An Anthracene-Based Hg2+ Fluorescent Probe with Dithioacetal: Simple Synthesis, High Selectivity and Sensitivity, and Dual-Mode Detection Capability
Abstract
:1. Introduction
2. Results and Discussion
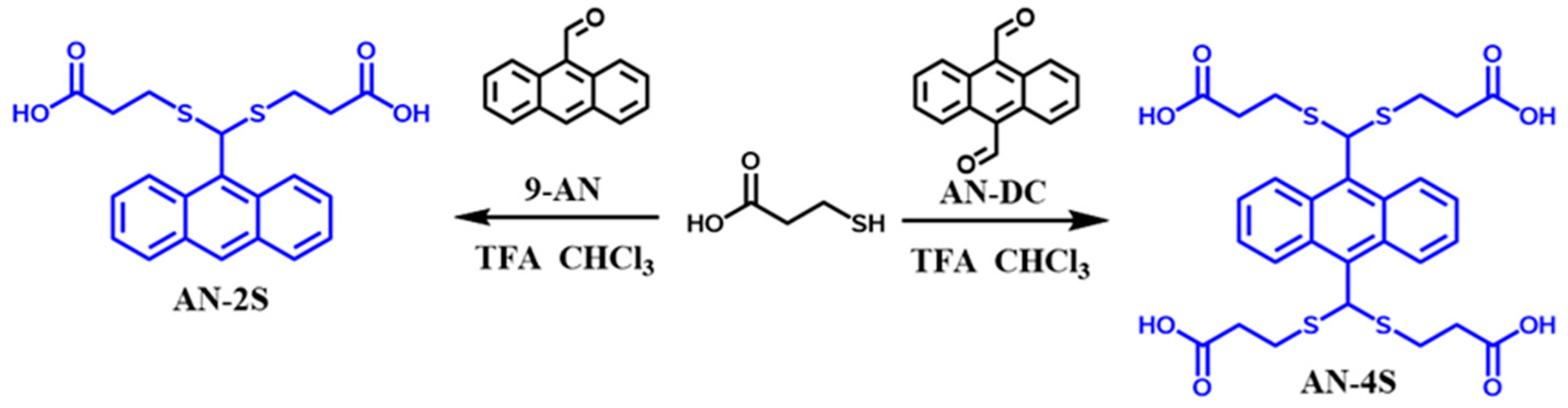
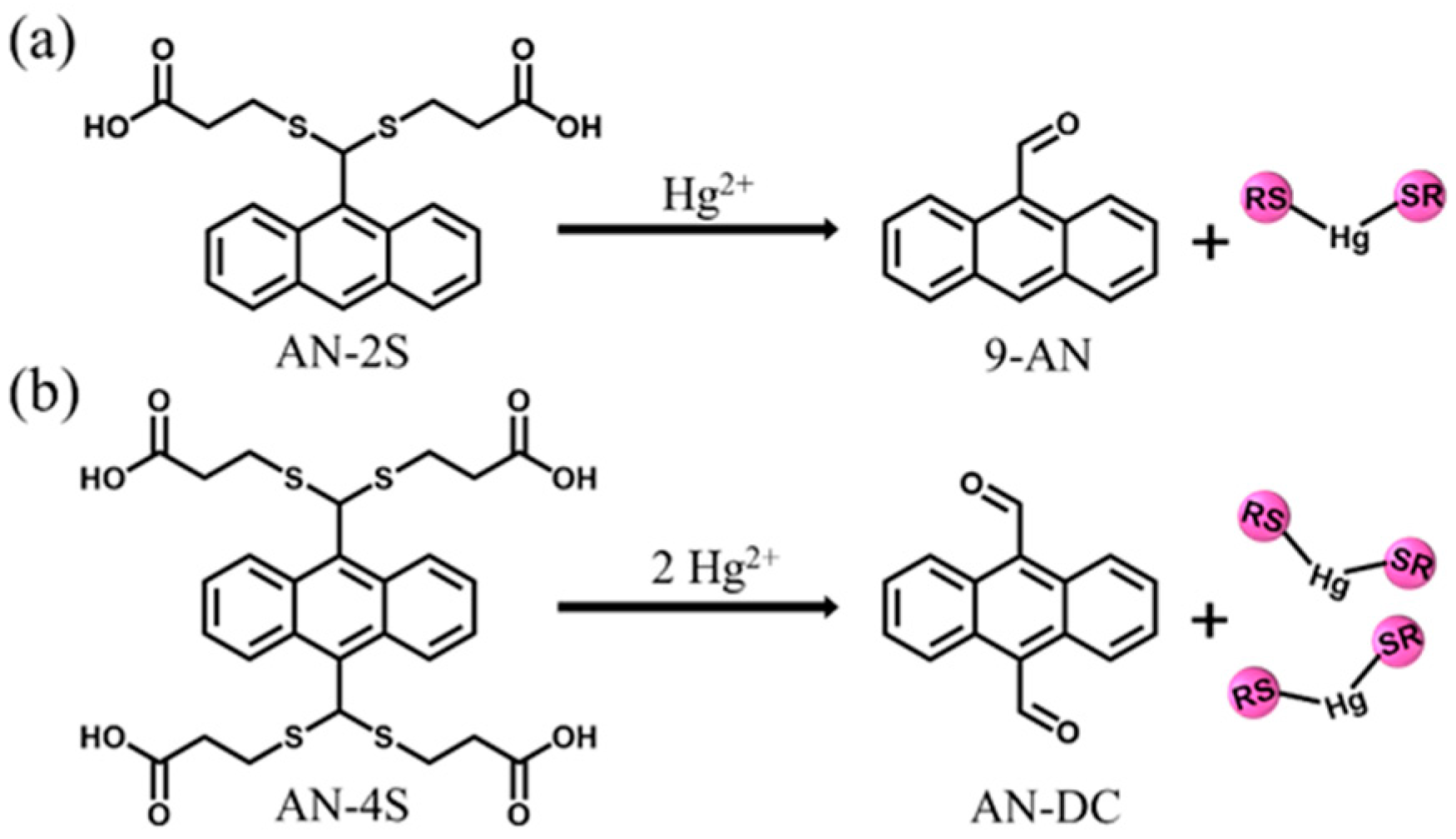
2.1. Synthesis of Water-Soluble AN-2S and AN-4S Probes
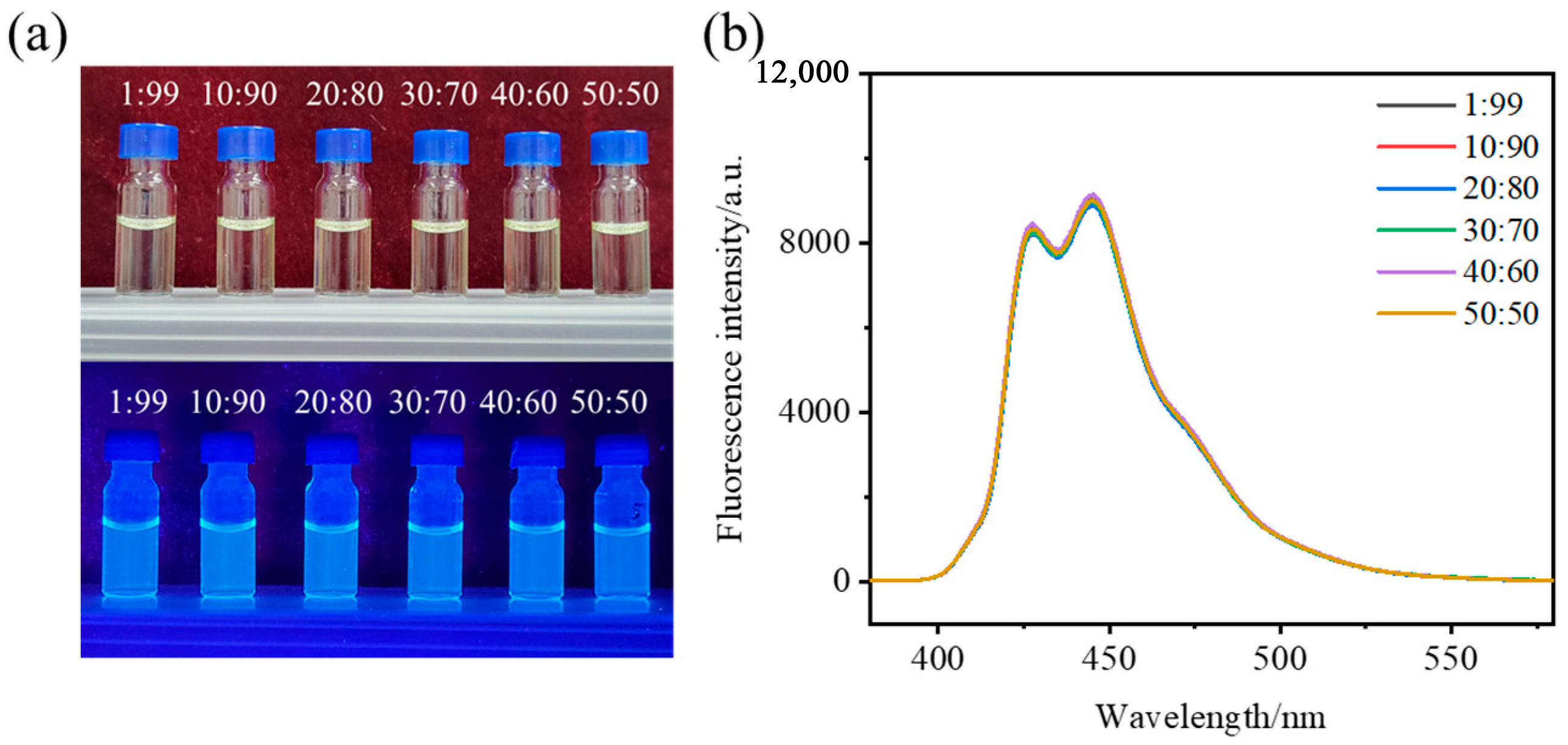
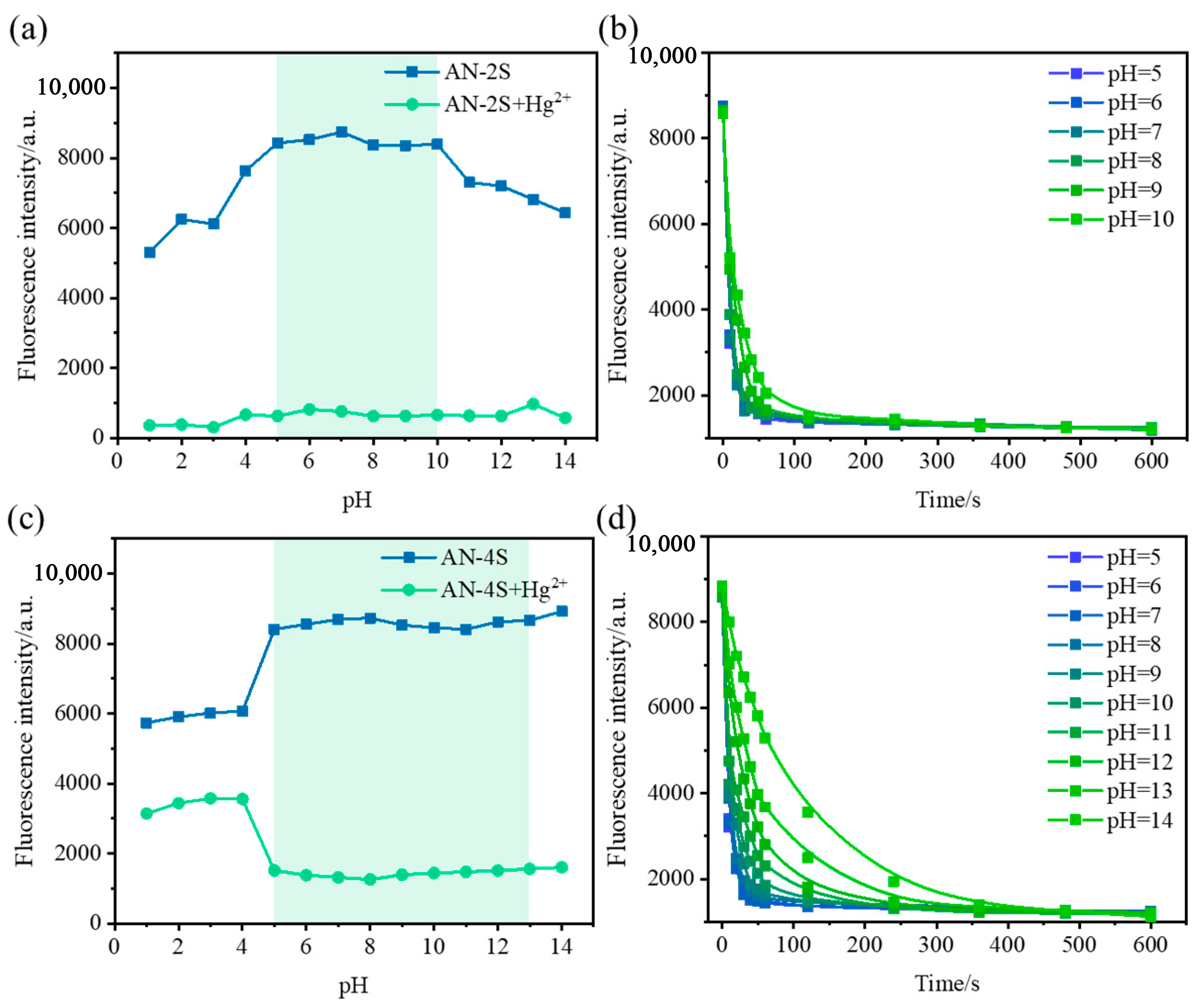
2.2. Solvent and Detection Mode Selection
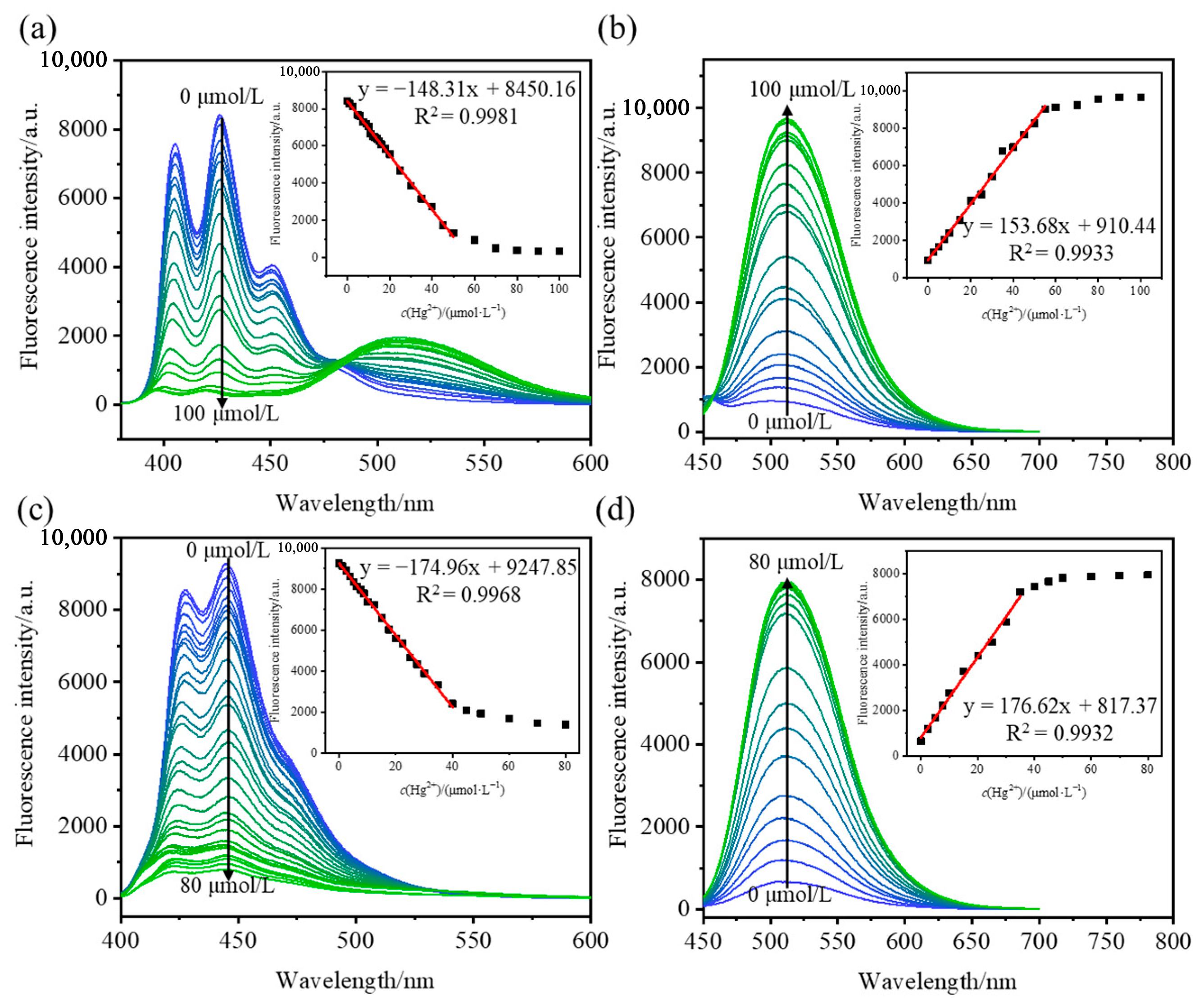
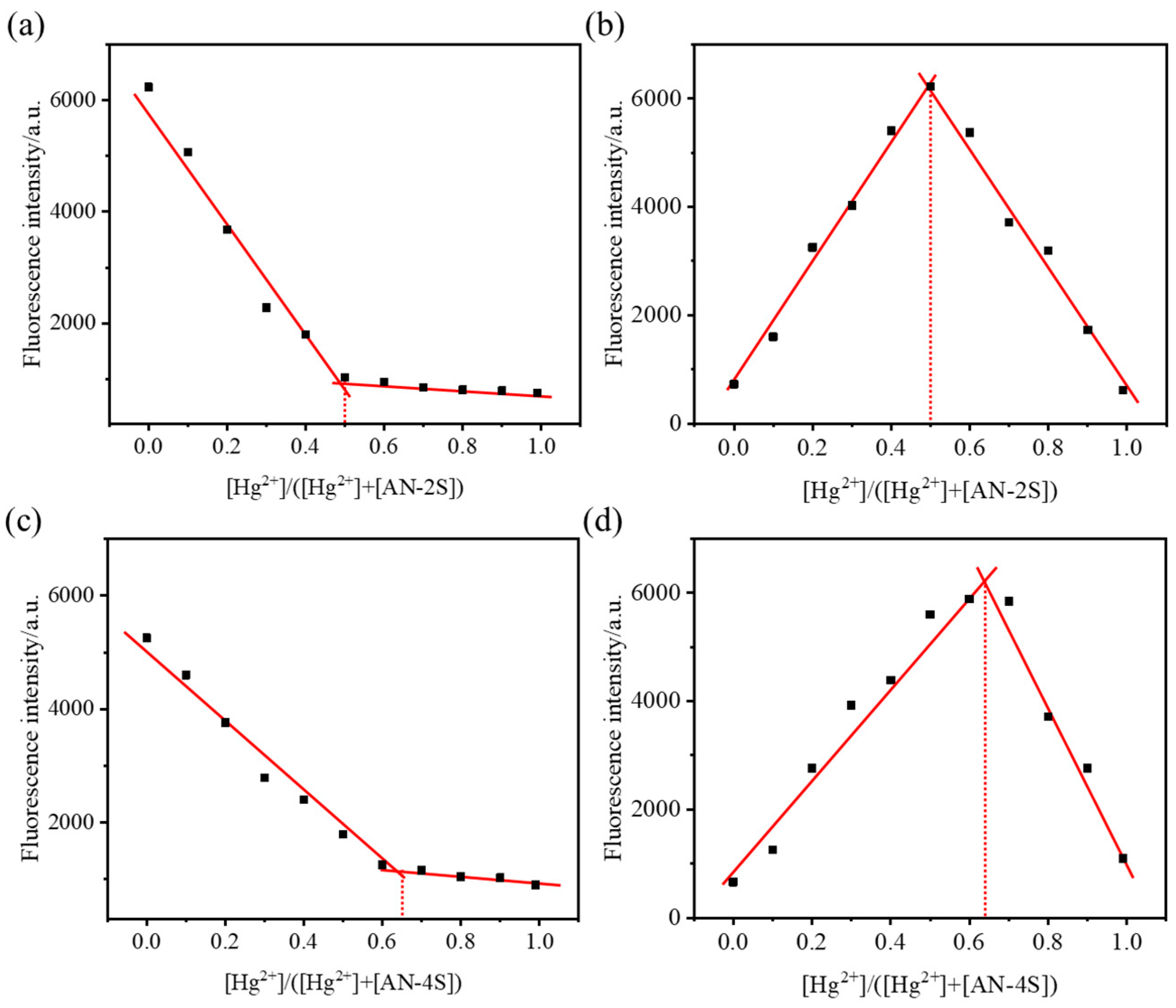
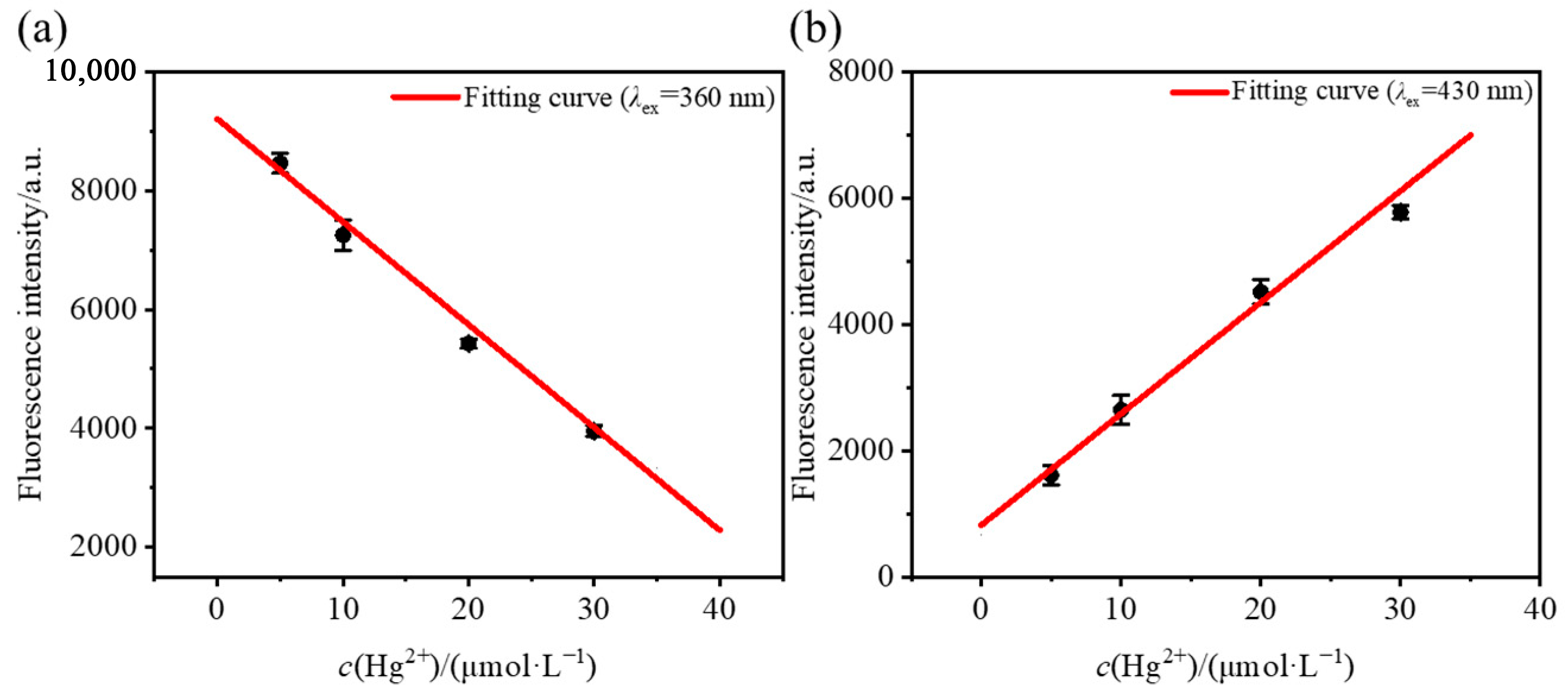
2.3. Fluorescence Titration Experiment
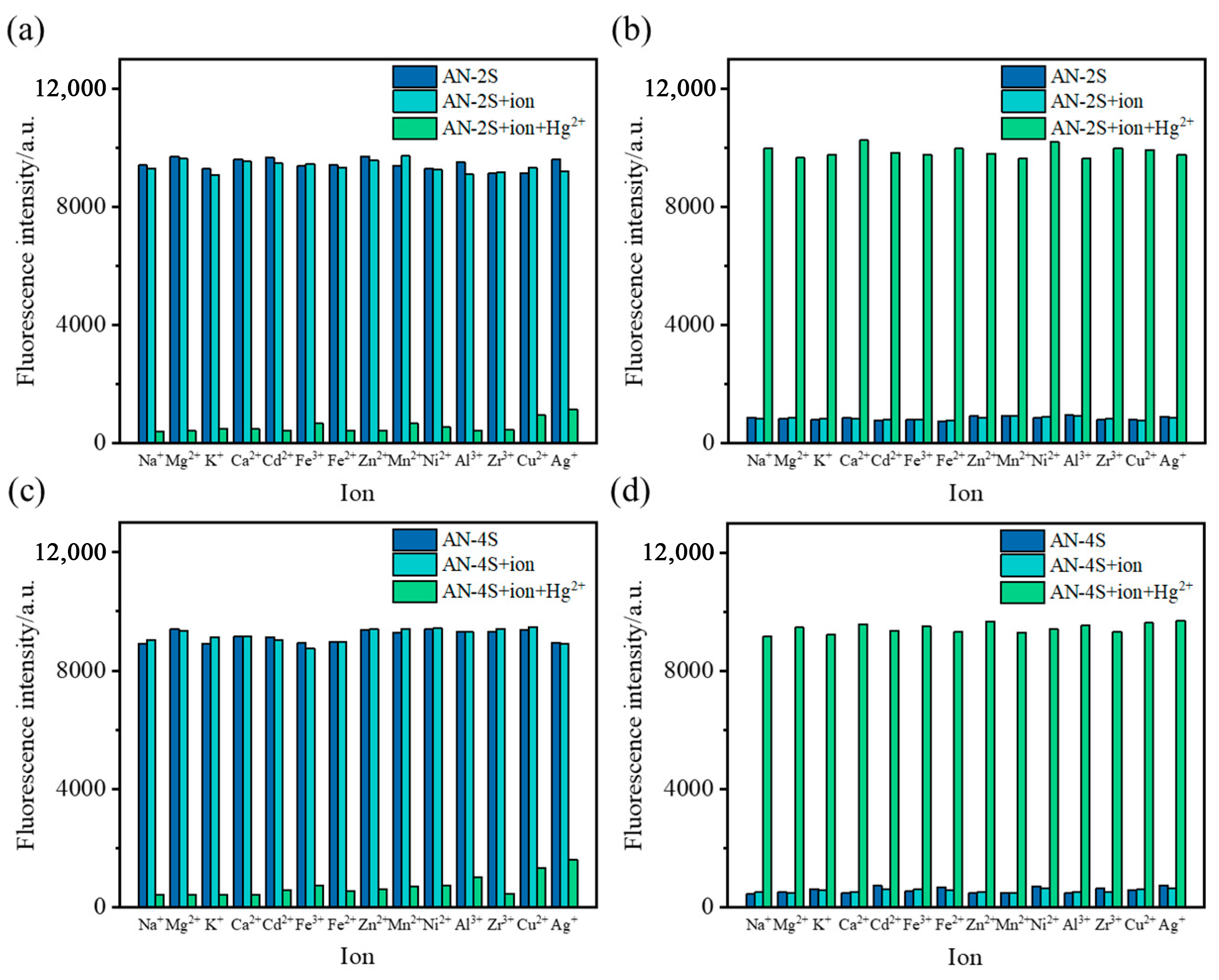
2.4. Selectivity and Anti-Interference Ability
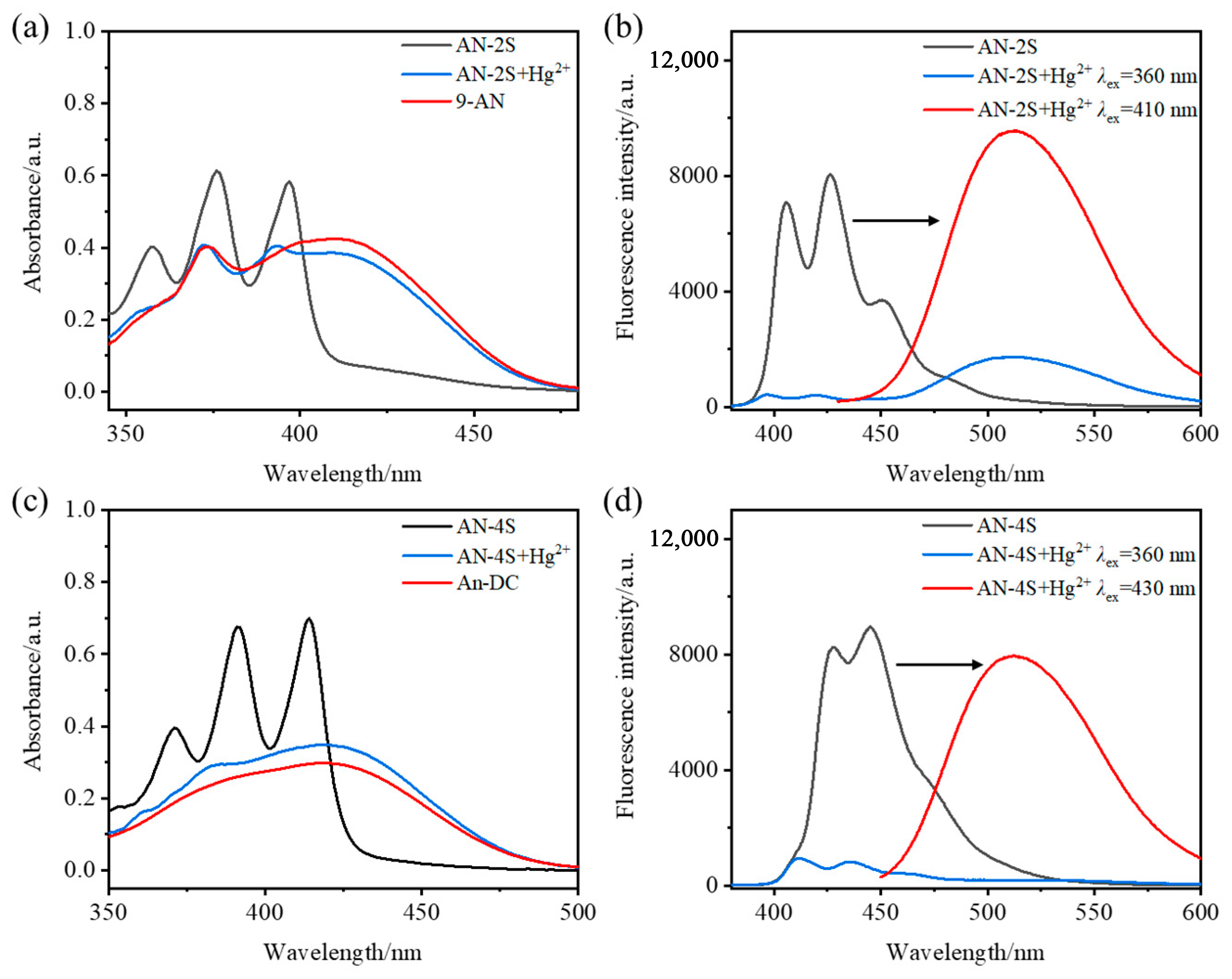
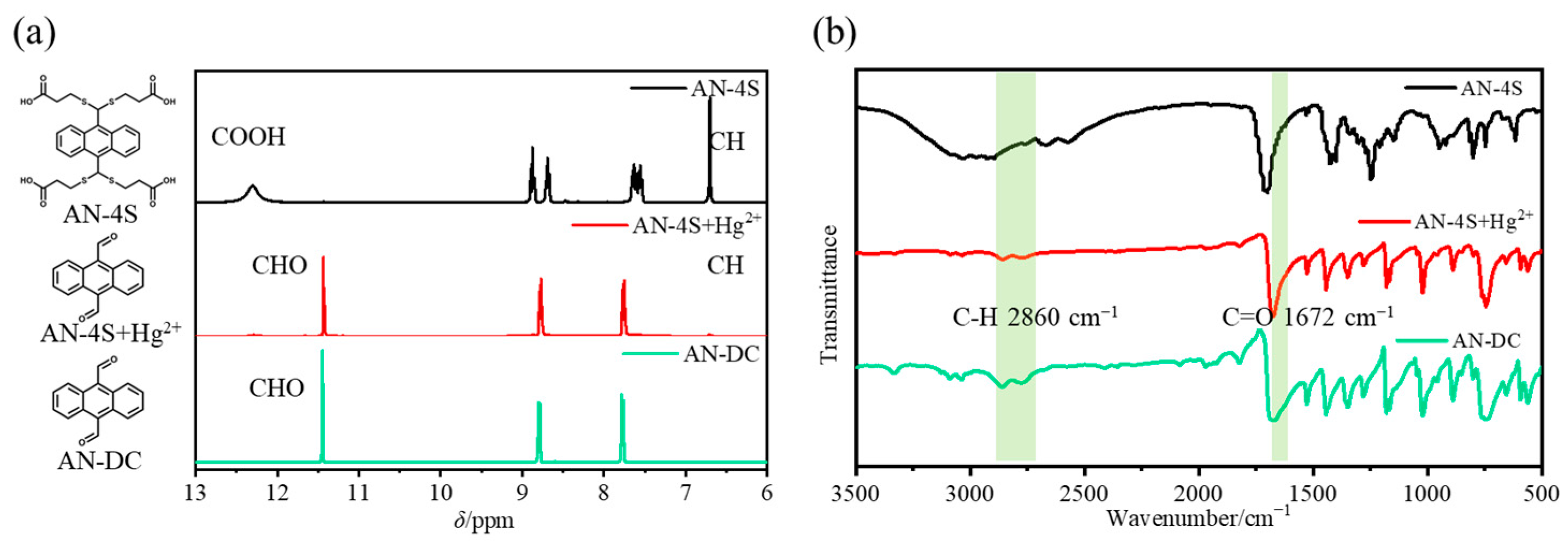
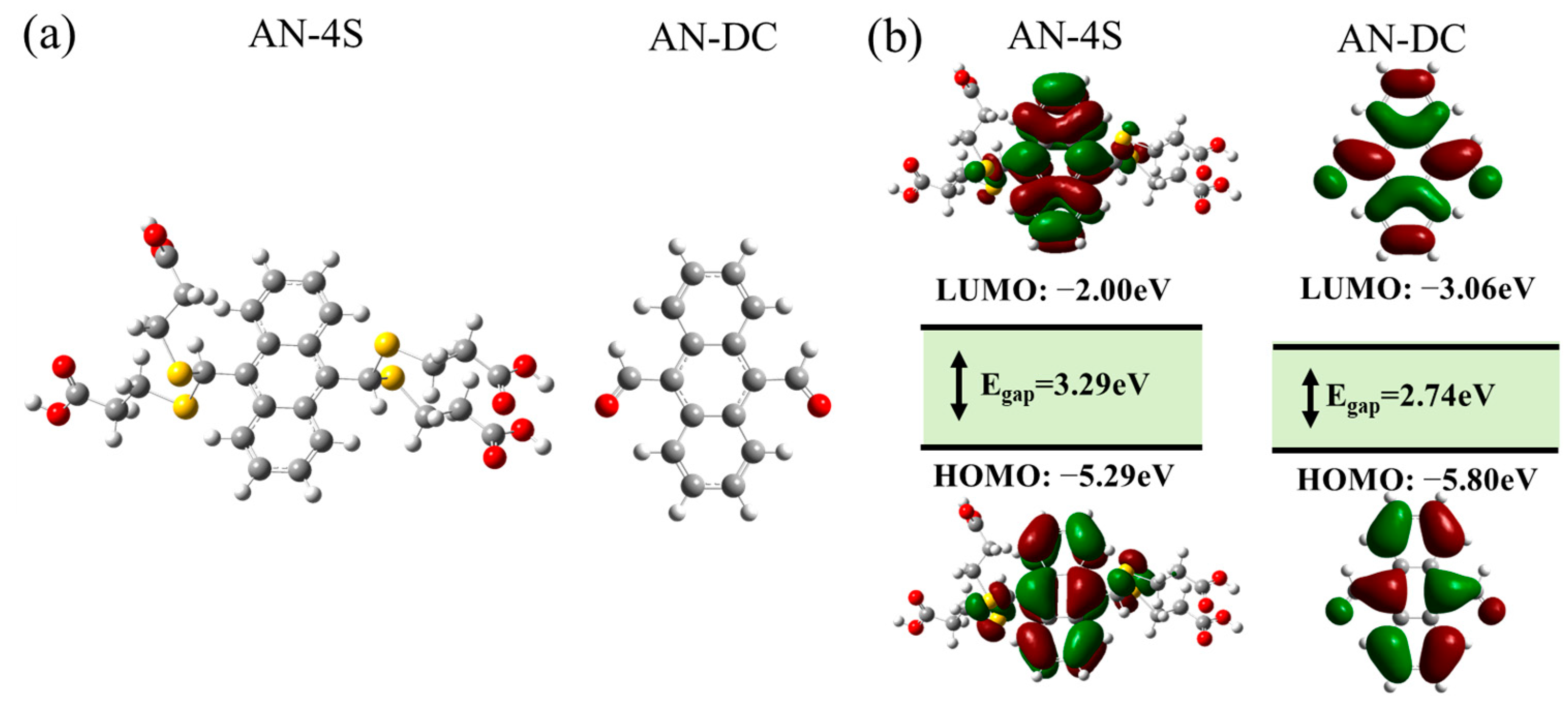
2.5. Response Mechanism
2.6. Practical Application
2.7. Comparison with Other Probes
3. Materials and Methods
3.1. Reagents and Instruments
3.2. One-Step Synthesis of Fluorescent AN-2S Probe
3.3. One-Step Synthesis of Fluorescent AN-4S Probe
3.4. Preparation of Test Samples
3.5. Preparation of Simulated Natural Water Samples
3.6. Preparation of Portable Test Strip
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 9-AN | 9-Anthraformaldehyde |
| AN-DC | 9, 10-Anthradialdehyde |
| TFA | Trifluoroacetic acid |
