Bimetallic Zinc-Iron-Modified Sugarcane Bagasse Biochar for Simultaneous Adsorption of Arsenic and Oxytetracycline from Wastewater
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization Analysis
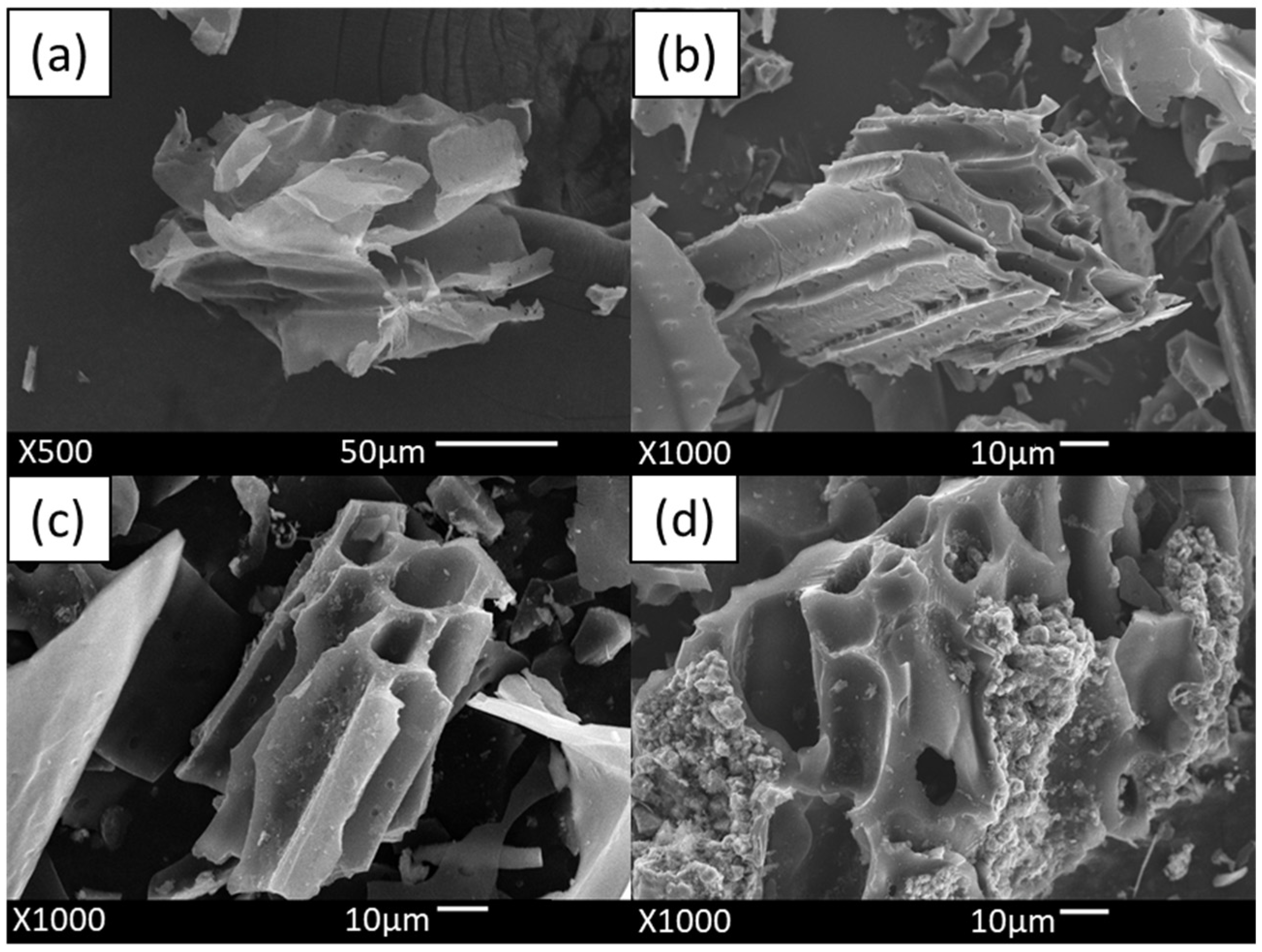
2.1.1. SEM Analysis Results
2.1.2. EDS Analysis Results
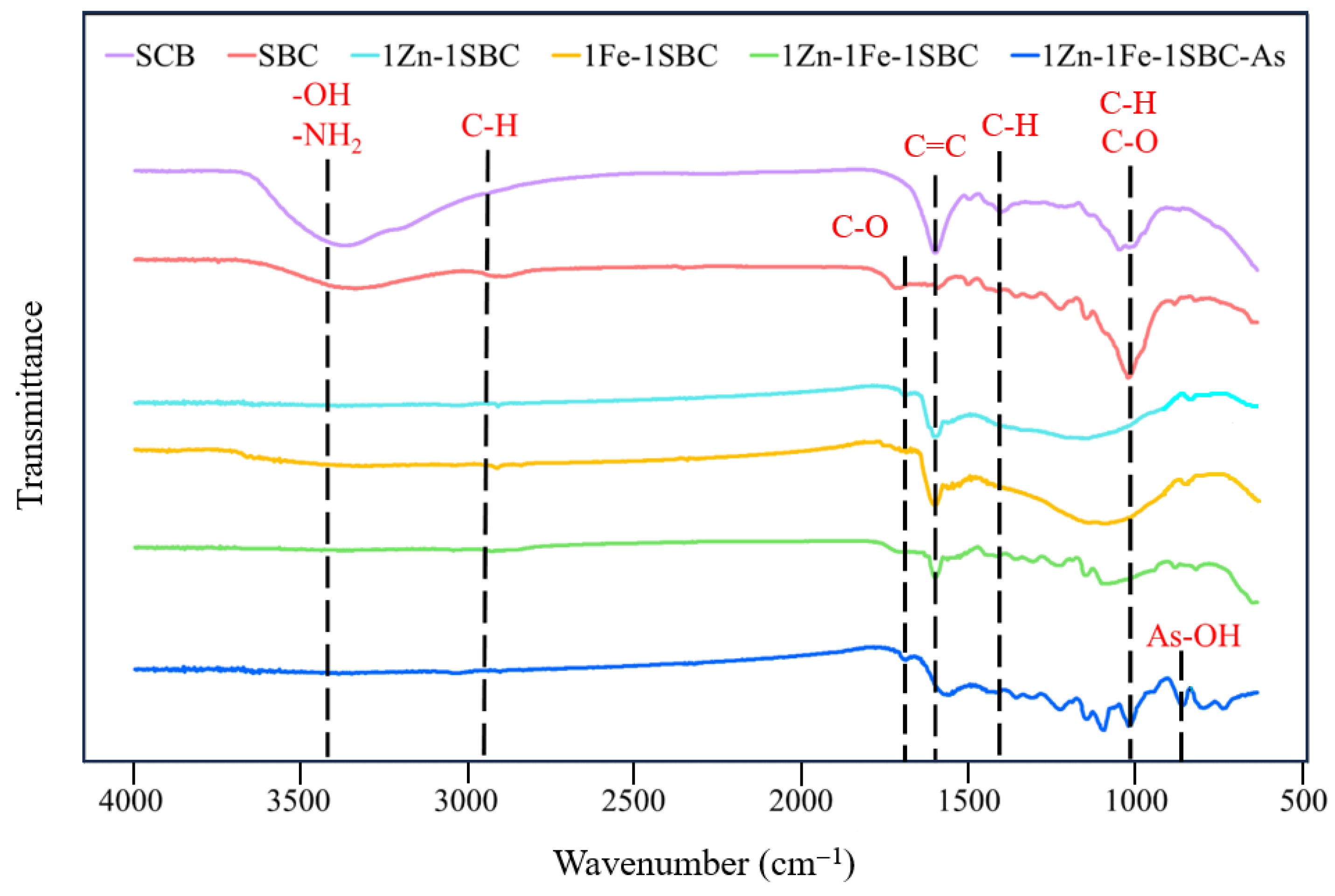
2.1.3. FTIR Analysis Results
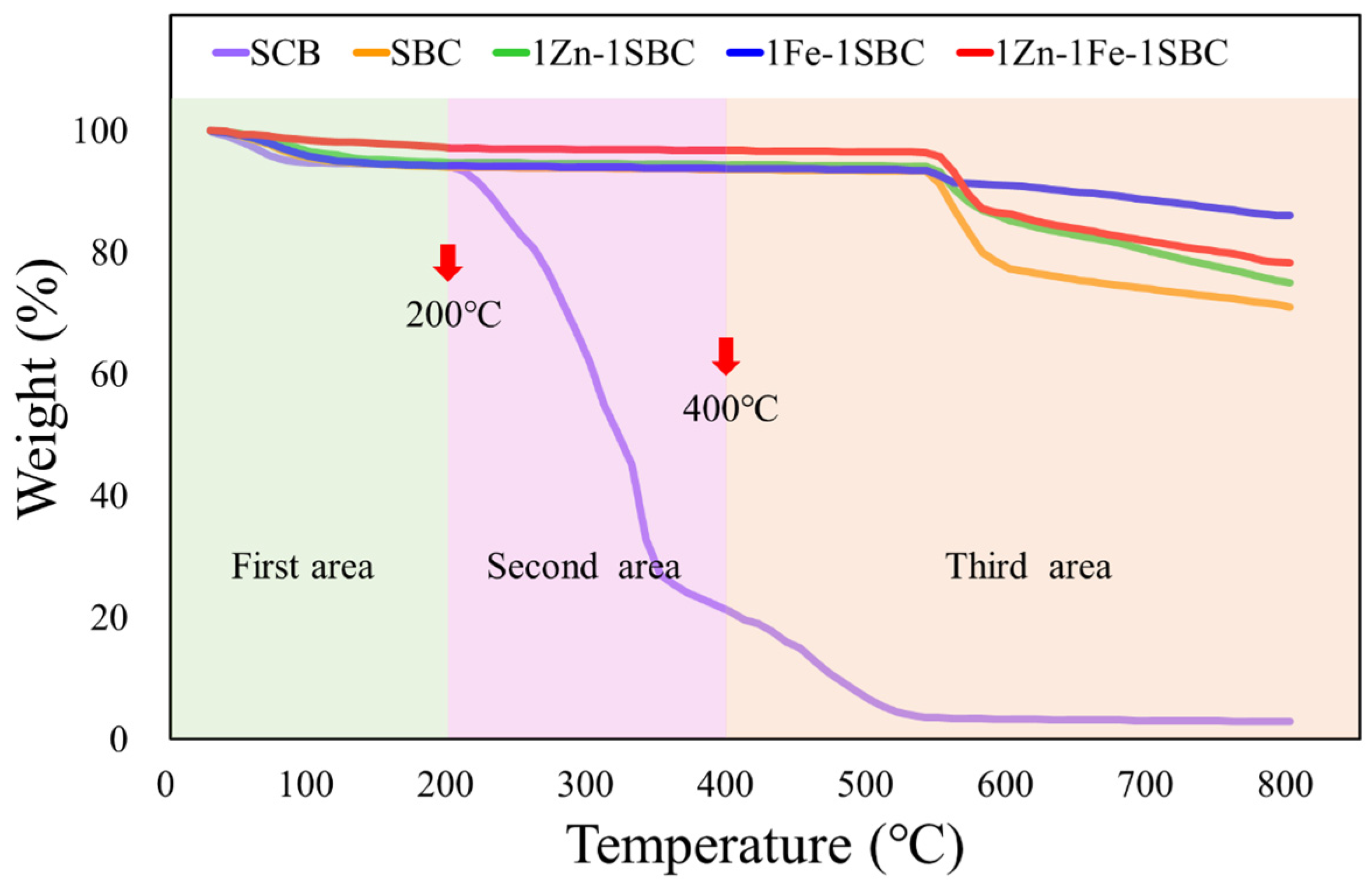
2.1.4. TGA Analysis Results
2.1.5. BET Analysis Results
2.1.6. XPS Analysis Results
2.2. Results of Simultaneous Adsorption of As(III) and OTC
2.2.1. Comparison of Different Biochars
2.2.2. Effect of Fe and Zn Content
2.2.3. Effect of pH
2.3. Isothermal Adsorption Model Correlation Results
2.4. Adsorption Kinetic Model Correlation Results
2.5. Thermodynamic Analysis Results
2.6. Arrhenius Model Analysis Results
2.7. Adsorption Mechanism of 1Zn-1Fe-1SBC for As(III) and OTC
2.7.1. Pore Blocking and Surface Effects
2.7.2. Formation of Hydrogen Bonds
2.7.3. Electrostatic Interactions
2.7.4. Surface Complexation
2.7.5. π-π Interactions
3. Materials and Methods
3.1. Preparation of Biochar
3.2. Preparation of Modified Biochar
3.2.1. Monometallic Modified Biochar
3.2.2. Bimetallic Modified Biochar
3.3. Material Characterization
3.4. Material Performance Valuation
3.5. Isothermal Adsorption Model
3.5.1. Langmuir Isotherm Model
3.5.2. Freundlich Isotherm Model
3.5.3. Temkin Isotherm Model
3.6. Adsorption Kinetics Models
3.6.1. Pseudo-First-Order Kinetics
3.6.2. Pseudo-Second-Order Kinetics
3.6.3. Intra-Particle Diffusion Model
3.7. Thermodynamic Model
3.8. Arrhenius Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fatoki, J.O.; Badmus, J.A. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. 2022, 5, 100052. [Google Scholar] [CrossRef]
- Yang, R.; Su, Y.; Aubrecht, K.B.; Wang, X.; Ma, H.G.; Grubbs, R.B.; Hsiao, B.S.; Chu, B. Thiol-functionalized chitin nanofibers for As (III) adsorption. Polymer. 2015, 60, 9–17. [Google Scholar] [CrossRef]
- Aposhian, H.V.; Maiorino, R.M.; Dart, R.C.; Perry, D.F. Urinary excretion of meso-2,3-dimercaptosuccinic acid in human subjects. Clin Pharmacol. Ther. 1989, 45, 520–526. [Google Scholar] [CrossRef]
- Mandal, P.; Debbarma, S.R.; Saha, A.; Ruj, B. Disposal problem of arsenic sludge generated during arsenic removal from drinking water. Procedia Environ. Sci. 2016, 35, 943–949. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Boxall, A.B.; Kolpin, D.W.; Leung, K.M.; Lai, R.W.; Galbán-Malagón, C.; Teta, C. Pharmaceutical pollution of the world’s rivers. Proc. Natl. Acad. Sci. USA 2022, 119, 2113947119. [Google Scholar] [CrossRef]
