In Situ Formation of WC/W2C Heterostructures on N-Doped Carbon for Deep Oxidative Desulfurization of Fuel Oil
Abstract
:1. Introduction
2. Results
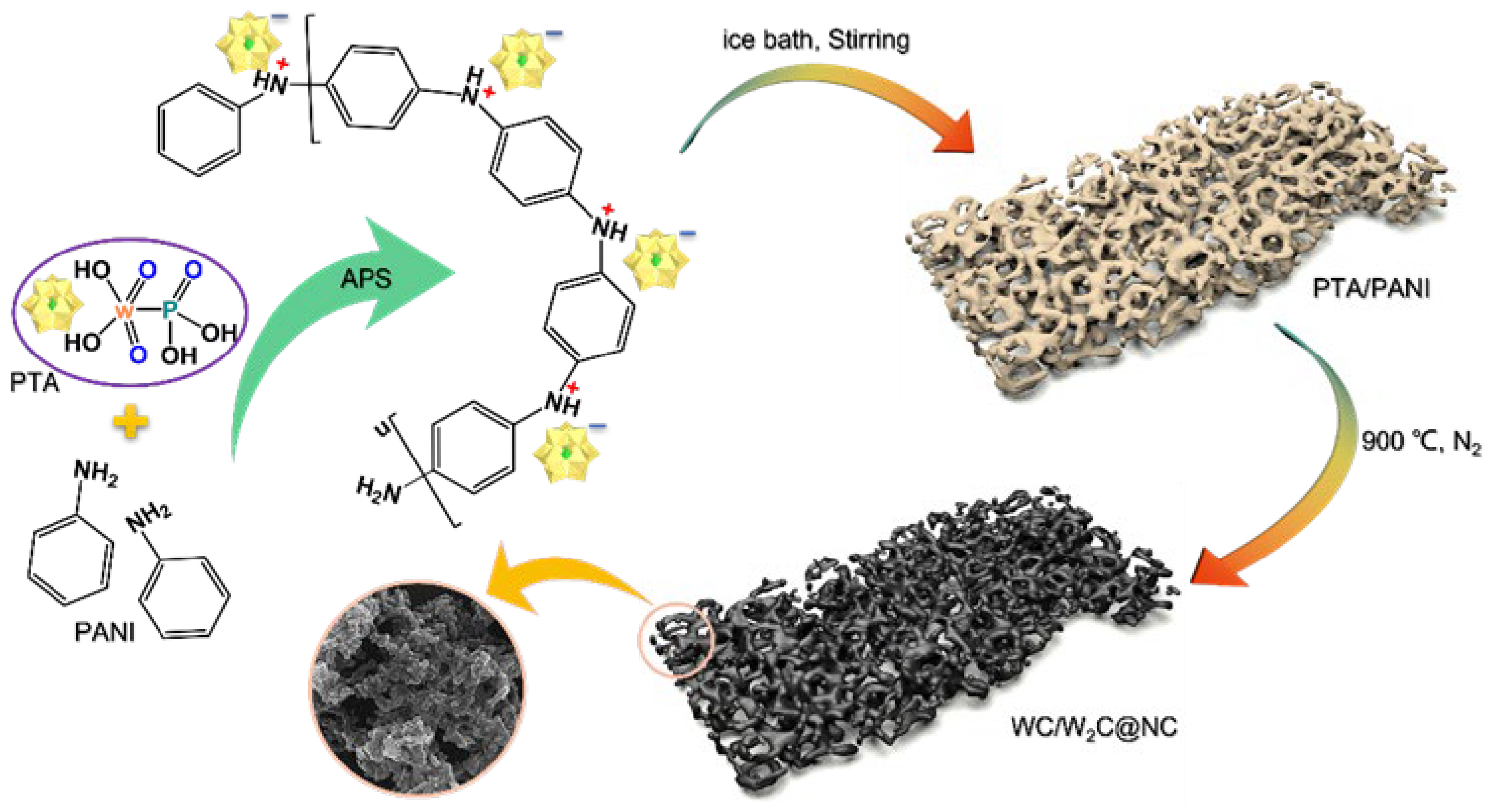
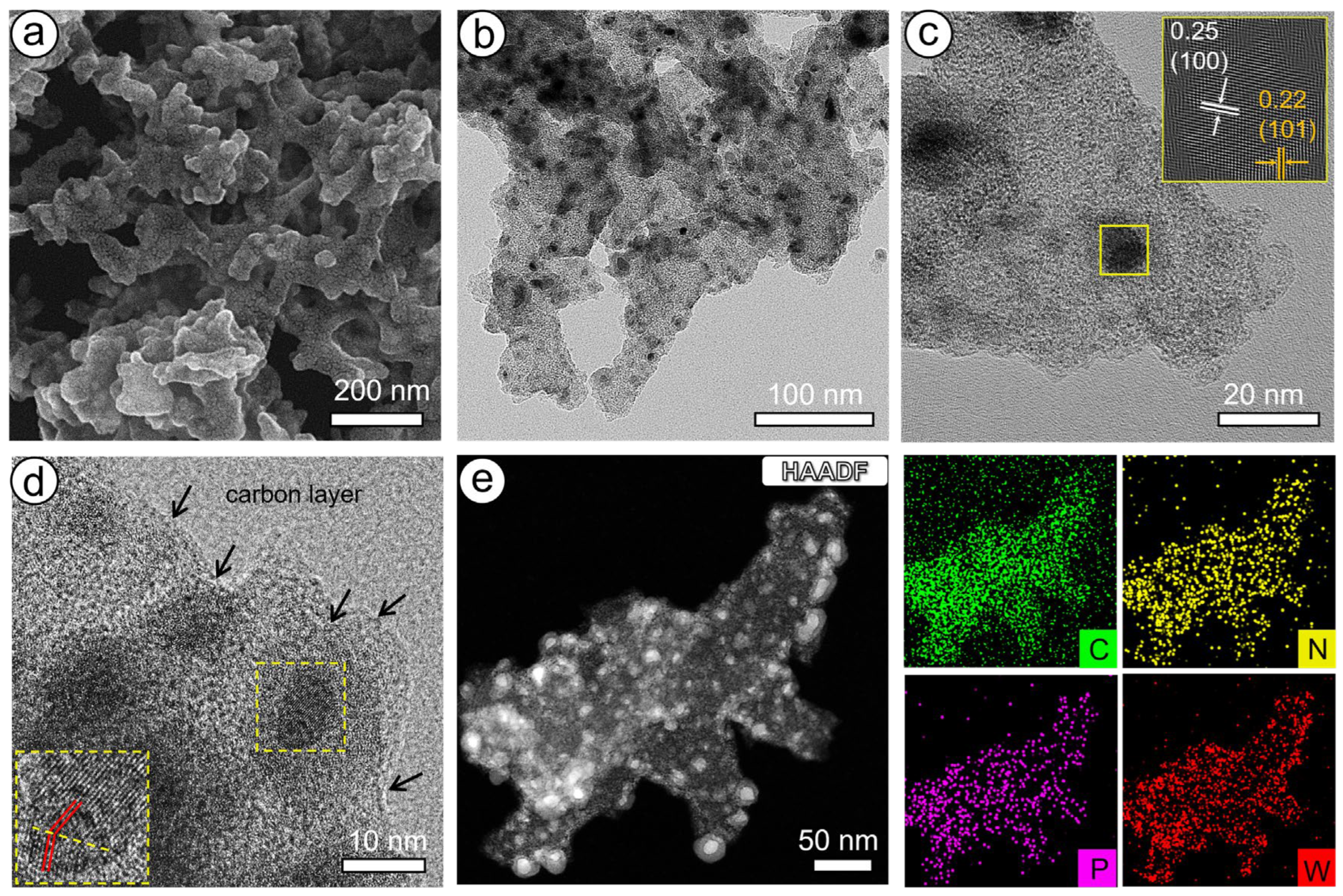
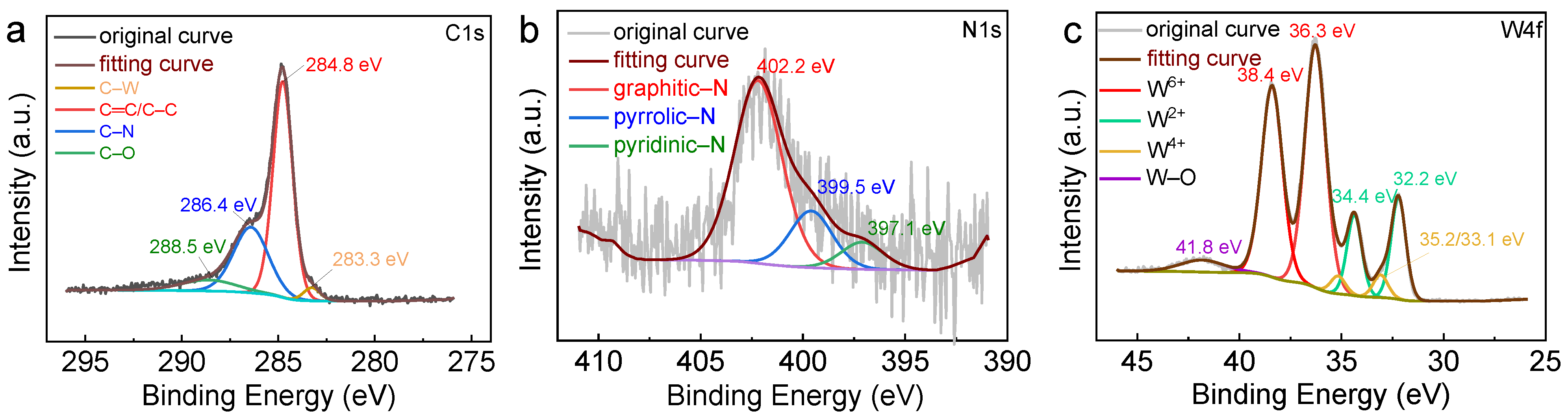
2.1. Synthesis and Characterization of Catalysts
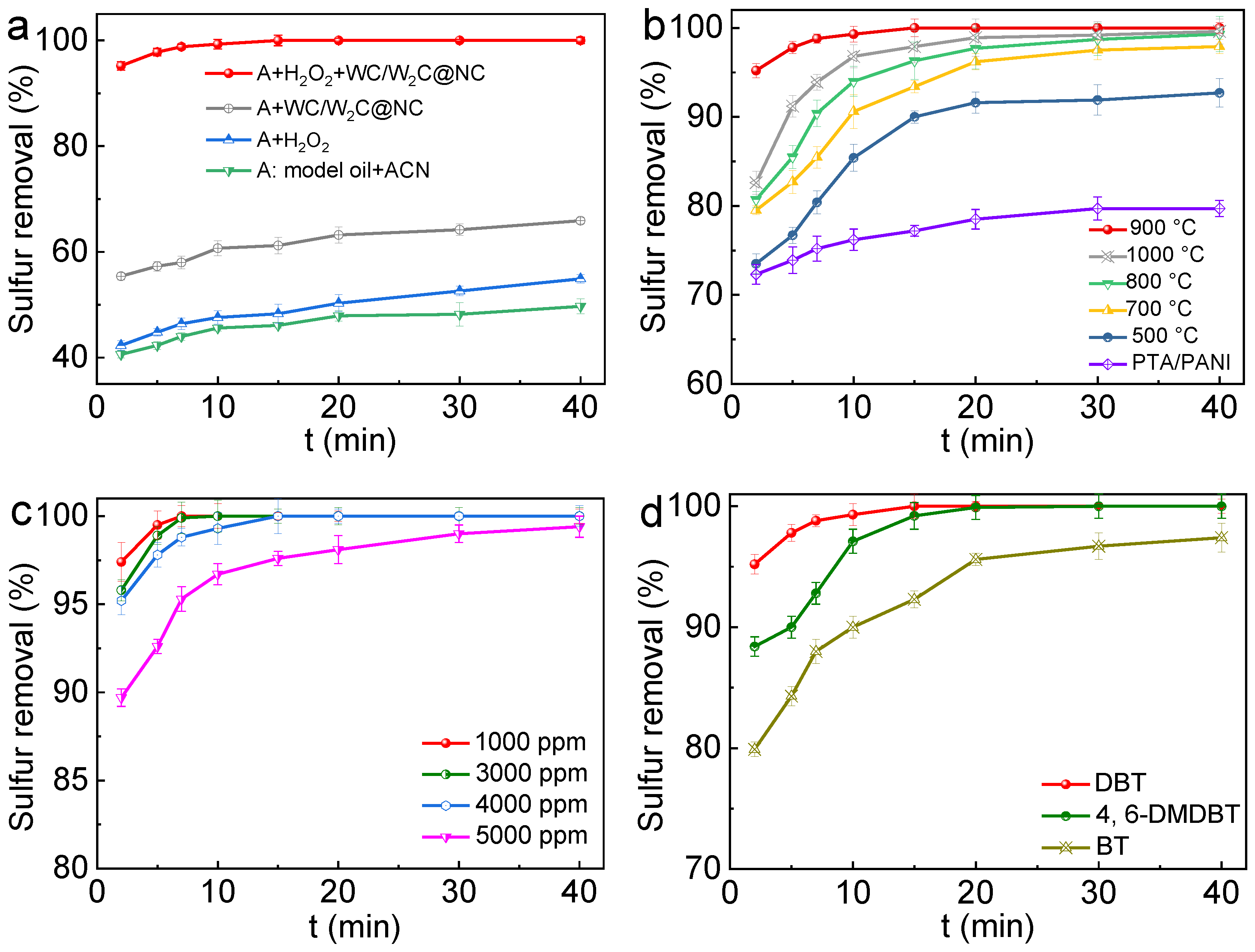
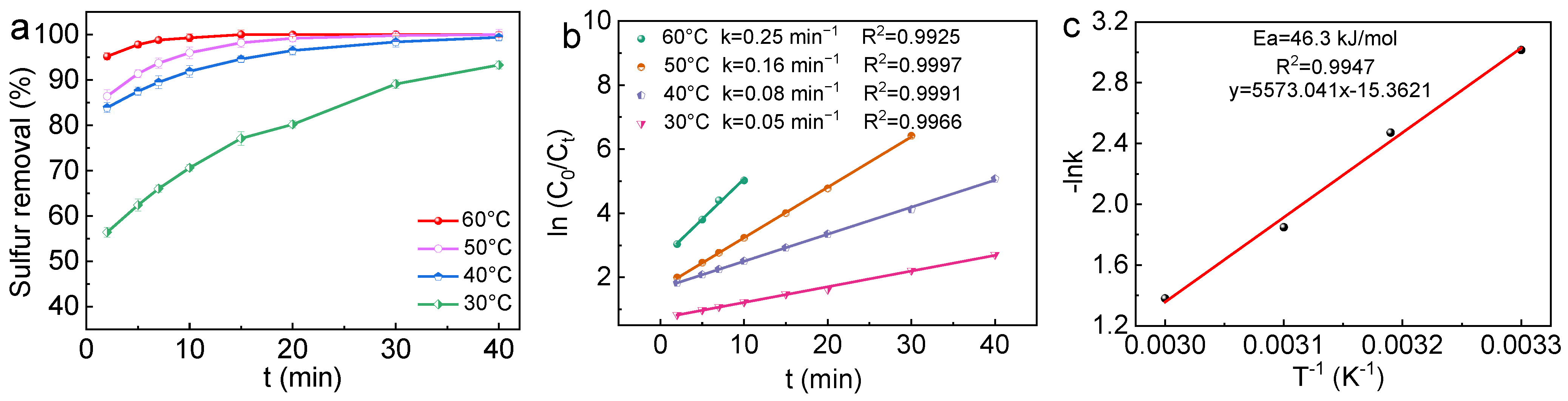
2.2. Evaluation of ODS Efficiencies of Catalysts
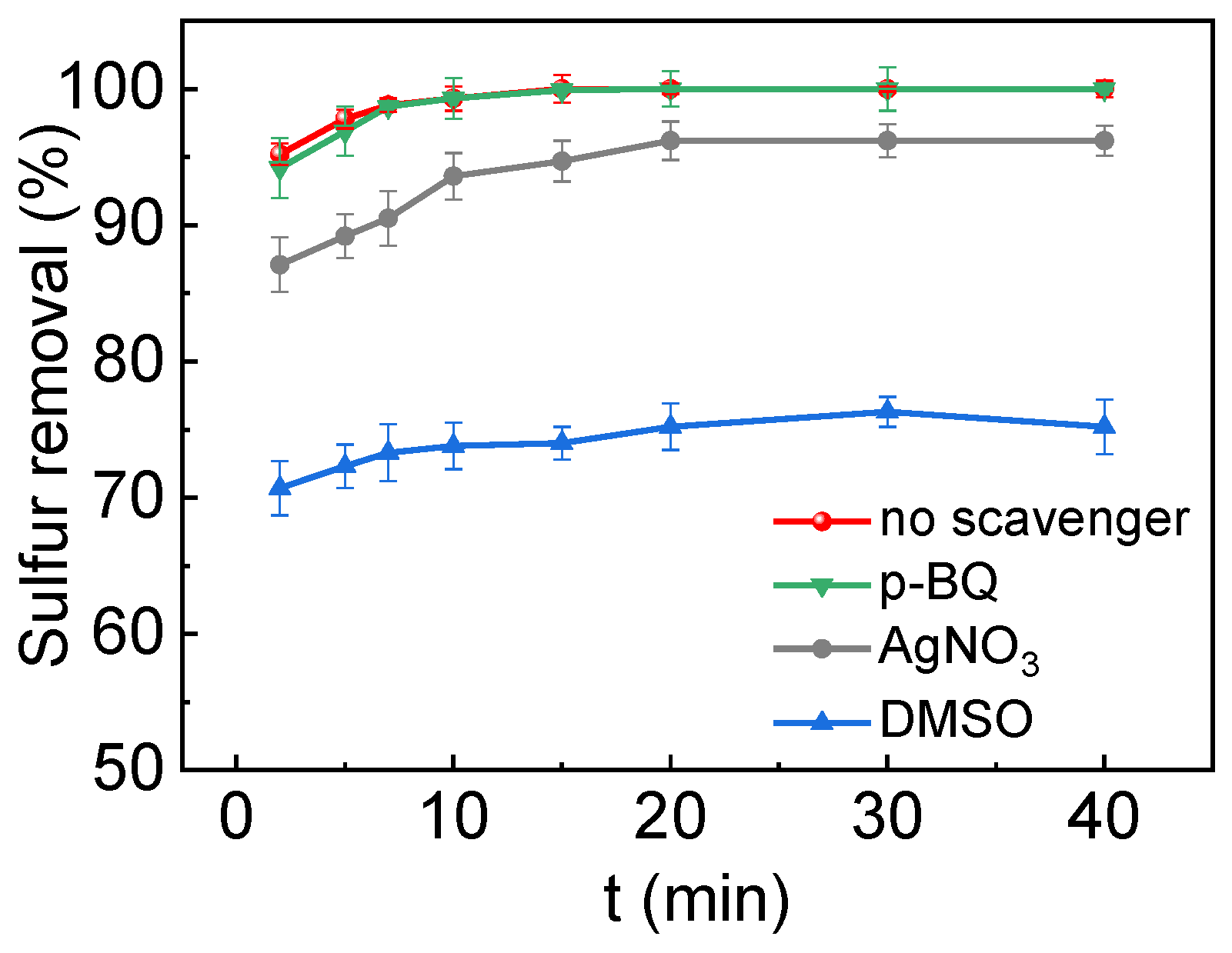
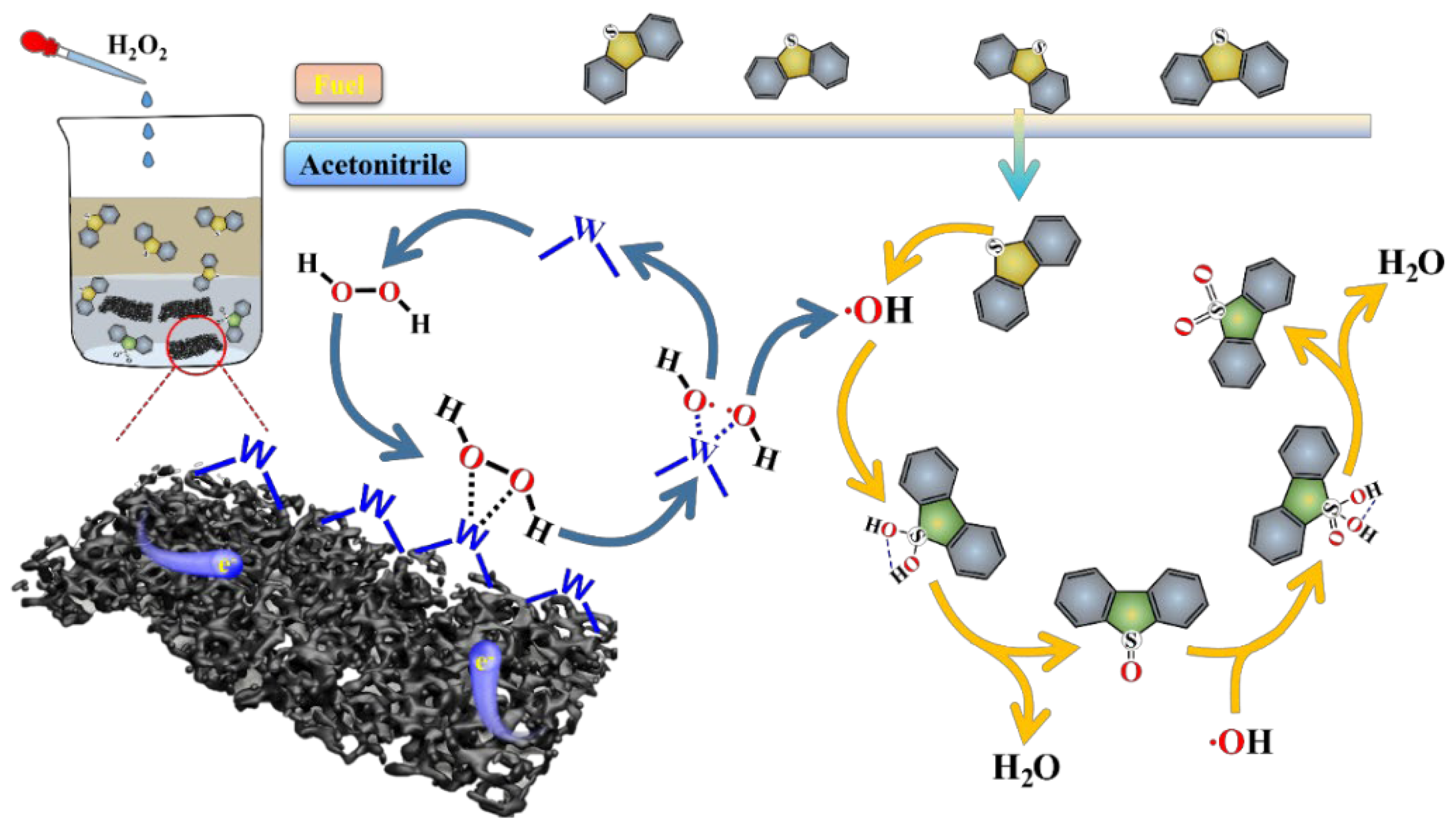
2.3. ODS Mechanism
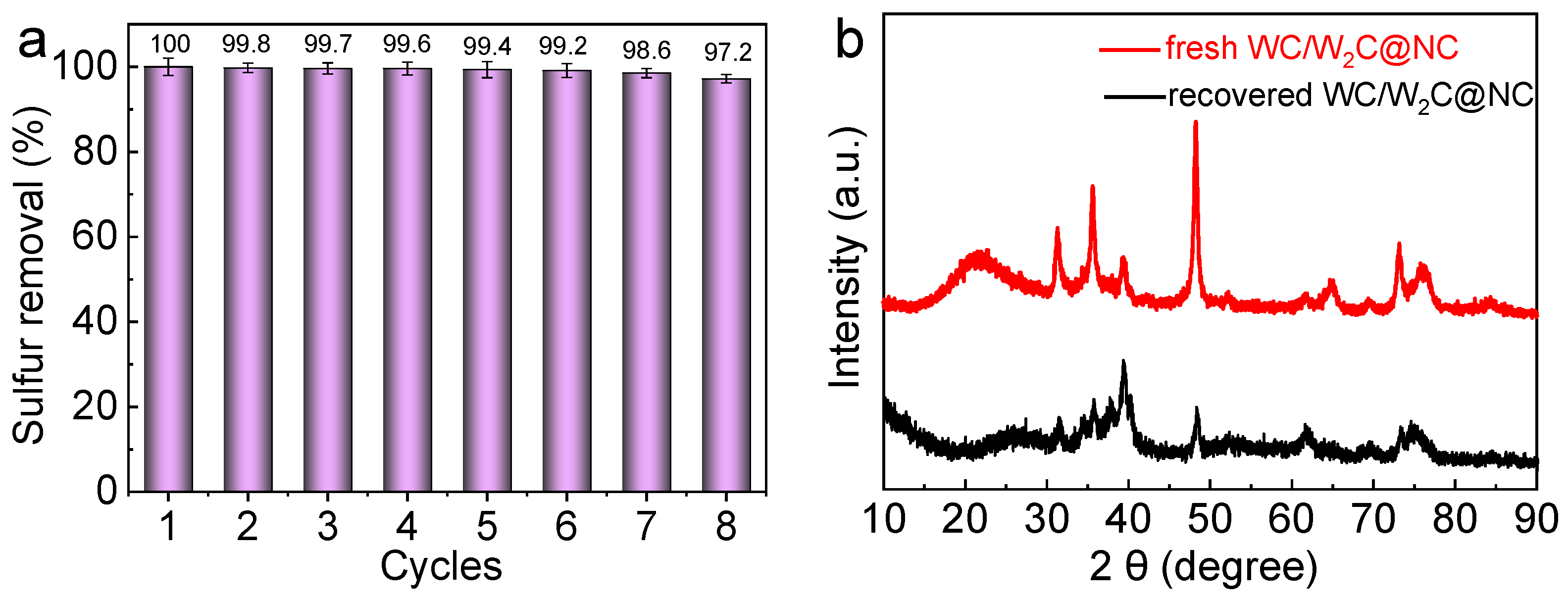
2.4. Recyclability and Reusability of Catalyst
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Evaluation of the Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajendran, A.; Cui, T.Y.; Fan, H.X.; Yang, Z.F.; Feng, J.; Li, W.Y. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Ye, G.; Zheng, M.; Zhang, Q.; Zhou, J.; Wu, L.; Wang, J. Defect-Mediated Synergistic Effect of POM/UiO-66(Zr) Host–Guest Catalysts for Robust Deep Desulfurization at Ambient Temperature. Small 2023, 19, 2301035. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.C.; Leo, P.; Santos-Vieira, I.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Improving Oxidative Catalytic Efficiency for Fuels Desulfurization Using Hybrid Materials Based in MOF-808@SBA-15. ChemCatChem 2024, 16, e202400355. [Google Scholar] [CrossRef]
- Bai, Y.; Xin, Y.; Liu, J.; Ma, L.; Li, G. Construction of H6PW9V3O40@rht-MOF-1 for deep oxidative desulfurization of fuel oil. Appl. Organomet. Chem. 2022, 36, e6633. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Yin, J.; Liu, X.; Qiu, W.; He, J.; Jiang, W.; Zhu, L.; Li, H.; Li, H. Mo-MOF-based ionanofluids for highly efficient extraction coupled catalytic oxidative desulfurization. Sep. Purif. Technol. 2025, 353, 128289. [Google Scholar] [CrossRef]
- Su, M.; Yang, L. Fe/Mn-MOFs with monocarboxylic acid-induced defects enhances the catalytic oxidation of calcium sulfite in desulfurization ash. Sep. Purif. Technol. 2025, 353, 128300. [Google Scholar] [CrossRef]