| LOD | Limit of detection |
| ICT | Intramolecular charge transfer |
| DFT | Density functional theory |
References
- Clarkson, T.W.; Magos, L.; Myers, G.J. The Toxicology of Mercury—Current Exposures and Clinical Manifestations. N. Engl. J. Med. 2003, 349, 1731. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L. The Toxicology of Mercury and Its Chemical Compounds. Crit. Rev. Toxicol. 2006, 36, 609. [Google Scholar] [CrossRef] [PubMed]
- Korbas, M.; Blechinger, S.R.; Krone, P.H.; Pickering, I.J.; George, G.N. Localizing Organomercury Uptake and Accumulation in Zebrafish Larvae at the Tissue and Cellular Level. Proc. Natl. Acad. Sci. USA 2008, 105, 12108–12112. [Google Scholar] [CrossRef]
- Magos, L.; Clarkson, T.W. Overview of the Clinical Toxicity of Mercury. Ann. Clin. Biochem. 2006, 43, 257. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Umesh, M.; Shanmugam, M.K.; Chakraborty, P.; Duhan, L.; Gummadi, S.N.; Pasrija, R.; Jayaraj, I.; Dasarahally Huligowda, L.K. A Retrospection on Mercury Contamination, Bioaccumulation, and Toxicity in Diverse Environments: Current Insights and Future Prospects. Sustainability 2023, 15, 13292. [Google Scholar] [CrossRef]