- Ghodsi, J.; Rafati, A.A.; Shoja, Y. First report on electrocatalytic oxidation of oxytetracycline by horse radish peroxidase: Application in developing a biosensor to oxytetracycline determination. Sens. Actuators B Chem. 2016, 224, 692–699. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, H.; Li, Y.; Liu, Z. Photodegradation of oxytetracycline in aqueous by 5A and 13X loaded with TiO2 under UV irradiation. J. Hazard. Mater. 2010, 176, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Pelaez, M.; Duan, X.; Deng, H.; O’Shea, K.; Fatta-Kassinos, D.; Dionysiou, D.D. Role of pH on photolytic and photocatalytic degradation of antibiotic oxytetracycline in aqueous solution under visible/solar light: Kinetics and mechanism studies. Appl. Catal. B-Environ. 2013, 134, 83–92. [Google Scholar] [CrossRef]
- Wang, J.; Pembleton, L.; Cogan, N.; Forster, J. Evidence for heterosis in italian ryegrass (Lolium multiflorum Lam.) based on inbreeding depression in F2 generation offspring from biparental crosses. Agronomy 2016, 6, 49. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, T. Mass flows and removal of antibiotics in two municipal wastewater treatment plants. Chemosphere 2011, 83, 1284–1289. [Google Scholar] [CrossRef]
- Zheng, S.; Qiu, X.; Chen, B.; Yu, X.; Liu, Z.; Zhong, G.; Li, H.; Chen, M.; Sun, G.; Huang, H.; et al. Antibiotics pollution in Jiulong River estuary: Source, distribution and bacterial resistance. Chemosphere 2011, 84, 1677–1685. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.; Wang, H.; Guo, C.; Qiu, H.; He, Y.; Zhang, Y.; Li, X.; Meng, W. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere 2015, 119, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Zhang, Y.; Gao, Y.; Tian, Z.; Yang, M. Anaerobic treatment of antibiotic production wastewater pretreated with enhanced hydrolysis: Simultaneous reduction of COD and ARGs. Water Res. 2017, 110, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.G. Hybrid method integrating adsorption and chemical precipitation of heavy metal ions on polymeric fiber surfaces for highly efficient water purification. Chemosphere 2024, 363, 142909. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, G.; Xie, H.; Zhai, Y.; Li, X. Advances and solutions in biological treatment for antibiotic wastewater with resistance genes: A review. J. Environ. Manag. 2024, 368, 122115. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wen, Q.; Chen, Z.; Yang, B.; Hu, H.Y. Different mobile counter ion types of magnetic ion-exchange resin coupling with ozonation treatment of the organic matter, antibiotic resistance genes, and pathogens in secondary effluent. J. Chem. Eng. 2024, 485, 149962. [Google Scholar] [CrossRef]
- Bhaskar, S.; Rashmi Shree, K.N.; Apoorva, K.V.; Sreenivasa, M.Y. Adsorption—Advanced oxidation process (AAOP) for the heavy metals and organic matter removal from leachate using combined filtration-Fenton’s and Photo-Fenton’s treatment. J. Environ. Manag. 2024, 371, 123009. [Google Scholar]