- Li, Y.X.; Shen, J.X.; Peng, S.S.; Zhang, J.K.; Wu, J.; Liu, X.Q.; Sun, L.B. Enhancing oxidation resistance of Cu(I) by tailoring microenvironment in zeolites for efficient adsorptive desulfurization. Nat. Commun. 2020, 11, 3206. [Google Scholar] [CrossRef]
- Dai, P.; Guo, M.; Ge, H.; Fan, C.; Li, X.; Liu, X.; Feng, J.; Li, R.; Tang, M. Constructing Ni/ZnMOx composite metal oxide adsorbents with excellent desulfurization capacity and thermal stability for reactive adsorption desulfurization. Fuel 2024, 372, 132201. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, Z.; Cheng, Z.L. Diatomite supported highly-dispersed ZnO/Zn-co-embedded ZIF-8 derived porous carbon composites for adsorption desulfurization. J. Hazard. Mater. 2024, 471, 134399. [Google Scholar] [CrossRef]
- Li, C.; Li, D.; Zou, S.; Li, Z.; Yin, J.; Wang, A.; Cui, Y.; Yao, Z.; Zhao, Q. Extraction desulfurization process of fuels with ammonium-based deep eutectic solvents. Green Chem. 2013, 15, 2793–2799. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, T.; Zhang, S.L.; Fan, Y.C.; Chen, B. Extractive desulfurization of fuels using trialkylamine-based protic ionic liquids. Sep. Purif. Technol. 2020, 231, 115923. [Google Scholar] [CrossRef]
- Kasiri Baboukani, Z.; Najafi Chermahini, A.; Farrokhpour, H. Photocatalytic oxidative desulfurization of a model fuel using S-doped TiO2/BiVO4 composites: A combination of experimental and theoretical study. J. Alloys Compd. 2024, 1002, 175478. [Google Scholar] [CrossRef]
- Xie, S.; Wang, D.; Deng, C.; Yang, L.; Bai, L.; Wei, D.; Yin, K.; Yang, H.; Xia, Z.; Chen, H. Enhanced photocatalytic aerobic oxidative desulfurization of diesel over FeMo6Ox nanoclusters decorated on CuS nanosheets. Appl. Catal. B-Environ. Energy 2024, 357, 124282. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, C.; Liu, Q.; Zang, M.; Liu, C.; Zhang, Y. Thermophilic biodesulfurization and its application in oil desulfurization. Appl. Microbiol. Biotechnol. 2018, 102, 9089–9103. [Google Scholar] [CrossRef] [PubMed]