- Saidon, N.B.; Szabó, R.; Budai, P.; Lehel, J. Trophic Transfer and Biomagnification Potential of Environmental Contaminants (Heavy Metals) in Aquatic Ecosystems. Environ. Pollut. 2024, 340, 122815. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Reinfelder, J.R.; Morel, F.M.M. Bioaccumulation of Nercury and Nethylmercury. Water Air Soil Pollut. 1995, 80, 915. [Google Scholar] [CrossRef]
- Harris, H.H.; Pickering, I.J.; George, G.N. The Chemical Form of Mercury in Fish. Science 2003, 301, 1203. [Google Scholar] [CrossRef]
- Kraepiel, A.M.L.; Keller, K.; Chin, H.B.; Malcolm, E.G.; Morel, F.M.M. Sources and Variations of Mercury in Tuna. Environ. Sci. Technol. 2003, 37, 5551. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wieckowska, A.; Willner, I. Optical Analysis of Hg2+ Ions by Oligonucleotide–Gold-Nanoparticle Hybrids and DNA-Based Machines. Angew. Chem. Int. Ed. 2008, 47, 3927–3931. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Y.; Qian, X. A Ratiometric Fluorescent Probe Based on FRET for Imaging Hg2+ Ions in Living Cells. Angew. Chem. Int. Ed. 2008, 47, 8025–8029. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, P.; Fjordside, S.; Hansen, M.G.; Petrova, M.B. Dietary Selenium Reduces Retention of Methyl Mercury in Freshwater Fish. Environ. Sci. Technol. 2011, 45, 9793–9798. [Google Scholar] [CrossRef]
- Zhang, M.; Bao, Y.-T.; Yang, W.; Xiao, H.-F.; Han, Z.-X.; Wu, X.; Yang, L. An Aurone-derived Low-molecular-weight Fluorescence Probe for the Detection of Hg2+ in Aqueous Solution and Living Cells. J. Heterocycl. Chem. 2018, 55, 1130–1135. [Google Scholar] [CrossRef]