- Zhu, Y.; Ma, J.; Zeng, S.; Li, X.; Lisak, G.; Chen, F. Advanced treatment of microplastics and antibiotic-containing wastewater using integrated modified dissolved air flotation and pulsed cavitation-impinging stream processes. J. Hazard Mater. Adv. 2022, 7, 100139. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Fan, Z.; Duan, P.; Wang, L.; Pan, J.; Gao, B. Redox potentials of sulfonamide antibiotics mediating the electron transfer process in single-atom Cu catalyst/peroxymonosulfate system: Selective removal mechanisms for sulfonamides. J. Hazard Mater. 2025, 485, 136880. [Google Scholar] [CrossRef]
- Chalenko, Y.; Shumyantseva, V.; Ermolaeva, S.; Archakov, A. Electrochemistry of Escherichia coli JM109: Direct electron transfer and antibiotic resistance. Biosens. Bioelectron. 2012, 32, 219–223. [Google Scholar] [CrossRef]
- Zhang, X.; Bhattacharya, T.; Wang, C.; Kumar, A.; Nidheesh, P.V. Straw-derived biochar for the removal of antibiotics from water: Adsorption and degradation mechanisms, recent advancements and challenges. Environ. Res. 2023, 237, 116998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, P.; Zha, X.; Xu, C.; Kang, S.; Zhou, M.; Wang, Y. Overview assessment of risk evaluation and treatment technologies for heavy metal pollution of water and soil. J. Clean. Prod. 2022, 379, 134043. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Peng, C.; Tan, J.; Wan, J.; Sun, P.; Li, Q.; Ji, X.; Zhang, Q.; Wu, Y. Recent advances of carbon-based nano zero valent iron for heavy metals remediation in soil and water: A critical review. J. Hazard. Mater. 2022, 426, 127993. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Mu, Y.; Ma, S.; Xu, S.; Mu, X. Remediation on antimony-contaminated soil from mine area using zero-valent-iron doped biochar and their effect on the bioavailability of antimony. Chemosphere 2024, 363, 143015. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Nguyen, N.T.; Duong, C.C.; Le, T.T.; Chen, S.S.; Chang, C.T. Adsorption configurations of iron complexes on As(III) adsorption over sludge biochar surface. J. Nanosci. Nanotechnol. 2021, 21, 5174–5180. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Cao, G.; Qiang, Y.; Lu, Y.; Qin, R.; Xu, W.; Xie, Y.; Mao, R. Effective removal of Pb (II) from wastewater by zinc-iron bimetallic oxide-modified walnut shell biochar: A combined experimental and DFT calculation approach. J. Environ. Manag. 2024, 370, 122757. [Google Scholar] [CrossRef]
- Lin, H.; Qiu, S.; Wu, Z.; Ye, X.; Liu, M. Fabrication of lignin-based biochar containing multi-metal ferrite and efficient removal for oxytetracycline hydrochloride. J. Clean. Prod. 2022, 331, 129885. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Mei, M.; Wang, T.; Chen, S.; Li, J. A Ca-rich biochar derived from food waste digestate with exceptional adsorption capacity for arsenic(III) removal via a cooperative mechanism. Sep. Purif. Technol. 2022, 295, 121359. [Google Scholar] [CrossRef]