- Alkhalili, B.E.; Yahya, A.; Abrahim, N.; Ganapathy, B. Biodesulfurization of Sour Crude Oil. Mod. Appl. Sci. 2017, 11, 104. [Google Scholar] [CrossRef]
- Xu, L.; Huang, H.; Xu, L.; Zhu, D.; Zhang, B.; He, J.; Li, H.; Jiang, W. Encapsulation of cobalt molybdate on modified hierarchical porous TS-1 zeolite catalyst for efficient oxidative desulfurization of fuel. Sep. Purif. Technol. 2025, 354, 129385. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, L.; Li, X.; Qiu, L.; Jiao, W.; Wang, R. In-situ fabrication of MoO2/MoxC heterojunction on rodlike carbon-coated zirconia for enhanced oxidative desulfurization of fuel oils. Sep. Purif. Technol. 2024, 349, 127726. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, P.; Xie, Z.; Jiao, W.; Wang, R. Magnetically lychee-like molybdenum carbide-supported N-doped carbon for the highly effective oxidative desulfurization of fuel oil. Fuel 2024, 375, 132585. [Google Scholar] [CrossRef]
- Zhao, S.N.; Lv, Y.; Li, X.; Jiao, W.Z.; Wang, R.X. Amorphous MoOx loaded on N-doped carbon-modified SiO2 for strongly enhanced oxidative desulfurization activity. Sep. Purif. Technol. 2024, 336, 126297. [Google Scholar] [CrossRef]
- Guo, J.; Li, B.; Zhao, D.; Chu, L.; Yang, H.; Huang, Z.; Liu, Z.; Yang, M.; Wang, G. Preparation of porous hollow spherical MoOX/C catalyst for efficient extraction and oxidative desulfurization. Chem. Eng. J. 2023, 474, 145853. [Google Scholar] [CrossRef]
- Bo, S.; Lin, H.; Zhang, J.; Liao, W.; Yang, K.; Su, T.; Lü, H.; Zhu, Z. In situ encapsulation of ultrasmall MoO3 nanoparticles into beta zeolite for oxidative desulfurization. Green Chem. 2024, 26, 2661–2672. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Jhung, S.H. Oxidative desulfurization of liquid fuel with tungsten-nitride@porous carbon, derived from MAF-6(Zn) loaded with phosphotungstic acid and melamine. Chem. Eng. J. 2021, 419, 129485. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zuo, P.; Wang, R.X.; Jiao, W.Z. Ultra-deep oxidative desulfurization of high sulfur liquid fuels with molybdenum carbide@carbon loaded on N-doped graphene. Fuel 2022, 324, 124534. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.F.; Lv, Y.; Ren, J.; Wang, R.X.; Jiao, W.Z. Oxidative desulfurization of liquid fuels catalyzed by W2C@C derived from metallophthalocyanine/phosphotungstic acid composites. Sep. Purif. Technol. 2022, 281, 119953. [Google Scholar] [CrossRef]