- Furdek Turk, M.; Senta, I.; Kniewald, G.; Mikac, N. Determination of organotin compounds (OTC) at low levels in seawater by solid-phase extraction (SPE) and gas chromatography-pulsed flame photometric detection (GC-PFPD). Int. J. Anal. Chem. 2018, 98, 1–15. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, C.; Shen, L.; Liu, J.; Xu, Z. Online anion exchange column preconcentration and high performance liquid chromatographic separation with inductively coupled plasma mass spectrometry detection for mercury speciation analysis. Anal. Chim. Acta 2014, 828, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Rahman, G.M.M.; Wolle, M.M.; Fahrenholz, T.; Kingston, H.M.S.; Pamuku, M. Measurement of Mercury Species in Whole Blood Using Speciated Isotope Dilution Methodology Integrated with Microwave-Enhanced Solubilization and Spike Equilibration, Headspace–Solid-Phase Microextraction, and GC-ICP-MS Analysis. Anal. Chem. 2014, 86, 6130–6137. [Google Scholar] [CrossRef]
- Leng, G.; Feng, L.; Li, S.-B.; Qian, S.; Dan, D.-Z. Determination of Mercury (Hg) in Sediment by a Sequential Injection (SI) System With Cold Vapor Generation Atomic Fluorescence Spectrometry (CVAFS) Detection After a Rapid and Mild Microwave-Assisted Digestion. Environ. Forensics 2013, 14, 9–15. [Google Scholar] [CrossRef]