- Xu, L.; Shu, Z.; Feng, L.; Zhou, J.; Li, T.; Zhao, Z.; Wang, W. Fresh biomass derived biochar with high-load zero-valent iron prepared in one step for efficient arsenic removal. J. Clean. Prod. 2022, 352, 131616. [Google Scholar] [CrossRef]
- de Almeida, S.G.; Tarelho, L.A.; Hauschild, T.; Costa, M.A.M.; Dussan, K.J. Biochar production from sugarcane biomass using slow pyrolysis: Characterization of the solid fraction. Chem. Eng. Process. Process Intensif. 2022, 179, 109054. [Google Scholar] [CrossRef]
- Naydenova, I.; Radoykova, T.; Petrova, T.; Sandov, O.; Valchev, I. Utilization perspectives of lignin biochar from industrial biomass residue. Molecules 2023, 28, 4842. [Google Scholar] [CrossRef]
- Jurczyk, S.; Andrzejewski, J.; Piasecki, A.; Musioł, M.; Rydz, J.; Kowalczuk, M. Mechanical and rheological evaluation of polyester-based composites containing biochar. Polymers 2024, 16, 1231. [Google Scholar] [CrossRef]
- Brinza, M.; Schröder, S.; Ababii, N.; Gronenberg, M.; Strunskus, T.; Pauporte, T.; Adelung, R.; Faupel, F.; Lupan, O. Two-in-one sensor based on PV4D4-coated TiO2 films for food spoilage detection and as a breath marker for several Diseases. Biosensors 2023, 13, 538. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Nishchak, O.Y.; Khaidarov, A.A.; Savchenko, N.F.; Pavlikov, A.V. Low-threshold field emission cathode based on heat-treated dehydrofluorinated polyvinylidene fluoride. J. Exp. Theor. Phys. 2022, 135, 844–852. [Google Scholar] [CrossRef]
- Chen, Q.; Qin, J.; Cheng, Z.; Huang, L.; Sun, P.; Chen, L.; Shen, G. Synthesis of a stable magnesium-impregnated biochar and its reduction of phosphorus leaching from soil. Chemosphere 2018, 199, 402–408. [Google Scholar] [CrossRef]
- Rago, Y.P.; Surroop, D.; Mohee, R. Assessing the potential of biofuel (biochar) production from food wastes through thermal treatment. Bioresour. Technol. 2018, 248, 258–264. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra A Practical Approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons: Newtown, CT, USA, 2006. [Google Scholar]
- Park, J.H.; Lee, J.H.; Lee, S.L.; Hwang, S.W.; Seo, D.C. Adsorption behavior of arsenic onto lignin-based biochar decorated with zinc. Colloid Surf. A 2021, 626, 127095. [Google Scholar] [CrossRef]
- Minaei, S.; Benis, K.Z.; McPhedran, K.N.; Soltan, J. Evaluation of a ZnCl2-modified biochar derived from activated sludge biomass for adsorption of sulfamethoxazole. Chem. Eng. Res. Des. 2023, 190, 407–420. [Google Scholar] [CrossRef]
- Cen, L.; Cheng, H.; Liu, Q.; Wang, S.; Wang, X. Arsenic release from arsenopyrite weathering in acid mine drainage: Kinetics, transformation, and effect of biochar. Environ. Int. 2022, 170, 107558. [Google Scholar] [CrossRef]
