0.0 | 3.7 ± 0.1 | 2.0 ± 0.2 | 2.1 ± 0.2 | 12.2 ± 0.2 | 20.1 ± 1.2 | 4.1 ± 0.1 | 7.5 ± 0.4 | ||||
| CT CL | 2.3 ± 0.1 | 3.5 ± 0.1 | 2.0 ± 0.2 | 2.1 ± 0.2 | 11.9 ± 0.2 | 21.0 ± 1.7 | 4.0 ± 0.2 | 6.9 ± 0.4 | ||||
| CT CH | 2.3 ± 0.0 | 3.5 ± 0.1 | 1.7 ± 0.2 | 2.1 ± 0.2 | 11.0 ± 0.6 | 20.6 ± 1.9 | 3.8 ± 0.2 | 7.0 ± 0.4 | ||||
| F | n.q. | 2.4 ± 0.1 | 3.5 ± 0.1 | n.q. | 2.0 ± 0.1 | 2.1 ± 0.2 | 4.8 ± 0.1 | 11.8 ± 0.6 | 20.5 ± 1.7 | n.q. | 4.1 ± 0.1 | 7.1 ± 0.4 |
| F ML | 2.4 ± 0.1 | 3.5 ± 0.1 | 1.9 ± 0.1 | 2.1 ± 0.2 | 11.9 ± 0.6 | 20.5 ± 1.8 | 4.0 ± 0.2 | 7.1 ± 0.4 | ||||
| F MH | 2.3 ± 0.1 | 3.6 ± 0.1 | 1.9 ± 0.1 | 2.2 ± 0.2 | 11.9 ± 0.7 | 20.8 ± 1.9 | 4.1 ± 0.2 | 7.0 ± 0.2 | ||||
| F CL | 2.3 ± 0.1 | 3.5 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.2 | 11.7 ± 0.6 | 21.2 ± 1.4 | 4.0 ± 0.1 | 7.1 ± 0.5 | ||||
| F CH | 2.2 ± 0.0 | 3.5 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.2 | 11.0 ± 0.5 | 21.2 ± 1.8 | 3.8 ± 0.2 | 7.1 ± 0.6 | ||||
| FG | n.q. | 5.9 ± 0.1 | 10.2 ± 0.1 | n.q. | 2.8 ± 0.1 | 2.8 ± 0.3 | 4.8 ± 0.1 | 21.4 ± 1.2 | 35.1 ± 1.2 | n.q. | 10.2 ± 0.7 | 25.3 ± 2.0 |
| FG ML | 6.3 ± 0.1 | 10.3 ± 0.3 | 2.9 ± 0.1 | 2.5 ± 0.1 | 22.3 ± 0.7 | 34.5 ± 1.5 | 10.8 ± 0.4 | 25.6 ± 1.8 | ||||
| FG MH | 6.3 ± 0.1 | 10.4 ± 0.1 | 2.9 ± 0.1 | 2.8 ± 0.3 | 22.6 ± 0.8 | 34.4 ± 1.7 | 11.7 ± 0.8 | 26.7 ± 1.8 | ||||
| FG CL | 6.2 ± 0.0 | 10.4 ± 0.2 | 2.8 ± 0.1 | 3.0 ± 0.3 | 22.3 ± 1.1 | 34.9 ± 2.0 | 11.2 ± 0.6 | 27.0 ± 0.9 | ||||
| FG CH | 6.3 ± 0.1 | 9.9 ± 0.2 | 2.9 ± 0.1 | 2.7 ± 0.2 | 22.6 ± 1.1 | 35.1 ± 3.2 | 11.2 ± 0.5 | 26.0 ± 2.0 | ||||
| FL | n.q. | 7.5 ± 0.1 | 12.7 ± 0.4 | n.q. | 2.7 ± 0.1 | 2.8 ± 0.3 | 5.0 ± 0.1 | 19.7 ± 1.0 | 32.6 ± 1.8 | n.q. | 12.2 ± 0.8 | 31.1 ± 2.0 |
| FL ML | 7.3 ± 0.1 | 12.9 ± 0.5 | 2.7 ± 0.1 | 2.8 ± 0.2 | 19.4 ± 1.3 | 32.3 ± 1.8 | 11.8 ± 0.6 | 31.4 ± 2.3 | ||||
| FL MH | 7.5 ± 0.1 | 12.9 ± 0.5 | 2.7 ± 0.0 | 2.8 ± 0.2 | 20.1 ± 0.8 | 32.5 ± 1.3 | 11.5 ± 0.6 | 30.8 ± 1.9 | ||||
| FL CL | 7.6 ± 0.1 | 12.6 ± 0.6 | 2.7 ± 0.1 | 2.8 ± 0.2 | 19.9 ± 1.1 | 31.9 ± 1.2 | 12.0 ± 0.7 | 29.2 ± 0.7 | ||||
| FL CH | 7.3 ± 0.1 | 12.5 ± 0.5 | 2.6 ± 0.1 | 2.7 ± 0.1 | 19.5 ± 1.2 | 31.8 ± 1.6 | 11.1 ± 0.5 | 30.4 ± 1.6 | ||||
| FC | n.q. | n.q. | n.q. | n.q. | 3.0 ± 0.1 | 2.5 ± 0.1 | 5.5 ± 0.1 | 33.5 ± 1.4 | 43.1 ± 1.4 | n.q. | 5.7 ± 0.3 | 8.5 ± 0.4 |