- Neupane, L.N.; Kim, J.M.; Lohani, C.R.; Lee, K.-H. Selective and sensitive ratiometric detection of Hg2+ in 100% aqueous solution with triazole-based dansyl probe. J. Mater. Chem. 2012, 22, 4003–4008. [Google Scholar] [CrossRef]
- Bozkurt, E.; Gul, H.I. Selective Fluorometric “Turn-off” Sensing for Hg2+ with Pyrazoline Compound and Its Application in Real Water Sample Analysis. Inorg. Chim. Acta 2020, 502, 119288. [Google Scholar] [CrossRef]
- Ye, F.; Liang, X.M.; Xu, K.-X.; Pang, X.-X.; Chai, Q.; Fu, Y. A Novel Dithiourea-Appended Naphthalimide “on-off” Fluorescent Probe for Detecting Hg2+ and Ag+ and Its Application in Cell Imaging. Talanta 2019, 200, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yu, X.; Zhang, X.; Jing, C.; Liu, T.; Hu, X.; Lu, S.; Uvdal, K.; Gao, H.W.; Hu, Z. Rapid Detection of Mercury (II) Ions and Water Content by a New Rhodamine B-based Fluorescent Chemosensor. Spectrochim. Acta A 2020, 241, 118657. [Google Scholar] [CrossRef]
- Zhu, N.; Xu, J.; Ma, Q.; Geng, Y.; Li, L.; Liu, S.; Liu, S.; Wang, G. Rhodamine-Based Fluorescent Probe for Highly Selective Determination of Hg2+. ACS Omega 2022, 7, 29236–29245. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, L.; Zhou, P. A rhodamine B-based Fluorescent Sensor toward Highly Selective Mercury (II) Ions Detection. Talanta 2016, 150, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.S.; Lafzi, F.; Bayindir, S.; Kilic, H.; Toprak, M. A Novel Rhodamine-Phenolphthalein Architecture for Selective Mercury Ion Detection in Aqueous Media. ChemPlusChem 2024, 89, e202300649. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Xu, H.; Liu, Y.; Tian, Y.; Tan, R.; Peng, Y.; Wang, Y.-W. A Chemodosimeter with High Selectivity for Ratiometric Detection of Mercury Ions in Buffer Solution. Tetrahedron Lett. 2023, 120, 154435. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, J.; Liu, S.; Mao, G.; Chen, B.; Hou, S.; Ma, Y.; Lian, Y. A Coumarin-naphthalimide-based Fluorescent Probe for the Ratiometric Detection of Hg2+ Utilizing an ICT-FRET Mechanism. J. Mol. Struct. 2025, 1324, 140966. [Google Scholar] [CrossRef]
- Orrillo, A.G.; Escalante, A.M.; Furlan, R.L.E. Dithioacetal Exchange: A New Reversible Reaction for Dynamic Combinatorial Chemistry. Chem. Eur. J. 2016, 22, 6746–6749. [Google Scholar] [CrossRef]
- Orrillo, A.G.; La-Venia, A.; Escalante, A.M.; Furlan, R.L.E. Rewiring Chemical Networks Based on Dynamic Dithioacetal and Disulfide Bonds. Chem. Eur. J. 2018, 24, 3141–3146. [Google Scholar] [CrossRef] [PubMed]