- Kang, J.K.; Seo, E.J.; Lee, C.G.; Park, S.J. Fe-loaded biochar obtained from food waste for enhanced phosphate adsorption and its adsorption mechanism study via spectroscopic and experimental approach. J. Environ. Chem. Eng. 2021, 9, 105751. [Google Scholar] [CrossRef]
- Marx, S.; Laubscher, A.N.E.; Bunt, J.R.; Venter, R.J.; Uwaoma, R.C.; Strydom, C.A. Evaluation of sugar cane bagasse hydrothermal liquefaction products for co-gasification with coal as green coal pellet production. Bioresour. Technol. Rep. 2023, 22, 101503. [Google Scholar] [CrossRef]
- Stylianou, M.; Laifi, T.; Bennici, S.; Dutournie, P.; Limousy, L.; Agapiou, A.; Zorpas, A.A. Tomato waste biochar in the framework of circular economy. Sci. Total Environ. 2023, 871, 161959. [Google Scholar] [CrossRef]
- Kaikiti, K.; Stylianou, M.; Agapiou, A. Development of food-origin biochars for the adsorption of selected volatile organic compounds (VOCs) for environmental matrices. Bioresour. Technol. 2021, 342, 125881. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.T.; Wang, X.F.; Xiang, S.; Ding, Y.; Zhao, D.Y.; Hu, M.; Zhao, J. Self-cleaning MnZn ferrite/biochar adsorbents for effective removal of tetracycline. Sci. Total Environ. 2022, 844, 157202. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gan, T.; Zhang, J.; Shi, Z.; Liu, Z.; Xiao, Z. High-capacity removal of oxytetracycline hydrochloride from wastewater via Mikania micrantha Kunth-derived biochar modified by Zn/Fe-layered double hydroxide. Bioresour. Technol. 2022, 361, 127646. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, W.; Zhang, H.; Bai, H.; Liu, K.; Zhang, J.; Zhu, W. Porous biochar derived from walnut shell as an efficient adsorbent for tetracycline removal. Bioresour. Technol. 2023, 383, 129213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, X.; Zhang, J.; Liu, Y.; Zhao, H.; Hu, J.; Zhao, J. Performance and mechanism of Sycamore flock based biochar in removing oxytetracycline hydrochloride. Bioresour. Technol. 2022, 350, 126884. [Google Scholar] [CrossRef]
- Yoon, K.; Cho, D.W.; Kwon, G.; Rinklebe, J.; Wang, H.; Song, H. Practical approach of As(V) adsorption by fabricating biochar with low basicity from FeCl3 and lignin. Chemosphere 2023, 329, 138665. [Google Scholar] [CrossRef]
- Zama, E.F.; Li, G.; Tang, Y.T.; Reid, B.J.; Ngwabie, N.M.; Sun, G.X. The removal of arsenic from solution through biochar-enhanced precipitation of calcium-arsenic derivatives. Environ. Pollut. 2022, 292, 118241. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.L.; Nguyen, N.T.; Chen, C.K.; Le, T.T.; Chen, S.S.; Chen, P.H. Preparation of metal modified onto biochar from hazardous waste for arsenic removal. J. Nanosci. Nanotechnol. 2021, 21, 3227–3236. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Liu, Y.; Zheng, Q.; Sun, G. A predictive risk model of groundwater arsenic contamination in China applied to the Huai River Basin, with a focus on the region’s cluster of elevated cancer mortalities. Appl. Geochem. 2017, 77, 178–183. [Google Scholar] [CrossRef]