| FC ML | 3.0 ± 0.1 | 2.6 ± 0.3 | 33.8 ± 1.8 | 41.5 ± 1.5 | 5.3 ± 0.2 | 8.6 ± 0.4 | ||||||
| FC MH | 2.9 ± 0.2 | 2.6 ± 0.3 | 34.7 ± 2.1 | 43.0 ± 2.1 | 5.3 ± 0.2 | 8.5 ± 0.8 | ||||||
| FC CL | 3.3 ± 0.3 | 2.8 ± 0.2 | 34.0 ± 2.5 | 42.1 ± 2.3 | 5.3 ± 0.2 | 8.6 ± 0.3 | ||||||
| FC CH | 3.2 ± 0.2 | 3.6 ± 0.4 | 33.0 ± 1.9 | 41.8 ± 2.1 | 5.3 ± 0.2 | 8.5 ± 0.3 | ||||||
| Time | A | B | C | A | B | B | A | B | C | A | B | C |
| Thiazole | 2,3,5-Trimethylpyrazine * | |||||||||||
| CT | n.q. | n.q. | 3.3 ± 0.4 | 4.6 ± 0.4 | 9.9 ± 0.5 | 12.8 ± 0.6 | ||||||
| CT ML | n.q. | 2.5 ± 0.3 | 8.3 ± 0.8 | 12.9 ± 0.9 | ||||||||
| CT MH | n.q. | 3.0 ± 0.4 | 11.7 ± 1.2 | 13.7 ± 0.9 | ||||||||
| CT CL | n.q. | 2.7 ± 0.3 | 10.3 ± 0.8 | 13.4 ± 0.4 | ||||||||
| CT CH | n.q. | 2.4 ± 0.3 | 8.4 ± 0.8 | 13.4 ± 1.2 | ||||||||
| F | n.q. | n.q. | 2.5 ± 0.3 | 4.6 ± 0.2 | 9.9 ± 1.1 | 12.7 ± 1.2 | ||||||
| F ML | n.q. | 2.5 ± 0.3 | 9.7 ± 0.8 | 11.8 ± 1.2 | ||||||||
| F MH | n.q. | 2.7 ± 0.3 | 10.0 ± 1.0 | 12.1 ± 0.9 | ||||||||
| F CL | n.q. | 2.6 ± 0.2 | 9.9 ± 0.9 | 11.9 ± 1.0 | ||||||||
| F CH | n.q. | 3.0 ± 0.3 | 10.2 ± 0.8 | 14.8 ± 1.1 | ||||||||
| FG | n.q. | 2.1 ± 0.1 | 3.4 ± 0.4 | 4.3 ± 0.3 | 17.8 ± 2.3 | 15.1 ± 1.3 | ||||||
| FG ML | 2.2 ± 0.1 | 3.3 ± 0.3 | 16.7 ± 1.9 | 14.0 ± 1.2 | ||||||||
| FG MH | 2.1 ± 0.1 | 3.3 ± 0.3 | 17.4 ± 3.1 | 17.5 ± 1.3 | ||||||||
| FG CL | 2.2 ± 0.1 | 3.1 ± 0.2 | 18.1 ± 1.6 | 18.9 ± 1.9 | ||||||||
| FG CH | 2.2 ± 0.1 | 3.1 ± 0.2 | 17.5 ± 1.4 | 17.5 ± 1.5 | ||||||||
| FL | n.q. | 2.3 ± 0.2 | 3.5 ± 0.4 | 4.1 ± 0.1 | 13.4 ± 0.9 | 12.3 ± 1.9 | ||||||
| FL ML | 2.4 ± 0.1 | 3.3 ± 0.3 | 14.0 ± 0.7 | 13.0 ± 0.9 | ||||||||
| FL MH | 2.4 ± 0.2 | 3.2 ± 0.2 | 13.3 ± 1.2 | 13.3 ± 0.9 | ||||||||
| FL CL | 2.5 ± 0.2 | 3.3 ± 0.5 | 12.1 ± 0.8 | 12.1 ± 1.4 | ||||||||
| FL CH | 2.4 ± 0.1 | 2.7 ± 0.2 | 13.2 ± 0.5 | 12.4 ± 0.3 | ||||||||
| FC | n.q. | 6.3 ± 0.7 | 18.8 ± 2.2 | 4.7 ± 0.1 | 5.9 ± 0.5 | 5.5 ± 0.4 | ||||||
| FC ML | 6.7 ± 0.7 | 21.4 ± 0.9 | 5.8 ± 0.3 | 5.3 ± 0.6 | ||||||||
| FC MH | 6.9 ± 0.8 | 19.2 ± 2.3 | 5.6 ± 0.5 | 5.8 ± 0.7 | ||||||||
| FC CL | 6.4 ± 0.7 | 19.0 ± 1.6 | 5.9 ± 0.5 | 5.6 ± 0.6 | ||||||||
| FC CH | 5.2 ± 0.3 | 18.3 ± 1.4 | 5.7 ± 0.3 | 5.3 ± 0.5 | ||||||||
| Time | A | B | C | A | B | C | ||||||