- Chen, W.F.; Muckerman, J.T.; Fujita, E. Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem. Commun. 2013, 49, 8896–8909. [Google Scholar] [CrossRef]
- Levy, R.B.; Boudart, M. Platinum-like behavior of tungsten carbide in surface catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef]
- Bi, M.L.; Guo, Y.; Wang, S.Y.; Zhang, B.X.; Jiang, Y.N.; Li, H.N.; Xu, Q.X.; Chen, L.D.; Zhao, Q.; Han, N. Titanium dioxide-modified nanosized TS-1 zeolite-supported phosphotungstic acid as a catalyst for deep catalytic oxidative desulfurization of fuel oil. New J. Chem. 2023, 47, 12027–12036. [Google Scholar] [CrossRef]
- Subhan, S.; Ur Rahman, A.; Yaseen, M.; Ur Rashid, H.; Ishaq, M.; Sahibzada, M.; Tong, Z. Ultra-fast and highly efficient catalytic oxidative desulfurization of dibenzothiophene at ambient temperature over low Mn loaded Co-Mo/Al2O3 and Ni-Mo/Al2O3 catalysts using NaClO as oxidant. Fuel 2019, 237, 793–805. [Google Scholar] [CrossRef]
- Lv, Y.; Jiao, J.; Wang, R.X.; Jiao, W.Z. Silicotungstic acid-supported C@SiO2 nanospheres as an efficient oxidative desulfurization catalyst. Chem. Eng. Sci. 2022, 248, 117225. [Google Scholar] [CrossRef]
- Mondol, M.M.H.; Kim, C.U.; Kim, C.M.; Jhung, S.H. Zirconium oxide@N-doped porous carbons with oxygen vacancies, derived from zirconium chloride embedded metal-azolate framework-6: A new oxidative desulfurization catalyst. Sep. Purif. Technol. 2023, 318, 123984. [Google Scholar] [CrossRef]
- Du, S.; Chen, H.-M.; Shen, H.-X.; Chen, J.; Li, C.-P.; Du, M. Hierarchically Nanoporous TS-1 Zeolites for Catalytic Oxidation Desulfurization of Liquid Fuels. ACS Appl. Nano Mater. 2020, 3, 9393–9400. [Google Scholar] [CrossRef]