- Xia, D.; Tan, F.; Zhang, C.; Jiang, X.; Chen, Z.; Li, H.; Wang, Y. ZnCl2-activated biochar from biogas residue facilitates aqueous As(III) removal. Appl. Surf. Sci. 2016, 377, 361–369. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Liang, Y.; Wu, T.; Chen, Y.; Yan, J.; Zhu, Y.; Ding, D. Adsorptive decontamination of antibiotics from livestock wastewater by using alkaline-modified biochar. Environ. Res. 2023, 226, 115676. [Google Scholar] [CrossRef]
- Norberto, J.; Benis, K.Z.; McPhedran, K.N.; Soltan, J. Microwave activated and iron engineered biochar for arsenic adsorption: Life cycle assessment and cost analysis. J. Environ. Chem. Eng. 2023, 11, 109904. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, X.; Fu, Q.; Hu, H.; Miao, F.; Huang, C.; Zhu, J. Renewable and efficient removal of arsenic from contaminated water by modified biochars derived from As-enriched plant. Bioresour. Technol. 2023, 387, 129680. [Google Scholar] [CrossRef]
- Fan, Y.; Su, J.; Xu, L.; Liu, S.; Hou, C.; Liu, Y.; Cao, S. Removal of oxytetracycline from wastewater by biochar modified with biosynthesized iron oxide nanoparticles and carbon nanotubes: Modification performance and adsorption mechanism. Environ. Res. 2023, 231, 116307. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Q.; Lv, Y.; Fu, H.; Liu, D.; Feng, Y.; Qu, H. Insights into the mechanism of peroxydisulfate activated by magnetic spinel CuFe2O4/SBC as a heterogeneous catalyst for bisphenol S degradation. Chem. Eng. J. 2021, 416, 129162. [Google Scholar] [CrossRef]
- Jin, X.; Li, H.; Zhu, X.; Li, N.; Owens, G.; Chen, Z. Enhanced removal of oxytetracycline from wastewater using bimetallic Fe/Ni nanoparticles combined with ZIF-8 nanocomposites. J. Environ. Manag. 2022, 318, 115526. [Google Scholar] [CrossRef]
- Sharma, A.; Varshney, M.; Shin, H.J.; Lee, B.H.; Chae, K.H.; Won, S.O. Effect of Cu insertion on structural, local electronic/atomic structure and photocatalyst properties of TiO2, ZnO and Ni(OH)2 nanostructures: XANES-EXAFS study. Mater. Chem. Phys. 2014, 191, 129–144. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.; Ge, C.; Müller, K.; Yu, H.; Huang, P.; Wang, H. Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems. Bioresour. Technol. 2018, 263, 385–392. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Wu, Z.; Gao, Y.; Li, X. Adsorption properties and mechanism of sepiolite modified by anionic and cationic surfactants on oxytetracycline from aqueous solutions. Sci. Total Environ. 2020, 708, 134409. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Concerning adsorption in solutions. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Venkatesha, T.G.; Nayaka, Y.A.; Chethana, B.K. Adsorption of Ponceau S from aqueous solution by MgO nanoparticles. Appl. Surf. Sci. 2013, 276, 620–627. [Google Scholar] [CrossRef]