| Benzaldehyde | 2-Acetyl-Furan | Furfural | 5-Methyl- Furfural | Homo- Furaneol | Furfuryl Ethyl Ether | Ethyl-2- Furoate | 2,3-Dihydro- Benzofuran | Thiazole | 2,3,5- Trimethyl- Pyrazine | |
|---|---|---|---|---|---|---|---|---|---|---|
| p-value | ||||||||||
| Time | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Metal | *** | n.s. | n.s. | n.s. | *** | * | n.s. | n.s. | n.s. | ** |
| Txmt | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Ti × M | *** | n.s. | n.s. | n.s. | n.s. | * | n.s. | n.s. | n.s. | * |
| Ti × Tx | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| M × Tx | *** | n.s. | n.s. | n.s. | n.s. | *** | n.s. | n.s. | ** | *** |
| Ti × M × Tx | *** | n.s. | n.s. | n.s. | n.s. | ** | n.s. | n.s. | n.s. | *** |
| F-statistic | ||||||||||
| Time | 5380.76 | 2589.59 | 70.34 | 4900.53 | 8295.08 | 0.30 | 11,228.25 | 4900.53 | 1499.79 | 2008.62 |
| Metal | 11.60 | 0.60 | 0.36 | 0.61 | 5.14 | 3.28 | 0.78 | 0.61 | 2.18 | 3.55 |
| Txmt | 49.55 | 1045.32 | 2510.63 | 3069.72 | 17,113.78 | 251.51 | 1558.62 | 3069.72 | 1789.62 | 491.83 |
| Ti × M | 4.16 | 1.35 | 0.98 | 0.33 | 1.01 | 2.47 | 1.17 | 0.33 | 1.54 | 2.53 |
| Ti × Tx | 24.93 | 195.77 | 140.09 | 774.28 | 1468.73 | 8.61 | 365.12 | 774.28 | 986.50 | 164.04 |
| M × Tx | 5.96 | 1.02 | 1.11 | 1.47 | 1.04 | 4.80 | 0.88 | 1.47 | 2.70 | 3.49 |
| Ti × M × Tx | 2.27 | 1.36 | 0.56 | 0.74 | 1.19 | 2.60 | 0.79 | 0.74 | 1.41 | 2.20 |
| Eta-squared (η2) | ||||||||||
| Time | 0.925 | 0.334 | 0.006 | 0.239 | 0.100 | 0.000 | 0.704 | 0.239 | 0.053 | 0.523 |
| Metal | 0.004 | 0.000 | 0.000 | 0.000 | 0.000 | 0.010 | 0.000 | 0.000 | 0.001 | 0.002 |
| Txmt | 0.017 | 0.540 | 0.939 | 0.600 | 0.826 | 0.755 | 0.196 | 0.600 | 0.727 | 0.256 |
| Ti × M | 0.003 | 0.001 | 0.000 | 0.000 | 0.000 | 0.007 | 0.000 | 0.000 | 0.000 | 0.003 |
| Ti × Tx | 0.017 | 0.101 | 0.039 | 0.151 | 0.071 | 0.026 | 0.092 | 0.151 | 0.200 | 0.171 |
| M × Tx | 0.008 | 0.002 | 0.002 | 0.001 | 0.000 | 0.058 | 0.000 | 0.001 | 0.004 | 0.007 |
| Ti × M × Tx | 0.006 | 0.003 | 0.001 | 0.001 | 0.000 | 0.031 | 0.001 | 0.001 | 0.001 | 0.009 |
| Chemical Parameter | Chardonnay Base Wine |
|---|---|
| pH | 2.94 ± 0.02 |
| TA (g/L) | 10.7 ± 0.1 |
| Alcohol (% v/v) | 10.4 ± 0.0 |
| Free SO2 (ppm) | 11 ± 1 |