- Evtushok, V.Y.; Podyacheva, O.Y.; Suboch, A.N.; Maksimchuk, N.V.; Stonkus, O.A.; Kibis, L.S.; Kholdeeva, O.A. H2O2-based selective oxidations by divanadium-substituted polyoxotungstate supported on nitrogen-doped carbon nanomaterials. Catal. Today 2020, 354, 196–203. [Google Scholar] [CrossRef]
- Cai, X.; Wang, Q.; Liu, Y.; Xie, J.; Long, Z.; Zhou, Y.; Wang, J. Hybrid of Polyoxometalate-Based Ionic Salt and N-Doped Carbon toward Reductant-Free Aerobic Hydroxylation of Benzene to Phenol. ACS Sustain. Chem. Eng. 2016, 4, 4986–4996. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Jiao, J.; Jiao, W.Z.; Wang, R.X. Oxidative desulfurization of model oil over the bowl-shaped N-doped carbon material loaded by the defective silicotungstic acid. Sep. Purif. Technol. 2023, 310, 123180. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zuo, P.; Yin, X.J.; Li, C.; Zhao, F.Y.; Shi, H.X.; Li, J.; Han, Y.; Wang, N.N.; Bai, X.X.; et al. Ultra-deep oxidative desulfurization of high sulfur liquid fuels using W/W2C heterostructured nanoparticles embedded on N-doped graphene. J. Environ. Chem. Eng. 2024, 12, 112173. [Google Scholar] [CrossRef]
- Chen, Z.; Gong, W.; Cong, S.; Wang, Z.; Song, G.; Pan, T.; Tang, X.; Chen, J.; Lu, W.; Zhao, Z. Eutectoid-structured WC/W2C heterostructures: A new platform for long-term alkaline hydrogen evolution reaction at low overpotentials. Nano Energy 2020, 68, 104335. [Google Scholar] [CrossRef]
- Wang, R.X.; Liu, Y.F.; Zuo, P.; Zhang, Z.D.; Lei, N.N.; Liu, Y.Q. Phthalocyanine-sensitized evolution of hydrogen and degradation of organic pollutants using polyoxometalate photocatalysts. Environ. Sci. Pollut. Res. 2020, 27, 18831–18842. [Google Scholar] [CrossRef]
- Li, L.X.; Liu, B.Y.; Wu, Z.W.; Yuan, X.; Luo, H.A. Preparation of Keggin-type mono-lacunary phosphotungstic-ammonium salt and its catalytic performance in ammoximation of cyclohexanone. Chem. Eng. J. 2015, 280, 670–676. [Google Scholar] [CrossRef]
- Jiao, J.; Zhou, X.Y.; Zhao, S.N.; Jiao, W.Z.; Wang, R.X. In situ highly dispersed loading of molybdenum dioxide with oxygen vacancies on N-doped graphene for enhanced oxidative desulfurization of fuel oil. J. Environ. Chem. Eng. 2023, 11, 109402. [Google Scholar] [CrossRef]