- Orrillo, A.G.; Escalante, A.M.; Furlan, R.L.E. Host Amplification in a Dithioacetal-Based Dynamic Covalent Library. Org. Lett. 2017, 19, 1446–1449. [Google Scholar] [CrossRef]
- Orrillo, A.G.; Escalante, A.M.; Martinez-Amezaga, M.; Cabezudo, I.; Furlan, R.L.E. Molecular Networks in Dynamic Multilevel Systems. Chem. Eur. J. 2019, 25, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.G.; Vijay, N.; Thirumalaivasan, N.; Velmathi, S.; Wu, S.P. Coumarin-based Hg2+ fluorescent probe: Fluorescence turn-on detection for Hg2+ bioimaging in living cells and zebrafish. Spectrochim. Acta A. 2019, 219, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Naha, S.; Thirumalaivasan, N.; Velmathi, S.; Wu, S.P. A novel nanomolar highly selective fluorescent probe for imaging mercury (II) in living cells and zebrafish. Sens. Actuat. B-Chem. 2018, 277, 673–678. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, C.; Xiao, Y.; Zhang, M.; Wang, Q.; Zheng, W.; Zhang, S. Preparation 2-(anthracen-9-yl)-1,3-dithiolane as a novel dual-channel AIE-active fluorescent probe for mercury (II) ion with excellent performance. J. Photochem. Photobiol. A 2019, 378, 142–146. [Google Scholar] [CrossRef]
- State Administration of China for Standardization, Standards for Drinking Water Quality (GB 5749-2022). Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=99E9C17E3547A3C0CE2FD1FFD9F2F7BE (accessed on 15 March 2022).