- Mahmood, T.; Aslam, M.; Naeem, A.; Siddique, T.; Din, S.U. Adsorption of As(III) form aqueous solution onto iron impregnated used tea activated carbon: Equilibrium, kinetic and thermodynamic study. J. Chil. Chem. Soc. 2018, 63, 3855–3866. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Guibal, E.; Milot, C.; Tobin, J.M. Metal-anion sorption by chitosan beads: Equilibrium and kinetic studies. Ind. Eng. Chem. Res. 1998, 37, 1454–1463. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Yazdani, M.; Salehi, E.; Zilabi, S.; Nikravesh, G. An insight into the analogy between solute-solvent binding energy and solubility of acid gases in ionic liquids: Thermodynamic modeling versus molecular dynamic simulation. J. Chem. Thermodyn. 2023, 184, 107092. [Google Scholar] [CrossRef]
- Kushwaha, R.; Singh, R.S.; Mohan, D. Comparative study for sorption of arsenic on peanut shell biochar and modified peanut shell biochar. Bioresour. Technol. 2023, 375, 128831. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhattacharya, T.; Vithanage, M. Valorization of waste biomass for biochar production and arsenic removal: A comparative assessment. Groundwater Sust. Dev. 2023, 22, 100972. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Dissociationswärme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte. Z. Phys. Chem. 1889, 4, 96–116. [Google Scholar] [CrossRef]
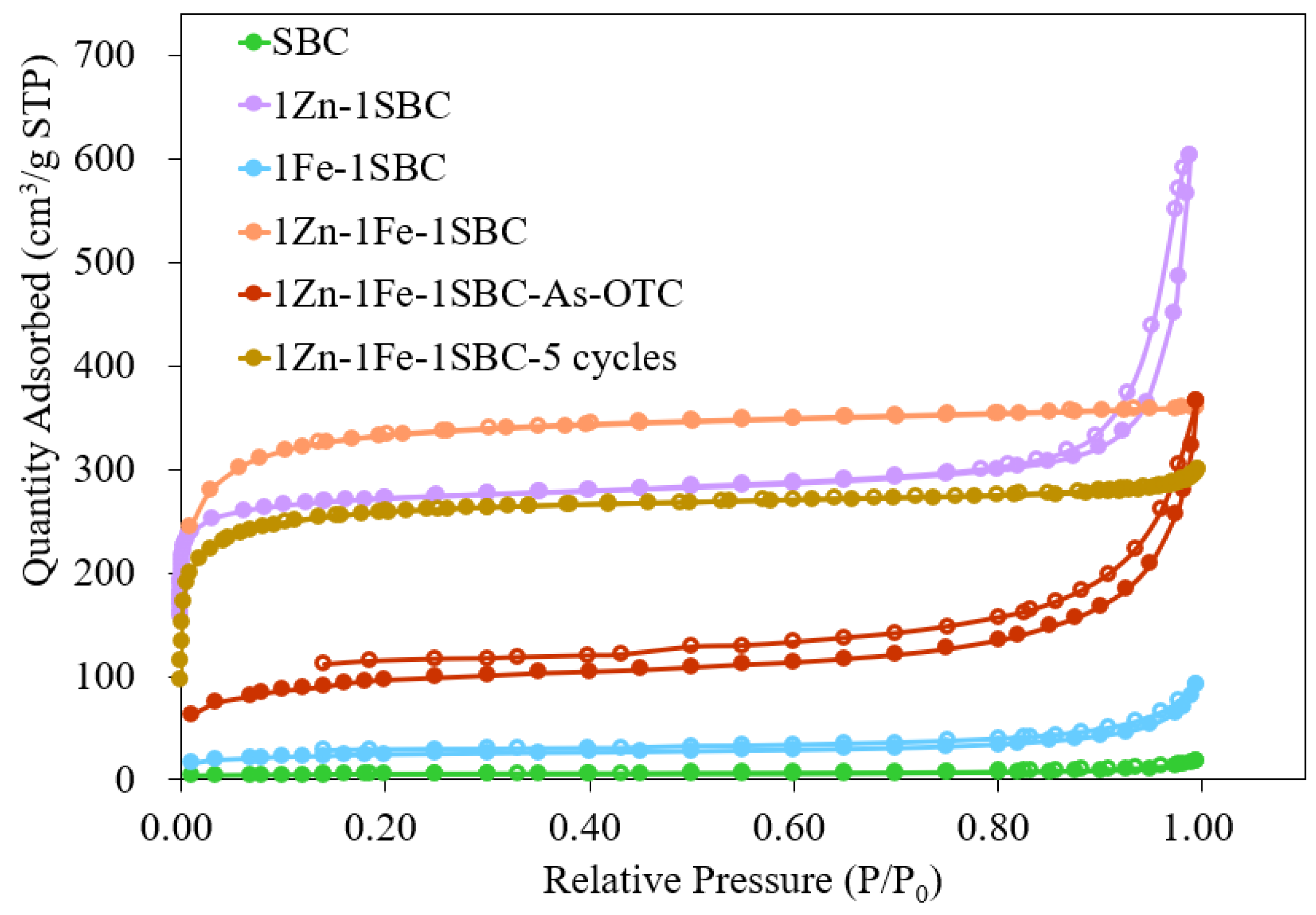
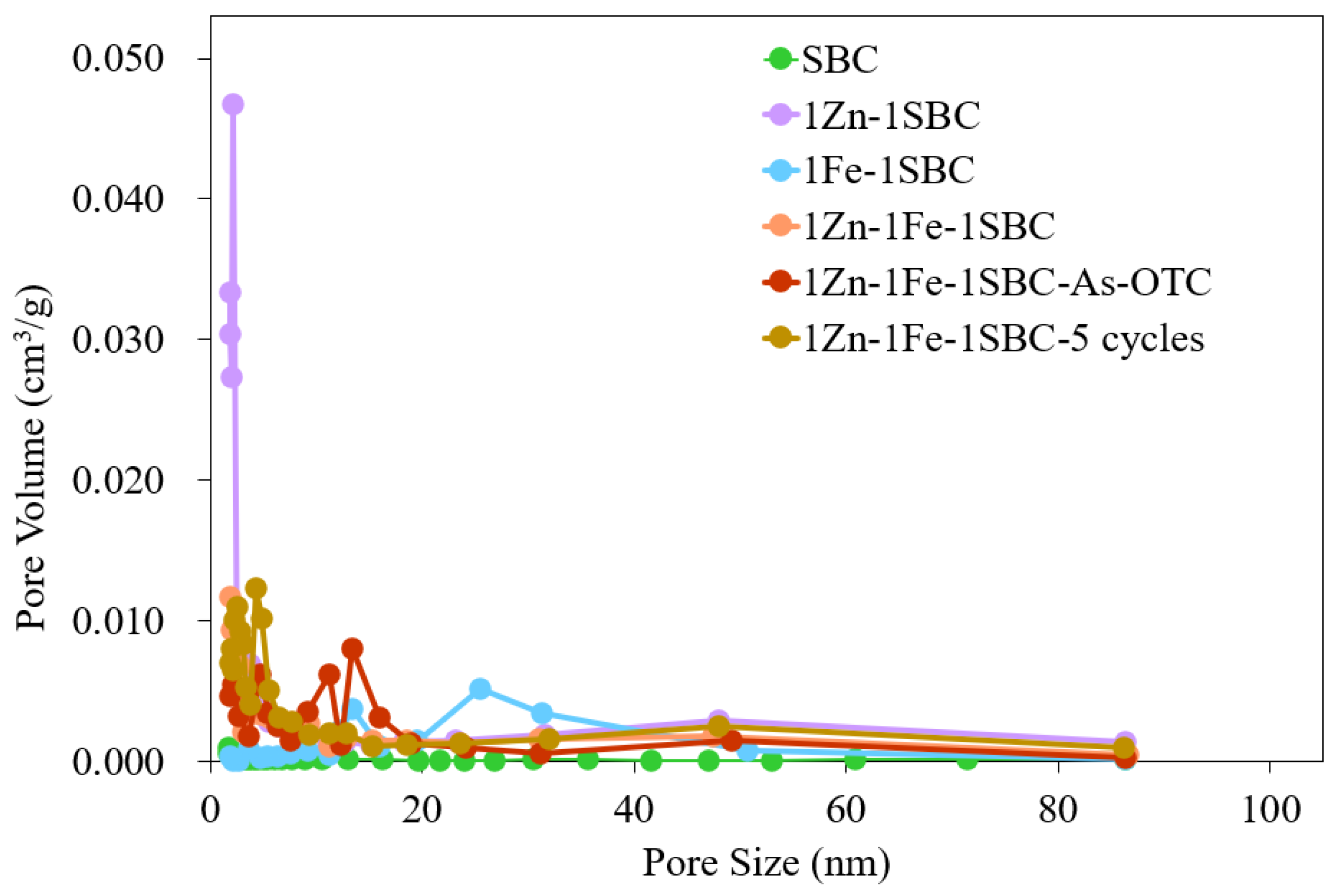


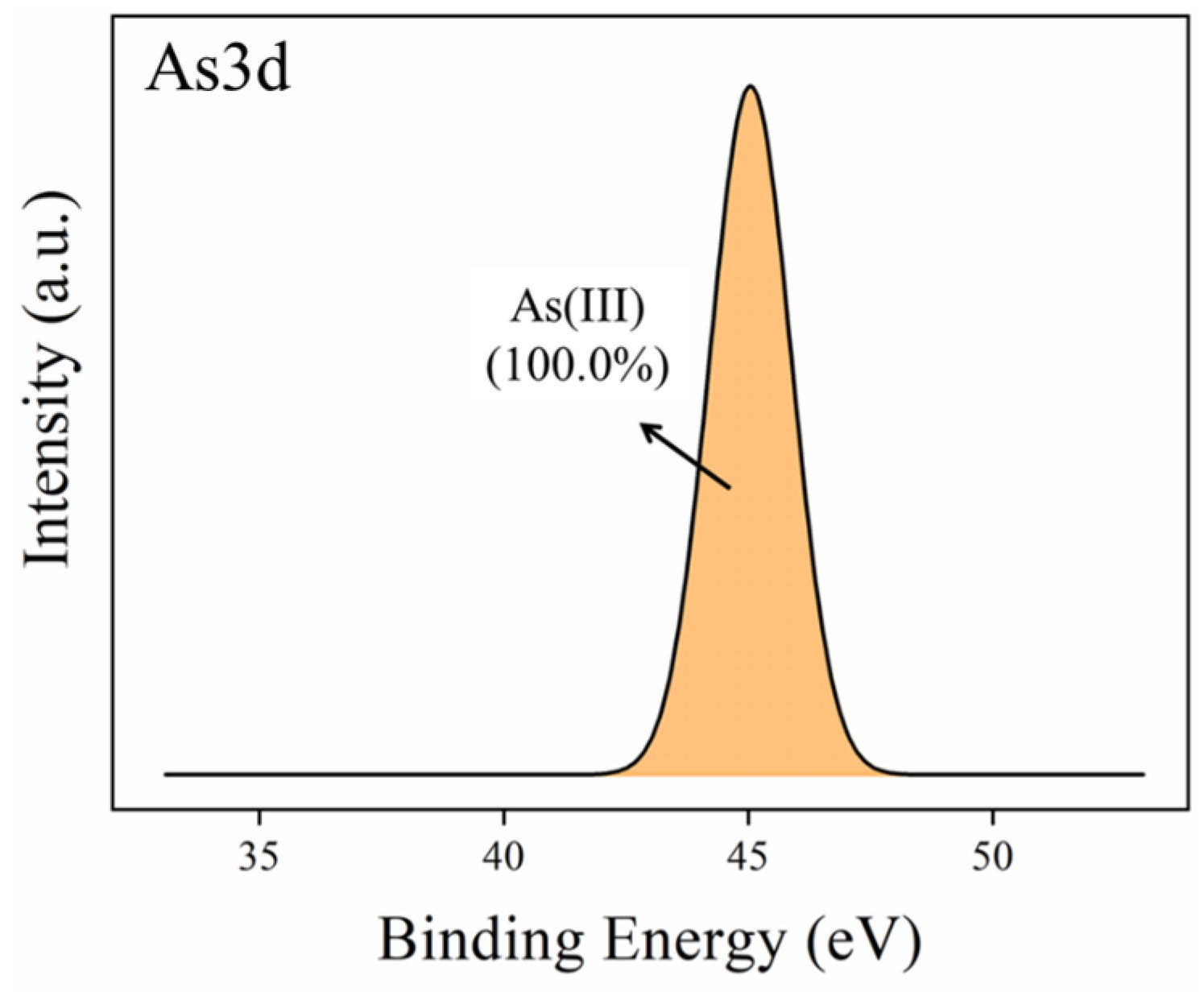
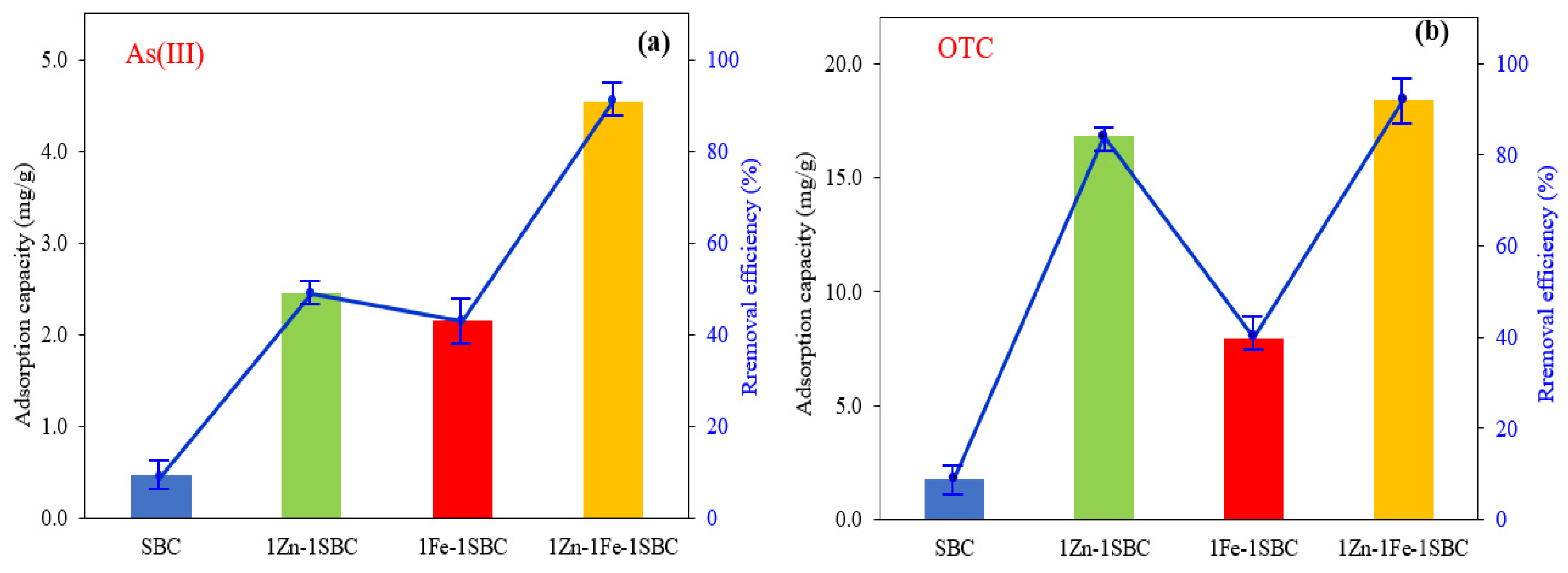


| Materials | Surface Area (m2 g−1) | Average Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| SBC | 16.8 | 0.02 | 4.65 |
| 1Zn-1SBC | 1314.5 | 0.64 | 1.92 |
| 1Fe-1SBC | 97.3 | 0.31 | 3.28 |
| 1Zn-1Fe-1SBC | 1136.8 | 0.55 | 1.98 |
| 1Zn-1Fe-1SBC-As-OTC | 945.7 | 0.43 | 3.05 |
| 1Zn-1Fe-1SBC-5 cycles | 989.5 | 0.45 | 2.31 |
| SCB | SBC | 1Zn-1Fe-1SBC | 1Zn-1Fe-1SBC-As-OTC | |||||
|---|---|---|---|---|---|---|---|---|
| W (%) | A (%) | W (%) | A (%) | W (%) | A (%) | W (%) | A (%) | |
| C (K) | 55.84 | 62.78 | 86.75 | 89.97 | 80.37 | 86.57 | 65.08 | 78.30 |
| O (K) | 44.04 | 37.14 | 12.38 | 9.74 | 15.17 | 12.26 | 19.01 | 17.17 |
| Si (K) | 0.12 | 0.08 | 0.87 | 0.39 | 0.26 | 0.12 | 1.52 | 0.78 |
| Zn (L) | - | - | - | - | 0.09 | 0.02 | 0.45 | 0.10 |
| Fe (K) | - | - | - | - | 4.11 | 1.03 | 13.86 | 3.63 |
| As (L) | - | - | - | - | - | - | 0.08 | 0.02 |
| N (K) | - | - | - | - | - | - | 0.40 | 0.01 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Model | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| KL (L mg−1) | Qm (mg g−1) | R2 | KF (L g−1) | n | R2 | KT (kJ mol−1) | AT (m3 mole−1) | R2 | |
| 1Zn-1Fe- 1SBC-As | 0.15 | 34.72 | 0.999 | 4.22 | 1.20 | 0.996 | 610.06 | 4.08 | 0.929 |