- Chen, M.; Wang, G.C.; Shao, L.L.; Yuan, Z.Y.; Qian, X.; Jing, Q.S.; Huang, Z.Y.; Xu, D.L.; Yang, S.X. Strategic Design of Vacancy-Enriched Fe1–xS Nanoparticles Anchored on Fe3C-Encapsulated and N-Doped Carbon Nanotube Hybrids for High-Efficiency Triiodide Reduction in Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 31208–31224. [Google Scholar] [CrossRef]
- Zhang, L.N.; Ma, Y.Y.; Lang, Z.L.; Wang, Y.H.; Khan, S.U.; Yan, G.; Tan, H.Q.; Zang, H.Y.; Li, Y.G. Ultrafine cable-like WC/W2C heterojunction nanowires covered by graphitic carbon towards highly efficient electrocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 15395–15403. [Google Scholar] [CrossRef]
- Liu, Y.F.; Yin, X.J.; Li, C.; Xie, Z.; Zhao, F.Y.; Li, J.; Hei, J.P.; Han, Y.; Wang, N.N.; Zuo, P. Defective silicotungstic acid-loaded magnetic floral N-doped carbon microspheres for ultra-fast oxidative desulfurization of high sulfur liquid fuels. Dalton Trans. 2023, 52, 17524–17537. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.D.; Uslamin, E.A.; Kosinov, N.; Hensen, E.J.M. Tuning the reactivity of molybdenum (oxy)carbide catalysts by the carburization degree: CO2 reduction and anisole hydrodeoxygenation. Catal. Sci. Technol. 2020, 10, 3635–3645. [Google Scholar] [CrossRef]
- Yan, G.; Wu, C.X.; Tan, H.Q.; Feng, X.J.; Yan, L.K.; Zang, H.Y.; Li, Y.G. N-Carbon coated P-W2C composite as efficient electrocatalyst for hydrogen evolution reactions over the whole pH range. J. Mater. Chem. A 2017, 5, 765–772. [Google Scholar] [CrossRef]
- Yang, J.B.; Wang, J.; Zhu, Y.H.; Yan, P.F.; Dong, Z.M.; Mei, H.; Xu, Y. Highly efficient C16-PW9/SiO2 designed based on trivacant tungstophosphate ([A-PW9O34]9−) for rapid oxidative desulfurization under mild conditions. Chem. Eng. Sci. 2024, 298, 120417. [Google Scholar] [CrossRef]
- Xiao, X.; Zhong, H.; Zheng, C.X.; Lu, M.L.; Zuo, X.X.; Nan, J.M. Deep oxidative desulfurization of dibenzothiophene using a flower-like WO3·H2O catalyst in an organic biphasic system. Chem. Eng. J. 2016, 304, 908–916. [Google Scholar] [CrossRef]