- Lei, S.; Meng, X.; Wang, L.; Zhou, J.; Qin, D.; Duan, H. A Naphthalimide-Based Fluorescent Probe for the Detection and Imaging of Mercury Ions in Living Cells. ChemistryOpen 2021, 10, 1116–1122. [Google Scholar] [CrossRef]
- Yu, C.; Li, X.; Yang, M.; Xie, Y.; Zhang, J. Detection of Silver and Mercury Ions Using Naphthalimide-Based Fluorescent Probe. Molecules 2024, 29, 5196. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Li, J.; Huang, Y.; Liu, H.; Lin, X.; Huang, L.; Li, D. Accurate and rapid mercury susceptibility detection in aquatic samples using fluorescent probe integrated rhodamine with pyridyl isothiocyanate. Spectrochim. Acta A 2024, 323, 124886. [Google Scholar] [CrossRef] [PubMed]
- Moha, R.; Gossen, V. USRCAT: Real-time ultrafast shape recognition with pharmacophoric constraints. J. Cheminform. 2012, 4, 27. [Google Scholar] [CrossRef]
- Becke, A.D. Density—functional thermochemistry. IV. A new dynamical correlation functional and implications for exact-exchange mixing. J. Chem. Phys. 1996, 104, 1040–1046. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Henriksson, A.; Sundbom, M. Dynamic polarizabilities of polyaromatic hydrocarbons using coupled-cluster linear response theory. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]

| Probe | Solvent (V(DMF):V(PBS)) | λex/λem (nm) | Detection Mode |
|---|---|---|---|
| AN-2S | 1:9 | 360/425 | Turn-off |
| AN-2S + Hg2+ | 1:9 | 410/510 | Turn-on |
| AN-4S | 1:99 | 360/445 | Turn-off |
| AN-4S + Hg2+ | 1:99 | 430/510 | Turn-on |
| Probe | Detection Mode | Linear Response Range (μmol/L) | σ | LOD (mol/L) |
|---|---|---|---|---|
| AN-2S | Turn-off | 0~45 | 12.31 | 2.5 × 10−7 |
| AN-2S | Turn-on | 0~45 | 4.99 | 9.8 × 10−8 |
| AN-4S | Turn-off | 0~40 | 16.83 | 2.3 × 10−7 |
| AN-4S | Turn-on | 0~40 | 2.92 | 4.9 × 10−8 |
| Sample | pH | λex/λem (nm) | Added Hg2+ Concentration (μmol/L) | Detected Hg2+ Concentration (μmol/L) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|---|---|
| Lake water | 6.8 | 360/445 | 5 | 4.51 | 90.20 | 1.98 |
| 10 | 11.49 | 114.90 | 3.45 | |||
| 20 | 21.49 | 107.45 | 2.39 | |||
| 30 | 30.45 | 101.50 | 1.96 | |||
| 430/510 | 5 | 4.48 | 89.60 | 2.82 | ||
| 10 | 10.33 | 103.30 | 3.74 | |||
| 20 | 20.89 | 104.45 | 4.25 | |||
| 30 | 28.02 | 101.61 | 1.77 |
| Probe | Solvent | LOD (mol/L) | cprobe (μnol/L) | Liner Range (μnol/L) | Application | |
|---|---|---|---|---|---|---|
| 1 [32] | 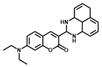 | 50% MeCN | 1.1 × 10−6 | 10 | 0~0.12 | Cell imaging |
| 2 [33] | 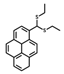 | 50% MeCN | 5.7 × 10−8 | 10 | - | Cell imaging |
| 3 [34] |  | 20% DMF | 2.3 × 10−8 | 10 | 1~10 | Cell imaging |
| 4 [36] | 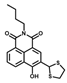 | 10% DMSO | 1.3 × 10−8 | 10 | 1~10 | Cell imaging |
| 5 [37] | 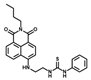 | 90% EtOH | 3.3 × 10−7 | 10 | 1~10 | Cell imaging |
| 6 [38] | 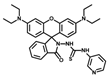 | 10% DMF | 1.3 × 10−7 | 10 | 0~10 | - |
| AN-2S | 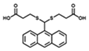 | 10% DMF | 2.5 × 10−7 (λex = 360 nm)/ 9.8 × 10−8 (λex = 410 nm) | 100 | 0~50 | Test strip |
| AN-4S | 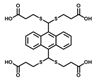 | 1% DMF | 2.3 × 10−7 (λex = 360 nm)/ 4.9 × 10−8 (λex = 410 nm) | 100 | 0~35 | Test strip |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Yan, Q. An Anthracene-Based Hg2+ Fluorescent Probe with Dithioacetal: Simple Synthesis, High Selectivity and Sensitivity, and Dual-Mode Detection Capability. Molecules 2025, 30, 561. https://doi.org/10.3390/molecules30030561
Ren H, Yan Q. An Anthracene-Based Hg2+ Fluorescent Probe with Dithioacetal: Simple Synthesis, High Selectivity and Sensitivity, and Dual-Mode Detection Capability. Molecules. 2025; 30(3):561. https://doi.org/10.3390/molecules30030561
Chicago/Turabian StyleRen, Hongli, and Qiang Yan. 2025. "An Anthracene-Based Hg2+ Fluorescent Probe with Dithioacetal: Simple Synthesis, High Selectivity and Sensitivity, and Dual-Mode Detection Capability" Molecules 30, no. 3: 561. https://doi.org/10.3390/molecules30030561
APA StyleRen, H., & Yan, Q. (2025). An Anthracene-Based Hg2+ Fluorescent Probe with Dithioacetal: Simple Synthesis, High Selectivity and Sensitivity, and Dual-Mode Detection Capability. Molecules, 30(3), 561. https://doi.org/10.3390/molecules30030561