| Total SO2 (ppm) | 83 ± 2 |
| A420 (mAU) | 3.4 ± 0.1 |
| Fructose (g/L) | 1.96 ± 0.01 |
| Glucose (g/L) | 0.35 ± 0.01 |
| Malic acid (g/L) | 4.93 ± 0.08 |
| Acetic acid (g/L) | 0.11 ± 0.00 |
| System † | Sugar | Amino Acid | Mg2+ (mg/L) | Ca2+ (mg/L) | ||
|---|---|---|---|---|---|---|
| CT | – | – | - | - | - | - |
| CT ML | 10 | - | - | - | ||
| CT MH | - | 50 | - | - | ||
| CT CL | - | - | 10 | - | ||
| CT CH | - | - | - | 50 | ||
| F | Fru 0.02 M (3603.20 mg/L) | – | - | - | - | - |
| F ML | 10 | - | - | - | ||
| F MH | - | 50 | - | - | ||
| F CL | - | - | 10 | - | ||
| F CH | - | - | - | 50 | ||
| FG | Gly 0.01 M (758.28 mg/L) | - | - | - | - | |
| FG ML | 10 | - | - | - | ||
| FG MH | - | 50 | - | - | ||
| FG CL | - | - | 10 | - | ||
| FG CH | - | - | - | 50 | ||
| FL | Lys 0.01 M (1491.73 mg/L) | - | - | - | - | |
| FL ML | 10 | - | - | - | ||
| FL MH | - | 50 | - | - | ||
| FL CL | - | - | 10 | - | ||
| FL CH | - | - | - | 50 | ||
| FC | Cys 0.01 M (1249.07 mg/L) | - | - | - | - | |
| FC ML | 10 | - | - | - | ||
| FC MH | - | 50 | - | - | ||
| FC CL | - | - | 10 | - | ||
| FC CH | - | - | - | 50 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charnock, H.M.; Pickering, G.J.; Kemp, B.S. Effect of Amino Acid, Sugar, Ca2+, and Mg2+ on Maillard Reaction-Associated Products in Modified Sparkling Base Wines During Accelerated Aging. Molecules 2025, 30, 535. https://doi.org/10.3390/molecules30030535
Charnock HM, Pickering GJ, Kemp BS. Effect of Amino Acid, Sugar, Ca2+, and Mg2+ on Maillard Reaction-Associated Products in Modified Sparkling Base Wines During Accelerated Aging. Molecules. 2025; 30(3):535. https://doi.org/10.3390/molecules30030535
Chicago/Turabian StyleCharnock, Hannah M., Gary J. Pickering, and Belinda S. Kemp. 2025. "Effect of Amino Acid, Sugar, Ca2+, and Mg2+ on Maillard Reaction-Associated Products in Modified Sparkling Base Wines During Accelerated Aging" Molecules 30, no. 3: 535. https://doi.org/10.3390/molecules30030535
APA StyleCharnock, H. M., Pickering, G. J., & Kemp, B. S. (2025). Effect of Amino Acid, Sugar, Ca2+, and Mg2+ on Maillard Reaction-Associated Products in Modified Sparkling Base Wines During Accelerated Aging. Molecules, 30(3), 535. https://doi.org/10.3390/molecules30030535