- Liu, Y.F.; Wang, F.; Lv, Y.; Yu, S.S.; Wang, R.X.; Jiao, W.Z. Three-dimensional graphene oxide covalently functionalized with Dawson-type polyoxotungstates for oxidative desulfurization of model fuels. Ind. Eng. Chem. Res. 2020, 60, 114–127. [Google Scholar] [CrossRef]
- Xing, X.X.; He, M.Y.; Guo, H.L.; Song, Y.J.; Zhu, W.S.; Pang, J.Y.; Bai, Y.; Dang, D.B. The design of molecularly dispersed tungstovanadate catalyst via ionic liquid linkers for oxidative desulfurization. Sep. Purif. Technol. 2025, 354, 129475. [Google Scholar] [CrossRef]
- Afsharpour, M.; Seifikar Gomi, L.; Elyasi, M. Novel metal-free N-doped bio-graphenes and their MoO3 bifunctional catalysts for ultra-deep oxidative desulfurization of heavy fuel. Sep. Purif. Technol. 2021, 274, 119014. [Google Scholar] [CrossRef]
- Sun, M.; Chen, W.C.; Zhao, L.; Wang, X.L.; Su, Z.M. A PTA@MIL-101(Cr)-diatomite composite as catalyst for efficient oxidative desulfurization. Inorg. Chem. Commun. 2018, 87, 30–35. [Google Scholar] [CrossRef]
- Afsharpour, M.; Kazemi, B. Magnetically recoverable MoO3-based catalyst promoted with W-doped bio-graphene as an effective catalyst in oxidative desulfurization of fuel. J. Mol. Liq. 2023, 380, 121693. [Google Scholar] [CrossRef]
- Ye, G.; Wang, H.; Chen, W.; Chu, H.; Wei, J.; Wang, D.; Wang, J.; Li, Y. In Situ Implanting of Single Tungsten Sites into Defective UiO-66(Zr) by Solvent-Free Route for Efficient Oxidative Desulfurization at Room Temperature. Angew. Chem. Int. Edit. 2021, 133, 20481–20487. [Google Scholar] [CrossRef]
- Astle, M.A.; Rance, G.A.; Loughlin, H.J.; Peters, T.D.; Khlobystov, A.N. Molybdenum Dioxide in Carbon Nanoreactors as a Catalytic Nanosponge for the Efficient Desulfurization of Liquid Fuels. Adv. Funct. Mater. 2019, 29, 1808092. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, X.M.; Yang, X.F.; Jiao, M.G.; Zhou, Z.; Zhang, M.H.; Wang, D.H.; Bu, X.H. Electronic structure of heterojunction MoO2/g-C3N4 catalyst for oxidative desulfurization. Appl. Catal. B Environ. 2018, 238, 263–273. [Google Scholar] [CrossRef]