| 1Zn-1Fe- 1SBC-OTC | 0.03 | 172.41 | 0.999 | 5.26 | 1.16 | 0.997 | 141.92 | 0.99 | 0.926 |
| Model | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|
| k1 | qe | R2 | k2 | qe | R2 | ||
| 1Zn-1Fe- 1SBC-AS | 1 ppm | 0.125 | 1.151 | 0.994 | 0.317 | 1.276 | 0.980 |
| 2 ppm | 0.083 | 2.154 | 0.986 | 0.135 | 2.423 | 0.968 | |
| 4 ppm | 0.061 | 3.626 | 0.948 | 0.090 | 4.114 | 0.976 | |
| 8 ppm | 0.051 | 5.577 | 0.886 | 0.078 | 6.325 | 0.986 | |
| 16 ppm | 0.043 | 9.442 | 0.845 | 0.054 | 10.132 | 0.989 | |
| 1Zn-1Fe- 1SBC-OTC | 1 ppm | 0.210 | 4.65 | 0.992 | 0.154 | 5.13 | 0.994 |
| 2 ppm | 0.142 | 8.75 | 0.973 | 0.059 | 10.1 | 0.990 | |
| 4 ppm | 0.078 | 13.1 | 0.936 | 0.033 | 17.5 | 0.989 | |
| 8 ppm | 0.054 | 23.3 | 0.894 | 0.019 | 27.5 | 0.987 | |
| 16 ppm | 0.067 | 45.2 | 0.973 | 0.007 | 50.5 | 0.970 | |
| Model | First Stage | Second Stage | Third Stage | |||
|---|---|---|---|---|---|---|
| K1 (mg/g min0.5) | R2 | K2 (mg/g min0.5) | R2 | K3 (mg/g min0.5) | R2 | |
| 1Zn-1Fe- 1SBC-AS | 0.922 | 0.997 | 0.411 | 0.993 | 0.116 | 0.997 |
| 1Zn-1Fe- 1SBC-OTC | 3.012 | 0.998 | 1.390 | 0.999 | 0.246 | 0.996 |
| Material | Temperature (K) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) |
|---|---|---|---|---|
| 1Zn-1Fe- 1SBC-As | 288 | −7.026 | 82.60 | 309.61 |
| 298 | −9.167 | |||
| 308 | −12.26 | |||
| 318 | −16.40 | |||
| 1Zn-1Fe- 1SBC-OTC | 288 | −10.49 | 84.45 | 329.60 |
| 298 | −13.47 | |||
| 308 | −17.64 | |||
| 318 | −20.07 |
| Material | Temperature (K) | k1 | Ea (kJ mol−1) | A (s−1) | R2 |
|---|---|---|---|---|---|
| 1Zn-1Fe- 1SBC-As | 288 | 0.020 | 21.82 | 32.92 | 0.954 |
| 298 | 0.024 | ||||
| 308 | 0.029 | ||||
| 318 | 0.041 | ||||
| 1Zn-1Fe- 1SBC-OTC | 288 | 0.021 | 26.07 | 754.3 | 0.985 |
| 298 | 0.027 | ||||
| 308 | 0.042 | ||||
| 318 | 0.052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.-T.; Lin, A.-B.; Chang, C.-T.; Hong, G.-B. Bimetallic Zinc-Iron-Modified Sugarcane Bagasse Biochar for Simultaneous Adsorption of Arsenic and Oxytetracycline from Wastewater. Molecules 2025, 30, 572. https://doi.org/10.3390/molecules30030572
Nguyen N-T, Lin A-B, Chang C-T, Hong G-B. Bimetallic Zinc-Iron-Modified Sugarcane Bagasse Biochar for Simultaneous Adsorption of Arsenic and Oxytetracycline from Wastewater. Molecules. 2025; 30(3):572. https://doi.org/10.3390/molecules30030572
Chicago/Turabian StyleNguyen, Nhat-Thien, An-Bang Lin, Chang-Tang Chang, and Gui-Bing Hong. 2025. "Bimetallic Zinc-Iron-Modified Sugarcane Bagasse Biochar for Simultaneous Adsorption of Arsenic and Oxytetracycline from Wastewater" Molecules 30, no. 3: 572. https://doi.org/10.3390/molecules30030572
APA StyleNguyen, N.-T., Lin, A.-B., Chang, C.-T., & Hong, G.-B. (2025). Bimetallic Zinc-Iron-Modified Sugarcane Bagasse Biochar for Simultaneous Adsorption of Arsenic and Oxytetracycline from Wastewater. Molecules, 30(3), 572. https://doi.org/10.3390/molecules30030572