| Catalysts | n (H2O2): n (S) | Dosage (g) | T (°C) | Initial S Conc. (ppm) | Time (min) | Removal Efficiency (%) | Reaction Rate Constant (k (min−1)) | Refs |
|---|---|---|---|---|---|---|---|---|
| C16-PW9/SiO2 | 3 | 0.01 | 50 | 500 | 100 | 100 | 0.24 | [45] |
| flower-like WO3·H2O | 8 | 0.13 | 70 | 4000 | 60 | 98.7 | 0.05 | [46] |
| [MIMPs]3PMo6W6O40 | 2.5 | 0.05 | 60 | 500 | 40 | 100 | [47] | |
| CNT@C4VW12 | 6 | 0.01 | 60 | 500 | 30 | 100 | - | [48] |
| W2C@C | 3 | 0.04 | 50 | 500 | 40 | 100 | 0.146 | [24] |
| MoO3/NGO | 4 | 0.05 | 60 | 500 | 160 | 95 | - | [49] |
| PTA@MIL-101(Cr) | 5 | 0.05 | 60 | 500 | 120 | 84.1 | - | [50] |
| VO-MoO2@NC | 4 | 0.08 | 70 | 500 | 40 | 100 | ||
| WC/W2C@NC | 2 | 0.05 | 60 | 4000 | 15 | 100 | 0.24 | This work |
| 2 | 0.05 | 60 | 3000 | 10 | 100 | |||
| 2 | 0.05 | 60 | 1000 | 7 | 100 | |||
| 2 | 0.05 | 60 | 5000 | 40 | 99.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, P.; Zhao, F.; Liu, F.; Hei, J.; Lv, G.; Huang, X.; Zhang, J.; Zhang, M.; Liu, Y.; Ma, T. In Situ Formation of WC/W2C Heterostructures on N-Doped Carbon for Deep Oxidative Desulfurization of Fuel Oil. Molecules 2025, 30, 617. https://doi.org/10.3390/molecules30030617
Zuo P, Zhao F, Liu F, Hei J, Lv G, Huang X, Zhang J, Zhang M, Liu Y, Ma T. In Situ Formation of WC/W2C Heterostructures on N-Doped Carbon for Deep Oxidative Desulfurization of Fuel Oil. Molecules. 2025; 30(3):617. https://doi.org/10.3390/molecules30030617
Chicago/Turabian StyleZuo, Peng, Fuyan Zhao, Fanfan Liu, Jinpei Hei, Guozheng Lv, Xianzong Huang, Jun Zhang, Meng Zhang, Yefeng Liu, and Tao Ma. 2025. "In Situ Formation of WC/W2C Heterostructures on N-Doped Carbon for Deep Oxidative Desulfurization of Fuel Oil" Molecules 30, no. 3: 617. https://doi.org/10.3390/molecules30030617
APA StyleZuo, P., Zhao, F., Liu, F., Hei, J., Lv, G., Huang, X., Zhang, J., Zhang, M., Liu, Y., & Ma, T. (2025). In Situ Formation of WC/W2C Heterostructures on N-Doped Carbon for Deep Oxidative Desulfurization of Fuel Oil. Molecules, 30(3), 617. https://doi.org/10.3390/molecules30030617








